Determinants of bird assemblage composition in riparian vegetation on sugarcane farms in the Queensland Wet Tropics
Anita F. Keir A , Richard G. Pearson A B and Robert A. Congdon AA College of Marine and Environmental Sciences, James Cook University, Townsville, Qld 4811, Australia.
B Corresponding author. Email: richard.pearson@jcu.edu.au
Pacific Conservation Biology 21(1) 60-73 https://doi.org/10.1071/PC14904
Submitted: 31 May 2014 Accepted: 3 October 2014 Published: 21 April 2015
Abstract
Remnant habitat patches in agricultural landscapes can contribute substantially to wildlife conservation. Understanding the main habitat variables that influence wildlife is important if these remnants are to be appropriately managed. We investigated relationships between the bird assemblages and characteristics of remnant riparian forest at 27 sites among sugarcane fields in the Queensland Wet Tropics bioregion. Sites within the remnant riparian zone had distinctly different bird assemblages from those of the forest, but provided habitat for many forest and generalist species. Width of the riparian vegetation and distance from source forest were the most important factors in explaining the bird assemblages in these remnant ribbons of vegetation. Gradual changes in assemblage composition occurred with increasing distance from source forest, with species of rainforest and dense vegetation being replaced by species of more open habitats, although increasing distance was confounded by decreasing riparian width. Species richness increased with width of the riparian zone, with high richness at the wide sites due to a mixture of open-habitat species typical of narrower sites and rainforest species typical of sites within intact forest, as a result of the greater similarity in vegetation characteristics between wide sites and the forest proper. The results demonstrate the habitat value for birds of remnant riparian vegetation in an agricultural landscape, supporting edge and open vegetation species with even narrow widths, but requiring substantial width (>90 m) to support specialists of the closed forest, the dominant original vegetation of the area.
Additional keywords: agriculture, Australia, diversity, rainforest, riparian width.
Introduction
Over the last 20 years or so there has been increasing research interest in the processes by which agriculture affects the ecology of birds (e.g. Ormerod and Watkinson 2000). Gaston et al. (2003) estimated that between a fifth and a quarter of bird numbers had been lost globally as a result of agriculture, while Green et al. (2005) showed that farming was the greatest extinction threat to birds. As only a small proportion of land is protected as nature reserves, conservation efforts need to address the biodiversity values of agricultural areas (Petit et al. 1999). There is a growing recognition of the importance of the diversity of ‘countryside’ elements to bird conservation (Ormerod and Watkinson 2000), and diversified agricultural systems may help buffer against extreme loss of diversity (Frishkoff et al. 2014). However, biodiversity is declining from land conversions of diverse, low-intensity agriculture to homogeneous, intensive production (Donald et al. 2001; Maron and Fitzsimons 2007).
Riparian zones (ribbons of streamside habitat) perform many important ecosystem functions. In agricultural landscapes, they may be left undeveloped because they are unsuitable for cultivation or for their geomorphologic, water quality and ecological roles. They often support higher floral and faunal diversity than surrounding habitats (Bentley and Catterall 1997; Woinarski et al. 2000; Catterall et al. 2001), and make important contributions to in-stream habitats through the input of litter and large woody debris and provision of shade (Cummins 1993; Pusey and Arthington 2003). They are a key landscape element for avian diversity and their restoration can greatly benefit bird assemblages in agricultural areas (Johnson et al. 2007; Bennett et al. 2014). These zones can contribute to conservation of forest wildlife, in contrast to the surrounding agricultural land, which can be unfavourable to most forest species (Forman and Godron 1986; Catterall 1993), depending on landscape context. While many studies have focused on the role of such remnant vegetation, others have recognised that many species use diverse ‘countryside’ elements within farmland, and emphasise the benefits of landscape heterogeneity for conservation (Haslem and Bennett 2008). Thus, while riparian zones have been shown to have high biodiversity values, the influence of landscape context on bird assemblages increases as the surrounding land use becomes more intensive (e.g. woodland to pasture to crop) (Martin et al. 2006). Remnant riparian ribbons can act both as patches of habitat in their own right and as corridors connecting larger patches. As they are often retained or restored to protect water quality and reduce erosion, there is potential for them to form part of larger networks of conservation reserves. In order to make improvements to the way riparian zones are managed, it is necessary to understand what factors might determine their value for wildlife.
Area of a patch is a major attribute that affects diversity (Forman 1995) and is determined in the riparian zone by the width and length of the vegetated strip. Since riparian zones are linear features, width may be of greater importance as it determines both the area of habitat available at any location along the stream, as well as the extent to which edge effects can permeate the strip. Edges attract species that may not occur in the forest proper (Moloney 2006), especially aggressive species such as miners and butcherbirds that can exclude smaller species (Major et al. 2001). Remnant attributes can therefore be more important than landscape attributes in determining the composition of bird communities.
In the Queensland Wet Tropics bioregion, in north-eastern Australia, many rainforest species can use remnants of forest as habitat and for dispersal (e.g. Isaacs 1994; Laurance and Laurance 1999), so riparian zones could play an important role in conservation in intensively farmed areas. This study was conducted in a lowland area of the Wet Tropics, where intensive sugarcane farming allows for little habitat diversity (Petit et al. 1999), and where riparian vegetation represents a large proportion of the remnant forest and could make a substantial contribution to off-reserve conservation. We aimed to identify factors that influence bird assemblages in riparian zones of sugarcane-growing areas by examining the relationships between the assemblages and characteristics of the riparian zone, including distance from the main contiguous forest and vegetation width. Such information is necessary for appropriate management of off-reserve habitats. The study comprised (1) a pilot survey to identify factors influencing bird assemblages in riparian forest, (2) an investigation of the effect of distance from remnant forest along a riparian corridor on the bird assemblage, and (3) an investigation of the effect of width of the riparian forest on the bird assemblage. The null hypotheses were that there would be no differences between the bird assemblages of riparian vegetation in sugarcane fields and in pristine forest, and that riparian width and distance from source forest would have no effect on assemblages.
Methods
Study area
The Queensland Wet Tropics bioregion contains the largest area of tropical rainforest in Australia and has great biological significance due to its high species diversity and endemicity (Webb 1987; Goosem et al. 1999). However, some vegetation types, such as those on the coastal lowlands, have been extensively cleared and developed for agriculture and are not represented within the World Heritage Area (Mackey et al. 1989). In 1983, it was estimated that some 56.9% of rainforest in the lowland plains had been cleared (Winter et al. 1987), and that figure has increased over the past three decades: for example, ~66.5% of the study area is now under agriculture (Pitt et al. 2007).
The study was centred on the town of Tully (17°56′S, 145°56′E). Sugarcane production is the dominant land use in this area, although banana plantations and cattle grazing are also common (Webb 1966). The lowlands are bordered to the east by the Coral Sea and to the west by forested mountain ranges, most of which are protected by World Heritage status (Werren 1993). The climate is warm tropical, with a summer wet season (November to March) and a drier cooler season (April to October), although rainfall occurs in all months. The region is one of the wettest in Australia, with average annual rainfall of 4170 mm in Tully; mean minimum and maximum air temperatures range from 15.1–24.0°C in July to 22.7–31.3°C in December (data from the Australian Bureau of Meteorology).
The riparian vegetation of the study area comprises mainly streamside remnants with some regrowth of the original closed rainforest (‘complex mesophyll vine forest’: Webb 1959) that is mostly less than 100 years old. The length of intact rainforest edge in the study area was 31 km, the area of intact lowland forest (to 100 m elevation) was 10.1 km2 (~39% of the study area), the total area of remnant riparian forest was 1.7 km2 (~6.6% of the study area) and the total length of forested riparian ribbons was 53.2 km. The total edge length of riparian ribbons was nominally double this value, but functionally it was probably much less because of the large proportion of narrow (~20 m) ribbon length that might be considered a single ‘edge’.
Study design and site selection
The study was undertaken during the early and mid dry season (April to August), 2003. Potential study sites were identified from topographic maps (1 : 50 000) of the study region (Mena Creek 8062-I, Tully 8062-II, Bilyana 8061-I). Approximately 100 ribbons of remnant riparian forest on sugarcane farms were classified according to several variables measured from these maps and from Google Earth©: they included maximum, minimum and mean width of the strip (width being the distance between the two vegetation edges, incorporating both banks and the stream); length of the strip; distance from large (>200 ha) blocks of remnant forest; connectivity (i.e. whether the strips formed part of a continuous corridor of vegetation that linked to these large forest blocks); and stream order. Only sites below 100 m altitude were considered and sites along major rivers were excluded, as the focus was on replicable lower-order streams.
For the pilot study, a preliminary analysis of frequency distributions of site characteristics across 58 sites (Appendix 1) allowed a replicated design for sites of different widths to be compared at constant length, and sites of different lengths to be compared at constant width (Table 1). Most sites were ‘connected’ (i.e. linked to the main forest), but a few ‘unconnected’ sites (isolated by 500–2750 m from the main forest by agricultural land: Appendix 1) were included to allow differences due to connectivity to be investigated. Three reference sites were located along streams within the main forest. Sites that had been modified recently or could not be accessed were excluded, and for some categories only two replicate sites were accessible (Table 1).
For the distance study, four streams that had at least 2 km of continuous riparian forest and that were connected to the main forest, were selected. For each stream, sites were selected at distances from the remnant forest of 50, 400, 800, 1600 and, for two streams only, 3200 m; four reference riparian sites in the main forest were also selected. For the width study, four sites were selected within width categories of <40 m, 50–70 m, and >80 m. To control for the effects of distance, these sites were situated at ~400 m from the main forest. The four reference sites in the main forest were also included.
Vegetation description
Rapid assessment of the main structural features of the vegetation followed Webb et al. (1976) and Walker and Hopkins (1990). The assessment was made along a 200-m length of vegetation at each site, in the same location where the bird surveys were conducted. Attributes recorded include estimates of canopy height, canopy cover, level of disturbance, abundance of weed species, and abundance of major life forms (Appendix 1). These features were chosen as they are related to differences in site quality (Webb 1959) and are readily observed without requiring precise measurement (Webb 1966), and because birds respond to habitat structure (MacNally 1990; Laurance et al. 1996). A four-point scale was used for recording most features (0, absent; 1, rare or inconspicuous; 2, occasional but conspicuous; 3, common), following Webb et al. (1976). Abundance of each species was estimated on a four-point scale, and the number of flowering/fruiting species at the site were recorded. Total fruit/flower abundance for each site was measured by summing all species’ abundance scores.
Bird surveys
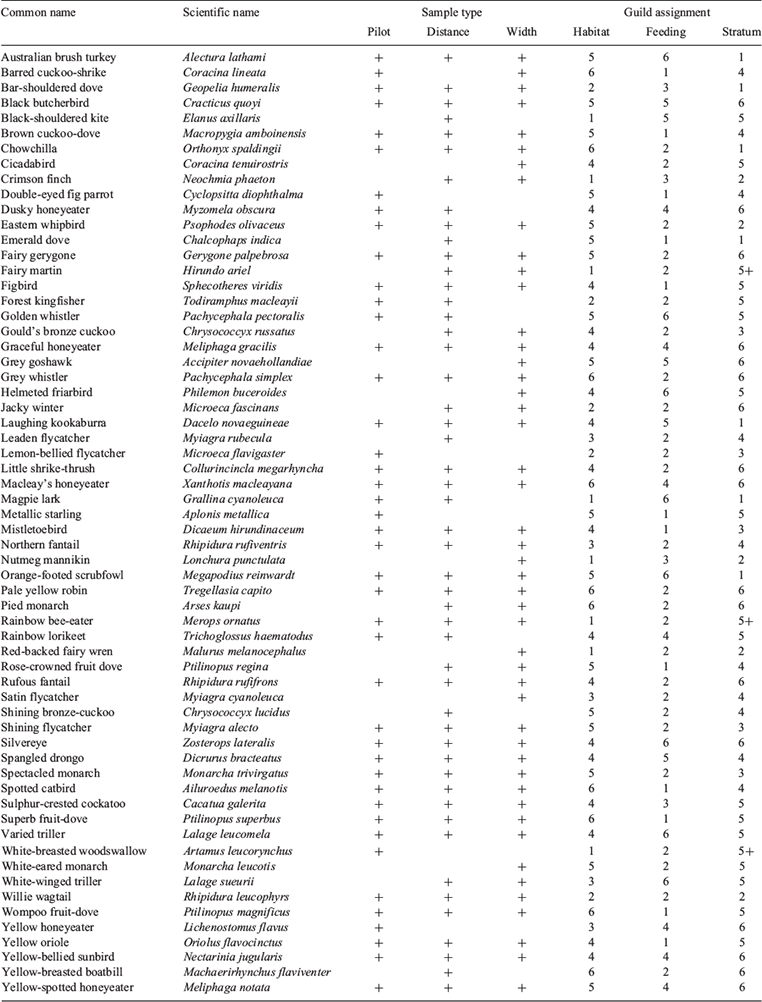
For the pilot study, birds were surveyed during a single census at each site (n = 27) in April 2003. Surveys were conducted between dawn and 0900 hours, when birds are most active (Bibby et al. 1992). For the distance (n = 22) and width (n = 16) studies, birds were censused twice at each site. Morning (dawn to 0900 hours) and afternoon (1600 to 1800 hours) censuses were performed at least four days apart, in June 2003 for the distance study and July–August 2003 for the width study. Rainy and windy days, which reduce bird activity and detectability (Bibby et al. 1992), were avoided. Birds were identified to species and counted. Common names of birds used here follow Pizzey and Knight (1999); Latin names are given in Appendix 2.
|
Line transects in the interior of each site were not possible because of the dense vegetation and often rugged topography, so censuses used line transects (Eberhardt 1978) along the edge of the riparian vegetation with point counts (Recher 1988) conducted within the riparian vegetation, about halfway between its edge and the stream. Five point counts were conducted at ~40-m intervals, perpendicular to the line transect. Each point count lasted 4 min, while 2 min was allowed to travel each 40-m interval, giving a total 28 min of search time for each census. A stopwatch was used so that all 28 min were spent actually searching for birds and did not include time spent walking into the strip for each point count or time spent identifying birds once spotted.
Analysis
Bird abundances for the width and distance studies were taken as the maximum number recorded for each species in the morning and afternoon censuses, to avoid double counting. Bird species were allocated to feeding, foraging-height and habitat guilds (Appendix 2). Feeding guilds were based on published diet descriptions, and allocated according to dominance in each species’ diet (1, frugivore; 2, insectivore; 3, granivore; 4, nectarivore; 5, predator; 6, generalist). Foraging-height groups were based on field observations from the present study and published information (1, ground; 2, ground and vegetation <2 m; 3, vegetation >2 m but below canopy; 4, vegetation >2 m including canopy; 5, canopy and above; 6, all strata). Habitat groupings were allocated following Moloney (2006) (1, open habitats; 2, open forest habitats, not rainforest; 3, woodland and open forest; 4, variety of habitats, including rainforest; 5, rainforest and other closed vegetation; 6, rainforest obligates, or species dependent on rainforest plants).
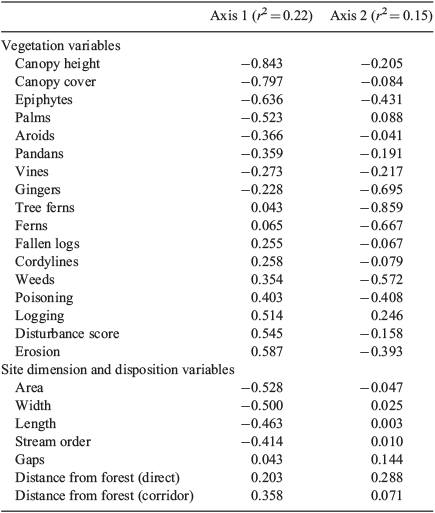
Principal Component Analysis (PCA) (using PC-ORD©: McCune and Mefford 1999) was used to investigate relationships between vegetation and site characteristics. The correlation coefficient was used as the distance measure to standardise the various metrics. Non-metric multidimensional scaling (NMDS, in PC-ORD) of bird abundances (log-transformed) was used to ordinate sites. Correlation coefficients were calculated between the bird-derived NMDS axis scores and site characteristics, including the vegetation PCA axis scores, which acted as measures of vegetation complexity. Bird assemblages were compared between groups of sites using a multiple-response permutation procedure (MRPP, in PC-ORD), which tests for differences between a priori groups, and does not require assumptions such as multivariate normality and homogeneity of variances (McCune and Mefford 1999). Groups of sites compared for the pilot study included reference, connected and unconnected sites, and the different length and width categories.
For the width and distance studies, species richness and total abundance were compared between groups of sites using one-way ANOVA or the Kruskal–Wallis K Samples Test, the non-parametric equivalent, if assumptions of normality or homogeneity of variances were not met (Francis 2001). Indicator species analysis (in PC-ORD) was used to identify species that characterised particular site groups, using the same distance or width categories. This method combines a species’ abundance with its frequency of occurrence to identify indicator species (McCune and Mefford 1999).
Correlation coefficients were calculated between width or distance and vegetation scores, richness and abundance of the bird guilds, and the bird assemblage as a whole. Regression analysis was performed between riparian width and bird species richness and abundance measures, using non-linear models of best fit in the Sigmaplot© 12 package. SPSS© 10.0 for Windows was used for other univariate analyses.
Results
Pilot survey
Vegetation
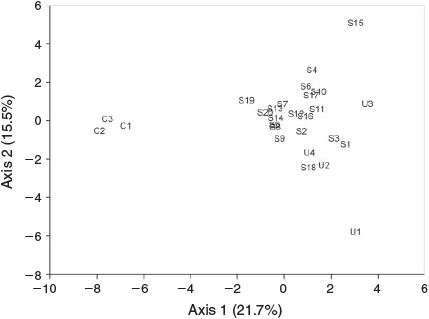
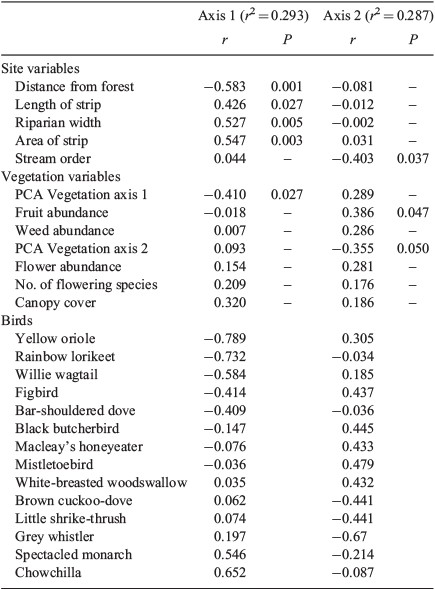
The vegetation at the study sites was complex mesophyll vine forest (Webb 1959), although sclerophyllous species occurred at site S14 (Acacia spp. and Melaleuca sp.) and site S15 (Acacia spp.). PCA differentiated sites on Axis 1 largely on the basis of canopy characteristics and several forest plant groups, contrasting intact forest and disturbed sites (Fig. 1, Table 2). The width, length and area of sites strongly correlated with this axis. On Axis 2, greatest loadings were on various forest features, and there were no correlations with site dimensions apart from stream order (Table 2). MRPP analysis confirmed that reference sites were significantly different from both connected sites (A = 0.110, P = 0.00025) and unconnected sites (A = 0.0253, P = 0.012), but unconnected and connected sites did not differ significantly in their vegetation (A = –0.0081, P = 0.652).
|
Birds
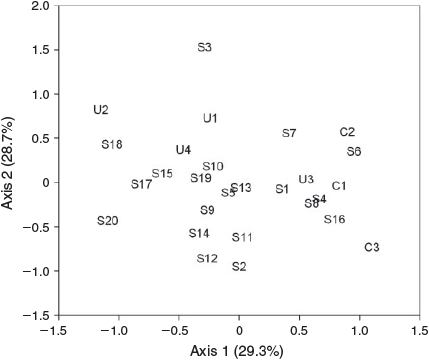
Forty-three bird species were recorded from the 27 sites (Appendix 2), with 7–16 species per sample. NMDS ordination of sites in species’ space showed separation between reference sites and most other sites on Axis 1 (Fig. 2), which correlated positively with width and negatively with distance from the forest and PCA Axis 1 (Table 3). It appears that these relationships were driven by the contrast between the reference sites and the rest as there was no significant relationship between Axis 1 and width when reference sites were removed (r = 0.118, P = 0.582). Birds correlating positively with this axis were the chowchilla and spectacled monarch, both closed-forest specialists (Habitat Guilds 5 and 6). Birds correlating negatively with this axis were characteristic of mixed forest or more open country (Habitat Guilds 2 and 4). Axis 2 correlated negatively with stream order and vegetation PCA Axis 2 and positively with fruit abundance; birds correlating positively with this axis included the fruit eaters (Feeding Guild 1), yellow oriole, figbird and mistletoebird. MRPP analysis identified significant differences between reference sites and unconnected sites (A = 0.2482, P = 0.0396), between reference and other sites (A = 0.0812, P = 0.0099), and between unconnected and other sites (A = 0.0599, P = 0.0268).
|
|
MRPP analysis of the three width classes (‘narrow’, <40 m; ‘mid’, 40–70 m; ‘wide’, >80 m) showed significant differences in bird assemblages (A = 0.030, P = 0.0281), but with the only significant pairwise difference between narrow and mid classes (A = 0.0363, P = 0.0298). Distance, area and length of the riparian strip were correlated with Axis 1 of the ordination (Table 3), although these relationships were again driven by the reference sites as the correlations were not significant when these sites were removed. The ordination generated using vegetation scores showed a similar pattern. Of the individual vegetation features measured, only the abundances of fruiting species were significantly correlated with axes of the ordination of sites based on the bird assemblage (Table 3).
Distance study
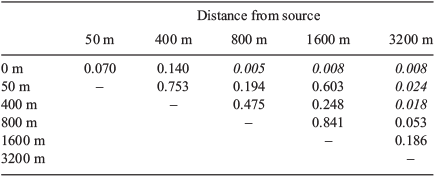
Forty-nine bird species were recorded from the 21 sites sampled for this study, with 8–17 species per sample. Kruskal–Wallis tests found no significant difference in species richness (χ2 = 6.425, P = 0.267) or total abundance (χ2 = 6.287, P = 0.279) between groups of sites in the different distance categories. However, MRPP showed differences in assemblage composition among all distance categories (A = 0.237, P = 0.0015). The results of comparisons between pairs of groups using MRPP showed a gradual change in bird assemblages with distance from forest (Table 4). Each distance category had similar bird assemblages to the two next distances in both directions, but was significantly different from sites further away (except the 50-m and 1600-m sites, which were similar). The PCA indicated that distance was negatively correlated with characteristics of intact forest such as canopy height and cover, and positively with disturbance features (Table 2), so it is difficult to ascribe differences in bird assemblages to distance per se.
|
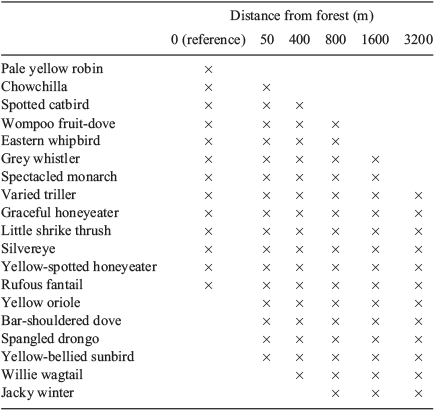
Some species showed clear patterns with distance (Table 5). For example, the pale yellow robin was the only species present at reference sites but not in the riparian remnants, and many species were found only at sites close to source forest (e.g. chowchilla, eastern whipbird, spotted catbird). Others, such as the willie wagtail and jacky winter, were found only at more distant sites. Indicator species analysis identified these two species as significant indicators of the furthest (3200 m) sites (P = 0.014 and P = 0.007), while the eastern whipbird was indicative of 50-m sites (P = 0.042). Many species occurred at all distances and at reference sites (e.g. rufous fantail, yellow-spotted honeyeater) (Table 5). Others, like the yellow-bellied sunbird, were absent from reference sites but were found across all distances in the remnant strips.
|
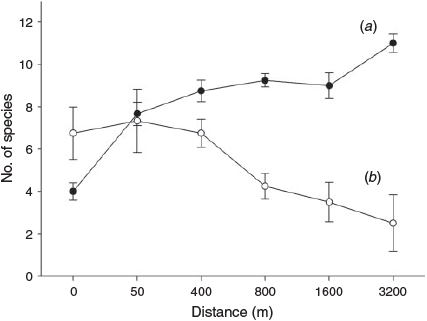
Neither species richness nor total abundance at each site were correlated with distance (r = –0.057, P = 0.807, and r = 0.012, P = 0.960, respectively), but distance effects were evident in some guilds: abundances of species in Habitat Guilds 1, 2 and 3 (more open habitats) increased significantly with distance from forest (r = 0.516, P = 0.017, r = 0.794, P < 0.001, and r = 0.451, P = 0.040, respectively); the abundance of Guild 4 species (habitat generalists) showed no correlation with distance (r = –0.060, P = 0.797); and the abundance of Habitat Guilds 5 and 6 (forest species) decreased with distance (r = –0.603, P = 0.005, and r = –0.492, P = 0.023). Thus, as distance from source forest increased, rainforest species decreased in abundance, while birds of more open habitats became more abundant (Fig. 3).
|
Width study
Forty-seven bird species were recorded across the 16 sites in this study, with between 8 and 19 species per sample. MRPP showed a significant difference among groups (reference, narrow, mid and wide) based on their bird assemblages (A = 0.245, P = 0.0007). However, comparisons between pairs of groups showed that while reference sites were different from all other groups (narrow: A = 0.292, P = 0.0077; mid: A = 0.301, P = 0.0076; wide: A = 0.185, P = 0.0084), bird assemblages at narrow, mid and wide sites were not significantly different. Species richness differed significantly between the four categories of sites (references, <40, 40–70 m, and >80 m widths) (one-way ANOVA: F3,12 = 16.337, P < 0.001). Tukey HSD post hoc analysis showed that reference and narrow (<40 m) groups did not differ from each other (P = 0.879), as both had relatively low richness, while mid (40–70 m) and wide (>80 m) classes, with higher species richness, also did not differ (P = 0.943).
Increasing width led to an initial increase then decline in the number of species in Habitat Guilds 1–4; there was a concomitant rise in the number of species in Guilds 5 and 6 (Fig. 4), which differed significantly between width categories (ANOVA: F3,12 = 8.709, P = 0.002). Species richness of Habitat Guilds 5 and 6 showed a significant linear relationship with riparian width in the remnant strips (excluding reference sites) (r = 0.893, P < 0.001). Species richness of birds in Habitat Guild 5 showed positive relationships with width (r = 0.652, P = 0.022), as did richness of Habitat Guild 6 species (r = 0.680, P = 0.015). Width itself correlated positively with characteristics of intact forest such as canopy height and cover and negatively with disturbance measures (Table 2). Other habitat guilds showed no significant relationship with width. Of the feeding and foraging stratum guilds, only insectivore richness correlated significantly with width (r = 0.720, P = 0.008).
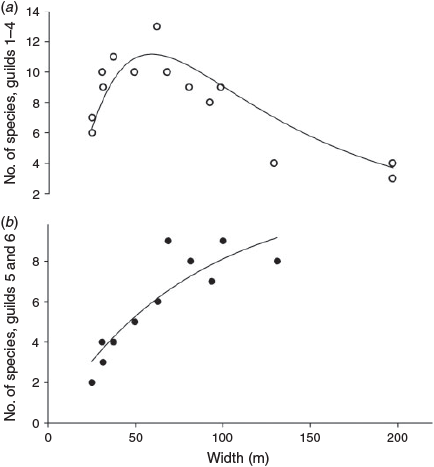
|
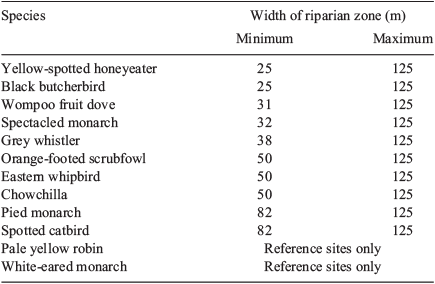
Indicator Species Analysis identified the pied monarch as a significant indicator of sites in the wide (>80 m) category (P = 0.029, Indicator value = 75). Although no other species were significant indicators, some did show identifiable patterns across the different classes of sites (Table 6). For example, the yellow-spotted honeyeater and the black butcherbird were found even at the narrowest sites, the eastern whipbird and the orange-footed scrubfowl occurred only at sites of 50 m or wider, the pied monarch was not found at sites less than 82 m wide, and the pale yellow robin and the white-eared monarch were found only at reference sites.
|
Discussion
Remnant riparian forest sites provided habitat for forest and generalist bird species, but had distinctly different assemblages from those of the reference sites in the intact forest. Bird assemblages in the remnant sites were influenced by width of the ribbon of forest and distance from the main forest. Assemblage composition changed progressively with distance, as birds of closed forest were replaced by species of more open habitats, although increasing distance was confounded by decreasing riparian width. Species richness was highest at the widest sites because of the presence of both closed-forest specialists and open-habitat or edge species. These contrasts were associated with differences in the vegetation, such as taller trees, greater canopy cover and fewer weeds at main forest (reference) sites. We note that this study was undertaken in the dry season; several further species would be expected to occur in the wet season (e.g. cuckoos) but, depending on their habitat preferences, would be expected to reflect the patterns described here.
While the vegetation was generally similar at connected and unconnected sites, vegetation changes may not yet be detectable as most remnants are much less than 100 years old. Some vegetation measures were related to width or distance – for example, canopy height and cover increased with width of the riparian zone. Weediness increased in narrower zones, probably because the large proportion of edge made them susceptible to disturbance and invasion (Panetta and Hopkins 1991), and because the decrease in canopy cover allowed more light through to the forest floor. Proliferation of edge habitat, facilitating intrusion by edge specialists, can deleteriously affect species that are reliant on large areas of forest (Moloney 2006).
Species diversity is typically lower in isolated patches than in patches connected by vegetation corridors (Harker et al. 1993), but no difference was found between the bird assemblages in unconnected and connected riparian sites in this study, even though a few specialist species did not appear in the unconnected sites. Their absence was probably masked in the analysis by the predominance of generalist species in the assemblages. Moloney (2006) made a similar observation for birds in remnant forest patches 50 km north of our study area, which he ascribed to the regular disturbance of the coastal forest by tropical cyclones.
Stream order may be important in determining wildlife assemblages in the riparian zone (Lock and Naiman 1998), and showed a negative relationship to the bird assemblages in this study, probably because stream order increased with distance from the main forest. We found that width of the riparian zone and distance from the main forest were the most important variables in explaining the bird assemblages. Distance from the main forest did not affect species richness or total abundance of birds, but did influence species composition, with forest species replaced by open-habitat species as distance from source forest increased. Species found at all distances were habitat generalists, such as the silvereye, which is known to use small and large habitat remnants (Evans et al. 1997), while species that occurred only close to or within intact forest were rainforest obligates or species that preferred dense vegetation; those recorded only at distant sites were non-rainforest species (Moloney 2006).
Increasing distance from source forest was unavoidably confounded by decreasing width of vegetation along the streams, making it difficult to separate the effects of distance from those of width. For example, distance and width both correlated strongly with Axes 1 and 2 of the PCA of sites by vegetation scores. However, bird species richness clearly increased with riparian width, as has been found elsewhere (e.g. Croonquist and Brooks 1993; Spackman and Hughes 1995). Narrow sites had few rainforest specialists, most species being of more open habitats, probably because most of the area of small remnants is edge habitat (Evans et al. 1997). This effect is likely to be exacerbated in narrow riparian strips which, because of their linear nature, have a large edge : area ratio.
Nevertheless, reference sites had lower richness than all but the narrowest riparian ribbons, because they lacked edge or open-habitat species. Similarly, Laurance et al. (1996) found that both habitat-generalist and non-rainforest species used regrowth, but rarely entered rainforest. The higher richness at wider remnant sites was due to the presence of rainforest species, typical of reference sites, and non-rainforest species found in narrower strips, as reported elsewhere (e.g. Fisher and Goldney 1997). There was an increase in rainforest species richness with riparian width, although overall species composition did not differ significantly between width classes, again because of the masking effect of the larger number of generalist species. Insectivores were the only feeding guild that showed any significant correlation with width, with both their richness and abundance increasing in wider riparian zones.
Changes in assemblages correlated with vegetation characteristics associated with changing width and distance, and were driven by the distribution patterns of individual species. Thus, forest specialist species were found only at wide sites close to source forest; some edge species were found only at more distant sites; and many generalist species occurred at all distances and at reference sites. These patterns clearly relate to traits and needs of individual species. Similarly, Teillard et al. (2014) found that the abundance of specialists was strongly correlated with habitat extent and negatively correlated with habitat heterogeneity whereas generalists were more abundant in landscapes with a higher proportion of arable land and high heterogeneity; and Major et al. (1999, 2001), like us, found that small insectivores were more likely to be found in large forest remnants because aggressive birds excluded the smaller species from smaller remnants. Viewing landscapes as a species-specific gradient of states of remaining habitat and condition is important in conservation management (Lindenmayer et al. 2003) and understanding species-specific responses to vegetation cover is important for predicting the effects of vegetation restoration (Cunningham et al. 2014). Targeting richness alone may miss the individual responses of species, thereby leading to inappropriate management action (Miller and Cale 2000).
Our results suggest some conservation value of remnant riparian vegetation in Wet Tropics agricultural landscapes, in agreement with studies elsewhere (e.g. eastern USA: Keller et al. 1993; Brazil: Lees and Peres 2008; northern Queensland: Isaacs 1994; New South Wales: Fisher and Goldney 1997). Only two species (the pale yellow robin and the white-eared monarch) recorded at reference sites were not also found at remnant riparian sites. However, we did not investigate birds’ survivorship or reproduction within sites, or passage through them, which are necessary to fully understand how to successfully restore fragmented environments (Marzluff and Ewing 2001). It is possible that riparian habitats are merely population sinks for specialist forest species, which would not persist without immigrants from source patches. However, while the riparian ribbons provided only about a tenth of the area of lowland forest in the study area, they provided a greater length of forest edge, which is substantial habitat for native edge and generalist species, no doubt boosting their population sizes in the region, and probably not acting as sinks for them.
The demonstrated influence of width of riparian vegetation, and probably its distance from intact source forest, provide a guide for restoration efforts. The wider the riparian strip, the more effective it is likely to be in reducing edge effects and sustaining characteristics of intact closed forest, which in combination can sustain closed forest biota, providing both habitat and linkages between larger patches (Tucker 2000). In agricultural landscapes there is a need for compromise, such that an optimal width will provide wildlife habitat while minimising the loss of productive land. This study indicated an optimal width of more than 90 m, but even at that width some forest species were not recorded. Comparable conclusions have been drawn from studies elsewhere – for example, recommended widths of riparian forests include 100 m in the eastern USA (Keller et al. 1993), at least 40–45 m in the western USA (Hagar 1999; Pearson and Manuwal 2001), more than 100 m on Vancouver Island (Shirley et al. 2005), and 90–140 m in Brazil (Bueno et al. 2012).
The problem of separating the effects of distance from source forest from those of riparian width could only be solved by examining wide riparian zones further from the source. In the region studied here, this would have been possible only by including sites along higher-order streams, which may have inherent differences in their vegetation or bird assemblages. If distant sites are unable to support forest-interior birds that are sensitive to habitat fragmentation, restoration efforts may best be focussed on extending existing forest blocks or riparian strips close to these blocks. It is probable, though, that at least some species will use wide riparian zones even if they are not close to large areas of source forest. Therefore, restoration of sufficiently wide riparian zones could help to restore connectivity across the landscape, while also providing a reservoir of rainforest bird diversity. Although it is not possible to determine from this study whether such corridors will assist movement of wildlife (Gregory and Beier 2014), movement of many species through riparian strips has been demonstrated for nearby upland sites on the Atherton Tablelands (Isaacs 1994), and it is clear that they will at least provide important habitat. Even smaller habitat patches and linkages between them have value for many open-vegetation species, so conservation efforts in these areas, focusing on forested riparian strips as an integral part of a larger network of conservation reserves, should be beneficial (Petit et al. 1999). Our conclusion for land management is that in the intensive farming landscape of sugarcane production, forest bird conservation can be greatly enhanced by long, preferably interconnecting, corridors of remnant or restored riparian forest of at least 90 m width, enhanced by active weed management.
Acknowledgements
We are pleased to acknowledge the assistance of the landowners who provided access to the sampling sites, and of Rodney and Patricia Keir who provided logistic support during field work. The project was supported with funding from the Sugar Research and Development Corporation. We thank M. Calver and two reviewers, whose constructive comments improved the manuscript considerably.
References
Bennett, A. F., Nimmo, D. G., and Radford, J. Q. (2014). Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments. Journal of Applied Ecology 51, 514–523.| Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments.Crossref | GoogleScholarGoogle Scholar |
Bentley, J. M., and Catterall, C. P. (1997). The use of bushland, corridors, and linear remnants by birds in southeastern Queensland, Australia. Conservation Biology 11, 1173–1189.
| The use of bushland, corridors, and linear remnants by birds in southeastern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Bibby, D. J., Burgess, N. D., and Hill, D. A. (1992). ‘Bird Census Techniques.’ (Academic Press: Toronto.)
Bueno, A. S., Bruno, R. S., Pimentel, T. P., Sanaiotti, T. M., and Magnusson, W. E. (2012). The width of riparian habitats for understory birds in an Amazonian forest. Ecological Applications 22, 722–734.
| The width of riparian habitats for understory birds in an Amazonian forest.Crossref | GoogleScholarGoogle Scholar | 22611867PubMed |
Catterall, C. P. (1993). The importance of riparian zones to terrestrial wildlife. In ‘Ecology and Management of Riparian Zones in Australia’. (Eds S. E. Bunn, B. J. Pusey and P. Price.) pp. 41–52. (Land and Water Resources Research and Development Corporation: Canberra.)
Catterall, C. P., Piper, S. D., Bunn, S. E., and Arthur, J. M. (2001). Flora and fauna assemblages vary with local topography in a subtropical eucalypt forest. Austral Ecology 26, 56–69.
Croonquist, M. J., and Brooks, R. P. (1993). Effects of habitat disturbance on bird communities in riparian corridors. Journal of Soil and Water Conservation 48, 65–72.
Cummins, K. W. (1993). Riparian stream linkages: in-stream issues. In ‘Ecology and Management of Riparian Zones in Australia’. (Eds S. E. Bunn, B. J. Pusey and P. Price.) pp. 5–20. (Land and Water Resources Research and Development Corporation: Canberra.)
Cunningham, R., Lindenmayer, D., Barton, P., Ikin, K., Crane, M., Michael, D., Okada, S., Gibbons, P., and Stein, J. (2014). Cross-sectional and temporal relationships between bird occupancy and vegetation cover at multiple spatial scales. Ecological Applications 24, 1275–1288.
| Cross-sectional and temporal relationships between bird occupancy and vegetation cover at multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Donald, P. F., Green, R. E., and Heath, M. F. (2001). Agricultural intensification and the collapse of Europe‘s farmland bird populations. Proceedings. Biological Sciences 268, 25–29.
| Agricultural intensification and the collapse of Europe‘s farmland bird populations.Crossref | GoogleScholarGoogle Scholar |
Eberhardt, L. L. (1978). Transect methods for population studies. Journal of Wildlife Management 42, 1–31.
| Transect methods for population studies.Crossref | GoogleScholarGoogle Scholar |
Evans, R., Catterall, C. P., and Brumm, G. V. (1997). The habitat value of extremely small bushland remnants to birds in Brisbane. The Sunbird 27, 38–44.
Fisher, A. M., and Goldney, D. C. (1997). Use by birds of riparian vegetation in an extensively fragmented landscape. Pacific Conservation Biology 3, 275–288.
Forman, R. T. T. (1995). ‘Land Mosaics: The Ecology of Landscapes and Regions.’ (Cambridge University Press: Cambridge.)
Forman, R. T. T., and Godron, M. (1986). ‘Landscape Ecology.’ (John Wiley & Sons: New York.)
Francis, G. (2001). ‘Introduction to SPSS for Windows, Versions 9.0 and 10.0 – With Notes For Studentware.’ (Pearson Education Australia: Sydney.)
Frishkoff, L. O., Karp, D. S., M’Gonigle, L. K., Mendenhall, C. D., Zook, J., Kremen, C., Hadly, E. A., and Daily, G. C. (2014). Loss of avian phylogenetic diversity in neotropical agricultural systems. Science 345, 1343–1346.
| Loss of avian phylogenetic diversity in neotropical agricultural systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsV2qtL%2FE&md5=a3e4787f93173cda104f0facf931b166CAS | 25214627PubMed |
Gaston, K. J., Blackburn, T. M., and Goldewijk, K. K. (2003). Habitat conversion and global avian biodiversity loss. Proceedings. Biological Sciences 270, 1293–1300.
| Habitat conversion and global avian biodiversity loss.Crossref | GoogleScholarGoogle Scholar |
Goosem, S. P., Morgan, G., and Kemp, J. E. (1999). Wet tropics. In ‘The Conservation Status of Queensland’s Bioregional Ecosystems’. (Eds P. S. Sattler and R. D. Williams.) pp. 1–72. (Environmental Protection Agency: Brisbane.)
Green, R. E., Cornell, S. J., Scharlemann, J. P. W., and Balmford, A. (2005). Farming and the fate of wild nature. Science 307, 550–555.
| Farming and the fate of wild nature.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmslOhtg%3D%3D&md5=30949a780d6e71a192fe2a160efcfa1aCAS | 15618485PubMed |
Gregory, A. J., and Beier, P. (2014). Response variables for evaluation of the effectiveness of conservation corridors. Conservation Biology 28, 689–695.
| Response variables for evaluation of the effectiveness of conservation corridors.Crossref | GoogleScholarGoogle Scholar | 24606549PubMed |
Hagar, J. C. (1999). Influence of riparian buffer width on bird assemblages in western Oregon. Journal of Wildlife Management 63, 484–496.
| Influence of riparian buffer width on bird assemblages in western Oregon.Crossref | GoogleScholarGoogle Scholar |
Harker, D., Evans, S., Evans, M., and Harker, K. (1993). ‘Landscape Restoration Handbook.’ (Lewis Publishers: Boca Raton, FL.)
Haslem, A., and Bennett, A. F. (2008). Birds in agricultural mosaics: the influence of landscape pattern and countryside heterogeneity. Ecological Applications 18, 185–196.
| Birds in agricultural mosaics: the influence of landscape pattern and countryside heterogeneity.Crossref | GoogleScholarGoogle Scholar | 18372565PubMed |
Isaacs, J. (1994). The riparian link: bird use of riparian vegetation in a fragmented rainforest landscape. M.Sc. Thesis, James Cook University, Townsville.
Johnson, M., Reich, P., and MacNally, R. (2007). Bird assemblages of a fragmented agricultural landscape and the relative importance of vegetation structure and landscape pattern. Wildlife Research 34, 185–193.
| Bird assemblages of a fragmented agricultural landscape and the relative importance of vegetation structure and landscape pattern.Crossref | GoogleScholarGoogle Scholar |
Keller, C. M. E., Robbins, C. S., and Hatfield, J. S. (1993). Avian communities in riparian forests of different widths in Maryland and Delaware. Wetlands 13, 137–144.
| Avian communities in riparian forests of different widths in Maryland and Delaware.Crossref | GoogleScholarGoogle Scholar |
Laurance, S. G. W., and Laurance, W. F. (1999). Tropical wildlife corridors: use of linear rainforest remnants by arboreal mammals. Biological Conservation 91, 231–239.
| Tropical wildlife corridors: use of linear rainforest remnants by arboreal mammals.Crossref | GoogleScholarGoogle Scholar |
Laurance, W. F., Gordon, C. E., and Perry, E. (1996). Structure of breeding bird communities in rainforest and regrowth forest in tropical Queensland. The Sunbird 26, 1–15.
Lees, A. C., and Peres, C. A. (2008). Conservation value of remnant riparian forest corridors of varying quality for Amazonian birds and mammals. Conservation Biology 22, 439–449.
| Conservation value of remnant riparian forest corridors of varying quality for Amazonian birds and mammals.Crossref | GoogleScholarGoogle Scholar | 18241239PubMed |
Lindenmayer, D. B., McIntyre, S., and Fischer, J. (2003). Birds in eucalypt and pine forests: landscape alteration and its implications for research models of faunal habitat use. Biological Conservation 110, 45–53.
| Birds in eucalypt and pine forests: landscape alteration and its implications for research models of faunal habitat use.Crossref | GoogleScholarGoogle Scholar |
Lock, P., and Naiman, R. J. (1998). Effects of stream size on bird community structure in coastal temperate forests of the Pacific Northwest, USA. Journal of Biogeography 25, 773–782.
| Effects of stream size on bird community structure in coastal temperate forests of the Pacific Northwest, USA.Crossref | GoogleScholarGoogle Scholar |
Mackey, B. G., Nix, H. A., Stein, J. A., Cork, S. E., and Bullen, F. T. (1989). Assessing the representativeness of the Wet Tropics of Queensland World Heritage property. Biological Conservation 50, 279–303.
| Assessing the representativeness of the Wet Tropics of Queensland World Heritage property.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R. C. (1990). The roles of floristics and physiognomy in avian community composition. Australian Journal of Ecology 15, 321–327.
| The roles of floristics and physiognomy in avian community composition.Crossref | GoogleScholarGoogle Scholar |
Major, R. E., Christie, F. J., Cowing, G., and Ivison, T. J. (1999). Elevated rates of predation on artificial nests in linear strips of habitat. Journal of Field Ornithology 70, 351–364.
Major, R. E., Christie, F. J., and Gowing, G. (2001). Influence of remnant and landscape attributes on Australian woodland bird communities. Biological Conservation 102, 47–66.
| Influence of remnant and landscape attributes on Australian woodland bird communities.Crossref | GoogleScholarGoogle Scholar |
Maron, M., and Fitzsimons, J. A. (2007). Agricultural intensification and loss of matrix habitat over 23 years in the west Wimmera, south-eastern Australia. Biological Conservation 135, 587–593.
| Agricultural intensification and loss of matrix habitat over 23 years in the west Wimmera, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Martin, T. G., McIntyre, S., Catterall, C. P., and Possingham, H. P. (2006). Is landscape context important for riparian conservation? Birds in grassy woodland. Biological Conservation 127, 201–214.
| Is landscape context important for riparian conservation? Birds in grassy woodland.Crossref | GoogleScholarGoogle Scholar |
Marzluff, J. M., and Ewing, K. (2001). Restoration of fragmented landscapes for the conservation of birds: a general framework and specific recommendations for urbanizing landscapes. Restoration Ecology 9, 280–292.
| Restoration of fragmented landscapes for the conservation of birds: a general framework and specific recommendations for urbanizing landscapes.Crossref | GoogleScholarGoogle Scholar |
McCune, B., and Mefford, M. J. (1999). ‘PC-ORD. Multivariate Analysis of Ecological Data, Version 4.’ (MjM Software Design: Gleneden Beach, OR.)
Miller, J. R., and Cale, P. (2000). Behavioral mechanisms and habitat use by birds in a fragmented agricultural landscape. Ecological Applications 10, 1732–1748.
| Behavioral mechanisms and habitat use by birds in a fragmented agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Moloney, J. (2006). The effects of habitat fragmentation on bird communities in a naturally disturbed environment: the Queensland Wet Tropics lowlands. Ph.D. Thesis, James Cook University, Townsville.
Ormerod, S. J., and Watkinson, A. R. (2000). Editors' introduction: birds and agriculture. Journal of Applied Ecology 37, 699–705.
| Editors' introduction: birds and agriculture.Crossref | GoogleScholarGoogle Scholar |
Panetta, F. D., and Hopkins, A. J. M. (1991). Weeds in corridors: invasion and management. In ‘Nature Conservation 2: The Role of Corridors’. (Eds D. A. Saunders and R. J. Hobbs.) pp. 341–351. (Surrey Beatty: Sydney.)
Pearson, S. F., and Manuwal, D. A. (2001). Breeding bird response to riparian buffer width in managed Pacific northwest douglas-fir forests. Ecological Applications 11, 840–853.
| Breeding bird response to riparian buffer width in managed Pacific northwest douglas-fir forests.Crossref | GoogleScholarGoogle Scholar |
Petit, L. J., Petit, D. R., Christian, D. G., and Powell, H. D. W. (1999). Bird communities of natural and modified habitats in Panama. Ecography 22, 292–304.
| Bird communities of natural and modified habitats in Panama.Crossref | GoogleScholarGoogle Scholar |
Pitt, G., Grounds, S., van den Berg, D., and Denham, R. (2007). Land use change mapping from 1999 to 2004 for the Tully River Catchment. Queensland Department of Natural Resources and Water.
Pizzey, G., and Knight, F. (1999). ‘Field Guide to the Birds of Australia.’ (Angus & Robertson: Sydney.)
Pusey, B. J., and Arthington, A. H. (2003). Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54, 1–16.
| Importance of the riparian zone to the conservation and management of freshwater fish: a review.Crossref | GoogleScholarGoogle Scholar |
Recher, H. F. (1988). Counting terrestrial birds. Australian Zoological Reviews 1, 25–45.
Shirley, S. M., James, N. M., and Smith, J. N. M. (2005). Bird community structure across riparian buffer strips of varying width in a coastal temperate forest. Biological Conservation 125, 475–489.
| Bird community structure across riparian buffer strips of varying width in a coastal temperate forest.Crossref | GoogleScholarGoogle Scholar |
Spackman, S. C., and Hughes, J. W. (1995). Assessment of minimum stream corridor width for biological conservation: species richness and distribution along mid-order streams in Vermont, USA. Biological Conservation 71, 325–332.
| Assessment of minimum stream corridor width for biological conservation: species richness and distribution along mid-order streams in Vermont, USA.Crossref | GoogleScholarGoogle Scholar |
Teillard, F., Antoniuccia, D., Jiguet, F., and Tichita, M. (2014). Contrasting distributions of grassland and arable birds in heterogenous farmlands: implications for conservation. Biological Conservation 176, 243–251.
| Contrasting distributions of grassland and arable birds in heterogenous farmlands: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Tucker, N. I. J. (2000). Linkage restoration: interpreting fragmentation theory for the design of a rainforest linkage in the humid Wet Tropics of north-eastern Queensland. Ecological Management & Restoration 1, 35–41.
| Linkage restoration: interpreting fragmentation theory for the design of a rainforest linkage in the humid Wet Tropics of north-eastern Queensland.Crossref | GoogleScholarGoogle Scholar |
Walker, J., and Hopkins, M. S. (1990). Vegetation. In ‘Australian Soil and Land Survey Field Handbook’. 2nd edn. (Eds R. C. McDonald, R. F. Isbell, J. G. Speight, J. Walker and M. S. Hopkins.) pp. 58–86. (Inkata Press: Melbourne.)
Webb, L. J. (1959). A physiognomic classification of Australian rainforests. Journal of Ecology 47, 551–570.
| A physiognomic classification of Australian rainforests.Crossref | GoogleScholarGoogle Scholar |
Webb, L. J. (1966). The identification and conservation of habitat-types in the wet tropical lowlands of north Queensland. Proceedings of the Royal Society of Queensland 78, 59–86.
Webb, L. J. (1987). Conservation status of the rainforest of north Queensland. In ‘The Rainforest Legacy: Australian National Rainforest Study. Vol. 1’. (Eds G. Werren and P. Kershaw.) pp. 153–158. (Australian Government Publishing Service: Canberra.)
Webb, L. J., Tracey, J. G., and Williams, W. T. (1976). The value of structural features in tropical rainforest typology. Australian Journal of Ecology 1, 3–28.
| The value of structural features in tropical rainforest typology.Crossref | GoogleScholarGoogle Scholar |
Werren, G. L. (1993). Conservation strategies for rare and threatened vertebrates of Australia’s Wet Tropics region. Memoirs of the Queensland Museum 34, 229–241.
Winter, J. W., Bell, F. C., and Pahl, L. I. (1987). Rainforest clearfelling in northeastern Australia. Proceedings of the Royal Society of Queensland 98, 41–57.
Woinarski, J. C. Z., Brock, C., Armstrong, M., Hempel, C., Cheal, D., and Brennan, K. (2000). Bird distribution in riparian vegetation in the extensive natural landscape of Australia‘s tropical savanna: a broad-scale survey and analysis of a distributional data base. Journal of Biogeography 27, 843–868.
| Bird distribution in riparian vegetation in the extensive natural landscape of Australia‘s tropical savanna: a broad-scale survey and analysis of a distributional data base.Crossref | GoogleScholarGoogle Scholar |