New approach for reproductive toxicity assessment: chromatoid bodies as a target for methylmercury and polychlorinated biphenyls in prepubertal male rats
M. S. Garcia A B , W. A. Orcini C , R. L. Peruquetti A C D * and J. E. Perobelli B E *
B E *
A School of Health Sciences, Sagrado Coração University, Rua Irmã Arminda, 10-50, Jd., Brasil, 17011-160, Bauru, São Paulo, Brazil.
B Experimental Toxicology Laboratory, Department of Marine Sciences, Federal University of São Paulo, Campus Baixada Santista, Rua Dr Carvalho de Mendonça, 144, Encruzilhada, 11070-102 Santos, SP, Brazil.
C Molecular Biology and Cytogenetics Laboratory, Sagrado Coração University, Rua Irmã Arminda, 10-50, Jd., Brasil, 17011-160, Bauru, São Paulo, Brazil.
D Office of the Associate Dean of Graduate Studies and Research, Sagrado Coração University, Rua Irmã Arminda, 10-50, Jd., Brasil, 17011-160, Bauru, São Paulo, Brazil.
E Corresponding author. Email: jperobelli@unifesp.br
Reproduction, Fertility and Development 32(10) 914-922 https://doi.org/10.1071/RD19447
Submitted: 6 December 2019 Accepted: 4 May 2020 Published: 11 June 2020
Abstract
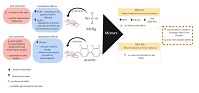
This study investigated the reproductive toxicity of methylmercury (MeHg) and Aroclor (Sigma-Aldrich), alone or in combination, following exposure of prepubertal male rats considering the chromatoid body (CB) as a potential target. The CB is an important molecular regulator of mammalian spermatogenesis, primarily during spermatid cytodifferentiation. Male Wistar rats were exposed to MeHg and/or Aroclor , according the following experimental design: control group, which was administered in corn oil (vehicle) only; MeHg-treated group, which was administered 0.5 mg kg−1 day−1 MeHg; Aroclor-treated group, which was administered 1 mg kg−1 day−1 Aroclor; Mix-LD, group which was administered a low-dose mixture of MeHg (0.05 mg kg−1 day−1) and Aroclor (0.1 mg kg−1 day−1); and Mix-HD group, which was administered a high-dose mixture of MeHg (0.5 mg kg−1 day−1) and Aroclor (1.0 mg kg−1 day−1). MeHg was diluted in distilled water and Aroclor was made up in corn oil (volume 1 mL kg−1). Rats were administered the different treatments from PND23 to PND53 by gavage, . The morphophysiology of CBs was analysed, together with aspects of steroid hormones status and regulation, just after the last treatment on PND53. In addition, the long-term effects on sperm parameters were assessed in adult animals. MeHg exposure increased mouse VASA homologue (MVH) protein levels in seminiferous tubules, possibly affecting the epigenetic status of germ cells. Aroclor produced morphological changes to CB assembly, which may explain the observed morphological defects to the sperm flagellum and the consequent decrease in sperm motility. There were no clear additive or synergistic effects between MeHg and Aroclor when administered in combination. In conclusion, this study demonstrates that MeHg and Aroclor have independent deleterious effects on the developing testis, causing molecular and morphological changes in CBs. To the best of our knowledge, this is the first study to show that CBs are targets for toxic agents.
Additional keywords: chemical mixtures, prepubertal exposure.
References
Alava, J. J., Cisneros-Montemayor, A. M., Sumaila, U. R., and Cheung, W. W. (2018). Projected amplification of food web bioaccumulation of MeHg and PCBs under climate change in the northeastern Pacific. Sci. Rep. 8, 13460.| Projected amplification of food web bioaccumulation of MeHg and PCBs under climate change in the northeastern Pacific.Crossref | GoogleScholarGoogle Scholar | 30194394PubMed |
Aly, H. A., Domènech, Ò., and Abdel-Naim, A. B. (2009). Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria. Food Chem. Toxicol. 47, 1733–1738.
| Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria.Crossref | GoogleScholarGoogle Scholar | 19306909PubMed |
Bełtowski, J., and Semczuk, A. (2010). Liver x receptor (lxr) and the reproductive system–a potential novel target for therapeutic intervention. Pharmacol. Rep. 62, 15–27.
| Liver x receptor (lxr) and the reproductive system–a potential novel target for therapeutic intervention.Crossref | GoogleScholarGoogle Scholar |
Chapin, R. E., Sloane, R. A., and Hasemann, J. K. (1997). The relationships among reproductive endpoints in Swiss mice, using the reproductive assessment by continuous breeding database. Fundam. Appl. Toxicol. 38, 129–142.
| The relationships among reproductive endpoints in Swiss mice, using the reproductive assessment by continuous breeding database.Crossref | GoogleScholarGoogle Scholar | 9299186PubMed |
de Pauli, L. F., Santos, E. G., Arcangelo, F. P. D., Orcini, W. A., and Peruquetti, R. L. (2017). Differential Expression of the Nucleolar Protein Fibrillarin During Mammalian Spermatogenesis and Its Probable Association With Chromatoid Body Components. Micron 94, 37–45.
| Differential Expression of the Nucleolar Protein Fibrillarin During Mammalian Spermatogenesis and Its Probable Association With Chromatoid Body Components.Crossref | GoogleScholarGoogle Scholar | 28027486PubMed |
de Mateo, S., and Sassone-Corsi, P. (2014). Regulation of spermatogenesis by small non-coding RNAs: role of the germ granule. Semin. Cell Dev. Biol. 29, 84–92.
| Regulation of spermatogenesis by small non-coding RNAs: role of the germ granule.Crossref | GoogleScholarGoogle Scholar | 24755166PubMed |
Desforges, J. P. W., Sonne, C., Levin, M., Siebert, U., De Guise, S., and Dietz, R. (2016). Immunotoxic effects of environmental pollutants in marine mammals. Environ. Int. 86, 126–139.
| Immunotoxic effects of environmental pollutants in marine mammals.Crossref | GoogleScholarGoogle Scholar |
Ender, C., and Meister, G. (2010). Argonaute proteins at a glance. J. Cell Sci. 123, 1819–1823.
| Argonaute proteins at a glance.Crossref | GoogleScholarGoogle Scholar | 20484662PubMed |
Falandysz, J., Rose, M., and Fernandes, A. R. (2012). Mixed poly-brominated/chlorinated biphenyls (PXBs): widespread food and environmental contaminants. Environ. Int. 44, 118–127.
| Mixed poly-brominated/chlorinated biphenyls (PXBs): widespread food and environmental contaminants.Crossref | GoogleScholarGoogle Scholar | 22483842PubMed |
Fernandes, G. S., Arena, A. C., Fernandez, C. D., Mercadante, A., Barbisan, L. F., and Kempinas, W. G. (2007). Reproductive effects in male rats exposed to diuron. Reprod. Toxicol. 23, 106–112.
| Reproductive effects in male rats exposed to diuron.Crossref | GoogleScholarGoogle Scholar | 17070669PubMed |
Filler, R. (1993). Methods for evaluation of rat epididymal sperm morphology. Methods in Toxicology. 3, 334–343.
| Methods for evaluation of rat epididymal sperm morphology.Crossref | GoogleScholarGoogle Scholar |
Fossato da Silva, D. A., Teixeira, C. T., Scarano, W. R., Favareto, A. P. A., Fernandez, C. D., Grotto, D., and Kempinas, W. D. G. (2011). Effects of methylmercury on male reproductive functions in Wistar rats. Reprod. Toxicol. 31, 431–439.
| Effects of methylmercury on male reproductive functions in Wistar rats.Crossref | GoogleScholarGoogle Scholar | 21262343PubMed |
Frenedoso da Silva, R., Missassi, G., dos Santos Borges, C., Silva de Paula, E., Hornos Carneiro, M. F., Grotto, D., and De Grava Kempinas, W. (2014). Phytoremediation potential of Maná-Cubiu (Solanum sessiliflorum Dunal) for the deleterious effects of methylmercury on the reproductive system of rats. BioMed Res. Int. 2014, 309631.
| Phytoremediation potential of Maná-Cubiu (Solanum sessiliflorum Dunal) for the deleterious effects of methylmercury on the reproductive system of rats.Crossref | GoogleScholarGoogle Scholar | 24772420PubMed |
Garcia, M. S., Constantino, D. H. J., Silva, A. P., and Perobelli, J. E. (2016). Fish pollutants MeHg and Aroclor cause permanent structural damage in male gonads and kidneys after prepubertal exposure. Int. J. Exp. Pathol. 97, 360–368.
| Fish pollutants MeHg and Aroclor cause permanent structural damage in male gonads and kidneys after prepubertal exposure.Crossref | GoogleScholarGoogle Scholar | 27917541PubMed |
Goncharov, A., Rej, R., Negoita, S., Schymura, M., Santiago-Rivera, A., Morse, G., and Carpenter, D. O. (2009). Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environ. Health Perspect. 117, 1454–1460.
| Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men.Crossref | GoogleScholarGoogle Scholar | 19750113PubMed |
Gray, L. E., Ostby, J., Marshall, R., and Andrews, J. (1993). Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure. Fundam. Appl. Toxicol. 20, 288–294.
| Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure.Crossref | GoogleScholarGoogle Scholar | 8504902PubMed |
Grotto, D., Valentini, J., Serpeloni, J. M., Monteiro, P. A. P., Latorraca, E. F., de Oliveira, R. S., and Barbosa, F. (2011). Evaluation of toxic effects of a diet containing fish contaminated with methylmercury in rats mimicking the exposure in the Amazon riverside population. Environ. Res. 111, 1074–1082.
| Evaluation of toxic effects of a diet containing fish contaminated with methylmercury in rats mimicking the exposure in the Amazon riverside population.Crossref | GoogleScholarGoogle Scholar | 22000760PubMed |
Guo, B. Q., Yan, C. H., Cai, S. Z., Yuan, X. B., and Shen, X. M. (2013). Low level prenatal exposure to methylmercury disrupts neuronal migration in the developing rat cerebral cortex. Toxicology 304, 57–68.
| Low level prenatal exposure to methylmercury disrupts neuronal migration in the developing rat cerebral cortex.Crossref | GoogleScholarGoogle Scholar | 23220560PubMed |
Haraguchi, C. M., Mabuchi, T., Hirata, S., Shoda, T., Hoshi, K., Akasaki, K., and Yokota, S. (2005). Chromatoid bodies: aggresome-like characteristics and degradation sites for organelles of spermiogenic cells. J. Histochem. Cytochem. 53, 455–465.
| Chromatoid bodies: aggresome-like characteristics and degradation sites for organelles of spermiogenic cells.Crossref | GoogleScholarGoogle Scholar | 15805420PubMed |
Horton, J. D., Goldstein, J. L., and Brown, M. S. (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131.
| SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver.Crossref | GoogleScholarGoogle Scholar | 11994399PubMed |
Jia, X., Xu, Y., Wu, W., Fan, Y., Wang, G., Zhang, T., and Su, W. (2017). Aroclor1254 disrupts the blood–testis barrier by promoting endocytosis and degradation of junction proteins via p38 MAPK pathway. Cell Death Dis. 8, e2823.
| Aroclor1254 disrupts the blood–testis barrier by promoting endocytosis and degradation of junction proteins via p38 MAPK pathway.Crossref | GoogleScholarGoogle Scholar | 28542131PubMed |
Joshi, D., Kumar, M. D., Kumar, S. A., and Sangeeta, S. (2014). Reversal of methylmercury-induced oxidative stress, lipid peroxidation, and DNA damage by the treatment of N-acetyl cysteine: a protective approach. J. Environ. Pathol. Toxicol. Oncol. 33, 167–182.
| Reversal of methylmercury-induced oxidative stress, lipid peroxidation, and DNA damage by the treatment of N-acetyl cysteine: a protective approach.Crossref | GoogleScholarGoogle Scholar | 24941299PubMed |
Kim, J. Y., Jung, H. J., and Yoon, M. (2015). Vasa (DDX4) is a putative marker for spermatogonia, spermatocytes and round spermatids in stallions. Reprod. Domest. Anim. 50, 1032–1038.
| Vasa (DDX4) is a putative marker for spermatogonia, spermatocytes and round spermatids in stallions.Crossref | GoogleScholarGoogle Scholar | 26482643PubMed |
Kotaja, N., and Sassone-Corsi, P. (2007). Opinion: the chromatoid body: a germ-cell-specific RNA-processing centre. Nat. Rev. Mol. Cell Biol. 8, 85.
| Opinion: the chromatoid body: a germ-cell-specific RNA-processing centre.Crossref | GoogleScholarGoogle Scholar | 17183363PubMed |
Mangelsdorf, I., Buschmann, J., and Orthen, B. (2003). Some aspects relating to the evaluation of the effects of chemicals on male fertility. Regul. Toxicol. Pharmacol. 37, 356–369.
| Some aspects relating to the evaluation of the effects of chemicals on male fertility.Crossref | GoogleScholarGoogle Scholar | 12758216PubMed |
Meeker, J. D. (2010). Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 66, 236–241.
| Exposure to environmental endocrine disrupting compounds and men’s health.Crossref | GoogleScholarGoogle Scholar | 20347536PubMed |
Meikar, O., and Kotaja, N. (2014). Isolation of chromatoid bodies from mouse testis as a rich source of short RNAs. Methods Mol. Biol. 1173, 11–25.
| Isolation of chromatoid bodies from mouse testis as a rich source of short RNAs.Crossref | GoogleScholarGoogle Scholar | 24920356PubMed |
Meikar, O., Da Ros, M., and Kotaja, N. (2013). Epigenetic regulation of male germ cell differentiation. Subcell. Biochem. 61, 119–138.
| Epigenetic regulation of male germ cell differentiation.Crossref | GoogleScholarGoogle Scholar | 23150249PubMed |
Moussa, H., Hachfi, L., Trimeche, M., Najjar, M. F., and Sakly, R. (2011). Accumulation of mercury and its effects on testicular functions in rats intoxicated orally by methylmercury. Andrologia 43, 23–27.
| Accumulation of mercury and its effects on testicular functions in rats intoxicated orally by methylmercury.Crossref | GoogleScholarGoogle Scholar | 21219378PubMed |
Needham, T. P., and Ghosh, U. (2019). Four decades since the ban, old urban wastewater treatment plant remains a dominant source of PCBs to the environment. Environ. Pollut. 246, 390–397.
| Four decades since the ban, old urban wastewater treatment plant remains a dominant source of PCBs to the environment.Crossref | GoogleScholarGoogle Scholar | 30577007PubMed |
Passos, C. J. S., Da Silva, D. S., Lemire, M., Fillion, M., Guimaraes, J. R. D., Lucotte, M., and Mergler, D. (2008). Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 18, 76–87.
| Daily mercury intake in fish-eating populations in the Brazilian Amazon.Crossref | GoogleScholarGoogle Scholar |
Perobelli, J. E. (2014). The male peripubertal phase as a developmental window for reproductive toxicology studies. Curr. Pharm. Des. 20, 5398–5415.
| The male peripubertal phase as a developmental window for reproductive toxicology studies.Crossref | GoogleScholarGoogle Scholar | 24502595PubMed |
Perobelli, J. E., Martinez, M. F., da Silva Franchi, C. A., Fernandez, C. D. B., Camargo, J. L. V. D., and Kempinas, W. D. G. (2010). Decreased sperm motility in rats orally exposed to single or mixed pesticides. J. Toxicol. Environ. Health A 73, 991–1002.
| Decreased sperm motility in rats orally exposed to single or mixed pesticides.Crossref | GoogleScholarGoogle Scholar | 20563933PubMed |
Robb, G. W., Amann, R. P., and Killian, G. J. (1978). Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 54, 103–107.
| Daily sperm production and epididymal sperm reserves of pubertal and adult rats.Crossref | GoogleScholarGoogle Scholar | 712697PubMed |
Santos, E. G., Silva, M. A., Amorim, R. P., de Souza Giordano, L., de Sales Silva, D., Rasmussen, L. T., and Peruquetti, R. L. (2018). Aging and Chromatoid Body Assembly: Are These Two Physiological Events Linked? Exp. Biol. Med. (Maywood) 243, 917–925.
| Aging and Chromatoid Body Assembly: Are These Two Physiological Events Linked?Crossref | GoogleScholarGoogle Scholar | 29958504PubMed |
Seed, J., Chapin, R. E., Clegg, E. D., Dostal, L. A., Foote, R. H., Hurtt, M. E., and Seyler, D. (1996). Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. Reprod. Toxicol. 10, 237–244.
| Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report.Crossref | GoogleScholarGoogle Scholar | 8738562PubMed |
Senthil Kumar, J., Banudevi, S., Sharmila, M., Murugesan, P., Srinivasan, N., Balasubramanian, K., Aruldhas, M. M., and Arunakaran, J. (2004). Effects of vitamin C and E on PCB (Aroclor 1254) induced oxidative stress, androgen binding protein and lactate in rat Sertoli cells. Reprod. Toxicol. 19, 201–208.
| Effects of vitamin C and E on PCB (Aroclor 1254) induced oxidative stress, androgen binding protein and lactate in rat Sertoli cells.Crossref | GoogleScholarGoogle Scholar | 15501385PubMed |
Siedlikowski, M., Bradley, M., Kubow, S., Goodrich, J. M., Franzblau, A., and Basu, N. (2016). Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: in vitro and epidemiological studies. Environ. Res. 149, 266–273.
| Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: in vitro and epidemiological studies.Crossref | GoogleScholarGoogle Scholar | 26896323PubMed |
Terzaghi, E., Zanardini, E., Morosini, C., Raspa, G., Borin, S., Mapelli, F., Vergani, L., and Di Guardo, A. (2018). Rhizoremediation half-lives of PCBs: role of congener composition, organic carbon forms, bioavailability, microbial activity, plant species and soil conditions, on the prediction of fate and persistence in soil. Sci. Total Environ. 612, 544–560.
| Rhizoremediation half-lives of PCBs: role of congener composition, organic carbon forms, bioavailability, microbial activity, plant species and soil conditions, on the prediction of fate and persistence in soil.Crossref | GoogleScholarGoogle Scholar | 28865272PubMed |
Toyooka, Y., Tsunekawa, N., Takahashi, Y., Matsui, Y., Satoh, M., and Noce, T. (2000). Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149.
| Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development.Crossref | GoogleScholarGoogle Scholar | 10781947PubMed |
US Environmental Protection Agency (EPA) (2011). Pubertal development and thyroid function in intact juvenile/peripubertal male rats assay. OCSPP Guideline 890.1500. Standard Evaluation Procedure (SEP). Available at https://www.epa.gov/sites/production/files/2015-07/documents/final_890.1500_male_purbertal_assay_sep_8.24.11.pdf [verified 22 May 2020].
Vieira, H. C., Morgado, F., Soares, A. M. V. M., and Abreu, S. N. (2015). Fish consumption recommendations to conform to current advice in regard to mercury intake. Environ. Sci. Pollut. Res. Int. 22, 9595–9602.
| Fish consumption recommendations to conform to current advice in regard to mercury intake.Crossref | GoogleScholarGoogle Scholar | 25948385PubMed |
Weber, R., Herold, C., Hollert, H., Kamphues, J., Ungemach, L., Blepp, M., and Ballschmiter, K. (2018). Life cycle of PCBs and contamination of the environment and of food products from animal origin. Environ. Sci. Pollut. Res. Int. 25, 16325–16343.
| Life cycle of PCBs and contamination of the environment and of food products from animal origin.Crossref | GoogleScholarGoogle Scholar | 29589245PubMed |
Wu, H., Hauser, R., Krawetz, S. A., and Pilsner, J. R. (2015). Environmental susceptibility of the sperm epigenome during windows of male germ cell development. Curr. Environ. Health Rep. 2, 356–366.
| Environmental susceptibility of the sperm epigenome during windows of male germ cell development.Crossref | GoogleScholarGoogle Scholar | 26362467PubMed |
Xu, X., and Newman, M. C. (2015). Mercury exposure as a function of fish consumption in two Asian communities in coastal Virginia, USA. Arch. Environ. Contam. Toxicol. 68, 462–475.
| Mercury exposure as a function of fish consumption in two Asian communities in coastal Virginia, USA.Crossref | GoogleScholarGoogle Scholar | 25430872PubMed |
Yadav, R. P., and Kotaja, N. (2014). Small RNAs in spermatogenesis. Mol. Cell. Endocrinol. 382, 498–508.
| Small RNAs in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 23632103PubMed |
Zalups, R. K. (2000). Molecular interactions with mercury in the kidney. Pharmacol. Rev. 52, 113–144.
| 10699157PubMed |


