256. Bovine oocyte vitrification in sodium free medium
R. T. Tecirlioglu A and A. J. French B CA Monash Immunology and Stem Cell Laboratories, Monash University, Clayton, VIC, Australia
B Centre for Reproduction and Development, Monash Institute of Medical Research, Monash University, Clayton, VIC, Australia
C CRC for Innovative Dairy Products, Melbourne, VIC, Australia
Reproduction, Fertility and Development 17(9) 103-103 https://doi.org/10.1071/SRB05Abs256
Submitted: 26 July 2005 Accepted: 26 July 2005 Published: 5 September 2005
Abstract
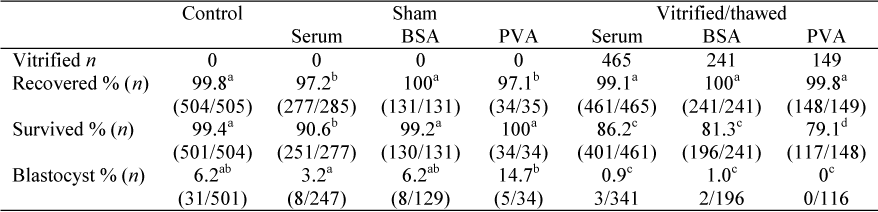
Vitrification has been widely developed for the cryopreservation of mammalian embryos. Despite considerable effort, the vitrification of immature bovine oocytes is less successful with poor survival post thawing and low fertilization rates and development to offspring. The MII oocyte is highly sensitive to temperature changes with cryopreservation resulting in high incidence of aneuploidy after fertilization. Recently the concentration of sodium ions has been implicated in vitrification-induced damage. This study investigated the effect of a low-sodium choline-based medium (CJ2) supplemented with either 10% bovine fetal serum (FBS), 0.8% bovine serum albumin (BSA) or 1% polyvinylalcohol (PVA) on the ability of in vitro matured metaphase II (MII) oocytes to survive vitrification. Cumulus removed MII matured oocytes were equilibrated in 10% ethylene glycol (EG) and 10% dimethylsulfoxide (DMSO) in CJ2 containing FBS, BSA or PVA for 5 minutes at 37ºC and then transferred into a vitrification solution composed of 20% EG, 20% DMSO and 0.6 M sucrose in CJ2. Twenty MII stage oocytes at a time were aspirated into the Gel Loader tip (GL-tip) with approximately 20 µL of vitrification solution, equilibrated 30 sec before plunging directly into liquid nitrogen and stored for 2 h. Oocytes were thawed rapidly and cryoprotectants removed by step-wise dilution in 0.25 M, 0.15 M, 0 M sucrose in TCM-199, 5 min each. Oocytes were then incubated in TCM-199 for 2 h before being stained with Hoechst-33342 and viewed under epi-florescence to determine survival. Developmental competence was determined by parthenogenetically activating (PA) surviving oocytes using calcium ionophore/6-dimethylaminopurine and cultured for 7 days in mSOF medium supplemented with 0.8% BSA. The recovery from vitrification procedure, survival post thawing and PA rates are summarized in the Table below. Vitrification in GL-tips allows efficient processing of MII oocytes with high rates of recovery. Overall, blastocyst development in this experiment was low in the control, sham groups and treated groups and that results may have been influenced by other factors (i.e. oocyte quality). Nevertheless, results demonstrate that reducing or eliminating sodium ions from the vitrification medium may protect the immature oocytes during vitrification or thawing and allow oocytes to be cryopreserved more effectively.