215 AN IMPROVED SYSTEM FOR THE IN VITRO PRODUCTION OF PORCINE EMBRYOS
J. M. Kelly A , A. Weaver B , D. O. Kleemann A , L. M. Frazer B , K. L. Kind B , W. H. van Wettere B and S. K. Walker CA South Australian Research and Development Institute, Turretfield Research Centre, Rosedale, SA, Australia;
B School of Animal and Veterinary Sciences, University of Adelaide, Roseworthy, SA, Australia;
C Embryo Technologies Pty Ltd., Adelaide, SA, Australia
Reproduction, Fertility and Development 23(1) 206-207 https://doi.org/10.1071/RDv23n1Ab215
Published: 7 December 2010
Abstract
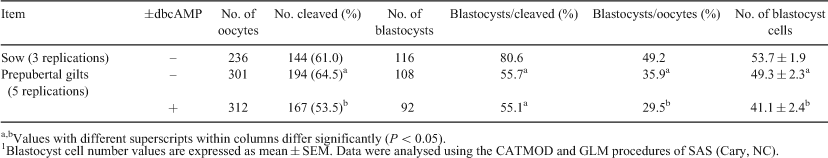
A reliable in vitro system for the production of porcine embryos (IVP) is important for use in basic research and in the reproductive technologies. Despite recent developments, blastocyst rates remain low compared with those obtained for most domestic species. Here, we report a porcine IVP system, based largely on our ovine and bovine systems (Walker et al. 1996 Biol. Reprod. 55, 703–708; Kelly et al. 2007 Reprod. Dom. Anim. 42, 577–582), that gives blastocyst development rates higher than previously reported. Abattoir-sourced ovaries were collected into PBS, and cumulus–oocyte complexes (COC) were aspirated (from 3- to 6-mm follicles) into HEPES–TCM-199 supplemented with 0.4% BSA. The COC (up to 30/well) were matured in 600 μL of maturation medium (TCM-199, 20% (vol/vol) porcine follicular fluid, FSH, LH, epidermal growth factor, cysteamine, and estradiol) for 42 h at 38.6°C in humidified 5% CO2. After 42 h, excess cumulus cells were removed and the COC were placed into modified Tris medium supplemented with BSA and caffeine (500 μL well–1). Sperm from pooled semen were washed in HEPES SOF supplemented with BSA, caffeine, and heparin and incubated for 45 min. Sperm were then centrifuged and the pellet was resuspended; a concentration of 5 × 105 sperm mL–1 was used. Oocytes and sperm were co-cultured for 6 h, cumulus cells were removed, and presumptive zygotes were placed into 600 μL of culture medium (SOF, BSA, and amino acids). Zygotes were cultured in 5% CO2:5% O2:90% N2, and cleavage and embryo development to Day 6 were assessed. Separate studies were conducted with COC from mature sows and from prepubertal gilts. In the latter, COC were exposed to ±dbcAMP for the first 22 h of the maturation period. Blastocyst rates in the sow were similar to those routinely produced in sheep and cows in our laboratory. Cleavage rates, mean blastocyst cell numbers, and incidence of polyspermy (assessed to be 10 to 12% in prepubertal gilts) were improvements on those generally reported (see Nagai et al. 2006 Front. Biosci. 11, 2565–2573 and Gil et al. 2010 Reprod. Dom. Anim. 45, 40–48 for general reviews). Observations indicate that the use of dbcAMP to regulate oocyte maturation had a negative effect on most parameters. Although blastocyst cell numbers are indicative of blastocyst quality, the significance of these findings can be validated only by transfer studies to determine embryo viability.
|
This work was partly supported by the Australian Cooperative Research Centre for an Internationally Competitive Pork Industry.


