Distribution, population structure, and management of a rare sandalwood (Santalum yasi, Santalaceae) in Fiji and Tonga
Ryan D. Huish A E , Tevita Faka’osi B , Heimuli Likiafu B , Joseva Mateboto C and Katherine H. Huish DA Biology Department, Hollins University, PO Box 9615, Roanoke, VA 24020, USA. Present address: Department of Natural Sciences, The University of Virginia's College at Wise, 1 College Avenue Wise, VA 24293, USA.
B Tongan Ministry of Agriculture and Food, Fisheries and Forests, PO Box 14, Nuku’alofa, Tonga.
C Department of Forestry, Government of Fiji, PO Box 2218, Suva, Fiji.
D c/o R. D. Huish, Department of Natural Sciences, The University of Virginia's College at Wise, 1 College Avenue Wise, VA 24293, USA.
E Corresponding author. Email: rhuish@gmail.com
Pacific Conservation Biology 21(1) 27-37 https://doi.org/10.1071/PC14902
Submitted: 14 February 2014 Accepted: 28 July 2014 Published: 1 May 2015
Abstract
The aromatic heartwood of Santalum yasi has been harvested extensively in Fiji and Tonga over the past two centuries for international trade in the medicinal, perfume, and incense industry and other cultural purposes. Field surveys and a review of historic and modern documents reveal a sparse and scattered distribution and indicate that the natural distribution of S. yasi has fluctuated over time, even declining to local extinction in the wild in some areas, while S. album has been introduced and naturalisation of S. yasi × S. album hybrids is evident. Population data collected along transects in three in situ S. yasi populations show discontinuous size-class structures, indicating regenerative stress. The population densities at study sites ranged from 19 to 63 adult trees (≥5 cm dbh) per hectare and less than two heartwood-bearing trees (≥15 cm dbh) per hectare. Though S. yasi trees may attain up to 40 cm dbh, no trees greater than 23 cm dbh were found in any of the studied populations. Low density and small size of adult trees and human-induced bole damage are suggestive of frequent, premature, and defensive harvesting patterns and indicate the need for ongoing adaptive comanagement in recognition of underlying economic and sociocultural pressures.
Additional keywords(or phrases): : adaptive comanagement, resource management, sustainable harvesting.
Introduction
The genus Santalum L. (Sandalwood) includes 15 extant species distributed on islands in the Pacific Ocean and Australia, South-east Asia, and India (Harbaugh and Baldwin 2007). Sandalwood is highly valued internationally for the aromatic heartwood. After a tree is felled, the heartwood is removed from the bole and roots and processed into the forms of hardwood, powder, and extracted oil. These products are used in a variety of ways for religious, cultural, perfumery, culinary, and medicinal purposes (Brennan and Merlin 1993; Ochi et al. 2005; Heuberger et al. 2006; Schnitzler et al. 2007; Burdock and Carabin 2008; Hansda 2009; Warnke et al. 2009; Kumar et al. 2012; Paulpandi et al. 2012; Subasinghe 2013). The extensive amount of human uses and consequent demand for sandalwood, in conjunction with its increasing rarity, make sandalwood one of the most valuable commercial trees in the world, and consequently subject to overharvesting pressures, threatening the sustainability of sandalwood resources (Brennan and Merlin 1993; Fox 2000; Kumar et al. 2012; Subasinghe 2013). Overharvesting has been cited as one of the major contributing factors leading to the extinction of S. fernandezianum F. Phil. in the early 20th century (World Conservation Monitoring Centre 1998b) and the listing of three other sandalwood species on the International Union for Conservation of Nature (IUCN) Red List of threatened species: S. album L., S. haleakalae Hbd., and S. macgregorii F. Muell. (Asian Regional Workshop 1998; World Conservation Monitoring Centre 1998a; Eddowes 1998). Other species of sandalwood have not yet been evaluated through the IUCN Red List criteria, though they may also warrant a threatened status.
Historically, most sandalwood products have been produced from the heartwood of S. album in South-east Asia and India, and this species has been the focus of most studies and government regulations. However, decreases in S. album resources have caused a widening gap between supply and demand, creating profitable market opportunities and increasing harvest pressure for alternative sandalwood species (Burfield 2005; Ananthapadmanabha 2013; Thomson 2013) including S. yasi Seem., from the Fiji and Tonga islands. Oil composition and content (McKinnell 1990; Doran et al. 2005), and cladistics (Harbaugh and Baldwin 2007) confirm S. yasi as a promising alternative to S. album when compared with other sandalwood species.
Santalum yasi – known in Fiji as yasi and in Tonga as ahi – is a small, hemiparasitic, monoecious tree reaching 10–15 m in height and more than 40 cm diameter at breast height (dbh) (Usumaki 1981; Jiko 2000). Fruiting for S. yasi may begin as early as 3–4 years, but it is not reliable until 7–10 years (Smith 1985; Bulai 1994; Thomson 2006b). The fruit is a reddish purple to almost black drupe. Sandalwood species are generally insect-pollinated (Kuijt 1969; Sedgley 1982) and the fruit is dispersed by frugivorous birds and bats (Veerendra and Padmanabha 1996). Heartwood formation is not well understood in S. yasi but generally begins after 10–15 years of growth or roughly at 15 cm dbh, though age and diameter are not always indicative of heartwood content or volume (Usumaki 1981). S. yasi is predominantly a subclimax species, filling the role of a pioneer species in recently disturbed areas, or as a late secondary species (Thomson 2006b).
Humans have valued S. yasi for centuries. Heartwood and live plants have been traded among Pacific islands as an intricate part of wedding and funeral ceremonies and as a commodity of political prestige among ruling chiefs (Vason 1810; Seemann 1869; Bennett 1932; Whistler 1991; Brennan and Merlin 1993). It was also one of the initial lures attracting Europeans into the South Pacific in the early 19th century when it became a major export (Shineberg 1967). The harvest of S. yasi has been almost exclusively from wild populations, following a characteristic cyclical pattern of short intense exploitation with subsequent periods of regeneration (Tuisese et al. 2000), and has faced ongoing regulation challenges (McKinnell 1990; Jiko 1993; Seruwaqa 1993; Kaufusi 1995; Thomson et al. 2002).
Despite the economic and cultural significance of S. yasi, minimal research has been conducted on the species. Peer-reviewed journal articles that cover S. yasi with any significant detail have been limited to information on morphology and embryology of the family (Paliwal 1956), oil quality evaluation of several sandalwoods (Howes et al. 2004; Doran et al. 2005), phylogenetic relationships of the Santalum genus (Harbaugh and Baldwin 2007; Harbaugh 2008), and an overview of research on sandalwood species (Subasinghe 2013). Unpublished work on S. yasi is limited and in some cases difficult to access. S. yasi, however, has been recognised as a priority species by the South Pacific Regional Initiative on Forest Genetic Resources (SPRIG) funded by the Australian Agency for International Development, which asserts the need for further research to inform management decisions (Tuisese et al. 2000). With the collaboration and insights of the Fiji and Tonga government forestry departments, this paper explores the distribution of S. yasi over time through a review of historic documentation and field surveys, and reports data on the size-class structure of three remnant in situ populations of S. yasi, and then discusses these results in the context of management strategies.
Methods
Distribution
Historical distribution for S. yasi in Fiji, Tonga, and other island groups was investigated through a review of documentation, including early accounts (De Ricci 1875; Horne 1881), floras (Endlicher 1836; Seemann 1869; Yuncker 1959; Smith 1985), herbarium specimens (15 herbaria from the Consortium of Pacific Herbaria (CPH 2014), the Australia National Herbarium (CANB 2013)), and various reports and management plans. Field surveys by the authors were also conducted from September to December 2006 on the three main islands of Fiji (Viti Levu, Vanua Levu, and Kadavu) and the three major island groups in Tonga (Tongatapu group, Vava’u group, Ha’apai group), which included ground observation and discussion with landowners, village leaders, and the proper representatives from the respective ministries of forests in Fiji and Tonga and academic institutions. Similar discussions were also carried out for the distribution of sandalwood on other islands. Documentation of the introduction and distribution of S. album and the hybrid S. album × yasi in Fiji and Tonga were investigated in like manner.
Population structure
Study site selection
During the investigation of distribution in Fiji and Tonga, S. yasi was found in many locations as scattered clusters or individuals. Three locations were found with rare concentrations of individuals, and these locations were selected for analysis of population structure. These in situ populations were in one location on Kadavu Island, Fiji, and two locations on ‘Eua Island, Tonga (Fig. 1).
|
Kadavu Island, Fiji. Kadavu (19°00′S, 178°13′E) is an island of volcanic origin, formed during the late Cenozoic to early Quaternary periods, reaching 807 m in elevation and lying 96 km south of Viti Levu, Fiji’s main island (Terry 1999). This steeply mountainous, oblong island, running east–west, covers over 400 km2 of land, of which ~83% is forested, with areas cleared for subsistence agriculture and pine plantations (Pinus caribaea Morelet) for lumber production (Duikoro and Tokairavua 2002). Kadavu soils consist primarily of ultisols (red-yellow podsols and humic latosols) with sandy and bouldery textures (Terry 1999). The study site, comprising 12 ha of lowland rainforest, was near the centre of the island, 2.5 km north-east of Muanisolo village (19°00′40″S, 178°15′34″E, 50 m elevation).
‘Eua Island, Tonga. ‘Eua (21°22′S, 174°56′W) is a mixed limestone/volcanic island, partially originating from Gondwana on the eastern margin of the continental Indian–Australian Plate 40 million years ago, ~20 km south-east of the main island of Tongatapu, Tonga (Mueller-Dombois and Fosberg 1998). ‘Eua has an oblong, triangular shape with a north–south orientation and an area of ~81 km2, reaching 312 m in elevation (Drake et al.1996). The soils of ‘Eua include mainly alfisols and mollisols, with entisols (coralline sands) along some coasts and inceptisols on the steep slopes (>30°) (Wilde and Hewitt 1983; Drake et al. 1996). Native forest covers much of the island, particularly along the steep ridges, plateaux, and regions along the coastline. Two separate study sites were selected here. The ‘Eua coastal site was a 16-ha, 100-m-wide strip of littoral forest between the village of Tufuvai and the further-inland village of Ha’atu’a, bordered on the west by the beach and on the east by agricultural land (21°21′S, 174°58′W, 1–5 m elevation). The ‘Eua highland site was a 24-ha area in Calophyllum-mixed-species upland rain forest (Mueller-Dombois and Fosberg 1998) ~2 km north-north-east of Houma village on the dissected slopes of the north-eastern ridge of the island (21°18′S, 174°55′W, 250 m elevation).
Transect methods
Size-class structure data were collected from September to October 2006 on Kadavu Island, Fiji, and from November to December 2006 on the two sites on ‘Eua Island, Tonga. Data were collected with the assistance of forestry officers and local village members and leaders following the methods of Peters (1994, 1996a, 1996b) and Hall and Bawa (1993). For each site, transects consisting of 10 m × 20 m contiguous plots of various lengths were set to cross through the population and to traverse topographical and climatic gradients (Hall and Bawa 1993). Transects ran parallel as determined by compass bearings, were georeferenced using GPS coordinates and spaced to meet the recommended 5% sample size (Peters 1996b). Measurement accuracy was maintained by correcting for slope using a clinometer and a prepared rope with corrected distance markers according to slope values (Peters 1996b).
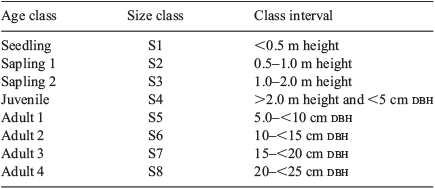
The size of S. yasi individuals found within each plot was measured according to height for young trees and diameter at breast height (dbh) for trees ≥5 cm dbh using tape measures and diameter tapes. Individuals in each population were classified into eight size-classes (Table 1) to allow the creation of size-class structure histograms (Peters 1996a). Size classes were based on the understanding of growth and age of a related species, S. austrocaledonicum Viell. from neighbouring New Caledonia and Vanuatu, which has similar size, life stages, and habitat preferences (Thomson 2006b).
|
Along the transects, other observations such as evidence of damage, disturbances, or invasive species were also recorded for each population. Where disturbance by feral pigs was observed, an analysis of the disturbance within the population was made by comparing plots with evidence of pig activity to plots without pig activity. Difference in seedling density and total regeneration density was analysed with the Chi-square test.
Results
Historical distribution
Fiji
The earliest distribution of S. yasi in Fiji is documented in harvesting accounts and by early botanists. In an early flora, Fiji was reported to be ‘abounding with rich forests of sandalwood’ (Endlicher 1836), with the large island of Vanua Levu – nicknamed ‘Sandalwood Island’ – reported to have the most plentiful S. yasi populations (Endlicher 1836; Seemann 1869; Horne 1881). In the early 19th century, trade and harvesting were intense but short-lived as mature trees became rare within 10 years (Shineberg 1967; Bulai 1994; Jiko 2000; Tuisese et al. 2000). By 1840, even a botanical specimen was difficult to find (De Ricci 1875). Horne (1881), in visiting the Bua district of Vanua Levu in 1878, found that the famous sandalwood had ‘almost disappeared’ with only a few scattered clusters of trees, some of which were in poor condition due to heavy forest undergrowth. These remaining S. yasi resources presumably recovered to some extent from the early exploitation, but were apparently still under stress in 1930 when an ‘order in council’ proscribed export only under permit, and then a prohibition on harvesting was declared in 1950 to allow for regeneration (Roth 1936; Jiko 1991). Roth describes the distribution of S. yasi in 1936 to be almost entirely confined to the Bua region. There were two herbarium specimens confirming its presence in the Bua region in 1938 (Parham 10115, Parham 10116 (SUVA 2014)), though it is unclear whether these specimens were collected from the wild or cultivated within the village. A follow-up resource survey conducted on Vanua Levu island by the Fijian Ministry of Forests in 1955 estimated no more than 200 S. yasi trees present in Bua Province, with less than 10 exceeding 30 cm dbh (Usumaki 1981; Jiko 1991).
Forestry officer J. T. Usumaki’s resource survey in 1981 reports increasing amounts of S. yasi in the Bua Province of Vanua Levu, though it was noted as being scattered over a large area and with low levels of regeneration. Usumaki recommended maintaining the harvest prohibition for at least another 10–20 years, but harvesting was reinstated in the mid 1980s with an accompanying regeneration levy (Jiko 1991; Tuisese et al. 2000). A fair population of S. yasi was reported by Smith (1985), Jiko (1993) and Tuisese et al. (2000) to be growing in the Bua, Cakaudrove, and Macuata provinces of Vanua Levu island, including the north-eastern part of the island near Udu point, and on the nearby Galoa and Tavea islands. Jiko (1993) notes that sandalwood used to be found in smaller stands along the western and northern coast of Vanua Levu island as far as Naduri, but those stands have since disappeared due to extensive harvesting and uncontrolled fire.
Soon after the turn of the century S. yasi was listed in Schedule 1 of Section 3 of the Endangered and Protected Species Act of 2002, which was drafted for the Fijian CITES report (Uluivuda 2002). This document defined S. yasi as an indigenous species ‘believed to be threatened with extinction’. Observation and inquiry to mataqali (village leaders) of villages in the Bua province of Vanua Levu in 2006 confirmed that S. yasi was virtually absent from forested areas, implying recent harvesting activity in these regions after the reports of Smith (1985), Jiko (1993) and Tuisese et al. (2000). A few small, naturalised pockets were found in disturbed sites near villages that had adult trees growing within their village yards. The absence of sandalwood in natural stands in this region was further confirmed in an inventory of forest trees in 2007 conducted by Fijian forestry officers.
The historical extent of S. yasi on other islands in Fiji is less documented. The limited information available, especially for outer island groups, may more likely be a function of remoteness of location rather than indicative of abundance or lack of abundance. On the main island of Viti Levu, herbarium collections from Davu in Tailevu were collected in 1937 (Savenaca 10117, Savenaca 10118 (SUVA)). Jiko (1993) reports the presence of S. yasi in the Western Division on the banks of the Colo West range from Tubainasolo to Nasaucoko, and some presence at Nakelo in the Central Division. One remaining stand in the Nausori Highlands was reported in 2000, but extensive harvesting and wildfires had reduced the stand to small pockets (Tuisese et al. 2000). In Smith’s flora, he noted S. yasi stands in parts of western Viti Levu in 1985; however, currently only S. album is present in that area.
Santalum yasi was reportedly brought to the island of Kadavu, a large island south of the main islands of Fiji, from Bua at the end of the 19th century, according to Kadavu residents. The earliest herbarium collection of S. yasi from Kadavu is in 1944 (Parham 10114 (SUVA)). The presence of S. yasi in several locations on Kadavu is mentioned by Jiko (1993). It was found in four villages and surrounding forests by Duikoro and Tokoariavau in 2002 in their enumeration report on S. yasi. The densest population they found was in the forests around Muanisolo village. This area has continued to have the densest population of S. yasi compared to other areas in Fiji and therefore became a chosen site for the population dynamics study for this research.
For the Lau island group, in the south-eastern part of the Fijian islands closest to Tonga, the earliest documentation of S. yasi is a herbarium collection taken in 1977 from Lakeba on the roadside near Tubou (Garnok-Jones 912 (CANB)). In 1984 Forestry officials reported a large stand of S. yasi on Onolevu Island and small pockets on Vanua Balavu, Lakeba, and Ono-i-lau islands (Tabunakawai and Chang 1984). Smith (1985) cites a collection from Oneata Island and Jiko (1993) also confirms the presence on the islands of Lakeba, Oneata, and Ono-i-lau.
Santalum album has been introduced to Fiji, though the timeline of its introduction is undocumented. Santalum album and S. album × S. yasi hybrids were found during the current survey in many locations across the main island of Viti Levu, including the Nausori Highlands and coastal areas, particularly along the north-western and western coasts. S. album and hybrids are also growing in forestry trial plots in Colo-i-suva and Nukurua, which are both north of the main city of Suva on the south-eastern side of the island. In the Lau island group, S. album was introduced in the 1980s to Lakeba Island according to local residents, and hybridisation has since occurred there as well. No evidence of S. album in Vanua Levu or Kadavu islands was found at the time this research was conducted.
Tonga
Abundance of S. yasi in Tonga attracted European traders in the early 19th century for short-lived, intensive harvesting (Bulai 1994; Tuisese et al. 2000). The extent and nature of population recovery and exploitation after this first European contact is uncertain because the next documentation on S. yasi in Tonga is Yuncker’s collections in the 1950s (Yuncker 1959). However, by 1979, concern for the supply of this valuable resource apparently spurred the Tongan government to implement the Forest Act of 1979 banning all S. yasi harvesting. Presumably, some level of regeneration occurred by 1990 when legal harvest resumed (MAFFF 2005).
On the main island of Tongatapu, collections of S. yasi were made by Yuncker in 1951 in four locations in coastal areas or thickets across the island (Yuncker 1959). Recently, Tuisese et al. (2000) report that S. yasi on the main island of Tongatapu is mainly found where it has been planted in village yards and agricultural bush allotments. The Wiser et al. (2002) publication on forest fragments in Tongatapu reports S. yasi as present in interior forest and interior late bush fallow and coastal forest and coastal late bush fallow. They note that these pockets of S. yasi contained more adults than juveniles. Inquiry and observation in 2006 confirm very sparsely scattered individuals in relic patches of forests and villages across Tongatapu.
The island of ‘Eua, south of Tongatapu and within the Tongatapu island group, was visited by Yuncker in 1953 and S. yasi was found in an old clearing behind Pangai village (Yuncker 1959). Ehrhart (1997) reports large numbers of small, young S. yasi trees, including plentiful regeneration. Tuisese et al. (2000) also noted S. yasi as very common on ‘Eua island, especially in secondary forests. Observation and data collection in 2006 for this research confirmed the densest populations of S. yasi in Tonga to be in the northern highlands and along the western coast of ‘Eua. These two in situ populations were therefore chosen for the population structure portion of this research.
Santalum yasi has also been documented in the Vava’u island group. On the main island of Vava’u, the presence of S. yasi is documented on Talau Mountain by Yuncker in 1953 (Yuncker 1959). Ehrhart in 1997 reports a stand of small trees. Observation and inquiry in 2006 confirmed the scattered presence of S. yasi close to some villages and sparsely in forests in Vava’u and other islands in the group including Hunga and Pangaimotu.
In the Ha’apai group, Yuncker cites a collection made along a road on Nomuka Island and near a beach on Lifuka Island in 1953 (Yuncker 1959). Ehrhart (1997) notes S. yasi to be occasionally found in the bush across various islands. Evans (2007) reports on widespread harvesting of S. yasi from Ha’ano Island in Ha’apai in the 1980s. Field surveys in 2006 confirmed the scattered presence of S. yasi close to some villages and sparsely in forests on many of the inhabited islands of Ha’apai.
In the northernmost Niue Island group, Yuncker’s flora cites a collection made in 1951 on a mountain ridge in Niuatoputapu (Yuncker 1959) but no other recent documentation has been made regarding S. yasi there. However, forestry officers report that resources there are scarce and young due to recent extensive harvesting.
The timing of the introduction of S. album to Tonga is unrecorded, but S. album is growing in forestry trial plots across the three main island groups, including in Tokomolo and Sia’atoutai on Tongatapu, the north-west highlands of Vava’u at Toafa, and on Foa Island in Ha’apai. S. album was also observed on ‘Eua Island near Pangai and Mata’aho, where it is becoming naturalised.
Other islands
The presence of S. yasi in other island nations in the south-west Pacific has been documented. There is a herbarium specimen collected from Vanuatu mentioned by Smith (1985), where Santalum austrocaledonicum is native. One collection of S. yasi was collected in Papua New Guinea in 1968 (Zieck s.n. (CANB)) where S. macgregorii is native. The island of Rotuma, a Fiji dependency 600 km north of Viti Levu, was planted with S. yasi and S. album by the Fiji Ministry of Forestry several decades ago. S. yasi and S. album have since died out there and the hybrids have become naturalised.
Santalum yasi has also been reported on Niue, an island country roughly 400 km east of Vava’u Island, Tonga (Thomson et al. 2005). This is likely a recent introduction because Smith (1902) denies the presence of ‘Tongan sandalwood’ in his article about Niue, and S. yasi is not included in the Flora of Niue (Yuncker 1943). In 1965, Sykes (1970) collected S. yasi from what he determined to be the only S. yasi tree on the island. Investigation by Thomson et al. (2005) confirms a small population growing west of Hakupu village on the south side of Niue Island.
Although trade with sandalwood occurred from Tonga and Fiji to neighbouring Samoa during pre- and post-European contact (Brennan and Merlin 1993), there is surprisingly only one record of it growing in Samoa near Falealupo (Brennan and Merlin 1993). A forestry officer in the district where this sample was found said that this specimen has since died and that he also once saw a tree come into the timber mill in the 1970s. He also reported that forestry trial plots containing sandalwood from Fiji and Vanuatu have been established in Samoa within the past decade. References for the traditional uses of asi (Samoan vernacular for sandalwood and apparent cognate of the Fijian ‘yasi’) are similar to the uses in Fiji and Tonga (Brennan and Merlin 1993) and are found in Samoan traditional songs (e.g. ‘Afoafouvale’s Song’: Kramer 1901). One unique use for asi in Samoa – and possibly offering clues to its historic presence in Samoa – is the use of asi leaves in family exhumation ceremonies called liutōfāga. Tamasese (2007) writes about this ceremony: ‘In Samoa, one of the essential ingredients for performing liutōfāga would be sandalwood and sandalwood leaves. This is evidenced in the Samoan word for funerals falelauasi, meaning the house that is lined with sandalwood leaves. Sandalwood, like incense, is one of the essences of Samoan culture, particularly Samoan spiritual culture’. It seems unlikely that this plant would have such cultural significance in Samoa if it were obtainable only through contact and trade with Tonga or Fiji – especially the use of its leaves – and hints towards either S. yasi being native to Samoa or an aboriginal introduction, with subsequent local extinction.
Population dynamics
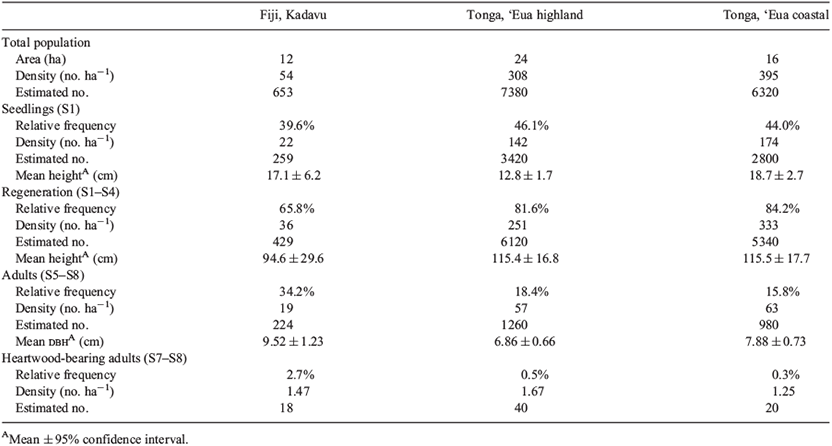
Population statistics and life-stage densities, relative frequencies, and mean height or mean dbh are summarised in Table 2. The Kadavu population had the lowest overall density and lowest relative frequency of regeneration. The ‘Eua coastal population had the densest population and the densest relative frequency of regeneration.
|
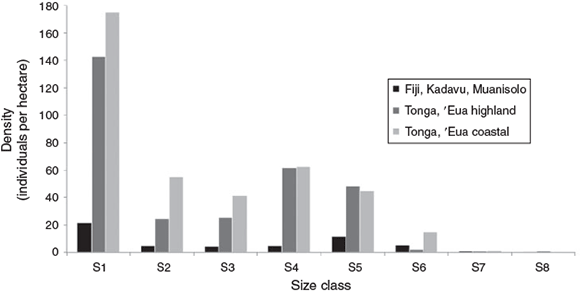
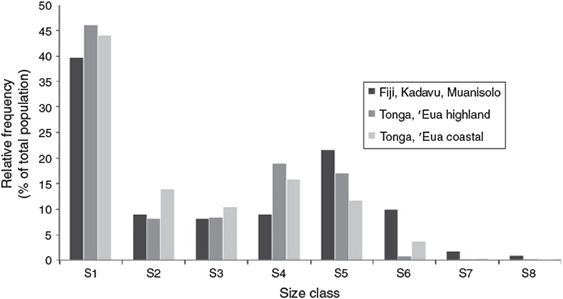
Size-class structure histograms were created for each of the S. yasi populations and compared by density (Fig. 2) and relative frequencies (Fig. 3). The histograms show discontinuous distribution in the sapling sizes (S2 and S3) for all three populations as well as discontinuity in juvenile sizes (S4) in the Kadavu population. These size-class structure density patterns are typical of populations with discontinuous or periodic recruitment and indicate regenerative stress (Peters 1996a). While natural variations and disturbances such as cyclones and forest community dynamics may play a part in the discontinuity, these patterns are a probable reflection of the cyclical harvesting of mature trees and the associated reduced seed production and regenerative capabilities over time.
|
|
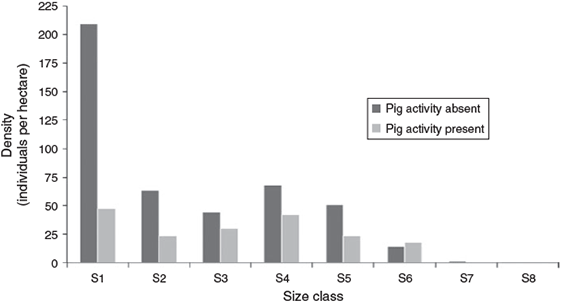
Soil disturbance due to feral pig (Sus scrofa domesticus Erxleben) activity was observed along the northern end of the ‘Eua coastal population near Tufuvai village. Within this population, a comparison of the plots where pig rooting was evident (8 plots) to plots without pig activity (30 plots) showed 77% fewer seedlings per hectare and 67% fewer young saplings (Fig. 4). This is a significantly lower seedling density (Chi-square test: χ2 = 9.891, d.f. = 1, P = 0.002) and significantly lower total regeneration density (Chi-square test: χ2 = 9.083, d.f. = 1, P = 0.002) for plots with evidence of pig rooting. The significance of these findings is limited because of the small sample size; however, pigs were also recognised by Wiser et al. (2002) to disturb soil and reduce ground cover in other forest areas in Tonga. Further study would help elucidate the influence pig activity has on regeneration of S. yasi.
|
Many introduced plant species have invaded the Pacific Island region (Richardson and Rejmanek 2011). Two of these species were observed growing in dense thickets bordering the ‘Eua S. yasi populations – yellow trumpet bush (Tecoma stans (L.) Juss. ex Kunth) at the ‘Eua coastal population and the guava tree (Psidium guajava L.) at the ‘Eua highland population. These may be of concern because no S. yasi individuals of any size class were found growing in these thickets.
Santalum yasi bole damage was frequently observed in the ‘Eua highland population. The damage was caused by the practice of preharvest checking for heartwood content by the removal of a wedge of wood with a machete to identify trees for potential harvest (Fig. 5). This type of tree damage was found in all of the early heartwood-forming adult individuals (S7 and S8), and 21% of the total adult (S5–S8) trees in this population. Of all the damaged trees noted, 82% were in the S4–S5 size classes, which are in the preheartwood development stages. This practice weakens the structural integrity of the tree and increases tree mortality due to wind damage, termites and microbial infections (Jiko 1991), and tree mortality due to this type of bole damage was also evident in the ‘Eua highland population. While the two ‘Eua populations did not differ significantly in total adult density, the high incidence of bole damage evident in the ‘Eua highland population may be a factor in the lower relative frequency of 10–15-cm dbh (S6) individuals compared to the ‘Eua coastal population seen in Fig. 3.

|
Discussion
The detailed extent of historic and modern distribution of S. yasi is unclear given the insufficiency of available documentation. However, the data presented considerably extend the previous understanding of the distribution and abundance of S. yasi. In nearly all locations in both Fiji and Tonga where S. yasi is present, it is sparse and scattered according to our field observations and the few available reports. The abundance of S. yasi on the main island of Vanua Levu in Fiji, for which the most documentation is available, has clearly fluctuated over time, declining to the point of local wild extinction or near extinction in many previously abundant areas. This emphasises the need for conservation and management efforts to focus on in situ populations.
The introduction of S. album to Fiji and Tonga could pose threats to S. yasi through their rapid hybridisation and the resulting competition for available habitat and hosts. The F1 hybrids are fertile and exhibit hybrid vigour, having been noted to grow considerably faster, form heartwood six to seven years earlier, and contain a much higher oil yield than either parent (Doran et al. 2005; Thomson 2006a). While these characteristics appear to be economically desirable, the introduction of S. album and the resulting hybridisation threatens the genetic integrity of the native S. yasi and creates a potential risk of infection with phytoplasmal sandal spike disease, which currently threatens S. album populations in India (Raychaudhuri and Varma 1988). Though sandal spike disease has not yet been identified in the Pacific islands and the susceptibility of S. yasi to the disease has not been established, hybrids could act as a bridge, allowing the disease to advance from S. album to S. yasi if the sandal spike disease is introduced to Fiji or Tonga, as has been seen in the spread of diseases via hybrid bridges between other plants (Brigham and Schwartz 2003). The extent of hybridisation between S. yasi and S. album needs to be confirmed urgently through DNA analysis, to better understand the source and rate of hybridisation and the level of threat to native populations. In the meantime, forestry officials may want to seriously consider following the precautionary principle by limiting S. album and other sandalwood species and removing them from forestry plots, seed lots, and other locations on outer island groups.
The discontinuous size class structure of each population indicates regenerative stress (Peters 1996a), and, in consideration of the long history of exploitation, is plausibly related to the loss of seed production by mature trees that are cyclically harvested. Regeneration is, therefore, a crucial aspect for future monitoring and study of in situ populations. Lack of seeds, and seedlings has also been an ongoing challenge for replanting and propagation efforts recognised by Jiko (1991, 2000) and Wiser et al. (1999) due to the scarcity and remote locations of reproductively mature trees (Thomson 2013). The distribution survey conducted reaffirms this scarcity in most locations across Fiji and Tonga. Fortunately, S. yasi has generally maintained a presence within villages on Vanua Levu that have historically been associated with abundant wild populations of S. yasi. These relic S. yasi individuals in villages and garden plots in Vanua Levu and many locations across Fiji and Tonga can potentially naturally reseed forested areas under favourable conditions. They can also provide a source of seed for propagation programs. The establishment of nurseries, seed lots, and plantations is important to the long-term management of S. yasi, and has begun over the past several decades in both Fiji and Tonga with limited initial success due to complex silviculture, limited resources, and damage due to cyclones (Jiko 1991; Seruwaqa 1993; Tuisese et al. 2000; Likiafu and Robson 2005). Beyond the complexity and expense of these efforts, nurseries, seed lots and plantations do not directly attend to remnant in situ populations and habitat that can provide a lower maintenance resource opportunity (Barrett and Fox 1994) and help preserve forest lands and plant products for continued traditional use. Preserving forest land is of particular concern for S. yasi habitat in Tonga, where pressure on land resources is increasing, and cash cropping developments have replaced much of the traditional subsistence farmland and nearly all indigenous forest land (Masikerei et al. 1996; Wiser et al. 2002).
Beyond human impact, natural variations and disturbances such as cyclones may also influence population dynamics of S. yasi. The ‘Eua coastal population had the densest population and the densest relative frequency of regeneration. S. yasi may have established more readily along the coast of ‘Eua due to periodic disturbance from tropical storms along coastal habitats and an abandoned village site nearby as S. yasi is noted to be a pioneer species that prefers disturbed habitat (Wiser et al. 2002; Thomson 2006b). Forest community dynamics may also influence population dynamics through competition with dense undergrowth and lianas. The Kadavu population had the lowest density and lowest relative frequency of regeneration, which may be associated with its notably dense forest habitat and steep topography. In situ management focused on reducing competition of non-host species may, therefore, be beneficial.
The size class structures in all three evaluated populations suggest frequent harvesting patterns, as identified by a low density of adult individuals and an absence of trees larger than 23 cm dbh, though S. yasi may potentially attain over 40 cm dbh (Jiko 2000). Further, the high percentage of adult trees damaged in the ‘Eua highland population due to preharvest checking for heartwood reflects premature harvesting patterns. These harvesting patterns and human-induced damage were recognised in Fiji as early as 1881 by Horne and was also recognised by Usumaki (1981) and can stem from various underlying pressures. Financial pressure is a major force for premature harvesting of S. yasi trees at minimal heartwood-forming sizes and further induces illegal harvesting when bans are in place (Evans 2007). The need for financial resources for community development was reported as a main impetus for the beginning of modern harvesting and exports from Fiji in the 1980s (Jiko 1991), despite forestry recommendations to delay harvesting for another 10–20 years to allow for further regeneration (Usumaki 1981; Tabunakawai and Chang 1984). Sociocultural pressure was a recognised factor in the defensive, widespread harvest of S. yasi from Ha’ano Island in Ha’apai, Tonga, over a period of two years in the 1980s (Evans 2007).
These underlying pressures, as well as limited man-power and funding, remote location of harvesting, and other factors cause difficulty for legislative enforcement, creating a large gap between the volume of S. yasi export allowed by permit and actual export volumes in Tonga (Likiafu 2008; Kaufusi 1995; MAFFF 2004, 2005, 2006, 2007). Legislation, therefore, does not seem to be a stand-alone solution for management of S. yasi.
Economic, social, cultural, and political factors affecting local resource-dependent groups need to be addressed as an essential component of sustainable management efforts (Berkes and Folke 1998; Turnbull 2004). These complex factors may be addressed through adaptive comanagement approaches, as discussed by Folke et al. (2002) and Olsson et al. (2004). Adaptive comanagement relies on shared, dynamic management between central government and local communities. Such cooperative management has been successfully applied to marine resource management in the South Pacific, including Fiji, as summarised by Johannes (2002) and may be further applied to management of S. yasi. Key components of successful cooperative management strategies identified by Johannes (2002) include communities perceiving a scarcity in their valuable resources, governments recognising and supporting traditional village-based authority and resource tenure, and regional/national government and non-government organisations providing improved conservation education and effective assistance and advice.
Rural communities in Fiji and Tonga are showing increasing interest in participating in management of S. yasi. Community-based management of the S. yasi population on Kadavu Island near Muanisolo village has experienced some success. Community members regulate harvesting practices and share stewardship over the trees by regularly monitoring and tending maturing trees, such as by removing competing species. Such management was benefited by the remote location of the village – which helped ensure resource tenure – and the assistance of a retired forestry officer who was one of the village leaders. This ongoing care and communal management may be a factor in why this S. yasi population contained the highest relative frequency of adults and heartwood-bearing trees, despite having the lowest population density of those studied. Proceeds from harvests have funded community development, including the building of the central village meeting hall. Communal benefit encourages protection of this valued resource and wise harvesting practices. Further research is being conducted by the authors on in situ sandalwood management strategies like these from the Asia-Pacific region to create a collaborative knowledge base. It is hoped that this will facilitate a cross-pollination of ideas, which can be tailored to local paradigms and customs and incorporated into coadaptive management strategies.
Conclusions
The data reported here reveal a scattered, sparse distribution of wild S. yasi and the relatively recent introduction of S. album and the naturalisation of their hybrids. The low density and discontinuous size class structure across all study sites may reflect frequent and excessive harvesting and identify regeneration as a crucial aspect for future monitoring and study. In situ populations can play an increasingly important role in the sustainable management of S. yasi as they represent lower maintenance resource opportunities that preserve diminishing forest habitat. While more research needs be done on S. yasi, these data emphasise the need for management to press forward despite limited biological and ecological information, and in recognition of the complex, underlying factors that surround its management. These underlying factors, such as economic and cultural pressures, may be addressed through adaptive comanagement in order to conserve this culturally and economically valuable species for future generations.
Acknowledgements
We sincerely thank the many forestry officers and community participants for their valuable support in developing and carrying out this project, as well as the New York Botanical Garden and the City University of New York for financial support.
References
Ananthapadmanabha, H. S. (2013). Indian sandalwood market trend production. In ‘Proceedings of International Sandalwood Symposium 2012’. (Eds M. Nageswara-Rao, J. R. Soneji, and D. T. Harbaugh Reynaud.) pp. 21–24. (Lulu Press, Inc.)Asian Regional Workshop (1998). Santalum album. In ‘IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2’. Available at www.iucnredlist.org [verified 30 November 2013].
Barrett, D. R., and Fox, J. E. D. (1994). Geographical distribution of Santalaceae and botanical characteristics of species in the genus Santalum. Sandalwood Workshop, New Caledonia, August 1994. Contribution No. 6. Canberra, ACIAR.
Bennett, G. (1932). An account of the sandal wood tree (Santalum), with observations on some of the botanical productions of the Sandwich Islands. Magazine of Natural History, Series I 5, 255–261.
Berkes, F., and Folke, C., (Eds.) (1998). ‘Linking Social and Ecological Systems. Management Practices and Social Mechanisms for Building Resilience.’ (Cambridge University Press: Cambridge.)
Brennan, P., and Merlin, M. (1993). Biogeography and traditional use of Santalum in the Pacific Region. In ‘Sandalwood in the Pacific Region. Proceedings of a symposium held on 2 June 1991 at the XVII Pacific Science Congress, Honolulu, Hawaii’. (Ed. F. H. McKinnell.) pp. 31–38. Australian Centre for International Agriculture Research Proceedings No. 49.
Brigham, C. A., and Schwartz, M. W. (2003). ‘Population Viability in Plants: Conservation, Management, and Modeling of Rare Plants.’ Ecological Studies Vol. 165. (Springer.)
Bulai, P. (1994). Sandalwood in Fiji. In ‘Sandalwood Seed, Nursery and Plantation Technology. Proceedings of a regional workshop for Pacific Island Countries, Noumea, New Caledonia’. (Eds L. Gerum, J. E. D. Fox and Y. Ehrhart.) pp. 167–172. RAS/92/361. Field Document No. 8. (UNDP/FAO South Pacific Forestry Development Programme: Suva Fiji.)
Burdock, G. A., and Carabin, I. G. (2008). Safety assessment of sandalwood oil (Santalum album L.). Food and Chemical Toxicology 46, 421–432.
| Safety assessment of sandalwood oil (Santalum album L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVOksLzM&md5=d515eebfb21f2ec5e248432426a76daeCAS | 17980948PubMed |
Burfield, T. (2005). Threatened and Vulnerable Aromatic Species. Cropwatch. Available at http://www.users.globalnet.co.uk/~nodice/new/magazine/crop7/cwatch7.htm [verified January 2014].
CANB (Australian National Herbarium) (2013). Australian National Herbarium Specimen Information Register (ANHSIR) database [extract at CANB, Excel file]. Australian National Herbarium: Canberra.
CPH (The Consortium of Pacific Herbaria) (2014). University of Hawai‘i [extract at CPH, Excel file]. Honolulu, HI.
De Ricci, J. H. (1875). ‘Fiji: Our New Province in the South Seas.’ (Edward Stanford: London.)
Doran, J., Thomson, L., Brophy, J. J., Goldsack, B., Bulai, P., Faka’osi, T., and Mokoia, T. (2005). Variation in heartwood oil composition of young sandalwood trees in the South Pacific (Santalum yasi, S. album and F1 hybrids in Fiji, and S. yasi in Tonga and Niue). In ‘Sandalwood Research Newsletter Issue 20’. (Ed. T. Page.) pp. 3–7.
Drake, D. R., Whistler, W. A., and Motley, T. J. (1996). Rain forest vegetation of ‘Eua Island, Kingdom of Tonga. New Zealand Journal of Botany 34, 65–77.
| Rain forest vegetation of ‘Eua Island, Kingdom of Tonga.Crossref | GoogleScholarGoogle Scholar |
Duikoro, J., and Tokairavua, J. (2002). Santalum yasi enumeration report on Kadavu Island. Forestry Department Report, Fiji.
Eddowes, P. J. (1998). Santalum macgregorii. In ‘IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2’. Available at www.iucnredlist.org [verified 29 November 2013].
Ehrhart, Y. (1997). Descriptions of some sandalwood tree populations in the South Pacific: consequences on the silviculture of these species and provenances. CIRAD-Foret/ New-Caledonia, Pouembout.
Endlicher, S. (1836). Flora of the South Sea Islands. Annalen des Wiener Museums der Naturgeschichte 1, 129–190.
Evans, M. (2007). Property, propriety, and ecology in contemporary Tonga. Human Organization 66, 22–27.
Folke, C., Carpenter, S., Elmqvist, T., et al. (2002). Resilience for sustainable development: building adaptive capacity in a world of transformations. International Council for Scientific Unions (ICSU), Rainbow Series No. 3, Paris. Available at http://www.sou.gov.se/mvb/pdf7resiliens.pdf
Fox, J. (2000). Sandalwood: the royal tree. Biologist (Columbus, Ohio) 47, 31–34.
| 1:STN:280:DC%2BD3M%2FptlOnsQ%3D%3D&md5=dd8b5e7c7895daaa9872aebc0ff35ee4CAS |
Hall, P., and Bawa, K. (1993). Methods to assess the impact of extraction of non-timber tropical forest products on plant populations. Economic Botany 47, 234–247.
| Methods to assess the impact of extraction of non-timber tropical forest products on plant populations.Crossref | GoogleScholarGoogle Scholar |
Hansda, R. (2009). The outlook for non-wood forest products in Asia and the Pacific. Working Paper No. APFSOSII/WP/2009/18, Food and Agriculture Organization of the United Nations regional office for Asia and the Pacific, Bangkok.
Harbaugh, D. T. (2008). Polyploid and hybrid origins of Pacific island sandalwoods (Santalum, Santalaceae) inferred from low-copy nuclear and flow cytometry data. International Journal of Plant Sciences 169, 677–685.
| Polyploid and hybrid origins of Pacific island sandalwoods (Santalum, Santalaceae) inferred from low-copy nuclear and flow cytometry data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVyktb4%3D&md5=55e12d26790babc0e9cb17a3a19fa7a2CAS |
Harbaugh, D. T., and Baldwin, B. G. (2007). Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. American Journal of Botany 94, 1028–1040.
| Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific.Crossref | GoogleScholarGoogle Scholar | 21636472PubMed |
Heuberger, E., Hongratanaworakit, T., and Buchbauer, G. (2006). East Indian sandalwood and α-santalol odor increase physiological and self-rated arousal in humans. Planta Medica 72, 792–800.
| East Indian sandalwood and α-santalol odor increase physiological and self-rated arousal in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Crs7g%3D&md5=1f422d9beab75b13c785eb266c7d6cccCAS | 16783696PubMed |
Horne, J. (1881). ‘A Year in Fiji: Or an Inquiry into the Botanical, Agricultural, and Economical Resources of the Colony.’ (Kessinger Publishing: Whitefish, MT.)
Howes, M. J. R., Simmonds, M. S. J., and Kite, G. C. (2004). Evaluation of the quality of sandalwood essential oils by gas chromatography–mass spectrometry. Journal of Chromatography. A 1028, 307–312.
| Evaluation of the quality of sandalwood essential oils by gas chromatography–mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFyntg%3D%3D&md5=87884f0391e2f19f3fc49a68c5802617CAS |
Jiko, L. (1991). Status and current interest in Sandalwood in Fiji. Report for the Department of Forests. Ministry of Agriculture, Fisheries and Forests, Fiji.
Jiko, L. (1993). Status and current interest in Sandalwood in Fiji. In ‘Sandalwood in the Pacific Region. Proceedings of a symposium held on 2 June 1991 at the XVII Pacific Science Congress, Honolulu, Hawaii’. (Ed. F. H. McKinnell.) pp. 14–19. Australian Centre for International Agriculture Research Proceedings No. 49.
Jiko, L. (2000). Status and current interest in sandalwood in Fiji. Sandalwood Research Newsletter 10, 1–3.
Johannes, R. E. (2002). The renaissance of community-based marine resource management in Oceania. Annual Review of Ecology and Systematics 33, 317–340.
| The renaissance of community-based marine resource management in Oceania.Crossref | GoogleScholarGoogle Scholar |
Kaufusi, S. (1995). The status of the sandalwood tree in Tonga. In ‘Sandalwood Seed, Nursery and Plantation Technology. Proceedings of a regional workshop for Pacific Island Countries. Noumea, New Caledonia’. (Eds L. Gerum, J. E. D. Fox and Y. Ehrhart.) pp. 179–180. (UNDP/FAO South Pacific Forestry Development Programme: Suva, Fiji.)
Kramer, A. (1901). ‘The Samoa Islands: Constitution, Pedigrees and Traditions.’ Translated by T. Verhaaren, 1999. (University of Hawai‘i Press: Honolulu, HI.)
Kuijt, J., (1969). ‘The Biology of Parasitic Flowering Plants.’ (University of California Press: Berkeley, CA.)
Kumar, A. N., Joshi, G., and Mohan Ram, H. Y. (2012). Sandalwood: history, uses, present status and the future. Current Science 103, 1408–1416.
Likiafu, H. (2008). Sandalwood policy statement. Ministry of Forests, Tonga. (Draft)
Likiafu, H., and Robson, K. (2005). Report on the development of sandalwood (Santalum) seed stands on Tongatapu and ‘Eua Milestone 62. An R&D partnership between Forestry Division, MAFF, Tonga & South Pacific Regional Initiative on Forest Genetic Resources, Queensland Government Department of Primary Industries and Fisheries.
MAFFF (2004). Annual report for The Ministry of Agriculture, Fisheries, Food and Forests (MAFFF). Quarantine Division, Tonga.
MAFFF (2005). Annual report for The Ministry of Agriculture, Fisheries, Food and Forests (MAFFF). Quarantine Division, Tonga.
MAFFF (2006). Annual report for The Ministry of Agriculture, Fisheries, Food and Forests (MAFFF). Quarantine Division, Tonga.
MAFFF (2007). Annual report for The Ministry of Agriculture, Fisheries, Food and Forests (MAFFF). Quarantine Division, Tonga.
Masikerei, K., Haanen, A., and Crocket, G. (1996). A co-ordinated approach to land information management. The Fiji Land Information System. Available at http://ces.iisc.ernet.in/energy/HC270799/LM/SUSLUP/Thema5/194/194.pdf [verified January 2009].
McKinnell, F. H. (1990). Status of management and silviculture research on sandalwood in Western Australia and Indonesia. In ‘Proceedings of the Symposium on Sandalwood in the Pacific, Honolulu, Hawai’i’. (Eds L. Hamilton and C. E. Conrad.) pp. 19–25. (USDA Forest Service, Pacific Southwest Research Station: Berkeley, CA.)
Mueller-Dombois, D., and Fosberg, F. R. (1998). ‘Vegetation of the Tropical Pacific Islands.’ (Springer.)
Ochi, T., Shibata, T., Higuti, T., Kodama, K., Kusumi, T., and Takaishi, Y. (2005). Anti Helicobacter pylori compounds from Santalum album. Journal of Natural Products 68, 819–824.
| Anti Helicobacter pylori compounds from Santalum album.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkslSltL8%3D&md5=0f2614af668d13bc01c4182870abb7afCAS | 15974602PubMed |
Olsson, P., Folke, C., and Berkes, F. (2004). Adaptive co-management for building resilience in social–ecological systems. Environmental Management 34, 75–90.
| Adaptive co-management for building resilience in social–ecological systems.Crossref | GoogleScholarGoogle Scholar | 15383875PubMed |
Paliwal, R. L. (1956). Morphological and embryological studies in some Santalaceae. Agra University Journal of Research, Science 5, 193–284.
Paulpandi, M., Kannan, S., Thangam, R., Kaveri, K., Gunasekaran, P., and Rejeeth, C. (2012). In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomedicine 19, 231–235.
| In vitro anti-viral effect of β-santalol against influenza viral replication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitlGis7w%3D&md5=498f1ede1335f2b72212f60d55ef88ccCAS | 22192867PubMed |
Peters, C. M. (1994). ‘Sustainable Harvest of Non-Timber Plant Resources in Tropical Moist Forest: An Ecological Primer.’ (Biodiversity Support Program Publication Series (consortium of World Wildlife Fund, The Nature Conservancy and World Resources Institute): Washington, DC.)
Peters, C. M. (1996a). The ecology and management of non-timber forest resources. Washington, DC. The World Bank Technical Paper No. 322, 1–157.
Peters, C. M. (1996b). Beyond nomenclature and use: a review of ecological methods for ethnobotanists. In ‘Selected Guidelines for Ethnobotancial Research: A Field Manual’. (Ed. M. N. Alexiades.) pp. 241–276. (New York Botanical Garden Press: New York.)
Raychaudhuri, S. P., and Varma, A. (1988). Sandal spike, the present situation. In ‘Tree Mycoplasmas and Mycoplasma Diseases’. (Ed. C. Hiruki.) pp. 33–55. (University of Alberta Press: Alberta.)
Richardson, D., and Rejmanek, M. (2011). Trees and shrubs as invasive alien species – a global review. Diversity & Distributions 17, 788–809.
| Trees and shrubs as invasive alien species – a global review.Crossref | GoogleScholarGoogle Scholar |
Roth, G. K. (1936). ‘Fiji: Handbook of the Colony.’ (Government Printing: Suva.)
Schnitzler, P., Koch, C., and Reichling, J. (2007). Susceptibility of drug resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop and sandalwood. Antimicrobial Agents and Chemotherapy 51, 1859–1862.
| Susceptibility of drug resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop and sandalwood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlt1yjtrc%3D&md5=f45d86e093dcff9dbd86613380137574CAS | 17353250PubMed |
Sedgley, M. (1982). Floral anatomy and pollen tube growth in the quandong (Santalum acuminatum (R.Br.) A.DC.). Australian Journal of Botany 30, 601–609.
| Floral anatomy and pollen tube growth in the quandong (Santalum acuminatum (R.Br.) A.DC.).Crossref | GoogleScholarGoogle Scholar |
Seemann, B. (1869). ‘Flora Vitiensis: A Description of the Plants of the Viti or Fiji Islands with an Account of their History, Uses, and Properties.’ (L. Reeve: London.)
Seruwaqa, N. L. (1993). Nawailevu Yasi Project Field Report. Report for the Department of Forests. Ministry of Agriculture, Fisheries and Forests, Fiji.
Shineberg, D. (1967). ‘They Came for Sandalwood: A Study of the Sandalwood Trade in the South-West Pacific, 1830–1865.’ (Melbourne University Press.)
Smith, S. P. (1902). Niue Island and its people. The Journal of the Polynesian Society (N. Z.) 11, 80–106.
Smith, A. C. (1985). ‘Flora Vitiensis Nova: a New Flora of Fiji. Vol 3.’ (Pacific Tropical Botanical Garden: Lawai, Kauai, Hawaii.)
Subasinghe, S. M. C. U. P. (2013). Sandalwood research: a global perspective. Journal of Tropical Forestry and Environment 3, 1–8.
SUVA (South Pacific Regional Herbarium) (2014). The Consortium of Pacific Herbarium (CPH) Database [extract at CPH, Excel file]. South Pacific Regional Herbarium, Honolulu.
Sykes, W. R. (1970). Contributions to the flora of Niue. New Zealand Department of Scientific and Industrial Research Bulletin, 200.
Tabunakawai, K., and Chang, A. (1984). Sandalwood Resource of the Ono-i-Lau Islands. Report for the Department of Forests. Ministry of Agriculture, Fisheries and Forests, Fiji.
Tamasese, T. A. T. (2007). In search of Tagaloa: Pulemelei, Samoan mythology and science. Archaeology in Oceania 42, 5–10.
Terry, J. P. (1999). Kadavu Island, Fiji: fluvial studies of a volcanic island in the humid tropical South Pacific. Singapore Journal of Tropical Geography 20, 86–98.
| Kadavu Island, Fiji: fluvial studies of a volcanic island in the humid tropical South Pacific.Crossref | GoogleScholarGoogle Scholar |
Thomson, L. (2006a). Low input breeding and genetic conservation of Pacific Island forest tree species. In ‘Proceedings from IUFRO Division 2 Joint Conference: Low Input Breeding and Genetic Conservation of Forest Tree Species’. pp. 208–215. (Antalya, Turkey.)
Thomson, L. (2006b). Santalum austrocaledonicum and S. yasi (sandalwood). ver. 2.1 In ‘Species Profiles of Pacific Island Agroforestry’. (Ed. C. R. Elevitch.) (Permanent Agriculture Resources: Holualoa, HI.)
Thomson, L. (2013). Update on sandalwood resources and trade in the South Pacific. In ‘Proceedings of the International Sandalwood Symposium 2012’. (Eds M. Nageswara-Rao, J. R. Soneji, D. T. Harbaugh Reynaud.) pp. 1–20. (Lulu Press: Raleigh, NC.)
Thomson, L., Robson, K., Waiqolo, I., Faka’osi, T., and Viji, I. (2002). Sandalwood work in SPRIG. In ‘Sandalwood Research Newsletter’. (Ed. T. Page.) 15, 7.
Thomson, L., Bulai, S., Ikitoelagi, M., and Mokoia, T. (2005). Management plan for ahi (Santalum yasi) on Niue. Technical report for the South Pacific Regional Initiative on Forest Genetic Resources, Phase 2, Milestone 65. 30 pp.
Tuisese, S., Evo, T., Bulai, P., Singh, K., Jiko, L., Faka’osi, T., Napa’a, S., Havea, M., and Thomson, L. (2000). A strategy for conserving, managing and better utilizing the genetic resources of Santalum yasi (sandalwood) in the Kingdom of Tonga and Republic of Fiji. Report prepared by Department of Forestry, Fiji and Forestry and Conservation Division, Tonga under South Pacific Regional Initiative on Forest Genetic Resources (SPRIG/AusAID). CSIRO Forestry and Forest Products, Canberra, Australia.
Turnbull, J. (2004). Explaining complexities of environmental management in developing countries: lessons from the Fiji Islands. The Geographical Journal 170, 64–77.
| Explaining complexities of environmental management in developing countries: lessons from the Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Uluivuda, J. I., (2002). Endangered and Protected Species Act 2002. Enacted by the Parliament of the Fiji Islands, 23 December 2002. Act No. 29.
Usumaki, J. T. (1981). A report on survey of sandalwood (Santalum yasi) resources in Bua Province, Vanua Levu. Report for the Department of Forests, Management Services Division, Colo-i-suva. Ministry of Agriculture, Fisheries and Forests, Fiji.
Vason, G. (1810). ‘An Authentic Narrative of Four Years’ Residence at Tonga-taboo, one of the Friendly Islands, in the South-sea.’ (Longman, Hurst, Rees, and Orme: London.)
Veerendra, H. C. S., and Padmanabha, H. S. A. (1996). The breeding system in sandal (Santalum album L.). Silvae Genetica 45, 188–190.
Warnke, P. H., Becker, S. T., Podschun, R., Sivananthan, S., Springer, I. N., et al. (2009). The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. Journal of Cranio-Maxillofacial Surgery 37, 392–397.
| The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections.Crossref | GoogleScholarGoogle Scholar | 19473851PubMed |
Whistler, W. A. (1991). ‘The Ethnobotany of Tonga: the Plants, their Tongan Names, and their Uses.’ Bishop Museum Bulletin in Botany No. 2. (Bishop Museum Press: Honolulu, HI.)
Wilde R. H., and Hewitt, A. E., (1983). Soils of ‘Eua Island, Kingdom of Tonga. New Zealand soil survey report No. 68.
Wiser, S. K., Burrows, L. E., Skyes, W. S., Drake, D. R., and Savage, T. J. (1999). A natural forest inventory of Tongatapu and nearby islands. Kingdom of Tonga. NZODA 1998/99 Forestry Project, Landcare Research New Zealand Ltd.
Wiser, S. K., Drake, D. R., Burrows, L. E., and Sykes, W. R. (2002). The potential for long-term persistence of forest fragments on Tongatapu, a large island in western Polynesia. Journal of Biogeography 29, 767–787.
| The potential for long-term persistence of forest fragments on Tongatapu, a large island in western Polynesia.Crossref | GoogleScholarGoogle Scholar |
World Conservation Monitoring Centre (1998a). Santalum haleakalae. In ‘IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2’. Available at www.iucnredlist.org [verified 30 November 2013].
World Conservation Monitoring Centre (1998b). Santalum fernandezianum. In ‘IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2’. Available at www.iucnredlist.org [verified 30 November 2013].
Yuncker, T. G. (1943). ‘The Flora of Niue Island.’ Bernice P. Bishop Museum Bulletin No. 178. (Bishop Museum Press: Honolulu, HI.)
Yuncker, T. G. (1959). ‘Plants of Tonga.’ Bishop Museum Bulletin No. 220. (Bishop Museum Press: Honolulu, HI.)


