Moving to the beat: a review of mammalian sperm motility regulation
Regina M. TurnerDepartment of Clinical Studies, Center for Animal Transgenesis and Germ Cell Research, University of Pennsylvania School of Veterinary Medicine, New Bolton Center, Kennett Square, PA 19348, USA. Email: rmturner@mail.vet.upenn.edu
Reproduction, Fertility and Development 18(2) 25-38 https://doi.org/10.1071/RD05120
Submitted: 21 September 2005 Accepted: 21 September 2005 Published: 14 December 2005
Abstract
Because it is generally accepted that a high percentage of poorly motile or immotile sperm will adversely affect male fertility, analysis of sperm motility is a central part of the evaluation of male fertility. In spite of its importance to fertility, poor sperm motility remains only a description of a pathology whose underlying cause is typically poorly understood. The present review is designed to bring the clinician up to date with the most current understanding of the mechanisms that regulate sperm motility and to raise questions about how aberrations in these mechanisms could be the underlying causes of this pathology.
Extra keywords: flagellum, male infertility, molecular genetics.
Introduction
It is generally accepted that good sperm motility is a central component of normal male fertility. Individuals with poorly motile or immotile sperm are typically infertile or sterile unless advanced assisted reproductive techniques are used. In the clinic, declaring an individual subfertile or infertile because of ‘poor sperm motility’ usually implies little or nothing about the actual pathogenesis of the problem. Other than a handful of single gene defects (largely described in humans and laboratory animals) and several more obvious causes of testicular damage, such as trauma, heat shock or a problem with handling or storing the sperm following ejaculation, we know relatively little about the likely myriad of mechanisms that may be involved in aberrant sperm motility. Thus, poor sperm motility remains predominantly a clinical sign of infertility, rather than a true diagnosis of the cause of infertility.
Because the root cause of asthenozoospermia (poor sperm motility) is usually not known, ‘treatments’ for this problem are non-specific and are predominantly focused on improving breeding management to minimise the requirements for sperm motility (e.g. breeding females close to ovulation or placing sperm in close proximity to the oocyte either via gamete intrafallopian transfer (GIFT) or in vitro fertilisation (IVF)). In the most extreme cases, intracytoplasmic sperm injection (ICSI) now allows the clinician to essentially bypass the requirement for sperm motility in those species for which ICSI has been proven effective in generating pregnancies.
However, the success of these costly and often inefficient assisted reproductive techniques only increases our need to better understand the genes and resultant proteins that are abnormal in these subfertile populations of sperm. Without this knowledge, we may inadvertently allow the transmission of genetic defects to future generations. A more complete understanding of the molecular processes that go into the creation of a motile sperm will enable us to address the issue of reduced motility and associated subfertility more effectively in the clinic. Eventually, poor sperm motility may be treatable or even cured by, for example, stimulating relevant signalling pathways or by genetic therapy, rather than by simply bypassing it with ICSI. Viewed from a different angle, knowledge of the molecules that are required to assemble and regulate a functional flagellum may allow us to intentionally disrupt the normal function of crucial, sperm-specific proteins and so may result in the development of a safe, effective male contraceptive.
The purpose of the present paper is to review our current understanding of the mechanisms that contribute to normal mammalian flagellar function and sperm motility. The goal is to challenge both researchers and clinicians to look more closely at the problem and, as a result, to uncover the pathologies that lie at its heart. The present review includes facts, some likelihoods and a few speculations. Much of the information summarised here was gained through the study of model systems, including mice, rats, hamsters and other species. However, the mechanisms underlying mammalian sperm function are often highly conserved and it is likely that many of the pathways identified in laboratory animal species function similarly in humans and domestic animals.
Two types of physiological mammalian sperm motility
Most mammalian sperm display two types of physiological motility: (1) activated motility, as is seen in freshly ejaculated sperm; and (2) hyperactivated motility, as is seen in most sperm recovered from the site of fertilisation (Katz and Yanagimachi 1980; Suarez and Osman 1987). The flagellum of an activated sperm generates a symmetrical, lower-amplitude waveform that drives the sperm in a more-or-less straight line in relatively non-viscous media, such as seminal plasma or semen extender. It is likely that this form of motility acts to aid in the propulsion of the sperm through the female reproductive tract.
Although some immotile sperm do reach the oviduct (probably as a result of contractions of the female reproductive tract), most sperm that lack activated motility fail to reach the uterotubal junction and so are incapable of in vivo fertilisation. This has been demonstrated clearly in rats (Gaddum-Rosse 1981) and is supported by strong clinical evidence from many mammalian species.
At some point after sperm reach the oviduct, the pattern of the flagellar beat changes to one that is asymmetric and of a higher amplitude. This is termed ‘hyperactivated motility’ and results in a circular or figure-8 trajectory in sperm suspended in seminal plasma or extender (Yanagimachi 1970, 1994; Ishijima et al. 2002). Recently, it was demonstrated that hyperactivated sperm swim in a relatively straight line when they are placed in the more viscous environment of the oviduct. In contrast, the flagellar beat of an activated sperm is insufficient to propel the sperm progressively through the oviduct. Based on this and other studies, it has been suggested that the physiological role of hyperactivated motility is to help sperm detach from the oviducal epithelium and move progressively through the oviducal environment to reach the site of fertilisation. In addition, hyperactivated motility may help the sperm to penetrate the egg vestments (Suarez et al. 1991; Stauss et al. 1995; Ho and Suarez 2001). In rodents, and probably in other species, hyperactivated sperm motility is also correlated with the ability of a sperm to fertilise an oocyte in vitro (Fraser and Quinn 1981; Boatman and Robbins 1991). Thus, the bulk of evidence suggests that a normal sperm must be able to acquire both activated motility in the caudal female reproductive tract and uterus and hyperactivated motility in the oviduct for it to have a reasonable chance of fertilising the egg. If these events do not occur at the proper times and in the proper places, male fertility will be significantly reduced. Although hyperactivated motility is often seen in association with the onset of capacitation, it has been shown that these two pathways are separate or divergent because hyperactivation and capacitation can also occur independently of one another (Olds-Clarke 1989; Marquez and Suarez 2004).
In the clinic, we are typically dealing with freshly ejaculated sperm. Therefore, we usually assess only activated motility. Unfortunately, there are no straightforward clinical methods to induce physiological hyperactivation and, thus, this form of motility is rarely examined in clinical samples. Because it is likely that some, if not all, species require hyperactivated motility for normal male fertility, our inability to efficiently assess this function when examining subfertile males represents one flaw in our examination technique.
Because in the clinical setting we deal predominantly with activated motility, the majority of the present review will discuss the mechanisms involved in the regulation of activated motility (heretofore referred to as ‘sperm motility’ or ‘motility’). Items relevant specifically to hyperactivated sperm motility will be pointed out when appropriate.
The right parts for the job
Normal flagellar ultrastructure is critical for function. In this regard, the four major ultrastructural subdivisions of the flagellum have been well described. These are the connecting piece, the mid-piece, the principal piece and the end-piece (Fig. 1; Fawcett 1975). The connecting piece is the short, most proximal portion of the flagellum that attaches to the implantation fossa of the nucleus in the sperm head. From the remnant of the centriole at this point, the axoneme extends throughout the length of all four subdivisions of the flagellum. The axoneme is made of a ring of nine microtubule doublets surrounding a central pair. Inner and outer dynein arms project from each of the outer nine doublets and these arms are responsible for generating the motive force of the flagellum. In addition, nine radial spokes, each originating from one of the nine outer microtubular doublet pairs, project inwards towards the central pair in a helical fashion (Fawcett 1975; Clermont et al. 1990).
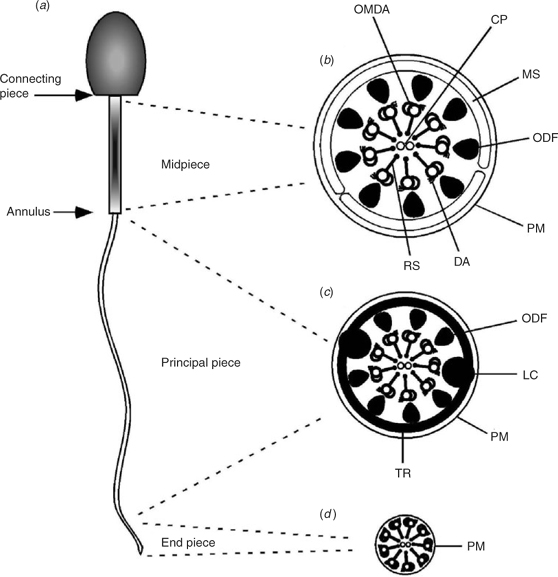
|
The mid-piece is the next most proximal flagellar component and comprises roughly one-quarter to one-third of the length of the flagellum. The mid-piece is defined by the presence of nine outer dense fibres (ODFs) surrounding each of the nine outer axonemal microtubule doublets and by a sheath of mitochondria that encloses the ODFs and the axoneme. The ODFs extend throughout the mid-piece and into the principal piece. The mitochondrial sheath (MS) is exclusive to the mid-piece. The annulus marks the termination of the mid-piece and the start of the next flagellar component, the principal piece. From the annulus, the principal piece extends along approximately two-thirds of the length of the flagellum.
At the start of the principal piece, the MS ends and two of the ODFs are replaced by the two longitudinal columns of the fibrous sheath (FS), thus reducing the number of ODFs in the principal piece from nine to seven. The columns of the FS run the length of the principal piece and are stabilised by circumferential ribs that surround the ODFs. The FS is the only structure that is exclusive to the principal piece. As the principal piece nears its termination, the ODFs and FS taper and eventually terminate. The short terminal section of the flagellum is called the end-piece and contains only the axoneme surrounded by the plasma membrane.
Axonemes are highly conserved in all ciliated and flagellated eukaryotic cells. The MS, ODFs and FS are accessory structures that are exclusive to the mammalian sperm flagellum. Although a few of the genes that code for the proteins that comprise each of these structures have been cloned and characterised, most remain only superficially described or remain unidentified altogether.
To get somewhere fast, you need a motor: the axoneme
The axoneme is the motor of all eukaryotic cilia and flagella. In green algae, over 200 axonemal and axoneme-associated proteins have been described and it is likely that mammalian axonemes are at least as complex. Defects in axonemal proteins can lead to abnormal assembly of the axoneme and/or aberrant motility (Luck et al. 1977; Dutcher 1995; Yagi and Kamiya 1995; Piperno et al. 1996; Smith and Lefebvre 1996). In mammalian sperm, the α- and β-tubulins are by far the most prominent axonemal proteins. Only a handful of other mammalian axonemal proteins have been well characterised.
Dyneins are the ‘motor’ proteins that project from the outer microtubular doublets of the axoneme. There are numerous dyneins that all are part of a large protein family (Porter and Johnson 1989; Holzbaur and Vallee 1994; Milisav 1998). When the dynein ATPase is activated, it causes sliding of adjacent outer axonemal doublet microtubules. As the doublets slide along one another, the flagellum bends (Gibbons and Rowe 1965; Tash and Means 1982).
Because axonemes are found in all flagellated and ciliated cells, axonemal disorders in mammals can result in clinical signs that involve ciliated cell types other than or in addition to sperm. A limited number of these disorders have been characterised in humans and some domestic animals. For example, axonemal defects can result in poor or absent sperm motility and associated infertility (as in primary ciliary dyskinesia Afzelius 1976; Narayan et al. 1994), deafness (if the disorder affects the auditory hair cells, as in Usher’s syndrome; Smith et al. 1994; Well et al. 1995), blindness (if the defect involves the modified ciliated cells (photoreceptor cells) of the retina, as in retinitis pigmentosa; Hunter et al. 1988; Humphries et al. 1992), chronic respiratory disease (if the defect involves ciliated cells of the respiratory tract, as in primary ciliary dyskinesia; Blouin et al. 2000) or some combination of these problems. Large-scale mutagenesis experiments have been performed on axonemal proteins in green algae and, thus, in this organism, many axonemal protein defects have been shown to result in flagellar immotility (Luck et al. 1977; Dutcher 1995).
Structural support for the motor: the outer dense fibres
The first suspected role of the ODFs was that of a passive, rigid support for the tail (Fawcett 1975). The successful cloning of several genes that code for keratin-like intermediate filament ODF proteins provides support for this hypothesis (Gastmann et al. 1993; Morales et al. 1994; Hoyer-Fender et al. 1995, 1998; Kim et al. 1995; Burmester and Hoyer-Fender 1996; Kierszenbaum et al. 1996; Tres and Kierszenbaum 1996; Brohmann et al. 1997; Shao et al. 1997; Schalles et al. 1998; Zarsky et al. 2003). To date, none of these ODF-specific genes has been targeted for mutation and, so, the true role of these proteins, and the role of the ODFs, remains somewhat speculative.
Motors need fuel: the mitochondrial sheath
In sperm, mitochondria are found only in the MS of the mid-piece. As in other cells, sperm mitochondria produce ATP through aerobic respiration. However, sperm mitochondria possess several unique proteins or protein isoforms that are not found in somatic mitochondria. In mice, these include sperm-specific isoforms of lactate dehydrogenase and hexokinase (Burgos et al. 1995; Bunch et al. 1998; Mori et al. 1998; Travis et al. 1998). Thus, defects in these sperm-specific proteins may result only in problems with sperm mitochondrial function, rather than with mitochondrial dysfunction in all cells.
Because aerobic respiration is typically required for a cell to survive, tests for mitochondrial function are usually highly correlated with viability assays. However, some mitochondrial markers, such as rhodamine 123, are able to identify sperm with varying degrees of mitochondrial function (Johnson et al. 1981; Windsor and White 1993; Troiano et al. 1998). Rather than being ‘all-or-none’ assays (as is the case with viability markers), some mitochondrial stains may be able to determine degrees of cell function. Thus, there may be some benefit to these assays over and above standard viability testing.
Also of interest, it has been shown that there are species-specific differences in the metabolic capabilities of mitochondria. This results in variations in the ability of sperm from different species to metabolise different substrates (Storey and Kayne 1980). It is possible that this variation has evolved as a result of species-specific differences in the substrate composition of oviducal fluids and helps explain why sperm from different species seem to have different requirements for semen extenders.
The sperm axonemal dyneins have a high requirement for ATP as an energy source for flagellar motility. However, sperm mitochondria are limited to the mid-piece, whereas the axonemal motor extends throughout the length of the flagellum. If mitochondrial ATP is the only source of fuel for the axoneme, then it would have to diffuse some distance to adequately supply the full length of the axoneme. Using the diffusion constant of ATP and a morphometric estimate of the volume of the mouse sperm flagellum, mathematical models have been developed (Du et al. 1994) that suggest that the ATP produced in the mid-piece is not sufficient to diffuse effectively along the length of the tail to meet the energy needs of the axonemal dynein ATPase (B. T. Storey, personal communication).
In addition, it has been shown, in mice, that if mitochondrial oxidative phosphorylation is defective, fertilisation can still occur, sperm still produce ATP (at lower levels than wild-type sperm) and sperm motility is still present, although reduced (Narisawa et al. 2002). These data suggest that mitochondrial oxidative phosphorylation is not the main source of ATP that supports flagellar motility. However, if this is the case, then what is the source of the fuel that supplies the distant axonemal dyneins? The answer appears to lie in a structure exclusive to the next section of the sperm flagellum, the principal piece.
Fuel, support and more: the fibrous sheath
Like the ODFs, the FS is thought to play a mechanical role in sperm motility by providing a rigid support for the flagellum and determining its planar beat (Fawcett 1975; Lindemann et al. 1992). The two longitudinal columns of the FS, together with the circumferential ribs, create an ‘I-beam’-like structure along which axonemal microtubules can slide. This sliding is cAMP dependent, suggesting that the cAMP-dependent kinase protein kinase A (PKA) is involved (Si and Okuno 1993, 1995).
In addition to its structural support role, the FS may play a more direct role in the regulation of flagellar motility because a growing number of proteins involved in motility signalling pathways and metabolism have been localised to the FS (Carrera et al. 1994; Bradley et al. 1996; Westhoff and Kamp 1997; Bunch et al. 1998; Miki and Eddy 1998; Mori et al. 1998; Travis et al. 1998; Turner et al. 1998, 1999; Nakamura et al. 1999; Fujita et al. 2000; Carr et al. 2001). It has also been suggested that at least one FS protein may act to protect sperm from oxidative stress, which could interfere with sperm motility or cause DNA damage (Fulcher et al. 1995). Based on these data, it now is widely believed that the FS serves as a scaffold and organising centre for multiple signalling and metabolic cascades that are critical for normal flagellar function (Turner et al. 1999; Miki et al. 2002; Eddy et al. 2003; Turner 2003).
The solution that sperm have developed to answer the need for a supply of ATP to the more distal segments of the axoneme apparently lies in the principal piece/FS. It appears that glycolysis is carried out along the length of the principal piece and that this, and not oxidative phosphorylation in the mid-piece, is the most important source of ATP for the tail. Several glycolytic enzymes, including hexokinase, lactate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase (GAPD-S), have been identified in the FS/principal piece of a growing number of mammalian species (Bradley et al. 1996; Westhoff and Kamp 1997; Bunch et al. 1998; Mori et al. 1998; Travis et al. 1998). In some cases, the isoforms of these enzymes are spermatogenic cell specific. In addition, all the glycolytic enzymes downstream of GAPD-S remain attached to the cytoskeleton, even after membrane removal, thus suggesting that they are components of either the FS or the ODFs (Storey and Kayne 1975). It has also been shown that mammalian sperm produce lactate from glucose under aerobic conditions (Storey and Kayne 1975). Adenosine triphosphate production through glycolysis is required for hyperactivated sperm motility (Hoshi et al. 1991; Urner and Sakkas 1996) and inhibition of oxidative phosphorylation does not block fertilisation (Fraser and Quinn 1981).
Formal proof of the importance of FS/principal piece glycolysis has been provided by studies of knockout mice. Targeted deletion of the sperm-specific GAPD-S, an enzyme that localises to the FS, results in male sterility in association with severe aberrations in sperm motility (Miki et al. 2004). Thus, it appears that mammals have solved the problem of ATP diffusion in the flagellum by providing a source of ATP right where it is needed most, through glycolysis along the length of the principal piece of the tail. Thus, glycolysis in the principal piece, but not necessarily oxidative phosphorylation in the mid-piece, is required for normal mammalian flagellar motility.
Sperm from different species have different abilities and requirements for carrying out glycolysis and oxidative phosphorylation. These differences are of profound importance when handling gametes in vitro. For example, glucose will inhibit capacitation in bull sperm, but is required for capacitation in mouse sperm. The role of glucose in sperm metabolism of other species remains under investigation (Quinn et al. 1995; Mahadevan et al. 1997; Barak et al. 1998; Williams and Ford 2001).
Moving to the beat
When the axonemal dynein arms become phosphorylated, the dynein ATPase is activated and the hydrolysis of ATP is converted into force (Tash 1989). The dynein arms then transiently interact with their adjacent microtubular doublets and generate a power stroke, thus causing the microtubules to slide past one another (Satir 1968; Summers and Gibbons 1971; Brokaw 1972, 1989; Shingyoji et al. 1977). Because the axoneme is anchored to the base of the sperm head, this sliding force is translated into a bend in the flagellum. Dephosphorylation of dynein by the calmodulin-dependent protein phosphatase calcineurin reverses this process.
Dynein produces a unidirectional force (Sale and Satir 1977). Thus, the generation of a normal axonemal bend requires that phosphorylation/dephosphorylation and the associated activation and inactivation of the dynein arms occur in an asynchronous manner both around the circumference and along the length of the axoneme (Wargo and Smith 2003). Each dynein arm interacts with its adjacent doublet, generates a stroke to force that doublet to bend, then releases the doublet so that the axoneme can return to its starting position and bend again in the opposite direction.
How is it all regulated?
The cAMP/PKA and calcium signalling pathways are generally recognised as the two signalling pathways that are most central to the regulation of mammalian sperm motility (Suarez et al. 1987; Tash and Means 1987; Lindemann and Goltz 1988; White and Aitken 1989; Brokaw 1991; Yanagimachi 1994; Ho et al. 2002). Heterotrimeric and small G-protein-mediated pathways, as well as pH changes, have also been implicated as playing roles in sperm motility, although these mechanisms are less well characterised in mature sperm (Hinsch et al. 1993; Yanagimachi 1994; Nakamura et al. 1999; Fujita et al. 2000; Carr et al. 2001; Wang et al. 2003).
cAMP/protein kinase A
The cAMP-dependent phosphorylation of flagellar proteins is at least partially responsible for the initiation and maintenance of activated sperm motility in mammals (Tash and Means 1982, 1983; San Agustin and Witman 1994). One of the phenotypes seen in mice with a targeted deletion of the sperm-specific isoform of the catalytic (C) subunit of the cAMP-dependent serine/threonine kinase PKA is male infertility that is highly correlated with poor sperm motility (Skalhegg et al. 2002). Thus, it is very likely that one mechanism of cAMP action is to affect sperm motility through activation of PKA.
Although PKA may work through multiple pathways to control flagellar function, one likely mechanism of its action is that serine/threonine phosphorylation of PKA target proteins results in activation of a downstream, as yet unidentified, tyrosine kinase or kinases whose targets are primarily located in the flagellum (Leclerc et al. 1996; Si and Olds-Clarke 2000). Tyrosine phosphorylation of a specific subset of flagellar proteins then results in motility (Fig. 2). To date, only a few of the protein targets for PKA phosphorylation in sperm have been identified (Tash and Bracho 1998). One known target is axonemal dynein and phosphorylation of this protein appears to be a critical regulatory point in the initiation of flagellar motility, as discussed above (Tash 1989).

|
Serine/threonine phosphatases probably balance the actions of the serine/threonine kinases. The resulting net amount of protein phosphorylation is one factor influencing the status of sperm motility (Tash 1989; Tash and Bracho 1994). In this regard, immotile primate sperm contain higher levels of protein phosphatase 1γ2 (the implication being less net phosphorylation) than do motile sperm and motility can be initiated in bovine caput epididymal sperm by inhibition of phosphatase activity (the implication being an increase in net phosphorylation; Smith et al. 1996; Vijayaraghavan et al. 1996).
In addition to regulating the PKA pathway, cAMP may also activate other signalling pathways in sperm and testes, including a cyclic nucleotide-gated ion channel and/or cAMP-mediated guanine nucleotide exchange factors (Burton et al. 1999; Fig. 2).
Tyrosine phosphorylation is also strongly associated with the onset of sperm motility (Tash and Bracho 1998) and is likely to be downstream of serine/threonine phosphorylation in the motility regulation pathway (Fig. 2). Some specific protein targets of tyrosine phosphorylation in sperm have been suggested and phosphorylation of these proteins has been closely linked to the onset of motility in bovine sperm (Vijayaraghavan et al. 1997b, 2000).
Tyrosine phosphorylation and dephosphorylation of flagellar proteins has also been linked to the onset and end, respectively, of hyperactivated sperm motility in primates and rodents (Chan et al. 1998; Mahony and Gwathmey 1999; Si and Okuno 1999). One of these phosphotyrosine-containing proteins is an A-kinase anchor protein (AKAP) called AKAP4 (Si 1999). The AKAPs are a family of proteins responsible for targeting PKA and other proteins to specific subcellular locations (Scott et al. 2000). Taken together, these data suggest that changes in AKAP-mediated protein targeting are involved in regulating hyperactivated sperm motility. Figure 2 summarises the known and suspected members of the PKA pathway in the flagellum.
Calcium signalling
Extracellular calcium is required for motility in most epididymal sperm samples and calcium is known to regulate both activated and hyperactivated motility (Suarez et al. 1987; Tash and Means 1987; Lindemann and Goltz 1988; White and Aitken 1989; Yanagimachi 1994; Ho et al. 2002). One mechanism by which calcium is directly linked to flagellar function is through its regulation of the atypical ‘soluble’ adenylyl cyclase (sAC), which generates cAMP to activate PKA. The sAC is required for sperm motility and is molecularly and biochemically distinct from the transmembrane adenylyl cyclases (tmACs), in part because sAC is uniquely sensitive to both bicarbonate and calcium (Buck et al. 1999; Chen et al. 2000; Wuttke et al. 2001; Jaiswal and Conti 2003; Litvin et al. 2003; Esposito et al. 2004; Liguori et al. 2004; Hess et al. 2005). Thus, through sAC, calcium signalling can be linked to PKA signalling as parts of a single pathway. However, there are also data to suggest that some calcium pathways are independent of PKA (see below and Fig. 2).
How sperm affect a rise in calcium within the flagellum is not completely clear. Intracellular calcium stores may be involved, particularly in the regulation of hyperactivated motility (Ho and Suarez 2003). However, other than the acrosome, there are no obvious intracellular calcium stores in sperm because, unlike somatic cells, there is no apparent endoplasmic reticulum within the flagellum. The redundant nuclear envelope of the sperm neck is one area that has been implicated as a source of intracellular flagellar calcium (Ho and Suarez 2003).
Regardless of the nature of intracellular stores, there is significant evidence to suggest that the passage of extracellular calcium through one or more membrane channels is another way in which sperm affect changes in intracellular calcium levels (Wiesner et al. 1998; Westenbroek and Babcock 1999; Wennemuth et al. 2000; Ren et al. 2001; Sakata et al. 2002; Quill et al. 2003; Fig. 2). Several voltage-gated calcium channel α1-subunits (i.e. pore-forming) have been identified in sperm (Lievano et al. 1996; Westenbroek and Babcock 1999; Wennemuth et al. 2000) and calcium channel activity has been found both in late-stage spermatogenic cells and mature sperm (Arnoult et al. 1996, 1998, 1999; Benoff 1998; Wennemuth et al. 2000). Voltage-gated calcium channels are known to be present on the sperm acrosome, where they are involved in regulating the acrosome reaction (Evans and Florman 2002). However, some calcium channel subunits also localise specifically to the flagellar principal piece, consistent with their having roles in the regulation of sperm motility (Westenbroek and Babcock 1999; Ren et al. 2001).
Cyclic nucleotide-gated (CNG) calcium channels are also present on the sperm flagellum and developing spermatogenic cells. Different subunits of these channels are present in different temporal and spatial patterns in sperm (Wiesner et al. 1998). These CNG channels may give rise to different patterns of calcium influx in different microdomains of the flagellum. Sperm CNG channels respond to both cGMP and cAMP (Wiesner et al. 1998). Although the CNG channels in sperm seem most sensitive to cGMP, their response to cAMP suggests that this second messenger could be linked to both of the key motility regulating pathways (PKA and calcium signalling; Fig. 2).
Identifying calcium channels on the sperm flagellum provides only indirect evidence that movement of extracellular calcium into the cell is involved in the regulation of motility. However, more direct evidence supporting a role for extracellular calcium in mammalian sperm motility also exists. Calcium increases flagellar wave asymmetry in permeabilised sperm and, eventually, at high enough levels, inhibits motility (Tash and Means 1982). In association with the decline in motility, a decrease in protein phosphorylation is observed. This decrease in phosphorylation is mediated by calmodulin (CaM) and the calcium/calmodulin-dependent phosphatase calcineurin (Tash and Means 1987; Tash et al. 1988).
‘Knockout’ studies also provide strong evidence that movement of extracellular calcium across the plasma membrane is required for sperm motility. The CatSper1 gated cation channel localises specifically to the principal piece of mature sperm and is required for cAMP-induced calcium influx into sperm. Targeted deletion of the CatSper1 gene results in ablation of the cAMP-stimulated rise in intracellular sperm calcium and results in male infertility in association with poor sperm motility (Ren et al. 2001). A related voltage-gated putative calcium channel (CatSper2) has also been described in the sperm flagellum (Quill et al. 2001). Targeted deletion of the CatSper2 gene results in male infertility that is associated with an absence of hyperactivated sperm motility (Quill et al. 2003). Thus, channel-mediated movement of extracellular calcium into the flagellum is required for both types of motility.
Finally, mice containing a targeted deletion of an unrelated voltage-dependent calcium channel, namely Cav2.3 (α1E), although fertile, demonstrated abnormalities in sperm intracellular calcium transients and differences in sperm linearity compared with wild-type sperm. These data provide further evidence that membrane channels are important in the regulation of flagellar function (Sakata et al. 2002).
Downstream components of calcium signalling
Calmodulin is a ubiquitous, highly conserved protein that serves as a classical intracellular calcium receptor (Means et al. 1982). At least some of the effects of calcium on the flagellum are likely to be achieved through CaM, because inhibition of CaM decreases sperm motility (White and Aitken 1989; Ahmad et al. 1995; Si and Olds-Clarke 2000). Components of the PKA pathway can restore motility to CaM-inhibited sperm if the glycolytic products pyruvate and lactate are present in the medium (our unpublished data). Therefore, either CaM is upstream of PKA in the sperm motility regulation pathway or the two pathways are independent but redundant. Interestingly, the effects of calcium on sAC are independent of CaM (Jaiswal and Conti 2003; Litvin et al. 2003). Therefore, although it is known that sAC is required for sperm motility (Esposito et al. 2004; Hess et al. 2005), and although sAC is regulated by Ca, the effect of CaM on motility is not achieved via this cyclase.
Taken together, these data suggest the existence of at least two different calcium pathways: one that is independent of CaM (e.g. sAC/PKA) and one that is not (Fig. 2). Alternatively, calcium could have an effect at two different points within the same pathway. Because deletion of the sAC gene results in immotile sperm, it is clear that calcium/CaM cannot compensate for the loss of sAC function. However, our unpublished data suggest that agonists of the PKA pathway can restore motility when CaM is inhibited. Thus, components of the sAC pathway can compensate for a loss of function in the calcium/CaM component of the pathway(s).
Calmodulin kinase (CaMK) is a downstream target of CaM. Isoforms of CaMK are present in the flagellum of mammalian sperm and CaMK inhibitors reduce sperm motility (Wu et al. 2000; Ignotz and Suarez 2005; Marin-Briggiler et al. 2005). Based on this, it seems likely that the effects of CaM on motility are achieved through one or more isoforms of CaMK in the flagellum (Fig. 2).
Other studies have linked CaM with the regulation of T-type calcium currents in sperm (Lopez-Gonzalez et al. 2001). Calcineurin, a calcium/CaM-dependent serine/threonine phosphatase, has also been implicated in the regulation of flagellar motility (Tash et al. 1988; Carrera et al. 1996). Thus, CaM/CaMK/calcineurin may represent components of a calcium-regulated sperm motility pathway in mammals.
Being in the right place at the right time
Sperm are highly compartmentalised cells. Proteins involved in the acrosome reaction must localise to the acrosome. Proteins involved in binding to the egg plasma membrane localise to the equatorial region. In the case of sperm motility, proteins directly required for sperm motility must localise specifically to the tail of mature sperm. One means by which proteins can be compartmentalised is through the use of AKAPs. The AKAPs are a family of proteins that function to tether PKA to specific subcellular regions. In the case of sperm motility, AKAPs tether PKA to the FS of the flagellum, thus restricting the scope of action of the kinase to within close proximity of motility related targets in the axoneme (Carrera et al. 1994; Mei et al. 1997; Miki and Eddy 1998; Mandal et al. 1999; Vijayaraghavan et al. 1999).
In this regard, the major protein of the mouse sperm FS is an AKAP called AKAP4 (Carrera et al. 1994). The AKAPs are now known to be major components of the FS/principal piece in a variety of mammalian species, suggesting that their role in flagellar function is evolutionarily conserved and, therefore, critical (Turner et al. 1998, 2005; Mandal et al. 1999; Moss et al. 1999; Jha and Shivaji 2002). Compartmentalisation of proteins to the flagellum via AKAPs is believed to ensure that the appropriate proteins find themselves in the right place at the right time to facilitate normal flagellar function (Fig. 2). In support of the importance of AKAP-based protein anchoring to flagellar function, mice containing a targeted mutation of the AKAP4 gene are infertile, in association with severely reduced sperm motility (Miki et al. 2002). In addition, specific inhibition of the anchoring of the type II regulatory subunit of PKA to AKAPs in sperm resulted in an arrest of bovine sperm motility (Vijayaraghavan et al. 1997a).
Interestingly, targeted deletion of the type II regulatory subunit of PKA, and associated loss of anchoring of the catalytic subunit of PKA, had no apparent affect on murine sperm motility or fertility (Burton et al. 1999). At first glance, these data appear to argue against a required role for PKA anchoring (presumably via AKAPs) and sperm motility. However, another possible conclusion is that other isoforms of PKA that are present in the flagellum may be able to compensate when anchoring of the type II regulatory subunit is lost.
In addition to the loss of sperm motility in AKAP4-knockout mice, it was reported that these mice also had a reduction in or a total loss of several proteins (in addition to PKA) from the flagellum (Miki et al. 2002). This finding suggests that, like other members of the AKAP family, AKAP4 is responsible for tethering several different proteins to a specific subcellular region. In this regard, other proteins have been implicated as being compartmentalised to the flagellum, and specifically to the FS, via interactions with anchoring proteins (Carr et al. 2001; Brown et al. 2003).
Interestingly, in somatic cells, members of the AKAP family scaffold both kinases and phosphatases to a single place within the cell (Coghlan et al. 1995; Klauck et al. 1996). If these anchoring proteins play similar roles in sperm, then they may serve as master organisers of the phosphorylation/dephosphorylation pathways that are required for the regulation of motility.
Aberrant sperm motility: what is going wrong?
We now have covered some of the physiological processes that are required for normal sperm motility. However, as clinicians, we often are asked to explain the reasons for aberrant sperm motility and associated male infertility. Currently, very little of the information gained from the basic studies described above has made its way into clinical practice. However, using this information, we can now intelligently speculate as to what may be going wrong in males with idiopathic asthenozoospermia.
Obviously, sperm with gross or ultrastructural morphological defects of the tail would be expected to have mechanical problems generating a normal flagellar beat. Typically, these abnormalities can be identified on routine morphological assessment of sperm or, in more specialised cases, using electron microscopy.
Because of the need for oxidative phosphorylation for basic cell maintenance, it is not hard to imagine that defects of sperm mitochondrial proteins that result in compromised ATP production would adversely affect sperm function. Assays for mitochondrial function may be useful in these cases. However, as described above, current evidence argues against an absolute requirement for mid-piece oxidative phosphorylation in sperm motility. In light of this, poor mitochondrial function may not be a primary cause of poor sperm motility. Clinical tests for normal principal piece glycolysis would probably be more directly relevant, if they could be developed.
Considering the complexity of the signalling pathways that must work properly to support sperm motility, it seems likely that any number of aberrations of these pathways could be responsible for naturally occurring cases of poor sperm motility. For example, problems with membrane calcium channels (as exhibited by sperm immotility in CatSper-knockout mice; Ren et al. 2001), problems with production of cAMP and thus defects in the PKA pathway (as exhibited by sperm immotility in sAC-knockout mice; Esposito et al. 2004) or problems with any of the pathway components (such as the reduced motility seen in sperm with inhibited CaM or CaMK; Si and Olds-Clarke 2000; Ignotz and Suarez 2005; Marin-Briggiler et al. 2005) have all been shown to negatively impact on sperm motility in mice and, in some cases, in other mammalian species. If similar problems occur naturally, then it seems highly likely that they will be manifested as clinical cases of sperm immotility and male subfertility. To date, the author is not aware of any clinical assays that have been developed to assess the functionality of signalling pathways in the flagellum. However, now that we have a base of knowledge on the molecular physiology of flagellar function, we should be able to make significant strides towards testing for potential problems in these pathways in our clinical cases and, hopefully, eventually towards providing specific treatments for these disorders.
Another slant to the importance of understanding the molecular workings of the flagellum is that once these pathways are understood, it may be possible to intentionally down-regulate flagellar function and so develop a safe and effective male contraceptive. Because so many sperm proteins are specialised and are not found in somatic cells, one could readily identify proteins that, when targeted, would ablate sperm motility without any undesired side effects.
Conclusions
Using our understanding of the molecular basis for sperm motility, we can now begin to imagine the number of genes and resulting proteins that must function normally to generate a motile sperm. These include proteins involved in sperm structure, flagellar assembly, calcium signalling, protein phosphorylation, metabolism and protein targeting. And we have not even addressed the genes involved in spermatogenesis (e.g. the deleted in azoospermia gene DAZ) or in the development of a normal reproductive tract (e.g. the cystic fibrosis transmembrane conductance regulator (CFTR) gene). Many questions remain and gaps in our knowledge must be filled before we can comprehensively address the problem of idiopathic asthenozoospermia. The information gained from the study of flagellar physiology will allow the clinician to be more informed when analysing clinical cases of asthenozoospermia and may eventually result in improved diagnostics and specific treatments for male infertility. Alternatively, it could lead us to the development of an effective and safe male contraceptive.
Acknowledgments
The author thanks the National Institutes of Health (HD01189-03), CONRAD Mellon Foundation (10100710) and the University of Pennsylvania Research Foundation for their support.
Afzelius, B. A. (1976). A human syndrome caused by immotile cilia. Science 193, 317–319.
| PubMed |
Zarsky, H. A. , Cheng, M. , and Van Der Hoorn, F. A. (2003). Novel RING finger protein OIP1 binds to conserved amino acid repeats in sperm tail protein ODF1. Biol. Reprod. 68, 543–552.
| Crossref | GoogleScholarGoogle Scholar | PubMed |



