24 DEVELOPMENT OF BOVINE CLONED EMBRYOS PRODUCED BY NUCLEAR TRANSFER OF EMBRYONIC CULTURED CELLS ISOLATED FROM SOMATIC CELL NUCLEAR TRANSFER BLASTOCYSTS
X. J. Bai A B , J. L. Yu B , M. Murakami B , Y. Zhang A and Y. J. Dong BA Institute of Bioengineering, Northwest Agriculture and Forestry University, Yangling, Shanxi, China;
B Center of Animal Embryo Engineering, Qingdao Agricultural University, Qingdao, Shandong, China
Reproduction, Fertility and Development 20(1) 92-92 https://doi.org/10.1071/RDv20n1Ab24
Published: 12 December 2007
Abstract
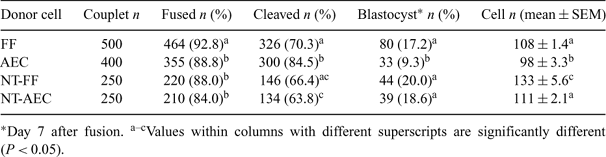
Embryonic stem (ES) cells derived from somatic cell nuclear transfer (NT) bovine embryos would increase the utility of the cow as a large animal model for human cell therapy. They would also be useful for studies of cell differentiation. Such cells exhibit full pluripotency, and cloned offspring were obtained from them following a second NT in mice, indicating that the reprogramming that produced pluripotent ES cells could be reversed (Wakayama et al. 2001 Science 292, 740–743). The objective of this study was to examine if there would be any beneficial effects of using somatic cell NT-derived embryonic cultured cells as donors for cloning in cattle. Cloned embryos were produced from a single cell line of bovine fetal fibroblasts (FF) and adult ear-tip cells (AEC) (passages 1 to 5) by NT, as described previously (Dong et al. 2004 Asian–Aust. J. Anim. Sci. 17, 168–173). NT embryos that reached the blastocyst stage were cultured separately to isolate embryonic cultured cells derived from FF (NT-FF) and AEC (NT-AEC) according to previous methods (Dong et al. 2003 Acta Genet. Sin. 30, 114–118). More than 80% of the generated embryonic cultured cells stained positive for alkaline phosphatase. Embryonic cells cultured for 7 to 35 days were used as the donor cells for NT in the NT-FF and NT-AEC groups. Cloned embryos were produced using individual cell lines of FF, AEC, NT-FF, and NT-AEC (passages 1 to 5, putative cell cycle stage of G0 or G1) as donor cells, and their development in vitro is summarized in Table 1. The FF and AEC groups include data from the initial round of NT. The rates of fusion and embryo development were compared by chi-square analysis. Duncan's multiple range test was used to compare the mean cell numbers of blastocysts. The percentage of embryos that developed into blastocysts was significantly higher (P < 0.05) in the FF group than in the AEC group. Interestingly, we observed that the developmental potential in vitro and the mean cell number of blastocysts tended to be higher in the NT-FF and NT-AEC groups than in the FF and AEC groups. A total of 15 and 6 good quality Day 7 embryos in the NT-FF and NT-AEC groups were nonsurgically transferred to 5 and 3 synchronized recipients (2 to 3 embryos/female), respectively. On Day 30 of gestation, 3 (60%) and 1 (33%) females in the NT-FF and NT-AEC groups, respectively, were diagnosed as pregnant via ultrasonography. One (20%) recipient cow in the NT-FF group remained pregnant at Day 60 of gestation, but lost the pregnancy by Day 90. These results suggest that cloning of bovine embryonic cultured cells generated from fetal and adult somatic cells by NT can produce transferable embryos and initiate pregnancies, although none of the pregnancies has developed beyond the first trimester at this time.
|


