The involvement of hypoxia-inducible factor 1α (HIF1α)-stabilising factors in steroidogenic acute regulatory (STAR) protein-dependent steroidogenesis in murine KK1 granulosa cells in vitro
Tina Gysin A and Mariusz P. Kowalewski A *
A *
A Institute of Veterinary Anatomy, Vetsuisse Faculty, University of Zurich (UZH), Zurich CH-8057, Switzerland.
Reproduction, Fertility and Development 33(18) 865-880 https://doi.org/10.1071/RD21170
Published online: 7 December 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
As a component of hypoxia-inducible factor 1 (HIF1)-complexes, HIF1α regulates the expression of steroidogenic acute regulatory (STAR) protein in granulosa cells. However, severe hypoxia or exaggeratedly expressed HIF1α have detrimental effects. HIF1α is regulated by factor inhibiting HIF (FIH), prolyl hydroxylases (PHD1, 2, 3) and von Hippel-Lindau (VHL) suppressor protein. In this study, the expression of FIH, PHD1, 2, 3 and VHL was investigated in murine ovaries and immortalised KK1 granulosa cells. We found FIH, VHL and PHD2 transcripts predominantly in growing tertiary follicles. Functional aspects were assessed in KK1 cells exposed to decreasing O2 (20%, 10%, 1%), by determining HIF1α, FIH, VHL, PHD1–3 and STAR expression. The main findings indicated gradually increasing PHD2 under lowered O2. Functional blocking of PHDs revealed biphasic effects on STAR expression; concomitantly with increasing HIF1α, STAR expression, which was initially induced, decreased significantly when HIF1α was strongly stabilised. Finally, PHD2 in particular might act as a specific regulator of HIF1α and, thereby, of STAR availability in granulosa cells.
Keywords: factor inhibiting HIF (FIH), granulosa cells, HIF1α, hypoxia-inducible factor 1 (HIF), prolyl hydroxylases (PHD1, 2, 3), steroidogenesis, steroidogenic acute regulatory (STAR) protein, von Hippel Lindau (VHL) suppressor.
Introduction
Enclosed in ovarian follicles and surrounded by a semi-permeable basal lamina, granulosa cells exert their roles in an environment characterised by a restricted vascular supply (Suzuki et al. 1998; Martelli et al. 2009; Rodgers and Irving-Rodgers 2010). Consequently, the provision of nutrients and oxygen (O2) for granulosa cells and the oocyte depends on diffusion and varies between different stages of folliculogenesis (Redding et al. 2008). A study of pigs has shown that the O2 tension of follicular fluid correlates negatively with follicular size (Basini et al. 2004). Following ovulation, the follicular basal lamina is breached and vascular supply spreads into the follicular cavity during the formation of the corpus luteum (CL). However, during early luteal development, angiogenesis falls behind the intense luteal cell proliferation and the O2 supply is further limited (Amselgruber et al. 1999). Similarly, during luteal regression, reduced vascular density leads again to hypoxia, thereby contributing to functional and structural luteolysis (Meidan et al. 2013; Nishimura and Okuda 2020). Cumulatively, hypoxia plays important roles in regulating reproductive events, including follicular and luteal function.
Hypoxia-inducible factor (HIF)-complexes are major transcriptional responders, activating the cell specific response to hypoxia (Semenza 1998; Semenza 2000). They are heterodimers consisting of α- and β-subunits connected at the helix–loop–helix (bHLH)-Per-Arnt-Sim domain (Wang and Semenza 1995; Jiang et al. 1996a). Only HIFα-expression is O2 dependent, whereas HIFβ is constitutively expressed (Huang et al. 1996; Semenza et al. 1996). Of the three known isoforms of HIFα, HIF1α is the best characterised and is expressed ubiquitously in mammalian tissues (Wenger et al. 1996, 1998). Its O2-dependent cellular availability is regulated enzymatically; it undergoes hydroxylation and degradation initiated by factor inhibiting HIF (FIH) and prolyl hydroxylases (PHDs), utilising O2 as a cosubstrate (Elkins et al. 2003). Accordingly, their hydroxylation abilities are limited in hypoxia. FIH hydroxylates the asparaginyl residue on HIF1α and inhibits the binding of CBP/P300, a transcriptional cofactor and stabiliser of dimerisation of HIF1-complexes (Mahon et al. 2001; Dames et al. 2002; Freedman et al. 2002). PHDs hydroxylate prolyl residues serving as binding sites for the von Hippel-Lindau (VHL) tumor suppressor protein (Bruick and McKnight 2001; Epstein et al. 2001). Once bound, VHL, which is a ubiquitin E3 ligase, marks HIF1α for proteasomal degradation (Ivan et al. 2001; Jaakkola et al. 2001). There are three known isoforms of PHDs in mammalian cells: PHD1 (EGLN2), PHD2 (EGLN1), and PHD3 (EGLN3) (Bruick and McKnight 2001; Epstein et al. 2001). Their expression varies among different tissues and cell types, with PHD2 having the highest and most widely spread expression, while PHD3 protein levels are below detectable limits in several cell types (Lieb et al. 2002; Oehme et al. 2002; Cioffi et al. 2003; Appelhoff et al. 2004). PHD2 seems to be the major isoform involved in regulating HIF1α (Berra et al. 2003; Appelhoff et al. 2004; Minamishima et al. 2008), whereas suppression of PHD1 and PHD3 expression had no effect on HIF1α in normoxia, silencing of PHD2 upregulated HIF1α levels in several cell types (Berra et al. 2003) and, in contrast with the other isoforms, its prenatal knockout in mice is lethal (Minamishima et al. 2008). Therefore, the PHD2 is considered a critical O2 sensor (Berra et al. 2003).
HIF1α also plays indispensable regulatory roles in the ovary. Studies of mice have shown that suppressing the DNA binding capacity of HIF1-complexes with echinomycin prevents ovulation (Kong et al. 2005; Kim et al. 2009). Further, the link between hypoxia and steroidogenesis has been previously established and HIF1α is involved in steroidogenic acute regulatory (STAR) protein-dependent steroidogenesis in granulosa cells (Jiang et al. 2011; Kowalewski et al. 2015; Fadhillah et al. 2017). STAR is a key factor regulating the provision of steroidogenic substrate to mitochondria and its function is rate-limiting for steroidogenesis (Clark et al. 1997). In vitro studies with granulosa cells demonstrated that blocking HIF1α activity with echinomycin, or decreasing its expression by siRNA, suppresses basal, and dbcAMP-stimulated STAR expression, both under moderately lowered (10%) and, importantly, ambient (20%) O2 tension (Kong et al. 2005; Kowalewski et al. 2015; Fadhillah et al. 2017). These findings show the need for transcriptionally active HIF1-complexes under 20% O2, strongly emphasising the importance of HIF1α for STAR-mediated steroidogenesis. They also suggest the possibility of O2-independent regulation of HIF1α in granulosa cells. Such mechanisms, mediated through FSH or inflammatory stimuli, have been indicated previously (Jiang et al. 1996b; Kim et al. 2009). Conversely, both severely lowered O2 and exaggerated stabilisation of HIF1α (caused by exposure to CoCl2) proved to be detrimental for STAR expression and steroidogenesis in the ovary and the testis (Koos and Feiertag 1989; Basini et al. 2004; Jiang et al. 2011; Kumar et al. 2014; Kowalewski et al. 2015; Fadhillah et al. 2017).
Cumulatively, these observations underline the need for precise regulation of FIH-, PHDs- and VHL-dependent regulation of HIF1α availability and activity for the proper steroidogenic function of granulosa cells, under both ambient and reduced O2 conditions. While this is becoming our working hypothesis, surprisingly, none of this has been shown previously for steroidogenic cells. Consequently, in the present study, we have used a well-characterised model, immortalised KK1 granulosa cells (Kananen et al. 1995; Kowalewski et al. 2009, 2015; Manna et al. 2009), to provide the first insights into the regulation of HIF1α-stabilising factors in steroidogenic cells. The presence of HIF1α stabilising machinery was also confirmed in murine ovaries and the respective mRNA localised. Using the steroidogenic model, the response of FIH, VHL and PHDs to different O2 conditions was assessed in vitro, and the possible effects on STAR protein expression were addressed.
Materials and methods
Tissue collection and preparation
Ovaries were collected from wild type not mutated wt/wt C57BL/6 mice (n = 3). This tissue was derived from other experiments performed at the University of Zurich with the permit number 042/2018, and was shared with us using the 3R (reduce, replace, refine) approach. The animals did not receive any treatment and were killed by overdose with pentobarbital. The tissue material was collected and processed immediately after the animals were killed. Isolated ovaries were trimmed of the surrounding tissues and either preserved for 24 h in RNAlater (Ambion Biotechnology GmbH, Wiesbaden, Germany) and subsequently frozen at −80°C until isolation of RNA, or fixed for 24 h in phosphate-buffered 4% formaldehyde and then paraffin-embedded, for in situ hybridisation studies.
Cell cultures and stimulation experiments
For all in vitro experiments, immortalised murine KK1 granulosa cells (kindly provided by Dr. Ilpo Huhtaniemi, Hammersmith Campus, Imperial College, London, UK) were used. All experiments were performed at least three times with cells from different passages. The KK1 cells are well-characterised and respond in a dose-dependent manner to stimulation with N6,2-dibutyryladenosine-3,5-cyclic monophosphate (dbcAMP/cAMP; Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) (Kananen et al. 1995; Kowalewski et al. 2009, 2010, 2015; Manna et al. 2009). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F-12 (1:1) containing 10% heat inactivated fetal bovine serum (FBS), 1% gentamycin (10 mg/mL) and sodium bicarbonate (NaHCO3, 1.125 g/L) in a humified incubator at 37°C, 20% O2, 5% CO2 (DMEM/F-12 and FBS were purchased from Thermo Fisher Scientific AG, Reinach, Switzerland; NaHCO3 from Sigma-Aldrich Chemie GmbH; and gentamycin from Chemie Brunschwig AG, Basel, Switzerland). Twenty-four hours prior to experiments, cells were trypsinised (Chemie Brunschwig AG), seeded on six-well plates to grow overnight to 75–85% confluence and preincubated under 20%, 10%, or 1% O2. On the day of the experiments, serum-containing medium was removed, cells were washed with sterile 1× phosphate buffered saline (PBS; Chemie Brunschwig AG), and serum-free medium (preconditioned 24 h prior to stimulation, according to the respective experimental conditions) was added for stimulation.
The cells were stimulated with dbcAMP in a hypoxic incubator for 6 h, the time point associated with the highest STAR, phospho(P)-STAR and progesterone (P4) output (Kowalewski et al. 2010, 2015). In blocking experiments, cells were preincubated with 5 nm echinomycin (Cayman Chemical Inc., Ann Arbor, MI, USA), or 25 μM, 50 μM, or 100 μM roxadustat (FG-4592; MedChemExpress, Monmouth Junction, NJ, USA) for 40 min prior to combined stimulation with dbcAMP. Echinomycin blocks the DNA-binding activity of HIF1α, but not its expression (Kong et al. 2005; Kim et al. 2009; Kowalewski et al. 2015; Turhan et al. 2021). Roxadustat is a prolyl hydroxylase inhibitor (Chen et al. 2019; Dhillon 2019), and was used within the concentrations recommended by the manufacturer.
African green monkey kidney cells (COS-1), referred to here as COS (Horowitz et al. 1983), served as controls, as recommended by the manufacturers of the antibodies used for the western blots. Unstimulated COS were incubated in DMEM with 10% FBS and 1% penicillin/streptomycin at 20% O2 and 1% O2 following the same experimental procedure as the KK1.
RNA isolation, reverse transcription and qualitative polymerase chain reaction (PCR)
TRIzol® reagent (Thermo Fisher Scientific) was used for total RNA isolation from mouse ovarian tissue and KK1 granulosa cells, following the manufacturer’s directions. The quantity and quality of isolated RNA was determined using a NanoDrop 200C® spectrophotometer (Thermo Fisher Scientific). Qualitative PCR was performed to confirm the presence of transcripts encoding for FIH, VHL and PHD1-3 in ovaries and KK1 cells. Therefore, reverse transcription (RT) using random hexamers as primers, and conventional hot start PCR reactions, were run in a Mastercycler® (Eppendorf AG, Hamburg, Germany) as previously described (Kowalewski et al. 2006b). After RT, complementary DNA (cDNA) was amplified using the AmpliTaq Gold™ DNA Polymerase with Gold Buffer and MgCl2 (Applied Biosystems by Thermo Fisher, Foster City, CA, USA). Self-designed primers (Table 1) were ordered from Microsynth AG (Balgach, Switzerland). The conditions for amplification were set as follows: 10 min at 95°C, 39 cycles of 1 min at 94°C, 1 min at 60°C and 2.5 min at 72°C, 10 min at 72°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), proved to be stably expressed under varying O2 concentration in KK1 (Kowalewski et al. 2015), served as control. Amplicons were visualised on ethidium bromide stained 2% agarose gel. The bands obtained were isolated using a QIAquick® Gel Extraction Kit (50) (Qiagen, Hilden, Germany) and commercially sequenced (Microsynth AG) to confirm the primers’ specificity.

|
In situ hybridisation (ISH)
Because the antibodies used for western blot analysis lacked the specificity required for immunohistochemistry, the localisation of factors involved in HIF1α-stabilisation (FIH, VHL, PHD2) in the ovary was assessed at the mRNA level by non-radioactive ISH, utilising the protocol described previously (Kowalewski et al. 2006a, 2008). Therefore, complementary RNA (cRNA) probes were synthesised by using homogenised murine ovarian tissue in PCR with primers specific for FIH, VHL and PHD2 (Table 1b). The PCR products were separated on a 2% agarose gel, stained with ethidium bromide and isolated using the QIAquick® Gel Extraction Kit (50) (Qiagen) followed by ligation into the pGEM-T plasmid (Promega, Dübendorf, Switzerland) and transformation of bacteria with the plasmid. Colonies containing the insert were selected and plasmids were purified using the PureYield™ Plasmid Miniprep System (Promega). Final products were submitted for sequencing to Microsynth. Restriction enzymes Nco1 and Not1 were used for linearising plasmids in the course of synthesis of sense- and antisense cRNA. The cRNAs synthesis and labelling were done by using the DIG-RNA labelling kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s manual. Dot-blot analyses were performed for semi-quantification of DIG-labelled ribo-probes in serial dilutions on positively charged nylon membranes (Roche Diagnostics). To perform non-radioactive ISH, paraffin-embedded mouse ovaries were cut into 2 μm-thick slices using a microtome and then placed on SuperFrost Plus microscope slides (Menzel-Gläser, Braunschweig, Germany). The sections were dewaxed, digested with proteinase K (70 μg/mL; Sigma-Aldrich) for 20 min to increase tissue permeability, and post-fixed with 4% paraformaldehyde. Hybridisation was performed overnight at 37°C in a formamide chamber. To detect the DIG-labelled cRNA, the sections were incubated with alkaline phosphatase-conjugated sheep anti-DIG Fab Fragments (diluted by 1:5000; Roche Diagnostics) in 1% ovine serum overnight at 4°C. The signals were visualised using 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (BCIP/NBT; Roche Diagnostics).
Western blot
Protein preparation and immunoblotting were performed as described previously (Gram et al. 2013). Briefly, after stimulation, cells were collected using Net-2-lysis buffer (50 mM Tris–HCl, pH 7.4, 300 mM NaCl, 0.05% Nonidet P-40) containing 10 μL/mL protease inhibitor cocktail (Sigma-Aldrich Chemie). Protein homogenates (20–30 μg total protein) were separated using 8–15% polyacrylamide gels and transferred onto methanol-activated polyvinylidene difluoride (PVDF) membranes at 100 volts (V) for 1 h. The membranes were blocked with 5% skimmed milk in PBS/0.25% Tween 20 (PBST) and incubated with specific antibodies, diluted in 2% skimmed milk/PBST (Table 2) at 4°C overnight. Beta-actin (ACTB) (Table 2) served as an internal loading control. Therefore, membranes were first stained for the target proteins and then reblotted and stained against the ACTB to semi-quantify the expression of the targets. Secondary antibodies (Table 2) were used for 1 h. The signals were visualised using SuperSignal™ West Pico or SuperSignal West Femto Chemiluminescent Substrate (Pierce Biotechnology by Thermo Fisher Scientific) to initiate signals in a ChemiDoc™ XRS+ with Image Lab™ Sofware (Bio-Rad Laboratories AG, Cressier, Siwtzerland) in the presence of Precision Plus Protein marker (Bio-Rad). The optical density of proteins was measured with ImageJ software (Wayne Rasband, National Institutes of Health, USA) and ACTB was used as the reference to calculate the standardised optical density (SOD) of protein bands.

|
Statistics
All experiments were repeated at least three times. Cells from different passages were used for each experiment and representative blots are shown. GraphPad 3.06 (GraphPad Software, San Diego, CA, USA) software was used for statistical analysis with one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparison post-test. The WB optical density results are presented as mean ± s.d. P < 0.05 was considered significant.
Results
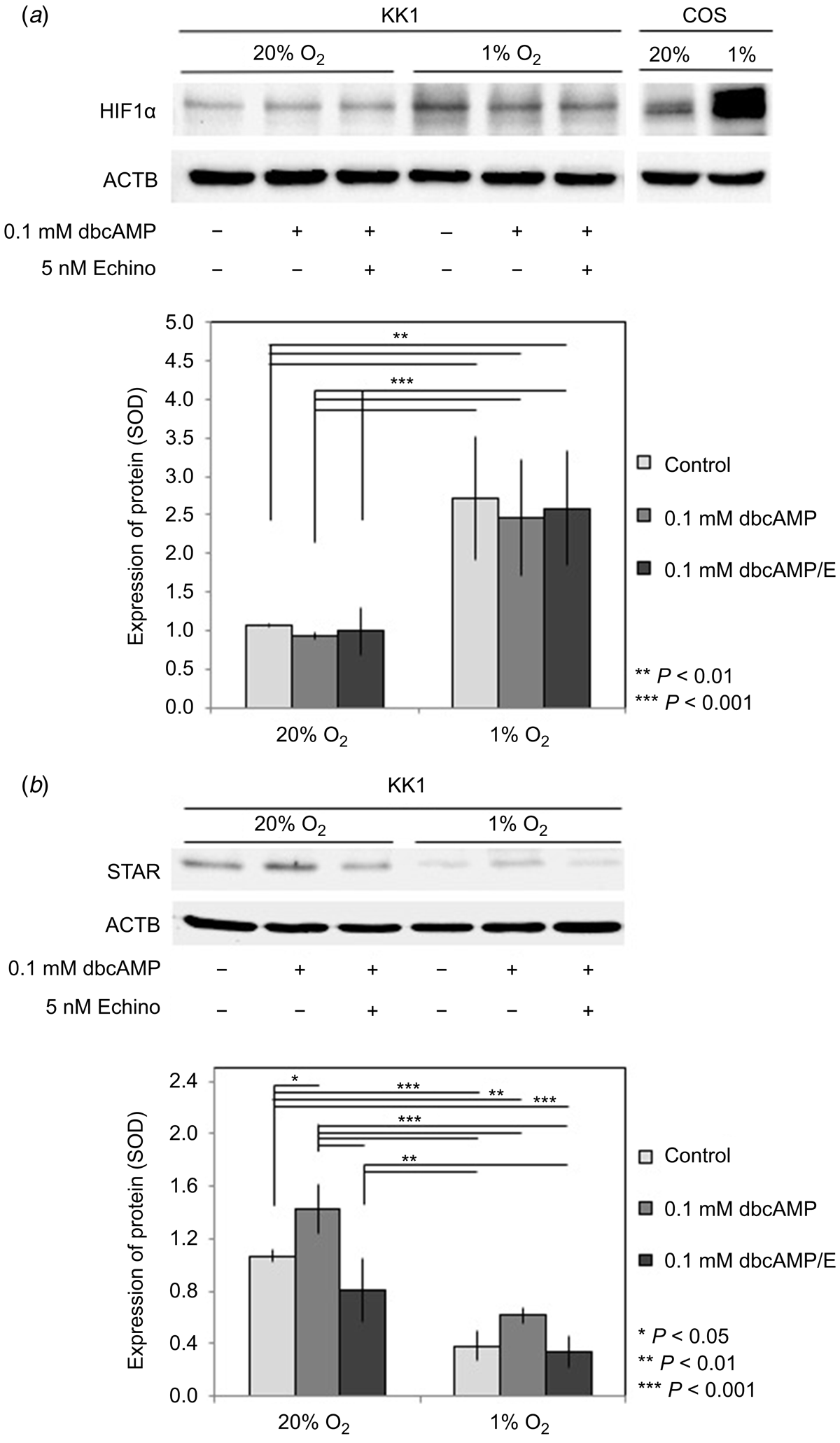
Effects of changing oxygen content and HIF1α activity on STAR protein expression
In order to verify the hypoxia-related stabilisation of HIF1α and its effects on STAR in KK1 granulosa cells (Fig. 1) (Kowalewski et al. 2015), control experiments were set up with cells exposed to 20% O2 and severely lowered (1%) O2, stimulated with a low background (0.1 mM) of dbcAMP in the presence/absence of echinomycin. Additionally, as for subsequent analyses, control experiments were performed with COS (Fig. 1) to verify the specificity of the anti-HIF1α antibody. Although dbcAMP and echinomycin did not affect the levels of HIF1α protein, strongly lowered O2 (1%) increased its expression significantly (P < 0.01 and P < 0.001 compared with control and treated cells respectively; details in Fig. 1a). As expected, the expression of STAR was induced by dbcAMP, and this positive effect was inhibited by echinomycin (Fig. 1b). Concomitant with the strongly induced HIF1α levels, 1% O2 had detrimental effects on STAR levels in the experimental groups, i.e. in control and treated cells (P < 0.05, for statistical details see Fig. 1b).
Expression and localisation of HIF1α-stabilising factors in KK1 granulosa cells and mouse ovarian tissue
Because FIH, VHL, PHD1, PHD2 and PHD3 are involved in the stabilisation of HIF1α, enabling cells to adapt to hypoxia by balancing the availability of HIF1α (Semenza 1998), we verified their mRNA and protein expression in murine ovaries (Fig. 2) and in the KK1 granulosa cells used as a steroidogenic model (Fig. 2). The basic expression of all factors was confirmed at the transcript level in both ovaries and KK1 cells (Fig. 2a). Similarly, all of the proteins were detected in ovaries by western blot, indicating that PHD2 had the highest abundance compared with lower levels of PHD1 and PHD3 (Fig. 2b). However, in KK1 cells, both PHD1 and PHD2 were abundantly expressed, while PHD3 expression seemed to be below the detection level (Fig. 2b). FIH and VHL were clearly detectable and abundantly expressed in both KK1 cells and ovaries. COS were used as positive controls for all antibodies, as recommended by the manufacturers and supported by the available literature (Duan et al. 1995; Huang et al. 1996; Bracken et al. 2006). They displayed the clearly detectable presence of FIH and PHD1, and apparently lower VHL, PHD2 and PHD3 content, pointing towards tissue/cell dependent expression of HIF1α stabilising factors (Fig. 2b).
The antibodies used for the western blots were found to be unsuitable for immunohistochemical detection in the localisation studies. Therefore, ISH was applied, allowing the localisation of the respective transcripts for FIH, VHL and PHD2. PHD2 was the most abundantly expressed PHD in the ovary (Fig. 2b), and has been shown to be the predominant isoform involved in the stabilisation of HIF1α in several systems (Berra et al. 2003; Minamishima et al. 2008; Katschinski 2009). Due to our use of the 3R approach to source ovarian tissue, an accurate staging of the ovaries was not possible prior to tissue collection. Nevertheless, all the functional structures characteristic of an adult ovary were found in histological sections. This included secondary and tertiary follicles of different sizes and luteal structures, mostly of mature and regressing CL (Fig. 3). The signals of all three factors were localised in the granulosa cells of growing follicles (Fig. 3). No quantification of signals intensity was performed. However, signals appeared weaker in the CL. Small, primordial and primary follicles as well as interstitium stained negatively and revealed a slight background staining only. The sense probes employed as negative controls (NC) remained unstained.
Effects of changing O2 content and functional suppression of HIF1-complexes’ activity on the expression of VHL, FIH and PHDs in KK1 cells
The effects of functional blocking of HIF1α-complexes on the expression of HIF1α stabilising factors were examined to evaluate the possible self-regulatory loops; control and dbcAMP-stimulated KK1 granulosa cells were used, in the presence of echinomycin under decreasing O2 content (20%, 10%, 1%) (Figs 4 and 5). Unstimulated COS cells were applied as positive controls under 20% and 1% O2, and did not show any changes in protein expression for VHL, FIH, PHD1, or PHD2. PHD3 responded to decreased O2 and seemed to be more highly represented under 1% O2 (Figs 4 and 5). Similarly, for KK1 cells, VHL, FIH and PHD1 did not change significantly in response to either dbcAMP or echinomycin, nor to changing O2 levels, and did not respond to changing O2 content (ANOVA P = 0.92, P = 0.96 and P = 0.8, for VH, FIH and PHD1, respectively) (Figs 4 and 5). They seemed, therefore, to be constitutively expressed. In contrast, the expression of PHD2 (Fig. 5b) increased gradually in response to decreasing O2 content; it was significantly upregulated at 10% O2 compared with 20% (P < 0.001), followed by a further increase towards 1% O2 (P < 0.001) (Fig. 5b). None of the treatments affected the responsiveness to decreasing O2, thus PHD2 did not respond to dbcAMP or echinomycin. As for PHD3, its expression was below detection limits in KK1 cells (Fig. 1), and remained unaffected in response to lowered O2 (Fig. 5c).
Effects of functional suppression of PHDs on HIF1α and STAR expression in KK1 granulosa cells under changing O2 content
The expression of HIF1α and STAR protein were assessed in KK1 cells treated with increasing dosages (25 μM, 50 μM and 100 μM) of roxadustat, a functional PHD blocker (Dhillon 2019) (Fig. 6). Echinomycin was included, additionally confirming its inhibitory effects and, thereby, the involvement of HIF1α in STAR expression under ambient O2 (Fig. 6b). Cells were exposed to 20% O2, allowing the maintenance of a low basal expression of HIF1α, which was clearly detectable in all experimental groups. Blocking of PHDs with roxadustat gradually stabilised HIF1α levels. Thus, its abundance was increased in cells treated with 25 μM roxadustat (P < 0.001), was further potentiated towards 50 μM roxadustat (P < 0.05, compared with 25 μM), and remained highly stabilised under 100 μM roxadustat treatment (Fig. 6a). As expected, STAR protein responded positively to dbcAMP treatment (P < 0.05) and displayed the highest levels in response to 25 μM roxadustat (P < 0.001 compared with untreated control, and P < 0.01 compared with 0.1 mM dbcAMP). However, the levels of STAR were apparently lower in cells treated with dbcAMP in the presence of 50 μM roxadustat, followed by strong suppression of its expression in cells treated with 100 μM roxadustat (P < 0.001 compared with 25 μM). Interestingly, there was also a significantly lower expression of STAR following functional suppression of HIF1α with echinomycin, when compared with cells in which HIF1α was stabilised with 25 μM roxadustat, showing the strongest STAR expression (Fig. 6b).
Discussion
The transcriptional activity of HIF1-complexes is required for adequate functionality of ovarian follicles, including the steroidogenic properties of granulosa cells (Kowalewski et al. 2015; Nishimura and Okuda 2015, 2020; Baddela et al. 2020). Their interaction with STAR plays fundamental roles in this process (Kowalewski et al. 2015; Nishimura and Okuda 2015). However, extremely high levels of HIF1α are detrimental, highlighting the need for regulation of its abundance under altered oxygenation conditions, also clearly indicated by in vitro studies utilising abundant (20%) O2 levels (Kowalewski et al. 2015; Fadhillah et al. 2017). In the present study, the expression and potential role of HIF1α regulating factors were examined in the murine model. Their expression was proved in the ovary, and in vitro experiments were applied to investigate their responsiveness to changing O2 saturation in KK1 granulosa cells.
The responsiveness of KK1 cells to changing O2 levels was examined by exposing them to higher and lower O2 (20% vs 1%) and verifying the HIF1α and STAR expression. In accordance with our previous findings (Kowalewski et al. 2015), strongly reduced O2 diminished the basal and dbcAMP-induced expression of STAR. At the same time, the effects of echinomycin confirmed that HIF1α is needed for adequate STAR activation. In addition, protein isolates from COS cells were included to test the specificity of the anti-HIF1α antibody, and showed their high responsiveness to lower O2, mirrored in a strong activation of HIF1α under severe hypoxia. Interestingly, although not the main focus of the study, the hypoxia-induced HIF1α expression in COS appeared to exceed those levels observed in KK1 cells under the same experimental conditions, pointing towards a cell/tissue specific response to hypoxia.
However, the present study focused on the expression and regulation of HIF stabilising factors (FIH, VHL, PHD1, PHD2, PHD3). The presence of their respective transcripts and proteins was investigated in ovaries and KK1 cells. While the expression of all targets was confirmed at the mRNA level in all tissue/cell types, the expression patterns and the apparent abundance of the respective proteins appeared to provide the first hints for possible regulatory mechanisms. In particular, the expression of PHDs differed strongly. While FIH and VHL were clearly detectable, only PHD2 seemed to be abundantly present in the ovary, apparently exceeding the levels of PHD1 and PHD3. In turn, PHD3 seemed to be under detection limits in KK1 cells, which presented abundant expression of PHD1 and PHD2. Thus, the isoform commonly strongly represented in ovary and KK1 cells was the PHD2, pointing towards its possible predominant role in these tissue/cells. This situation differed, however, from what has been observed in COS, in which FIH and PHD1 seemed to be the predominant HIF1α regulators. The individual, tissue specific distribution of PHDs, and the prominence of PHD2 compared with PHD3, have also been observed in other biological targets, for example, in Pc-3 cells (human prostate adenocarcinoma) or human kidney (Hirsilä et al. 2003; Appelhoff et al. 2004). Moreover, HIFα specificity has been postulated for PHD2 and PHD3, implying their selective affinity to HIF1α or HIF2α respectively (Appelhoff et al. 2004). Whether this applies to the ovary is not known, as the expression and potential roles of HIF2α in the ovarian cellular compartments have not yet been investigated. However, compared to HIF1α, the expression of HIF2α appears to be targeted to certain cell types (Wiesener et al. 2003; Loboda et al. 2010). Regarding reproductive organs, HIF1α has been described as being constitutively expressed in testicular Leydig cells (Palladino et al. 2011). The lack of detectable levels of PHD3 in KK1 cells compared with the ovary could relate to the cellular source of the protein within the organ, with whole ovarian isolates presenting relatively low, yet detectable amounts of the protein. Conversely in COS, PHD3 seemed to be activated by lower O2 content.
Next, we felt prompted to investigate the cellular distribution of VHL, FIH and PHD2 in the ovary. PHD2 was selected based on our observations, supported by the previously available information emphasising its role as the predominant regulator of HIF1α (Berra et al. 2003; Wiesener et al. 2003; Katschinski 2009; Mollenhauer et al. 2012). ISH indicated that granulosa cells of growing follicles were the main cellular target of all factors. This is an important finding implying an active local regulation of HIF1α availability within the ovary, possibly preventing its exaggeratedly high levels and thereby supporting the underlying hypothesis of the present study. Interestingly, the regressing CL in particular seemed to stain more weakly, supporting the higher availability of functionally active HIF1α during luteal regression observed in other species, for example, in the cow or dog (Nishimura et al. 2006; Sousa et al. 2016; Nishimura and Okuda 2020).
By balancing HIF1α expression in an O2-dependent manner, factors stabilising HIF1α act in a tissue and cell type specific manner (Los et al. 1996; Berra et al. 2003; Appelhoff et al. 2004; Soilleux et al. 2005). It was, thus, interesting to observe the stably expressed levels of VHL, FIH and PHD1 in KK1 granulosa cells under changing O2 saturation. Neither dbcAMP, nor echinomycin-mediated modulation of HIF1-complexes activity, affected their levels, which seemed to be constitutively and abundantly present. Based on this observation, it could be concluded that the FIH- and PHD1-mediated hydroxylation of HIF1α in granulosa cells depends predominantly on O2 availability; the less oxygen is available, the more HIF1α is stabilised due to inactivation of FIH and PHD1 (Semenza 1998). In this context, the ubiquitination of HIF1α by VHL depends on PHD activity, whereas decreased functionality of FIH allows the cofactor CBP to attract HIF1-complexes to their transcriptional targets.
It was interesting and of functional importance to see the gradual increase of PHD2 in granulosa cells following decreasing O2 saturation. This expression pattern resembled the previously published HIF1α levels observed in KK1 cells under progressively decreasing O2 concentrations (Kowalewski et al. 2015) and led us to hypothesise that PHD2 could be the major O2 responsive regulator of HIF1α in granulosa cells. Being induced by decreasing O2 saturation, PHD2 might act as a specific regulator preventing exaggeratedly high HIF1α levels and thereby protecting the cells from its possible inhibitory effect. Such backup mechanisms, highlighting the importance of intracellular protection mechanism against toxic levels of HIF1α, have been previously indicated in other systems. Thus, blocking of PHD2 expression in HeLa cells at ambient (20%) O2 resulted in initially increased HIF1α expression that diminished over time with progressively decreasing HIF1α levels (Berra et al. 2003). Although the exact molecular mechanisms are not fully understood, the existence of such backup mechanisms underlines the complexity and importance of balancing HIF1α in steroidogenic cells. As for other factors, the expression of PHD2 remained stable under exposure to dbcAMP and echinomycin. This differs from what has been observed in other cell types, for example, HeLa cells, in which the hypoxia-dependent increased expression of PHD2 was inhibited by blocking HIF1α expression with siRNA (Berra et al. 2003), or in rat C6 glioma cells (D’Angelo et al. 2003), and points towards cell type- or species-specific regulation of PHD2 expression.
Nevertheless, taking into account its responsiveness to changing O2 content, and its abundance in ovarian isolates compared with other isoforms, PHD2 appears to be a major player regulating the stabilisation of HIF1α in granulosa cells. These findings corroborate observations made in other cell and tissue types (Berra et al. 2003; Appelhoff et al. 2004; Minamishima et al. 2008).
Finally, in order to gain a deeper functional insight into the role of PHDs in the HIF1α-dependent expression of STAR, experiments utilising roxadustat, a specific functional blocker of PHDs, were performed. Roxadustat was initially developed to treat anemia caused by chronic kidney disease in humans (Dhillon 2019). It increases HIF1α levels, followed by transcription of HIF1 target genes, including erythropoietin (Besarab et al. 2015). Roxadustat is not selective to different PHD isoforms. KK1 cells responded in a dose-dependent manner to the treatment by presenting increasing HIF1α levels under ambient O2, clearly indicating the involvement of PHDs in the maintenance of HIF1α in steroidogenic granulosa cells. Most importantly, constituting one of the most important observations from the present study, these effects were accompanied by a biphasic response in STAR protein expression. It was significantly upregulated in response to lower concentrations of roxadustat, to above the levels detected in cells treated with dbcAMP only, apparently mimicking the positive effects of moderate hypoxia on STAR expression described previously (Kowalewski et al. 2015). Conversely, enhanced stabilisation of HIF1α with higher dosages of the compound, significantly reduced STAR to basal levels, i.e. significantly lower than those observed in dbcAMP-treated cells, resembling the effects of strongly lowered (1%) O2, confirmed in the present study. Consequently, it became obvious that the more HIF1α is stabilised, crossing the not yet defined threshold, the less STAR is present in steroidogenic cells (Fig. 7). The underlying molecular mechanisms need further clarification. Similar effects on steroid synthesis, resulting from overexpression of HIF1α, were shown in luteinised bovine granulosa cells (Jiang et al. 1996b) or canine lutein cells (Sousa et al. 2016) treated with CoCl2. The latter is a non-specific blocker of HIF1α degradation, affecting the functionality of PHDs, FIH and VHL, leading to extremely high intracellular HIF1α levels (Muñoz-Sánchez and Chánez-Cárdenas 2019).
Conclusions
Cumulatively, the present study shows the ovarian expression of factors regulating cellular availability of HIF1α and indicates the importance of regulating its expression in steroidogenic cells. Although studies utilising primary cell cultures are needed to support the present findings, by actively responding to changing oxygenation conditions, PHD2 might be the major player, among the PHDs, involved in stabilising HIF1α in granulosa cells. It could be involved in a self-regulatory loop protecting these cells from detrimentally high levels of HIF1α (Fig. 7). PHD-dependent maintenance of HIF1α regulates STAR availability, and when overexpressed HIF1α downregulates it, impeding steroidogenic cell function. Nevertheless, HIF1-complexes are required for efficient STAR synthesis (Fig. 7). Thus, our conclusion that balancing HIF1α levels is crucial for the steroidogenic function of granulosa and, presumably, other steroidogenic cells is of translational value.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This project was supported by the Vetsuisse Faculty of Zurich via the Institute of Veterinary Anatomy.
Author contributions
TG was involved in developing the concept of the study, experimental design, generating data, analysis and interpretation of data, and drafting the original manuscript. MPK designed and supervised the project, was involved in analysis and interpretation of the data, and editing and revising the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
Authors are thankful to Dr. Sharon Mortimer (Oozoa Biomedical, Inc., Vancouver, Canada) for careful editing of the manuscript and Ricardo Fernandez Rubia for excellent technical support.
References
Amselgruber, WM, Schafer, M, and Sinowatz, F (1999). Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anatomia, Histologia, Embryologia 28, 157–166.| Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study.Crossref | GoogleScholarGoogle Scholar | 10458020PubMed |
Appelhoff, RJ, Tian, YM, Raval, RR, Turley, H, Harris, AL, Pugh, CW, Ratcliffe, PJ, and Gleadle, JM (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. Journal of Biological Chemistry 279, 38458–38465.
| Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor.Crossref | GoogleScholarGoogle Scholar |
Baddela, VS, Sharma, A, Michaelis, M, and Vanselow, J (2020). HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Scientific Reports 10, 3906.
| HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells.Crossref | GoogleScholarGoogle Scholar | 32127571PubMed |
Basini, G, Bianco, F, Grasselli, F, Tirelli, M, Bussolati, S, and Tamanini, C (2004). The effects of reduced oxygen tension on swine granulosa cell. Regulatory Peptides 120, 69–75.
| The effects of reduced oxygen tension on swine granulosa cell.Crossref | GoogleScholarGoogle Scholar | 15177922PubMed |
Berra, E, Benizri, E, Ginouvès, A, Volmat, V, Roux, D, and Pouyssègur, J (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO Journal 22, 4082–4090.
| HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia.Crossref | GoogleScholarGoogle Scholar |
Besarab, A, Provenzano, R, Hertel, J, Zabaneh, R, Klaus, SJ, Lee, T, Leong, R, Hemmerich, S, Yu, KHP, and Neff, TB (2015). Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrology Dialysis Transplantation 30, 1665–1673.
| Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients.Crossref | GoogleScholarGoogle Scholar |
Bracken, CP, Fedele, AO, Linke, S, Balrak, W, Lisy, K, Whitelaw, ML, and Peet, DJ (2006). Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. Journal of Biological Chemistry 281, 22575–22585.
| Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment.Crossref | GoogleScholarGoogle Scholar |
Bruick, RK, and McKnight, SL (2001). A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340.
| A conserved family of prolyl-4-hydroxylases that modify HIF.Crossref | GoogleScholarGoogle Scholar | 11598268PubMed |
Chen, N, Hao, C, Liu, BC, Lin, H, Wang, C, Xing, C, Liang, X, Jiang, G, Liu, Z, Li, X, Zuo, L, Luo, L, Wang, J, Zhao, MH, Liu, Z, Cai, GY, Hao, L, Leong, R, Wang, C, Liu, C, Neff, T, Szczech, L, and Yu, KHP (2019). Roxadustat treatment for anemia in patients undergoing long-term dialysis. New England Journal of Medicine 381, 1011–1022.
| Roxadustat treatment for anemia in patients undergoing long-term dialysis.Crossref | GoogleScholarGoogle Scholar |
Cioffi, CL, Liu, XQ, Kosinski, PA, Garay, M, and Bowen, BR (2003). Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochemical and Biophysical Research Communications 303, 947–953.
| Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells.Crossref | GoogleScholarGoogle Scholar | 12670503PubMed |
Clark, BJ, Wells, J, King, SR, and Stocco, DM (1994). . Characterization of the steroidogenic acuteregulatory protein (StAR). Journal of Biological Chemistry 269, 28314–28322.
Clark, BJ, Combs, R, Hales, KH, Hales, DB, and Stocco, DM (1997). Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells. Endocrinology 138, 4893–4901.
| Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells.Crossref | GoogleScholarGoogle Scholar | 9348220PubMed |
D’Angelo, G, Duplan, E, Boyer, N, Vigne, P, and Frelin, C (2003). Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. Journal of Biological Chemistry 278, 38183–38187.
| Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation.Crossref | GoogleScholarGoogle Scholar |
Dames, SA, Martinez-Yamout, M, De Guzman, RN, Dyson, HJ, and Wright, PE (2002). Structural basis for Hif-1 alpha/CBP recognition in the cellular hypoxic response. Proceedings of the National Academy of Sciences 99, 5271–5276.
| Structural basis for Hif-1 alpha/CBP recognition in the cellular hypoxic response.Crossref | GoogleScholarGoogle Scholar |
Dhillon, S (2019). Roxadustat: first global approval. Drugs 79, 563–572.
| Roxadustat: first global approval.Crossref | GoogleScholarGoogle Scholar | 30805897PubMed |
Duan, DR, Humphrey, JS, Chen, DY, Weng, Y, Sukegawa, J, Lee, S, Gnarra, JR, Linehan, WM, and Klausner, RD (1995). Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations. Proceedings of the National Academy of Sciences 92, 6459–6463.
| Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations.Crossref | GoogleScholarGoogle Scholar |
Elkins, JM, Hewitson, KS, McNeill, LA, Seibel, JF, Schlemminger, I, Pugh, CW, Ratcliffe, PJ, and Schofield, CJ (2003). Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1 alpha. Journal of Biological Chemistry 278, 1802–1806.
| Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1 alpha.Crossref | GoogleScholarGoogle Scholar |
Epstein, AC, Gleadle, JM, McNeill, LA, Hewitson, KS, O’Rourke, J, Mole, DR, Mukherji, M, Metzen, E, Wilson, MI, Dhanda, A, Tian, YM, Masson, N, Hamilton, DL, Jaakkola, P, Barstead, R, Hodgkin, J, Maxwell, PH, Pugh, CW, Schofield, CJ, and Ratcliffe, PJ (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54.
| C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation.Crossref | GoogleScholarGoogle Scholar | 11595184PubMed |
Freedman, SJ, Sun, ZYJ, Poy, F, Kung, AL, Livingston, DM, Wagner, G, and Eck, MJ (2002). Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proceedings of the National Academy of Sciences 99, 5367–5372.
| Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha.Crossref | GoogleScholarGoogle Scholar |
Gram, A, Büchler, U, Boos, A, Hoffmann, B, and Kowalewski, MP (2013). Biosynthesis and degradation of canine placental prostaglandins: prepartum changes in expression and function of prostaglandin F2alpha-synthase (PGFS, AKR1C3) and 15-hydroxyprostaglandin dehydrogenase (HPGD). Biology of Reproduction 89, 2.
| Biosynthesis and degradation of canine placental prostaglandins: prepartum changes in expression and function of prostaglandin F2alpha-synthase (PGFS, AKR1C3) and 15-hydroxyprostaglandin dehydrogenase (HPGD).Crossref | GoogleScholarGoogle Scholar | 23677986PubMed |
Hirsilä, M, Koivunen, P, Gunzler, V, Kivirikko, KI, and Myllyharju, J (2003). Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. Journal of Biological Chemistry 278, 30772–30780.
| Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor.Crossref | GoogleScholarGoogle Scholar |
Horowitz, M, Cepko, CL, and Sharp, PA (1983). Expression of chimeric genes in the early region of SV40. Journal of Molecular and Applied Genetics 2, 147–159.
| 6192195PubMed |
Huang, LE, Arany, Z, Livingston, DM, and Bunn, HF (1996). Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. Journal of Biological Chemistry 271, 32253–32259.
| Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit.Crossref | GoogleScholarGoogle Scholar |
Ivan, M, Kondo, K, Yang, H, Kim, W, Valiando, J, Ohh, M, Salic, A, Asara, JM, Lane, WS, and Kaelin, WG (2001). HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468.
| HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing.Crossref | GoogleScholarGoogle Scholar | 11292862PubMed |
Jaakkola, P, Mole, DR, Tian, YM, Wilson, MI, Gielbert, J, Gaskell, SJ, von Kriegsheim, A, Hebestreit, HF, Mukherji, M, Schofield, CJ, Maxwell, PH, Pugh, CW, and Ratcliffe, PJ (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472.
| Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation.Crossref | GoogleScholarGoogle Scholar | 11292861PubMed |
Jiang, BH, Rue, E, Wang, GL, Roe, R, and Semenza, GL (1996a). Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. Journal of Biological Chemistry 271, 17771–17778.
| Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1.Crossref | GoogleScholarGoogle Scholar |
Jiang, BH, Semenza, GL, Bauer, C, and Marti, HH (1996b). Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. American Journal of Physiology 271, C1172–C1180.
| Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension.Crossref | GoogleScholarGoogle Scholar |
Jiang, YF, Tsui, KH, Wang, PH, Lin, CW, Wang, JY, Hsu, MC, Chen, YC, and Chiu, CH (2011). Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum. Animal Reproduction Science 129, 152–161.
| Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum.Crossref | GoogleScholarGoogle Scholar | 22226573PubMed |
Kananen, K, Markkula, M, Rainio, E, Su, JG, Hsueh, AJ, and Huhtaniemi, IT (1995). Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoter/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Molecular Endocrinology 9, 616–627.
| Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoter/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines.Crossref | GoogleScholarGoogle Scholar | 7565808PubMed |
Katschinski, DM (2009). In vivo functions of the prolyl-4-hydroxylase domain oxygen sensors: direct route to the treatment of anaemia and the protection of ischaemic tissues. Acta Physiologica 195, 407–414.
| In vivo functions of the prolyl-4-hydroxylase domain oxygen sensors: direct route to the treatment of anaemia and the protection of ischaemic tissues.Crossref | GoogleScholarGoogle Scholar | 19183336PubMed |
Kim, J, Bagchi, IC, and Bagchi, MK (2009). Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 150, 3392–3400.
| Signaling by hypoxia-inducible factors is critical for ovulation in mice.Crossref | GoogleScholarGoogle Scholar | 19325003PubMed |
Kong, D, Park, EJ, Stephen, AG, Calvani, M, Cardellina, JH, Monks, A, Fisher, RJ, Shoemaker, RH, and Melillo, G (2005). Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Research 65, 9047–9055.
| Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity.Crossref | GoogleScholarGoogle Scholar | 16204079PubMed |
Koos, RD, and Feiertag, MA (1989). The effect of reduced oxygen tension on progesterone accumulation in rat granulosa cell cultures. Steroids 54, 553–562.
| The effect of reduced oxygen tension on progesterone accumulation in rat granulosa cell cultures.Crossref | GoogleScholarGoogle Scholar | 2515620PubMed |
Kowalewski, MP, Dyson, MT, Boos, A, and Stocco, DM (2010). Vasoactive intestinal peptide (VIP)-mediated expression and function of steroidogenic acute regulatory protein (StAR) in granulosa cells. Molecular and Cellular Endocrinology 328, 93–103.
| Vasoactive intestinal peptide (VIP)-mediated expression and function of steroidogenic acute regulatory protein (StAR) in granulosa cells.Crossref | GoogleScholarGoogle Scholar | 20655982PubMed |
Kowalewski, MP, Dyson, MT, Manna, PR, and Stocco, DM (2009). Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reproduction, Fertility, and Development 21, 909–922.
| Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression.Crossref | GoogleScholarGoogle Scholar | 19698295PubMed |
Kowalewski, MP, Gram, A, and Boos, A (2015). The role of hypoxia and HIF1alpha in the regulation of STAR-mediated steroidogenesis in granulosa cells. Molecular and Cellular Endocrinology 401, 35–44.
| The role of hypoxia and HIF1alpha in the regulation of STAR-mediated steroidogenesis in granulosa cells.Crossref | GoogleScholarGoogle Scholar | 25478925PubMed |
Kowalewski, MP, Mason, JI, Howie, AF, Morley, SD, Schuler, G, and Hoffmann, B (2006a). Characterization of the canine 3beta-hydroxysteroid dehydrogenase and its expression in the corpus luteum during diestrus. Journal of Steroid Biochemistry and Molecular Biology 101, 254–262.
| Characterization of the canine 3beta-hydroxysteroid dehydrogenase and its expression in the corpus luteum during diestrus.Crossref | GoogleScholarGoogle Scholar |
Kowalewski, MP, Mutembei, HM, and Hoffmann, B (2008). Canine prostaglandin F2alpha receptor (FP) and prostaglandin F2alpha synthase (PGFS): molecular cloning and expression in the corpus luteum. Animal Reproduction Science 107, 161–175.
| Canine prostaglandin F2alpha receptor (FP) and prostaglandin F2alpha synthase (PGFS): molecular cloning and expression in the corpus luteum.Crossref | GoogleScholarGoogle Scholar | 17689894PubMed |
Kowalewski, MP, Schuler, G, Taubert, A, Engel, E, and Hoffmann, B (2006b). Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology 66, 1423–1430.
| Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus.Crossref | GoogleScholarGoogle Scholar | 16481032PubMed |
Kumar, A, Rani, L, and Dhole, B (2014). Role of oxygen in the regulation of Leydig tumor derived MA-10 cell steroid production: the effect of cobalt chloride. Systems Biology in Reproductive Medicine 60, 112–118.
| Role of oxygen in the regulation of Leydig tumor derived MA-10 cell steroid production: the effect of cobalt chloride.Crossref | GoogleScholarGoogle Scholar | 24328340PubMed |
Lieb, ME, Menzies, K, Moschella, MC, Ni, R, and Taubman, MB (2002). Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochemistry and Cell Biology 80, 421–426.
| Mammalian EGLN genes have distinct patterns of mRNA expression and regulation.Crossref | GoogleScholarGoogle Scholar | 12234095PubMed |
Loboda, A, Jozkowicz, A, and Dulak, J (2010). HIF-1 and HIF-2 transcription factors—similar but not identical. Molecules and Cells 29, 435–442.
| HIF-1 and HIF-2 transcription factors—similar but not identical.Crossref | GoogleScholarGoogle Scholar | 20396958PubMed |
Los, M, Jansen, GH, Kaelin, WG, Lips, CJ, Blijham, GH, and Voest, EE (1996). Expression pattern of the von Hippel-Lindau protein in human tissues. Laboratory Investigation 75, 231–238.
| 8765323PubMed |
Mahon, PC, Hirota, K, and Semenza, GL (2001). FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes & Development 15, 2675–2686.
| FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity.Crossref | GoogleScholarGoogle Scholar |
Manna, PR, Huhtaniemi, IT, and Stocco, DM (2009). Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology 150, 3308–3317.
| Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells.Crossref | GoogleScholarGoogle Scholar | 19282384PubMed |
Martelli, A, Palmerini, MG, Russo, V, Rinaldi, C, Bernabò, N, Di Giacinto, O, Berardinelli, P, Nottola, SA, Macchiarelli, G, and Barboni, B (2009). Blood vessel remodeling in pig ovarian follicles during the periovulatory period: an immunohistochemistry and SEM-corrosion casting study. Reproductive Biology and Endocrinology 7, 72.
| Blood vessel remodeling in pig ovarian follicles during the periovulatory period: an immunohistochemistry and SEM-corrosion casting study.Crossref | GoogleScholarGoogle Scholar | 19607713PubMed |
Meidan, R, Klipper, E, Zalman, Y, and Yalu, R (2013). The role of hypoxia-induced genes in ovarian angiogenesis. Reproduction, Fertility and Development 25, 343–350.
| The role of hypoxia-induced genes in ovarian angiogenesis.Crossref | GoogleScholarGoogle Scholar |
Minamishima, YA, Moslehi, J, Bardeesy, N, Cullen, D, Bronson, RT, and Kaelin, WG (2008). Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood 111, 3236–3244.
| Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure.Crossref | GoogleScholarGoogle Scholar | 18096761PubMed |
Mollenhauer, M, Kiss, J, Dudda, J, Kirchberg, J, Rahbari, N, Radhakrishnan, P, Niemietz, T, Rausch, V, Weitz, J, and Schneider, M (2012). Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy. Langenbeck’s Archives of Surgery 397, 1313–1322.
| Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy.Crossref | GoogleScholarGoogle Scholar | 22961008PubMed |
Muñoz-Sánchez, J, and Chánez-Cárdenas, ME (2019). The use of cobalt chloride as a chemical hypoxia model. Journal of Applied Toxicology 39, 556–570.
| The use of cobalt chloride as a chemical hypoxia model.Crossref | GoogleScholarGoogle Scholar | 30484873PubMed |
Nishimura, R, and Okuda, K (2015). Multiple roles of hypoxia in ovarian function: roles of hypoxia-inducible factor-related and -unrelated signals during the luteal phase. Reproduction, Fertility and Development 28, 1479–1486.
Nishimura, R, and Okuda, K (2020). Multiple roles of hypoxia in bovine corpus luteum. The Journal of Reproduction and Development 66, 307–310.
| Multiple roles of hypoxia in bovine corpus luteum.Crossref | GoogleScholarGoogle Scholar | 32249240PubMed |
Nishimura, R, Sakumoto, R, Tatsukawa, Y, Acosta, TJ, and Okuda, K (2006). Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum. Endocrinology 147, 4273–4280.
| Oxygen concentration is an important factor for modulating progesterone synthesis in bovine corpus luteum.Crossref | GoogleScholarGoogle Scholar | 16740971PubMed |
Oehme, F, Ellinghaus, P, Kolkhof, P, Smith, TJ, Ramakrishnan, S, Hütter, J, Schramm, M, and Flamme, I (2002). Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochemical and Biophysical Research Communications 296, 343–349.
| Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors.Crossref | GoogleScholarGoogle Scholar | 12163023PubMed |
Palladino, MA, Pirlamarla, PR, McNamara, J, Sottas, CM, Korah, N, Hardy, MP, Hales, DB, and Hermo, L (2011). Normoxic expression of hypoxia-inducible factor 1 in rat Leydig cells in vivo and in vitro. Journal of Andrology 32, 307–323.
| Normoxic expression of hypoxia-inducible factor 1 in rat Leydig cells in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar | 20966422PubMed |
Redding, GP, Bronlund, JE, and Hart, AL (2008). Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reproduction, Fertility and Development 20, 408–417.
| Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling.Crossref | GoogleScholarGoogle Scholar |
Rodgers, RJ, and Irving-Rodgers, HF (2010). Formation of the ovarian follicular antrum and follicular fluid. Biology of Reproduction 82, 1021–1029.
| Formation of the ovarian follicular antrum and follicular fluid.Crossref | GoogleScholarGoogle Scholar | 20164441PubMed |
Semenza, GL (1998). Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Current Opinion in Genetics & Development 8, 588–594.
| Hypoxia-inducible factor 1: master regulator of O2 homeostasis.Crossref | GoogleScholarGoogle Scholar |
Semenza, GL (2000). HIF-1: mediator of physiological and pathophysiological responses to hypoxia. Journal of Applied Physiology 88, 1474–1480.
| HIF-1: mediator of physiological and pathophysiological responses to hypoxia.Crossref | GoogleScholarGoogle Scholar | 10749844PubMed |
Semenza, GL, Jiang, BH, Leung, SW, Passantino, R, Concordet, JP, Maire, P, and Giallongo, A (1996). Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. The Journal of Biological Chemistry 271, 32529–32537.
| Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1.Crossref | GoogleScholarGoogle Scholar | 8955077PubMed |
Soilleux, EJ, Turley, H, Tian, YM, Pugh, CW, Gatter, KC, and Harris, AL (2005). Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors PHD-1, PHD-2, PHD-3 and FIH in normal and neoplastic human tissues. Histopathology 47, 602–610.
| Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors PHD-1, PHD-2, PHD-3 and FIH in normal and neoplastic human tissues.Crossref | GoogleScholarGoogle Scholar | 16324198PubMed |
Sousa, LMMdC, Silva, RdS, Fonseca, VUd, Leandro, RM, Di Vincenzo, TS, Alves-Wagner, AB, Machado, UF, and Papa, PdC (2016). Is the canine corpus luteum an insulin-sensitive tissue? Journal of Endocrinology 231, 223–233.
| Is the canine corpus luteum an insulin-sensitive tissue?Crossref | GoogleScholarGoogle Scholar |
Suzuki, T, Sasano, H, Takaya, R, Fukaya, T, Yajima, A, and Nagura, H (1998). Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Human Reproduction 13, 953–959.
| Cyclic changes of vasculature and vascular phenotypes in normal human ovaries.Crossref | GoogleScholarGoogle Scholar | 9619553PubMed |
Turhan, A, Pereira, MT, Schuler, G, Bleul, U, and Kowalewski, MP (2021). Hypoxia-inducible factor (HIF1alpha) inhibition modulates cumulus cell function and affects bovine oocyte maturation in vitro. Biology of Reproduction 104, 479–491.
| Hypoxia-inducible factor (HIF1alpha) inhibition modulates cumulus cell function and affects bovine oocyte maturation in vitro.Crossref | GoogleScholarGoogle Scholar | 33095229PubMed |
Wang, GL, and Semenza, GL (1995). Purification and characterization of hypoxia-inducible factor 1. The Journal of Biological Chemistry 270, 1230–1237.
| Purification and characterization of hypoxia-inducible factor 1.Crossref | GoogleScholarGoogle Scholar | 7836384PubMed |
Wenger, RH, Rolfs, A, Marti, HH, Guénet, JL, and Gassmann, M (1996). Nucleotide sequence, chromosomal assignment and mRNA expression of mouse hypoxia-inducible factor-1 alpha. Biochemical and Biophysical Research Communications 223, 54–59.
| Nucleotide sequence, chromosomal assignment and mRNA expression of mouse hypoxia-inducible factor-1 alpha.Crossref | GoogleScholarGoogle Scholar | 8660378PubMed |
Wenger, RH, Rolfs, A, Spielmann, P, Zimmermann, DR, and Gassmann, M (1998). Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood 91, 3471–3480.
| Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter.Crossref | GoogleScholarGoogle Scholar | 9558407PubMed |
Wiesener, MS, Jürgensen, JS, Rosenberger, C, Scholze, CK, Hörstrup, JH, Warnecke, C, Mandriota, S, Bechmann, I, Frei, UA, Pugh, CW, Ratcliffe, PJ, Bachmann, S, Maxwell, PH, and Eckardt, KU (2003). Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. The FASEB Journal 17, 271–273.
| Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs.Crossref | GoogleScholarGoogle Scholar | 12490539PubMed |
Fadhillah Fadhillah (2017). Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells. Journal of Reproduction and Development 63, 75–85.
| Hypoxia-inducible factor 1 mediates hypoxia-enhanced synthesis of progesterone during luteinization of granulosa cells.Crossref | GoogleScholarGoogle Scholar |