Transformation of diphenylarsinic acid and related compounds in groundwater: production of thiol-containing arsenicals
Kunichika Nakamiya A B C , Mitsuha Yoshikane B , Hosoya Tomoko A and Yasuyuki Shibata AA National Institute for Environmental Studies, Center for Environmental Measurement and Analysis, Onogawa 16-2, Tsukuba, Ibaraki 305-8506, Japan.
B Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, 277-8563 Chiba, Japan.
C Corresponding author. Email: nakamiya.kunichika@nies.go.jp
Environmental Chemistry 10(1) 17-21 https://doi.org/10.1071/EN12110
Submitted: 30 July 2012 Accepted: 13 December 2012 Published: 27 February 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Environmental context. Contamination of groundwater by arsenic compounds used in chemical warfare research is a recently discovered environmental problem in Japan. We report evidence that the arsenic compounds originally present in the groundwater are transforming to thio-arsenic compounds of currently unknown environmental fate.
Abstract. During routine analyses of groundwater samples contaminated with diphenylarsinic acid (DPAA) at Kamisu, Ibaraki Prefecture, Japan, we obtained data indicating that unknown arsenic compounds accounted for up to 75 % of the total arsenic in some of the samples. Results from using liquid chromatography in combination with elemental mass spectrometry and tandem molecular mass spectrometry suggested that two of the main unknown peaks were diphenyldithioarsinic acid and methylphenyldithioarsinic acid. These assignments were later confirmed by comparison with chemically synthesised compounds. A potential transformation scheme for DPAA in the environment is proposed based on the derivatives identified in the environmental samples.
In 2003, pollution incidents associated with use of an organoarsenical, diphenylarsinic acid (DPAA, Fig. 1), occurred at three locations in Ibaraki and Kanagawa prefectures, Japan (press release from the Ministry of Environment, Tokyo (in Japanese), http://www.env.go.jp/chemi/gas_inform/, accessed 2012). In Kanagawa Prefecture, DPAA pollution in groundwater was found at two sites where chemical warfare research had been conducted more than half a century ago. In Ibaraki Prefecture, however, DPAA pollution has apparently occurred more recently, and a suspected source of pollution, several large blocks of concrete contaminated with DPAA (see photos at http://www.env.go.jp/chemi/report/h17-11/02.pdf, accessed 16 January 2013), were later found near the polluted area. In Kamisu, Ibaraki Prefecture, people who drank contaminated groundwater suffered toxic effects such as abnormal peristalsis, spasms and motor dysfunction.[1]
|
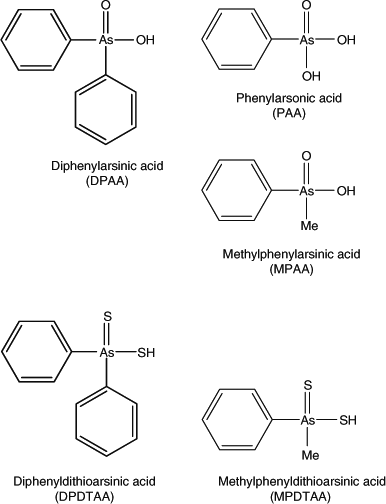
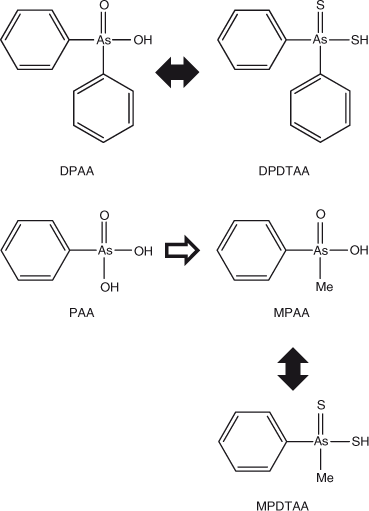
Immediately after recognising the adverse health effects associated with these pollution incidents, the government took action to identify the source of pollution and to monitor and elucidate the processes that were associated with the underground transport of the pollutants. Analyses of human samples (urine, blood) for DPAA were made to identify people who were exposed to this compound, and a toxicological evaluation and toxicokinetic study of DPAA and the related compounds phenylarsonic acid (PAA) and methylphenylarsinic acid (MPAA, Fig. 1) was carried out. Research activities have included the development of analytical techniques,[2] monitoring of the concentrations of DPAA and its transformation products in human urine and groundwater,[3,4] studies of the absorption and metabolism of DPAA by plants,[5,6] studies of the toxicodynamics of DPAA in experimental animals[7,8] and studies aimed at elucidating the mechanism of DPAA’s toxicity.[9]
During recent measurements using liquid chromatography–inductively coupled plasma–mass spectrometry (LC-ICP-MS) of DPAA and related arsenicals in groundwater samples, we detected unknown arsenic species that appeared to be derived from DPAA. We report here the identification of these new arsenic compounds in groundwater samples.
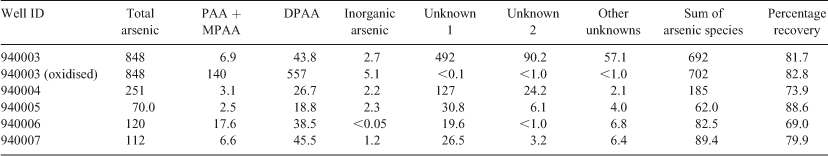
We investigated the well water samples using a combination of inductively coupled plasma–atomic emission spectrometry (ICP-AES), LC-ICP-MS and liquid chromatography–tandem molecular mass spectrometry (LC-MS-MS). Although unknown arsenic species were shown to be present in the water from all five wells tested (Table 1), the concentrations of the two major unknowns were highest in well 940003; consequently, this well was chosen for further investigation.
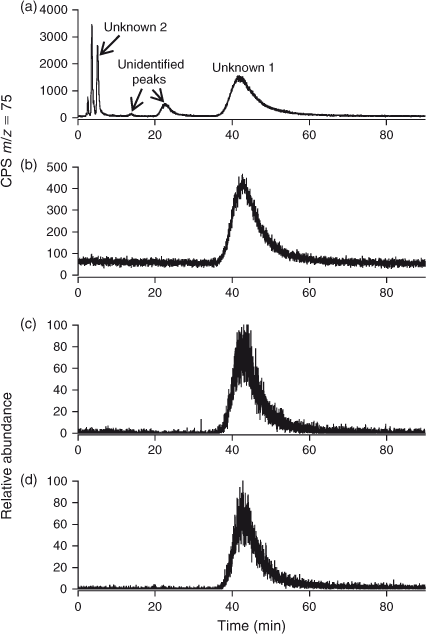
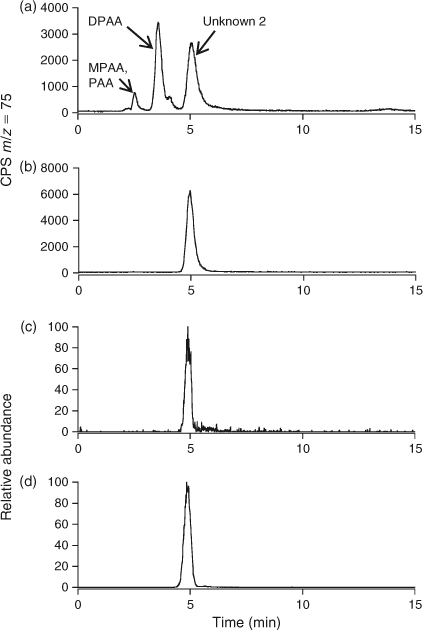
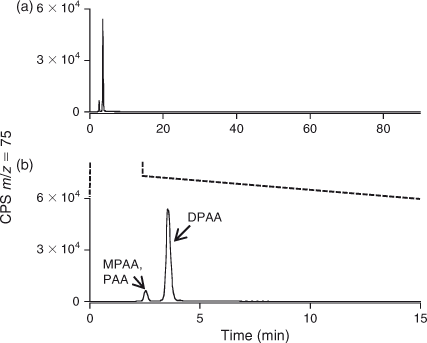
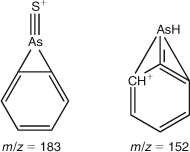
Preliminary analysis indicated the presence of two arsenic-containing unknowns with molecular masses of 294 and 232 (m/z 295 and 233 for the [M + H]+ ion), which appeared to slowly convert into DPAA and MPAA respectively. For example, when the sample was allowed to naturally oxidise (4 months at 4 °C), and was then re-analysed by LC-ICP-MS, the unknown peaks disappeared with a large increase in the size of the peaks for DPAA and MPAA–PAA (these last two arsenicals are not separated under our LC conditions) Fig. 2. These observations suggested the presence of thio-arsenicals where the oxygen atom on arsenic had been substituted with a sulfur atom. Thus, we synthesised the thio derivatives of DPAA and MPAA, namely diphenyldithioarsinic acid (DPDTAA) and methylphenyldithioarsinic acid (MPDTAA), and confirmed their presence in well water by matching LC-ICP-MS and LC-MS-MS data (Figs 3, 4). For LC-MS-MS analyses, we used selected reaction monitoring mode by choosing the protonated molecular ions at m/z 295 (DPDTAA) or m/z 233 (MPDTAA), fragmenting and detecting the common, and most intense, product ion at m/z 183. A second significant common product ion had m/z 152; possible structures are shown in Fig. 5.
|
|
|
|
There were several other minor unidentified peaks in the samples (Fig. 3a). These peaks also disappeared when the sample was allowed to naturally oxidise, suggesting that they might also be thio-arsenicals (Fig. 2). Possibly, they are mono-thio derivatives of DPAA and related compounds, but the small quantities of these compounds precluded their identification by LC-MS-MS (Table 1).
Although the methylation of arsenic leading to natural alkylarsenic compounds has been thoroughly studied,[10] there have been few methylation studies of man-made arylarsenic compounds. The microbial methylation of PAA was first reported by Cullen et al.[11] and we have observed the methylation of DPAA by isolated microorganisms.[12] Recently, the thiolation of inorganic arsenic and alkyl arsenic compounds has also been reported.[13,14] There are no published data and only limited published information about the thiolation of arylarsenic compounds. Preliminary studies had indicated that there were unknown arylarsenic compounds in soil contaminated with DPAA (T. Kaise, unpubl. data, 2004), and thiolation of DPAA and related compounds has been suggested in field soil (K. Baba, unpubl. data, 2009) and in Kanagawa Prefecture well water (K. Nakamiya and Y. Shibata, unpubl. data, 2009). Here we show that two previously unknown arylarsenic compounds in groundwater were DPDTAA and MPDTAA.
The discovery that DPAA and related compounds in groundwater can be transformed into thio derivatives is relevant to determining the fate of arsenicals in the environment, and the consequent health effects of these compounds. The origin of the sulfur in DPDTAA and MPDTAA in groundwater has also not yet been clarified.[13] Usually H2S derived from microorganisms and volcanic gas is responsible for the thiolation of arsenic compounds.[13] Originally, DPAA and PAA were deposited with garbage in landfills at Kamisu. Thus, hydrogen sulfide derived from fermentation reactions mediated by microorganisms in the garbage may be promoting the thiolation of DPAA and PAA; suggested biosynthesis schemes of DPDTAA and MPDTAA are shown in Fig. 6.
|
In summary, we identified the novel environmental contaminants DPDTAA and MPDTAA in groundwater at Kamisu, Ibaraki, Japan, and propose pathways for their formation in the environment.
Experimental details
Water samples were drawn from five wells in Kamisu at depths ranging from 10–30 m using a hand pump. Samples were filtered (0.2-µm Minisart membrane; Sartorius AG, Goettingen, Germany) within 2 days and stored at 4 °C until analysis, which took place within 3 days. To investigate the oxidised forms of the unknown species, one sample from well 940003 was stored at 4 °C for 4 months without filtration whereupon it underwent natural oxidation by dissolved oxygen; it was then filtered and analysed by LC-ICP-MS.
Total arsenic measurements were made by ICP-AES with a 61Etrace (Thermo Jarrell Ash Corp., Franklin, MA, USA). LC-ICP-MS measurements were made with an Agilent 1100 LC (Agilent Technologies Inc., Tokyo, Japan) that was coupled to an Agilent 7500c ICP-MS operated in the collision cell mode (He, 3.3 mL min–1). LC-MS-MS measurements were performed with an Agilent 1200 LC system coupled to an Applied Biosystems 4000-Qtrap (AB SCIEX, Foster City, CA, USA) operated in the positive-ion electrospray ionisation (ESI) mode with a spray voltage of 5.5 kV, sheath gas of 552 kPa, auxiliary gas of 276 kPa and heated capillary temperature of 500 °C. The declustering potential and collision energy were respectively set at 60 and 35 V. LC separations were performed on a gel permeation column as detailed in the legends to Figs 2–4. High-resolution mass spectra were recorded on an Agilent 6224 Accurate-Mass TOF-MS; for characterisation of standards, crystals of the potassium salts of DPDTAA and MPDTAA were dissolved in water and directly injected into the mass spectrometer. NMR spectra were recorded on a JEOL ECA 800 spectrometer (JEOL, Tokyo, Japan); chemical shifts are reported in parts per million relative to a trimethylsilane standard.
DPDTAA and MPDTAA were respectively prepared[15] from DPAA and MPAA by treating the compounds with H2S gas generated from MgCl2 and NaHS.[16] In brief, we added 1 g of DPAA, 0.22 g of potassium hydroxide, and 12 mL of ethanol to a vial (40 × 130 mm, Nichiden-rika Glass Co., Ltd, Hyogo, Japan) with a septum cap (PTFE/silicone, 20-mm septa, Supelco, Bellefonte, PA, USA) and an aluminium seal. To remove air, we applied a vacuum to the headspace until the ethanol began to boil, and then injected ~200 mL of H2S (atmospheric pressure) into each vial through a needle while the vial was warmed with gentle stirring at 60 °C in a water bath. The vial was further stirred for 3 h at 60 °C with further occasional injection of H2S. The ethanol was evaporated, the residue was dissolved in a small amount of methanol and diethyl ether was added whereupon white crystals separated, which were dried over phosphorus pentoxide. The yield of DPDTAA was 0.67 g (53 %) with a melting point of 238–243 °C. 1H NMR δH (800 MHz, D2O) 7.37 (t, 4H), 7.42 (t, 2H), 7.76 (d, 4H); 13C NMR δC (200 MHz, D2O) 129.0 (m-), 129.3 (o-), 130.9 (p-), 141.8 (C–As). High-resolution mass spectrometry: m/z [M + H]+: found 294.9597; calculated for C12H10AsS2 294.9596. We prepared MPDTAA in the same manner as DPDTAA, but with a 70 : 30 mixture of ethanol : water as solvent. The yield was 0.52 g (45 %) with a melting point of 227 °C. 1H NMR δH (800 MHz, CD3OD) 1.98 (s, 3H), 7.37 (t, 1H), 7.42 (t, 2H), 8.05 (d, 2H); 13C NMR δC (200 MHz, CD3OD) 30.4 (methyl), 127.9 (m-), 129.0 (o-), 129.6 (p-), 144.7 (C–As). High-resolution mass spectrometry: m/z [M + H]+: found 232.9435; calculated for C7H8AsS2 232.9439.
Acknowledgements
This study was conducted as part of the monitoring and research activities of the Ministry of the Environment, Japan. The authors thank Dr Naoto Shimizu (Agilent Technologies Inc.) for his help with the analysis of the unknown arsenic compounds using high resolution mass spectrometry.
References
[1] K. Ishii, A. Tamaoka, F. Otsuka, N. Iwasaki, K. Shin, A. Matsui, G. Endo, Y. Kumagai, T. Ishii, S. Shoji, T. Ogata, M. Ishizaki, M. Doi, N. Shimojo, Diphenylarsinic acid poisoning from chemical weapons in Kamisu, Japan Ann. Neurol. 2004, 56, 741.| Diphenylarsinic acid poisoning from chemical weapons in Kamisu, JapanCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVCgsLbJ&md5=839530813f5a5222d48b7c01e1d5e7aeCAS |
[2] K. Nakamiya, Y. Shibata, H. Ito, J. S. Edmonds, M. Morita, Synthesis of phenyl arsenic analytical standards related to contaminated well water in Kamisu, Ibaraki, Japan Appl. Organomet. Chem. 2005, 19, 282.
| Synthesis of phenyl arsenic analytical standards related to contaminated well water in Kamisu, Ibaraki, JapanCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWguro%3D&md5=0f1b7a656055877dde3dd1ec50d9bae2CAS |
[3] Y. Shibata, K. Tsuzuku, S. Komori, C. Umedzu, H. Imai, M. Morita, Analysis of diphenylarsinic acid in human and environmental samples by HPLC-ICP-MS Appl. Organomet. Chem. 2005, 19, 276.
| Analysis of diphenylarsinic acid in human and environmental samples by HPLC-ICP-MSCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWgur0%3D&md5=a268199325db8c83f393ea6073194103CAS |
[4] K. Kinoshita, Y. Shida, C. Sakuma, M. Ishizaki, K. Kiso, O. Shikino, H. Ito, M. Morita, T. Ochi, T. Kaise, Determination of diphenylarsinic acid and phenylarsonic acid, the degradation products of organoarsenic chemical warfare agents, in well water by HPLC-ICP-MS Appl. Organomet. Chem. 2005, 19, 287.
| Determination of diphenylarsinic acid and phenylarsonic acid, the degradation products of organoarsenic chemical warfare agents, in well water by HPLC-ICP-MSCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVWgurs%3D&md5=12ca714a7feb37ffe81e22bacca8ee22CAS |
[5] T. Arao, Y. Maejima, K. Baba, Uptake of aromatic arsenicals from soil contaminated with diphenylarsinic acid by rice Environ. Sci. Technol. 2009, 43, 1097.
| Uptake of aromatic arsenicals from soil contaminated with diphenylarsinic acid by riceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotlKguw%3D%3D&md5=916dfc0892bf6c082977ed809c4f7f24CAS |
[6] S. Ueno, T. Kitamura, M. Nakamura, K. Ozone, Y. Shibata, M. Ishizaki, Determination of diphenylarsinic acid in plants and biological samples by HPLC/MS/MS using a stable isotope labeled compound Bunseki Kagaku 2006, 55, 41.
| Determination of diphenylarsinic acid in plants and biological samples by HPLC/MS/MS using a stable isotope labeled compoundCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvValug%3D%3D&md5=1f78a555931677f67d773c08a72060eeCAS |
[7] T. Ochi, K. Kinoshita, T. Suzuki, K. Miyazaki, A. Noguchi, T. Kaise, The role of glutathione on the cytotoxic effects and cellular uptake of diphenylarsinic acid, a degradation product of chemical warfare agents Arch. Toxicol. 2006, 80, 486.
| The role of glutathione on the cytotoxic effects and cellular uptake of diphenylarsinic acid, a degradation product of chemical warfare agentsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmtlaisrs%3D&md5=5a08ca5b192880b1aa31e20ae5e20342CAS |
[8] K. Kita, T. Ochi, Down-regulation of glutaminase C in human hepatocarcinoma cell by diphenylarsinic acid, a degradation product of chemical warfare agents Toxicol. Appl. Pharmacol. 2007, 220, 262.
| Down-regulation of glutaminase C in human hepatocarcinoma cell by diphenylarsinic acid, a degradation product of chemical warfare agentsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkslersbs%3D&md5=f6699c0ae0422642e8c608b66280fa29CAS |
[9] Y. Kobayashi, T. Negishi, A. Mizumura, T. Watanabe, S. Hirano, Distribution and excretion of arsenic in cynomolgus monkey following repeated administration of diphenylarsinic acid Arch. Toxicol. 2008, 82, 553.
| Distribution and excretion of arsenic in cynomolgus monkey following repeated administration of diphenylarsinic acidCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpt12rs7c%3D&md5=038269eae3162abad1fd3ab75e911f7cCAS |
[10] R. Bentley, T. G. Chasteen, Microbial methylation of metalloids: arsenic, antimony, and bismuth Microbiol. Mol. Biol. Rev. 2002, 66, 250.
| Microbial methylation of metalloids: arsenic, antimony, and bismuthCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltFSltrs%3D&md5=55502b93319fece027938872dc1784fbCAS |
[11] W. R. Cullen, A. E. Erdman, B. C. McBride, A. W. Pickett, The identification of dimethylphenylarsine as a microbial metabolite using a simple method of chemofocusing J. Microbiol. Methods 1983, 1, 297.
| The identification of dimethylphenylarsine as a microbial metabolite using a simple method of chemofocusingCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtFOnsLs%3D&md5=11f0245e896debea408e74d9f6ea6559CAS |
[12] K. Nakamiya, T. Nakayama, H. Ito, J. S. Edmonds, Y. Shibata, M. Morita, Degradation of arylarsenic compounds by microorganisms FEMS Micro. Boil. Lett. 2007, 274, 184.
| Degradation of arylarsenic compounds by microorganismsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVaisLnF&md5=7509f9cb5007b85bfdb09cc8d465f6ecCAS |
[13] D. Wallschläger, J. London, Determination of methylated arsenic–sulfur compounds in groundwater Environ. Sci. Technol. 2008, 42, 228.
| Determination of methylated arsenic–sulfur compounds in groundwaterCrossref | GoogleScholarGoogle Scholar |
[14] R. A. Diaz-Bone, T. Van de Wiele, Biotransformation of metal(loid)s by intestinal microorganisms Pure Appl. Chem. 2010, 82, 409.
| Biotransformation of metal(loid)s by intestinal microorganismsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVShsLc%3D&md5=f40b0e9be5598bf6af0c479d2d57ddc1CAS |
[15] A. Müller, P. Werle, Darstellung, Elektronen- und Schwingungsspektren von Kaliumdiphenyldithioarsinat und Phenyldithioarsinato-komplexen Chem. Ber. 1971, 104, 3782.
| Darstellung, Elektronen- und Schwingungsspektren von Kaliumdiphenyldithioarsinat und Phenyldithioarsinato-komplexenCrossref | GoogleScholarGoogle Scholar |
[16] G. Brauer, Hydrogen sulfide, in Handbook of Preparative Inorganic Chemistry 1963, pp. 344–346 (Academic Press Inc. Ltd: London).
[17] J. S. Edmonds, T. Nakayama, T. Kondo, M. Morita, Diasteoisomerism of thiol complexes of arsenic acids and pseudoasymmetry of arsenic: a 1H and 13C NMR study Magn. Reson. Chem. 2006, 44, 151.
| Diasteoisomerism of thiol complexes of arsenic acids and pseudoasymmetry of arsenic: a 1H and 13C NMR studyCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht12rtrs%3D&md5=6481e5f0e53e99cf70b5d290d7b02141CAS |