Effects of freezing and activation on membrane quality and DNA damage in Xenopus tropicalis and Xenopus laevis spermatozoa
S. Morrow A , J. Gosálvez B , C. López-Fernández B , F. Arroyo B , W. V. Holt C and M. J. Guille A DA School of Biological Sciences and European Xenopus Resource Centre, The University of Portsmouth, Portsmouth, PO1 2DT, UK.
B Department of Biology, Genetics Unit, The Autonomous University of Madrid, 20849 Madrid, Spain.
C Academic Department of Reproductive and Developmental Medicine, The University of Sheffield, Sheffield, S10 2SF, UK.
D Corresponding author. Email: matthew.guille@port.ac.uk
Reproduction, Fertility and Development 29(8) 1556-1566 https://doi.org/10.1071/RD16190
Submitted: 10 April 2015 Accepted: 4 July 2016 Published: 3 October 2016
Journal Compilation © Published Open Access CC BY 2017 Open Access CC BY-NC-ND
Abstract
There is growing concern over the effect of sperm cryopreservation on DNA integrity and the subsequent development of offspring generated from this cryopreserved material. In the present study, membrane integrity and DNA stability of Xenopus laevis and Xenopus tropicalis spermatozoa were evaluated in response to cryopreservation with or without activation, a process that happens upon exposure to water to spermatozoa of some aquatic species. A dye exclusion assay revealed that sperm plasma membrane integrity in both species decreased after freezing, more so for X. laevis than X. tropicalis spermatozoa. The sperm chromatin dispersion (SCD) test showed that for both X. tropicalis and X. laevis, activated frozen spermatozoa produced the highest levels of DNA fragmentation compared with all fresh samples and frozen non-activated samples (P < 0.05). Understanding the nature of DNA and membrane damage that occurs in cryopreserved spermatozoa from Xenopus species represents the first step in exploiting these powerful model organisms to understand the developmental consequences of fertilising with cryopreservation-damaged spermatozoa.
Additional keywords: cryopreservation, DNA fragmentation, sperm chromatin dispersion.
Introduction
In the face of the current global amphibian extinction crisis, sperm cryopreservation represents a key potential strategy for supporting threatened populations and enabling the future re-establishment of recently extinct species (Clulow et al. 2014). There is also increasing interest in the use of sperm cryopreservation for storing genetically altered lines of amphibians, especially Xenopus laevis and Xenopus tropicalis, given their growing importance as biomedical models (Khokha 2012; O’Neill and Ricardo 2013; Pratt and Khakhalin 2013; Schmitt et al. 2014). Such storage strategies enhance animal welfare by reducing the number of animals held and have a positive effect on the cost-effectiveness of biomedical research. Although cryopreserved amphibian spermatozoa recover motility and can fertilise eggs in vitro (Beesley et al. 1998; Browne et al. 1998; Michael and Jones 2004; Sargent and Mohun 2005), success rates are variable and species dependent.
Previous studies have reported that between 5% and 10% of X. laevis spermatozoa are potentially motile following the freeze–thaw process and show fertility rates ranging from 36% to 81%. Despite the decrease in post-thaw survival, sufficient spermatozoa were recovered to generate mutant genotypes (Sargent and Mohun 2005). This is increasingly important because a wide range of mutants is now available in this model organism (e.g. Fish et al. 2014; Nakayama et al. 2015; Shi et al. 2015) that provides cost-effective access to a very wide range of experimental approaches while sharing much of the genome structure of humans (Hellsten et al. 2010). More recent efforts to optimise cryopreservation of X. laevis spermatozoa based on a matrix devised to evaluate the various stages of the freezing protocol yielded encouraging results (Mansour et al. 2009). However, there was a reduction in fertilisation rate (from 82% to 60%) and hatching rate (from 60% to 48%) from fresh to frozen samples. These decreases strongly suggest that cryopreserved amphibian spermatozoa have undergone damage that reduces the success of embryonic development; despite this, little is known about the nature and extent of such damage.
DNA fragmentation is an inherent feature of DNA condensation during spermiogenesis (Meistrich et al. 2003), but it can also be caused by external factors (González-Marín et al. 2012) (Wright et al. 2014). There is increasing evidence that the processes involved in sperm cryopreservation, which include exposure to various chemicals as well as temperature and osmotic excursions, induce sperm DNA fragmentation (Kopeika et al. 2015). Therefore, the consequences for genome integrity as a result of the freeze–thaw process are of great concern given the importance of an intact genome for successful embryonic development. For example, inhibition of DNA repair in trout embryos derived from DNA-damaged spermatozoa revealed differential expression of genes involved in growth and repair, nervous system development, morphogenesis and cell differentiation (Fernández-Díez et al. 2015). Furthermore, trout embryos obtained from frozen spermatozoa had an increased number of deaths and altered mRNA levels of genes involved in growth and morphogenesis, including insulin growth factor receptor 1a (Igfr1a), growth factor 1 (Gh1) and insulin 1 (Ins1), suggesting a direct effect of fertilising with DNA cryodamaged spermatozoa (Pérez-Cerezales et al. 2011). In the loach, fertilisation with cryopreserved spermatozoa in the presence of a DNA repair inhibitor also revealed extensive embryonic death and axial defects in the surviving embryos (Kopeika et al. 2004). It is therefore clear that cryopreservation of spermatozoa can directly affect development, but in order to understand the mechanisms affected by sperm cryopreservation in detail it is necessary to work in a well-defined embryo model system.
The processes and mechanisms that govern embryonic development in Xenopus are well understood and the usefulness of this model has improved since the genomes of both species have become available (Hellsten et al. 2010; Karpinka et al. 2015). Xenopus are particularly suited to these studies because of the large numbers of embryos that can be produced synchronously and develop externally, and because they are readily analysed at various stages of development both phenotypically and by high-throughput biochemical analysis (Harland and Grainger 2011). More studies are urgently needed to shed light on the developmental consequences of cryoinduced DNA damage and Xenopus provides an ideal platform to do so. The present study represents an important first step towards this objective.
The aim of the present study was to test, in Xenopus, how sperm freezing and subsequent activation for IVF modify both the integrity of the sperm plasma membrane and the quality of the DNA. We hypothesised not only that cooling and freezing would induce DNA damage, but also that the extent of DNA damage would be exacerbated by motility activation. The practical use of frozen–thawed spermatozoa at the European Xenopus Research Centre (EXRC) has suggested that the effectiveness of cryopreservation differs between X. laevis and X. tropicalis, and one of the objectives of the present study was therefore to investigate and document these differences systematically. Species differences in sperm cryopreservation success occur frequently and sometimes provide useful insights into the mechanisms of cryoinjury (Holt 2000).
Materials and methods
Animals and recovery of spermatozoa
All the experiments described herein were performed under the appropriate national legislation. Xenopus were maintained in a recirculating aquarium system (Tecniplast) and fed at least once daily with high-protein trout pellets; X. laevis were maintained at 18°C and X. tropicalis were maintained at 25°C. Four male X. tropicalis and four male X. laevis sourced from the EXRC were killed by terminal anaesthesia in ethyl-m-aminobenzoate (Sigma Aldrich) followed by destruction of the brain. Testes were removed and placed in 1.0× modified Barth’s saline (MBS; Gurdon 1977). To obtain fresh sperm samples from an X. tropicalis male, a single testis was macerated in 250 µL of 0.1× MBS and diluted with an equal volume of either 0.1× MBS or distilled water (Sigma-Aldrich), for non-activated or activated samples respectively, to a final concentration of approximately 10 × 106 spermatozoa mL–1. To obtain fresh sperm samples from an X. laevis male, a quarter of a testis was used in the same way but was diluted with 1.0× or 0.1× MBS for non-activated or activated samples respectively.
Experimental design
Analyses of sperm plasma membrane integrity and DNA fragmentation were performed within 2 min of sperm collection, using activated and non-activated sperm samples from each male (T0). Four biological replicates were performed side by side using a random sampling order and 300 spermatozoa were analysed on each slide. Analyses were repeated after incubation for 4 h (T4) or 24 h (T24) at room temperature. From each male, a separate portion of testis was removed, spermatozoa were extracted and then cryopreserved (see Sperm Freezing and Thawing section below) and the same analyses were performed.
Cryoprotective solution
Cryoprotective solution was made by first dispersing one chicken egg yolk (~15 mL) in an equal volume of distilled water and then diluting to 20% v/v in solution containing 0.4 M sucrose, 10 mM NaHCO3 and 2 mM pentoxyfylline. Aliquots of cryoprotective solution were then centrifuged at 10 000g for 20 min at 10°C in an Eppendorf 5810R centrifuge with an F-34-6-38 rotor. The pellets were discarded and the supernatants were divided into 500-µL aliquots then frozen and stored at –20°C (method adapted from the Harland Laboratory; http://tropicalis.berkeley.edu/home/obtaining_embryos/sperm-freezing/sperm-freeze.html, accessed 1 October 2013).
Sperm freezing and thawing
A single X. tropicalis testis or approximately one-quarter of an X. laevis testis was transferred to an Eppendorf tube in 0.5 mL Leibovitz-15 (L-15) medium (Sigma-Aldrich) supplemented with 10% calf serum (Sigma-Aldrich) and 2 mM l-glutamine; it was then dissociated by gentle application of an Eppendorf pestle. Ice-cold cryoprotective solution was added to ice-cold sperm macerates in a ratio of 1 : 1 and gently mixed, and 250 µL samples were pipetted into 0.5-mL thin-walled polypropylene Eppendorf tubes (Fisher Scientific). The tubes were placed in a polystyrene box covered with aluminium foil and placed directly into the –80°C freezer for at least 24 h. This was the most effective of the three published methods (Sargent and Mohun 2005; Mansour et al. 2009; Harland lab protocol, above) we have tested when measured by healthy tadpole production. After a minimum of 24 h, sperm samples were removed from the –80°C freezer and held by hand to defrost them. X. tropicalis samples were activated with 250 µL sterile nuclease-free water and X. laevis samples were activated with 250 µL of 0.1× MBS before analysis (method adapted from the Harland Laboratory; http://tropicalis.berkeley.edu/home/obtaining_embryos/sperm-freezing/sperm-freeze.html, accessed 1 October 2013).
Plasma membrane integrity
Sperm plasma membrane integrity was assessed using propidium iodide (PI; Yániz et al. 2013). Each sample was diluted to 1.6 × 106 cells mL–1; then, 8 µL was pipetted onto the surface of a glass slide and mixed with 1 µL acridine orange (Sigma Aldrich) and PI (Sigma Aldrich), each at a stock concentration of 1 mg mL–1. Acridine orange, which has an emission maximum of 525 nm (green) when bound to DNA, permeates all spermatozoa. However, this is displaced by PI in spermatozoa with damaged plasma membranes, which then fluoresce red (emission maximum 617 nm). Three hundred spermatozoa were manually counted per sample using a Leica DMRB epifluorescence microscope (Leica Microsystems) equipped with single-band fluorescence block filter for green (fluorescein isothiocyanate (FITC) equivalent) and red (Cy3 equivalent) fluorescence. The proportion of spermatozoa stained green was then calculated.
Sperm chromatin dispersion test
In the present study the sperm chromatin dispersion (SCD) test was performed using the Sperm-Halomax kit (Halotech) adapted for Xenopus, with the following protocol. Molten 50-µL aliquots of low melting point agarose maintained at 35°C were mixed with 20-µL portions of sperm suspension. Aliquots (10-µL) of the sperm–agarose mixture were then pipetted onto the surface of a pretreated glass slide (provided in the Sperm-Halomax kit) and covered with a glass coverslip (18 × 18 mm). Gentle pressure was applied to the coverslip to ensure the formation of a thin, even microgel. The slides were then placed onto a prechilled (4°C) metallic tray and placed in the refrigerator at 4°C for at least 5 min. The slides were then taken out of the refrigerator, the coverslips removed and the slides placed horizontally into a bath of lysing solution provided in the Sperm-Halomax kit for 5 min to lyse the spermatozoa and deproteinise the DNA. The slides were then removed from the lysis solution and placed in a bath of distilled water for 5 min, followed by subsequent washes in 70% and 100% ethanol for 2 min each time to dehydrate the microgels. The slides were then finally left to air dry. Equal parts of SYBR green (Sigma Aldrich) and VectaShield mounting medium (Vector Laboratories) were added to the slides, which were visualised using fluorescent microscopy. Three hundred spermatozoa were counted per sample using a Leica DMRB epifluorescence microscope (Leica Microsystems) equipped with single-band fluorescence block filter for green (FITC equivalent) and red (Cy3 equivalent) fluorescence. The maximum excitation wavelength of SYBR green is 497 nm and the fluorescence emission of SYBR green-stained DNA is 520nm. The proportion of spermatozoa showing DNA fragmentation was calculated.
In situ nick translation
For in situ nick translation (ISNT), spermatozoa were diluted to 10 × 106 cells mL–1 and embedded into an agarose microgel on a pretreated slide as described above for the SCD test using slides and lysis solution from the Sperm-Halomax kit. Once the slides had been dehydrated in the ethanol series and dried, they were washed in phosphate-buffered saline (PBS) for 5 min and then incubated in DNA polymerase reaction buffer (10 mM Tris-HCl, 5 mM MgCl2, 7.5 mM dithiothreitol, pH 7.5) for 10 min at 37°C. Then, 100 µL reaction buffer containing 25 units DNA-polymerase I Large (Klenow) Fragment (Invitrogen) and 20 µM of each nucleotide, including biotin-16-dUTP, was pipetted onto the slide, covered with a plastic coverslip and incubated in a moist chamber for 30 min at 37°C. As a negative control, the same procedure was repeated on another microgel containing spermatozoa from the same male but without the DNA polymerase I. The slides were then washed three times in PBS for 5 min each time and then dehydrated in sequential 70%, 90% and 100% ethanol baths and air dried. To detect the incorporation of biotin-16-dUTP, slides were incubated with streptavidin Alexa Fluor 488 conjugate (Life Technologies) diluted 1 : 500 for 30 min at 21°C and then washed three times in PBS to remove excess streptavidin Alexa 488. The maximum excitation wavelength of Alexa Fluor 488 is 493 nm, with a fluorescence emission of 519 nm. Slides were then counterstained with PI (2 µg mL–1). When PI is bound to nucleic acids, the fluorescence excitation maximum is 535 nm and the emission maximum is 617 nm. Fluorescence images were captured using a Zeiss Axiomager ZI fluorescence microscope equipped with a Hamamatsu digital camera (C4742-95) with single-band fluorescence block filters for green (FITC equivalent) and red (Cy3 equivalent) to detect fluorescence. Areas of interest were captured using Velocity 4 software (Improvision; PerkinElmer). Three hundred spermatozoa were assayed and counted for each treatment.
Statistical analysis
Statistical analyses were performed using STATISTICA for Windows (Statsoft UK). Effects were examined using analysis of variance (ANOVA) and specific contrasts were examined within the ANOVA using orthogonal polynomial coefficients. Where data did not exhibit normal distribution, the non-parametric Kruskal–Wallis test was used for statistical analysis. Relationships between membrane integrity, DNA fragmentation and sperm freezing were examined using analysis of covariance (ANCOVA; vassarstats.net, accessed 21 May 2014). All data are shown as the mean ± s.e.m.
Results
Assessment of sperm DNA quality in response to stressors requires the dynamic evaluation of DNA damage. This approach of assessing DNA damage over time to create a ‘profile’ indicative of DNA stability can reveal damage that is cryptic and/or occurring at levels not easily detectable at the time the spermatozoa are extracted or thawed (Gosálvez et al. 2009, 2011a). Thus, more reliable comparisons between individuals or groups can be drawn and this approach was adopted throughout.
Plasma membrane integrity
The dye exclusion test was able to distinguish effectively between spermatozoa with intact or damaged membranes in X. tropicalis (Fig. 1a). Spermatozoa with damaged membranes also showed swelling (e.g. Fig. 1b, white arrow). We also detected a morphological abnormality in the form of macrocephaly, suggesting a polyploid spermatozoon (e.g. Fig. 1b, yellow arrow). More significant progressive swelling and diffuse staining was observed in spermatozoa stained red (Fig. 1c, d). Although the number of spermatozoa with poor membrane integrity was higher in frozen and activated samples (see below) than in controls, there were no clear morphological differences between the samples or between species (data not shown).
|
Sperm activation status had little or no effect on the detrimental effects of freezing and thawing (F1,36 = 3.77; P > 0.05) and, at T0, the extent of freezing-induced plasma membrane damage was significant, regardless of activation status (F1,18 = 27.95 (P < 0.001) and F1,18 = 15.01 (P = 0.001) for non-activated and activated spermatozoa respectively). Therefore, activation status was disregarded for the purposes of further data analysis.
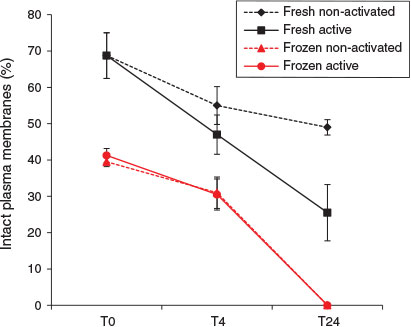
Sperm plasma membrane integrity was significantly reduced in X. tropicalis after freezing and thawing (Fig. 2); mean values at T0 for fresh and frozen–thawed spermatozoa were 68.8 ± 4.1% and 40.4 ± 1.1% respectively (F1,8 = 370.7; P < 0.001). Equivalent mean values for the entire 24 h period were 52.3 ± 3.7% and 23.7 ± 3.7% for fresh and frozen–thawed spermatozoa respectively (F1,46 = 20.9; P < 0.001). Membrane integrity of fresh and frozen–thawed spermatozoa declined at equivalent rates over the first 4 h of incubation, as indicated by the lack of significant interaction between percentage membrane integrity and freezing during that period (F1,28 = 1.62; P > 0.05), but after 24 h all the frozen–thawed spermatozoa exhibited plasma membrane damage.
|
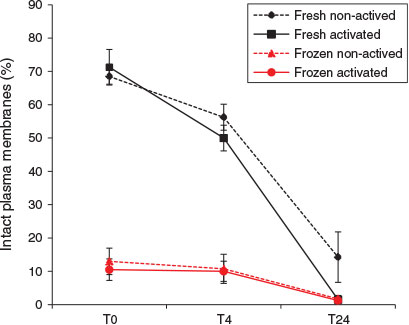
Plasma membrane integrity of X. laevis spermatozoa was also reduced by freezing and thawing (Fig. 3). At T0 the proportion of frozen–thawed spermatozoa with an intact plasma membrane (median 10%) was nearly one-seventh that of fresh spermatozoa (median 68.5%; Kruskal–Wallis H = 11.31, d.f. = 1, n = 16; P < 0.001). A similar difference was also observed at T4 (median percentage of fresh and frozen–thawed spermatozoa with an intact plasma membrane 52.5% and 7.5% respectively; Kruskal–Wallis H = 11.31, d.f. = 1, n = 16; P < 0.001). No statistically significant difference in the effects of freeze–thawing was observed between activated and non-activated spermatozoa.
|
Validation of the SCD test through ISNT
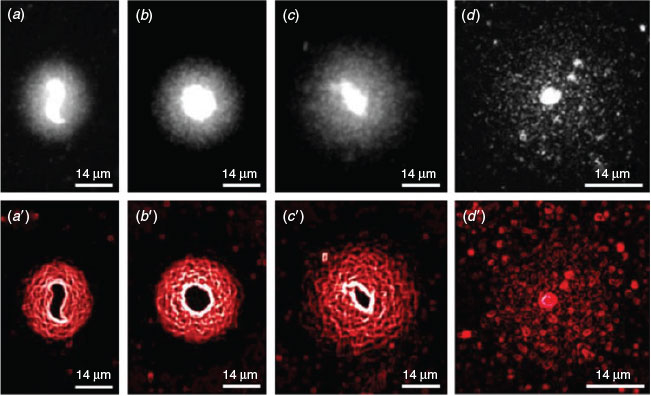
ISNT was used to label the ends of DNA strand breaks and therefore to confirm the presence of DNA fragmentation observed in the SCD test. Incorporated nucleotides bound to streptavidin-labelled fluorescein incorporated at the 3′-OH ends of single-strand breaks (SSBs) and double-strand breaks (DSBs) produce green fluorescence and, when merged with images showing the same sample stained with PI, frozen–thawed spermatozoa exhibiting fragmented DNA can be identified by their green–yellow halo (Fig. 4a–f, a′–f′). As a negative control, DNA polymerase I was omitted from the reaction, resulting in no incorporation of labelled nucleotides, as shown by a lack of green fluorescence (Fig. 4g–l). Notably, green fluorescence can be detected in the nuclear core of spermatozoa that do not exhibit a halo of fragmented DNA (Fig. 4 e, f, e′, f′).
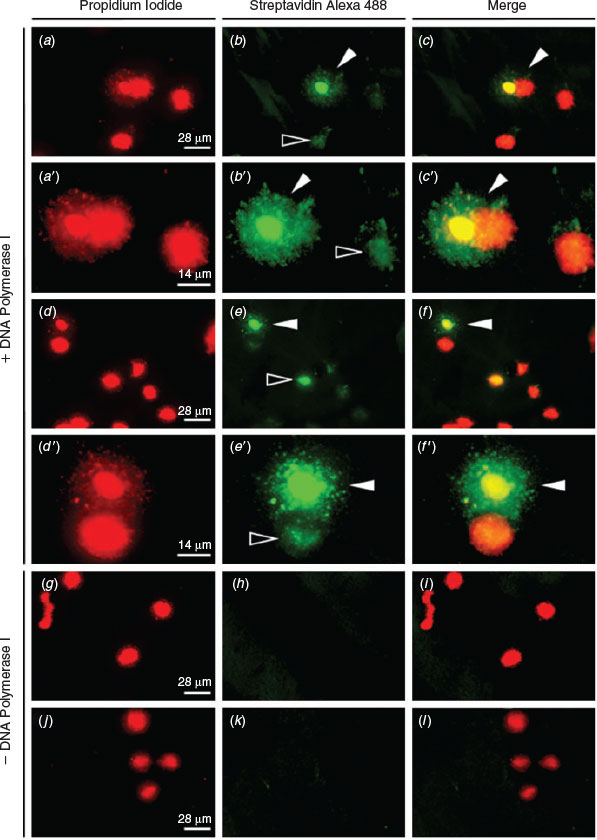
|
DNA fragmentation and sperm morphology
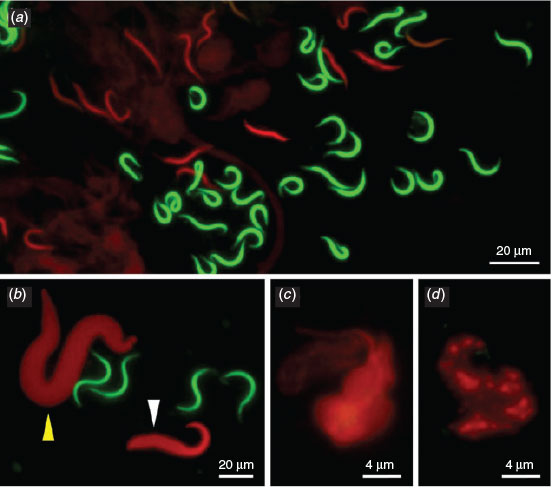
Sperm DNA fragmentation assessment through the SCD test revealed four main morphotypes. Sperm morphotypes (SM) 1 and 2 (Fig. 5a, b) showed no DNA fragmentation, but loss of the classic elongated crescent core of the sperm head was observed in SM-2. The chromatin surrounding the sperm head was still highly organised and restricted to the immediate periphery. SM-3 showed a low level of DNA fragmentation, with the chromatin dispersing further, thus creating a larger halo (Fig. 5c). The final morphotype, SM-4, shows a high level of DNA fragmentation with complete loss of the chromatin ring and with highly dispersed fragments of DNA (Fig. 5d). Omission of DNA polymerase I as a control prevented the incorporation of biotin (Fig. 5g–l). In some cases, the halos produced in the SM-4 morphotype were difficult to identify because of the highly dispersed fragments of DNA and reduced size of the nuclear core (Fig. 6; cf. cells indicated by yellow and white arrows). This may have produced underestimates of the number of spermatozoa with highly damaged DNA at later time points.
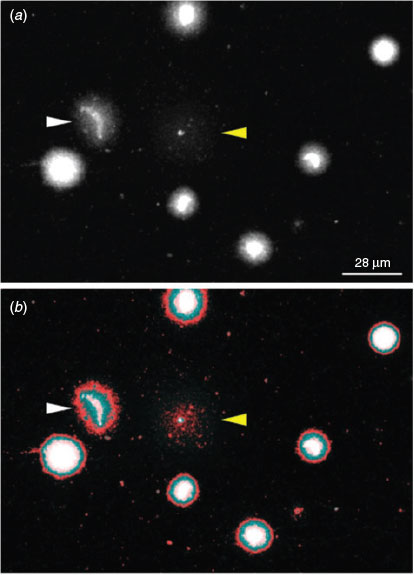
|
At T0, the activation status of spermatozoa did not affect the rate of DNA fragmentation. However, after 4 h incubation, activated spermatozoa showed a significantly higher DNA fragmentation rate than non-activated spermatozoa (regardless of the freeze–thawing treatment; F1,12 = 8.69; P = 0.012). An elevated DNA fragmentation rate due to freeze–thawing was also detectable after 4 h incubation (F1,12 = 10.08; P = 0.008), but no significant effects of freeze–thawing on DNA fragmentation were observed at either T0 or T24 (Figs 7, 8).
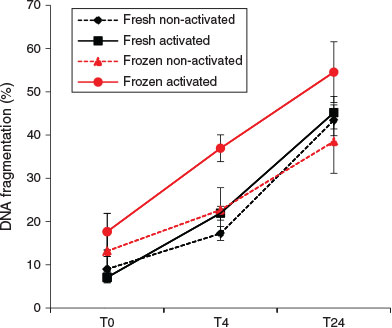
|
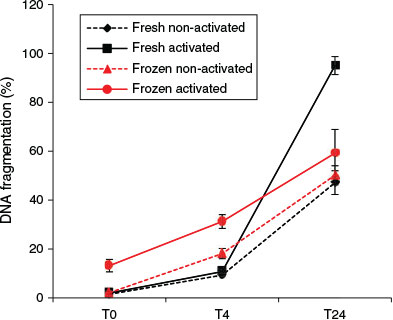
|
The data showed significant interactions between the effects of freezing and sperm activation at all the time points examined. Activated spermatozoa always exhibited higher proportions of DNA fragmentation. Freeze–thawing induced significant increases in percentage DNA fragmentation at T0 (F1,12 = 18.11; P = 0.001) and T4 (F1,12 = 61.9; P < 0.001), but paradoxically the reverse was observed at T24. This T24 result is considered to be an artefact (see Discussion).
Combined effect of freezing on membrane integrity and DNA quality
Data for X. laevis (r2 = 0.7; P < 0.001) and X. tropicalis (r2 = 0.48; P = 0.014) revealed significant negative correlations between DNA fragmentation and sperm plasma membrane integrity. These data are the mean of the activated and non-activated samples, which showed no difference from one another. Spermatozoa with plasma membrane damage showed higher levels of DNA fragmentation, as indicated by the regression lines in Fig. 9. One-way ANCOVA for the combined effect of freezing on membrane integrity and DNA fragmentation showed that the regression lines for fresh and frozen spermatozoa did not depart significantly from being parallel in either species.
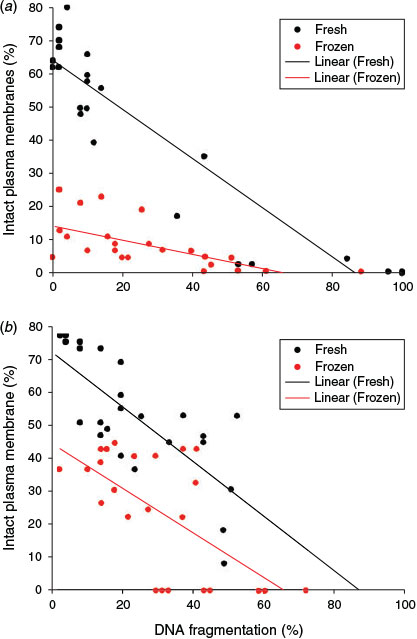
|
Discussion
In the present study we demonstrated detrimental effects on DNA quality in the spermatozoa of X. tropicalis and X. laevis caused by two treatments: cryopreservation and activation. A simple dye exclusion test revealed that the freeze–thaw process causes significantly more damage in X. laevis than X. tropicalis spermatozoa at T0. The plasma membrane integrity of both fresh and frozen samples from both species continued to decrease over time, with frozen samples reaching a point of complete loss of viable spermatozoa by 24 h. Throughout the time course we did not observe a significant effect of activation on membrane integrity. Because even minor sperm damage is revealed during prolonged incubation (Johnston et al. 2012), there is therefore no evidence that activation contributes to sperm death within the time it takes for fertilisation to occur. However, cryopreservation caused very significant damage: a 30% decrease in plasma membrane integrity for X. tropicalis and a 60% decrease for X. laevis. The X. laevis data in the present study are very similar to those obtained by Mansour et al. (2009), with both studies reporting approximately 70% spermatozoa with intact membranes from fresh samples and the maximum 20% for frozen samples in the present study falling into the range reported by Mansour et al. (2009) of 7–50%. To our knowledge, this is the first description of sperm plasma membrane integrity measurement for X. tropicalis.
In addition to detecting damaging effects on plasma membrane integrity, the present study has shown a significant detrimental effect of cryopreservation on DNA quality in both Xenopus species. The sperm DNA fragmentation index was determined by the SCD test, which, in turn, was validated by ISNT through incorporation of fluorescently labelled nucleotides into SSBs and DSBs. At T0, the most relevant for fertilisation use, there is a significant decrease in DNA integrity in frozen activated X. laevis samples compared with all other treatments. Because this is the sample that would be used for fertilisation, it is clear that the DNA damage observed is relevant to the use of such frozen–thawed spermatozoa. Although in this case we were able to detect DNA damage at T0, DNA damage levels can sometimes be very low or cryptic at T0, but potentially significant nonetheless. Effects may not be seen until 4 h or later; hence, we measured DNA stability over time. Overall, a cryopreservation and activation-induced increase in DNA damage was observed over the 24-h period for both species. For X. laevis at T24, activation alone significantly enhanced DNA damage in fresh sperm samples. Although there was no significant DNA damage enhancement detected at T0 for X. tropicalis, cryptic damage was detected at later times; thus, for both species, there is strong evidence that when frozen–thawed and activated spermatozoa are used to fertilise eggs, they contain damaged DNA. Although the SCD assay has already been validated in X. laevis (Pollock et al. 2015), we can find no evidence of data from either Xenopus or other amphibia with which to compare the data reported here.
Although the exact mechanisms that underlie the process of cryoinduced DNA damage are not fully understood, reactive oxygen species (ROS) formed during cryopreservation have been widely implicated in inducing DNA damage, both in fish (Li et al. 2010) and in mammalian spermatozoa (Lopes et al. 1998; Bennetts and Aitken 2005; Gosálvez et al. 2014). An alternative explanation to the oxidative stress mechanism is the enhancement of pre-existing DNA damage, defects in DNA repair enzymes (Zribi et al. 2010) or activation of caspase and apoptosis (Paasch et al. 2004; Said et al. 2010). Because the delay between the sperm extraction process and necessary preparation steps for analysis was kept to a minimum and did not exceed 2 min, the likely explanations for the enhancement of DNA damage described here are limited.
The molecular differences that underpin the response of spermatozoa to cryopreservation largely remain to be explored, but spermatozoa of species with low DNA protamination have increased vulnerability to oxidative damage (Gosálvez et al. 2011b). There is growing consideration for the developmental consequences that could potentially arise from such scenarios. For example, genome regions that are not associated with protamines may permit ‘easy’ access to transcriptional machinery after fertilisation, but may also confer an increased predisposition to iatrogenic assault resulting in DNA fragmentation of key embryonic genes. Indeed, it has been demonstrated in trout spermatozoa that positional differences are a key factor in determining sensitivity to oxidative damage and that certain genes can function as good biomarkers for cryoinduced damage, such as Hox genes (González-Rojo et al. 2014).
X. laevis lack protamine disulfide bonds but adopt an alternative chromatin-stabilising mechanism where alkali labile sites (ALS) produce stretches of highly repetitive and highly sensitive single-stranded DNA that heavily condenses the chromatin during spermatogenesis (Cortés-Gutiérrez et al. 2008). Because Xenopus are external fertilizers, it is thought that this serves as a key mechanism for ensuring that the integrity of Xenopus spermatozoa is not compromised during transit to the eggs. ALS may explain SM-2, where the distinct corkscrew shape of the sperm head is lost but no DNA fragmentation is detected. This was also apparent in the ISNT assay, where spermatozoa that did not exhibit morphotypes showing halos of DNA fragments had still incorporated fluorescent-labelled nucleotides, signifying the incorporation of nucleotides within the nuclear core at SSBs in ALS (Pollock et al. 2015). Indeed, from a functional and evolutionary standpoint, it is not surprising that activation of spermatozoa and the subsequent increase in DNA breakage following their release into the surrounding aquatic environment before fertilisation may diminish the quality of the DNA. It is possible that this serves as a mechanism to ensure that only spermatozoa that have spent a minimal amount of time exposed to the external and potentially detrimental environment can fertilise eggs.
This interpretation is dependent on the underlying assumption that spermatozoa with high DNA fragmentation are likely to have low fertility rates or less capacity to fertilise. Indeed, for X. laevis there is a strong correlation between high DNA fragmentation and poor membrane integrity. This therefore represents a strong selection pressure on reproductive performance. Interestingly, the data for X. tropicalis show that there is a population of sperm samples that has a higher proportion of intact plasma membranes than X. laevis. Furthermore, these sperm samples show levels of DNA fragmentation that exceed 30%. This suggests that frozen X. tropicalis spermatozoa, which have fragmented DNA, better retain their capacity to fertilise compared with X. laevis spermatozoa and that there is an increased probability of DNA-fragmented X. tropicalis spermatozoa fertilising an egg. Indeed, even fertilisation by spermatozoa with DNA fragmentation levels lower than 30% and with intact membranes could still have significant developmental consequences. It may not necessarily be the quantity of DNA damage alone, but also the nature of the DNA damage (i.e. SSB or DSB) and location within the genome that determines any detrimental effect on embryo development. For example, DNA damage does not affect normal development in intracytoplasmic sperm injection-derived pig embryos (Men et al. 2013), but there are reports that the opposite is true in humans (for a review, see Zini et al. 2009).
In conclusion, the present study has shown that the freeze–thaw process induces significant reductions in sperm membrane and DNA quality in both species of Xenopus and that activation of frozen spermatozoa enhances the detrimental effects on DNA integrity. In future, a combination of high-throughput transcriptome analysis and more classical embryological techniques applied to Xenopus embryos produced using cryopreserved spermatozoa would determine the possible existence of DNA damage hotspots and begin to identify molecular pathways compromised in such embryos.
Acknowledgements
Work in the European Xenopus Resource Centre is funded by the Wellcome Trust (101480/Z) and Biotechnology and Biological Sciences Research Council (BB/K019988/1). S. Morrow was the recipient of an Natural Environment Research Council CASE studentship in collaboration with the Institute of Zoology., London, UK. The authors are grateful to Trent Garner for suggesting improvements to the manuscript.
References
Beesley, S. G., Costanzo, J. P., and Lee, R. E. (1998). Cryopreservation of spermatozoa from freeze-tolerant and -intolerant anurans. Cryobiology 37, 155–162.| Cryopreservation of spermatozoa from freeze-tolerant and -intolerant anurans.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1cvktlWqsA%3D%3D&md5=7081c871c4709c21509fc2b9d5d2a99bCAS | 9769166PubMed |
Bennetts, L. E., and Aitken, R. J. (2005). A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol. Reprod. Dev. 71, 77–87.
| A comparative study of oxidative DNA damage in mammalian spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtVOqsrk%3D&md5=5decf0ae7e95b9956b055714cc703deaCAS | 15736137PubMed |
Browne, R. K., Clulow, J., Mahony, M., and Clark, A. (1998). Successful recovery of motility and fertility of cryopreserved cane toad (Bufo marinus) sperm. Cryobiology 37, 339–345.
| Successful recovery of motility and fertility of cryopreserved cane toad (Bufo marinus) sperm.Crossref | GoogleScholarGoogle Scholar | 9917350PubMed |
Clulow, J., Trudeau, V. L., and Kouba, A. J. (2014). Amphibian declines in the twenty-first century: why we need assisted reproductive technologies. Adv. Exp. Med. Biol. 753, 275–316.
| Amphibian declines in the twenty-first century: why we need assisted reproductive technologies.Crossref | GoogleScholarGoogle Scholar | 25091914PubMed |
Cortés-Gutiérrez, E. I., Dávila-Rodríguez, M. I., López-Fernández, C., Fernández, J. L., and Gosálvez, J. (2008). Alkali-labile sites in sperm cells from Sus and Ovis species. Int. J. Androl. 31, 354–363.
| Alkali-labile sites in sperm cells from Sus and Ovis species.Crossref | GoogleScholarGoogle Scholar | 17651406PubMed |
Fernández-Díez, C., González-Rojo, S., Montfort, J., Le Cam, A., Bobe, J., Robles, V., Pérez-Cerezales, S., and Herráez, M. P. (2015). Inhibition of zygotic DNA repair: transcriptome analysis of the offspring in trout (Oncorhynchus mykiss). Reproduction 149, 101–111.
| Inhibition of zygotic DNA repair: transcriptome analysis of the offspring in trout (Oncorhynchus mykiss).Crossref | GoogleScholarGoogle Scholar | 25433028PubMed |
Fish, M. B., Nakayama, T., Fisher, M., Hirsch, N., Cox, A., Reeder, R., Carruthers, S., Hall, A., Stemple, D. L., and Grainger, R. M. (2014). Xenopus mutant reveals necessity of rax for specifying the eye field which otherwise forms tissue with telencephalic and diencephalic character. Dev. Biol. 395, 317–330.
| Xenopus mutant reveals necessity of rax for specifying the eye field which otherwise forms tissue with telencephalic and diencephalic character.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFynsrnE&md5=a468d7d09dca06d7b9b9222456129bd8CAS | 25224223PubMed |
González-Marín, C., Gosálvez, J., and Roy, R. (2012). Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int. J. Mol. Sci. 13, 14 026–14 052.
| Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells.Crossref | GoogleScholarGoogle Scholar |
González-Rojo, S., Fernández-Díez, C., Guerra, S. M., Robles, V., and Herraez, M. P. (2014). Differential gene susceptibility to sperm DNA damage: analysis of developmental key genes in trout. PLoS One 9, e114161.
| Differential gene susceptibility to sperm DNA damage: analysis of developmental key genes in trout.Crossref | GoogleScholarGoogle Scholar | 25479606PubMed |
Gosálvez, J., Cortés-Gutiérrez, E. I., Nuñez, R., Fernández, J. L., Caballero, P., López-Fernández, C., and Holt, W. V. (2009). A dynamic assessment of sperm DNA fragmentation versus sperm viability in proven fertile human donors. Fertil. Steril. 92, 1915–1919.
| A dynamic assessment of sperm DNA fragmentation versus sperm viability in proven fertile human donors.Crossref | GoogleScholarGoogle Scholar | 18980765PubMed |
Gosálvez, J., López-Fernández, C., Fernández, J. L., Gouraud, A., and Holt, W. V. (2011a). Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 78, 951–961.
| Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species.Crossref | GoogleScholarGoogle Scholar | 21919111PubMed |
Gosálvez, J., Ramirez, M. A., López-Fernández, C., Crespo, F., Evans, K. M., Kjelland, M. E., and Moreno, J. F. (2011b). Sex-sorted bovine spermatozoa and DNA damage: I. Static features. Theriogenology 75, 197–205.
| Sex-sorted bovine spermatozoa and DNA damage: I. Static features.Crossref | GoogleScholarGoogle Scholar | 20932559PubMed |
Gosálvez, J., Holt, W. V., and Johnston, S. D. (2014). Sperm DNA fragmentation and its role in wildlife conservation. Adv. Exp. Med. Biol. 753, 357–384.
| Sperm DNA fragmentation and its role in wildlife conservation.Crossref | GoogleScholarGoogle Scholar | 25091917PubMed |
Gurdon, J. B. (1977). Methods for nuclear transplantation in amphibia. Methods Cell Biol. 16, 125–139.
| Methods for nuclear transplantation in amphibia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXlsVyjt7c%3D&md5=edff58b791337b1abc374a700498fc3eCAS | 329056PubMed |
Harland, R. M., and Grainger, R. M. (2011). Xenopus research: metamorphosed by genetics and genomics. Trends Genet. 27, 507–515.
| Xenopus research: metamorphosed by genetics and genomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVKhur7K&md5=123f4c51f39fbd57ea90ddff43182070CAS | 21963197PubMed |
Hellsten, U., Harland, R. M., Gilchrist, M. J., Hendrix, D., Jurka, J., Kapitonov, V., Ovcharenko, I., Putnam, N. H., Shu, S., Taher, L., et al. (2010). The genome of the Western clawed frog Xenopus tropicalis. Science 328, 633–636.
| The genome of the Western clawed frog Xenopus tropicalis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlt1Crt70%3D&md5=59f6918eaa0ed6c85218501c9848e1b1CAS | 20431018PubMed |
Holt, W. V. (2000). Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology 53, 47–58.
| Fundamental aspects of sperm cryobiology: the importance of species and individual differences.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7pvFagtA%3D%3D&md5=026e3d4311245704c504bc37328f8d05CAS | 10735061PubMed |
Johnston, S. D., Zee, Y. P., López-Fernández, C., and Gosálvez, J. (2012). The effect of chilled storage and cryopreservation on the sperm DNA fragmentation dynamics of a captive population of koalas. J. Androl. 33, 1007–1015.
| The effect of chilled storage and cryopreservation on the sperm DNA fragmentation dynamics of a captive population of koalas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFSqt7%2FM&md5=091b8fb40f25fd87c4c9bc67b6256717CAS | 22282436PubMed |
Karpinka, J. B., Fortriede, J. D., Burns, K. A., James-Zorn, C., Ponferrada, V. G., Lee, J., Karimi, K., Zorn, A. M., and Vize, P. D. (2015). Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 43, D756–D763.
| Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes.Crossref | GoogleScholarGoogle Scholar | 25313157PubMed |
Khokha, M. K. (2012). Xenopus white papers and resources: folding functional genomics and genetics into the frog. Genesis (New York, N.Y.: 2000) 50, 133–142.
| Xenopus white papers and resources: folding functional genomics and genetics into the frog.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFygtLc%3D&md5=1babcc827f856a8dcde2678873ea4b43CAS |
Kopeika, J., Kopeika, E., Zhang, T., Rawson, D. M., and Holt, W. V. (2004). Effect of DNA repair inhibitor (3-aminobenzamide) on genetic stability of loach (Misgurnus fossilis) embryos derived from cryopreserved sperm. Theriogenology 61, 1661–1673.
| Effect of DNA repair inhibitor (3-aminobenzamide) on genetic stability of loach (Misgurnus fossilis) embryos derived from cryopreserved sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhslWnu7w%3D&md5=4f2ed5906848b14a6098901db676523bCAS | 15019462PubMed |
Kopeika, J., Thornhill, A., and Khalaf, Y. (2015). The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update 21, 209–227.
| The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence.Crossref | GoogleScholarGoogle Scholar | 25519143PubMed |
Li, P., Li, Z.-H., Dzyuba, B., Hulak, M., Rodina, M., and Linhart, O. (2010). Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques. Biol. Reprod. 83, 852–858.
| Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio, L.) sperm caused by cryopreservation techniques.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGls7%2FK&md5=ab2767c5792174d7eca873992d03e4a5CAS | 20668258PubMed |
Lopes, S., Jurisicova, A., Sun, J. G., and Casper, R. F. (1998). Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum. Reprod. 13, 896–900.
| Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjs12gtbo%3D&md5=411c4bbfcf36849dba5590b22056a0e1CAS | 9619544PubMed |
Mansour, N., Lahnsteiner, F., and Patzner, R. A. (2009). Optimization of the cryopreservation of African clawed frog (Xenopus laevis) sperm. Theriogenology 72, 1221–1228.
| Optimization of the cryopreservation of African clawed frog (Xenopus laevis) sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCgtr3J&md5=4225a6d70e1101216b43eba047adc4e8CAS | 19766299PubMed |
Meistrich, M. L., Mohapatra, B., Shirley, C. R., and Zhao, M. (2003). Roles of transition nuclear proteins in spermiogenesis. Chromosoma 111, 483–488.
| Roles of transition nuclear proteins in spermiogenesis.Crossref | GoogleScholarGoogle Scholar | 12743712PubMed |
Men, N. T., Kikuchi, K., Nakai, M., Fukuda, A., Tanihara, F., Noguchi, J., Kaneko, H., Linh, N. V., Nguyen, B. X., Nagai, T., and Tajima, A. (2013). Effect of trehalose on DNA integrity of freeze-dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection. Theriogenology 80, 1033–1044.
| Effect of trehalose on DNA integrity of freeze-dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVKktLvN&md5=99e3a797d2118a0a302e10d7a5392958CAS | 24041826PubMed |
Michael, S. F., and Jones, C. (2004). Cryopreservation of spermatozoa of the terrestrial Puerto Rican frog, Eleutherodactylus coqui. Cryobiology 48, 90–94.
| Cryopreservation of spermatozoa of the terrestrial Puerto Rican frog, Eleutherodactylus coqui.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXht1yiurg%3D&md5=b29454023d8a3f39e23e9dc9056c2526CAS | 14969686PubMed |
Nakayama, T., Fisher, M., Nakajima, K., Odeleye, A. O., Zimmerman, K. B., Fish, M. B., Yaoita, Y., Chojnowski, J. L., Lauderdale, J. D., Netland, P. A., and Grainger, R. M. (2015). Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev. Biol. 408, 328–344.
| Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjs1Gqu7g%3D&md5=dba935d03eae58fbb79f4ce817eb84adCAS | 25724657PubMed |
O’Neill, A. C., and Ricardo, S. D. (2013). Human kidney cell reprogramming: applications for disease modeling and personalized medicine. J. Am. Soc. Nephrol. 24, 1347–1356.
| Human kidney cell reprogramming: applications for disease modeling and personalized medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFCqsr3N&md5=f1e9b94b4448b37455ff38672dad43f3CAS | 23949797PubMed |
Paasch, U., Sharma, R. K., Gupta, A. K., Grunewald, S., Mascha, E. J., Thomas, A. J., et al. (2004). Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol. Reprod. 71, 1828–1837.
| Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVWgsr%2FF&md5=e863b2dcca52220c9fa21bec63a68dc2CAS | 15286043PubMed |
Pérez-Cerezales, S., Gutiérrez-Adán, A., Martínez-Páramo, S., Beirão, J., and Herráez, M. P. (2011). Altered gene transcription and telomere length in trout embryo and larvae obtained with DNA cryodamaged sperm. Theriogenology 76, 1234–1245.
| Altered gene transcription and telomere length in trout embryo and larvae obtained with DNA cryodamaged sperm.Crossref | GoogleScholarGoogle Scholar | 21741697PubMed |
Pollock, K., Gosálvez, J., Arroyo, F., López-Fernández, C., Guille, M., Noble, A., and Johnston, S. D. (2015). Validation of the sperm chromatin dispersion (SCD) test in the Amphibian Xenopus laevis using in situ nick translation and comet assay. Reprod. Fertil. Dev. 27, 1168–1174.
| Validation of the sperm chromatin dispersion (SCD) test in the Amphibian Xenopus laevis using in situ nick translation and comet assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1Glu7jI&md5=f841cbf8fbe967b20234e46b6a1a05e3CAS | 25482041PubMed |
Pratt, K. G., and Khakhalin, A. S. (2013). Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets. Dis. Model. Mech. 6, 1057–1065.
| Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets.Crossref | GoogleScholarGoogle Scholar | 23929939PubMed |
Said, T. M., Gaglani, A., and Agarwal, A. (2010). Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 21, 456–462.
| Implication of apoptosis in sperm cryoinjury.Crossref | GoogleScholarGoogle Scholar | 20800544PubMed |
Sargent, M. G., and Mohun, T. J. (2005). Cryopreservation of sperm of Xenopus laevis and Xenopus tropicalis. Genesis (New York, N.Y.: 2000) 41, 41–46.
| Cryopreservation of sperm of Xenopus laevis and Xenopus tropicalis.Crossref | GoogleScholarGoogle Scholar |
Schmitt, S. M., Gull, M., and Brändli, A. W. (2014). Engineering Xenopus embryos for phenotypic drug discovery screening. Adv. Drug Deliv. Rev. 69–70, 225–246.
| Engineering Xenopus embryos for phenotypic drug discovery screening.Crossref | GoogleScholarGoogle Scholar | 24576445PubMed |
Shi, Z., Wang, F., Cui, Y., Liu, Z., Guo, X., Zhang, Y., Deng, Y., Zhao, H., and Chen, Y. (2015). Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis. FASEB J. 29, 4914–4923.
| Heritable CRISPR/Cas9-mediated targeted integration in Xenopus tropicalis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xns1Cis7w%3D&md5=415deabfeda1b6a00296bbeecab2ff51CAS | 26268927PubMed |
Wright, C., Milne, S., and Leeson, H. (2014). Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 28, 684–703.
| Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtlajsLc%3D&md5=dcd63d5c6b64658833124e5d28c2e4d1CAS | 24745838PubMed |
Yániz, J. L., Palacín, I., Vicente-Fiel, S., Gosalvez, J., López-Fernández, C., and Santolaria, P. (2013). Comparison of membrane-permeant fluorescent probes for sperm viability assessment in the ram. Reproduction in Domestic Animals 48, 598–603.
| Comparison of membrane-permeant fluorescent probes for sperm viability assessment in the ram.Crossref | GoogleScholarGoogle Scholar | 23293961PubMed |
Zini, A., San Gabriel, M., and Baazeem, A. (2009). Antioxidants and sperm DNA damage: a clinical perspective. J. Assist. Reprod. Genet. 26, 427–432.
| Antioxidants and sperm DNA damage: a clinical perspective.Crossref | GoogleScholarGoogle Scholar | 19768529PubMed |
Zribi, N., Feki Chakroun, N., El Euch, H., Gargouri, J., Bahloul, A., and Ammar Keskes, L. (2010). Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 93, 159–166.
| Effects of cryopreservation on human sperm deoxyribonucleic acid integrity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXit1Wmt74%3D&md5=136a066bf66ea16ce8a5c537d0980fa7CAS | 19027111PubMed |