319 OXYGEN CONSUMPTION OF BOVINE CUMULUS CELLS AND OOCYTES CULTURED IN DIFFERENT CULTURE SYSTEMS FOR OOCYTE MATURATION
H. Abe A , H. Shiku B , S. Aoyagi C , T. Matsue B and H. Hoshi DA Tohoku University Biomedical Engineering Research Organization, Sendai, Japan
B Graduate School of Environmental Studies, Tohoku University, Sendai, Japan
C Hokuto Denko Corporation, Tokyo, Japan
D Research Institute for the Functional Peptides, Yamagata, Japan
Reproduction, Fertility and Development 18(2) 267-267 https://doi.org/10.1071/RDv18n2Ab319
Published: 14 December 2005
Abstract
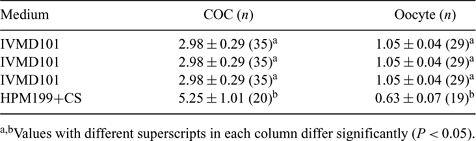
Oxygen consumption is a ubiquitous parameter that can provide valuable information on metabolic mechanisms and on oocyte and embryo quality. Recently, we succeeded in non-invasively and quantitatively determining oxygen consumption of individual bovine embryos by scanning electrochemical microscopy (SECM). The aim of this study was to assess by SECM the oxygen consumption of bovine cumulus cells and oocytes cultured in serum-free and serum-supplemented media for oocyte maturation. Bovine cumulus–oocyte complexes (COCs) were obtained from ovarian follicles 2–6 mm in diameter. COCs were cultured in IVMD101 medium for serum-free culture and HPM199 medium supplemented with 5% calf serum (HPM199+CS) for serum-supplemented culture in a humidified atmosphere of 5% CO2 in air (20% O2) at 38.5°C for 24 h. Oxygen consumption by single COCs was non-invasively quantified by a SECM measuring system (Abe et al. 2004 J. Mamm. Ova Res. 21, 22). After the measurements, COCs were treated with 0.5% pronase to completely remove the cumulus cells. The oxygen consumption of single denuded oocyte was measured by SECM. Some COCs and oocytes were prepared for transmission electron microscopy. Oxygen consumption has been monitored in COCs and oocytes cultured in IVMD101 and HPM199+CS media for oocyte maturation (Table 1). Oxygen consumption rates of the immature COCs and denuded oocytes (immediately upon recovery from ovary: control) were 6.91 and 0.70 (×10−14 mol s−1), respectively. In serum-free culture (IVMD101), an increase in oxygen consumption rate was found in oocytes, whereas the oxygen consumption of COCs decreased during oocyte maturation. On the other hand, the oxygen consumption of COCs and oocytes cultured in serum-supplemented medium (HPM199+CS) were not change compared with that of controls. Electron microscopic study demonstrated that the mitochondria moved from a peripheral location in the ooplasm to an even spatial distribution in the oocytes cultured in IVMD101 medium, whereas many of the mitochondria in oocytes cultured in HPM199+CS were distributed in the peripheral region of the ooplasm after oocyte maturation. These results suggest that the respiration activity of bovine cumulus cells and oocytes changed during oocyte maturation, and the respiration activity and ultrastructural features of oocytes may affect the culture conditions. The SECM procedures may provide valuable information on oocyte quality and culture conditions for oocyte maturation.
|


