Relationships among felt scale insects (Hemiptera : Coccoidea : Eriococcidae) of southern beech, Nothofagus (Nothofagaceae), with the first descriptions of Australian species of the Nothofagus-feeding genus Madarococcus Hoy
Nate B. Hardy A E , Penny J. Gullan A , Rosa C. Henderson B and Lyn G. Cook C DA Department of Entomology, University of California, One Shields Avenue, Davis, California 95616-8584, USA.
B Landcare Research, Private Bag 92170, Auckland, New Zealand.
C School of Botany and Zoology, The Australian National University, Canberra, ACT 0200, Australia.
D Present address: School of Integrative Biology, The University of Queensland, Brisbane 4072, Australia.
E Corresponding author. Email: nbhardy@gmail.com
Invertebrate Systematics 22(3) 365-405 https://doi.org/10.1071/IS07032
Submitted: 11 July 2007 Accepted: 28 February 2008 Published: 18 June 2008
Abstract
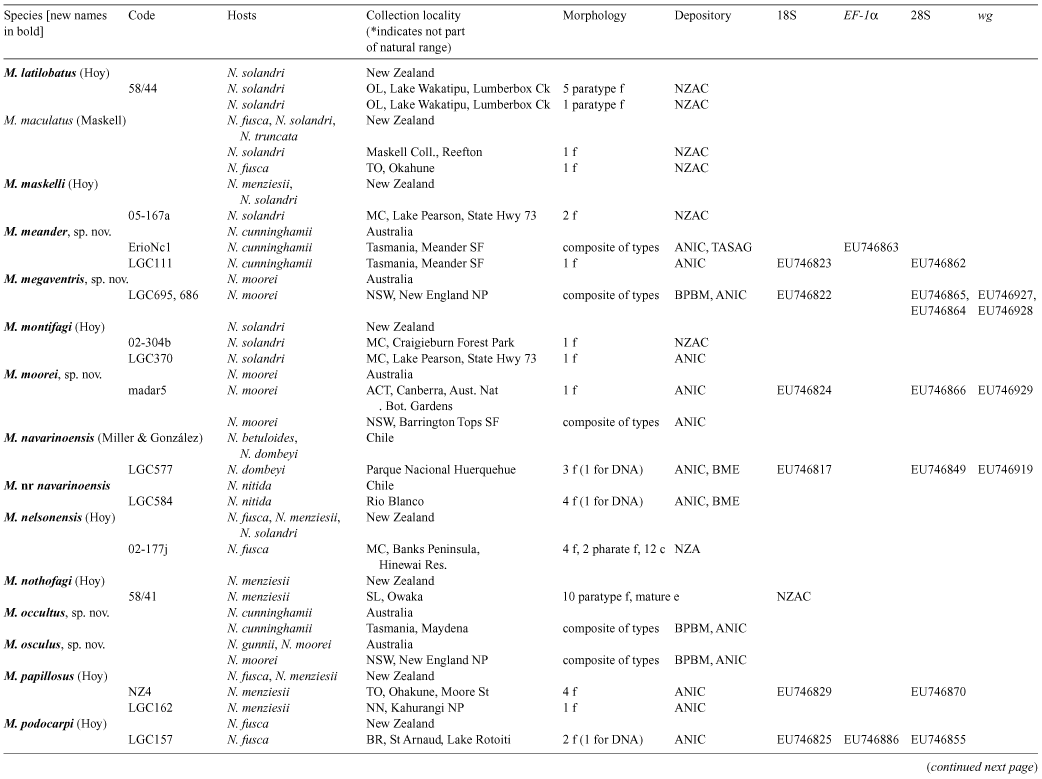
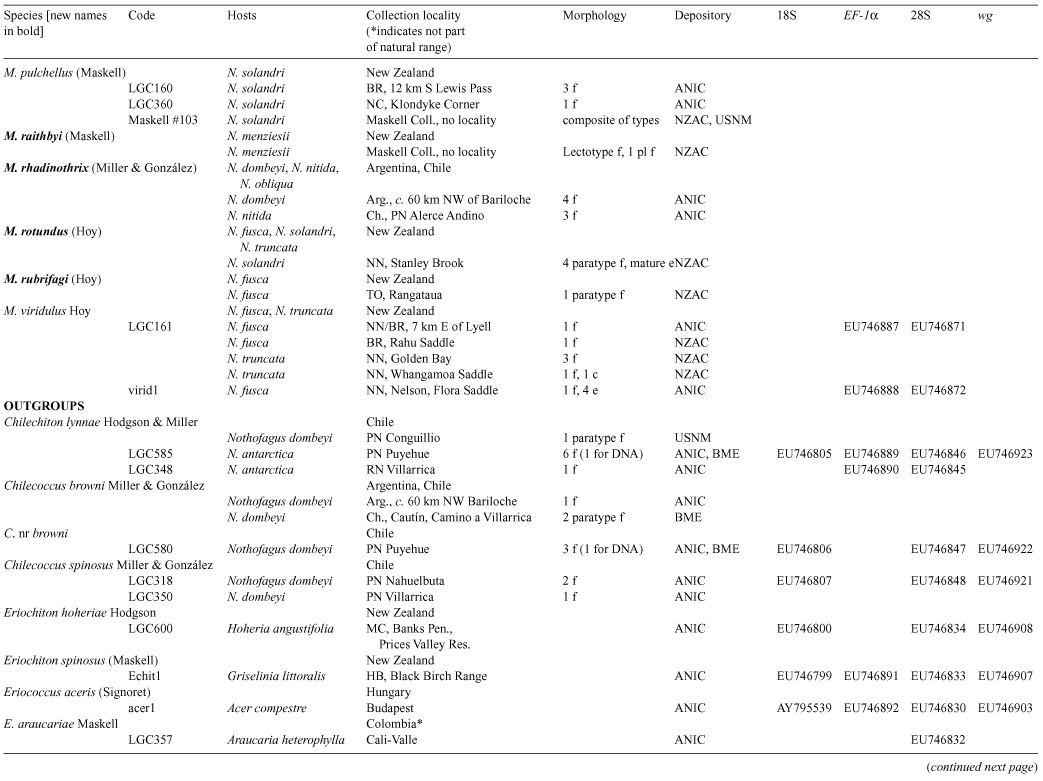
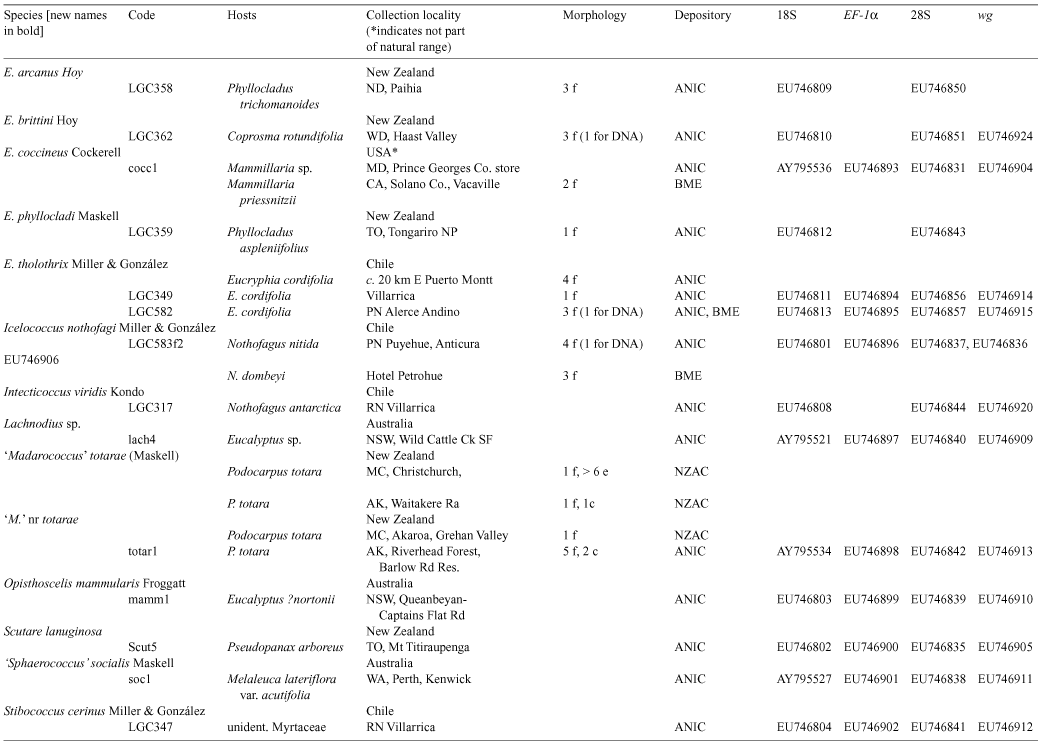
Species of southern beech (Nothofagus) have been studied extensively because of their importance in understanding southern hemisphere biogeography. Nothofagus species support a diverse assemblage of insect herbivores, including more than 30 described species of felt scales (Eriococcidae). We reconstructed the phylogeny of the Nothofagus-feeding felt scales with nucleotide sequence data and morphology. All but one of the exclusively Nothofagus-feeding species included in the analyses were recovered as a monophyletic group. This clade comprised the genera Chilechiton Hodgson & Miller, Chilecoccus Miller & González, Intecticoccus Kondo, Madarococcus Hoy (except for M. totorae Hoy), Sisyrococcus Hoy and several species of the genus Eriococcus Targioni Tozzetti. The genera Eriococcus and Madarococcus were not recovered as monophyletic. Here we revise Madarococcus. We expand the concept of the genus, provide a key to the adult females of the 31 species of Madarococcus and, for each named species, provide revised synonymies and any new collection or taxonomic information. We recognise the genus from Australia for the first time and describe the adult females of six new Australian species: Madarococcus cunninghamii Hardy & Gullan, sp. nov.; M. meander Hardy & Gullan, sp. nov.; M. megaventris Hardy & Gullan, sp. nov.; M. moorei Hardy & Gullan, sp. nov.; M. occultus Hardy & Gullan, sp. nov., and M. osculus Hardy & Gullan, sp. nov. We also describe the first-instar nymphs of M. cunninghamii, sp. nov., M. meander, sp. nov. and M. moorei, sp. nov. We transfer 17 species into Madarococcus from Eriococcus: M. argentifagi (Hoy), comb. nov.; M. cavellii (Maskell), comb. nov.; M. chilensis (Miller & González), comb. nov.; M. detectus (Hoy), comb. nov.; M. eurythrix (Miller & González), comb. nov.; M. fagicorticis (Maskell), comb. nov.; M. hispidus (Hoy), comb. nov.; M. latilobatus (Hoy), comb. nov.; M. maskelli, (Hoy), comb. nov.; M. montifagi (Hoy), comb. nov.; M. navarinoensis (Miller & González), comb. nov.; M. nelsonensis (Hoy), comb. nov.; M. nothofagi (Hoy), comb. nov.; M. podocarpi (Hoy), comb. nov.; M. raithbyi (Maskell), comb. nov.; M. rotundus (Hoy), comb. nov. and M. rubrifagi (Hoy), comb. nov. We transfer two species from Sisyrococcus into Madarococcus: M. intermedius (Maskell), comb. nov. and M. papillosus (Hoy), comb. nov. One species, M. totarae (Maskell), is excluded from Madarococcus, but cannot at present be placed in another genus and is listed as ‘M.’ totarae incertae sedis. We report the first collection of an eriococcid, M. osculus, sp. nov., on the deciduous beech, Nothofagus gunnii. With respect to biogeography, the results of our phylogenetic analysis are congruent with those obtained from recent analysis of Nothofagus; Australian and New Zealand species of Madarococcus form a monophyletic group to the exclusion of the South American species, suggesting that long-distance dispersal has played an important role in shaping the distributions of both the Nothofagus-feeding felt scales and their hosts.
Acknowledgements
We gratefully acknowledge the contributions to Australian entomology made by the late Jack Beardsley, who collected specimens of each of the six Australian species described in this paper. We thank Cathy Young (Department of Primary Industries and Water, Tasmania) and Jane Keble-Williams (University of Tasmania) for the loan of Tasmanian eriococcids from Nothofagus. Dug Miller kindly provided information on the USNM holdings of Maskell specimens and arranged the loan of some type specimens. Jon Martin arranged the loan of and provided some information on BMNH specimens of Madarococcus. David Morris kindly provided sequence data for one collection. Many people assisted this research by collecting specimens. We especially thank Nicholas Martin who provided many eriococcid specimens to NZAC, Mike Crisp and David Morris for collecting galls on Nothofagus in Australia, and Demian Kondo for collecting the Chilean specimens used for the DNA work. We acknowledge the Chilean National Forests Corporation (CONAF) for collecting permits (held by PJG in 1996, E. Arias in 2003, T. Kondo in 2006). The Australian National Botanic Gardens (ANBG), Canberra, allowed PJG to collect scale insects and Stuart Donaldson (ANBG) provided assistance. Peter Cranston accompanied PJG on many field trips to collect Nothofagus-feeding eriococcids and Robert Hoare assisted PJG with fieldwork in New Zealand. Graeme Henderson accompanied RCH on field trips to collect scale insects in New Zealand. The following agencies/departments also provided collecting permits to PJG, LGC, RCH and their collaborators: the former Department of Parks, Wildlife and Heritage, and the current Department of Primary Industries and Water, Tasmania; New South Wales National Parks and Wildlife Service; State Forests of New South Wales; and the Department of Conservation, New Zealand. This work was supported by an Australian Biological Resources Study grant (1995–1998) to PJG; a National Science Foundation PEET grant (DEB-0118718) and by Hatch funding from the California Agricultural Experiment Station to PJG; the Foundation for Research, Science and Technology, New Zealand, under contract CO9X0501 to RCH; and an ARC Discovery Project grant to LGC.
Belshaw R., Quicke D. L. J.
(1997) A molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae). Molecular Phylogenetics and Evolution 7, 281–293.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
[Verified 25 June 2007.]
Brower A. V. Z., DeSalle R.
(1998) Patterns of mitochondrial versus nuclear DNA sequence divergence among nymphalid butterflies: the utility of wingless as a source of characters for phylogenetic inference. Insect Molecular Biology 7, 73–82.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
[Verified 6 July 2007]
Miller D. R., González R. H.
(1975) A taxonomic analysis of the Eriococcidae of Chile. Revista Chilena de Entomologia 9, 131–163.

Miller D. R., McKenzie H. L.
(1967) A systematic study of Ovaticoccus Kloet and its relatives, with a key to North American genera of Eriococcidae (Homoptera: Coccoidea: Eriococcidae). Hilgardia 38, 471–539.

Posada D., Crandall K. A.
(1998) Modeltest: testing the model of DNA substitution. Bioinformatics (Oxford England) 14, 817–818.
| Crossref | GoogleScholarGoogle Scholar |

Ronquist F., Huelsenbeck J. P.
(2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

SanMartín I., Ronquist F.
(2004) Southern Hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology 53, 216–243.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Springer M. S.,
Teeling E. C.,
Madsen O.,
Stanhope M. J., de Jong W. W.
(2001) Integrated fossil and molecular data reconstruct bat echolocation. Proceedings of the National Academy of Sciences of the United States of America 98, 6241–6246.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sunnucks P., Hales D. F.
(1996) Numerous Transposed sequences of mitochondrial chytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae). Molecular Biology and Evolution 17, 510–524..

Tautz D.,
Hancock J. M.,
Webb D. A.,
Tautz C., Dover G. A.
(1988) Complete sequences of the rRNA genes of Drosophila melanogaster. Molecular Biology and Evolution 5, 366–376.
| PubMed |

von Dohlen C. D., Moran N. A.
(1995) Molecular phylogeny of the Homoptera: a paraphyletic taxon. Journal of Molecular Evolution 41, 211–223.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wiens J. J.
(2003) Missing data, incomplete taxa, and phylogenetic accuracy Systematic Biology 52, 528–538.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Williams D. J.
(1985a) Some South American scale insects (Homoptera: Coccoidea) on Nothofagus. Journal of Natural History 19, 249–258.
| Crossref | GoogleScholarGoogle Scholar |

Williams D. J.
(1985b) The British and some other European Eriococcidae (Homoptera: Coccoidea). Bulletin of the British Museum Natural History [Entomology] 51, 347–393.

Williams D. J.
(2007) Scale insects of the families Asterolecaniidae and Eriococcidae (Hemiptera: Coccoidea) in New Caledonia. Journal of Natural History 41, 1343–1366.
| Crossref | GoogleScholarGoogle Scholar |

|
|
|