CASA-Mot technology: how results are affected by the frame rate and counting chamber
Daznia Bompart A E , Almudena García-Molina A , Anthony Valverde B C , Carina Caldeira A B , Jesús Yániz D , Manuel Núñez de Murga B and Carles Soler A BA Proiser R+D, Avenuenida Catedrático Agustín Escardino, 9, Building 3 (CUE), Floor 1, 46980, Paterna, Spain.
B University of Valencia, Department of Cellular Biology, Functional Biology and Physical Anthropology, Campus Burjassot, C/ Dr Moliner, 50, 46100, Burjassot, Spain.
C Technological Institute of Costa Rica, San Carlos Campus, School of Agronomy, 223-21001 Alajuela, Costa Rica.
D TECNOGAM Research Group, Environmental Sciences Institute (IUCA), Department of Animal Production and Food Sciences, University of Zaragoza, 50013, Huesca, Spain.
E Corresponding author. Email: daznia.bompart@proiser.com
Reproduction, Fertility and Development 30(6) 810-819 https://doi.org/10.1071/RD17551
Submitted: 25 October 2017 Accepted: 18 January 2018 Published: 4 April 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
For over 30 years, CASA-Mot technology has been used for kinematic analysis of sperm motility in different mammalian species, but insufficient attention has been paid to the technical limitations of commercial computer-aided sperm analysis (CASA) systems. Counting chamber type and frame rate are two of the most important aspects to be taken into account. Counting chambers can be disposable or reusable, with different depths. In human semen analysis, reusable chambers with a depth of 10 µm are the most frequently used, whereas for most farm animal species it is more common to use disposable chambers with a depth of 20 µm . The frame rate was previously limited by the hardware, although changes in the number of images collected could lead to significant variations in some kinematic parameters, mainly in curvilinear velocity (VCL). A frame rate of 60 frames s−1 is widely considered to be the minimum necessary for satisfactory results. However, the frame rate is species specific and must be defined in each experimental condition. In conclusion, we show that the optimal combination of frame rate and counting chamber type and depth should be defined for each species and experimental condition in order to obtain reliable results.
Additional keywords: computer-aided sperm analysis (CASA) system, kinematic, motility.
Semen analysis
Traditional analysis of semen samples includes the assessment of concentration, motility and morphology, and is the basic tool for determining male fertility (Chong et al. 1983; Overstreet 1984; Budworth et al. 1988; Hirai et al. 2001; McPherson et al. 2014). The most common way to evaluate semen quality is based on subjective observations, which require a great deal of time and experience. For this reason, they often lack consistency. It seems that the first trials to define a standard for semen analysis (Falk and Kaufman 1950; MacLeod and Gold 1951; Barták 1971; MacLeod 1971; American Fertility Society 1980; Belsey et al. 1980) were commonly ignored, incorrectly followed or not read at all (Chong et al. 1983). Unfortunately, this problem still persists, and many different criteria continue to be used even now, leading to considerable confusion and a complete lack of possible comparison between laboratories. The most reputed institutions have endeavoured to define universal standards for semen analysis (WHO 2010; see also previous version of Kvist and Björndahl 2002; Barratt et al. 2011). However, in the end, these standards as still not correctly followed or possibly not even used at all.
Semen evaluation has two main purposes, one related to male fertility (both in humans and other species) and the other for optimising the production of insemination doses for livestock breeding (Hansen 2014). In terms of human male fertility, it has been shown that, in humans, problems conceiving in approximately 50% of couples are related to the male partner (Kumar and Singh 2015). Even if the final causes of infertility are complex and involve the female partner, performing semen analysis continues to be the easiest way to evaluate what is happening with the male part of the problem. Regarding seminal dose production, inaccurate estimates of sperm concentration along with a incorrect interpretation of the spermiogram can lead to faulty insemination doses. All of this can jeopardise ejaculate optimisation, fertility and fecundity (Jequier and Ukombe 1983; Mortimer et al. 1986; Douglas-Hamilton et al. 2005a; Kathiravan et al. 2011; Amann and Waberski 2014).
Since the outset of CASA-Mot technology, various studies have dealt with the aim of defining optimal set-ups for use of the systems (Katz and Davis 1987). However, these studies only referred to some species. The aim of this paper is to review the effect of frame rate and counting chamber type and depth on the results of CASA-Mot in a variety of mammalian species.
History of sperm kinematic analysis
During the second part of the last century, several attempts were made to develop an objective method for evaluating sperm motility that were initially based on flagellar motility alone (Taylor 1951, 1952; Hancock 1953; Rothschild 1953; Gray 1955, 1958; Gray and Hancock 1955; Rikmenspoel and van Herpen 1957; Brokaw 1965, 1970, 1972; Fray et al. 1972; Blum and Lubliner 1973; Denehy 1975; Yundt et al. 1975; Katz and Dott 1975; Cosson 1996). These trials later focused on head position using the following techniques: kinemicrography (van Duijin et al. 1971), photography (Glover 1968; Janick and MacLeod 1970; Elliot et al. 1973; Revell and Wood 1978), spectrophotometry (Atherton 1975; Cooke and Hallet 1976; Atherton et al. 1978; Majumder and Chakrabarti 1984), Laser light scattering (Dubois et al. 1975; Shimizu and Matsumoto 1977; Mitsukawa 1979), videomicrography (Tash et al. 1986; Katz and Overstreet 1981; O’Connor et al. 1981; Samuels and van der Horst 1986), cinematography (Katz et al. 1978; David et al. 1981), haemocytometry (Ishii et al. 1977), photomicrography (Katz and Dott 1975; Makler 1978a; Overstreet et al. 1979; Amann and Hammerstedt 1980; Bartoov et al. 1981; Aitken et al. 1982, 1985) and stroboscopy ( Cosson et al. 1985). These approaches were time consuming and of little use for diagnostic purposes. Nevertheless, these results offer interesting results and provide a strong background upon which current methods are based.
Era of CASA technology
With the introduction of computer-aided sperm analysis (CASA) technology at the end of the 1970s, the intention was to overcome these problems and many approaches were developed that were both scientific and clinical (Liu and Warme 1977; Katz and Overstreet 1981; Walker et al. 1982; Acott et al. 1983; Suarez et al. 1983; Schoëvaërt-Brossault 1984; Holt et al. 1985; Katz et al. 1985; Mathur et al. 1986; Katz and Davis 1987; Ginsburg et al. 1988; Aanesen and Bendvold 1989; Johnson et al. 1990, 1996a, 1996b). All these efforts greatly improved the significance of semen analysis in determining fertility (Aitken et al. 1994; MacLeod and Irvine 1995) and the seminal doses produced for AI programs (in the case of domestic animals; Hansen 2014). It should be considered that there are different technologies inside the general concept of CASA-Mot systems (Amann and Waberski 2014). However, herein we focus only on the one that is based on head centroid position to define sperm kinematics.
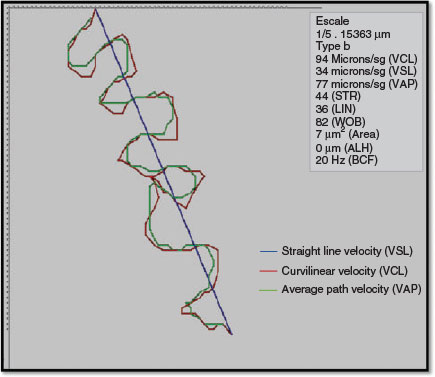
The use of CASA systems provides increasingly accurate and more quantitative information (Fig. 1) than using classical assessment (David et al. 1981; Serres et al. 1984; Verstegen et al. 2002; Chantler et al. 2004; Didion 2008) and reduces intertechnician variation in the estimation of sperm concentration, regardless of the type and depth of counting chamber used (Johnson et al. 1996a, 1996b; Lu et al. 2007).
|
Unfortunately, the high price of these systems, the lack of homogeneous results between different commercial equipment and the fact that these systems were basically only used to replicate subjective analysis, instead of using the great battery of data obtained, has limited the widespread deployment of this kind of technology.
However, CASA instruments are not ‘ready-to-use’ robots, and the reliability of their results depends largely on the expertise and training of the user (Holt et al. 1994). Unfortunately, this technology is still accepted uncritically by many of its users, reducing the feasibility of the results obtained (Kraemer et al. 1998). As a consequence, a fully and well-designed definition of accuracy and limits of each commercial CASA system is needed for its correct use (Vantman et al. 1988; Mortimer 1990; Davis and Katz 1992; Kraemer et al. 1998; Verstegen et al. 2002; Björndahl 2011; Palacios et al. 2012; Simonik et al. 2015).
The basic principle behind microscopy-based CASA-Mot systems is that a series of successive images of motile spermatozoa is acquired and analysed (within a field of view; Elliot et al. 1973; Jecht and Russo 1973; Katz and Dott 1975; Jouannet et al. 1977; Liu and Warme 1977; Overstreet et al. 1979; Amann and Hammerstedt 1980; Stephens et al. 1988; Holt and Palomo 1996). As noted previously, the CASA-Mot image analysis technique is based on the assessment of centroid-based values that are well correlated with the flagellar movement patterns. Therefore, although centroid-derived kinematic measurements are used for the definition of sperm movement, it is understood that these values are secondary indicators of aspects of flagellar movement (Mortimer et al. 1997).
Nevertheless, CASA-Mot systems do not follow one common standard and the motility reports generated with one commercial system cannot be compared directly with those obtained using other systems (Gill et al. 1988; Knuth and Nieschlag 1988; Vantman et al. 1988; Jasko et al. 1990; Olds-Clarke et al. 1990; Hoogewijs et al. 2012). The effects of technical settings (Smith and England 2001; Rijsselaere et al. 2003), such as frame rate, the number of frames analysed and counting chamber, are some of the factors affecting the final results. Sperm concentration and the semen diluents used are also important (Rijsselaere et al. 2003; Contri et al. 2010).
Following on from this, the final results could be affected by two different sources: (1) hardware and computational variations between systems; and (2) non-computational issues, such as specimen preparation and microscopic technique (Holt et al. 1994). One of the non-computational variables is related to sampling. Regarding this, and given that spermatozoa are not uniformly distributed in seminal plasma or other diluting fluids, the number of randomly selected fields needed to achieve a good level of precision must be defined (Vantman et al. 1988).
Two recent papers have reviewed not only the history of the CASA-Mot systems, but also their future perspectives (Amann and Waberski 2014; Mortimer et al. 2015). The review by Amann and Waberski (2014) is devoted to animal production, whereas that of Mortimer et al. (2015) is more focussed on human samples. The literature reporting semen analysis describes a wide variety of methodologies both in terms of CASA-Mot settings (Loomis and Graham 2008; Waite et al. 2008; Ortega-Ferrusola et al. 2009) and in the use of different counting chambers in combination with CASA systems (Ortega-Ferrusola et al. 2009).
Effects of frame rate on CASA-Mot results
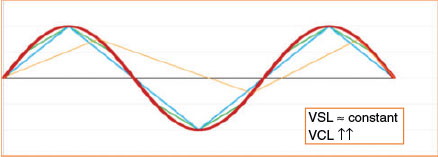
Many systems use standard video image acquisition rates (expressed in frames s−1; Hz) and are limited by hardware and software capabilities, with the most common previously being 16, 25, 30, 50 or 60 frames s−1 (Holt et al. 2007; Contri et al. 2010). The effect of frame rate on cell track recognition was pointed out even when manual image analysis was first introduced for semen evaluation (Mack et al. 1988). The increased quality of both hardware and software enables us to currently work with higher frame rates. However, conflicting results can arise when studies use different frame rates, even when using the same device (Morris et al. 1996; Rijsselaere et al. 2003). Results in different species confirm the observation of increasing velocity parameters as the frame rate increases (Mortimer et al. 1988; Mortimer and Swan 1995; Castellini et al. 2011; Gallego et al. 2013), and this is particularly important when hyperactivation needs to be evaluated (Morales et al. 1988; Mortimer and Swan 1995; Mortimer et al. 1997, 1988). The most sensitive parameter is the curvilinear velocity (VCL), whereas the least sensitive one must be the straight line velocity (VSL). The former is calculated using centroid positions in each image, whereas the latter indicates only the link between the first and the last points of the track (Fig. 2). In previous papers, it was observed that straightness (the relationship between both velocities (VSL/VCL)) also increased with increasing frame rate (Morris et al. 1996). The amplitude of lateral head displacement (ALH) is reduced at higher frame rates (Morris et al. 1996; Rijsselaere et al. 2003), but even this parameter is greatly affected by the algorithm used for its characterisation. In contrast, the beat cross frequency (BCF) increases greatly at higher frame rates (Rijsselaere et al. 2003).
|
Effect of counting chamber type on CASA-Mot results
CASA-Mot technology requires the use of specific counting chambers and it is necessary to understand the proprieties associated with each type of chamber (Le Lannou et al. 1992; Massányi et al. 2008; Hoogewijs et al. 2012). There are two general physical principles for charging the chambers: (1) by capillary action in most disposable chambers; and (2) by droplet displacement in reusable chambers (Coetzee and Menkveld 2001; Del Gallego et al. 2017). In the case of some reusable chambers based on drop displacement (e.g. Makler chamber (Sefi Medical Instruments); Matson et al. 1999), the time involved in placing the cover affects the results by increasing the apparent concentration the longer it takes to place the cover; this does not happen with chambers (e.g. Spermtrack (Proiser R+D); Soler et al. 2012). This could be related to the glass composition of the chambers and the number of ions exposed at the surface of the glass, or to the swim-up process of cells moving to the centre of the drop.
Another factor that may introduce errors is the volume used. Because the reusable chambers are usually circular, the volume required to fill them is defined by the area (πr2) multiplied by depth. In common practice, this is not calculated, producing an excessive volume that is displaced outside the plateau and leading to a false distribution of the cells. This makes it necessary to define the exact volume to be used in each chamber (Fig. 3).
In the case of disposable chambers, most of the commonly used disposable chambers include a cover slide that is attached using different kinds of glue. At the manufacturing level, the glue is used not only to design the shape of the counting chamber, but also to define its depth, depending on the height of the glue (Fig. 4).
|
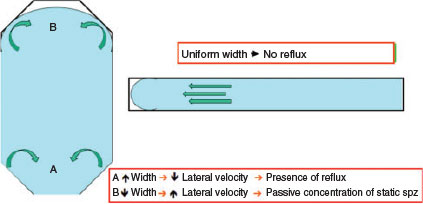
Thus, samples inside counting chambers are charged by capillary progress between the two layers (slide and cover), under a force derived from the surface tension between the fluid and the glass, with a meniscus forming at the leading edge. This is known as the laminar Poiseuille flow (Douglas-Hamilton et al. 2005b). This leads to a transverse lifting force, causing the particles to migrate perpendicular to the direction of the flow, which results in an uneven distribution of suspended particles throughout the sample (Vasseur and Cox 1976). This was described by Segré and Silberberg (1962a, 1962b) and is known as the SS effect (Douglas Hamilton et al. 2005a). The SS effect can affect the final result depending on sample viscosity, with the final result being that particles exhibit a tendency to become concentrated at the meniscus (Douglas-Hamilton et al. 2005a, 2005b).
The design of most chambers is such that there are changes in the width and shape of the space where the sample is distributed, which implies variations in the capillary forces when the semen is moving on, causing non-uniform distribution of spermatozoa (Douglas-Hamilton et al. 2005a, 2005b; Ibănescu et al. 2016). In contrast, when the chamber design is based on a thin parallel space, this problem is solved and the distribution of spermatozoa is uniform along the length of drop displacement (Soler et al. 2012, 2014; Del Gallego et al. 2017; Fig. 4).
Most capillary-filled chambers produce an underestimation of concentration due to the fact that the cells tend to accumulate at the edges, with a lower density in the centre where the concentration is evaluated (particularly with some CASA systems; Amann and Waberski 2014).
That different chamber designs affect both the final distribution of spermatozoa inside the chamber and their motility characteristics has been demonstrated for the boar (Christensen et al. 2005), cattle (Prathalingam et al. 2006; Contri et al. 2010; Lenz et al. 2011; Gloria et al. 2013), goats (Del Gallego et al. 2017), humans (Tomlinson et al. 2001; Soler et al. 2012; Peng et al. 2015), rabbits (Massányi et al. 2008), sheep (Palacín et al. 2013) and stallion (Len et al. 2010; Spizziri et al. 2010; Hoogewijs et al. 2012). This must be taken into account when comparing different studies.
The traditional gold standard for sperm concentration was defined by the use of haemocytometers, such as Bürker and Neubauer, despite there being discrepancies between them (Johnson et al. 1996a; Mahmoud et al. 1997). In general, there are significant differences in the results obtained after using these classical haemocytometers and the counting chambers used for CASA systems (Rijsselaere et al. 2003). This discrepancy could be attributed to many factors. First, in statistical terms, calculating the concentration by taking an aliquot from the original sample is only an estimate of the true concentration (Coetzee and Menkveld 2001). The volume analysed with both types of chambers is very different. In haemocytometers, the height of the space is 100 μm, whereas the height of sperm counting chambers is 10 or 20 μm. This means that the volume in sperm counting chambers, which is one-fifth to one-tenth that in the reference standard (haemocytometers), is also less representative of the entire sample. This can produce an underestimation of the number of cells present in the sample (Rijsselaere et al. 2003).
Conversely, others have observed an increase in concentration values using CASA systems that could be related to the inclusion of fragmented tracks as a consequence of cell crossing (Knuth and Nieschlag 1988; Vantman et al. 1988; Chan et al. 1989; Neuwinger et al. 1990) or the incorrect recognition of some non-sperm structures (e.g. lipid or protein globules, other cells) identified as sperm cells.
Finally, it is not possible to know whether one chamber is better than another. However, it is necessary to rationally analyse differences between both the chambers themselves and the results obtained given that differences do, indeed, exist (Kuster 2005).
To this end, it was shown that chambers based on capillary filling (e.g. MicroCell Chamber (Vitrolife)) resulted in similar sperm concentrations in human samples when samples were analysed both manually and with a CASA-Mot system to those obtained using a haemocytometer, whereas the concentration obtained using a drop-displacement chamber (Makler; both manual and CASA-Mot analysis) was significantly greater (Johnson et al. 1996a; Bailey et al. 2007).
Good examples of the analysis of these kinds of differences between drop-displacement and capillary chambers have been performed in the ram (Palacín et al. 2013) and goat (Del Gallego et al. 2017). In these papers, the effect of chamber charging on motility and kinematics was investigated, with the results showing that the drop-displacement chambers resulted in higher parameter values than obtained with capillary chambers. However, it is necessary to point out that ‘higher’ does not necessarily mean ‘better’, merely ‘different’ (Contri et al. 2010; Palacín et al. 2013; Del Gallego et al. 2017). Other studies have not shown differences between different counting chambers, in either mammals (boar; Gączarzewicz 2015) or fish (eel; Gallego et al. 2013).
It was proposed that one of the possible explanations for the lower sperm motility could be the toxicity of chemical substances present in the capillary-filled chambers (Gloria et al. 2013), which could be species specific.
Effect of counting chamber depth effect on CASA-Mot results
The natural movement of spermatozoa is helical in nature and, depending on the species and physiological status, considerable space is needed to develop this kind of motility correctly (Kraemer et al. 1998; Soler et al. 2018).
Despite this, in the case of human spermatozoa, the reduced amplitude of movement, the relatively short flagellum and the high viscosity of seminal plasma enabled us to use chambers with a depth of 10 µm without involving extensive modifications, even if the resultant movement is only two-dimensional instead of three-dimensional (Makler 1978b). However, when the reusable Spermtrack counting chamber was used, there were differences in human samples in sperm concentration and total motility comparing chambers with depths of 10 and 20 μm (Makler 1978b). This difference was not observed when ISASD4C (Proiser R+D) chambers (depths 10, 16 and 20 μm) were used, which also demonstrates the importance of the charging method on the final results (Soler et al. 2012). The effects of the capacitation medium on human samples also differ considerably between chambers with depths of 10 and 20 µm, indicating that the latter is much more suitable (Le Lannou et al. 1992).
In the veterinary literature, the depth of the sperm suspension for motility analysis is variable and frequently left unmentioned (Hoogewijs et al. 2012). In species such as the goat, progressive motility and cell velocities were higher with capillary-loading chambers with depths of 20 than 10 μm (Del Gallego et al. 2017). However, these results were not confirmed in other species, including human (Le Lannou et al. 1992; Soler et al. 2012) and hamster (Shivaji et al. 1995), which could be related to the fact that the spermatozoa of different species exhibit different motility patterns.
The SS effect mentioned above is also dependent on the depth of the chambers, because the filling velocity is proportional to depth and decreases as the meniscus penetrates the chamber. Thus, shallow chambers fill more slowly than deeper chambers, providing a possible explanation for differences between chambers of different depths (Kuster 2005) in the sense that the deeper the chamber, the better the uniform distribution.
Sampling in the counting chamber
The final question regarding counting chamber sampling refers to the variability between field analysis with drop- and capillary-filled chambers. Sperm motility was similar in all fields analysed in drop-filled Spermtrack chambers (Soler et al. 2012; Del Gallego et al. 2017). When capillary-filled chambers were used, variations in the distribution of spermatozoa along the chamber were different for different species. No changes were observed for human (Soler et al. 2012) and bull (Nöthling and dos Santos 2012) spermatozoa, but in other species, such as the fox, sperm motility was reduced in some individuals in the outermost region (from the deposition line) while remaining constant in others (Soler et al. 2014). In contrast, in goats, motility increased with the distance to the deposition point (Del Gallego et al. 2017).
The differences observed between counting chambers (with results varying with depth and charging method) imply that correlation studies are needed to translate results from one type of chamber to another to overcome the problem of different results being generated. This work must be based on good protocols, defining suitable sampling methods and the total number of cells analysed. This is particularly important when results of semen analysis are used for the calculation of commercial semen doses because, if it is not taken into account, the number of doses can differ markedly depending on the chamber used (Hoogewijs et al. 2012).
The new generation of CASA-Mot systems based on laser microscopy allows the use of counting chambers with a depth >20 µm (i.e. the maximum depth could be 100 µm). It has been shown that kinematic values obtained in chambers with a depth of 100 µm are significantly higher than those obtained using chambers with a depth of 10 or 20 µm, compared to the optical microscope (Soler et al. 2018).
In conclusion, the use of standardised procedures is not sufficient to guarantee reliable kinematic parameters with the use of CASA-Mot systems (in general with all CASA technology). Optimal analytical conditions need to be defined, including proper training for the technicians who must understand why one procedure is more appropriate than another. Subsequently, a well-defined quality control analysis is needed in order to obtain good scientific and good clinical results.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors’ work reported herein was supported, in part, by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) (Grant AGL2014-52775-P) and the Government of Aragon- European Social Fund (DGA-FSE). CC has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie project IMPRESS (GA No. 642893). AV is granted by the CONICIT and MICITT, Costa Rica.
References
Aanesen, A., and Bendvold, E. (1989). The CellsSoft computerized semen analysis system. I. Consistency of measurements and stability of results in relation to sample size analyzed. Andrologia 21, 559–567.| The CellsSoft computerized semen analysis system. I. Consistency of measurements and stability of results in relation to sample size analyzed.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c7ksFKjug%3D%3D&md5=747abb16e07c9692934d7a53fb59d24fCAS |
Acott, T. S., Katz, D. F., and Hoskins, D. D. (1983). Movement characteristics of bovine epididymal spermatozoa: effects of forward motility protein and epididymal maturation. Biol. Reprod. 29, 389–399.
| Movement characteristics of bovine epididymal spermatozoa: effects of forward motility protein and epididymal maturation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2FltlGmsg%3D%3D&md5=ec072d882f4b6bd4921597bdb7f5b48fCAS |
Aitken, R. J., Best, F. S. M., Richardson, D. W., Djahanbakhch, O., Mortimer, D., Templeton, A. A., and Lees, M. M. (1982). An analysis of sperm function in cases of unexplained infertility: conventional criteria, movement characteristics, and fertilizing capacity. Fertil. Steril. 38, 212–221.
| An analysis of sperm function in cases of unexplained infertility: conventional criteria, movement characteristics, and fertilizing capacity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL383mt1Wlug%3D%3D&md5=7c4ec9f84a6b6bb0ed9711a511f6f11dCAS |
Aitken, R. J., Sutton, M., Warner, P., and Richardson, D. W. (1985). Relationship between the movement characteristics of human spermatozoa and their ability to penetrate cervical mucus and zona-free hamster oocytes. J. Reprod. Fertil. 73, 441–449.
| Relationship between the movement characteristics of human spermatozoa and their ability to penetrate cervical mucus and zona-free hamster oocytes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M7osFCjsg%3D%3D&md5=8a4f10cc40f1a4c9056215450e8c11b8CAS |
Aitken, J., Buckingham, D., and Harkiss, D. (1994). Analysis of the extent to which sperm movement can predict the results of ionophore-enhanced functional assays of the acrosome reaction and sperm–oocyte fusion. Hum. Reprod. 9, 1867–1874.
| Analysis of the extent to which sperm movement can predict the results of ionophore-enhanced functional assays of the acrosome reaction and sperm–oocyte fusion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXitFaitbo%3D&md5=765e065c2152e292e24779f53a5c2a71CAS |
Amann, R. P., and Hammerstedt, R. H. (1980). Validation of a system for computerized measurement of spermatozoal velocity and percentage of motile sperm. Biol. Reprod. 23, 647–656.
| Validation of a system for computerized measurement of spermatozoal velocity and percentage of motile sperm.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M%2FoslWjtg%3D%3D&md5=fddf0a14626b0a9c5968c4136bbedd70CAS |
Amann, R. P., and Waberski, D. (2014). Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 81, 5–17.
| Computer-assisted sperm analysis (CASA): capabilities and potential developments.Crossref | GoogleScholarGoogle Scholar |
American Fertility Society (AFS). (1980). ‘How to Organize a Basic Study of the Infertile Couple.’ (AFS: Birmingham, AL.)
Atherton, R. W. (1975). An objective method for evaluating Angus and Hereford sperm motility. Int. J. Fertil. 20, 109–112.
| 1:STN:280:DyaE287ktFyksQ%3D%3D&md5=a35cfc5b54ccb4f3da918d0e20743f34CAS |
Atherton, R. W., Radany, E. W., and Polakoski, K. L. (1978). Spectrophotometric quantitation of mammalian spermatozoon motility. Biol. Reprod. 18, 624–628.
| Spectrophotometric quantitation of mammalian spermatozoon motility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1c7oslKitA%3D%3D&md5=6ab2f0475491a7cffba5896cf5573033CAS |
Bailey, E., Fenning, N., Chamberlain, S., Devlin, L., Hopkinson, J., and Tomlinson, M. (2007). Validation of sperm counting methods using limits of agreement. J. Androl. 28, 364–373.
| Validation of sperm counting methods using limits of agreement.Crossref | GoogleScholarGoogle Scholar |
Barratt, C. L. R., Björndahl, L., Menkveld, R., and Mortimer, D. (2011). ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance. Hum. Reprod. 26, 3207–3212.
| ESHRE special interest group for andrology basic semen analysis course: a continued focus on accuracy, quality, efficiency and clinical relevance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MboslyksA%3D%3D&md5=de480160faac30da251c2a96a09f52fdCAS |
Barták, V. (1971). Sperm velocity test in clinical practice. Int. J. Fertil. 16, 107–112.
Bartoov, B., Kalay, D., and Mayevsky, A. (1981). Sperm motility analyzer (SMA), a practical tool of motility and cell concentration determinations in artificial insemination centers. Theriogenology 15, 173–182.
| Sperm motility analyzer (SMA), a practical tool of motility and cell concentration determinations in artificial insemination centers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvVemsg%3D%3D&md5=874dbf6c2a238f6428c3e714f4b9cf1fCAS |
Belsey, M. A., Eliasson, R., Gallegos, A. J., Moghissi, K. S., Paulsen, C. H., and Prasad, M. R. N. (Eds) (1980). ‘Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction.’ (World Health Organization: Singapore.)
Björndahl, L. (2011). What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen results. Hum. Fertil. (Camb.) 14, 179–186.
| What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen results.Crossref | GoogleScholarGoogle Scholar |
Blum, J. J., and Lubliner, J. (1973). Biophysics of flagellar motility. Annu. Rev. Biophys. Bioeng. 2, 181–219.
| Biophysics of flagellar motility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXltVWksb8%3D&md5=f421bf2a758e3b2d10d235980d1f687bCAS |
Brokaw, C. J. (1965). Non-sinusoidal bending wave of sperm flagella. J. Exp. Biol. 43, 155–169.
| 1:STN:280:DyaF287jvFejtw%3D%3D&md5=ebf063f6457d0d410a3abf6a51c5eb50CAS |
Brokaw, C. J. (1970). Bending moments in free swimming flagella. J. Exp. Biol. 53, 445–464.
| 1:STN:280:DyaE3M%2Fjslaitg%3D%3D&md5=0aac8335a8a028730d69ff1616fa31beCAS |
Brokaw, C. J. (1972). Computer simulation of flagellar movement. I. Demonstration of stable bend propagation and bend initiation by sliding filament model. Biophys. J. 12, 564–586.
| Computer simulation of flagellar movement. I. Demonstration of stable bend propagation and bend initiation by sliding filament model.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE387psFaruw%3D%3D&md5=93086d40415c73a03dbc969d62b68458CAS |
Budworth, P. R., Amann, R. P., and Chapman, P. L. (1988). Relationships between computerized measurements of motion of frozen–thawed bull spermatozoa and fertility. J. Androl. 9, 41–54.
| Relationships between computerized measurements of motion of frozen–thawed bull spermatozoa and fertility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7ltlGitw%3D%3D&md5=5379fa85f3bfc9cb9905370a4efb0a90CAS |
Castellini, C., Dal Bosco, A., Ruggeri, S., and Collodel, G. (2011). What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil. Steril. 96, 24–27.
| What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis?Crossref | GoogleScholarGoogle Scholar |
Chan, S. Y. W., Wang, B. L., Song, T., Lo, A., Tsoi, W. L., and Leung, J. (1989). Computer assisted image analysis of sperm concentration in human semen before and after swim up preparation: comparison with assessment of hemocytometer. Int. J. Androl. 12, 339–345.
| Computer assisted image analysis of sperm concentration in human semen before and after swim up preparation: comparison with assessment of hemocytometer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c%2FnslKqtg%3D%3D&md5=1855c73adf341769229edb5fe3758113CAS |
Chantler, E., Abraham-Peskir, J., and Roberts, C. (2004). Consistent presence of two normally distributed sperm subpopulations within normozoospermic human semen: a kinematic study. Int. J. Androl. 27, 350–359.
| Consistent presence of two normally distributed sperm subpopulations within normozoospermic human semen: a kinematic study.Crossref | GoogleScholarGoogle Scholar |
Chong, A. P., Walters, C. A., and Weinrieb, S. A. (1983). The neglected laboratory test: the semen analysis. J. Androl. 4, 280–282.
| The neglected laboratory test: the semen analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2FgslOlsQ%3D%3D&md5=4da13d63ff7572d3637393933a6e5b6bCAS |
Christensen, P., Stryhn, H., and Hansen, C. (2005). Discrepancies in the determination of sperm concentration using Burker-Turk, Thoma and Makler counting chambers. Theriogenology 63, 992–1003.
| Discrepancies in the determination of sperm concentration using Burker-Turk, Thoma and Makler counting chambers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2Fot1Kmsw%3D%3D&md5=2777ed1586a7bf54d7efbb4d409173aaCAS |
Coetzee, K., and Menkveld, R. (2001). Validation of a new disposable counting chamber. Arch. Androl. 47, 153–156.
| Validation of a new disposable counting chamber.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mrgs1Cksw%3D%3D&md5=e1e91c56c0078a3b66293acb96b63bbbCAS |
Contri, A., Valorz, C., Faustini, M., Wegher, L., and Carluccio, A. (2010). Effect of semen preparation on CASA motility results in cryopreserved bull spermatozoa. Theriogenology 74, 424–435.
| Effect of semen preparation on CASA motility results in cryopreserved bull spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Cooke, D. F., and Hallet, F. R. (1976). Motility evaluation of bull spermatozoa by photon correlation spectroscopy. J. Mechanochem. Cell Motil. 3, 219–223.
| 1:STN:280:DyaE2s7ms1OrsA%3D%3D&md5=0a1ab0dc84fedc1a10b5af653abee57dCAS |
Cosson, J. (1996). A moving image of flagella: news and views on the mechanisms involved in axonemal beating. Cell Biol. Int. 20, 83–94.
| A moving image of flagella: news and views on the mechanisms involved in axonemal beating.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FovFantg%3D%3D&md5=1e259967d1a3de2324692e1908328367CAS |
Cosson, M.-P., Billard, R., Gatti, J.-L., and Christen, R. (1985). Rapid and quantitative assessment of trout spermatozoa motility using stroboscopy. Aquaculture 46, 71–75.
| Rapid and quantitative assessment of trout spermatozoa motility using stroboscopy.Crossref | GoogleScholarGoogle Scholar |
David, G., Serres, C., and Jouannet, P. (1981). Kinematics of human spermatozoa. Gamete Res. 4, 83–95.
| Kinematics of human spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Davis, R. O., and Katz, D. F. (1992). Standardization and comparability of CASA instruments. J. Androl. 13, 81–86.
| 1:STN:280:DyaK383gt1Skuw%3D%3D&md5=2de3514672c54039c2bef1c5948cfefeCAS |
Del Gallego, R., Sadeghi, S., Blasco, E., Soler, C., Yániz, J. L., and Silvestre, M. A. (2017). Effect of chamber characteristics, loading and analysis time on motility and kinetic variables analysed with the CASA-Mot system in goat sperm. Anim. Reprod. Sci. 177, 97–104.
| Effect of chamber characteristics, loading and analysis time on motility and kinetic variables analysed with the CASA-Mot system in goat sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXjs1yrsA%3D%3D&md5=6988e0a11fc96d6258b3822d9ee5e190CAS |
Denehy, M. A. (1975). The propulsion of nonrotating ram and oyster spermatozoa. Biol. Reprod. 13, 17–29.
| The propulsion of nonrotating ram and oyster spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE287mtFKjtg%3D%3D&md5=9b8658e4172feceb9a840079e6a02f92CAS |
Didion, B. A. (2008). Computer assisted semen analysis and its utility for profiling boar semen samples. Theriogenology 70, 1374–1376.
| Computer assisted semen analysis and its utility for profiling boar semen samples.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cnmvFGktw%3D%3D&md5=6de5c0c1cd731013e68e520aa461144fCAS |
Douglas-Hamilton, D. H., Smith, N. C., Kuster, C. E., Vermeiden, J. P. W., and Althouse, G. C. (2005a). Particle distribution in low-volume capillary-loaded chambers. J. Androl. 26, 107–114.
Douglas-Hamilton, D. H., Smith, N. C., Kuster, C. E., Vermeiden, J. P. W., and Althouse, G. C. (2005b). Capillary-loaded particle fluid dynamics: effect on estimation of sperm concentration. J. Androl. 26, 115–122.
Dubois, M., Jouannet, P., Berge, P., Volochine, B., Serres, C., and David, G. (1975). Methode et appareillage de mesure objective de la mobilite des spermatozoides humans. Ann. Phys. Biol. Med. 9, 19–41.
Elliot, F. I., Sherman, J. K., Elliot, E. J., and Sullivan, J. J. (1973). A photo method of measuring sperm motility. J. Anim. Sci. 37, 310.
Falk, H. C., and Kaufman, S. A. (1950). What constitutes a normal semen? Fertil. Steril. 1, 489–503.
| What constitutes a normal semen?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG3M%2FhtlKmtQ%3D%3D&md5=9e8624b793e1edd1746724c766d98961CAS |
Fray, C. S., Hoffer, A., and Fawcett, D. (1972). A re-examination of motility patterns of rat spermatozoa. Anat. Rec. 173, 301–307.
| A re-examination of motility patterns of rat spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE383hvFSqtw%3D%3D&md5=15687d404095fd75b0eff943cb68de50CAS |
Gączarzewicz, D. (2015). Influence of chamber type integrated with computer-assisted semen analysis (CASA) system on the results of boar semen evaluation. Pol. J. Vet. Sci. 18, 817–824.
| Influence of chamber type integrated with computer-assisted semen analysis (CASA) system on the results of boar semen evaluation.Crossref | GoogleScholarGoogle Scholar |
Gallego, V., Carneiro, P. C. F., Mazzeo, I., Vílchez, M. C., Peñaranda, D. S., Soler, C., Pérez, L., and Asturiano, J. F. (2013). Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software. Theriogenology 79, 1034–1040.
| Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svjslSktQ%3D%3D&md5=ec9534b50819c2ff1ab02a6511b237efCAS |
Gill, H. S., Van Arsdalen, K., Hypolote, J., Levin, R. M., and Ruzich, J. V. (1988). Comparative study of two computerized semen motility analyzers. Andrologia 20, 433–440.
| Comparative study of two computerized semen motility analyzers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2Fos1KltA%3D%3D&md5=f4ec85a48ee64ba16d9e3c98e8abb396CAS |
Ginsburg, K. A., Moghissi, K. S., and Abel, E. L. (1988). Computer assisted human semen analysis sampling errors and reproducibility. J. Androl. 9, 82–90.
| Computer assisted human semen analysis sampling errors and reproducibility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3ls1ynug%3D%3D&md5=66162a6c6e5e4c4ce58815f6057db5bbCAS |
Gloria, A., Carluccio, A., Contri, A., Wegher, L., Valorz, C., and Robbe, D. (2013). The effect of the chamber on kinetic results in cryopreserved bull spermatozoa. Andrology 1, 879–885.
| The effect of the chamber on kinetic results in cryopreserved bull spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sbksVaiuw%3D%3D&md5=e8ccdcd2782c5381d0649827f73873ceCAS |
Glover, F. A. (1968). Physical method of measuring the mobility of bull sperm. Nature 219, 1263.
| Physical method of measuring the mobility of bull sperm.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1cvhtFGhtw%3D%3D&md5=813cf18f4b7ba3d1eafa32d5ab00d731CAS |
Gray, J. (1955). The movement of sea-urchin spermatozoa. J. Exp. Biol. 32, 775–801.
Gray, J. (1958). The movement of spermatozoa of the bull. J. Exp. Biol. 35, 96–108.
Gray, J., and Hancock, G. J. (1955). The propulsion of sea urchin spermatozoa. J. Exp. Biol. 32, 802–814.
Hancock, C. J. (1953). The self-propulsion of microscopic organisms through liquids. Proc. R. Soc. Lond. A Math. Phys. Sci. 217, 96–121.
| The self-propulsion of microscopic organisms through liquids.Crossref | GoogleScholarGoogle Scholar |
Hansen, P. J. (2014). Current and future reproductive technologies and world food production. Adv. Exp. Med. Biol. 752, 1–22.
| Current and future reproductive technologies and world food production.Crossref | GoogleScholarGoogle Scholar |
Hirai, M., Boersma, A., Hoeflich, A., Wolf, E., Foll, J., Aumüller, T. R., and Braun, J. (2001). Objectively measured sperm motility and sperm head morphometry in boars (Sus scrofa): relation to fertility and seminal plasma growth factors. J. Androl. 22, 104–110.
| 1:CAS:528:DC%2BD3MXkvFyjtw%3D%3D&md5=215a1bbe92c536bc197279ea8f592255CAS |
Holt, W. V., and Palomo, M. J. (1996). Optimization of a continuous real-time computerized semen analysis system for ram sperm motility assessment, and evaluation of four methods of semen preparation. Reprod. Fertil. Dev. 8, 219–230.
| Optimization of a continuous real-time computerized semen analysis system for ram sperm motility assessment, and evaluation of four methods of semen preparation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28zivFWjug%3D%3D&md5=49559196c5869b2ba742a37a7c110426CAS |
Holt, W. V., Moore, H. D. M., and Hillier, S. G. (1985). Computer assisted measurement of sperm swimming speed in human semen: correlation of results with in vitro fertilization assays. Fertil. Steril. 44, 112–119.
| Computer assisted measurement of sperm swimming speed in human semen: correlation of results with in vitro fertilization assays.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M3jtFaqsQ%3D%3D&md5=02f8d23eb9f79be46dddfd03d836c657CAS |
Holt, W., Watson, P., Curry, M., and Holt, C. (1994). Reproducibility of computer-aided semen analysis: comparison of five different systems used in a practical workshop. Fertil. Steril. 62, 1277–1282.
| Reproducibility of computer-aided semen analysis: comparison of five different systems used in a practical workshop.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2Fks1CitA%3D%3D&md5=8d1544391f50793c9f20d9a319555897CAS |
Holt, W. V., O’Brien, J., and Abaigar, T. (2007). Applications and interpretation of computer-assisted sperm analyses and sperm sorting methods in assisted breeding and comparative research. Reprod. Fertil. Dev. 19, 709–718.
| Applications and interpretation of computer-assisted sperm analyses and sperm sorting methods in assisted breeding and comparative research.Crossref | GoogleScholarGoogle Scholar |
Hoogewijs, M. K., de Vliegher, S. P., Govaere, J. L., de Schauwer, C., de Kruif, A., and van Soom, A. (2012). Influence of counting chamber type on CASA outcomes of equine semen analysis. Equine Vet. J. 44, 542–549.
| Influence of counting chamber type on CASA outcomes of equine semen analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38zosFChtA%3D%3D&md5=dcc9f4120c955cced26798fc4f06a033CAS |
Ibănescu, I., Leiding, C., Ciorneri, S. G., Rosca, P., Sfartz, I., and Drugociu, D. (2016). Differences in CASA output according to the chamber type when analyzing frozen–thawed bull sperm. Anim. Reprod. Sci. 166, 72–79.
| Differences in CASA output according to the chamber type when analyzing frozen–thawed bull sperm.Crossref | GoogleScholarGoogle Scholar |
Ishii, N., Mitsukawa, S., and Shirai, M. (1977). Sperm motile efficiency. Andrologia 9, 55–62.
| Sperm motile efficiency.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s7nvFyiug%3D%3D&md5=7995b468e6c61f1360a4fa3f49e03544CAS |
Janick, J., and MacLeod, J. (1970). The measurement of human spermatozoan motility. Fertil. Steril. 21, 140–146.
| The measurement of human spermatozoan motility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3c7htF2rsg%3D%3D&md5=98acf226fe52b36f4fb705cf771c33b8CAS |
Jasko, D. J., Lein, D. H., and Foote, R. H. (1990). A comparison of two computer-assisted semen analysis instruments for the evaluation of sperm motion characteristics in the stallion. J. Androl. 11, 453–459.
| 1:STN:280:DyaK3M%2Fnt1WjsA%3D%3D&md5=56cdc974071b189bd295b69a0107543aCAS |
Jecht, E. W., and Russo, J. J. (1973). A system for the quantitative analysis of human sperm motility. Andrologie 5, 215–221.
| A system for the quantitative analysis of human sperm motility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2c%2Fmt1WjtA%3D%3D&md5=0666c3c5d53710562e0d01f1073e3945CAS |
Jequier, A. M., and Ukombe, E. B. (1983). Errors inherent in the performance of routine semen analysis. Br. J. Urol. 55, 434–436.
| Errors inherent in the performance of routine semen analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s3osVSqsQ%3D%3D&md5=66a68bc55d6f6e4dc7869e9f1ada71c4CAS |
Johnson, J. E., Boone, W. R., and Shapiro, S. S. (1990). Determination of the precision of an automated semen analyzer. Lab. Med. 21, 33–38.
| Determination of the precision of an automated semen analyzer.Crossref | GoogleScholarGoogle Scholar |
Johnson, J. E., Boone, W. R., and Blackhurst, D. W. (1996a). Manual versus computer-automated semen analyses. Part I. Comparison of counting chambers. Fertil. Steril. 65, 150–155.
| Manual versus computer-automated semen analyses. Part I. Comparison of counting chambers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287gvFSnuw%3D%3D&md5=25c7fab50c48f98d285aec22548b795bCAS |
Johnson, J. E., Boone, W. R., and Blackhurst, D. W. (1996b). Manual versus computer-automated semen analyses. Part II. Determination of the working range of a computer-automated semen analyzer. Fertil. Steril. 65, 156–159.
| Manual versus computer-automated semen analyses. Part II. Determination of the working range of a computer-automated semen analyzer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287gvFSmsg%3D%3D&md5=5d1560a5b8eff06f417b5f6376fa2d5cCAS |
Jouannet, P., Volochine, B., Deguent, P., Serres, C., and David, G. (1977). Light scattering determination of various characteristic parameters of spermatozoa motility in a series of human sperm. Andrologia 9, 36–49.
| Light scattering determination of various characteristic parameters of spermatozoa motility in a series of human sperm.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s7nvFyitA%3D%3D&md5=f0dedadf984abacb40a026af374300ddCAS |
Kathiravan, P., Kalatharan, J., Karthikeya, G., Rengarajan, K., and Kadrivel, G. (2011). Objective sperm motion analysis to assess dairy bull fertility using computer-aided system. A review. Reprod. Domest. Anim. 46, 165–172.
| Objective sperm motion analysis to assess dairy bull fertility using computer-aided system. A review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itlOgtQ%3D%3D&md5=effc5d565fbfe074039ee5f3ac2d4ee2CAS |
Katz, D. F., and Davis, R. O. (1987). Automatic analysis of human sperm motion. J. Androl. 8, 170–181.
| Automatic analysis of human sperm motion.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3nvFCmsQ%3D%3D&md5=4713a6aeef4c3a285e645b602f1c6809CAS |
Katz, D. F., and Dott, H. M. (1975). Methods of measuring swimming speed of spermatozoa. J. Reprod. Fertil. 45, 263–272.
| Methods of measuring swimming speed of spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE28%2Fpslegsg%3D%3D&md5=091340ea80be1f1a9b80f84bba81ecb8CAS |
Katz, D. F., and Overstreet, J. W. (1981). Sperm motility assessment by video-micrography. Fertil. Steril. 35, 188–193.
| Sperm motility assessment by video-micrography.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7jvV2gsQ%3D%3D&md5=335d442616039826e26b994f3e4e7bb6CAS |
Katz, D. F., Mills, R. N., and Prichett, T. R. (1978). The movement of human spermatozoa in cervical mucus. J. Reprod. Fertil. 53, 259–265.
| The movement of human spermatozoa in cervical mucus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M%2Fgslegsg%3D%3D&md5=ba7a52e0aa729613613bbbca99a9b628CAS |
Katz, D. F., Davis, R. O., Delandmeter, B., and Overstreet, J. W. (1985). Real-time analysis of sperm motion using automatic video image digitization. Comput. Methods Programs Biomed. 21, 173–182.
| Real-time analysis of sperm motion using automatic video image digitization.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL287jsVGgug%3D%3D&md5=1e0f3f77b6772578536a705f3aff64adCAS |
Knuth, U. A., and Nieschlag, E. (1988). Comparison of computerized semen analysis with the conventional procedure in 322 patients. Fertil. Steril. 49, 881–885.
| Comparison of computerized semen analysis with the conventional procedure in 322 patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7ps1Gjsg%3D%3D&md5=04cdef14663302a2633f69461ea08f9aCAS |
Kraemer, M., Fillion, C., Martin-Pont, B., and Auger, J. (1998). Factors influencing human sperm kinematic measurements by the Celltrak computer-assisted sperm analysis system. Hum. Reprod. 13, 611–619.
| Factors influencing human sperm kinematic measurements by the Celltrak computer-assisted sperm analysis system.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3jt1WgsQ%3D%3D&md5=60954729224a61eae823bdb7f3feeb54CAS |
Kumar, N., and Singh, A. K. (2015). Trends of male factor infertility, an important cause of infertility: a review of literature. J. Hum. Reprod. Sci. 8, 191–196.
| Trends of male factor infertility, an important cause of infertility: a review of literature.Crossref | GoogleScholarGoogle Scholar |
Kuster, C. (2005). Sperm concentration determination between hemacytometric and CASA systems: why they can be different? Theriogenology 64, 614–617.
| Sperm concentration determination between hemacytometric and CASA systems: why they can be different?Crossref | GoogleScholarGoogle Scholar |
Kvist, U., and Björndahl, L. (2002). ‘Manual on Basic Semen Analysis.’ (European Society of Human Reproduction and Embryology (ESHRE)).
Le Lannou, D., Griveau, J. F., Le Pichon, J. P., and Quero, J. C. (1992). Effects of chamber on the motion pattern of human spermatozoa in semen or in capacitating medium. Hum. Reprod. 7, 1417–1421.
| Effects of chamber on the motion pattern of human spermatozoa in semen or in capacitating medium.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s7nvFGgsA%3D%3D&md5=0b655e762975a648d814185d2624ef10CAS |
Len, J. A., Jenkins, J. A., Eilts, B. E., Pacamonti, D. L., Lyle, S. K., and Hosgood, G. (2010). Immediate and delayed (after cooling) effects of centrifugation on equine sperm. Theriogenology 73, 225–231.
| Immediate and delayed (after cooling) effects of centrifugation on equine sperm.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MfhvVygtQ%3D%3D&md5=b4989e545c4f5821226270d88cec5c13CAS |
Lenz, R. W., Kjelland, M. E., Vonderhaar, K., Swannack, T. M., and Moreno, J. F. (2011). A comparison of bovine seminal quality assessments using different viewing chambers with a computer-assisted semen analyzer. J. Anim. Sci. 89, 383–388.
| A comparison of bovine seminal quality assessments using different viewing chambers with a computer-assisted semen analyzer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvFSnur4%3D&md5=d8394f3ee5261113d086b9ddb0951ee3CAS |
Liu, Y. T., and Warme, P. K. (1977). Computerized evaluation of sperm cell motility. Comput. Biomed. Res. 10, 127–138.
| Computerized evaluation of sperm cell motility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s7mtFaguw%3D%3D&md5=dcc23f81af34ce51d60492b6bb2fed2aCAS |
Loomis, P. R., and Graham, J. K. (2008). Commercial semen freezing: individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod. Sci. 105, 119–128.
| Commercial semen freezing: individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXislSmtLk%3D&md5=2b0913b1038f6da5390ff4295565cc4aCAS |
Lu, J. C., Xu, H. R., Chen, F., and Huang, Y. F. (2007). Primary investigations on the quality control for semen analysis in Nanjing city. Zhonghua Nan Ke Xue 13, 37–41.
| 1:CAS:528:DC%2BD1cXmsFWnu78%3D&md5=3702024138d466eb03c8f1c19163fecdCAS |
Mack, S. O., Wolf, D. P., and Tasj, J. S. (1988). Quantitation of specific parameters of motility in large number of human sperm by digital image processing. Biol. Reprod. 38, 270–281.
| Quantitation of specific parameters of motility in large number of human sperm by digital image processing.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7ptlajsQ%3D%3D&md5=441d613894f7fb03937369a70baba0a5CAS |
MacLeod, J. (1971). Human male infertility. Obstet. Gynecol. Surv. 26, 335.
| Human male infertility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2M%2Fot1ygug%3D%3D&md5=3b0c1e2cacf4dc9e52884854b887f3d4CAS |
MacLeod, J., and Gold, R. Z. (1951). The male factor in fertility and infertility: spermatozoan counts in 1000 men of known fertility and 1000 cases of infertile marriages. J. Urol. 66, 436–449.
| The male factor in fertility and infertility: spermatozoan counts in 1000 men of known fertility and 1000 cases of infertile marriages.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG3M%2FptFGhsQ%3D%3D&md5=2fbdfaf9feaf2b0820353b0d7367acfdCAS |
MacLeod, I. C., and Irvine, D. S. (1995). The predictive value of computer-assisted semen analysis in the context of a donor insemination programme. Hum. Reprod. 10, 580–586.
| The predictive value of computer-assisted semen analysis in the context of a donor insemination programme.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzgtFKktg%3D%3D&md5=a9a2d592632a0ac917474f595cb83692CAS |
Mahmoud, A. M. A., Depoorter, B., Piens, N., and Comhaire, F. H. (1997). The performance of 10 different methods for the estimation of sperm concentration. Fertil. Steril. 68, 340–345.
| The performance of 10 different methods for the estimation of sperm concentration.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szosVGquw%3D%3D&md5=292ed08ddd721c13af00a60a93774f25CAS |
Majumder, G. C., and Chakrabarti, C. K. (1984). A simple spectrophotometric method of assay of forward motility of goat spermatozoa. J. Reprod. Fertil. 70, 235–241.
| A simple spectrophotometric method of assay of forward motility of goat spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c7hsVKnug%3D%3D&md5=37c09b9ca6f82857c95676c0f3d89a88CAS |
Makler, A. (1978a). A new multiple exposure photography method for objective human spermatozoal motility determination. Fertil. Steril. 30, 192–199.
| A new multiple exposure photography method for objective human spermatozoal motility determination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1c3jvFWktQ%3D%3D&md5=3f3e353a0cd8f26b872d4ddeb740085aCAS |
Makler, A. (1978b). A new chamber for rapid sperm count and motility estimation. Fertil. Steril. 30, 313–318.
| A new chamber for rapid sperm count and motility estimation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M%2FksFOnsg%3D%3D&md5=d11e5dcf986ba2d2ded3ceb38fa3b22eCAS |
Massányi, P., Chrenek, P., Lukáč, N., Makarevich, A. V., Ostro, A., Živčak, J., and Bulla, J. (2008). Comparison of different evaluation chambers for analysis of rabbit spermatozoa motility parameters using CASA system. Slovak J. Anim. Sci. 2, 60–66.
Mathur, S., Carlton, M., Ziegler, J., Rust, P. F., and Williamson, H. O. (1986). A computerized sperm motion analysis. Fertil. Steril. 46, 484–488.
| 1:STN:280:DyaL28zgtFamsQ%3D%3D&md5=35beeb1274f1279aeab94268858260aaCAS |
Matson, P., Irving, J., Zuvela, E., and Hughes, R. (1999). Delay in the application of the cover glass is a potential source of error in the Makler counting chamber. Fertil. Steril. 72, 559–561.
| 1:STN:280:DyaK1MvkslWhtA%3D%3D&md5=03cc12bbe533969093c8d1989d6e2998CAS |
McPherson, F. J., Nielsen, S. G., and Chenoweth, P. J. (2014). Semen effects on insemination outcomes in sows. Anim. Reprod. Sci. 151, 28–33.
| Semen effects on insemination outcomes in sows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2Mzks1ygtw%3D%3D&md5=b2ccc105acf0d28eb0fc96dc05c90c9dCAS |
Mitsukawa, S. (1979). A new method for determining sperm motility: the clinical application of sperm motile efficiency. Jpn J Urol 70, 1221–1231.
| A new method for determining sperm motility: the clinical application of sperm motile efficiency.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c7hvVGnsw%3D%3D&md5=bbb95287d2a12d81df90a73393de1c83CAS |
Morales, P., Overstreet, J. W., and Katz, D. F. (1988). Changes in human sperm motion during capacitation in vitro. J. Reprod. Fertil. 83, 119–128.
| Changes in human sperm motion during capacitation in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3os1Gjug%3D%3D&md5=dc69c91a2e0751fb32b600097b368545CAS |
Morris, A. R., Coutts, J. R. T., and Roberston, L. (1996). A detailed study of the effect of videoframe rates of 25, 30 and 60 Hertz on human sperm movement characteristics. Hum. Reprod. 11, 304–310.
| A detailed study of the effect of videoframe rates of 25, 30 and 60 Hertz on human sperm movement characteristics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283ls12itw%3D%3D&md5=5ebf602414d17c07d23f33747dfdf335CAS |
Mortimer, D. (1990). Objective analysis of sperm motility and kinematics. In ‘Handbook of the Laboratory Diagnosis and Treatment of Infertility’. (Eds B. A. Keel and B. W. Webster.) pp. 97–133. (CRC Press: Boca Raton, FL.)
Mortimer, S. T., and Swan, M. A. (1995). Kinematics of capacitating human spermatozoa analysed at 60 Hz. Hum. Reprod. 10, 873–879.
| Kinematics of capacitating human spermatozoa analysed at 60 Hz.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MznvVeqtQ%3D%3D&md5=06d6a2f9502a67d3c8caea85d36b8321CAS |
Mortimer, D., Shu, M. A., and Tan, R. (1986). Standardization and quality control of sperm concentration and sperm motility counts in semen analysis. Hum. Reprod. 1, 299–303.
| Standardization and quality control of sperm concentration and sperm motility counts in semen analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s7ms1Kmtw%3D%3D&md5=a2fb7eca97ed2a706858766e2712c251CAS |
Mortimer, D., Goel, N., and Shu, M. A. (1988). Evaluation of the CellSoft automated semen analysis system in a routine laboratory setting. Fertil. Steril. 50, 960–968.
| Evaluation of the CellSoft automated semen analysis system in a routine laboratory setting.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2FnvVKmtg%3D%3D&md5=a40178ab86996311dd9ffcbc4f1cccd0CAS |
Mortimer, S. T., Schoëvaërt, D., Swan, M. A., and Mortimer, D. (1997). Quantitative observations of flagellar motility of capacitating human spermatozoa. Hum. Reprod. 12, 1006–1012.
| Quantitative observations of flagellar motility of capacitating human spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2szjsl2rtg%3D%3D&md5=470fc88b74b2c9ccc6bd8a5ed3326551CAS |
Mortimer, S. T., van der Horst, G., and Mortimer, D. (2015). The future of computer-aided sperm analysis. Asian J. Androl. 17, 545–553.
| The future of computer-aided sperm analysis.Crossref | GoogleScholarGoogle Scholar |
Neuwinger, J., Knuth, U. A., and Nieschlag, E. (1990). Evaluation of the Hamilton–Thorne motility analyser for routine semen analysis in a fertility clinic. Int. J. Androl. 13, 100–109.
| Evaluation of the Hamilton–Thorne motility analyser for routine semen analysis in a fertility clinic.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c3msVSmtQ%3D%3D&md5=a41e3aaec45131c2b5f1da7288d03cc4CAS |
Nöthling, J. O., and dos Santos, I. P. (2012). Which fields under coverslip should one assess to estimate sperm motility? Theriogenology 77, 1686–1697.
| Which fields under coverslip should one assess to estimate sperm motility?Crossref | GoogleScholarGoogle Scholar |
O’Connor, M. T., Amann, R. P., and Saacke, R. G. (1981). Comparisons of computer evaluations of spermatozoal motility with standard laboratory tests and their use for predicting fertility. J. Anim. Sci. 53, 1368–1376.
| Comparisons of computer evaluations of spermatozoal motility with standard laboratory tests and their use for predicting fertility.Crossref | GoogleScholarGoogle Scholar |
Olds-Clarke, P., Baer, H. M., and Gerber, W. L. (1990). Human sperm motion analysis by automatic (Hamilton–Thorn Motility Analyzer) and manual (Image-80) digitization systems. J. Androl. 11, 52–58.
| 1:STN:280:DyaK3c7os1Chuw%3D%3D&md5=9fc80d257bb9cd29030f8726513d3173CAS |
Ortega-Ferrusola, C., Macías García, B., Suárez Rama, V., Gallardo-Bolaños, J. M., González-Fernández, L., Tapia, J. A., Rodríguez-Martínez, H., and Peña, F. J. (2009). Identification of sperm subpopulations in stallion ejaculates: changes after cryopreservation and comparison with traditional statistics. Reprod. Domest. Anim. 44, 419–423.
| Identification of sperm subpopulations in stallion ejaculates: changes after cryopreservation and comparison with traditional statistics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MrisFWqsQ%3D%3D&md5=d4961601a6b38d7aae1e4961e4311f64CAS |
Overstreet, J. W. (1984). Laboratory tests for human male reproductive risk assessment. Teratog. Carcinog. Mutagen. 4, 67–82.
| Laboratory tests for human male reproductive risk assessment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c7nt12jug%3D%3D&md5=eb83ecdf8f42ad653a75fbf5cbdc43e0CAS |
Overstreet, J. W., Katz, D. F., Hanson, F. W., and Fonesca, J. R. (1979). A simple inexpensive method for objective assessment of human sperm movement characteristics. Fertil. Steril. 31, 162–172.
| A simple inexpensive method for objective assessment of human sperm movement characteristics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M7gslGntQ%3D%3D&md5=84d52e3e2a8e1867077c7bfeb6ee9394CAS |
Palacín, I., Vicente-Fiel, S., Santolaria, P., and Yániz, J. L. (2013). Standardization of CASA sperm motility assessment in the ram. Small Rumin. Res. 112, 128–135.
| Standardization of CASA sperm motility assessment in the ram.Crossref | GoogleScholarGoogle Scholar |
Palacios, E. R., Clavero, A., Gonzalvo, M. C., Rosales, A., Mozas, J., Martínez, L., Ramírez, J. P., Björndahl, L., Morancho-Zaragoza, J., Fernández-Pardo, E., and Castilla, J. A. (2012). Acceptable variability in external quality assessment programmes for basic semen analysis. Hum. Reprod. 27, 314–322.
| Acceptable variability in external quality assessment programmes for basic semen analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC387ksVKlsw%3D%3D&md5=871e9c385bcd9db766ff3002ee1f5f93CAS |
Peng, N., Zou, X., Li, L., Peng, N., Zou, X., and Li, L. (2015). Comparison of different counting chambers using a computer-assisted semen analyzer. Syst Biol Reprod Med 61, 307–313.
Prathalingam, N. S., Holt, W. W., Revell, S. G., Jones, S., and Watson, P. F. (2006). The precision and accuracy of six different methods to determine sperm concentration. J. Androl. 27, 257–262.
| The precision and accuracy of six different methods to determine sperm concentration.Crossref | GoogleScholarGoogle Scholar |
Revell, S. G., and Wood, P. D. P. (1978). A photographic method for the measurement of motility of bull spermatozoa. J. Reprod. Fertil. 54, 123–126.
| A photographic method for the measurement of motility of bull spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M%2FltVegtQ%3D%3D&md5=0318b4ae370e135f309720e5e7b80c51CAS |
Rijsselaere, T., Van Soom, A., Maes, D., and de Kruif, A. (2003). Effect of technical settings on canine semen motility parameters measured by the Hamilton–Thorne analyzer. Theriogenology 60, 1553–1568.
| Effect of technical settings on canine semen motility parameters measured by the Hamilton–Thorne analyzer.Crossref | GoogleScholarGoogle Scholar |
Rikmenspoel, R., and van Herpen, G. (1957). Photoelectric and cinematographic measurements of the motility of bull sperm cells. Phys. Med. Biol. 2, 54–63.
| Photoelectric and cinematographic measurements of the motility of bull sperm cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG1c%2FislWmtA%3D%3D&md5=0e5a431f19ab5f5a835130d0911b424cCAS |
Rothschild, L. (1953). A new method of measuring sperm speeds. Nature 171, 512–513.
| A new method of measuring sperm speeds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG3s%2FnvFGntg%3D%3D&md5=a7d320186c2e9f50ff418584a65b12c4CAS |
Samuels, J. S., and van der Horst, G. (1986). Sperm motility analysis by means of frame lapse videography. Arch. Androl. 17, 151–155.
| Sperm motility analysis by means of frame lapse videography.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s7lsFGrsQ%3D%3D&md5=c456a8240a4f384ecb101b3d7d682b83CAS |
Schoëvaërt-Brossault, D. (1984). Automated analysis of human sperm motility. Comput. Biomed. Res. 17, 362–375.
| Automated analysis of human sperm motility.Crossref | GoogleScholarGoogle Scholar |
Segré, G., and Silberberg, A. (1962a). Behaviour of macroscopic rigid spheres in Poiseuille flow. Part 1. Determination of local concentration by statistical analysis of particle passages through crossed light beams. J. Fluid Mech. 14, 115–135.
| Behaviour of macroscopic rigid spheres in Poiseuille flow. Part 1. Determination of local concentration by statistical analysis of particle passages through crossed light beams.Crossref | GoogleScholarGoogle Scholar |
Segré, G., and Silberberg, A. (1962b). Behaviour of macroscopic rigid spheres in Poiseuille flow. Part 2. Experimental results and interpretation. J. Fluid Mech. 14, 136–157.
| Behaviour of macroscopic rigid spheres in Poiseuille flow. Part 2. Experimental results and interpretation.Crossref | GoogleScholarGoogle Scholar |
Serres, C., Fenneux, D., Jouannet, P., and David, G. (1984). Influence of the flagellar development and propagation on the human sperm movement in seminal plasma. Gamete Res. 9, 183–195.
| Influence of the flagellar development and propagation on the human sperm movement in seminal plasma.Crossref | GoogleScholarGoogle Scholar |
Shimizu, H., and Matsumoto, G. (1977). Light scattering study on motile spermatozoa. IEEE Trans. Biomed. Eng. 24, 153–157.
| Light scattering study on motile spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s3kvVansw%3D%3D&md5=e71f93465dfac4620f828a1c735fdb34CAS |
Shivaji, S., Peedicayil, J., and Devi, L. G. (1995). Analysis of the motility parameters of in vitro hyperactivated hamster spermatozoa. Mol. Reprod. Dev. 42, 233–247.
| Analysis of the motility parameters of in vitro hyperactivated hamster spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXosVWqtrw%3D&md5=817771145f13c568dd1daaf3834eeab5CAS |
Simonik, O., Sichtar, J., Krejcarkova, A., Rajmon, R., Stadnik, L., Beran, J., Dolezalova, M., and Biniova, Z. (2015). Computer assisted sperm analysis – the relationship to bull field fertility, possible errors and their impact on outputs: a review. Indian J. Anim. Sci. 85, 3–11.
Smith, S. C., and England, G. C. W. (2001). Effect of technical settings and semen handling upon motility characteristics of dog spermatozoa measured by computer-aided sperm analysis. J. Reprod. Fertil. Suppl. 57, 151–159.
| 1:STN:280:DC%2BD38%2Fmt1SrtQ%3D%3D&md5=388192dc3745f71fe22171b800eb2056CAS |
Soler, C., Fuentes, M. C., Sancho, M., García, A., and Núñez de Murga, M. (2012). Effect of counting chamber on seminal parameters, analyzing with the ISASv1®. Rev. Int. Androl. 10, 132–138.
| Effect of counting chamber on seminal parameters, analyzing with the ISASv1®.Crossref | GoogleScholarGoogle Scholar |
Soler, C., García, A., Contell, J., Segervall, J., and Sancho, M. (2014). Kinematics and subpopulations’ structure definition of blue fox (Alopex lagopus) sperm motility using the ISASV1 CASA system. Reprod. Domest. Anim. 49, 560–567.
| Kinematics and subpopulations’ structure definition of blue fox (Alopex lagopus) sperm motility using the ISASV1 CASA system.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cjosFSltA%3D%3D&md5=b010021ffe63f8d2c5b85ae4b5a131a1CAS |
Soler, C., Picazo-Bueno, J. Á., Micó, V., Valverde, A., Bompart, D., Blasco, F. J., Alvarez, J. G., and García-Molina, A. (2018). Effect of counting chamber depth on the accuracy of lensless microscopy for the assessment of boar sperm motility. Reprod. Fertil. Dev. , .
| Effect of counting chamber depth on the accuracy of lensless microscopy for the assessment of boar sperm motility.Crossref | GoogleScholarGoogle Scholar |
Spizziri, B. E., Fox, M. H., Bruemmeer, J. E., Squires, E. L., and Graham, J. K. (2010). Cholesterol-loaded-cyclodextrins and the fertility potential of stallion spermatozoa. Anim. Reprod. Sci. 118, 255–264.
| Cholesterol-loaded-cyclodextrins and the fertility potential of stallion spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGlurs%3D&md5=7cf9a178a3e66c8087c9ea73eb336ed1CAS |
Stephens, D. T., Hickman, R., and Hoskins, D. D. (1988). Description, validation, and performance characteristics of a new computer-automated sperm motility analysis system. Biol. Reprod. 38, 577–586.
| Description, validation, and performance characteristics of a new computer-automated sperm motility analysis system.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3ktFSrtw%3D%3D&md5=63602974e30e388831d7363f540446e3CAS |
Suarez, S. S., Katz, D. F., and Overstreeet, J. W. (1983). Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization. Biol. Reprod. 29, 1277–1287.
| Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2FotVKkuw%3D%3D&md5=9542d74dfd276d66c82f4acef4e536a6CAS |
Tash, J. S., Hidaka, H., and Means, A. R. (1986). Axokinin phosphorylation by cAMP-dependent protein kinase is sufficient for activation of sperm flagellar movement. J. Cell Biol. 103, 649–655.
| Axokinin phosphorylation by cAMP-dependent protein kinase is sufficient for activation of sperm flagellar movement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XltlWhu7Y%3D&md5=cf4488ed77098add41ca3d80bd719b19CAS |
Taylor, G. I. (1951). Analysis of the swimming of microscopic organisms. Proc. R. Soc. Lond. A Math. Phys. Sci. 209, 447–461.
| Analysis of the swimming of microscopic organisms.Crossref | GoogleScholarGoogle Scholar |
Taylor, G. (1952). The action of waving cylindrical tails in propelling microscopic organisms. Proc. Royal. Soc. A. 211, 224–239.
Tomlinson, M., Turner, J., Powell, G., and Sakas, D. (2001). One-step disposable chambers for sperm concentration and motility assessment: how do they compare with the World Health Organization’s recommended methods? Hum. Reprod. 16, 121–124.
| One-step disposable chambers for sperm concentration and motility assessment: how do they compare with the World Health Organization’s recommended methods?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7ktVertQ%3D%3D&md5=bf144c6f128c01f38c977792e390f0a7CAS |
van Duijin, C., Van Vorrst, C., and Freund, M. (1971). Movement characteristics of human spermatozoa analyzed from kinemicrographs. Eur. J. Obstet. Gynecol. Reprod. Biol. 1, 121–135.
| Movement characteristics of human spermatozoa analyzed from kinemicrographs.Crossref | GoogleScholarGoogle Scholar |
Vantman, D., Koukoulis, G., Dennison, L., Zinaman, M., and Sherins, R. (1988). Computer-assisted semen analysis: evaluation of method and assessment of the influence of sperm concentration on linear velocity determination. Fertil. Steril. 49, 510–515.
| Computer-assisted semen analysis: evaluation of method and assessment of the influence of sperm concentration on linear velocity determination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7jslKrsw%3D%3D&md5=21ea4e0146b6652a05c1c1f1cbc22ecdCAS |
Vasseur, P., and Cox, R. G. (1976). The lateral migration of a spherical particle in two-dimensional shear flows. J. Fluid Mech. 78, 385–413.
| The lateral migration of a spherical particle in two-dimensional shear flows.Crossref | GoogleScholarGoogle Scholar |
Verstegen, J., Iguer-ouada, M., and Onclin, K. (2002). Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 57, 149–179.
| Computer assisted semen analyzers in andrology research and veterinary practice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2Fksl2mtA%3D%3D&md5=f7df33962b8de34ef0fd69f0834e96fcCAS |
Waite, J. A., Love, C. C., Brinsko, S. P., Teague, S. R., Salazar, J. L., Mancil, S. S., and Varner, D. D. (2008). Factors impacting equine sperm recovery rate and quality following cushioned centrifugation. Theriogenology 70, 704–714.
| Factors impacting equine sperm recovery rate and quality following cushioned centrifugation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvovVGgtg%3D%3D&md5=59dfef32f829501e68b3d5851657a132CAS |
Walker, J. S., Wient, H., and Freund, M. (1982). A comparison of subjective and objective sperm motility evaluation. J. Androl. 3, 184–192.
| A comparison of subjective and objective sperm motility evaluation.Crossref | GoogleScholarGoogle Scholar |
World Health Organization (WHO) (2010). ‘WHO Laboratory Manual for the Examination and Processing of Human Semen.’ 5th edn. (WHO: Geneva.)
Yundt, A. P., Shak, W. J., and Lardner, T. J. (1975). Applicability of hydrodynamic analyses of spermatozoan motion. J. Exp. Biol. 62, 27–41.
| 1:STN:280:DyaE2M3ht1Ohsw%3D%3D&md5=e73c9deb55ce9e03900a5a2426e5231eCAS |



