Dry storage of sperm: applications in primates and domestic animals
Stuart A. MeyersDepartment of Anatomy, Physiology, and Cell Biology, School of Veterinary Medicine, 1 Shields Ave, University of California, Davis, CA 95616, USA. Email: smeyers@ucdavis.edu
Reproduction, Fertility and Development 18(2) 1-5 https://doi.org/10.1071/RD05116
Submitted: 21 September 2005 Accepted: 21 September 2005 Published: 14 December 2005
Abstract
Cryopreservation of spermatozoa, oocytes and embryos, as well as somatic cells or cell lines for cloning from cells, are all options for the long-term storage of unique genotypes and endangered species. Spermatozoal cryopreservation and storage currently require liquid nitrogen or ultralow refrigeration-based methods for long- or short-term storage, which requires routine maintenance and extensive space requirements. The preservation of stem cells also has strict requirements for long-term storage to maintain genetic integrity. Dessicated (lyopreserved) sperm and stem cells will provide an unprecedented type of long-term storage without the need for expensive and burdensome cryogenic conditions. Experiments were conducted to determine an effective intracellular concentration of the lyoprotectant trehalose. High-pressure liquid chromatography studies revealed that trehalose can be incorporated into mature sperm cells as well as spermatogonial stem cells from rhesus monkeys. In addition, using fourier transform infrared spectroscopy, we determined that thermotropic phase transitions for fresh ejaculates from rhesus monkey and stallion sperm occurred at 10–15, 33–37 and 55–59°C. Preliminary studies in our laboratory have indicated that spermatogonial stem cells can be dried to <3 g g−1 water and maintain viability following rehydration. Studies in our laboratory have provided preliminary results suggesting that the desiccated storage of sperm and spermatogonial stem cells may be a viable alternative to conventional cryopreservation.
Introduction
Preservation of mammalian cells
The long-term storage of numerous mammalian somatic and germ cell types has been accomplished using cryopreservation, usually in liquid nitrogen (cryogenic tanks) or in other subzero environments (−80°C ultralow-type freezer). Many cell types do not tolerate frozen storage above −80°C and undergo severe deterioration, with subsequent lethal damage. However, in the presence of cryoprotectants (CPA) that are, to some degree, cytotoxic, many cell types can survive low-temperature storage. The process of cryopreservation has profound effects on cells, many of which result in sublethal damage to the cells and a subsequent reduction of function. Some haematopoietic and human embryonic stem cell lines and gametes undergo sublethal and lethal changes associated with the complex interaction of low temperature, cryopreservation reagent, osmotic and oxidative balance, solute and electrolyte balance and ice crystallisation (Reubinoff et al. 2001; Buchanan et al. 2004). As a routine consequence of cryogenic storage, approximately 25–75% of cells stored this way are lost owing to necrotic and apoptotic cell death and this is dependent on cell type, freezing rate and CPA (Fuller 2004). With appropriate CPA, freezing damage can be minimised. However, high concentrations of CPA can cause osmotic and toxic cell damage (Arakawa et al. 1990; Anchordoguy et al. 1992). Thus, CPA addition and removal can have long-lasting cellular effects. This is particularly troubling for gamete preservation and alternative CPAs have been studied extensively.
Anhydrobiosis and cellular injury
Numerous plant and animal species have the ability to survive almost complete dehydration. This life without water, or anhydrobiosis, is dependent on a series of complex physiological adaptations, but is primarily dependent upon the accumulation of disaccharides in the cells of the organism during drying. Anhydrobiotic animals accumulate the disaccharide trehalose (Crowe et al. 1984, 1997; Womersley and Ching 1989; Westh and Ramlov 1991), whereas the dehydration-resistant tissues of certain plants, such as seeds and pollen grains, accumulate sucrose (Koster and Leopold 1988; Hoekstra et al. 1992, 2001). When accumulated, these sugars may be in excess of 20–50% of the dry weight of these organisms.
The drying of cells usually leads to damage of cellular membranes and proteins, which often results in cell death. The removal of intracellular water causes changes in molecular interactions. When cell membranes are dehydrated, the water molecules that help to maintain the spacing between the polar phospholipid headgroups are removed. This results in the lipid fatty acid chains and polar head groups coming closer to each other, towards membrane collapse, and an increase in the membrane phase transition temperature (Tm) as the membrane changes from a biologically active fluid phase to the gel phase (Crowe et al. 1992, 1997). Subsequently, as water is added back during rehydration, the membrane undergoes another phase transition, resulting in leakage of soluble cell contents through each membrane (Leslie et al. 1994; Crowe et al. 1997).
Trehalose, a disaccharide found in high concentrations in many desiccation-tolerant animals and plants (Crowe et al. 1984, 1992; Gadd et al. 1987; Westh and Ramlov 1991; Bianchi et al. 1993; Drennan et al. 1993; Crowe and Crowe 2000), has been the lyoprotectant of choice for many cellular dehydration studies (Puhlev et al. 2001; Wolkers et al. 2001; Acker et al. 2002) owing to its ability to replace the hydrogen-bonded water molecules and depress the phase transition in dehydrated samples (Crowe and Crowe 1988; Harrigan et al. 1990; Leslie et al. 1994; Tsvetkova et al. 1998). Trehalose also has a high glass transition temperature (Tg; Koster et al. 1994, 2000; Sun et al. 1996; Crowe et al. 1997; Buitink et al. 1998) owing to the stability of the glycosidic bond (Kacurakova and Mathlouthi 1996; Schebor et al. 1999). The high glass transition state for trehalose allows living cells to be placed into a static glassy state at ambient temperature following the removal of cellular water. This glassy state is characterised by a very high viscosity, which serves to inhibit the cellular chemical, biological and physical processes that lead to cell deterioration and death. Although the protective effect of trehalose during drying has been demonstrated in many cellular and non-cellular biological materials, its role during the dehydration of stem cells, and spermatogenic cells in particular, has not been shown conclusively (Gordon et al. 2001; Puhlev et al. 2001).
Because it appears that sugars need to be present on both sides of a cell’s membrane to provide optimal protection, any sugar used in cryoprotection must be capable of entering the cell’s interior through the plasma membrane (Chen et al. 2001). The limited ability of trehalose to cross cell membranes is an obstacle to a cell’s ability to utilise trehalose. Several strategies to improve the mammalian cell’s ability to take up trehalose have been reported, including a genetic engineering approach, in which a Staphylococcus α-haemolysin was used to porate mammalian fibroblasts and keratinocytes for trehalose uptake (Eroglu et al. 2000), trehalose microinjection (Eroglu et al. 2002) and thermal poration (Beattie et al. 1997). It has recently been shown that mesenchymal stem cells can take up trehalose from the external environment by fluid phase endocytosis, reaching internal trehalose concentrations in the range 20–30 mm (Oliver et al. 2004), which has been shown to protect human platelets during freeze-drying and storage (Wolkers et al. 2001, 2002).
Preservation of cells in the dry state
The dehydrated storage of cells represents an alternative to cryopreservation and has already been shown to be effective for the storage of human blood platelets at room temperature for up to 2 years, during which time recovery and response to thrombin remained essentially unchanged (Wolkers et al. 2001, 2002). Efforts to dry nucleated cells have also been reported (Guo et al. 2000; Bloom et al. 2001; Gordon et al. 2001; Puhlev et al. 2001; Acker et al. 2002; Bhowmick et al. 2003), but achieving consistent results of highly viable, physiologically active cells following dehydration to low water content remains elusive.
Several studies reported in the literature from 1976 and 1981 contained reports of freeze-dried bull spermatozoa (Larson and Graham 1976; Jeyendran et al. 1981), although no fertility results were presented associated with dried sperm. Rather, a small percentage of motility and fertility was reported by Larson and Graham (1976) associated with some degree of decreased moisture content. Mature spermatozoa have been completely dessicated, stored and, following intracytoplasmic sperm injection (ICSI), have resulted in embryonic development in mice, rabbits, pigs and cattle (Wakayama and Yanagimachi 1998; Keskintepe et al. 2002) or live offspring in mice, rabbits, rats and fish (Wakayama and Yanagimachi 1998; Ward et al. 2003; Liu et al. 2004; Hirabayashi et al. 2005; Kaneko and Nakagata 2005; Poleo et al. 2005). These studies used freeze-dried sperm that were non-motile and non-viable, but the spermatozoal ultrastructure was evidently intact (Kusakabe et al. 2001).
Dessication of cells for dry storage has been achieved using freeze-drying (lyophilisation) or convective drying in ambient environments. Both approaches rely on the removal of cellular water to achieve the viscous or glassy state, also considered to be the vitrified state. Vitrification usually refers to cells or tissues in the fully hydrated state, although it can also occur in dehydrated samples. In freeze-drying cells, the sample is usually first frozen to subzero temperatures and then the water is sublimated. Substantial amounts of water (approximately 0.3 g water per g dry weight) are known to be associated with proteins, carbohydrates and membranes in cells. Some researchers consider this water to be non-freezable and that, if this water is frozen, it may play a significant role in tissue damage. Crowe (2004) has suggested that freezing and dehydration are fundamentally different processes in that dehydration removes the water of hydration of proteins and membranes, whereas freezing under the conditions used for cryopreservation leaves that water unfrozen. Furthermore, the solute requirements for stabilising biological structures in the dry and frozen states are different; all solutes that stabilise such structures in the dry state are also good cryopreservation agents. The reverse is not true. For example, glycerol and dimethylsulfoxide (DMSO), the most commonly used cryopreservation agents, are ineffective in preserving dry biomaterials.
Recent studies
Spermatozoa
Recent investigations in our laboratory have focused on the ability of non-human primate, cattle and horse sperm to undergo trehalose loading and subsequent dehydration. Using fourier transform infrared spectroscopy (FTIR), we determined that thermotropic phase transitions for fresh ejaculates from rhesus monkey and stallion sperm occurred at 10–15, 33–37 and 55–59°C. Similar results have been observed for bovine spermatozoa (S. A. Meyers et al., unpublished results) in our laboratory. These data suggest that the plasma membrane likely becomes leaky owing to lipid rearrangement and the phase transition temperatures could be used to enhance uptake by diffusion of trehalose.
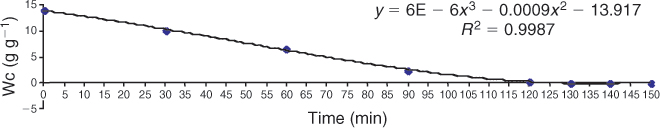
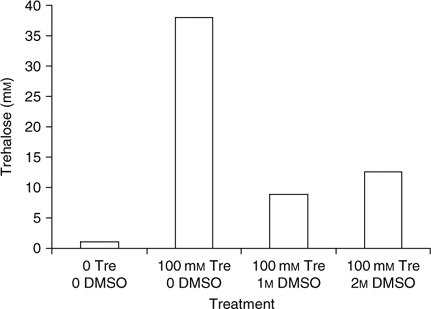
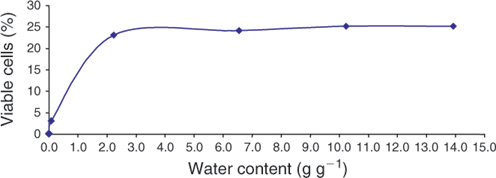
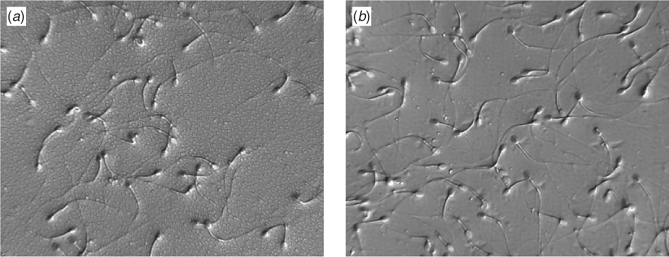
High-pressure liquid chromatography (HPLC) was used to measure intracellular trehalose concentrations. Figure 1 demonstrates that spermatozoal trehalose concentrations approach 35% loading efficiency for these cells. The addition of DMSO failed to enhance trehalose uptake. Subsequent studies used these loading conditions to obtain intracellular trehalose concentrations that are similar to those seen in trehalose-loaded human platelets (Wolkers et al. 2002). Sperm were then loaded with trehalose and dried in a vacuum oven at ambient temperature for up to 150 min in order to assess the temperature-independent ability for trehalose-loaded and control sperm to undergo drying and to determine water content by gravimetric methods. Figure 2 demonstrates the drying curve thus obtained. Sperm viability and membrane integrity were determined using propidium iodide in cells sampled during drying (Fig. 3). Figure 4 demonstrates the microscopic appearance of sperm cells vacuum-dried with and without exogenous trehalose added to the medium.
|
|
|
Spermatogonial stem cells
We have determined that spermatogonial stem cells can be isolated from sexually immature rhesus macaques (S. A. Meyers et al., unpublished observations). Spermatogonial stem cells were isolated using an enzymatic testicular dispersion method from immature rhesus males (aged from 3 months to 2 years) using sequential enzymatic digestion of prepubertal monkey testes. This method reveals cell preparations with relatively few cell types and excludes interstitial cells. These isolated cell preparations were cryopreserved or cultured for up to 3 weeks in both KSOM and Dulbecco’s modified Eagle’s medium (DMEM) with minimal cell loss or visible degenerative changes. Using thermal poration at 37°C, at which temperature the plasma membrane is most pliable owing to a phase transition at 37°C, our data indicated that trehalose was taken up by a significant population of incubated testicular cells. As determined by HPLC, trehalose concentrations in these cells reached levels of 30–75 mm and this was dependent on extracellular trehalose concentration. The loading efficiency of the disaccharide was reduced at higher concentrations and this supports the concept that these cells may endocytose trehalose.
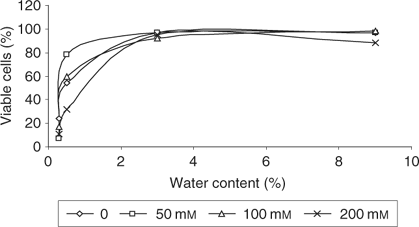
Following the isolation of spermatogonial cells, cells were plated overnight in DMEM containing 5% fetal bovine serum (FBS; c-DMEM) in a 34°C/5% CO2 incubator containing 0, 50, 100 and 200 mm trehalose. Trypsin/EDTA was added for 3 min at 34°C to lift the cells from the flask. Then, a 50-μL drop was placed in the centre of a 1.5-mL microcentrifuge tube cap. The caps were placed in a 30°C vacuum oven under vacuum (21 inches Hg). Duplicate tubes were removed from the vacuum oven at various times. At each time point, one cap was rehydrated using c-DMEM for a final volume of 150 μL and viability was determined by propidium iodide exclusion. The other cap was weighed and then dried completely overnight in the vacuum oven at (+)80°C for determination of water content. Figure 5 demonstrates that spermatogonial stem cells (SSCs) maintain viability below 1 g H2O g−1 dry weight and that 50% viability is seen at different water contents, depending on trehalose exposure. Although the data do not clearly indicate that higher trehalose levels are coincident with higher cell viability, the data show that, with loading at 50 mm trehalose, cell viability is greatest at very low water content (below 0.5 g H2O g−1). Clearly, more studies will be required to determine the effects of trehalose on cell dehydration. These studies described herein provide a strong indication that SSCs may tolerate drying in some form, similar to that shown for mesenchymal stem cells and non-nucleated platelets.
|
Conclusions
Although cell desiccation removes the water of hydration for proteins and cell membrane lipids and results in the subsequent physical collapse of a cell’s architecture, protection using the disaccharide trehalose may permit partial or complete drying without complete loss of function. Recent studies indicate that spermatogonial stem cells (diploid premeiotic cells) may tolerate at least partial dehydration below 1 g g−1 water content and retain in vitro viability. Spermatozoa seem to have more stringent requirements for intracellular water and have not been preserved with significant motility at this time.
Acker, J. P. , Fowler, A. , Layman, B. , Cheney, S. , and Toner, M. (2002). Survival of desiccated mammalian cells: beneficial effects of isotonic media. Cell Preserv. Tech. 1, 129–140.
| Crossref | GoogleScholarGoogle Scholar |
Crowe, J. H. , and Crowe, L. M. (1988). Trehalose and dry dipalmitoylphosphatidylcholine revisited. Biochim. Biophys. Acta 946, 193–201.
| PubMed |

Crowe, J. H. , and Crowe, L. M. (2000). Preservation of mammalian cells – learning nature’s tricks. Nat. Biotechnol. 18, 145–146.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Crowe, J. H. , Crowe, L. M. , and Chapman, D. (1984). Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223, 701–703.

Crowe, J. H. , Hoekstra, F. A. , and Crowe, L. M. (1992). Anhydrobiosis. Ann. Rev. Physiol. 54, 579–599.
| Crossref | GoogleScholarGoogle Scholar |

Crowe, J. H. , Oliver, A. E. , Hoekstra, F. A. , and Crowe, L. M. (1997). Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology 35, 20–30.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Drennan, P. M. , Smith, M. T. , Goldsworthy, D. , and van Staden, J. (1993). The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. J. Plant Physiol. 142, 493–496.

Eroglu, A. , Russo, M. J. , Bieganski, R. , Fowler, A. , Cheley, S. , Bayley, H. , and Toner, M. (2000). Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 18, 163–167.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Eroglu, A. , Toner, M. , and Toth, T. L. (2002). Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil. Steril. 77, 152–158.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Fuller, B. J. (2004). Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters 25, 375–388.
| PubMed |

Gadd, G. M. , Chalmers, K. , and Reed, R. H. (1987). The role of trehalose in dehydration resistance of Sacchromyces cerevisiae. FEMS Microbiol. Lett. 48, 249–254.
| Crossref | GoogleScholarGoogle Scholar |

Gordon, S. L. , Oppenheimer, S. R. , Mackay, A. M. , Brunnabend, J. , Puhlev, I. , and Levine, F. (2001). Recovery of human mesenchymal stem cells following dehydration and rehydration. Cryobiology 43, 182–187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Guo, N. , Puhlev, I. , Brown, D. R. , Mansbridge, J. , and Levine, F. (2000). Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 18, 168–171.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Harrigan, P. R. , Madden, T. D. , and Cullis, P. R. (1990). Protection of liposomes during dehydration or freezing. Chem. Phys. Lipids 52, 139–149.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hirabayashi, M. , Kato, M. , Ito, J. , and Hochi, S. (2005). Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads. Zygote 13, 79–85.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hoekstra, F. A. , Crowe, J. H. , Crowe, L. M. , Vanroekel, T. , and Vermeer, E. (1992). Do phospholipids and sucrose determine membrane phase-transitions in dehydrating pollen species. Plant Cell Environ. 15, 601–606.

Hoekstra, F. A. , Golovina, E. A. , and Buitink, J. (2001). Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6, 431–438.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Jeyendran, R. S. , Graham, E. F. , and Schmehl, M. K. (1981). Fertility of dehydrated bull sperm. Cryobiology 18, 292–300.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kacurakova, M. , and Mathlouthi, M. (1996). FTIR and laser-Raman spectra of oligosaccharides in water: characterization of the glycosidic bond. Carbohydr. Res. 284, 145–157.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kaneko, T. , and Nakagata, N. (2005). Relation between storage temperature and fertilizing ability of freeze-dried mouse spermatozoa. Comp. Med. 55, 140–144.
| PubMed |

Keskintepe, L. , Pacholczyk, G. , Machnicka, A. , Norris, K. , Curuk, M. A. , Khan, I. , and Brackett, B. G. (2002). Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol. Reprod. 67, 409–415.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Koster, K. L. , and Leopold, A. C. (1988). Sugars and desiccation tolerance in seeds. Plant Physiol. 88, 829–832.

Koster, K. L. , Webb, M. S. , Bryant, G. , and Lynch, D. V. (1994). Interactions between soluble sugars and POPC during dehydration: vitrification of sugars alters the phase behavior of the phospholipids. Biochim. Biophys. Acta 1193, 143–150.
| PubMed |

Koster, K. L. , Lei, Y. P. , Anderson, M. , Martin, S. , and Bryant, G. (2000). Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys. J. 78, 1932–1946.
| PubMed |

Kusakabe, H. , Szczygiel, M. A. , Whittingham, D. G. , and Yanagimachi, R. (2001). Maintenance of genetic integrity in frozen and freeze-dried mouse spermatozoa. Proc. Natl Acad. Sci. USA 98, 13 501–13 506.
| Crossref | GoogleScholarGoogle Scholar |

Larson, E. V. , and Graham, E. F. (1976). Freeze-drying of spermatozoa. Dev. Biol. Stand. 36, 343–348.
| PubMed |

Leslie, S. B. , Teter, S. A. , Crowe, L. M. , and Crowe, J. H. (1994). Trehalose lowers membrane phase transitions in dry yeast cells. Biochim. Biophys. Acta 1192, 7–13.
| PubMed |

Liu, J. L. , Kusakabe, H. , Chang, C. C. , Suzuki, H. , and Schmidt, D. W. , et al. (2004). Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol. Reprod. 70, 1776–1781.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Oliver, A. E. , Jamil, K. , Crowe, J. H. , and Tablin, F. (2004). Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv. Technol. 2, 35–49.
| Crossref | GoogleScholarGoogle Scholar |

Poleo, G. A. , Godke, R. R. , and Tiersch, T. R. (2005). Intracytoplasmic sperm injection using cryopreserved, fixed, and freeze-dried sperm in eggs of Nile tilapia. Biotechnology 7, 104–111.

Puhlev, I. , Guo, N. , Brown, D. R. , and Levine, F. (2001). Desiccation tolerance in human cells. Cryobiology 42, 207–217.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Reubinoff, B. E. , Pera, M. F. , Vajta, G. , and Trounson, A. E. (2001). Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum. Reprod. 16, 2187–2194.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Schebor, C. , Burin, L. , del Pilar Buera, M. , and Chirife, J. (1999). Stability to hydrolysis and browning of trehalose, sucrose and raffinose in low mositure systems in relation to their use as protectants of dry biomaterials. Lebensm. Wissensch. Technol. 32, 481–485.
| Crossref | GoogleScholarGoogle Scholar |

Sun, W. Q. , Leopold, A. C. , and Crowe, J. H. (1996). Stability of dry liposomes in sugar glasses. Biophys. J. 70, 1769–1776.
| PubMed |

Tsvetkova, N. M. , Phillips, B. L. , Crowe, L. M. , Crowe, J. H. , and Risbud, S. H. (1998). Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys. J. 75, 2947–2955.
| PubMed |

Wakayama, T. , and Yanagimachi, R. (1998). Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 16, 639–641.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Ward, M. A. , Kaneko, T. , Kusakabe, H. , Biggers, J. D. , Whittingham, D. G. , and Yanagimachi, R. (2003). Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol. Reprod. 69, 2100–2108.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Westh, P. , and Ramlov, H. (1991). Trehalose accumulation in the tardigrade Adorybiotus coronifer during anhydrobiosis. J. Exp. Zool. 258, 303–311.
| Crossref | GoogleScholarGoogle Scholar |

Wolkers, W. F. , Walker, N. J. , Tablin, F. , and Crowe, J. H. (2001). Human platelets loaded with trehalose survive freeze-drying. Cryobiology 42, 79–87.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Wolkers, W. F. , Walker, N. J. , Tamari, Y. , Tablin, F. , and Crowe, J. H. (2002). Towards a clinical application of freeze-dried human platelets. Cell Preserv. Technol. 1, 175–188.
| Crossref | GoogleScholarGoogle Scholar |

Womersley, C. , and Ching, C. (1989). Natural dehydration regimes as a prerequisite for the successful induction of anhydrobiosis in the nematode Rotylenchulus reniformis. J. Exp. Biol. 143, 359–372.
| PubMed |