Molecular cloning, characterisation and expression analysis of the vitellogenin genes vtgAo1 and vtgC during ovarian development in Chinese hook snout carp Opsariichthys bidens
Huayu Jiang A * , Daojun Tang A * , Xinming Gao A * , Chenwen Lin A , Binbin Feng A , Chen Du A , Shan Jin A and Junquan Zhu A B
A B
A Key Laboratory of Applied Marine Biotechnology by the Ministry of Education, School of Marine Sciences, Ningbo University, Ningbo, Zhejiang 315211, PR China.
B Corresponding author. Email: zhujunquan@nbu.edu.cn
Reproduction, Fertility and Development 33(7) 455-465 https://doi.org/10.1071/RD20294
Submitted: 9 November 2020 Accepted: 17 February 2021 Published: 16 April 2021
Abstract
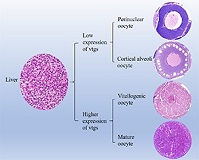
Vitellogenesis is essential for oocyte maturation. Vitellogenin (Vtg), a yolk precursor protein, plays an important role in oogenesis and vitellogenesis. Chinese hook snout carp Opsariichthys bidens is an economically important freshwater fish in China whose reproductive and developmental biology are not well understood. In this study, we undertook histological analysis to examine ovary development and oogenesis in O. bidens. The ovaries were divided into Stages II–V and oocytes were divided into perinuclear oocytes, cortical alveoli oocytes, vitellogenic oocytes and mature oocytes. Full-length cDNA sequences were cloned of two vtg genes from the liver of O. bidens, namely Ob-vtgAo1 and Ob-vtgC. Ob-vtgAo1 and Ob-vtgC cDNA are made up of 4136 and 4392 bases respectively and encode proteins containing 1335 and 1250 amino acids respectively. Ob-vtgAo1 contains three yolk protein domains: lipovitellin heavy chain (LvH), phosvitin (Pv) and lipovitellin light chain (LvL), whereas Ob-VtgC contains LvH and LvL, which are incomplete Vtgs. Ob-vtgAo1 and Ob-vtgC mRNA expression was significantly higher in the liver of O. bidens than in all other tissues. In oocytes of Stage II–III ovaries, yolk granules are almost absent and ovarian and hepatic Ob-vtgAo1 and Ob-vtgC expression is low. At Stage IV, the oocyte is filled with yolk granules and ovarian and hepatic Ob-vtgAo1 and Ob-vtgC expression is significantly increased. Collectively, these findings help us better understand vitellogenesis in O. bidens.
Keywords: Chinese hook snout carp, molecular cloning, oogenesis, Opsariichthys bidens, vitellogenesis, vitellogenin, Vtg.
References
Agnese, M., Verderame, M., Meo, E., Prisco, M., Rosati, L., Limatola, E., Del, G. R., Aceto, S., and Andreuccetti, P. (2013). A network system for vitellogenin synthesis in the mussel Mytilus galloprovincialis (L.). J. Cell. Physiol. 228, 547–555.| A network system for vitellogenin synthesis in the mussel Mytilus galloprovincialis (L.).Crossref | GoogleScholarGoogle Scholar | 22806185PubMed |
Amano, H., Fujita, T., Hiramatsu, N., Shimizu, M., Sawaguchi, S., Matsubara, T., Kagawa, H., Nagae, M., Sullivan, C., and Hara, A. (2007). Egg yolk proteins in gray mullet (Mugil cephalus): purification and classification of multiple lipovitellins and other vitellogenin-derived yolk proteins and molecular cloning of the parent vitellogenin genes. J. Exp. Zool. A. Ecol. Genet. Physiol. 307, 324–341.
| Egg yolk proteins in gray mullet (Mugil cephalus): purification and classification of multiple lipovitellins and other vitellogenin-derived yolk proteins and molecular cloning of the parent vitellogenin genes.Crossref | GoogleScholarGoogle Scholar | 17480036PubMed |
Ando, S., and Hatano, M. (1991). Distribution of carotenoids in the eggs from four species of salmonids. Comp. Biochem. Physiol. B 99, 341–344.
| Distribution of carotenoids in the eggs from four species of salmonids.Crossref | GoogleScholarGoogle Scholar | 1764913PubMed |
Arocha, F. (2002). Oocyte development and maturity classification of swordfish from the north‐western Atlantic. J. Fish Biol. 60, 13–27.
| Oocyte development and maturity classification of swordfish from the north‐western Atlantic.Crossref | GoogleScholarGoogle Scholar |
Azuma, M., Irie, T., and Seki, T. (1993). Retinals and retinols induced by estrogen in the blood plasma of Xenopus laevis. J. Exp. Biol. 178, 89–96.
| 8315372PubMed |
Babin, P. J. (1992). Binding of thyroxine and 3,5,3′-triiodothyronine to trout plasma lipoproteins. Am. J. Physiol. 262, E712–720.
| Binding of thyroxine and 3,5,3′-triiodothyronine to trout plasma lipoproteins.Crossref | GoogleScholarGoogle Scholar | 1590381PubMed |
Babin, P. J., Bogerd, J., Kooiman, F. P., Van Marrewijk, W. J. A., and Van der Horst, D. J. (1999). Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor. J. Mol. Evol. 49, 150–160.
| Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor.Crossref | GoogleScholarGoogle Scholar | 10368443PubMed |
Baker, M. E. (1988). Invertebrate vitellogenin is homologous to human von Willebrand factor. Biochem. J. 256, 1059–1061.
| Invertebrate vitellogenin is homologous to human von Willebrand factor.Crossref | GoogleScholarGoogle Scholar | 3265622PubMed |
Brooks, J. M., and Wessel, G. (2002). The major yolk protein in sea urchins is a transferrin-like, iron binding protein. Dev. Biol. 245, 1–12.
| The major yolk protein in sea urchins is a transferrin-like, iron binding protein.Crossref | GoogleScholarGoogle Scholar | 11969251PubMed |
Byrne, B. M., Gruber, M., and Ab, G. (1989). The evolution of egg yolk proteins. Prog. Biophys. Mol. Biol. 53, 33–69.
| The evolution of egg yolk proteins.Crossref | GoogleScholarGoogle Scholar | 2682782PubMed |
Cerdà, J., Fabra, M., and Raldúa, D. (2007). Physiological and molecular basis of fish oocyte hydration. In ‘The Fish Oocyte.’ (Eds Babin P.J., Cerdà J., Lubzens E.) (Springer, Dordrecht.)
Finn, R. N. (2007). Vertebrate yolk complexes and the functional implications of phosvitins and other subdomains in vitellogenins. Biol. Reprod. 76, 926–935.
| Vertebrate yolk complexes and the functional implications of phosvitins and other subdomains in vitellogenins.Crossref | GoogleScholarGoogle Scholar | 17314313PubMed |
Finn, R. N., and Kristoffersen, B. A. (2007). Vertebrate vitellogenin gene duplication in relation to the ‘3R hypothesis’: Correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS One 2, e169.
| Vertebrate vitellogenin gene duplication in relation to the ‘3R hypothesis’: Correlation to the pelagic egg and the oceanic radiation of teleosts.Crossref | GoogleScholarGoogle Scholar | 17245445PubMed |
Gao, X. M., Zhou, Y., Zhang, D. D., Hou, C. C., and Zhu, J. Q. (2019). Multiple vitellogenin genes (vtgs) in large yellow croaker (Larimichthys crocea): molecular characterization and expression pattern analysis during ovarian development. Fish Physiol. Biochem. 45, 829–848.
| Multiple vitellogenin genes (vtgs) in large yellow croaker (Larimichthys crocea): molecular characterization and expression pattern analysis during ovarian development.Crossref | GoogleScholarGoogle Scholar | 30843140PubMed |
Garcia, J., Munro, E. M., Monte, M., Fourrier, M., Whitelaw, J., Smail, D., and Ellis, A. (2010). Atlantic salmon (Salmo salar L.) serum vitellogenin neutralises infectivity of infectious pancreatic necrosis virus (IPNV). Fish Shellfish Immunol. 29, 293–297.
| Atlantic salmon (Salmo salar L.) serum vitellogenin neutralises infectivity of infectious pancreatic necrosis virus (IPNV).Crossref | GoogleScholarGoogle Scholar | 20420921PubMed |
Hara, A., Hiramatsu, N., and Fujita, T. (2016). Vitellogenesis and choriogenesis in fishes. Fish. Sci. 82, 187–202.
| Vitellogenesis and choriogenesis in fishes.Crossref | GoogleScholarGoogle Scholar |
Kang, B. J., Jung, J. H., Lee, J. M., Lim, S. G., Saito, H., Kim, M. H., Kim, Y. J., Saigusa, M., and Han, C. H. (2007). Structural and expression analyses of two vitellogenin genes in the carp, Cyprinus carpio. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 148, 445–453.
| Structural and expression analyses of two vitellogenin genes in the carp, Cyprinus carpio.Crossref | GoogleScholarGoogle Scholar | 17804271PubMed |
Kong, H. J., Kim, J. L., Moon, J. Y., Kim, W. J., Kim, H. S., Park, J. Y., Cho, H. K., and An, C. M. (2014). Characterization, expression profile, and promoter analysis of the Rhodeus uyekii vitellogenin Ao1 gene. Int. J. Mol. Sci. 15, 18804–18818.
| Characterization, expression profile, and promoter analysis of the Rhodeus uyekii vitellogenin Ao1 gene.Crossref | GoogleScholarGoogle Scholar | 25329620PubMed |
Lambert, L. A., Perri, H., and Meehan, T. J. (2005a). Evolution of duplications in the transferrin family of proteins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140, 11–25.
| Evolution of duplications in the transferrin family of proteins.Crossref | GoogleScholarGoogle Scholar | 15621505PubMed |
Lambert, L. A., Perri, H., Halbrooks, P., and Mason, A. (2005b). Evolution of the transferrin family: Conservation of residues associated with iron and anion binding. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 142, 129–141.
| Evolution of the transferrin family: Conservation of residues associated with iron and anion binding.Crossref | GoogleScholarGoogle Scholar | 16111909PubMed |
Li, Z., Zhang, S. C., Zhang, J., Liu, M., and Liu, Z. H. (2009). Vitellogenin is a cidal factor capable of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid. Mol. Immunol. 46, 3232–3239.
| Vitellogenin is a cidal factor capable of killing bacteria via interaction with lipopolysaccharide and lipoteichoic acid.Crossref | GoogleScholarGoogle Scholar | 19729202PubMed |
Liu, Q. H., Zhang, S. C., Li, Z. J., and Gao, C. R. (2009). Characterization of a pattern recognition molecule vitellogenin from carp (Cyprinus carpio). Immunobiology 214, 257–267.
| Characterization of a pattern recognition molecule vitellogenin from carp (Cyprinus carpio).Crossref | GoogleScholarGoogle Scholar | 19327543PubMed |
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method.Crossref | GoogleScholarGoogle Scholar | 11846609PubMed |
Montorzi, M., Falchuk, K. H., and Vallee, B. L. (1994). Xenopus laevis vitellogenin is a zinc protein. Biochem. Biophys. Res. Commun. 200, 1407–1413.
| Xenopus laevis vitellogenin is a zinc protein.Crossref | GoogleScholarGoogle Scholar | 8185593PubMed |
Montorzi, M., Falchuk, K., and Vallee, B. (1995). Vitellogenin and lipovitellin: zinc proteins of Xenopus laevis oocytes. Biochemistry 34, 10851–10858.
| Vitellogenin and lipovitellin: zinc proteins of Xenopus laevis oocytes.Crossref | GoogleScholarGoogle Scholar | 7662665PubMed |
Mori, H., Nakagawa, M., Soyano, K., and Koya, Y. (2003). Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fish. Sci. 69, 910–923.
| Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity.Crossref | GoogleScholarGoogle Scholar |
Munoz, M., Casadevall, M., and Bonet, S. (2002). The ovarian morphology of Scorpaena notata shows a specialized mode of oviparity. J. Fish Biol. 61, 877–887.
| The ovarian morphology of Scorpaena notata shows a specialized mode of oviparity.Crossref | GoogleScholarGoogle Scholar |
Pan, M. L., Bell, W., and Telfer, W. (1969). Vitellogenic blood protein synthesis by insect fat body. Science 165, 393–394.
| Vitellogenic blood protein synthesis by insect fat body.Crossref | GoogleScholarGoogle Scholar | 17809522PubMed |
Reading, B. J., Hiramatsu, N., Sawaguchi, S., Matsubara, T., Hara, A., Lively, M. O., and Sullivan, C. V. (2008). Conserved and variant molecular and functional features of multiple egg yolk precursor proteins (vitellogenins) in white perch (Morone americana) and other teleosts. Mar. Biotechnol. (NY) 11, 169–187.
| Conserved and variant molecular and functional features of multiple egg yolk precursor proteins (vitellogenins) in white perch (Morone americana) and other teleosts.Crossref | GoogleScholarGoogle Scholar | 18766402PubMed |
Reading, B. J., Sullivan, C. V., and Schilling, J. (2017). ‘Vitellogenesis in Fishes.’ (Elsevier Ltd.)
Sawaguchi, S., Koya, Y., Yoshizaki, N., Ohkubo, N., Andoh, T., Hiramatsu, N., Sullivan, C., Hara, A., and Matsubara, T. (2005). Multiple vitellogenins (Vgs) in mosquitofish (Gambusia affinis): identification and characterization of three functional vg genes and their circulating and yolk protein products. Biol. Reprod. 72, 1045–1060.
| Multiple vitellogenins (Vgs) in mosquitofish (Gambusia affinis): identification and characterization of three functional vg genes and their circulating and yolk protein products.Crossref | GoogleScholarGoogle Scholar | 15616220PubMed |
Sun, C., Hu, L. L., Liu, S. S., Hu, G. B., and Zhang, S. C. (2013). Antiviral activity of phosvitin from zebrafish Danio rerio. Dev. Comp. Immunol. 40, 28–34.
| Antiviral activity of phosvitin from zebrafish Danio rerio.Crossref | GoogleScholarGoogle Scholar | 23305746PubMed |
Tong, Z., Li, L., Pawar, R., and Zhang, S. (2009). Vitellogenin is an acute phase protein with bacterial-binding and inhibiting activities. Immunobiology 215, 898–902.
| Vitellogenin is an acute phase protein with bacterial-binding and inhibiting activities.Crossref | GoogleScholarGoogle Scholar | 20006406PubMed |
Trichet, V., Buisine, N., Mouchel, N., Morán, P., Pendás, A. M., Le Pennec, J. P., and Wolff, J. (2000). Genomic analysis of the vitellogenin locus in rainbow trout (Oncorhynchus mykiss) reveals a complex history of gene amplification and retroposon activity. Mol. Gen. Genet. 263, 828–837.
| Genomic analysis of the vitellogenin locus in rainbow trout (Oncorhynchus mykiss) reveals a complex history of gene amplification and retroposon activity.Crossref | GoogleScholarGoogle Scholar | 10905350PubMed |
Wang, H., Yan, T., Tan, J. T. T., and Gong, Z. Y. (2000). Zebrafish vitellogenin gene (vg3) encodes a novel vitellogenin without a phosvitin domain and may represent a primitive vertebrate vitellogenin gene. Gene 256, 303–310.
| Zebrafish vitellogenin gene (vg3) encodes a novel vitellogenin without a phosvitin domain and may represent a primitive vertebrate vitellogenin gene.Crossref | GoogleScholarGoogle Scholar | 11054560PubMed |
Wang, H., Tan, J. T. T., Emelyanov, A., Korzh, V., and Gong, Z. Y. (2005). Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene 356, 91–100.
| Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio.Crossref | GoogleScholarGoogle Scholar | 15979250PubMed |
Wang, R., Gao, Y., Zhang, L. H., Zhang, Y., Fang, Z. Q., He, J. G., Zhang, W. M., and Ma, G. Z. (2010). Cloning, expression, and induction by 17-β estradiol (E2) of a vitellogenin gene in the white cloud mountain minnow Tanichthys albonubes. Fish Physiol. Biochem. 36, 157–164.
| Cloning, expression, and induction by 17-β estradiol (E2) of a vitellogenin gene in the white cloud mountain minnow Tanichthys albonubes.Crossref | GoogleScholarGoogle Scholar | 20467857PubMed |
Wildner, D. D., Grier, H., and Quagio-Grassiotto, I. (2013). Female germ cell renewal during the annual reproductive cycle in Ostariophysians fish. Theriogenology 79, 709–724.
| Female germ cell renewal during the annual reproductive cycle in Ostariophysians fish.Crossref | GoogleScholarGoogle Scholar | 23317762PubMed |
Williams, V. N., Reading, B. J., Amano, H., Hiramatsu, N., Schilling, J., Salger, S., Islam Williams, T., Gross, K., and Sullivan, C. (2014a). Proportional accumulation of yolk proteins derived from multiple vitellogenins is precisely regulated during vitellogenesis in striped bass (Morone saxatilis). J Exp. Zool. A Ecol. Genet. Physiol. 321, 301–315.
| Proportional accumulation of yolk proteins derived from multiple vitellogenins is precisely regulated during vitellogenesis in striped bass (Morone saxatilis).Crossref | GoogleScholarGoogle Scholar | 24648375PubMed |
Williams, V. N., Reading, B. J., Hiramatsu, N., Amano, H., Glassbrook, N., Hara, A., and Sullivan, C. V. (2014b). Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: molecular characterization and processing during oocyte growth and maturation. Fish Physiol. Biochem. 40, 395–415.
| Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: molecular characterization and processing during oocyte growth and maturation.Crossref | GoogleScholarGoogle Scholar | 24005815PubMed |
Xue, R., Wang, X. Y., Xu, S. H., Liu, Y. F., Feng, C. C., Zhao, C. Y., Liu, Q. H., and Li, J. (2018). Expression profile and localization of vitellogenin mRNA and protein during ovarian development in turbot (Scophthalmus maximus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 226, 53–63.
| Expression profile and localization of vitellogenin mRNA and protein during ovarian development in turbot (Scophthalmus maximus).Crossref | GoogleScholarGoogle Scholar | 30114525PubMed |


