Epigenetics and developmental programming of welfare and production traits in farm animals
K. D. Sinclair A F , K. M. D. Rutherford B , J. M. Wallace C , J. M. Brameld A , R. Stöger A , R. Alberio A , D. Sweetman A , D. S. Gardner A , V. E. A. Perry A , C. L. Adam C , C. J. Ashworth D , J. E. Robinson E and C. M. Dwyer BA Schools of Biosciences and Veterinary Medicine and Sciences, University of Nottingham, Sutton Bonington, Leicestershire, LE12 5RD, UK.
B Animal Behaviour and Welfare team, SRUC, West Mains Road, Edinburgh EH9 3JG, UK.
C Rowett Institute of Nutrition and Health, University of Aberdeen, Foresterhill, Aberdeen, AB25 2ZD, UK.
D The Roslin Institute and R(D)SVS, University of Edinburgh, Easter Bush, Midlothian, EH25 9RG, UK.
E College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, UK.
F Corresponding author. Email: kevin.sinclair@nottingham.ac.uk
Reproduction, Fertility and Development 28(10) 1443-1478 https://doi.org/10.1071/RD16102
Submitted: 2 March 2016 Accepted: 6 June 2016 Published: 21 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
The concept that postnatal health and development can be influenced by events that occur in utero originated from epidemiological studies in humans supported by numerous mechanistic (including epigenetic) studies in a variety of model species. Referred to as the ‘developmental origins of health and disease’ or ‘DOHaD’ hypothesis, the primary focus of large-animal studies until quite recently had been biomedical. Attention has since turned towards traits of commercial importance in farm animals. Herein we review the evidence that prenatal risk factors, including suboptimal parental nutrition, gestational stress, exposure to environmental chemicals and advanced breeding technologies, can determine traits such as postnatal growth, feed efficiency, milk yield, carcass composition, animal welfare and reproductive potential. We consider the role of epigenetic and cytoplasmic mechanisms of inheritance, and discuss implications for livestock production and future research endeavours. We conclude that although the concept is proven for several traits, issues relating to effect size, and hence commercial importance, remain. Studies have also invariably been conducted under controlled experimental conditions, frequently assessing single risk factors, thereby limiting their translational value for livestock production. We propose concerted international research efforts that consider multiple, concurrent stressors to better represent effects of contemporary animal production systems.
Additional keywords: behaviour, fertility, fetal programming, lactation, livestock, nutrition, stress.
Introduction
The concept that developmental processes in utero can predispose offspring to certain chronic diseases in later life, including cancer and various metabolic and cardiovascular diseases, arose following publication of the pioneering retrospective cohort studies on human subjects conducted by David Barker and colleagues at the University of Southampton. Initial studies correlated the incidence of infant mortality to deaths in adults attributable to bronchitis, stomach cancer and rheumatic heart disease (Barker and Osmond 1986). Later studies linked suboptimal in utero development, culminating in low birthweight, to hypertension in children (Barker and Osmond 1988); their findings on death by coronary heart disease in adult men were published in The Lancet the following year (Barker et al. 1989). These and related observations gave rise to what became known as ‘The Barker Hypothesis’, now more commonly referred to as the ‘Developmental Origins of Health and Disease’ or DOHaD. Since then, a plethora of studies has been conducted both in humans and in a variety of animal model species, including farm animals, that support these initial observations, and these have been the subject of extensive review and meta-analysis in recent years (e.g. McMillen and Robinson 2005; Gluckman et al. 2008; Fowler et al. 2012; Thayer et al. 2012; Langley-Evans 2013; Steegers-Theunissen et al. 2013).
There are several important issues to emerge from these studies that are considered in the present article, including: (1) the nature of environmental exposure (e.g. maternal stress, parental nutrition, environmental chemicals and assisted reproduction); (2) the stage of development at time of exposure (e.g. early vs late pregnancy and infancy); (3) developmental legacy (e.g. chronic diseases, cognitive abilities, growth, fertility and productivity); and (4) underpinning epigenetic mechanisms and transgenerational inheritance. Importantly, several studies have considered the longer-term consequences of early life events on physiological processes linked to animal production. There is evidence in farm animals that maternal nutrition, stress or illness during pregnancy can affect in utero development, with implications for later health and productivity. Similarly, there is evidence that offspring development from birth to the onset of puberty can have long-lasting effects for traits of economic importance. However, uncertainty regarding effect size remains and underlying epigenetic mechanisms are poorly understood compared with other species.
With the foregoing discussion in mind, this article extends beyond earlier reviews that considered specific aspects of ‘developmental programming’, such as intrauterine growth restriction (IUGR; Wu et al. 2006) and consequences for lifetime fecundity (Gardner et al. 2008), to provide a contemporary and comprehensive account of the extent to which in utero development can determine lifetime productivity and health in domestic species. It considers in detail how early life events, including parental nutrition, maternal stress and exposure to environmental chemicals, impinge on long-term growth, development, productivity and health of offspring. It begins with a contemporary account of our understanding of epigenetic mechanisms as they affect development and concludes with an assessment of the implications for current systems of livestock production.
Epigenetic mechanisms of embryonic and fetal programming
Cloning of Dolly the sheep demonstrated that virtually every cell within an adult mammal retains the entire genetic information (i.e. the whole genome) of the organism. It also made clear that not all the genetic code is used by the many different cell types of an animal. That is, a mammary cell and a neuron are ‘programmed’ to selectively retrieve different genetic information encoded within the genome. Such cell type-specific ‘programming’ is established during normal development and involves epigenetic mechanisms. A contemporary description of epigenetics states that it is ‘the study of mitotically and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence’ (Riggs et al. 1996). Epigenetic processes program gene expression patterns and thereby uphold cell identity without altering or mutating the DNA. Such states in gene activity can be inherited through many cell divisions. Cells in adult tissues have the capacity to carry memories of embryonic development (Hon et al. 2013) or even from past generations.
Two classic memory systems exist in mammals that are based on epigenetic programming of the genome: (1) DNA methylation; and (2) chromatin and histone modifications. In addition, non-coding RNAs have emerged as functional molecules that can initiate and guide epigenetic changes in both DNA and histones (for a review, see Sabin et al. 2013). A detailed description of each of these mechanisms is provided in the Supplementary Material available for this paper and illustrated in Fig. S1.
Recent advances in epigenetics
Although cytosine modifications in mammalian genomes are generally thought to occur in a DNA sequence context of cytosine–phosphorous–guanine (CpG) dinucleotides, there are notable exceptions. Prior to high-throughput sequencing, prevalent non-CpG methylation of cytosines had been detected in mouse embryonic stem cells at cytosine–phosphorous–adenine (CpA) and, to a lesser extent, at cytosine–phosphorous–thymine (CpT) sites (Ramsahoye et al. 2000). More recent data confirm and extend this finding, demonstrating that non-CpG methylation is also present during male germ cell development (Ichiyanagi et al. 2013), in oocytes (Shirane et al. 2013) and is enriched within gene bodies of highly transcribed genes in both fetal and adult mouse brain (Lister et al. 2013). Because mammals appear to lack enzymes that copy asymmetric non-CpG marks, it is currently not clear how this type of modification could contribute to the propagation of epigenetic states established as a result of fetal programming events.
The study of RNA methylation is also an emerging field related to ‘traditional’ epigenetics and may prove relevant for our mechanistic understanding of fetal programming. Two modifications on bases located internally of RNA molecules, N6-methyladenosine (m6A) and 5-methylcytosine (5-meC), are now considered to have important roles, albeit their specific biological functions are only starting to be determined. For example, m6A is a reversible base modification that can be removed by fat mass and obesity associated (FTO), an m6A-demethylase genetically associated with obesity and the control of energy homeostasis. How such RNA modifications may be able to contribute to heritable epigenetic phenotypes remains to be shown (for a review, see Liu and Jia 2014).
Resetting the epigenome during mammalian development
Two major epigenetic reprogramming events take place during early embryo development. The first event occurs right at the onset of development (Fig. S2). Soon after fertilisation, DNA from the spermatozoon and egg undergo an extensive chromatin remodelling in a process that begins by the formation of two pronuclei containing highly decondensed DNA. The open chromatin configuration resulting from the decondensation of sperm DNA facilitates the assembly of new nucleosomes in the male pronucleus and entails the replacement of protamines by histones. These newly incorporated histones acquire specific modifications during the first cleavage divisions. These modifications include marks indicative of transcriptional activation (e.g. histone H3K9 acetylation (ac) and H3K4 trimethylation (me3)) as well as other marks (e.g. H3K9 me3) enriched in transcriptionally inactive regions. This array of new histone marks establishes a chromatin landscape that will ensure the timely expression of developmental genes when the major zygotic genome activation takes place after several cell divisions. Similarly, the maternal genome undergoes remodelling of chromatin marks, but this follows different kinetics to that of the male pronucleus (Morgan et al. 2005). One of the best characterised epigenetic marks is DNA methylation. Sperm DNA, which is more methylated than oocyte DNA (Kobayashi et al. 2012; Smallwood and Kelsey 2012) undergoes active demethylation during the first cell cycle. This process is driven, in part, by ten-eleven translocation (TET) methylcytosine dioxygenase 3 (TeT3), which catalyses the conversion of methylated cytosines into hydroxymethyl cytosines (Gu et al. 2010; Iqbal et al. 2011) before the start of DNA replication (Wossidlo et al. 2010). Paternal DNA demethylation is an essential step in early development, because most TeT3 mutant embryos do not survive development to term (Gu et al. 2010). However, the maternal genome undergoes passive DNA demethylation by dilution during mitotic divisions and by the concurrent exclusion of de novo DNA methyltransferase (Dnmt) from the nucleus of early blastomeres (Carlson et al. 1992). However, the global DNA demethylation observed in the preimplantation embryo excludes certain regions of the genome. Indeed, imprinted genes are protected from this DNA demethylation activity, and recent evidence shows that the maternal and paternal imprints are protected by different mechanisms (Nakamura et al. 2012). The extensive demethylation between the zygote and the morula stage prepares the chromatin of the totipotent blastomeres for the segregation of the lineages that will contribute to the formation of the conceptus. Although the complexity of these changes is just beginning to be understood, it is well known that this process is indispensable for ensuring normal embryo development. This is demonstrated by experiments where mutation of histone-modifying enzymes or Dnmt in embryos leads to severe abnormalities or death (Li et al. 1992; Peters et al. 2001; Posfai et al. 2012). This indicates that remodelling during early stages of development is of critical importance for resetting the epigenome in preparation for establishment of new programs of differentiation during lineage commitment. Importantly, the kinetics described above for rodents have also been observed in embryos of different domestic animals, including cattle, suggesting that these mechanisms are conserved across mammals (Lepikhov et al. 2008; Maalouf et al. 2008).
The second major wave of epigenetic reprogramming takes place in the germline (Fig. S2). The embryonic precursors of mature gametes, or primordial germ cells (PGC), are first located at the base of the allantois from where they will initiate their migration to their final destination, the gonadal ridges. In large mammals this period extends between 2 and 8 weeks of development. It is here that environmental perturbations can have long-lasting effects on offspring. Indeed, during this period PGC undergo extensive reprogramming of their epigenome, characterised by dynamic changes in histone modifications (loss of H3K9 mono and dimethylation (me1/2) and gain of H3K27 me3 and H3K4 dimethylation (me2)), genome-wide DNA demethylation (including imprinted genes) and reactivation of the X chromosome in females (Saitou and Yamaji 2012). However, recent investigations show that some retrotransposable elements (such as intracisternal A-type particles or IAPs) escape reprogramming in germ cells (Popp et al. 2010), a mechanism that probably evolved to prevent parasitic sequences moving within the genome. Importantly, the resistance to reprogramming by these sequences can lead to phenotypic inheritance between generations (Morgan et al. 1999; Daxinger and Whitelaw 2012). This initial reprogramming resulting in the resetting of chromatin marks is followed by the differential re-establishment of imprints in male and female gametes. In males, paternal imprints are re-established in mitotically arrested gonocytes before birth. However, in females, imprints are re-established after birth during follicle growth. The mechanisms of germline reprogramming have been characterised primarily in rodents, but studies in large mammals (i.e. human and pig) show that the overall equivalent kinetics are similar, although some of the changes occur in a more protracted manner, consistent with slower development compared with rodents (Hyldig et al. 2011; Gkountela et al. 2013).
It is thought that this highly complex and extensive remodelling of the PGC epigenome is critical for preventing the inheritance of epimutations acquired by the parental DNA. Having a detailed understanding of the dynamic changes that occur during epigenetic reprogramming of the germline in large mammals will inform as to the periods of increased susceptibility of PGC to environmental effects and their potential effects in offspring.
Gender differences mediated by epigenetics
Differences in gene expression have been observed between male and female preimplantation embryos (Kobayashi et al. 2006; Bermejo-Alvarez et al. 2010; for a review, see Gardner et al. 2009). This type of sexual dimorphism appears to be a hormone-independent cell phenotype and affects both autosomal and X chromosome-linked genes. For example, one-third of transcribed protein-coding genes analysed (~2900 transcripts) show sex-specific differences in in vitro-generated bovine blastocysts (Bermejo-Alvarez et al. 2010). Paternal imprinting of the bovine X chromosome could partly explain the upregulated expression of X-linked genes in normal female blastocysts, because parthenogenetic embryos, which carry two maternal X chromosomes, were found to have lower transcript levels of representative X-encoded genes, such as BEX1, CAPN6, BEX2, SRPX2, and UBE2A (Bermejo-Alvarez et al. 2010). Moreover, the activity of the two X chromosomes in female blastocysts appears to affect the expression of autosomal genes, leading to gender-specific transcript differences (for a review, see Wijchers and Festenstein 2011). Female mouse embryonic stem cells with a deficiency of the DNMT3-like methyltransferase (DNMT3L–/–) lose genomic DNA methylation patterns more rapidly than their male DNMT3L–/– embryonic stem cell counterparts (Ooi et al. 2010). Altered nutrition during gametogenesis and preimplantation development, shown to modulate DNA methylation patterns (e.g. Sinclair et al. 2007), may augment sexual dimorphism of gene expression patterns and thereby contribute to more pronounced gender differences in adult animals.
Epigenetics and developmental programming
Given that the genetic code does not vary between cell types, it follows that epigenetic mechanisms evolved in multicellular organisms to allow cell-lineage specific gene expression (Jablonka 1994). How these mechanisms combine to facilitate cellular differentiation is incompletely characterised but, with the advent of contemporary deep-sequencing and related technologies, developmental epigenetics has become a highly active field of biology, so that our understanding of these processes is likely to improve rapidly in the very near future.
Much attention to date has focused on the role of tissue-specific differentially methylated regions of DNA, particularly those that reside within CpG islands (CGIs). These may be associated with annotated gene transcription start sites or lie within or between genes. Illingworth et al. (2008) demonstrated tissue-specific methylation in several CGIs associated with developmentally important genes, including homeobox (HOX) and paired box (PAX) family members in humans. More recently, these authors showed that DNA methylation was more likely to occur at CGIs within gene bodies during the early stages of lineage specification and to be associated with gene silencing (Deaton et al. 2011). Such regions may be potential targets for environmentally induced epigenetic regulation and, as such, form the mechanistic basis of programming of lifelong health, productivity and fertility in animals.
Inter(trans)generational inheritance
Epigenetic inheritance is frequently touted as the mechanism by which traits acquired in one generation are passed onto the next (for a review, see Grossniklaus et al. 2013; Aiken and Ozanne 2014). Jirtle and Skinner (2007) explained that for epigenetic modifications to chromatin to be considered a plausible mechanism for inheritance of phenotypic change, then effects need to persist to at least the F3 generation. The reason is that when an F0 gestating female is exposed to environmental stimuli, both the F1 embryo and F2 generation germline are also directly exposed. For this reason, neither parental nor, indeed, grandparental effects need have an epigenetic basis, although pup licking and grooming behaviour in rats can lead to epigenetic modifications at the glucocorticoid gene promoter in offspring (Weaver et al. 2004). It follows that much of our knowledge of transgenerational epigenetic inheritance in mammals pertains largely to inbred mice, where maternally or paternally induced transmission can come in the form of covalent modifications to DNA and histone methylation (e.g. Padmanabhan et al. 2013; Wei et al. 2014; Siklenka et al. 2015) and altered RNA expression (Wagner et al. 2008), often in a breeding scheme-dependent manner (Yuan et al. 2015). Not surprisingly, evidence for similar effects occurring in long-lived and out-bred farm animals is scarce, leading González-Recio et al. (2015) to question its importance in livestock production. However, some recent tantalising (i.e. not quite statistically significant) data in the pig indicate that F0 boars fed diets enriched in one-carbon metabolites (including methionine, choline, vitamin B12 and folate) sired F1 boars that, in turn, sired F2 pigs that produced leaner carcasses associated with global changes in gene expression and epigenetic modifications to at least one of these genes (Braunschweig et al. 2012). In addition, evidence of transgenerational transmission of attenuated stress reactivity in male offspring, due to early life stress, has been demonstrated, albeit in domestic chickens (Goerlich et al. 2012).
At this point in the discussion it is worth noting that prions represent a class of protein that can be inherited across generations independently of chromosomes. Pioneering studies with prion proteins (PrP) in lower eukaryotes (i.e. yeast and filamentous fungi) have revealed that they can act as ‘epigenetic’ elements and can account, at least in part, for non-Mendelian patterns of inheritance for several traits (Hofmann et al. 2013). Mammalian prions share many common features with their counterparts in yeast, but their function and patterns of inheritance are less well known. Nevertheless, they have been shown to regulate pluripotency in mouse embryonic cells and contribute to their differentiation into neural progenitor cells (Peralta et al. 2011; Miranda et al. 2013). Importantly, normal PrP (PrPC) are also present in bovine oocytes and pre-elongation embryos (Peralta et al. 2012), although again their function is poorly understood.
Understandably, prions have received bad press over the past two decades because of the involvement of a misfolded form of this protein (designated PrPSc) in bovine spongiform encephalopathy (BSE) and variant Creutzfeldt–Jakob disease (vCJD; Ironside 2012). Intergenerational inheritance (i.e. vertical transmission) of these aberrant variants that lead to scrapie in sheep can occur across the placenta from around mid-gestation in genetically susceptible dams and fetuses (Wrathall et al. 2008). However, the consensus formed from results of AI and embryo transfer experiments in livestock suggests that transmissible spongiform encephalopathies (SEs) are unlikely to be spread by semen or the prehatching embryo (i.e. less than Day 7). Nevertheless, the foregoing discussion highlights the importance of the inheritance of cytoplasmic factors (that include mitochondria) in addition to nuclear chromatin at the point of fertilisation. It also indicates that the maternal environment can influence fetal development in a manner that leads to the intergenerational transmission of phenotypes, and that this may be independent of direct epigenetic effects.
Hologenome concept and development
The hologenome concept in the context of developmental programming states that environmental factors, such as diet, can alter the microbiota in such a way as to not only benefit the holobiont (host plus all micro-organisms) in the short term but, through transmission to offspring, have long-lasting multigenerational effects (Rosenberg and Zilber-Rosenberg 2011). This line of thinking is comparatively new and, in mammals, largely untested. Under normal conditions the cooperation between the microbiota and host generally leads to improved fitness of the holobiont. For the host, this includes protection against infectious disease, development and function of innate and adaptive immune systems (particularly in the gut), vitamin synthesis (including B vitamins, such as cobalamin and folate) and protection against certain cancers and ‘metabolic syndrome’ (Kau et al. 2011). Indeed, Ross et al. (2013) used metagenomic data derived from high-throughput deep sequencing to predict inflammatory bowel disease status and body mass index in humans; they used the same approach as Ross et al. (2013) to predict enteric methane production in cattle.
In mammals, microbial symbionts are initially transmitted vertically to offspring through the birth canal (e.g. reported differences in human infant microbiota between vaginal and Caesarean deliveries), subsequently from milk (e.g. differences between breast- vs formula-fed infants) and from close physical maternal contact and the surrounding environment (Kaplan et al. 2011; Kau et al. 2011). In human medicine, current interests primarily concern developmental programming of the immune system, whereas in ruminants the current primary driver for research into microbiota–host interactions lies in methanogenesis and greenhouse gas emissions (Morgavi et al. 2010), where emerging evidence in sheep and goats indicates that the population of methanogens in the rumen may be acquired from a very young age (Gagen et al. 2012; Abecia et al. 2013). There is clearly considerable scope to extend these emerging ideas and data in all species to investigate long-term developmental programming in offspring, adaptive responses to changing environments and associated intergenerational inheritance.
Programming of body composition
To understand how nutritional and epigenetic factors during early development affect body composition, it is necessary to consider the origins and developmental trajectories of the cells that form muscle and adipose tissue. The processes that drive muscle formation, myogenesis, have been well studied in several systems, whereas adipogenesis, the formation of fat cells (adipocytes), is less well characterised.
Skeletal muscle
Myogenesis is the process whereby pluripotent embryonic cells become committed to the muscle cell lineage, proliferate and finally fuse together (i.e. differentiate) to form large multinuclear cells called myotubes (in in vitro studies) or muscle fibres (in in vivo studies). The processes and regulatory factors involved are very similar across mammals, birds and fish, but the timing when they occur can be quite different. There are numerous reviews (e.g. Brameld and Daniel 2008; Rehfeldt et al. 2011b) describing this process and the factors that regulate it, including cross-species comparisons (Rehfeldt et al. 2011a), and only a brief summary is provided here.
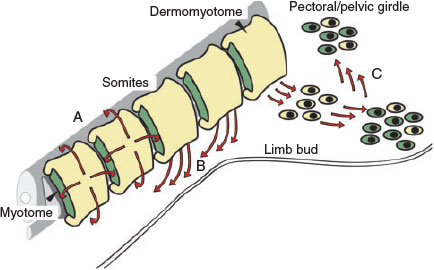
In vertebrates, muscles of the trunk and limbs are derived from somites (segmented embryonic structures) that also produce vertebrae, ribs, tendons and dermis. Within somites, muscle progenitor cells, known as myoblasts, are specified and begin to differentiate in the primary myotome, where they fuse to form multinucleated functional adult muscle fibres. Several genes are known to be required for this process. Specification and proliferation of myoblasts depends on the transcription factors Pax3 and Pax7, whereas myogenic regulatory factors (MRFs), a group of related muscle-specific transcription factors, are involved in both specification of myoblasts and the later stages of myogenic differentiation (Maltin et al. 2001; Buckingham 2007; Mok and Sweetman 2011; Sweetman 2012).
Within the embryo, different muscle groups follow distinct developmental routes (Buckingham and Vincent 2009). Trunk and back muscles are derived directly from the primary myotome, which extends into the regions where the adult muscles will be located and form the adult muscular pattern. Other muscles, such as the limb muscles, come from myoblasts that delaminate from somites and then migrate into the developing limbs (Fig. 1). This includes some muscles of the pelvic and pectoral girdles that develop from limb muscle cells that, having migrated into the limbs, then migrate out again to populate these regions. This is known as the ‘in–out mechanism’ (Evans et al. 2006; Valasek et al. 2011). Understanding the different origins and developmental processes that generate various muscle types will be important in designing interventions that target these muscles.
|
During fibre formation, myoblasts exit the cell cycle and fuse to form the multinuclear fibres containing all the contractile proteins and structures required for muscle function. Fibre formation occurs in two or three phases depending on species. The initial phase of differentiation and fusion of myoblasts generates the primary muscle fibres. These provide the scaffold on which the adult musculature is built and so are responsible for generating the mature muscle pattern. Following formation of primary fibres, further rounds of differentiation and fusion occur such that secondary muscle fibres form around each primary fibre, with tertiary fibres also forming between the secondary fibres in some larger mammals, including sheep (Wilson et al. 1992). The main difference between species is the time at which these phases of fibre formation take place, with fibre formation in most large mammals (including sheep, cattle and humans) completed by the middle of gestation; in pigs, formation completes towards late gestation, whereas in poultry and small mammals (e.g. rats) fibre formation continues for a limited period after hatching or birth (see Brameld and Daniel 2008; Rehfeldt et al. 2011a).
Hence, muscle fibre number in all large animal species is believed to be set around the time of birth and subsequent muscle growth is driven by increases in fibre size (i.e. hypertrophy) rather than number (Buttery et al. 2000). Because of this, it is critical to understand how changes during development can influence muscle fibre number, because changing the number of fibres may directly affect meat quality and yield. The central question underlying approaches to maximise muscle production is how to control the switch from the early, proliferative myoblast to the differentiated muscle fibre. Increasing myoblast number by increasing their proliferation rate or delaying their differentiation has the potential to increase muscle fibre numbers and therefore muscle mass in adult animals.
Another important consideration in muscle fibres is the distinction between fast and slow fibres. It is thought that primary fibres initially become slow fibres, whereas secondary and tertiary fibres initially become fast fibres. In adult muscle, fast fibres tend to be larger, especially if they are frequently used, but this size difference is not clear in younger animals. It appears that this initial relationship between primary–secondary and slow–fast fibres is lost as the animal develops, probably because the fibres are able to change type and the proportions of fast and slow fibres can affect muscle mass. Unlike rodents, where individual muscles are classified as fast or slow, in large mammals all muscles are mixed-fibre types and the relative proportions of the fibre types in each muscle can be altered during development or adult life.
Muscle fibre number
The number of muscle fibres that form in various animal species can be altered via genetic (e.g. double muscling in cattle) or environmental (e.g. maternal nutrition or administration of hormones) factors, but only if the environmental insults take place at specific times during gestation (see Brameld and Daniel 2008; Rehfeldt et al. 2011a, 2011b). Tables S4 and S5 summarise studies investigating the effects of maternal nutrition on muscle fibre formation in sheep and pigs. The effects of nutritional insults on the pregnant ewe or sow during the critical period of muscle development (early or early–mid gestation) can be detected in young offspring (late gestation fetuses or neonates), but these effects tend to be lost (or are too difficult to detect) in older sheep and pigs (with the exception being of runt pigs). The main effect observed in neonates or fetuses is a change in the number of secondary fibres formed and/or the ratio of secondary fibres to primary fibres (often determined as the fast : slow ratio). Because fibre formation is thought to be complete at birth, it may be predicted that the changes in fibre number would be permanent and therefore affect subsequent carcass quality, particularly lean muscle mass. However, this does not appear to be the case, but this may be due to the capacity for skeletal muscle to adapt during postnatal growth via changes in fibre type and metabolism or difficulty or issues with measurements in larger muscles from older animals. For example, we assume that all fibres extend the full length of the muscle and therefore measurements made across the middle are representative of the whole muscle, but we know that some fibres terminate in the middle of the muscle in several species, including cattle and pigs (Swatland and Cassens 1972), meaning that this assumption is, at best, tenuous. It is also worth mentioning that the few studies that have taken lambs to market weight or beyond have all provided good-quality diets during the postnatal growth period. It is not known whether the animals would still be able to compensate or adapt if they were on a relatively poor diet or were challenged in some other way. Hence, the permanency of the number of fibres after birth is unclear.
Muscle mass or size
Although there appear to be no long-term effects of maternal nutrition on muscle fibre number and/or diameter (in sheep or pigs), there are suggestions that prenatal environmental factors can affect certain measures of lean muscle mass and/or carcass composition. The effects on various measures of adiposity are the focus of the next section, but some studies have observed differences in muscle-related carcass measurements, for example, differences in muscle cross-sectional area (CSA) at slaughter. However, these effects tend to be largely dependent on the sex of the animals, with effects only seen in males or females at a particular stage of their growth (Micke et al. 2010a, 2011a). Therefore, it is unclear how permanent these effects are or what mechanisms are involved, although sex steroids (oestrogens and/or androgens) may play a role. Effects specific to maternal nutrition during pregnancy are difficult to detect because they may affect the postnatal nutritional status of the dam and nutrition of the offspring as well. However, studies performed on cattle, as part of the Australian Beef Cooperative Research Centre, used a factorial experimental design to separate pregnancy- and lactation-specific effects (see Robinson et al. 2013). Importantly, the findings suggest that the effects of maternal nutrition during pregnancy and lactation are additive and a lifetime approach should be taken rather than simply investigating the effects at a particular stage of development (Robinson et al. 2013).
Genetic regulation of muscle growth
Because muscle formation and growth is complex, involving numerous biological processes, not surprisingly there are many genes involved. One area that has been particularly interesting is the genetics relating to signalling molecules involved in myogenesis. Experiments in various model systems, such as mice and chickens, have uncovered a range of signals that can affect the rate of myoblast proliferation, differentiation and fibre type (Duprez 2002). However, the exact mechanisms that lead to muscle growth, even for very well-characterised signals with long-established roles, such as the insulin-like growth factor (IGF) family (Schiaffino and Mammucari 2011), remain to be established.
One of the most important examples of this type of molecule is myostatin, a secreted member of the transforming growth factor (TGF)-β family that negatively regulates muscle growth. Myostatin mutations have been identified in many animal lines selected for high muscle growth (Lee 2004), such as Belgian blue cattle (McPherron and Lee 1997), elite sheep (Tellam et al. 2012) and high-growth chickens (Bhattacharya and Chatterjee 2013). Loss-of-function mutations in myostatin increase both muscle fibre number and size. Interestingly, at least some of these effects take place in the developing fetus and are associated with increased rates of muscle cell proliferation and delayed differentiation (Gerrard and Grant 1994), producing increased muscle fibre number at birth. These are the same mechanisms as those proposed for the effects of maternal nutrition and environmental factors on muscle fibre number in the developing fetus (see above). In Texel sheep, a mutation has been identified in the 3′ untranslated region (UTR) of the myostatin (Mstn) mRNA that creates a binding site for the muscle-specific microRNA miR-1/206. This leads to muscle-specific downregulation of myostatin protein levels and increased muscle mass (Clop et al. 2006). A transgenic sheep line has recently been generated with artificial RNA interference (RNAi) that mimics this effect, and these sheep also show increased muscle mass (Hu et al. 2013a). However, there are also some breeds of cattle (Smith et al. 2000) and pigs (Jiang et al. 2002a, 2002b) with similar or other mutations in the Mstn gene that do not show the double-muscling phenotype, suggesting that other factors are also involved.
Another signalling pathway with direct relevance to animal production is seen in callipyge sheep. These sheep have a complex genotype with a mutation in an imprinted regulatory region that leads to increased expression of DLK1, part of the Notch/Delta signalling pathway. Overexpression of DLK1 in skeletal muscle increases muscle mass (Davis et al. 2004) and DLK1 is also upregulated in broiler chickens (Shin et al. 2009), suggesting that it may be a good target for intervention in various species. It appears that the callipyge phenotype relates primarily to changes in postnatal rather than prenatal muscle growth, but it does have a complex inheritance pattern (see Georges et al. 2003). However, callipyge sheep produce tough meat (Koohmaraie et al. 1995), thought to be due to decreased protein degradation pre- and postmortem because of increased levels of calpastatin, the endogenous inhibitor of the calpain proteolytic enzymes.
Epigenetic programming of muscle development
There appear to be no studies investigating whether the effects of environmental factors on muscle fibre formation are associated with epigenetic changes (e.g. DNA methylation patterns). The main hypothesis investigated in this area relates to genetic mutations or environmental insults altering the rates and/or timing of muscle cell proliferation and differentiation (see Brameld and Daniel 2008), so it is unclear whether epigenetic mechanisms are involved.
Although there are limited data from large animals, there have been many studies on epigenetic control of muscle development in cell culture and model animal species. Expression of myogenin is a key step in myogenic differentiation, and changes in DNA methylation patterns at this locus have been identified as differentiation proceeds and this gene is induced (Fuso et al. 2010; Palacios et al. 2010). DNA binding of MyoD and induction of muscle-determining genes is also regulated by epigenetic changes to binding sites in the promoters of its target genes (Fong et al. 2012). Dynamic changes in DNA modification regulate myogenic commitment and differentiation (Tsumagari et al. 2013), and global changes in DNA methylation patterns have been mapped in fast- and slow-growing strains of chicken, evidence for a direct epigenetic effect on muscle growth (Hu et al. 2013b). It is also apparent that long non-coding RNAs play an important role in regulating muscle growth, at least in part by controlling muscle-specific promoter activity (Mousavi et al. 2013). The interplay between muscle-specific gene transcription, epigenetic regulation and chromosomal dynamics is at the forefront of current research in myogenesis and is likely to have profound effects on production as these results are translated to farm animals.
Interim conclusions
The molecular mechanisms that drive muscle formation are reasonably well defined. It is clear that different muscles use specific variations from the standard muscle developmental program. Of particular interest is determining how maternal effects can affect this process in particular muscles, especially in terms of how proliferation versus differentiation is determined, how primary and secondary muscle fibre formation is altered by these cell fate decisions, how fibre type is regulated and whether these processes can be influenced to enhance production. The data would appear to suggest relatively small (if any) long-term effects of maternal nutrition on muscle fibre number or on the percentage of fibre types in sheep, but all studies have provided good-quality diets during postnatal growth. Whether the same would be true if lambs were subjected to poor(er)-quality diets or other challenges during postnatal growth is not known.
Evidence for programming of body fat
In contrast with muscles, the developmental processes leading to the formation of mature adipose tissue cells (adipocytes) are poorly understood; however, some recent work has given insights into this process (Billon et al. 2008; Berry et al. 2013). In general, adipocytes can be divided into two types: white and brown. Brown adipose tissue (BAT) adipocytes contain numerous small lipid droplets, have large numbers of mitochondria and provide the main mechanism for maintaining body temperature via heat production in cold-exposed rodents. White adipose tissue (WAT) adipocytes contain a single large fat droplet and are the classical fat cell type used for long-term storage of excess energy in the form of triacylglycerol (TAG). Although brown fat has been predominantly associated with young animals (e.g. newborn lambs), BAT deposits have also been identified in adult animals (Billon and Dani 2012). This division into WAT and BAT has been questioned recently, and animals raised in cold conditions also have extensive brown-like adipocytes in their WAT fat depots, with such cells now referred to as either BRITE or beige adipose cells (Wu et al. 2012) It is unclear whether these cells are white cells that have changed their phenotype or whether they are brown cells that form in the WAT from a separate stem cell population or a mixture of both (Liu et al. 2013).
BAT, but not WAT, is derived from early myoblasts (Seale et al. 2008), so shares an origin with muscle cells, but the source of WAT adipocyte precursor cells remains largely unknown. Some neck WAT depots are colonised by neural crest cells (Billon et al. 2007), migratory multipotent cells from the dorsal neural tube, but very little is known about the origins and signals that regulate WAT adipocyte formation. As a result, there are also fewer well-established molecular markers of specific developmental stages of adipocyte cell formation available to inform studies of adipose development. Indeed, those molecular markers that have been identified as transcriptional regulators of adipogenesis (e.g. CCAAT-enhancer-binding protein (C/EBP) α, C/EBPβ, peroxisome proliferator-activated receptor (PPAR) γ) are common to both BAT and WAT adipocytes. In all cases, these factors are involved in the differentiation of the proliferative precursor cells (pre-adipocytes) into terminally differentiated (non-proliferative) adipocytes. The main differences identified (primarily in rodent studies) are that BAT adipocytes tend to have higher expression of genes relating to mitochondrial biogenesis and oxidative metabolism (e.g. PPARγ coactivator-1α (PGC1α)), with the only BAT-specific protein being uncoupling protein (UCP)-1, the key mitochondrial protein involved in the heat-generating properties of BAT. Nonetheless, key genes that regulate adipose tissue development and function are reported to be active and sensitive to maternal undernutrition in the perirenal fat depot of mid-gestation sheep fetuses (Wallace et al. 2014b). Sex-specific differences in adipose gene expression emerge by late gestation and dominate in early postnatal life, reflecting the significantly greater adiposity in females versus males (Wallace et al. 2014b, 2015). Sex-specific differences in adipose gene expression in different fat depots have also been reported in adult cattle (Micke et al. 2011b) following early gestational maternal dietary perturbation.
Body fat or adiposity
Measures of adiposity tend to go in the opposite direction to measures of lean or muscle mass. For example, double muscling in Belgian blue cattle is associated with reduced body fat, as well as increased muscle mass and an increased number of muscle fibres. Several studies have investigated the effects of maternal nutrition on various measures of adiposity, including back fat thickness, individual adipose tissue depot weights, carcass and/or muscle lipid content and total body fat. Table S6 summarises studies published in sheep. The magnitude and direction of the effect observed is dependent on the age of the offspring and the timing of the nutritional insult during gestation. In relatively young (late fetal or early neonatal) offspring (up to ~77 days of gestation), adiposity tends to go in the direction you would expect, with reduced nutrition resulting in reduced adiposity and vice versa (Muhlhausler et al. 2006; Luther et al. 2007). After the neonatal period, and up to and including the period when lambs in the UK reach normal ‘market weight’ (i.e. up to 4 or 5 months), little to no additional programming effects are observed, although by this time females already consistently have higher adiposity measures than males. After this time (6 months plus), a few studies (Daniel et al. 2007; Ford et al. 2007; Sinclair et al. 2007; Jaquiery et al. 2012) have demonstrated that maternal food restriction, particularly during early gestation (conception to 80 days of gestation), increased measures of adiposity, particularly in male offspring. Furthermore, both over- and undernutrition for the last 6 weeks of gestation predisposed abdominal (but not subcutaneous) adiposity in lambs at 6 months of age, despite differential effects on birthweight and on postnatal glucose–insulin metabolism (Khanal et al. 2014, 2015). These sheep studies suggest that there may indeed be long-term ‘programming’ of adiposity, particularly in the normally leaner males, and that this is not necessarily associated with differences in birthweight. In contrast, there are several studies (Louey et al. 2005; De Blasio et al. 2007; Wallace et al. 2011b; Hancock et al. 2012) suggesting that low birthweight, often as a consequence of placental insufficiency, is associated with increased adiposity in both young and old offspring. Studies in runted pigs (IUGR) show not only fewer muscle fibres in later life compared with their high birthweight littermates, but also increased adipose tissue depot weights and reduced postnatal growth rates (see Brameld et al. 2003). Increased adiposity in runted pigs is thought to be due to increased numbers of small-diameter adipocytes in the various fat depots. Hence, undernutrition during pregnancy in pigs is associated with increased adiposity of progeny in later life, but the sensitive time for the effect is still unclear. There is also one cattle study (Long et al. 2010a) suggesting that overnutrition throughout gestation results in increased adiposity in older offspring (at 22 vs 19 months). Cattle studies by Micke et al. (2010a, 2011a, 2011b) show that the maternal diet during the first and second trimesters alters growth and carcass development, including fat deposition, from weaning through to slaughter at 22 months of age in a sex-specific manner. One of the main problems in trying to draw conclusions from these various studies is the variability in the timing of the nutritional insult and the age at which the offspring are studied.
Energy balance
Although there does appear to be some evidence of long-term programming of adiposity, the mechanisms for this are far from clear. Whether this apparent programming is via a direct effect on adipocytes and their development is not known. Unlike muscle fibres, there is no evidence to suggest that the number of adipocytes (or precursor cells) may be set at some stage of life. Indeed, it would be counterintuitive that this would occur, because the main function of WAT adipocytes is to store excess fatty acids from the blood because high levels of circulating free fatty acids are toxic. Therefore, the mechanism(s) for effects on body fat are more likely to involve long-term changes in energy balance, involving changes in whole-body energy expenditure (e.g. basal metabolic rate or BMR) and/or appetite regulation. The effects of environmental insults on appetite regulation in the offspring are the focus of the next section.
There are very few (if any) studies in this area that have directly measured energy expenditure, BMR or heat production. However one study (Daniel et al. 2007) showed increased adiposity of adult offspring in response to maternal undernutrition, with no significant changes in food intake, implying that a difference in energy expenditure may be involved. Interestingly, a very similar study (George et al. 2012) observed no effect of maternal undernutrition on whole-body fat or perirenal (PR) and omental (OM) depot weights in 6-year-old ewes, despite increased bodyweight, food intake and feed efficiency, suggesting that energy expenditure may be altered. Whether such changes in metabolism and/or energy expenditure relate to skeletal muscle (which accounts for a major proportion of BMR because of its mass), BAT (a highly metabolic, heat-generating tissue in rodents) and/or other tissues (e.g. the gut has a very rapid turnover) is not known. It is interesting to note that comparing high- and low-feed efficiency or residual feed intake (RFI) in sheep (Sharifabadi et al. 2012) indicates reduced mitochondrial respiration in muscles from the more efficient animals. This appears to involve reduced BMR and is associated with genes encoding mitochondrial proteins, suggesting that oxidative metabolism and/or efficiency of ATP synthesis may be key. Once again, the important tissues would seem to be skeletal muscle and BAT, but more work is needed to investigate this further.
Epigenetic programming of body fat
As in the programming of skeletal muscle, there are few studies that have investigated a role for epigenetics in programming adiposity in sheep or cattle. Male rat offspring of overfed, obese mothers upregulate lipogenic pathways and adipogenic regulators in WAT, with associated changes in DNA methylation at key sites (Borengasser et al. 2013). It is also known that adipocyte differentiation is regulated by the transcription factors C/EBP and PPARγ and that the recruitment and activity of these molecules to chromatin requires epigenetic changes to histones and DNA methylation patterns (Cristancho and Lazar 2011). This is strongly suggestive of altered adipocyte commitment and differentiation via epigenetic mechanisms and deserves further study in livestock species, particularly in mapping changes during normal adipocyte formation and their functional significance in executing the adipocyte transcriptional program.
Interim conclusions
As with muscle development, the central question is how adipose tissue development is affected by in utero influences. However, because adipogenesis is not as well understood as myogenesis, there is still a need to clarify the underlying biology to identify markers and cellular processes to study this system in vivo. We know that different depots grow at different stages of development, with differences observed between different species. For example, in sheep the PR depot grows fairly early, being present in young neonatal lambs, whereas the subcutaneous depot only really grows much later in adult sheep, but the opposite is observed in pigs. Because the factors regulating adipogenesis, lipogenesis and lipolysis appear to be the same in all adipose tissue depots, we still have no real understanding of how this differential in fat depot growth or development is regulated, or whether it may be altered by prenatal environment or nutrition.
Evidence for programming of appetite regulation
The central nervous system, in particular the hypothalamic region of the brain, plays a pivotal role in the control of voluntary food intake and appetite drive in mammals. The activity of these neural pathways is modulated by factors circulating in the bloodstream that provide information on the body’s nutritional status. Under normal conditions, adequate nutritional intake is thereby achieved for basal metabolic requirements, growth, reproduction and appropriate deposition of energy stores as fat. Because these neural and feedback pathways develop in early life, it is pertinent to examine the extent to which the adult phenotype may be altered or programmed by early life challenges.
The mature hypothalamic arcuate nucleus produces both appetite-stimulating (orexigenic) neuropeptides, primarily neuropeptide Y (NPY) and agouti-related peptide (AGRP), and appetite-suppressing (anorexigenic) neuropeptides, primarily pro-opiomelanocortin (POMC) gene product and cocaine- and amphetamine-regulated transcript (CART). The output of these neuropeptides is able to respond appropriately to a range of peripheral nutrient and hormonal metabolic signals, most notably the adipose-derived hormone leptin, and neuronal projections from the arcuate nucleus to other hypothalamic regions, such as the paraventricular nucleus (PVN), are important in mediating their effects (Schwartz et al. 2000). Central regulation of appetite has been largely studied in the context of human obesity (e.g. Dhillo 2007) rather than livestock.
Hypothalamus
In livestock, these neural pathways develop early in fetal life, with the hypothalamus being morphologically distinct by the end of the first third of gestation. Gene expression for the primary appetite-regulating hypothalamic neuropeptides is seen in the fetal sheep arcuate nucleus from early (Day 50; C. L. Adam, P. A. Williams and J. M. Wallace, unpubl. obs.) to mid-gestation (Day 81; Adam et al. 2008) onwards (Days 110–130; Mühlhäusler et al. 2005; term = 145 days), and evidence is emerging that expression levels may be affected by changes in the prenatal nutritional environment. The postnatal persistence of such changes in gene expression may contribute to the programming of an altered adult appetitive phenotype, and this hypothesis forms the basis for many investigations into the fetal origins of human obesity (Muhlhausler and Ong 2011). However, most such investigations use laboratory rodents in which the hypothalamus is relatively immature at birth and the extrapolation of findings to larger mammals needs to recognise the temporal differences in development between altricial (rodent) and precocious (livestock) species. Therefore, the present overview focuses on findings from sheep.
The fetus relies passively on transplacental transfer of nutrients (primarily glucose) from the maternal circulation for its nutrition, and fetal nutritional status can affect the developing hypothalamic appetite-regulating circuitry (Table S7). Hypothalamic NPY and AGRP (orexigenic) are increased in late-gestation sheep fetuses of undernourished mothers (Warnes et al. 1998; Adam et al. 2015) and anorexigenic CART gene expression is decreased in late-gestation IUGR sheep fetuses in overnourished adolescent mothers (Adam et al. 2011b). Conversely, late-gestation intrafetal glucose infusion increased anorexigenic POMC gene expression (Mühlhäusler et al. 2005). In mid-gestation, POMC gene expression was positively correlated with fetal glycaemia (Adam et al. 2008), but maternal overnutrition or obesity had no effect on hypothalamic levels of orexigenic or anorexigenic neuropeptides in ovine fetuses (Breton et al. 2011).
The foregoing suggests that relative expression levels of appetite-regulatory hypothalamic neuropeptides are sensitive in sheep to prenatal nutrition, but the key question is whether these changes persist to affect their appetite-regulatory actions postnatally (Table S6). Maternal overnutrition in late gestation resulted in increased POMC gene expression in the arcuate nucleus of lambs at Postnatal Day (PND) 30 (Muhlhausler et al. 2006), whereas maternal food restriction in early gestation decreased hypothalamic NPY expression at PND7 (Sebert et al. 2009). However, no effects on hypothalamic gene expression levels were seen in obese 1-year-old offspring following early gestation maternal food restriction (Sebert et al. 2009) or in 11-week-old low-birthweight lambs following IUGR (Adam et al. 2013). Importantly, however, this latter study highlighted a major effect of gender, with orexigenic genes predominating in males and anorexigenic genes predominating in females, linked closely to the sex differences in body composition (adiposity) and consequent metabolic hormone status (leptinaemia; Adam et al. 2013; Wallace et al. 2014a).
Metabolic hormones that regulate the hypothalamic appetite circuits in adults also control their development (Bouret 2013). Notably, leptin determines patterns of neurogenesis, axon growth and synaptic plasticity in the developing hypothalamus, especially during a discrete developmental period soon after birth in rodents (Bouret and Simerly 2007). It is not known exactly when this developmental period occurs in more precocious larger mammals, like sheep and cattle, but it is likely to be prenatal given the greater maturity of the hypothalamus at birth (Grayson et al. 2010). Leptin secretion is initiated in the later stages of gestation in sheep and cattle following significant adipose tissue deposition, and therefore fetal nutrition and growth will be critical in this regard. Late-gestation sheep fetuses with increased adiposity had both increased leptinaemia and increased hypothalamic expression of the leptin receptor (Adam et al. 2011a), and indeed the adipose–hypothalamic axis is thought to be critical to the developmental programming of hypothalamic feeding circuits (Horvath and Bruning 2006; Breton et al. 2011). Thus, leptin plays an important neurotrophic role in early life and elevated circulating leptin in lambs soon after birth does not appear to be anorexigenic (De Blasio et al. 2010) However, plasma leptin is regulated predominantly by nutrition in preruminant lambs (Ehrhardt et al. 2003), and by 5–6 months of age adult-like anorexigenic actions of leptin are seen in sheep given leptin administered into the hypothalamus, regardless of birthweight or gender (Adam et al. 2011b).
Epigenetic programming of appetite regulation
There are very limited published data on epigenetic changes in central appetite-regulating pathways in farm animals. Periconceptional undernutrition led to hypomethylation of the POMC promoter, although it did not change POMC or NPY gene expression, in the late-gestation fetal sheep hypothalamus (Stevens et al. 2010); this was further exacerbated by twinning and the consequent additional nutritional challenge of placental restriction (Begum et al. 2012). Because the rodent hypothalamic POMC promoter region is a key target of epigenetic changes following perinatal nutritional manipulations (Coupe et al. 2010), this clearly warrants further investigation in livestock species.
Food intake and appetite
Studies of appetite (voluntary food intake) in offspring from nutritionally perturbed ovine pregnancies have produced variable results depending on the age at study, postnatal management and nature of the perturbation (Table S7). Following late-gestation maternal overnutrition, lambs had increased appetite for the first 3 weeks but not at 4 weeks of age (Muhlhausler et al. 2006), whereas IUGR lambs from pregnancies characterised by placental insufficiency (carunclectomy) also had increased feeding activity at 2 weeks of age (De Blasio et al. 2007). Lamb birthweight was unaltered in the foregoing studies, whereas there was no effect on suckling activity in 3-week-old IUGR lambs with low birthweight from overnourished adolescent placentally insufficient pregnancies (Adam et al. 2013). Others have reported no effect of low birthweight on food intake in the first 5 weeks of life (Vilette and Theriez 1981, 1983). However, low-birthweight lambs consume more food to achieve a given liveweight because it takes them longer to achieve it; thus, low birthweight lambs consumed 13% or 20% more than normal birthweight lambs when artificially reared rapidly or slowly respectively to 20 kg (Greenwood et al. 1998). Nonetheless, Vilette and Theriez (1981) reported that food intake from weaning to 35 kg was not related to birthweight, Sibbald and Davidson (1998) found that food intake from weaning to 2 years of age was not affected by moderate maternal nutrient restriction in late gestation and consequent low birthweight and Daniel et al. (2007) describe no effect on food intake by lambs up to 17 and 24 weeks of age after severe maternal food restriction in early gestation. In longer-term studies, following early gestation maternal food restriction, Sebert et al. (2009) reported no effect on appetite in obese 1-year-old offspring, but George et al. (2012) reported increased appetite drive in obese female 6-year-old offspring. Conversely, Long et al. (2010a) reported increased appetite at 19 months of age in the offspring of overnourished obese mothers.
Data from cattle vary. Micke et al. (2015) reported that protein intake in the first and second trimesters has sexually dimorphic effects on progeny appetite and postnatal growth pathways and that this is associated with altered circulating thyroid hormone and leptin concentrations in the progeny. In contrast, no differences in food intake (at 26–30 months of age) were attributed to wide differences in prenatal growth and birthweight (Greenwood and Cafe 2007). Low-birthweight cattle at a given age eat less than normal birthweight counterparts, but not when intakes are adjusted for current bodyweight; similarly, twin cattle tend to eat less food than singletons at a given age by virtue of their smaller size (de Rose and Wilton 1991).
Interim conclusions
Nutritional challenges in utero alter the developing hypothalamic appetite-regulatory circuits in fetal cattle and sheep, but there is a lack of evidence for the persistence and functional significance of such changes for food intake in current animal production systems. However, emerging data on sensitivity to epigenetic changes by the promoter of the anorexigenic POMC gene could be of lasting significance for appetite drive and deserves further study in livestock species.
Mammary gland development and lactation
Knight and Sorensen (2001) provided a comprehensive, critical and insightful review of how mammary gland development can be influenced by events that occur during both fetal and neonatal periods, as well as during the much better characterised peripubertal period. The earlier two periods of development determine mammary ductal outgrowth and subsequent proliferative activity. It follows that these fetal and neonatal periods could be sensitive to maternal nutrition; they are certainly sensitive to the prevailing endocrine milieu, in particular to circulating levels of testosterone and oestrogens. For example, in litter-bearing species, such as the pig, the number of teats is inversely related to the male : female ratio (e.g. Drickamer et al. 1999). However, it is widely recognised that most mammary development occurs in the dam during pregnancy. Although such responses fall out with the scope of this article, it is of note that in monotocous species, such as the cow, the fetus can influence maternal mammary development during pregnancy (e.g. fetal sex determines subsequent lactational performance of the gestating dam; Hinde et al. 2014).
In utero development and subsequent milk yields
Few studies considered the effects of in utero environment on daughter lactational performance before the review of Knight and Sorensen (2001), which led these authors to focus more on mouse models of mammogenesis, environmental oestrogens and breast cancer. Indeed, much of what we currently know concerning the embryonic origins of mammary gland development pertains to the mouse, where the process appears to be fairly autonomous (for a detailed contemporary review, see Macias and Hink 2012). However, several studies have emerged that have assessed the effects of in utero development on daughter milk yields in dairy cows. Some also considered effects beyond those directly associated with mammogenesis to include ‘programmed’ metabolic regulation of nutrient partitioning towards the mammary gland during subsequent daughter lactations (Bach 2012). Some report minor negative effects of maternal milk yield immediately before and during pregnancy on daughter milk yields (Banos et al. 2007; Berry et al. 2008). However, the small effect size reported probably explains why other ‘lesser-powered’ studies failed to observe such effects (Bach 2012). Similarly, more recent data from González-Recio et al. (2012) reported a small but statistically significant effect of maternal lactation during pregnancy on daughter milk yields (~50 kg reduction over 305 days). Perhaps unsurprisingly the negative effects of maternal lactation on daughter performance in that study were greatest for high-yielding and older (third vs second vs first parity) cows. Nevertheless, the practical significance of these reductions in daughter performance (of between 0.6% and 0.9% lactational yields) for contemporary high-yielding dairy cows is questionable.
Similarly in sheep, Paten et al. (2013) failed to convincingly demonstrate that maternal diet during either early or late pregnancy had any significant effect on daughter milk yields. The same group had previously demonstrated very modest negative effects of overfeeding (i.e. ad libitum feeding vs maintenance) during pregnancy on daughter milk yields (van der Linden et al. 2009), although in other studies by this laboratory effects of pregnancy nutrition on fetal mammary gland development varied between studies (Blair et al. 2010). It is not easy in these types of study to separate effects of feeding level and nutritional status during pregnancy from those during subsequent lactation, because there are inevitably carryover effects of ewe body condition that will determine her subsequent lactational performance and daughter growth rates. Furthermore, suckling animals are seldom ever able to express their full genetic potential for milk production because yields are dictated by the appetite of their young. Consequently, residual capacity can compensate for varying nutrient supply and offspring demands during lactation. Interestingly, however, the birthweights of grandoffspring were greater for the maintenance than ad libitum-fed groups in the study of Blair et al. (2010). Because lamb size and growth rate will also affect milk yield in suckled dams, it follows that the modest ‘programming’ effect reported may not have been on the mammary gland per se, but rather on the reproductive tract of gestating females.
As for cattle and sheep, mammary gland development in the pig is initiated during the early stages of fetal development (from around Day 23 of gestation), and involves intricate signalling between the epithelial cells of the mammary buds, which go on to form milk lines, and the surrounding mesenchymal cells; molecular details for this species are only just coming to light (Chomwisarutkun et al. 2012). However, other than the aforementioned effect of fetal sex ratio on teat number in gilts, the authors are unaware of any studies that have investigated in utero programming of mammary gland development in this species.
Prepubertal development and subsequent milk yields
There is compelling evidence in cattle that, in contrast with the in utero period, season (i.e. photoperiod), plane of nutrition and growth rate during the prepubertal period can each impinge on the development of the mammary gland in a manner that will determine milk yields during subsequent lactations (Robinson et al. 1999; Dahl et al. 2012). The curvilinear nature of the relationship between weight gain during the rearing period and mammary gland development is such that reductions of around 10% in milk yields have been reported, particularly in first-lactation dairy heifers that were either under- or overfed during the peripubertal period. Although our understanding of the endocrinology of compensatory growth is incomplete, the establishment of so-called ‘stair-step’ patterns of feeding for dairy heifers (Park et al. 1987) arose from our knowledge of the timing and extent of this phenomenon during prepubertal development. This pattern of feeding subjects heifers to short (3- to 4-month) periods of restricted growth interspersed with short (2-month) periods of compensatory growth to allow relatively high mean prepubertal growth rates to be achieved with no detrimental effects on mammary gland development or fertility.
Attempts to replicate these findings in pigs have met with mixed results. There is evidence of both positive (Crenshaw et al. 1989) and negative (Farmer et al. 2012a) effects of ‘stair-step’ feeding on mammary gland development, but with little effect on piglet performance (Farmer et al. 2012b). It is difficult to reconcile these mixed results in pigs with the more clear-cut findings in cattle, other than to observe that in the studies cited above there was no evidence of compensatory growth in restricted–overfed gilts, which, at the end of the experimental periods, were lighter than matched control-fed gilts.
Positive allometric mammary gland growth occurs between 1 and 5 months of age in ewe lambs associated with rapid parenchymal growth before the onset of seasonally induced puberty. In this regard, sheep differ somewhat from the other major farm animal species (including goats) in that ovariectomy of prepubertal ewe lambs has no effect on mammary gland development during this period (Wallace 1953), although exogenous oestrogen (i.e. 0.1 mg kg–1 day–1 17β-oestradiol for 7 days) enhanced epithelial cell proliferation (Ellis et al. 1998). However, as for cattle, there is evidence in sheep that rapid weight gain before puberty can impair mammary gland development and subsequent milk production in ewe lambs (McCann et al. 1989).
Programming of fertility
Successful reproduction and fertility are central to the financial success of livestock enterprises and have their origins in fetal life. Crucially, in females, the resting reserve of primordial follicles that determines lifetime supply of potentially fertilisable oocytes (eggs) is established before birth and cannot be replenished thereafter (Erickson 1966a, 1966b; McNatty et al. 1995). In contrast, males continuously produce new spermatozoa after puberty, but the number of Sertoli cells, which are the primary determinant of sperm production and testes size in adulthood, is determined by proliferation during the fetal, neonatal and peripubertal periods (Sharpe et al. 2003). Thus, the developing reproductive axis and its hormonal control systems are potentially susceptible to the range of environmental programming stimuli detailed above.
Female offspring: sheep
Table S8 details the effects of early life nutrition on the developing reproductive axis and on adult fertility in sheep. In sheep (gestation length ~145 days) the overwhelming evidence relates to maternal undernutrition in adult ewes, typically 0.5- to 0.7-fold maintenance, compared with controls nourished to meet the needs for fetal growth. Exposures during pregnancy are either limited to the known key periods of gonadal development or span the entire gestation. Where endpoints were assessed in fetal life, the consensus is one of delayed germ cell degeneration or delayed ovarian follicular development, as measured by elaboration of the granulosa cell layer (Borwick et al. 1997; Rae et al. 2001). Altered proliferation and apoptosis within the developing ovary may be the root cause (Lea et al. 2006; Grazul-Bilska et al. 2009). In all cases, the effects of maternal undernutrition were independent of fetal body growth. In contrast, compelling reductions in primordial follicle number (80% less) were evident in fetuses destined to be growth restricted at birth (Da Silva et al. 2002, 2003). Although the adolescent dams were overnourished (approximately twofold maintenance), in this paradigm competition for nutrients between the growing mother and conceptus results in restricted placental development and a major reduction in uteroplacental blood flow–fetal nutrient supply from mid-gestation onwards (Wallace 2011). Accordingly, by late gestation, fetal ovarian follicle population size was positively correlated with placental and fetal weight.
None of these aforementioned prenatal nutritional manipulations affects the onset of puberty in spring-born females, at least when fed ad libitum after birth to ensure they exceed the critical weight required to respond to photoperiodic cues at their first breeding season (Da Silva et al. 2001; Kotsampasi et al. 2009). Similarly, there is little evidence of a robust effect of prenatal nutrition on the postnatal function of the hypothalamic–pituitary–axis in that baseline and gonadotrophin-releasing hormone (GnRH)-stimulated gonadotrophin secretion are largely unperturbed at puberty and in adulthood (Borwick et al. 2003; Kotsampasi et al. 2009). This suggests that the prenatal nutritional programming of lifetime fertility in females primarily has its origins within the ovary and/or uterus. At its simplest, this could be manifest as a reduction in: (1) ovulation rate (OR), directly reflecting a diminished ovarian follicle reserve; (2) embryo survival, variously reflecting poor oocyte quality, fertilisation failure or inability to progress beyond the maternal recognition of pregnancy stage; and/or (3) reduction in litter size due to failure to implant or limited uterine capacity. There is supporting evidence to substantiate these possibilities, but more than one factor is likely involved. Maternal undernutrition from conception to Day 95 of gestation was associated with a modest (20%) reduction in the natural OR of female offspring expressed at a single time point within their second breeding season (Rae et al. 2002a). Ovulation rate clearly sets the upper limit of eggs shed by the ovary in any cycle, but whether this effect on OR was sustained throughout the life course or translated into a reduction in litter size is unknown. In contrast, when maternal undernutrition was limited to the first 35 days of gestation, there was no effect on the naturally occurring OR of the female offspring measured on seven occasions during the first two breeding seasons or following mild ovarian stimulation with exogenous hormones on one occasion during the second (Parr et al. 1986). Similarly, the OR of female offspring at the end of a 3-year breeding life (corrected for premating adiposity) was not affected by maternal nutrient supplementation throughout the second two-thirds of pregnancy (Gunn et al. 1995). Effects on OR may not limit litter size until late in a female’s reproductive life when her ovarian reserve becomes exhausted. Theoretically, the ovarian follicle population may become limiting earlier in pedigree females repeatedly superovulated as part of genetic improvement programs, but this has not been tested.
On sheep farms, fertility is largely recorded as pregnancy rate and litter size. Again, the available evidence is contradictory. When assessed on a single occasion following a synchronised mating in the first season, pregnancy rate and litter size of female offspring were not affected by diverse nutritional exposures (under vs overnutrition) during early or mid-pregnancy (Munoz et al. 2009). In contrast, a major reduction in pregnancy rate following hand mating and a 45-day breeding period were reported in a small study of 2-year-old females previously exposed to maternal undernutrition between 28 and 78 days gestation (Long et al. 2010b). A more robust assessment of the effect of early life nutrition on fertility is provided when large numbers of females are studied repeatedly. Accordingly, Gunn et al. (1995) studied the effect of supplementing maternal nutrition during the last 100 days of pregnancy or during the first 100 days of lactation on female offspring fertility over three lambing seasons. Relative to the unsupplemented controls, both periods of supplementation were associated with a higher lifetime incidence of multiple births, with lactation > pregnancy due to reduced barrenness and ewe mortality. Similarly, there were fewer lambs born to female offspring over a period spanning up to eight pregnancies, when stocking density was high (low available nutrition) from conception to weaning, but only when stocking density was also high in adult life (Langlands et al. 1984). This suggests that negative effects of prenatal undernutrition may not be revealed unless nutrient availability at breeding is also marginal. Within genotypes, birthweight is a useful indicator of in utero fetal nutrient supply that can be readily measured on-farm. Intriguingly, data from performance-recorded Suffolk flocks reveal that females born at both birthweight extremes (2 s.d. above or below the mean birthweight) had lower litter size during a median of three further pregnancies (Gardner et al. 2009). This effect was independent of offspring growth rate from birth to 8 weeks of age, but growth rates in these pedigree females were high across the board, reflecting intensive nutritional inputs common in such flocks. In contrast, a lower lifetime incidence of multiple births has been reported for female offspring who, with their mothers, were exposed to poor pasture from birth to weaning to restrict offspring growth (Rhind et al. 1998). These lifetime studies were low intensity and not designed to investigate mechanisms, but reductions in litter size are likely to involve, in part, increased embryo or fetal mortality. In the absence of experimental assessments of litter size in relation to ovulation and fertilisation rates, embryo quality and pregnancy rates within a single study, it is pertinent that maternal undernutrition during mid-pregnancy increased markers of DNA damage in fetal oogonia (Murdoch et al. 2003), whereas blastocyst production in vitro from prepubertal ewe lambs was highest when they had been exposed to high rather than low maternal nutrition during mid–late pregnancy (Kelly et al. 2005). Conceptus survival is also dependent on appropriate uterine development and capacity (Vallet et al. 2013). Although under normal circumstances it is assumed that the capacity of the uterus and placentas of ruminants to provide fetal support exceeds the number of fetuses present, it is intriguing to note a modest but significant reduction in the number of uterine caruncles, and hence potential placentomes, in low birthweight female lambs (Aitken et al. 2003).
Female offspring: cattle
The effect of early life nutrition on aspects of reproduction in beef and dairy cattle are summarised in Table S9. In cattle (gestation length ~285 days), data relating prenatal nutritional exposure to altered fetal ovarian development are scarce. Nevertheless, it is well established that the number of antral follicles present at all stages after birth is a direct reflection of the ovarian follicle reserve established prenatally (Evans et al. 2012). Thus, birthweight (as a proxy for fetal nutrient supply) was positively associated with the antral follicle count (AFC) in a large population of neonatal beef calves that died due to dystocia, and in adult heifers examined by ultrasound (Cushman et al. 2009). Reduced ovarian weight and large follicle diameter at 30 months is reported following slow prenatal growth rates (Wilkins et al. 2006), whereas a general reduction in all follicle types was evident following exposure to a low- then high-protein diet during the first two-thirds of gestation (Sullivan et al. 2009). A direct effect of maternal undernutrition during the first third of gestation on the ovarian follicle reserve has been documented using serial ultrasound (five occasions) during prepubertal and adult life (Mossa et al. 2013). The robust decrease in follicle number reported is associated with increased maternal testosterone during dietary restriction and was independent of calf birthweight and postnatal growth. Further, in vitro data suggest that ovarian steroids are potent negative regulators of follicle formation and, as such, environmental factors that alter steroid production in the dams and/or fetus may affect the size of the ovarian follicle reserve (Fortune et al. 2013).
Similar to sheep, prenatal maternal nutrition did not affect the onset of puberty in beef cattle (Martin et al. 2007; Sullivan et al. 2009; Mossa et al. 2013), but higher offspring pregnancy rates were observed following protein supplementation in late pregnancy (Martin et al. 2007) and when the AFC before breeding was high (Cushman et al. 2009). In contrast, manipulation of the post-weaning diet in beef heifers did not affect AFC or overall pregnancy rate (Eborn et al. 2013). In cattle, there is a lack of data linking prenatal nutrition and offspring reproductive performance. Although dairy cows with a high AFC are threefold more likely to become pregnant by the end of the breeding period (Mossa et al. 2012), there are no data directly linking birthweight and AFC in dairy breeds. Moreover, size at birth did not affect fertility in the first lactation and low birthweight was, in part, protective against abnormal ovarian cycles in the second service period (Swali and Wathes 2006). In contrast, growth data obtained from 17 UK dairy farms suggest that low postnatal growth rates increase age at first breeding and calving (Brickell et al. 2009).
Male offspring: sheep and cattle
Relative to females, there is a dearth of information on the effect of early life nutrition on male offspring fertility (Tables S8, S9). The single bovine study shows FSH levels and testis volume were increased in the bull calf by first-trimester protein restriction (Sullivan et al. 2010). In sheep, when endpoints were assessed in mid-pregnancy, the consensus is that neither maternal undernutrition nor reduced fetal nutrient supply affect Sertoli cell number, the number of seminiferous cords or basal pituitary gonadotrophin secretion (Rae et al. 2002b; Da Silva et al. 2003; Andrade et al. 2013). However, in the newborn lamb, the number of seminiferous cords and Sertoli cells was reduced following maternal undernutrition from mid-pregnancy onwards and was associated with a modest reduction in birthweight (12% reduction) relative to the adequately nourished control group (Bielli et al. 2002). In addition, male lambs that were severely growth restricted in utero (47% reduction) as a result of overnourishing their adolescent dams exhibited slower absolute postnatal growth rates, delayed age at puberty, lower testosterone concentrations and reduced testicular volume per unit liveweight between 28 and 35 weeks of age (Da Silva et al. 2001). Because Sertoli cells set the ceiling for sperm production, and continue to proliferate until puberty, it is likely that poor prenatal growth followed by a delayed attainment of the pubertal liveweight threshold could well impact ram libido and sperm production and quality, particularly if rams are used in their first breeding season. However, this has not been tested directly. By contrast, in studies where maternal nutrition is restricted during the first two-thirds of gestation and lamb birthweight and postnatal growth are unaffected, there is no evidence of a long-term effect on the onset of puberty (Kotsampasi et al. 2009) or on indices of semen quality in adult life (Rae et al. 2002a).
Male and female offspring: pigs
For the pig industry, studies assessing the effect of varying maternal nutrition have traditionally focussed on the dam’s OR, embryo survival, litter size and rebreeding interval, with scant regard for possible effects on the fertility of the offspring themselves. Indeed, because the former important indices of a dam’s productivity are remarkably independent of macronutrient intake during pregnancy (Anderson et al. 1979; Quesnel et al. 2010), it is unlikely that the developing reproductive axis of the fetus or neonate would be significantly perturbed, but this has not been evaluated. However, as a litter bearing-species, there is potential for variation in intrauterine nutrition and the gonadal phenotype of IUGR or ‘runt’ piglets at birth suggests the potential at least for impaired fertility in the longer term if selected for breeding. Accordingly, comparison of growth-restricted versus normally grown piglets from the same litter revealed delayed follicular development in the former group when examined within 24 h of birth (Da Silva-Buttkus et al. 2003). IUGR can also be a litter characteristic, typically if driven by high ORs and good to moderate early embryonic survival in higher-parity sows (Vonnahme et al. 2002). The resulting low litter average birthweight affects absolute numbers of germ, Sertoli and Leydig cells in males at 5 days of age, and this could have implications for stud boar performance (Smit et al. 2013).
Effect of environmental chemicals
Environmental chemicals, including endocrine-disrupting compounds (EDCs) can adversely program components of the reproductive axis (brain–pituitary–gonad–uterus), altering important aspects of offspring fertility and causing financial loss to livestock producers (Rhind et al. 2003; Rhind 2005). These chemicals largely come from industrial processes, domestic effluents and agricultural practices. Much of the data relating to the reproductive actions of EDCs are derived from epidemiological studies of wildlife species and from rodent studies involving supra-environmental exposures. Care must be taken in interpreting the results of these studies and to ensure that an environmentally relevant dose of a compound(s) is administered. There is a lack of information in farm animal species: most current knowledge comes from sheep and involves small numbers of subjects, with even less information for cattle and pigs. Detailing the effect of EDCs is challenging because they do not necessarily comprise one chemical substance but mixtures, in which the EDCs that are present at relatively low concentrations may carry a low level of risk alone but may cause significant physiological disruption when combined in the real-life environment (Bellingham et al. 2009). These mixtures include sewage sludge (SS), a by-product of wastewater treatment from domestic, industrial and agricultural sources commonly spread on pastures that are then grazed by domestic ruminants (Rhind et al. 2011). As such, SS is one of the most relevant EDCs in the context of this review. The fetal and neonatal stages are particularly sensitive to EDCs and exposure during these critical windows of development can affect the reproductive axis in a sexually differentiated manner, as discussed below. Although very few studies have explored the fetomaternal exchanges of EDCs, Rhind et al. (2010) found no correlations between the maternal and fetal burdens at Days 55 and 110 of gestation in the livers of sheep exposed to environmentally relevant concentrations of sewage sludge. Studies on bisphenol A (BPA) in the same species show that the conjugation capabilities of the fetoplacental unit are low in early gestation (around Day 55) but higher at a later date (around Day 130), indicating that exposure to the EDC is high during the earlier developmental window (Corbel et al. 2013, 2015). The present review concentrates on five EDCs: BPA, octylphenol (OP), methyoxychlor (MXC), polychlorinated biphenyls (PCBs) and SS. Main effects are summarised in Table S10.
Effects of EDCs in the hypothalamus
Reproduction is ultimately controlled by approximately 2000 neurons in the hypothalamic preoptic area of the brain that synthesise and secrete GnRH (Dees and McArthur 1981; Lehman et al. 1986). These neurons are regulated by neurotransmitter and neuropeptide systems that convey information about an animal’s internal and external environment, including its steroidal status (Fig. 2). GnRH neurons do not possess oestrogen, progesterone or androgen receptors (Herbison et al. 1993) and therefore information must be relayed by steroid-receptive neural systems, including kisspeptin and galanin. Many of the EDCs act as steroid mimetics, exerting their actions via classic steroid receptors in reproductive tissue. EDCs can reduce the population of GnRH neurons and GnRH receptors (SS: Bellingham et al. 2010; BPA and MXC: Mahoney and Padmanabhan 2010). Whether reductions in GnRH synthetic capacity affect reproduction in females is unclear because relatively small amounts of GnRH are needed to trigger ovulation (Bowen et al. 1998). However, a lack of GnRH receptors may alter signalling pathways and reduce pituitary LH and FSH secretion, with downstream effects on fertility. EDCs also alter oestrogen receptor (ER) α and ERβ expression in the female sheep hypothalamus (ESR1 and ESR2 respectively; Mahoney and Padmanabhan 2010). Specifically, female fetuses exposed to BPA have increased ESR1 gene expression in adulthood, whereas ESR2 gene expression is reduced by exposure to both BPA and MXC. Changes in steroid receptor abundance may alter feedback mechanisms responsible for ovulation, potentially altering mating behaviour and fertility (Mahoney and Padmanabhan 2010), and this hypothesis has been investigated by Salloum et al. (2013) for BPA and MXC. In addition, specific oestrogen-receptive neural populations that regulate GnRH secretion are altered by EDCs, namely kisspeptin, its receptor and galanin receptors 1–3 (Bellingham et al. 2009, 2010). Reductions in these neural populations were observed in 110-day male and female fetuses, although whether these neural networks remain perturbed after birth and into adulthood is unexplored (Bellingham et al. 2009, 2010). After birth, both kisspeptin and galanin are important for the timing of puberty, ovulation and receptivity in farm animals (Caraty et al. 2012); therefore, reductions in these neurotransmitter systems may potentially reduce reproductive performance in sheep. Data on the hypothalamic (and pituitary) effects of EDCs in the pig are lacking and could be an avenue for further study.
|
Effects of EDCs on pituitary gonadotrophins
The gonadotrophins LH and FSH are important in females for ovarian development and function, as well as to promote ovulation, and in males to promote testicular development and spermatogenesis. Exposure of male sheep fetuses to OP from Day 70 of gestation until birth suppressed FSHβ gene expression and the percentage of FSH-immunoreactive (FSH-ir) cells in the pituitary gland, whereas LH gene expression and cell numbers were unaffected (Sweeney et al. 2000). These pituitary effects were associated with altered testicular function (see below). In the female fetus, populations of pituitary gonadotrophs are reduced following exposure to SS (Bellingham et al. 2009), as is the percentage of cells double labelled for kisspeptin and LHβ and those labelled for ESR1. In relation to gonadotrophin secretion per se, FSH release is suppressed by OP exposure in fetal animals (Sweeney et al. 2000), whereas episodic LH is inhibited by both short- and long-term administration of BPA to prepubertal ewe lambs (Evans et al. 2004; Collet et al. 2010). It is worth noting that the suppressive actions of BPA on LH pulses depend on the dose, and high doses were required to replicate the actions of exogenous oestradiol (Collet et al. 2010). Conversely, GnRH-stimulated LH release was higher in prepubertal female lambs exposed to PCB 118 (but not PCB 153) during gestation, indicative of selective PCB modulation of the responsiveness of the pituitary gland to hypothalamic stimulation (Kraugerud et al. 2012). Both PCBs altered follicular dynamics in these lambs (see below) and may reflect an indirect effect on gonadotrophin release or a direct effect on the ovary.
Effects of EDCs on ovarian and uterine function
EDC exposure during germ cell formation may cause permanent reductions in reproductive function in adulthood. Importantly, changes in the germline could be passed to later generations, as shown in rodents (Skinner et al. 2010). There are well-documented actions of EDCs on the morphology and development of the sheep ovary during fetal and early postnatal life. SS exposure alters ovarian dynamics in 110-day fetuses, reducing oocyte density, advancing follicle development and increasing a pro-apoptotic protein (Bax) key to normal ovarian development (Fowler et al. 2008). Similarly, exposure to PCB 118 and PCB 153 throughout gestation altered follicular dynamics in prepubertal animals 60 days after birth. PCB 153 increased the number of primary follicles, whereas PCB 118 increased the sum of secondary, early antral and antral follicles (Kraugerud et al. 2012). Although the long-term consequences of such enhanced development are unknown, Kraugerud et al. (2012) suggest that increased recruitment from the primordial follicle pool could lead to premature ovarian failure. Fetuses exposed to BPA from Day 30 of gestation until necropsy at Day 65 or 90 exhibit reduced ovarian steroidogenic gene and miRNA expression for several genes central to successful gonadal differentiation and folliculogenesis (Veiga-Lopez et al. 2013). These changes in gene expression may also underlie, in part, the altered reproductive endocrine function reported in prenatally BPA-exposed ewes during their first breeding season (Savabieasfahani et al. 2006). Exposure to relatively low doses of BPA (50 or 0.5 µg kg–1) during the first 2 weeks of life also has profound actions on follicular dynamics of the 30-day-old ewe lamb. Specifically, ovarian weight is lower in BPA-exposed animals and the number of primordial follicles is reduced as primordial follicle recruitment is accelerated. However, there is also an increase in the number of atretic antral follicles (Rivera et al. 2011). Other physiological changes were also noted, including a reduced response of oestrogen to stimulation with FSH and changes in steroid receptors (Rivera et al. 2015), which, in conjunction, may lead to future subfertility. The onset of puberty was not affected by prenatal BPA or MXC exposure in the latter study. In contrast, when females were exposed to OP for varying periods from Day 70 of gestation to weaning at 4 months, the onset of puberty was advanced by 3 weeks with an associated increase in first breeding season duration (Wright et al. 2002). As well as EDC-induced changes in ovarian function, changes have been noted at the uterocervical level. Specifically, BPA (but not OP) exposure of ovariectomised ewe lambs for 6 weeks from 4 weeks of age increased uterine weight (due, in part, to endometrial oedema), increased keratinisation of the cervical epithelium and altered the distribution of oestrogen receptors in the uterine endometrium. Further work will be necessary to determine whether these pathological changes affect the ability of ovary-intact ewes to carry viable lambs (Morrison et al. 2003).
IVF studies have explored the effects of PCBs on the maturation of pig oocytes. Although there was no effect on the number of cleaved oocytes, the number of zygotes that became blastocysts was substantially reduced (Brevini et al. 2004; Favetta et al. 2012). Further exposure of porcine oocytes and/or embryos to OP or organochlorines (OCs) variously altered oocyte viability, fertilisation rate and morula development (Campagna et al. 2002, 2008; Ducolomb et al. 2009).
In cattle, several studies have demonstrated negative effects of organochlorines, OPs, BPA and PCB on the developmental competence of the oocyte when added to the culture media during IVM procedures (Tiemann et al. 1996; Alm et al. 1998; Pocar et al. 2001a, 2001b; Ferris et al. 2015). Ferris et al. (2015) used environmentally relevant concentrations of BPA that fell below the estimated lowest observed adverse effect level (LOAEL) to determine environmental chemicals (EC) actions on oocyte maturation. Exposure altered the sex ratio, decreased the percentage of males, increased cellular apoptosis, decreased embryo quality and developmental potential and altered the expression of key developmental genes. Despite these limited in vitro studies, there are no corresponding data following EDC exposure in vivo, and this is clearly required if the relevance to beef and dairy fertility is to be ascertained. Indeed, for all three species, there is a complete dearth of information on the programming effects of EDCs on crucial aspects of female offspring reproductive function, including ovulation and fertilisation rates, embryo quality, pregnancy rates and lifetime litter size. A further potential target for research would be with high-yielding dairy cows in negative energy balance, where EDCs could exacerbate negative effects on reproductive function with associated productivity and welfare consequences.
Effects of EDCs on testicular function
OPs have been shown to disrupt testicular development and function in sheep following prenatal and early postnatal exposure. Maternal exposure of dams to OP from Day 70 of gestation until birth resulted in decreased testicular weight and a reduction in Sertoli cell number at birth (Sweeney et al. 2000), but an equivalent exposure did not affect semen quantity, quality or IVM or IVF characteristics at 12 months of age (Sweeney et al. 2007). In contrast, when males were exposed to OP from birth to weaning (but not from Day 70 of gestation to weaning), the number of morphologically abnormal spermatozoa was increased by 12% (Sweeney et al. 2007). The effect of this small increase in the proportion of abnormal spermatozoa on ram fertility in field conditions is unknown. Prenatal and early postnatal exposure to SS has also been shown to disrupt testicular function. In fetal life (at Day 110 of gestation), long-term maternal SS exposure resulted in a major attenuation of fetal testicular development associated with reduced testicular hormone secretion (Paul et al. 2005). When exposure was continued until weaning at 4 months and tissues were collected at 19 months, approximately half the exposed rams had testes that appeared to be morphologically normal, whereas the remainder had major reductions in germ cell number and a greater number of Sertoli cell-only tubules (Bellingham et al. 2012). This observation is important because it indicates that specific individuals may be more susceptible to EDC disruption than others and reinforces that population data may underestimate the problems associated with EDC exposure. Moreover, it suggests that identifying at-risk or affected individuals on-farm could be a challenge.
In pigs, sperm viability and motility were impaired when directly exposed to increasing concentrations of the OPs malathion and diazinon in vitro, possibly due to attenuated energy release by the mitochondria (Betancourt et al. 2006). Similar effects were observed when pig spermatozoa were cultured in IVF medium containing a mixture of 15 organochlorines (Campagna et al. 2002).
Evidence of epigenetic involvement
Our understanding of the molecular mechanisms that underlie the prenatal programming of domestic livestock fertility is in its infancy. Epigenetic marks are candidates for bearing the memory of early life exposure and both PGCs (the direct progenitors of spermatozoa or oocytes) and the preimplantation embryo are subjected to intense epigenetic modifications (Seisenberger et al. 2013). As discussed earlier, DNA methylation in germ cells is erased and new DNA methylation is acquired during multiplication of spermatogonia in the male (fetal event), whereas in females it primarily takes place during follicle or oocyte growth and maturation (after puberty and before each ovulation). Thus, the key times when offspring are potentially susceptible to epigenetic modification is likely to be gender specific, with males theoretically being more sensitive than females during fetal life (Dupont et al. 2012).
Variations in the maternal, placental and fetal hormonal milieu during intrauterine development are postulated as epigenetic signals (Fowden et al. 2010). Because the glucocorticoids and reproductive steroids are highly sensitive to maternal nutrition (Fowden et al. 2010; Wallace et al. 2011a) and EDCs have major oestrogenic properties (Fowler et al. 2012), they are clearly important candidates in this respect. However, to date there is no direct link between these putative environmental cues and epigenetic modifications that relate to a fertility phenotype in farm species, primarily because these mechanisms have not been investigated to any great extent. In contrast, there is compelling evidence from rodents that at least some of the effects of environmental chemicals, such as diethylstilbestrol (DES) (Alworth et al. 2002) and the isoflavonoid phytoestrogen genistein (Tang et al. 2008), are mediated via changes to DNA methylation. Anway et al. (2005) went further to demonstrate male germline-mediated epigenetic transgenerational effects of the anti-androgenic compound vinclozolin (an agricultural fungicide) and the oestrogenic compound MXC on gonadal sex determination, which persisted to the F3 generation in the rat.
Interim conclusions
There is reasonable evidence to support a role for early life nutrition in the programming of specific aspects of female offspring fertility. In sheep, the most robust effects to date relate to the negative effect of low fetal nutrient supply on the number of ovarian follicles. However, even this may not limit litter size until relatively late in a female’s breeding life or until she experiences a second environmental challenge, such as poor nutrition or repeated superovulation in adult life. A similarly robust effect of poor prenatal nutrition on the ovarian follicle reserve in beef cattle is also emerging and, in both species, low birthweight may be a useful proxy marker of the individuals most likely to be perturbed. In contrast, in male offspring, the negative effect of poor prenatal nutrient supply is likely to be confined to the first breeding season and of little consequence to fertility thereafter. To date, the most worrying implication of EDC research relates to the higher incidence of spermatogenic abnormalities in male offspring. Further, although early life exposure to EDCs clearly affects the reproductive axis at the brain, pituitary and ovarian levels, the effect on female offspring fertility and fecundity has simply not been examined in the relevant farm species.
Effects of advanced (assisted) reproductive technologies
Compared with AI, the transfer of in vivo-derived (IVD) and in vitro-produced (IVP) embryos is largely restricted in cattle to breeding within elite or nucleus herds. Recent estimates place the number of embryos transferred globally at just under 1 million (International Embryo Transfer Society (IETS) 2012). The number of IVP embryos transferred has quadrupled over the past decade and now constitutes 40% of total embryos transferred in cattle. One driver for the current surge in interest in IVP embryos is the prospect of genomic selection of Day 7 embryos (Lauri et al. 2013). This can significantly reduce the breeding interval (and so increase response to selection) by virtue of the fact that selection occurs at Day 7 of gestation and not at term. The use of sex-sorted spermatozoa for IVF can also reduce wastage and associated costs that arise from the production of unwanted (usually male) calves.
Developments and refinements to bovine IVP methodologies since 1982 led to the global commercialisation of this technology by the mid to late 1990s. However, the first reports of developmental anomalies leading to the birth of large calves and associated obstetric complications following the transfer of IVP embryos arose soon thereafter. These observations were later reported in sheep, and collectively became known as the large offspring syndrome (LOS). It is beyond the scope of the present article to review this topic in detail; this has been done elsewhere (e.g. Young et al. 1998; Sinclair et al. 2000; van Wagtendonk-de Leeuw et al. 2000; Farin et al. 2006). However, the study of in utero and postnatal development following the transfer of IVP and cloned embryos (which undergo a period of in vitro culture) has served to highlight the sensitivity of the periconception period, and the earliest stages of mammalian development, to environmental influences. Given that from fertilisation to hatching (at around Day 7 or Day 8 of gestation) ruminant embryos contain a large population of pluripotent cells, it follows that many of the altered phenotypes described in offspring (discussed next) may have arisen, at least in part, as a consequence of epigenetic alterations to DNA and associated proteins in several developmentally important genes (discussed later).
Long-term postnatal consequences of ART
Differences in birthweight between calves or lambs conceived naturally or by AI, and calves or lambs derived from IVP embryos, normally disappear at around 6–12 months of age (Wilson et al. 1992; Walker et al. 1996), indicating that in utero overgrowth is transient and does not persist postnatally, although in the study of McEvoy et al. (1998), oversized calves at birth derived from IVP embryos had abnormally large hearts when slaughtered at just over 1 year of age. Anecdotal evidence based on a limited number of observations from some of the early studies indicated that IVP-derived offspring of the same genotype may be more muscular. Subsequent studies in both cattle and sheep confirmed that primary muscle fibre CSA was increased, as was the ratio of secondary to primary muscle fibres, in late-gestation fetuses (Maxfield et al. 1998; Crosier et al. 2002). These effects in sheep were associated with a shift in the temporal expression of myogenic factor 5 (Myf-5), a member of the MyoD gene family responsible for inducing mesodermal precursor cells to differentiate into myoblasts and to proliferate, both under the influence of Sonic hedgehog and Wnt-1 (Maltin et al. 2001). In contrast, there was no effect of embryo source on expression of Myf-5, MyoD or myogenin (MYOG) in skeletal muscle of Day 222 bovine fetuses; instead, there was a reduction in expression of mysotatin (MSTN; Crosier et al. 2002). Loss of function of this TGF-β family member is known to lead to muscle hypertrophy in cattle and sheep (Rodgers and Garikipati 2008), the origins of which occur prenatally. These observations are remarkable because the initiating factors during IVP would have had to act on the population of pluripotent cells that constitute the preimplantation embryo, reinforcing earlier statements in this article that the earliest stages of mammalian development are particularly sensitive to environmental perturbation.
Few studies have formally evaluated carcass and muscle characteristics of offspring conceived with IVP embryos, and none of these was designed specifically to address the issue of whether the IVP process itself altered these traits. Patterson et al. (1993) assessed carcass characteristics of single and twin beef calves derived from IVP embryos and concluded that, following embryo transfer (ET), viable twins have similar beef-producing potential to single-born calves. Similarly, Amen et al. (2007) used IVP and ET to produce reciprocal crosses of Bos indicus and Bos taurus cattle in order to assess carcass and meat traits, but no assessment of independent effects of assisted reproductive technology (ART) could be made. The study of Sinclair et al. (1995) is often cited (wrongly) as providing evidence that the process of IVP itself leads to increased carcass weights with greater yields of saleable meat. Carcass and saleable meat yields were greater for IVP–ET-derived offspring than for AI controls, but this was almost certainly due to the selection or breeding process and not the IVP of embryos per se.
Similarly, until very recently no studies have assessed long-term effects of IVP–ET on subsequent offspring fertility and milk yield. However, a large study (comprising 426 ET recipients) in Florida, involving the transfer of fresh or frozen–thawed and sexed IVP embryos versus AI in Holstein cows, found no effects of ART on pregnancy rates to first service or daily milk yields during first lactation in resultant female offspring (Bonilla et al. 2014). Consequently, it is unlikely that these traits would be affected in beef cattle.
Long-term effects of reproductive cloning
Prenatal losses, obstetric complications and postnatal morbidity associated with reproductive cloning generally fall under the heading of LOS and have been reviewed elsewhere (e.g. Young et al. 1998; Chavatte-Palmer et al. 2000) and will not be considered further herein, except to state that the incidence and severity of pregnancy losses, obstetric complications and neonatal morbidity are usually greater for pregnancies generated from embryonic cell NT embryos (Garry et al. 1996), but particularly from somatic cell nuclear transfer (SCNT) embryos (Hill et al. 1999; Chavatte-Palmer et al. 2004), than with either IVP or IVD embryos. Attention instead is focused on the health and productivity of cloned offspring. Again, this topic has been extensively reviewed elsewhere (Norman and Walsh 2004; Takahashi and Yoshihiko 2004; Tome et al. 2004; Heyman et al. 2007; Laible et al. 2007; Rudenko and Matheson 2007; Rudenko et al. 2007; Watanabe and Nagai 2008), driven largely by statutory requirements of various government agencies (e.g. US Federal Drug Agency, European Food Standards Authority) to ensure that cloning does not compromise animal welfare and that food products from cloned animals are safe for human consumption. The available evidence indicates that SCNT cloned offspring that survive to puberty are generally healthy and that the composition and nutritive value of milk and meat products from cloned (non-transgenic) livestock do not differ from that of animals conceived naturally. However, there does appear to be subtle differences in muscle fibre contractile types (i.e. more slow-twitch oxidative relative to fast-twitch glycolytic fibres) in young cloned heifers (at around 8 months of age), but again, as with general issues regarding animal health, these compositional differences in muscle subsequently disappear following the onset of puberty and are not evident in cattle >12 months of age (Jurie et al. 2009).
Epigenetic programming of long-term development
It became apparent early on that many of the LOS features resembled those of naturally occurring overgrowth syndromes in humans (e.g. Beckwith–Wiedemann syndrome (BWS)) that are associated with errors in an imprinted cluster of genes on human chromosome 11 (Sinclair et al. 2000). This led to the discovery that LOS in sheep was due, at least in part, to a loss of imprinting and expression of the gene encoding the IGF-2 receptor (IGF2R) in a range of tissues, but particularly those emanating from the mesodermal lineage (Young et al. 2001). This loss of imprinting arose as a consequence of loss of DNA methylation in the second intron differentially methylated region (DMR) of that gene. Loss of methylation at this DMR, and a conserved DMR located upstream of the ovine H19 gene (H19, imprinted maternally expressed transcript (non-protein coding)), was prevalent among SCNT cloned lambs (Young et al. 2003) and SCNT cloned calves (Smith et al. 2012), leading to biallelic expression of these imprinted genes.
Germline epigenetic marks are established in a parent-specific manner in a small subset of genes (current maximum best estimates in the mouse are between 300 and 400; Kelsey and Bartolomei 2012) that facilitate tissue and developmental stage-specific monoallelic expression of affected genes following fertilisation. These processes are best understood in the mouse (Cedar and Bergman 2009; Tomizawa et al. 2012), with only limited data available in ruminants (Thurston et al. 2008). The dynamics of imprint establishment during gametogenesis and early embryogenesis (coincident with procedures used in ART) are such that, depending on the precise timing and nature of the procedural insult, it is highly probable that different combinations of imprinted genes may be affected to a greater or lesser extent. Thus, Bebbere et al. (2013) failed to detect differences in allelic expression ratios and IGF2R second intron DMR methylation in the few (n = 4) overgrown IVP fetuses studied compared with normal weight IVP and in vivo conceived fetuses at Day 80 of gestation in the cow. Instead, Chen Cárdenas et al. (2013) found a loss of imprinting leading to biallelic expression of KCNQ1OT1 (KCNQ1 oppositie strand/antisense transcript 1, the gene most misregulated in BWS) in bovine LOS fetuses derived from IVP embryos, and that this is associated with a loss of methylation at the KvDMR1 (KCNQ1 differentially methylated region 1) on the maternal allele. This observation confirmed an earlier report of abnormal hypomethylation of KvDMR1 and expression of KCNQ1OT1 in two of seven SCNT cloned calves and one of two IVP-derived calves (Hori et al. 2010). Although generally highly conserved, there are also recognisable differences in imprinting within eutherian mammals that could account for many such differences between species, differential imprinting at the IGF2R locus being a case in point (Das et al. 2009; Renfree et al. 2013).
The emerging picture is further complicated by the fact that many of these imprinted genes are polymorphic. In taurine cattle, for example, single-nucleotide polymorphisms (SNPs) are known to exist in at least seven imprinted genes (including IGF2R) and to be associated with several commercially important traits, including those associated with fertility (e.g. gestation length, calving difficulty, perinatal mortality), milk yield (e.g. protein percentage, somatic cell counts) and growth (e.g. carcass weight, conformation, rump depth; Magee et al. 2010; Berkowicz et al. 2011). There is also some evidence of allelic switching of imprinted IGF2R in SCNT cloned bovine fetuses where the paternal allele is imprinted in one tissue whereas the maternal allele is imprinted in another tissue (Suteevun-Phermthai et al. 2009). These potentially confounding factors could account for at least some of the discrepancies and apparent stochastic effects observed in aberrant genomic imprinting patterns between studies that frequently report effects in only small numbers of animals. Differences in imprinting between fetal and placental tissues could also account for some of this variation.
Finally, although the focus of most research has understandably been directed towards errors in genomic printing following ART in both animals and humans, it is highly likely that the epigenetic status, and possibly expression, of many more non-imprinted genes are also affected (Grace and Sinclair 2009). Surprisingly, this hypothesis has never fully been tested. Some groups (e.g. Santos et al. 2010) have used immunofluorescent techniques to visually quantify global 5-methyl-cytidine staining to assess effects of ART procedures on DNA methylation in embryos, but this method lacks sensitivity, cannot identify locus-specific changes in methylation and possesses other methodological limitations (Li and O’Neill 2012). However, in a microarray analysis of 1536 CpG sites in just over 700 genes, Katari et al. (2009) found that imprinted loci were no more or less likely to be differentially methylated than non-imprinted loci. DNA in that study was extracted from human cord blood and placentas from term pregnancies established naturally or following IVF. More recent data in humans and mice, where genome-wide DNA methylation was assessed by methylated DNA immunoprecipitation (MeDIP) array), confirm epigenetic differences in promoter methylation of non-imprinted genes between offspring conceived naturally or by IVF (Li et al. 2011; Oliver et al. 2012). These studies are somewhat preliminary in that they were limited by scale and/or tissues sampled (e.g. peripheral blood in the human study). The platforms used are also somewhat insensitive compared with contemporary deep-sequencing approaches that can provide single base pair resolution.
Interim conclusions
Sweeping changes to DNA methylation and chromatin remodelling take place in gametes and in the preimplantation embryo during the normal course of development, rendering these cells particularly vulnerable to environmentally induced epigenetic modifications to DNA as can occur during ART, leading to problems such as LOS. It is very likely that a broader but more subtle range of aberrant phenotypes than have been described to date manifest following the use of these technologies, but that under normal conditions of commercial livestock production these go unnoticed. The major adverse phenotypes include pregnancy failure following ET and an increased but variable incidence of obstetrical complications during parturition. The latter effects occur less frequently and more sporadically these days for reasons that, although not fully understood, may be due to refinements to oocyte and/or embryo culture media that now avoid the use of serum and somatic support cells. However, there is scant information on the longer-term effects of ART on farmed livestock. The available evidence indicates that most ART offspring that reach puberty are, for the most part, normal. However, in beef cattle at least, genomic imprinting significantly contributes to the genetic variance of several commercially important traits, with estimated proportions of between 8% and 25% of total additive genetic variance (Neugebauer et al. 2010). It remains to be determined whether procedures used in ART may affect these traits to any great extent.
Animal behaviour and welfare
In contrast with earlier sections in this review, few studies into animal behaviour and welfare provide evidence for epigenetic regulation at the molecular level. Nonetheless, we describe studies that have shown an effect of prenatal factors on response outcomes, some of which are long lasting in their effects (although many have not been studied over prolonged periods) as evidence for where these effects may be governed by epigenetic changes. Responses of interest are those that alter the ability of the animal to respond to the environment in which they are managed, either through behavioural adaptation, stress physiology and responsiveness or immune responses, and hence disease susceptibility. These effects are of relevance to the animal itself, as well as to the producer, because they can affect mortality and morbidity, disease susceptibility, reactiveness of animals to common on-farm practices (e.g. vaccination) and ease of handling. The prenatal and early postnatal period is of importance in defining how individuals respond to their environment throughout life. This has been the subject of several recent reviews in farm livestock demonstrating the important role that variation in maternal state can have on progeny health and welfare (Greenwood et al. 2010; Rutherford et al. 2012; Merlot et al. 2013). Prenatal stress or suboptimal maternal nutrition have both been shown to affect how well offspring cope with their social, physical and infectious environments.
Effect of the prenatal environment on birthweight and mortality
Perhaps the best described, and unambiguous, effects on welfare (and productivity) are the effects of early life events on the survival of offspring. Many studies have investigated the causes of offspring mortality, particularly in sheep and pigs, but some also in cattle, and demonstrate that preterm delivery, low birthweight, dystocia, poor behavioural development in early life and an inability to adjust to postnatal life (e.g. impaired ability to thermoregulate) all contribute to an increased risk of mortality. Many of these risk factors have their origins in prenatal development.
The single greatest contributor to lamb mortality is birthweight (Dwyer et al. 2003), although the relationship is not linear, with very low and very heavy lambs having high mortality. Numerous studies consistently show that maternal undernutrition of the ewe in late pregnancy (after Day 100) reduces lamb birthweight (for a review, see Rooke et al. 2015). Undernutrition before Day 100 has variable effects across different studies: severe early undernutrition has a marked effect on birthweight and mortality (Vincent et al. 1985); and moderate undernutrition in early to mid-gestation generally does not affect birthweight, except in young and growing females (Munoz et al. 2009) or ewes selected or adapted for a well-fed environment (Burt et al. 2007; Rooke et al. 2010). In these studies mortality was generally not affected if birthweight was not affected, although the study of Rooke et al. (2010) reports increased lamb mortality with early undernutrition (to Day 90 of gestation), even in the absence of an effect on birthweight. Conversely maternal overnutrition of ewe lambs results in a severe reduction in lamb weight, as the mother partitions nutrients towards the growth of her own tissues at the expense of the placenta and fetus (Wallace et al. 2011b). Reduced bodyweight is associated with slower behavioural development (Dwyer et al. 2003), with delays in reaching the udder and suckling that contribute to the higher mortality of low-birthweight lambs (Dwyer et al. 2001). In humans, an association between birthweight, neurobehavioural developments and epigenetic regulation of placental HSD11B2 (hydroxysteroid 11-beta dehydrogenase 2) has recently been suggested (Marsit et al. 2015) and similar mechanisms may be relevant to farmed livestock. A role for the placental epigenome in regulating birthweight in the sheep has been suggested (Fowden et al. 2010). Shearing pregnant ewes has consistently been reported to increase lamb birthweight, particularly if conducted during early to mid-gestation (Figure S3). Associated with this response are increases in maternal feed intake, gestation length and maternal plasma glucose concentrations (Symonds et al. 1988; Morris et al. 2000; Keady and Hanrahan 2009; Banchero et al. 2010). Although shearing is associated with a robust stress response in the ewe, these data suggest that its primary effect on birthweight is through increased feed intake, and hence nutrition of the developing lamb.
Other late-gestation treatments of the ewe that are associated solely with stress (social isolation or aversive handling by humans) have also reported an increase in birthweight (Roussel et al. 2004; Hild et al. 2011). In goats, aversive handling in pregnancy led to placental alterations and increased fetal loss compared with gentle or minimal handling (Baxter et al. 2016), whereas positive handling improved neonatal behavioural development. In addition, shearing studies in late gestation that did not elicit an increase in birthweight have been reported to improve lamb behavioural progression to sucking (Banchero et al. 2010), although none of the maternal stress studies reported changes in lamb survival. The mechanisms underlying these effects are largely unknown.
In cattle, the major contributor to calf mortality is dystocia, which is also a risk factor for calf morbidity and mortality in later life (for a review, see Arnott et al. 2012). The main cause of calving difficulty is fetal–pelvic disproportion, which is likely to be related, at least in part, to prenatal nutrition (Micke et al. 2010b), although studies of stillborn calves suggest that prenatal factors also contribute to the likelihood that calves will not survive the birth process (Barrier et al. 2013). Studies of maternal nutrition in cattle are far less numerous than in sheep and, generally, the effects of maternal nutritional restriction and calf weight are variable. The effects differ dependent on timing, duration and severity of the dietary insult, as well as the parity of the dams. Some studies have found no effect (Arnott et al. 2012). In contrast, other studies have reported a reduction in calf birthweight as a consequence of maternal undernutrition during the second and/or third trimester of pregnancy (Warrington et al. 1988; Houghton et al. 1990; Freetly et al. 2000; Cafe et al. 2006; Micke et al. 2010a, 2010c). In general, reducing calf birthweight by means of maternal dietary restriction does not reduce the incidence of dystocia or calf morbidity. This is due to both effects on maternal physiology (Micke et al. 2015) and changes to the immune status of the calf and colostrum (McGee et al. 2006). However, in a few studies cow nutrition has been linked to calf mortality (e.g. when cows were kept on an energy-restricted diet during late gestation leading to reduced birthweight; Corah et al. 1975).
Calf birthweight and survival can be affected by environmental conditions, independent of the nutritional status of the mother. Both heat (Collier et al. 1982) and cold (Andreoli et al. 1988) exposure in gestation are reported to reduce calf birthweight, as well as gestation length (Table S1), which is associated with increased calf mortality and morbidity (Azzam et al. 1993). Drought exposure of pregnant cattle can profoundly affect offspring development and survival (Arnott et al. 2012). In particular, this has been associated with a condition in beef calves termed congenital chondrodystrophy of unknown origin, where failure in long bone growth results in disproportionate dwarfism, breathing difficulties and perinatal death. This condition seems to occur as a result of maternal malnutrition in early gestation as a consequence of severely reduced rainfall (White et al. 2010b). As for sheep, the potential role of epigenetic mechanisms in these effects have not been demonstrated.
In pig production, average mortality is high (commonly between 10% and 20%) and most deaths are due to stillbirth, crushing by the sow or starvation (Edwards and Baxter 2015). However, the underlying causes of these different types of death are a complex and multifactorial interplay of maternal and progeny biology and human management. IUGR, as demonstrated by altered body proportionality (Baxter et al. 2008), places piglets at risk for increased mortality. One important prenatal cause of IUGR is large litter size. Average litter size has increased markedly in many domestic pig populations because of genetic selection schemes. In pig breeds that do not naturally produce large litters, selection for increased litter size has resulted in a decrease in average birthweight, increased birthweight variability within litters and a consequential increase in still births and postnatal mortality (for a review, see Rutherford et al. 2013).
Maternal stress during gestation has also been shown to affect piglet birthweight, although no clear pattern has emerged. For example, maternal stress may decrease (Kranendonk et al. 2006a), increase (Otten et al. 2007) or have no effect (Rutherford et al. 2009) on piglet birthweight. Similarly, direct impacts of prenatal stress (PNS) on piglet survival (Tuchscherer et al. 2002) are the exception rather than the rule.
In rodents, there is evidence for epigenetic regulation in the expression of maternal behaviour, which contributes to offspring survival. For example, Cameron et al. (2008) show that epigenetic regulation of the promoter region of estrogen receptor 1 (ERα) occurs following exposure to high or low levels of maternal care. This then affects the expression of maternal care subsequently expressed in adulthood. Although variations in the behaviour of livestock mothers and their offspring have been observed, and linked to survival (e.g. Dwyer 2008), evidence for epigenetic effects has not yet been investigated.
Effect of the fetal environment on stress responsiveness
Common farm husbandry practices, such as restraint, handling and transportation, are associated with stress responses indicating activation of the hypothalamo–pituitary–adrenal (HPA) axis (e.g. Roussel et al. 2006; Roussel-Huchette et al. 2008). The HPA axis is a hormonal cascade involving production of corticotropin-releasing hormone (CRH) in the paraventricular nucleus of the hypothalamus, which stimulates release of adrenocorticotrophin (ACTH) from the pituitary, which further stimulates release of glucocorticoids (cortisol or corticosterone in some species) from the adrenal gland. Glucocorticoids have a multifaceted role in the body, as witnessed by a glucocorticoid receptor in every cell of the body, but their primary role is to mobilise energy reserves during periods of low nutrient intake, acting in a reciprocal fashion with insulin, which stores nutrients during times of nutritional plenty.
In rodents, prenatal stress (classically induced by restraining the mother regularly over the last half to one-third of pregnancy) is known to induce long-lasting changes in the HPA axis of the offspring, characterised predominantly by increased HPA axis reactivity (Henry et al. 1994). This elicits behavioural traits, such as high anxiety and depressive-like behaviour, and impaired memory for hippocampus-dependent tasks (e.g. spatial tasks; Darnaudery and Maccari 2008). Many studies report sex-dependent effects, with males typically showing an increase in anxiety after prenatal stress and dysmasculinised behaviours, whereas females show converse behavioural responses (Zuena et al. 2008). The release of glucocorticoids by the stressed mother, which can cross the placenta to affect the developing offspring, appears to mediate these responses (Mesquita et al. 2009; Harris and Seckl 2011) and changes to over 700 genes in the fetus, in a region- and sex-specific manner, have been reported (Mychasiuk et al. 2011). Epigenetic alterations in the offspring brain are known to follow prenatal stress (Gudsnuk and Champagne 2012), accompanied by changes in the development of neurogenesis, particularly in the hippocampus (Korosi et al. 2012). Very recently, evidence has also emerged for an effect of paternal stress on the stress reactivity of his offspring in rats (Mychasiuk et al. 2013): stress of males during spermatogenesis before conception reduced learning responses and reduced stress responsiveness of male offspring, and had sex-specific effects on DNA methylation patterns.
In farm animals, a detailed mechanistic understanding of the effects of maternal stress is lacking. However, the clear effect of maternal, and paternal, stress on the subsequent behaviour and stress responsiveness of the offspring in rodents suggests that similar mechanisms may operate in livestock. To date, experimental studies have focused on assessing HPA axis function (e.g. release of cortisol and/or ACTH) or the altered behavioural stress responsiveness of the offspring either in response to pharmacological challenge or when exposed to an environmental or social stressor. Details of the effect on HPA axis function in lambs that have been observed following maternal undernutrition during pregnancy, or following maternal stress, are provided in Table S2. Responses are variable (due, in part, to inconsistency between studies in time at which the offspring were assessed), but do suggest some sex-specific variation, as seen in young adult sheep (e.g. Gardner et al. 2006), and differences in responses when lambs are younger or older. Studies in cattle are very scarce, but Lay et al. (1997) showed that repeated transportation of Brahman cows during gestation increased the cortisol response of the progeny to an acute restraint stress.
Otten et al. (2015) reviewed prenatal stress studies in pigs and concluded that fetal brain regions relating to HPA function appear highly vulnerable to maternal stress and many studies have demonstrated changes in HPA function in postnatal life as a consequence of PNS (e.g. Otten et al. 2010). Social stress experienced during pregnancy has been shown to increase expression of corticotropin-releasing hormone (CRF) mRNA in the amygdala and paraventricular nucleus of the hypothalamus, as well as altering the ratio of CRF1 and CRF2 receptors in the amygdala (Jarvis et al. 2006; Rutherford et al. 2014). Artificial (restraint) stress has also been shown to increase numbers of glucocorticoid receptors in the hippocampus and reduce them in the hypothalamus (Kanitz et al. 2003).
Effect of the fetal environment on offspring behaviour
Unlike studies into effects on stress physiology, which frequently report no effect, effects on offspring behaviour are more frequently reported with studies of gestational undernutrition and maternal stress in pregnancy inducing altered behavioural reactivity (Table S3). However, some studies report an increased behavioural reactivity (e.g. Erhard and Rhind 2004; Roussel et al. 2004), which, in keeping with rodent studies, was more pronounced in males than females. Other studies suggest that lambs are less reactive to stress (e.g. Roussel-Huchette et al. 2008; Hernandez et al. 2010).
In pigs, the effect of PNS on progeny behaviour has been more widely studied. Across several studies (for a review, see Otten et al. 2015), maternal stress has been found to have a negative effect on various behavioural domains important for piglet productivity and welfare, including pain responses (Rutherford et al. 2009), reactivity (Kranendonk et al. 2006b; Otten et al. 2007), social behaviour (Jarvis et al. 2006; Kranendonk et al. 2006b) and maternal behaviour (Jarvis et al. 2006; Rutherford et al. 2014). In the latter study (Rutherford et al. 2014), impairment of maternal behaviour had a significant effect on survival in the next generation; under conditions (free farrowing) where gilts could fully express their maternal behaviour, litters born to mothers that had been prenatally stressed had threefold the mortality of litters born to control mothers.
As described above, similar changes in responsiveness in rodents have been shown to be accompanied by alterations in epigenetic regulation, leading Brien et al. (2014) to conclude from their review of the literature that epigenetic effects of early environment on brain function and behaviour appear to be universal in mammals. Thus, although these effects have not yet been demonstrated in livestock species, the available evidence suggests that epigenetic regulation is involved.
Immune function and health
Although most studies addressing fetal programming of immune competence have focused on laboratory species, there are compelling reasons to believe that the fetuses of livestock species may be more susceptible to the effects of the maternal environment. The immune systems of mammals that give birth to precocious offspring, such as sheep, cattle and pigs, develop predominantly in utero and hence represent a potential target for fetal programming. For example, in sheep, the development of the thymus commences in early gestation and CD5 T cells appear by Day 35 and are soon followed by CD8 and CD4 T cells (Cronjé 2003). Circulation of lymphocytes begins between Days 70 and 75 of fetal life and, by Day 80, the cellular and immunohistological appearance of the ovine thymus is identical to the postnatal thymus (Cahill et al. 1999). By parturition, the central and peripheral lymphoid systems of the ovine fetus are at an advanced stage of development (Cunningham et al. 1999). In pigs, most components of the immune system are present at birth, but are functionally underdeveloped (Le Dividich et al. 2005).
Passive immunity in neonates is predominantly acquired through the uptake and absorption of immunoglobulins in colostrum, rather than placental transfer. Changes to both the quantity (Hammer et al. 2011) and the micronutrient composition (for a review, see Rooke et al. 2008) of the diet offered to the pregnant ewe can affect serum IgG content in her lambs. Such effects could be mediated by altered composition of colostrum and/or the amount ingested by the neonate, or the ability of the neonatal gut to absorb immunoglobulins. For example, low periconception levels of cobalt or vitamin B12 reduced serum IgG in 2- and 4-week-old lambs (Fisher and Macpherson 1991), whereas some studies report an increase in lamb plasma IgG concentrations following vitamin E supplementation before lambing (Gentry et al. 1992). Excess intakes of minerals during pregnancy do not alter colostrum IgG content, but impair immunoglobulin absorption in lambs, and this appears to be largely attributable to the iodine component of the mineral mix (Boland et al. 2005). Generally, undernutrition during early (Muñoz et al. 2009) or late (Hammer et al. 2011) pregnancy increased lamb plasma IgG concentrations at birth and 24 h of age respectively. The study of Hammer et al. (2011) is of particular interest because lambs were removed from their mothers before suckling and fed colostrum replacer, and so the differences in lamb IgG absorption were independent of maternal colostrum production. An increase in serum IgG levels in lambs born to undernourished ewes appears counterintuitive, but speculation about underlying mechanisms is difficult given the absence of data on colostrum composition, intake or lamb plasma volume. Tuchscherer et al. (2012) reported that the protein composition of isocaloric sow diets fed throughout gestation affected piglet immunoglobulin levels on Day 1 of life, with piglets born to sows fed a high-protein (30%) diet having lower serum IgG and IgM levels than piglets born to sows fed an adequate (12.1%) protein diet. Piglets born to sows fed a low (6.5%) or the high-protein diet had lower serum IgA than those receiving the adequate protein diet. However, these differences did not persist.
In cattle, heifers born from cows exposed to natural summer heat stress during the last 45 days of pregnancy had lower serum concentrations of IgG after colostrum consumption than heifers born to mothers receiving a cooling treatment, despite similar colostrum IgG concentrations and feeding levels (Tao et al. 2012). Stress induced by restraining pregnant sows for 5 min a day for the last 5 weeks of pregnancy did not affect sow colostrum IgG concentrations, but piglet blood IgG was reduced (Tuchscherer et al. 2002), suggesting that prenatal stress may have affected the ability of piglets to ingest colostrum. It has been suggested that changes in the profile of maternal glucocorticoids following maternal stress may accelerate neonatal gut maturation (Merlot et al. 2013), leading to reduced immunoglobulin absorption.
Measures of acquired immune function of offspring whose mothers were subjected to different pregnancy regimens are varied, with differences between breeds and breeding seasons, and it is difficult to consolidate current findings. For example, in one year of a study conducted over two consecutive years in which Scottish blackface and Suffolk ewes received either maintenance or 75% maintenance rations during Days 1–90 of pregnancy, the offspring of underfed Suffolk, but not underfed Scottish blackface, ewes had higher strongyle faecal egg counts at weaning age (Rooke et al. 2010). The differences between breeding seasons could be attributed to a range of factors, including differences in lamb postnatal growth and grazing behaviour. It is of interest that the breed by maternal nutrition interaction affected lamb spleen and thymus weight (Ashworth et al. 2014), because the thymus has been proposed as a possible mediator of the effect of prenatal nutrition on immune competence in later life (Cronjé 2003). In pigs, prenatal stress reduced piglet thymus size at birth (Tuchscherer et al. 2002), the CD4+ : CD8+ T cell ratio on Day 4 and blood lymphocyte and granulocyte numbers between Days 26 and 60 of life (Couret et al. 2009), and increased the magnitude of proinflammatory cytokine responses to a lipopolysaccharide challenge in 36-day-old pigs (Collier et al. 2011). In cattle, heat stress during late gestation reduced blood lymphocyte proliferation in female offspring until 56 days of age (Tao et al. 2012), providing evidence that a specific prenatal treatment can affect both passive and acquired immune function in offspring. This had previously been observed in sheep, where a maternal periconceptional diet deficient in elemental cobalt and sulphur altered both the innate and acquired immune function of offspring at 12 months of age (Sinclair et al. 2007). Relative to matched controls, both the acute-phase (serum haptoglobin) and adaptive (serum IgG) responses to purified ovalbumin in Quil A adjuvant were increased in 12-month-old lambs from ewes fed a cobalt- and sulphur-deficient diet before and around the time of mating.
Prenatal affects on welfare: interim conclusions
Variations in the prenatal environment, as dictated by the management of the pregnant mother, can contribute to animal welfare outcomes in livestock. Research in cattle is limited with regard to health and welfare outcomes of maternal challenges during gestation. Negative effects on birthweight may suggest possible postnatal problems, but direct demonstrations of welfare deficits are rare. Periods of severe undernutrition during gestation can have very obvious negative effects on health and welfare (e.g. chondrodystrophy studies), but little is known about more subtle and commercially relevant effects. In sheep, experience of maternal stress during pregnancy can lead to apparently improved welfare status in offspring. Shearing during pregnancy has well-known beneficial effects on birthweight. Other repeated stress treatments have also been shown to cause changes in progeny that can be interpreted as being positive. Although behaviour- and welfare-related PNS studies in farm animals demonstrate that progeny can be affected by maternal stress throughout their lifetime, the literature to date has been largely focused on hypothesis-generating studies, with numerous outcome parameters. To progress this area further will require more focus on studies that examine parameters putatively affecting how PNS responses are manifest under commercial production systems. In particular, studies to date have largely failed to compare the outcome of challenges to different genotypes in the same study, or assess the effect of progeny changes under different postnatal conditions. Both aspects of this area will be important in future for determining the biological basis of prenatally induced changes, and particularly for assessing the applied relevance to agriculture. In the rodent literature, similar effects to those outlined here for gestational stress have been associated with epigenetic or programming effects, but this mechanistic understanding of the effects has largely been unexplored in livestock species.
Implications for livestock production and future research
There are several traits that have not been considered fully in the target species and aspects of normal agricultural practice that could have an effect on prenatal development but have never been assessed. In particular, those relating to animal management and potential for psychological stress, such as aspects of housing, social stress from interactions with conspecifics, have been shown to be very influential in pigs but rarely assessed in ruminants. Studies have almost invariably been performed under controlled experimental conditions and translating these findings to the vast array of global livestock production systems is problematic. Furthermore, many of these studies have focused on developing our mechanistic understanding of the underlying biology (including epigenetics) rather than clearly establishing commercially relevant adult phenotypes. This reflects the nature of funding over the past two decades, which has largely come from research councils and charities that have a clear biomedical slant. Consequently, there are larger bodies of data on sheep and pigs than on cattle. Finally, in experimental studies, frequently only single factors are assessed, with other effects being tightly controlled. In reality, animals are exposed to multiple, concurrent stressors (e.g. undernutrition, high stocking density and thermal challenge), which can have a greater effect than when applied individually.
With these caveats in mind, traits of commercial importance that can be influenced by fetal development include dystocia and neonatal survival, growth rate and feed conversion, offspring health and disease susceptibility, saleable meat yield and quality, behavioural traits associated with ease of handling and reproductive potential, including fertility and litter size. However, issues surrounding effect size and, consequently, significance remain. A case in point is development of the fetal mammary gland and lifetime lactational performance. In addition, in all but the most extreme cases of maternal malnutrition during pregnancy, there is little evidence that muscle development can be manipulated in utero in a manner that would lead to permanent and measurable differences in carcass and saleable meat yields in slaughtered offspring. In contrast, although less is known about the developmental processes that lead to the formation of mature adipose tissues, it appears that these depots in offspring may be affected by maternal diet during pregnancy to a much greater extent than is the case for muscle.
There is huge gap in our understanding of how basic mechanisms regulating appetite can be programmed in utero. This could have huge implications for reducing greenhouse gas emissions and for improving residual feed intake. In contrast, there is compelling evidence that exposure to environmental chemicals (as can occur on sewage sludge-treated pastures) during in utero development can affect components of the reproductive axis. Male fetuses are more sensitive than female fetuses, with defects in testis development and sperm production being reported in ram lambs at 20 months of age. However, consequences for subsequent flock fertility remain to be ascertained.
No convincing picture is emerging of a detrimental effect of maternal nutrition on subsequent behavioural reactivity in offspring, although several studies have reported changes in responsiveness. However, whether this is associated with poorer welfare or increased difficulty in handling has not been clearly demonstrated. Finally, as we enter an era of genomic evaluation of farm livestock, we should be mindful of potentially adverse developmental outcomes following the use of advanced reproductive technologies. These technologies can lead to sporadic, but severe, developmental problems in offspring. Consequently, systems (both on-farm and (inter)nationally) should be instigated to record pregnancy outcomes, including obstetric complications and neonatal mortality, in order to firmly establish the nature and extent of any issues arising following ET. The advent of ‘precision farming’ and electronic identification, behavioural and physiological monitoring systems should facilitate such follow-up studies in future.
Acknowledgements
Financial support for composing this article was obtained from the Agriculture and Horticulture Development Board (AHDB, Beef and Lamb), UK. Concept of review was also initiated from discussions originating from EU COST Action FA1201, Epiconcept: Epigenetics and Periconception Environment.
References
Abecia, L., Martin-Garcia, A. I., Martinez, G., Newbold, C. J., and Yanez-Ruiz, D. R. (2013). Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning. J. Anim. Sci. 91, 4832–4840.| Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1SqtLvE&md5=87a6d573047c3fcd53055d802abdaf6bCAS | 23965388PubMed |
Adam, C. L., Findlay, P. A., Chanet, A., Aitken, R. P., Milne, J. S., and Wallace, J. M. (2008). Expression of energy balance regulatory genes in the developing ovine fetal hypothalamus at midgestation and the influence of hyperglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1895–R1900.
| Expression of energy balance regulatory genes in the developing ovine fetal hypothalamus at midgestation and the influence of hyperglycemia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVarsrg%3D&md5=269eaf1ce40985f77331e11deba66592CAS | 18417651PubMed |
Adam, C. L., Bake, T., Findlay, P. A., Milne, J. S., Aitken, R. P., and Wallace, J. M. (2011a). Effects of altered glucose supply and adiposity on expression of hypothalamic energy balance regulatory genes in late gestation growth restricted ovine fetuses. Int. J. Dev. Neurosci. 29, 775–781.
| Effects of altered glucose supply and adiposity on expression of hypothalamic energy balance regulatory genes in late gestation growth restricted ovine fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht12jur3I&md5=f88f2228e1d67b975ebd075fc924987bCAS | 21616134PubMed |
Adam, C. L., Findlay, P. A., Aitken, R. P., Milne, J. S., and Wallace, J. M. (2011b). Influence of birth weight, sex, age and adiposity on central leptin and insulin sensitivity in young growing sheep, as indicated by changes in voluntary food intake. Proc. Nutr. Soc. 70, E382.
| Influence of birth weight, sex, age and adiposity on central leptin and insulin sensitivity in young growing sheep, as indicated by changes in voluntary food intake.Crossref | GoogleScholarGoogle Scholar |
Adam, C. L., Bake, T., Findlay, P. A., Milne, J. S., Aitken, R. P., and Wallace, J. M. (2013). Impact of birth weight and gender on early postnatal hypothalamic energy balance regulatory gene expression in the young lamb. Int. J. Dev. Neurosci. 31, 608–615.
| Impact of birth weight and gender on early postnatal hypothalamic energy balance regulatory gene expression in the young lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslKqs7fK&md5=46d2bcf4cf31a977187d22ac609bf375CAS | 23932904PubMed |
Adam, C. L., Williams, P. A., Milne, J. S., Aitken, R. P., and Wallace, J. M. (2015). Orexigenic gene expression in late gestation ovine fetal hypothalamus is sensitive to maternal undernutrition and realimentation. J. Neuroendocrinol. 27, 765–771.
| Orexigenic gene expression in late gestation ovine fetal hypothalamus is sensitive to maternal undernutrition and realimentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFWrtbbI&md5=cdc5e6411dbe02faa98c44fb0d44ef9eCAS | 26212239PubMed |
Aiken, C. E., and Ozanne, S. E. (2014). Transgenerational developmental programming. Hum. Reprod. Update 20, 63–75.
| Transgenerational developmental programming.Crossref | GoogleScholarGoogle Scholar | 24082037PubMed |
Aitken, R. P., Milne, J. S., and Wallace, J. M. (2003). The impact of prenatal growth restriction on the onset of puberty, ovulation rate and uterine capacity in sheep. Pediatr. Res. 6, 34A.
Alm, H., Torner, H., Tiemann, U., and Kanitz, W. (1998). Influence of organochlorine pesticides on maturation and postfertilization development of bovine oocytes in vitro. Reprod. Toxicol. 12, 559–563.
| Influence of organochlorine pesticides on maturation and postfertilization development of bovine oocytes in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtVKqu7o%3D&md5=ea7457b1dd38febf9b64a44a1a4911eaCAS | 9763248PubMed |
Alworth, L. C., Howdeshell, K. L., Ruhlen, R. L., Day, J. K., Lubahn, D. B., Huang, T. H., Besch-Williford, C. L., and vom Saal, F. S. (2002). Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol. Appl. Pharmacol. 183, 10–22.
| Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xms1Wmt7Y%3D&md5=5befa92fcc1785b49116875abff84f03CAS | 12217638PubMed |
Amen, T. S., Herring, A. D., Sanders, J. O., and Gill, C. A. (2007). Evaluation of reciprocal differences in Bos indicus x Bos taurus backcross calves produced through embryo transfer: I. Birth and weaning traits. J. Anim. Sci. 85, 365–372.
| Evaluation of reciprocal differences in Bos indicus x Bos taurus backcross calves produced through embryo transfer: I. Birth and weaning traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1elsrg%3D&md5=63a9c8ae52518303fe70f922a8c4b7c4CAS | 17235021PubMed |
Anderson, L. L., Hard, D. L., and Kertiles, L. P. (1979). Progesterone secretion and fetal development during prolonged starvation in the pig. Am. J. Physiol. 236, E335–E341.
| 1:CAS:528:DyaE1MXitValt74%3D&md5=aa7b2972806eeea92f0cb9936120db4eCAS | 434195PubMed |
Andrade, L. P., Rhind, S. M., Rae, M. T., Kyle, C. E., Jowett, J., and Lea, R. G. (2013). Maternal undernutrition does not alter Sertoli cell numbers or the expression of key developmental markers in the mid-gestation ovine fetal testis. J. Negat. Results Biomed. 12, 2.
| Maternal undernutrition does not alter Sertoli cell numbers or the expression of key developmental markers in the mid-gestation ovine fetal testis.Crossref | GoogleScholarGoogle Scholar | 23295129PubMed |
Andreoli, K. M., Minton, J. E., Spire, M. F., and Schalles, R. R. (1988). Influence of Prepartum Exposure of Beef Heifers to Winter Weather on Concentrations of Plasma Energy-Yielding Substrates, Serum Hormones and Birth-Weight of Calves. Theriogenology 29, 631–642.
| Influence of Prepartum Exposure of Beef Heifers to Winter Weather on Concentrations of Plasma Energy-Yielding Substrates, Serum Hormones and Birth-Weight of Calves.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvFWmsw%3D%3D&md5=843ff8b2477ed7bdd15884973b577b3dCAS | 16726385PubMed |
Anway, M. D., Cupp, A. S., Uzumcu, M., and Skinner, M. K. (2005). Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469.
| Epigenetic transgenerational actions of endocrine disruptors and male fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXks1eqsbY%3D&md5=e251b584dd05f99f9d8360f8a0641ba6CAS | 15933200PubMed |
Arnott, G., Roberts, D., Rooke, J. A., Turner, S. P., Lawrence, A. B., and Rutherford, K. M. (2012). Board invited review: The importance of the gestation period for welfare of calves: maternal stressors and difficult births. J. Anim. Sci. 90, 5021–5034.
| Board invited review: The importance of the gestation period for welfare of calves: maternal stressors and difficult births.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXisFSjs7c%3D&md5=06e3c9082cf571637a7e5ae77753fc70CAS | 22952359PubMed |
Ashworth, C. J., Hogg, C. O., Matheson, S., Dwyer, C. M., and Rooke, J. A. (2014). Breed, but not maternal under nutrition, affects markers of immune status in lambs. Adv. Anim. Biosci. 5, 154.
Azzam, S. M., Kinder, J. E., Nielsen, M. K., Werth, L. A., Gregory, K. E., Cundiff, L. V., and Koch, R. M. (1993). Environmental-Effects on Neonatal-Mortality of Beef-Calves. J. Anim. Sci. 71, 282–290.
| 1:STN:280:DyaK3s7nvFehsA%3D%3D&md5=31a49ec99204ccd3413058aa1afa117aCAS | 8440645PubMed |
Bach, A. (2012). Nourishing and managing the dam and postnatal calf for optimal lactation, reproduction and immunity. J. Anim. Sci. 90, 1835–1845.
| Nourishing and managing the dam and postnatal calf for optimal lactation, reproduction and immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsFWgsbo%3D&md5=933aa297c111b062f93c832a4243f715CAS | 21926322PubMed |
Banchero, G., Vazquez, A., Montossi, F., de Barbieri, I., and Quintans, G. (2010). Pre-partum shearing of ewes under pastoral conditions improves the early vigour of both single and twin lambs. Anim. Prod. Sci. 50, 309–314.
| Pre-partum shearing of ewes under pastoral conditions improves the early vigour of both single and twin lambs.Crossref | GoogleScholarGoogle Scholar |
Banos, G., Brotherstone, S., and Coffey, M. P. (2007). Prenatal maternal effects on body condition score, female fertility, and milk yield of dairy cows. J. Dairy Sci. 90, 3490–3499.
| Prenatal maternal effects on body condition score, female fertility, and milk yield of dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlCksbw%3D&md5=d66090adec68c4bb8f82829ee35d73d8CAS | 17582133PubMed |
Barker, D. J., and Osmond, C. (1986). Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 327, 1077–1081.
| Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales.Crossref | GoogleScholarGoogle Scholar |
Barker, D. J., and Osmond, C. (1988). Low birth weight and hypertension. BMJ 297, 134–135.
| Low birth weight and hypertension.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1czhtVaisA%3D%3D&md5=e9d88334c831b93afb50470eb8c648bbCAS | 3408942PubMed |
Barker, D. J., Winter, P. D., Osmond, C., Margetts, B., and Simmonds, S. J. (1989). Weight in infancy and death from ischaemic heart disease. Lancet 334, 577–580.
| Weight in infancy and death from ischaemic heart disease.Crossref | GoogleScholarGoogle Scholar |
Barrier, A. C., Haskell, M. J., Birch, S., Bagnall, A., Bell, D. J., Dickinson, J., Macrae, A. I., and Dwyer, C. M. (2013). The impact of dystocia on dairy calf health, welfare, performance and survival. Vet. J. 195, 86–90.
| The impact of dystocia on dairy calf health, welfare, performance and survival.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bnslSnug%3D%3D&md5=b1986d34f424857ca19b8bb0ebfe67c4CAS | 22985606PubMed |
Baxter, E. M., Jarvis, S., D’Eath, R. B., Ross, D. W., Robson, S. K., Farish, M., Nevison, I. M., Lawrence, A. B., and Edwards, S. A. (2008). Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69, 773–783.
| Investigating the behavioural and physiological indicators of neonatal survival in pigs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3js1yhsA%3D%3D&md5=3eb6d2175e884e75fdddc5fc9c1b7a73CAS | 18242685PubMed |
Baxter, E. M., Mulligan, J., Hall, S. A., Donbavand, J. E., Zanella, A. J., and Dwyer, C. M. (2016). Positive and negative gestational handling influences placental traits and mother-offspring behavior in dairy goats. Physiol. Behav. 157, 129–138.
| Positive and negative gestational handling influences placental traits and mother-offspring behavior in dairy goats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xitl2hsrg%3D&md5=530e2ce2cf30fc859fd5da90216f984bCAS | 26850289PubMed |
Bebbere, D., Bauersachs, S., Furst, R. W., Reichenbach, H. D., Reichenbach, M., Medugorac, I., Ulbrich, S. E., Wolf, E., Ledda, S., and Hiendleder, S. (2013). Tissue-specific and minor inter-individual variation in imprinting of IGF2R is a common feature of Bos taurus Concepti and not correlated with fetal weight. PLoS One 8, e59564.
| Tissue-specific and minor inter-individual variation in imprinting of IGF2R is a common feature of Bos taurus Concepti and not correlated with fetal weight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmtlSnu7w%3D&md5=7948fa7feee03b721d16f312662ae891CAS | 23593146PubMed |
Begum, G., Stevens, A., Smith, E. B., Connor, K., Challis, J. R., Bloomfield, F., and White, A. (2012). Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 26, 1694–1703.
| Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsVCht70%3D&md5=80408a67bc82aeb274fc2382d53c8b1fCAS | 22223754PubMed |
Bellingham, M., Fowler, P. A., Amezaga, M. R., Rhind, S. M., Cotinot, C., Mandon-Pepin, B., Sharpe, R. M., and Evans, N. P. (2009). Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ. Health Perspect. 117, 1556–1562.
| Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCjtbvN&md5=2d79c98a8bb30d8b838e4e6487ff772cCAS | 20019906PubMed |
Bellingham, M., Fowler, P. A., Amezaga, M. R., Whitelaw, C. M., Rhind, S. M., Cotinot, C., Mandon-Pepin, B., Sharpe, R. M., and Evans, N. P. (2010). Foetal hypothalamic and pituitary expression of gonadotrophin-releasing hormone and galanin systems is disturbed by exposure to sewage sludge chemicals via maternal ingestion. J. Neuroendocrinol. 22, 527–533.
| Foetal hypothalamic and pituitary expression of gonadotrophin-releasing hormone and galanin systems is disturbed by exposure to sewage sludge chemicals via maternal ingestion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvFCmsbo%3D&md5=4a36d71377053825a2b2a3017834cf6cCAS | 20236231PubMed |
Bellingham, M., McKinnell, C., Fowler, P. A., Amezaga, M. R., Zhang, Z., Rhind, S. M., Cotinot, C., Mandon-Pepin, B., Evans, N. P., and Sharpe, R. M. (2012). Foetal and post-natal exposure of sheep to sewage sludge chemicals disrupts sperm production in adulthood in a subset of animals. Int. J. Androl. 35, 317–329.
| Foetal and post-natal exposure of sheep to sewage sludge chemicals disrupts sperm production in adulthood in a subset of animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFCisrbK&md5=179cd759ec4bae4e780032ae0eab7a33CAS | 22150464PubMed |
Berkowicz, E. W., Magee, D. A., Sikora, K. M., Berry, D. P., Howard, D. J., Mullen, M. P., Evans, R. D., Spillane, C., and MacHugh, D. E. (2011). Single nucleotide polymorphisms at the imprinted bovine insulin-like growth factor 2 (IGF2) locus are associated with dairy performance in Irish Holstein-Friesian cattle. J. Dairy Res. 78, 1–8.
| Single nucleotide polymorphisms at the imprinted bovine insulin-like growth factor 2 (IGF2) locus are associated with dairy performance in Irish Holstein-Friesian cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFylug%3D%3D&md5=f06010a6f786ee1dd56b072761559b24CAS | 20822563PubMed |
Bermejo-Alvarez, P., Rizos, D., Rath, D., Lonergan, P., and Gutierrez-Adan, A. (2010). Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc. Natl. Acad. Sci. USA 107, 3394–3399.
| Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFymtbo%3D&md5=efd126a965fd9904445fa7ad3eb1424cCAS | 20133684PubMed |
Berry, D. P., Lonergan, P., Butler, S. T., Cromie, A. R., Fair, T., Mossa, F., and Evans, A. C. O. (2008). Negative influence of high maternal milk production before and after conception on offspring survival, and milk production in dairy cattle. J. Dairy Sci. 91, 329–337.
| Negative influence of high maternal milk production before and after conception on offspring survival, and milk production in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVGisA%3D%3D&md5=2e090fc34baaf35989641b4419b3565eCAS | 18096955PubMed |
Berry, D. C., Stenesen, D., Zeve, D., and Graff, J. M. (2013). The developmental origins of adipose tissue. Development 140, 3939–3949.
| The developmental origins of adipose tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslSmsrbJ&md5=1041b80af8212e6b171e4ee384671fd8CAS | 24046315PubMed |
Betancourt, M., Resendiz, A., and Fierro, E. C. (2006). Effect of two insecticides and two herbicides on the porcine sperm motility patterns using computer-assisted semen analysis (CASA) in vitro. Reprod. Toxicol. 22, 508–512.
| Effect of two insecticides and two herbicides on the porcine sperm motility patterns using computer-assisted semen analysis (CASA) in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xps1Cjt7k%3D&md5=73eb66c290bc329ad66e79a6225f28f8CAS | 16713176PubMed |
Bhattacharya, T. K., and Chatterjee, R. N. (2013). Polymorphism of the myostatin gene and its association with growth traits in chicken. Poult. Sci. 92, 910–915.
| Polymorphism of the myostatin gene and its association with growth traits in chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsV2ktL4%3D&md5=03e20431d31f921ec67415e2c5234476CAS | 23472013PubMed |
Bielli, A., Pérez, R., Pedrana, G., Milton, J. T. B., Lopez, A., Blackberry, M. A., Duncombe, G., Rodriguez-Martinez, H., and Martin, G. B. (2002). Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb. Reprod. Fertil. Dev. 14, 333–337.
| Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb.Crossref | GoogleScholarGoogle Scholar |
Billon, N., and Dani, C. (2012). Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. Rep. 8, 55–66.
| Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVCrtrg%3D&md5=a229e492164b436c9a38e4948b03ae86CAS |
Billon, N., Iannarelli, P., Monteiro, M. C., Glavieux-Pardanaud, C., Richardson, W. D., Kessaris, N., Dani, C., and Dupin, E. (2007). The generation of adipocytes by the neural crest. Development 134, 2283–2292.
| The generation of adipocytes by the neural crest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns1ygsro%3D&md5=88e0ff19067b1b4e68ffc9193a78eaadCAS | 17507398PubMed |
Billon, N., Monteiro, M. C., and Dani, C. (2008). Developmental origin of adipocytes: new insights into a pending question. Biol. Cell 100, 563–575.
| Developmental origin of adipocytes: new insights into a pending question.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFamsLrM&md5=29adc6b76d5b405b175dda30b714e02bCAS | 18793119PubMed |
Blair, H. T., Jenkinson, C. M. C., Peterson, S. W., Kenyon, P. R., van der Lindon, D. S., Davenport, L. C., Mackenzie, D. D. S., Morris, S. T., and Firth, E. C. (2010). Dam and granddam feeding during pregnancy in sheep affects milk supply in offspring and reproductive performance in grand-offspring. J. Anim. Sci. 88, E40–E50.
| Dam and granddam feeding during pregnancy in sheep affects milk supply in offspring and reproductive performance in grand-offspring.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3pslyrtQ%3D%3D&md5=a2c55a82d08c427a49bc5a0444d650f4CAS | 19966171PubMed |
Boland, T. M., Guinan, M., Brophy, P. O., Callan, J. J., Quinn, P. J., Nowakowski, P., and Crosby, T. F. (2005). The effect of varying levels of mineral and iodine supplementation to ewes during late pregnancy on serum immunoglobulin G concentrations in their progeny. Anim. Sci. 80, 209–218.
| The effect of varying levels of mineral and iodine supplementation to ewes during late pregnancy on serum immunoglobulin G concentrations in their progeny.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktVOnsrk%3D&md5=ab2c53c3aedbfa57c97af6e064bac3f4CAS |
Bonilla, L., Block, J., Denicol, A. C., and Hansen, P. J. (2014). Consequences of transfer of an in vitro-produced embryo for the dam and resultant calf. J. Dairy Sci. 97, 229–239.
| Consequences of transfer of an in vitro-produced embryo for the dam and resultant calf.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslClt77I&md5=61b039f39f3f79677b83fe12f92e2886CAS | 24210495PubMed |
Borengasser, S. J., Zhong, Y., Kang, P., Lindsey, F., Ronis, M. J., Badger, T. M., Gomez-Acevedo, H., and Shankar, K. (2013). Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 154, 4113–4125.
| Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs12qsLfE&md5=b59e41f938eeaf3d6de4d7e5fecf2b5fCAS | 23959936PubMed |
Borwick, S. C., Rhind, S. M., McMillen, S. R., and Racey, P. A. (1997). Effect of undernutrition of ewes from the time of mating on fetal ovarian development in mid gestation. Reprod. Fertil. Dev. 9, 711–715.
| Effect of undernutrition of ewes from the time of mating on fetal ovarian development in mid gestation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3osV2nuw%3D%3D&md5=6461fa3f4ad82f0c724c89c66707870bCAS | 9623491PubMed |
Borwick, S. C., Rae, M. T., Brooks, J., McNeilly, A. S., Racey, P. A., and Rhind, S. M. (2003). Undernutrition of ewe lambs in utero and in early post-natal life does not affect hypothalamic–pituitary function in adulthood. Anim. Reprod. Sci. 77, 61–70.
| Undernutrition of ewe lambs in utero and in early post-natal life does not affect hypothalamic–pituitary function in adulthood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlGiu7c%3D&md5=8fe066904000a5f332de7db41b16d6ebCAS | 12654528PubMed |
Bouret, S. G. (2013). Organizational actions of metabolic hormones. Front. Neuroendocrinol. 34, 18–26.
| Organizational actions of metabolic hormones.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitFKmtLo%3D&md5=9206e7438f1562ca3e7a2629e7d68ebfCAS | 23357643PubMed |
Bouret, S. G., and Simerly, R. B. (2007). Development of leptin-sensitive circuits. J. Neuroendocrinol. 19, 575–582.
| Development of leptin-sensitive circuits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXoslKqurs%3D&md5=0169acb346c8ff2637a30c64aecf74e6CAS | 17620099PubMed |
Bowen, J. M., Dahl, G. E., Evans, N. P., Thrun, L. A., Wang, Y., Brown, M. B., and Karsch, F. J. (1998). Importance of the gonadotropin-releasing hormone (GnRH) surge for induction of the preovulatory luteinizing hormone surge of the ewe: dose–response relationship and excess of GnRH. Endocrinology 139, 588–595.
| 1:CAS:528:DyaK1cXns1Onuw%3D%3D&md5=abec8748a379794835b80083a1e42de1CAS | 9449629PubMed |
Brameld, J. M., and Daniel, Z. C. T. R. (2008). In utero effects on livestock muscle development and body composition. Aust. J. Exp. Agric. 48, 921–929.
| In utero effects on livestock muscle development and body composition.Crossref | GoogleScholarGoogle Scholar |
Brameld, J. M., Fahey, A. J., Langley-Evans, S. C., and Buttery, P. J. (2003). Nutritional and hormonal control of muscle growth and fat deposition. Arch. Tierz. Dummerstorf 46, 143–156.
Braunschweig, M., Jagannathan, V., Gutzwiller, A., and Bee, G. (2012). Investigations on transgenerational epigenetic response down the male line in F2 pigs. PLoS One 7, e30583.
| Investigations on transgenerational epigenetic response down the male line in F2 pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFWisL4%3D&md5=98c1bf503c4ffa40ef10dae53066e7ecCAS | 22359544PubMed |
Breton, A. B., Cockrum, R. R., Austin, K. J., Cammack, K. M., Ford, S. P., Hess, B. W., Moss, G. E., Nathanielsz, P. W., and Alexander, B. M. (2011). Hypothalamic expression of genes for appetite regulators and estrogen alpha, estrogen beta and leptin receptors in obese dams and their fetuses. Animal 5, 1944–1948.
| Hypothalamic expression of genes for appetite regulators and estrogen alpha, estrogen beta and leptin receptors in obese dams and their fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVCgtbvK&md5=75347a53d7ee57bd7a66f3ebdb8bcc75CAS | 22440471PubMed |
Brevini, T. A., Vassena, R., Paffoni, A., Francisci, C., Fascio, U., and Gandolfi, F. (2004). Exposure of pig oocytes to PCBs during in vitro maturation: effects on developmental competence, cytoplasmic remodelling and communication with cumulus cells. Eur. J. Histochem. 48, 347–356.
| 1:STN:280:DC%2BD2M%2FovVCguw%3D%3D&md5=59cc234ec4daa72cc5a947280eb47382CAS | 15718200PubMed |
Brickell, J. S., McGowan, M. M., and Wathes, D. C. (2009). Effect of management factors and blood metabolites during the rearing period on growth in dairy heifers on UK farms. Domest. Anim. Endocrinol. 36, 67–81.
| Effect of management factors and blood metabolites during the rearing period on growth in dairy heifers on UK farms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ahtA%3D%3D&md5=d64db22d0efb5f21beedd5f4f0f2acefCAS | 19059748PubMed |
Brien, F. D., Cloete, S. W. P., Fogarty, N. M., Greeff, J. C., Hebart, M. L., Hiendleder, S., Hocking Edwards, J. E., Kelly, J. M., Kind, K. L., Kleeman, D. O., Plush, K. L., and Miller, D. R. (2014). A review of genetic and epigenetic factors affecting lamb survival. Anim. Prod. Sci. 54, 667–693.
| A review of genetic and epigenetic factors affecting lamb survival.Crossref | GoogleScholarGoogle Scholar |
Buckingham, M. (2007). Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 330, 530–533.
| Skeletal muscle progenitor cells and the role of Pax genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnslGmtLc%3D&md5=19bf31150071f1bc12641dc003a19ce9CAS | 17631448PubMed |
Buckingham, M., and Vincent, S. D. (2009). Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 19, 444–453.
| Distinct and dynamic myogenic populations in the vertebrate embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCrsb7E&md5=d6984517b35c6b1f30af99f6032834b1CAS | 19762225PubMed |
Burt, B. E., Hess, B. W., Nathanielsz, P. W., and Ford, S. P. (2007). Flock differences in the impact of maternal dietary restriction on offspring growth and glucose tolerance in female offspring. Soc. Reprod. Fertil. Suppl. 64, 411–424.
| 1:CAS:528:DC%2BD1cXpvVyrsb0%3D&md5=1e4af84f2b78f85076167b54aba53c80CAS | 17491162PubMed |
Buttery, P. J., Brameld, J. M., and Dawson, J. M. (2000). Control and manipulation of hyperplasia and hypertrophy in muscle tissue. In ‘Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction’. (Ed. P. B. Cronje.) pp. 237–254. (CAB International.)
Cafe, L. M., Hennessy, D. W., Hearnshaw, H., Morris, S. T., and Greenwood, P. L. (2006). Influences of nutrition during pregnancy and lactation on birthweights and growth to weaning of calves sired by Piedmontese and Wagyu bulls. Aust. J. Exp. Agric. 46, 245–255.
| Influences of nutrition during pregnancy and lactation on birthweights and growth to weaning of calves sired by Piedmontese and Wagyu bulls.Crossref | GoogleScholarGoogle Scholar |
Cahill, R. N. P., Kimpton, W. G., Washington, E. A., Holder, J. E., and Cunningham, C. P. (1999). The ontogeny of T cell recirculation during foetal life. Semin. Immunol. 11, 105–114.
| The ontogeny of T cell recirculation during foetal life.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3mtlCjuw%3D%3D&md5=f179413cc82e35b842f02786c3569714CAS |
Cameron, N. M., Shahrokh, D., Del Corpo, A., Dhir, S. K., Szyf, M., Champagne, F. A., and Meaney, M. J. (2008). Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J. Neuroendocrinol. 20, 795–801.
| Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1Cju7k%3D&md5=a08f965db43bd65ce8a0f239c928d391CAS | 18513204PubMed |
Campagna, C., Sirad, M. A., Ayotte, P., and Bailey, J. L. (2001). Impaired maturation, fertilization and embryonic development of porcine oocytes following exposure to an environmentally relevant organochlorine mixture. Biol. Reprod. 65, 554–560.
| Impaired maturation, fertilization and embryonic development of porcine oocytes following exposure to an environmentally relevant organochlorine mixture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXls1Wns78%3D&md5=a77ee67febc78336123417ea667cd4e3CAS | 11466225PubMed |
Campagna, C., Guillemette, C., Paradis, R., Sirard, M.-A., and Ayotte, P. (2002). An environmentally relevant organochlorine mixture impairs sperm function and embryo development in the porcine model. Biol. Reprod. 67, 80–87.
| An environmentally relevant organochlorine mixture impairs sperm function and embryo development in the porcine model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvV2it7w%3D&md5=b74c37ea7e0a3f790fbac27a0cd19ebdCAS | 12080002PubMed |
Campagna, C., Ayotte, P., Sirard, M. A., and Bailey, J. L. (2008). An environmentally relevant mixture of organochlorines, their metabolites and effects on preimplantation development of porcine embryos. Reprod. Toxicol. 25, 361–366.
| An environmentally relevant mixture of organochlorines, their metabolites and effects on preimplantation development of porcine embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtlyhsL8%3D&md5=5a764cdd4194b0dae4eb41678ed702eeCAS | 18479888PubMed |
Caraty, A., Decourt, C., Briant, C., and Beltramo, M. (2012). Kisspeptins and the reproductive axis: potential applications to manage reproduction in farm animals. Domest. Anim. Endocrinol. 43, 95–102.
| Kisspeptins and the reproductive axis: potential applications to manage reproduction in farm animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtVCitr8%3D&md5=be68b99502b9b1c4a1e4146ae8acf801CAS | 22533939PubMed |
Carlson, L. L., Page, A. W., and Bestor, T. H. (1992). Properties and localization of DNA methyltransferase in preimplantation mouse embryos: implications for genomic imprinting. Genes Dev. 6, 2536–2541.
| Properties and localization of DNA methyltransferase in preimplantation mouse embryos: implications for genomic imprinting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXnslOqtg%3D%3D&md5=d997e1d2eac0cd36f7ea68203e1fb778CAS | 1340468PubMed |
Cedar, H., and Bergman, Y. (2009). Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 10, 295–304.
| Linking DNA methylation and histone modification: patterns and paradigms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksF2rsL0%3D&md5=098b9fce53ad95d5ba49d7c8a7fef7f4CAS | 19308066PubMed |
Chavatte-Palmer, P., Heyman, Y., and Renard, J. P. (2000). Cloning and associated physiopathology of gestation. Gynecol. Obstet. Fertil. 28, 633–642.
| 1:STN:280:DC%2BD3M%2Fkt1enug%3D%3D&md5=48cb4f28ca4babc9549a7b335d6f0cedCAS | 11075501PubMed |
Chavatte-Palmer, P., Remy, D., Cordonnier, N., Richard, C., Issenman, H., Laigre, P., Heyman, Y., and Mialot, J. P. (2004). Health status of cloned cattle at different ages. Cloning Stem Cells 6, 94–100.
| Health status of cloned cattle at different ages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFelurw%3D&md5=46cc6e10d05c7d343efab640b546dbadCAS | 15268782PubMed |
Chen Cárdenas, S. M., Mayer, N., Romanini, M. C., Rolando, A. N., Liaudat, A. C., Brun, N., Vivas, A., Gauna, H. F., and Rodríguez, N. (2013). Reproductive response in offspring male rats exposed to prenatal stress and to early postnatal stimulation. Int. J. Morphol. 31, 754–764.
| Reproductive response in offspring male rats exposed to prenatal stress and to early postnatal stimulation.Crossref | GoogleScholarGoogle Scholar |
Chomwisarutkun, K., Murani, E., Ponsuksili, S., and Wimmers, K. (2012). Gene expression analaysis of mammary tissue during fetal bud formation and growth in two pig breeds – indications of prenatal initiation of postnatal phenotypic differences. BMC Dev. Biol. 12, 13–29.
| Gene expression analaysis of mammary tissue during fetal bud formation and growth in two pig breeds – indications of prenatal initiation of postnatal phenotypic differences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1SksLk%3D&md5=78e6f6cad15e1eb229ddec0222a07b3cCAS | 22537077PubMed |
Clop, A., Marcq, F., Takeda, H., Pirottin, D., Tordoir, X., Bibe, B., Bouix, J., Caiment, F., Elsen, J. M., Eychenne, F., Larzul, C., Laville, E., Meish, F., Milenkovic, D., Tobin, J., Charlier, C., and Georges, M. (2006). A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 38, 813–818.
| A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFyrurk%3D&md5=fba6e7a51b1c20341d431cfe220d6d0eCAS | 16751773PubMed |
Collet, S. H., Picard-Hagen, N., Viguie, C., Lacroix, M. Z., Toutain, P. L., and Gayrard, V. (2010). Estrogenicity of bisphenol A: a concentration–effect relationship on luteinizing hormone secretion in a sensitive model of prepubertal lamb. Toxicol. Sci. 117, 54–62.
| Estrogenicity of bisphenol A: a concentration–effect relationship on luteinizing hormone secretion in a sensitive model of prepubertal lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGrtLbL&md5=daa3ae6fa1c25e8a1cebbb17e95158aeCAS | 20566471PubMed |
Collier, R. J., Doelger, S. G., Head, H. H., Thatcher, W. W., and Wilcox, C. J. (1982). Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J. Anim. Sci. 54, 309–319.
| 1:CAS:528:DyaL38XhtF2qu7s%3D&md5=4a13d6a302f13435f4636b8a247f6143CAS | 7076593PubMed |
Collier, C. T., Williams, P. N., Carroll, J. A., Welsh, T. H., and Laurenz, J. C. (2011). Effect of maternal restraint stress during gestation on temporal lipopolysaccharide-induced neuroendocrine and immune responses of progeny. Domest. Anim. Endocrinol. 40, 40–50.
| Effect of maternal restraint stress during gestation on temporal lipopolysaccharide-induced neuroendocrine and immune responses of progeny.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsV2gu7fP&md5=ac3e8ad34e592a863a215a5b1fff335eCAS | 20932703PubMed |
Corah, L. R., Dunn, T. G., and Kaltenbach, C. C. (1975). Influence of prepartum nutrition on the reproductive performance of beef females and the performance of their progeny. J. Anim. Sci. 41, 819–824.
| 1:STN:280:DyaE28%2FgsFaqtA%3D%3D&md5=e0fe3ab4a614066c918be75b22e36b74CAS | 1158813PubMed |
Corbel, T., Gayrard, V., Vigue, C., Puel, S., Lecroix, M. Z., Toutain, P.-L., and Picard-Hagen, N. (2013). Bisphenol A disposition in the sheep maternal-placental-fetal unit: mechanisms determining fetal internal exposure. Biol. Reprod. 89, 11.
| Bisphenol A disposition in the sheep maternal-placental-fetal unit: mechanisms determining fetal internal exposure.Crossref | GoogleScholarGoogle Scholar | 23699389PubMed |
Corbel, T., Perdu, E., Gayrard, V., Puel, S., Lacroix, M. Z., Viguie, C., Toutain, P.-L., Zalko, D., and Picard-Hagen, N. (2015). Conjugation and deconjugation reactions within the feto-pacental compartment in a sheep model; a key factor defining Bisphenol A fetal exposure. Drug Metab. Dispos. 43, 467–476.
| Conjugation and deconjugation reactions within the feto-pacental compartment in a sheep model; a key factor defining Bisphenol A fetal exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXktF2mur0%3D&md5=f67b3ed3b4f57a350b1ea1a346f0fb59CAS | 25576162PubMed |
Coupé, B., Amarger, V., Grit, I., Benani, A., and Parnet, P. (2010). Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 151, 702–713.
| Nutritional programming affects hypothalamic organization and early response to leptin.Crossref | GoogleScholarGoogle Scholar | 20016030PubMed |
Couret, D., Jamin, A., Kuntz-Simon, G., Prunier, A., and Merlot, E. (2009). Maternal stress during late pregnancy has moderate but long-lasting effects on the immune system of the piglets. Vet. Immunol. Immunopathol. 131, 17–24.
| Maternal stress during late pregnancy has moderate but long-lasting effects on the immune system of the piglets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVSit77K&md5=c78939f09e2508873d2cf980693a1af4CAS | 19362376PubMed |
Crenshaw, J. D., Park, C. S., Swantek, P. M., Keller, W. L., and Zimprich, R. C. (1989). ‘Phased-Feeding Scheme for Developing Gilts (ND1783 and ND1787).’ (Agriculture Experimental Station, North Dakota State University: Fargo.)
Cristancho, A. G., and Lazar, M. A. (2011). Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734.
| Forming functional fat: a growing understanding of adipocyte differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1anu7%2FN&md5=fca3b215a6636d6a2c379996d14ebb77CAS | 21952300PubMed |
Cronjé, P. B. (2003). Foetal programming of immune competence. Aust. J. Exp. Agric. 43, 1427–1430.
| Foetal programming of immune competence.Crossref | GoogleScholarGoogle Scholar |
Crosier, A. E., Farin, C. E., Rodriguez, K. F., Blondin, P., Alexander, J. E., and Farin, P. W. (2002). Development of skeletal muscle and expression of candidate genes in bovine fetuses from embryos produced in vivo or in vitro. Biol. Reprod. 67, 401–408.
| Development of skeletal muscle and expression of candidate genes in bovine fetuses from embryos produced in vivo or in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsFKqtr0%3D&md5=809df4bfca126c6454928f96cc34742aCAS | 12135873PubMed |
Cunningham, C. P., Cahill, R. N. P., Washington, E. A., Holder, J. E., Twohig, J. P., and Kimpton, W. G. (1999). Regulation of T cell homeostasis during fetal and early postnatal life. Vet. Immunol. Immunopathol. 72, 175–181.
| Regulation of T cell homeostasis during fetal and early postnatal life.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnsl2ltb8%3D&md5=176d37b03a72a481d33a081aecfebc41CAS | 10614507PubMed |
Cushman, R. A., Allan, M. F., Kuehn, L. A., Snelling, W. M., Cupp, A. S., and Freetly, H. C. (2009). Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight. J. Anim. Sci. 87, 1971–1980.
| Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1eju7w%3D&md5=3bece7ec0e42622cfc623e00b75dcb9fCAS | 19286826PubMed |
Da Silva, P., Aitken, R. P., Rhind, S. M., Racey, P. A., and Wallace, J. M. (2001). Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction 122, 375–383.
| Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntVGksLY%3D&md5=c4962867d3603fc5a6ff16cef9655315CAS | 11597303PubMed |
Da Silva, P., Aitken, R. P., Rhind, S. M., Racey, P. A., and Wallace, J. M. (2002). Impact of maternal nutrition during pregnancy on pituitary gonadotrophin gene expression and ovarian development in growth-restricted and normally grown late gestation sheep fetuses. Reproduction 123, 769–777.
| Impact of maternal nutrition during pregnancy on pituitary gonadotrophin gene expression and ovarian development in growth-restricted and normally grown late gestation sheep fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvVCiur8%3D&md5=9677445a213d4496d059b0acce1330afCAS | 12052231PubMed |
Da Silva, P., Aitken, R. P., Rhind, S. M., Racey, P. A., and Wallace, J. M. (2003). Effect of maternal overnutrition during pregnancy on pituitary gonadotrophin gene expression and gonadal morphology in female and male foetal sheep at Day 103 of gestation. Placenta 24, 248–257.
| Effect of maternal overnutrition during pregnancy on pituitary gonadotrophin gene expression and gonadal morphology in female and male foetal sheep at Day 103 of gestation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlOltA%3D%3D&md5=f45e19d09cd4a954e24d560ee70393fbCAS | 12566252PubMed |
Da Silva-Buttkus, P., van den Hurk, R., te Velde, E. R., and Taverne, M. A. M. (2003). Ovarian development in intrauterine growth-retarded and normally developed piglets originating from the same litter. Reproduction 126, 249–258.
| Ovarian development in intrauterine growth-retarded and normally developed piglets originating from the same litter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXntFyjtrs%3D&md5=57b4ecfce5aa8d8230a03af127debc7bCAS | 12887281PubMed |
Dahl, G. E., Tao, S., and Thompson, I. M. (2012). Effects of photoperiod on mammary gland development and lactation. J. Anim. Sci. 90, 755–760.
| Effects of photoperiod on mammary gland development and lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFSitbw%3D&md5=45729be26e7782838ff49d969198c663CAS | 21984715PubMed |
Daniel, Z. C., Brameld, J. M., Craigon, J., Scollan, N. D., and Buttery, P. J. (2007). Effect of maternal dietary restriction during pregnancy on lamb carcass characteristics and muscle fiber composition. J. Anim. Sci. 85, 1565–1576.
| Effect of maternal dietary restriction during pregnancy on lamb carcass characteristics and muscle fiber composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1antLc%3D&md5=3a0a433988cd92e130a1005e9177b248CAS | 17296773PubMed |
Darnaudéry, M., and Maccari, S. (2008). Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Brain Res. Rev. 57, 571–585.
| Epigenetic programming of the stress response in male and female rats by prenatal restraint stress.Crossref | GoogleScholarGoogle Scholar |
Das, R., Hampton, D. D., and Jirtle, R. L. (2009). Imprinting evolution and human health. Mamm. Genome 20, 563–572.
| Imprinting evolution and human health.Crossref | GoogleScholarGoogle Scholar | 19830403PubMed |
Davis, E., Jensen, C. H., Schroder, H. D., Farnir, F., Shay-Hadfield, T., Kliem, A., Cockett, N., Georges, M., and Charlier, C. (2004). Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype. Curr. Biol. 14, 1858–1862.
| Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovFant7g%3D&md5=5228d62257f90a186b943fff2dd1d2d1CAS | 15498495PubMed |
Daxinger, L., and Whitelaw, E. (2012). Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 13, 153–162.
| 1:CAS:528:DC%2BC38XhsVOru7c%3D&md5=791224fb726eeb88c5ab60e20c00698aCAS | 22290458PubMed |
De Blasio, M. J., Gatford, K. L., Robinson, J. S., and Owens, J. A. (2007). Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R875–R886.
| Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjt1Gmsr8%3D&md5=a406478e3f3b0ea45c979c27ea13b532CAS | 17023666PubMed |
De Blasio, M. J., Blache, D., Gatford, K. L., Robinson, J. S., and Owens, J. A. (2010). Placental restriction increases adipose leptin gene expression and plasma leptin and alters their relationship to feeding activity in the young lamb. Pediatr. Res. 67, 603–608.
| Placental restriction increases adipose leptin gene expression and plasma leptin and alters their relationship to feeding activity in the young lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFKktr0%3D&md5=39b3831f02fa8acbac526bcdd64afaa2CAS | 20220548PubMed |
de Rose, E. P., and Wilton, J. W. (1991). Productivity and profitability of twin births in beef cattle. J. Anim. Sci. 69, 3085–3093.
| 1:STN:280:DyaK3MzntlahtQ%3D%3D&md5=ad88136eba881165e23fcc4b4727b401CAS | 1894545PubMed |
Deaton, A. M., Webb, S., Kerr, A. R., Illingworth, R. S., Guy, J., Andrews, R., and Bird, A. (2011). Cell type-specific DNA methylation at intragenic CpG islands in the immune system. Genome Res. 21, 1074–1086.
| Cell type-specific DNA methylation at intragenic CpG islands in the immune system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVKktbY%3D&md5=6dc4c8a4a5b589dce4beab5810b3e243CAS | 21628449PubMed |
Dees, W. L., and McArthur, N. H. (1981). Immunohistochemical localization of gonadotropin releasing hormone (GnRH) in the bovine hypothalamus and infundibulum. Anat. Rec. 200, 281–285.
| Immunohistochemical localization of gonadotropin releasing hormone (GnRH) in the bovine hypothalamus and infundibulum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkslOmsb8%3D&md5=7c028212ed605104fb747b4c754ca89fCAS | 7023278PubMed |
Dhillo, W. S. (2007). Appetite regulation: an overview. Thyroid 17, 433–445.
| Appetite regulation: an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVSlsr0%3D&md5=082c6c7dea1d715203381cc001e9faebCAS | 17542673PubMed |
Drickamer, L. C., Rosenthal, T. L., and Arthur, R. D. (1999). Factors affecting the number of teats in pigs. J. Reprod. Fertil. 115, 97–100.
| Factors affecting the number of teats in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhs12is7s%3D&md5=8930ed67c84d55d532dbb56186eaceeaCAS | 10341727PubMed |
Ducolomb, Y., Casas, E., Valdez, A., Gonzalez, G., Altamirano-Lozano, M., and Betancourt, M. (2009). In vitro effect of malathion and diazinon on oocyte fertilization and embryo development in porcine. Cell Biol. Toxicol. 25, 623–633.
| In vitro effect of malathion and diazinon on oocyte fertilization and embryo development in porcine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlKhtLnL&md5=1e5132d142375ef5df6bfd6892606f3aCAS | 19148769PubMed |
Dupont, C., Cordier, A. G., Junien, C., Mandon-Pepin, B., Levy, R., and Chavatte-Palmer, P. (2012). Maternal environment and the reproductive function of the offspring. Theriogenology 78, 1405–1414.
| Maternal environment and the reproductive function of the offspring.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bhs1emug%3D%3D&md5=52fc1bef7de20583eefb57725b91d634CAS | 22925651PubMed |
Duprez, D. (2002). Signals regulating muscle formation in the limb during embryonic development. Int. J. Dev. Biol. 46, 915–925.
| 1:CAS:528:DC%2BD38XptlKhs7w%3D&md5=c0f03f41f0cdb4d13e0868947abcfe47CAS | 12455629PubMed |
Dwyer, C. M. (2008). Genetic and physiological determinants of maternal behavior and lamb survival: implications for low-input sheep management. J. Anim. Sci. 86, E246–E258.
| Genetic and physiological determinants of maternal behavior and lamb survival: implications for low-input sheep management.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3lsVyntg%3D%3D&md5=0c5e7d9c6c9f7981785a2a25c88766ecCAS | 17709772PubMed |
Dwyer, C. M., Lawrence, A. B., and Bishop, S. C. (2001). The effects of selection for lean tissue content on maternal and neonatal lamb behaviours in Scottish blackface sheep. Anim. Sci. 72, 555–571.
Dwyer, C. M., Lawrence, A. B., Bishop, S. C., and Lewis, M. (2003). Ewe–lamb bonding behaviours at birth are affected by maternal undernutrition in pregnancy. Br. J. Nutr. 89, 123–136.
| Ewe–lamb bonding behaviours at birth are affected by maternal undernutrition in pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsFWlsr0%3D&md5=8ece812a0c21e4966edd6327458a7168CAS | 12568672PubMed |
Eborn, D. R., Cushman, R. A., and Echternkamp, S. E. (2013). Effect of postweaning diet on ovarian development and fertility in replacement beef heifers. J. Anim. Sci. 91, 4168–4179.
| Effect of postweaning diet on ovarian development and fertility in replacement beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVOlsbrN&md5=01df914a5cd9594daa798cc4217ef089CAS | 23881678PubMed |
Edwards, S. A., and Baxter, E. M. (2015). Piglet mortality: causes and prevention. In ‘The Gestating and Lactating Sow’. (Ed. C. Farmer.) pp. 253–278. (Wageningen Academic Publishers: Wageningen.)
Ehrhardt, R. A., Greenwood, P. L., Bell, A. W., and Boisclair, Y. R. (2003). Plasma leptin is regulated predominantly by nutrition in preruminant lambs. J. Nutr. 133, 4196–4201.
| 1:CAS:528:DC%2BD3sXpslGmurs%3D&md5=54e3fcfc87e77a0e06314582506736e5CAS | 14652371PubMed |
Ellis, S., McFadden, T. B., and Akers, R. M. (1998). Prepubertal ovine mammary gland development is unaffected by ovariectomy. Domest. Anim. Endocrinol. 15, 217–225.
| Prepubertal ovine mammary gland development is unaffected by ovariectomy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkslSns7k%3D&md5=11a96d7f50241e3618adc2a2645cb502CAS | 9673454PubMed |
Erhard, H. W., and Rhind, S. M. (2004). Prenatal and postnatal exposure to environmental pollutants in sewage sludge alters emotional reactivity and exploratory behaviour in sheep. Sci. Total Environ. 332, 101–108.
| Prenatal and postnatal exposure to environmental pollutants in sewage sludge alters emotional reactivity and exploratory behaviour in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntFWksb4%3D&md5=94ded7527ffb658fcf92186cd09fcf76CAS | 15336895PubMed |
Erickson, B. H. (1966a). Development and senescence of the postnatal bovine ovary. J. Anim. Sci. 25, 800–805.
| 1:STN:280:DyaF2s7ntlWhsw%3D%3D&md5=3f0968413b39826d628e0e8ab90f9c93CAS | 6007918PubMed |
Erickson, B. H. (1966b). Development and radioresponse of the prenatal bovine ovary. J. Reprod. Fertil. 11, 97–105.
| Development and radioresponse of the prenatal bovine ovary.Crossref | GoogleScholarGoogle Scholar |
Evans, N. P., North, T., Dye, S., and Sweeney, T. (2004). Differential effects of the endocrine-disrupting compounds bisphenol-A and octylphenol on gonadotropin secretion, in prepubertal ewe lambs. Domest. Anim. Endocrinol. 26, 61–73.
| Differential effects of the endocrine-disrupting compounds bisphenol-A and octylphenol on gonadotropin secretion, in prepubertal ewe lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFSgtw%3D%3D&md5=2f2ba182d41a3b720074dee721c098b4CAS | 14732453PubMed |
Evans, D. J. R., Valasek, P., Schmidt, C., and Patel, K. (2006). Skeletal muscle translocation in vertebrates. Anat. Embryol. (Berl.) 211, 43–50.
| Skeletal muscle translocation in vertebrates.Crossref | GoogleScholarGoogle Scholar |
Evans, A., Mossa, F., Walsh, S. W., Scheetz, D., Jimenez-Krassel, F., Ireland, J. L. H., Smith, G. W., and Ireland, J. J. (2012). Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod. Domest. Anim. 47, 31–37.
| Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring.Crossref | GoogleScholarGoogle Scholar | 22827347PubMed |
Farin, P. W., Piedrahita, J. A., and Farin, C. E. (2006). Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology 65, 178–191.
| Errors in development of fetuses and placentas from in vitro-produced bovine embryos.Crossref | GoogleScholarGoogle Scholar | 16266745PubMed |
Farmer, C., Palin, M. F., and Martel-Kennes, Y. (2012a). Impact of diet deprivation and subsequent over-allowance during prepuberty. Part 1. Effects on growth performance, metabolite status, and mammary gland development in gilts. J. Anim. Sci. 90, 863–871.
| Impact of diet deprivation and subsequent over-allowance during prepuberty. Part 1. Effects on growth performance, metabolite status, and mammary gland development in gilts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFSiur0%3D&md5=6a4e8d87e112c04e914425eaf22fc07eCAS | 22003231PubMed |
Farmer, C., Palin, M. F., and Martel-Kennes, Y. (2012b). Impact of diet deprivation and subsequent over-allowance during prepuberty. Part 2. Effects on mammary gland development and lactation performance of sows. J. Anim. Sci. 90, 872–880.
| Impact of diet deprivation and subsequent over-allowance during prepuberty. Part 2. Effects on mammary gland development and lactation performance of sows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFSiuro%3D&md5=5479518f35207315b92b6130517b741dCAS | 22021808PubMed |
Favetta, L. A., Villagómez, D. A., Iannuzzi, L., Di Meo, G., Webb, A., Crain, S., and King, W. A. (2012). Disorders of sexual development and abnormal early development in domestic food-producing mammals: the role of chromosome abnormalities, environment and stress factors. Sex Dev. 6, 18–32.
| Disorders of sexual development and abnormal early development in domestic food-producing mammals: the role of chromosome abnormalities, environment and stress factors.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383js1ynsw%3D%3D&md5=b7cb76284da792ff1655819a0471c6c5CAS | 22024933PubMed |
Ferris, J., Favetta, L. A., and King, W. A. (2015). Bisphenol A exposure and oocyte maturation in vitro results in spindle abnormalities and chromosome misalignment in Bos taurus. Cytogenet. Genome Res. 145, 50–58.
| Bisphenol A exposure and oocyte maturation in vitro results in spindle abnormalities and chromosome misalignment in Bos taurus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOms7nL&md5=7663221d5cb873d209e6a77df21768b1CAS | 25871885PubMed |
Fisher, G. E. J., and Macpherson, A. (1991). Effect of cobalt deficiency in the pregnant ewe on reproductive-performance and lamb viability. Res. Vet. Sci. 50, 319–327.
| Effect of cobalt deficiency in the pregnant ewe on reproductive-performance and lamb viability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmtVKks7k%3D&md5=3c9257899599afc7694e30163e253883CAS |
Fong, A. P., Yao, Z., Zhong, J. W., Cao, Y., Ruzzo, W. L., Gentleman, R. C., and Tapscott, S. J. (2012). Genetic and epigenetic determinants of neurogenesis and myogenesis. Dev. Cell 22, 721–735.
| Genetic and epigenetic determinants of neurogenesis and myogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XksFehsbo%3D&md5=2d09a5435c694d1a290ff0ca501f2bcaCAS | 22445365PubMed |
Ford, S. P., Hess, B. W., Schwope, M. M., Nijland, M. J., Gilbert, J. S., Vonnahme, K. A., Means, W. J., Han, H., and Nathanielsz, P. W. (2007). Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 85, 1285–1294.
| Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksF2hur8%3D&md5=82cf655fa27f52d644f5508e48c49ddcCAS | 17224460PubMed |
Fortune, J. E., Yang, M. Y., Allen, J. J., and Herrick, S. L. (2013). Triennial Reproduction Symposium: the ovarian follicular reserve in cattle: what regulates its formation and size? J. Anim. Sci. 91, 3041–3050.
| Triennial Reproduction Symposium: the ovarian follicular reserve in cattle: what regulates its formation and size?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFChsL3I&md5=02c49b692716f182c607f9206e4518c9CAS | 23736047PubMed |
Fowden, A. L., Ward, J. W., Wooding, F. B., and Forhead, A. J. (2010). Developmental programming of the ovine placenta. Soc. Reprod. Fertil. Suppl. 67, 41–57.
| 1:STN:280:DC%2BC3MnosFegsA%3D%3D&md5=73fb6ca77c758de741266327771b7ac3CAS | 21755662PubMed |
Fowler, P. A., Dora, N. J., McFerran, H., Amezaga, M. R., Miller, D. W., Lea, R. G., Cash, P., McNeilly, A. S., Evans, N. P., Cotinot, C., Sharpe, R. M., and Rhind, S. M. (2008). In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol. Hum. Reprod. 14, 269–280.
| In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvFWks7Y%3D&md5=8c11b38b4abf9a5fd4e566aba15e63fbCAS | 18436539PubMed |
Fowler, P. A., Bellingham, M., Sinclair, K. D., Evans, N. P., Pocar, P., Fischer, B., Schaedlich, K., Schmidt, J. S., Amezaga, M. R., Bhattacharya, S., Rhind, S. M., and O’Shaughnessy, P. J. (2012). Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 355, 231–239.
| Impact of endocrine-disrupting compounds (EDCs) on female reproductive health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsFGnsbY%3D&md5=8ebe370a67c29584f291a1171bfc82d2CAS | 22061620PubMed |
Freetly, H. C., Ferrell, C. L., and Jenkins, T. G. (2000). Timing of realimentation of mature cows that were feed-restricted during pregnancy influences calf birth weights and growth rates. J. Anim. Sci. 78, 2790–2796.
| 1:CAS:528:DC%2BD3cXnslKrtL4%3D&md5=91cdbe17bfbc76499d7a6e847325a46eCAS | 11063300PubMed |
Fuso, A., Ferraguti, G., Grandoni, F., Ruggeri, R., Scarpa, S., Strom, R., and Lucarelli, M. (2010). Early demethylation of non-CpG, CpC-rich, elements in the myogenin 5′-flanking region: a priming effect on the spreading of active demethylation. Cell Cycle 9, 3965–3976.
| Early demethylation of non-CpG, CpC-rich, elements in the myogenin 5′-flanking region: a priming effect on the spreading of active demethylation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVent7k%3D&md5=3465533c2920718a3472728b6e314be8CAS | 20935518PubMed |
Gagen, E. J., Mosoni, P., Denman, S. E., Al Jassim, R., McSweeney, C. S., and Forano, E. (2012). Methanogen colonisation does not significantly alter acetogen diversity in lambs isolated 17 h after birth and raised aseptically. Microb. Ecol. 64, 628–640.
| Methanogen colonisation does not significantly alter acetogen diversity in lambs isolated 17 h after birth and raised aseptically.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWgtLjF&md5=88950f509631031ebb89094b6b6756eeCAS | 22383121PubMed |
Gardner, D. S., Van Bon, B. W. M., Dandrea, J., Goddard, P. J., May, S. F., Wilson, V., Stephenson, T., and Symonds, M. E. (2006). Effect of periconceptional undernutrition and gender on hypothalamic–pituitary–adrenal axis function in young adult sheep. J. Endocrinol. 190, 203–212.
| Effect of periconceptional undernutrition and gender on hypothalamic–pituitary–adrenal axis function in young adult sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Wjtrc%3D&md5=54c90eaf0c7fb5ada04ec45b22967110CAS | 16899555PubMed |
Gardner, D. S., Lea, R. G., and Sinclair, K. D. (2008). Developmental programming of reproduction and fertility: what is the evidence? Animal 2, 1128–1134.
| Developmental programming of reproduction and fertility: what is the evidence?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vptFSisQ%3D%3D&md5=5437a2041fd9d3e5e6ce9c1264b45942CAS | 22443724PubMed |
Gardner, D. S., Ozanne, S. E., and Sinclair, K. D. (2009). Effect of the early-life nutritional environment on fecundity and fertility of mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3419–3427.
| Effect of the early-life nutritional environment on fecundity and fertility of mammals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MnpsFalsA%3D%3D&md5=51f398e2f616d13b9f3e92b26a004f55CAS | 19833652PubMed |
Gardner, D. K., Larman, M. G., and Thouas, G. A. (2010). Sex-related physiology of the preimplantation embryo. Mol. Hum. Reprod. 16, 539–547.
| Sex-related physiology of the preimplantation embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlegt7s%3D&md5=adcaad1c20f6846e1a58de68e49f4cb1CAS | 20501630PubMed |
Garry, F. B., Adams, R., McCann, J. P., and Odde, K. G. (1996). Postnatal characteristics of calves produced by nuclear transfer cloning. Theriogenology 45, 141–152.
| Postnatal characteristics of calves produced by nuclear transfer cloning.Crossref | GoogleScholarGoogle Scholar |
Gentry, P. C., Ross, T. T., Oetting, B. C., and Birch, K. D. (1992). Effects of supplemental d-α-tocopherol on preweaning lamb performance, serum and colostrum tocopherol levels and immunoglobulin G titers. Sheep Res. J. 8, 95–100.
George, L. A., Zhang, L., Tuersunjiang, N., Ma, Y., Long, N. M., Uthlaut, A. B., Smith, D. T., Nathanielsz, P. W., and Ford, S. P. (2012). Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R795–R804.
| Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmvFSjsL8%3D&md5=f2b21b8977dfdb693aac3baaeab9cf86CAS | 22277936PubMed |
Georges, M., Charlier, C., and Cockett, N. (2003). The callipyge locus: evidence for the trans interaction of reciprocally imprinted genes. Trends Genet. 19, 248–252.
| The callipyge locus: evidence for the trans interaction of reciprocally imprinted genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFeqtrY%3D&md5=f68f72a9ac8173281907e92dc9ab1d9dCAS | 12711215PubMed |
Gerrard, D. E., and Grant, A. L. (1994). Insulin-like growth factor-II expression in developing skeletal-muscle of double muscled and normal cattle. Domest. Anim. Endocrinol. 11, 339–347.
| Insulin-like growth factor-II expression in developing skeletal-muscle of double muscled and normal cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXitlKhsbw%3D&md5=04bd065e03db4eebb6144dc34320bd4cCAS | 7828428PubMed |
Gkountela, S., Li, Z., Vincent, J. J., Zhang, K. X., Chen, A., Pellegrini, M., and Clark, A. T. (2013). The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat. Cell Biol. 15, 113–122.
| The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVChtb3L&md5=8f8346c90abd5470b8be5f7a60909582CAS | 23242216PubMed |
Gluckman, P. D., Hanson, M. A., Cooper, C., and Thornburg, K. L. (2008). Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73.
| Effect of in utero and early-life conditions on adult health and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotFSit7o%3D&md5=651aaeb2724a3b7990f326f486c0e439CAS | 18596274PubMed |
Goerlich, V. C., Natt, D., Elfwing, M., Macdonald, B., and Jensen, P. (2012). Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm. Behav. 61, 711–718.
| Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvVSgsbk%3D&md5=6e9572f34b116e513ccadef92d338a00CAS | 22465454PubMed |
González-Recio, O., Ugarte, E., and Bach, A. (2012). Trans-generational effect of maternal lactation during pregnancy: a Holstein cow model. PLoS One 7, e51816.
| Trans-generational effect of maternal lactation during pregnancy: a Holstein cow model.Crossref | GoogleScholarGoogle Scholar | 23284777PubMed |
González-Recio, O., Toro, M. A., and Bach, A. (2015). Past, present, and future of epigenetics applied to livestock breeding. Front. Genet. 6, 305.
| Past, present, and future of epigenetics applied to livestock breeding.Crossref | GoogleScholarGoogle Scholar | 26442117PubMed |
Grace, K. S., and Sinclair, K. D. (2009). Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin. Reprod. Med. 27, 409–416.
| Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOis7zK&md5=0865967ae9ddee89961a31e2bea82d2bCAS | 19711251PubMed |
Grayson, B. E., Kievit, P., Smith, M. S., and Grove, K. L. (2010). Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations. Front. Neuroendocrinol. 31, 16–31.
| Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1WgsbrF&md5=95af3d9e17b8d015cca4e4ab2c49f87bCAS | 19822169PubMed |
Grazul-Bilska, A. T., Caton, J. S., Arndt, W., Burchill, K., Thorson, C., Borowczyk, E., Bilski, J. J., Redmer, D. A., Reynolds, L. P., and Vonnahme, K. A. (2009). Cellular proliferation and vascularization in ovine fetal ovaries: effects of undernutrition and selenium in maternal diet. Reproduction 137, 699–707.
| Cellular proliferation and vascularization in ovine fetal ovaries: effects of undernutrition and selenium in maternal diet.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosl2ntbo%3D&md5=f42cac3a7993eb18f0e48d1dc2369fbaCAS | 19129369PubMed |
Greenwood, P. L., and Cafe, L. M. (2007). Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production. Animal 1, 1283–1296.
| Prenatal and pre-weaning growth and nutrition of cattle: long-term consequences for beef production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vpt1Wmtg%3D%3D&md5=c4d249549b8ffafe52afdb7f1660efe1CAS | 22444884PubMed |
Greenwood, P. L., Hunt, A. S., Hermanson, J. W., and Bell, A. W. (1998). Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J. Anim. Sci. 76, 2354–2367.
| 1:CAS:528:DyaK1cXmsV2jt7k%3D&md5=8a08315cf9bd4e8569860f3fd3d8b4b8CAS | 9781492PubMed |
Greenwood, P., Thompson, A., and Ford, S. (2010). Postnatal consequences of the maternal environment and of growth during prenatal life for productivity of ruminants. In ‘Managing the Prenatal Environment to Enhance Livestock Productivity’. (Eds P. L. Greenwood, A. W. Bell, P. E. Vercoe and G. J. Viljoen.) pp. 3–36. (Springer Netherlands.)
Grossniklaus, U., Kelly, W. G., Ferguson-Smith, A. C., Pembrey, M., and Lindquist, S. (2013). Transgenerational epigenetic inheritance: how important is it? Nat. Rev. Genet. 14, 228–235.
| Transgenerational epigenetic inheritance: how important is it?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXis1ajsbo%3D&md5=b3dc2fdb0f7232e6ddebedb3528e8c3cCAS | 23416892PubMed |
Gu, H., Bock, C., Mikkelsen, T. S., Jager, N., Smith, Z. D., Tomazou, E., Gnirke, A., Lander, E. S., and Meissner, A. (2010). Genome-scale DNA methylation mapping of clinical samples at single-nucleotide resolution. Nat. Methods 7, 133–136.
| Genome-scale DNA methylation mapping of clinical samples at single-nucleotide resolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjsVaktQ%3D%3D&md5=6391e5f76997604316b73772cfdf92c5CAS | 20062050PubMed |
Gudsnuk, K., and Champagne, F. A. (2012). Epigenetic influence of stress and the social environment. ILAR J. 53, 279–288.
| Epigenetic influence of stress and the social environment.Crossref | GoogleScholarGoogle Scholar | 23744967PubMed |
Gunn, R. G., Sim, D. A., and Hunter, E. A. (1995). Effects of nutrition in utero and in early life on the subsequent lifetime reproductive performance of Scottish blackface ewes in two management systems. Anim. Sci. 60, 223–230.
| Effects of nutrition in utero and in early life on the subsequent lifetime reproductive performance of Scottish blackface ewes in two management systems.Crossref | GoogleScholarGoogle Scholar |
Hammer, C. J., Thorson, J. F., Meyer, A. M., Redmer, D. A., Luther, J. S., Neville, T. L., Reed, J. J., Reynolds, L. P., Caton, J. S., and Vonnahme, K. A. (2011). Effects of maternal selenium supply and plane of nutrition during gestation on passive transfer of immunity and health in neonatal lambs. J. Anim. Sci. 89, 3690–3698.
| Effects of maternal selenium supply and plane of nutrition during gestation on passive transfer of immunity and health in neonatal lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqsrvP&md5=a5ca8c7498970e945f09ce4073bb42a1CAS | 21724938PubMed |
Hancock, S. N., Oliver, M. H., McLean, C., Jaquiery, A. L., and Bloomfield, F. H. (2012). Size at birth and adult fat mass in twin sheep are determined in early gestation. J. Physiol. 590, 1273–1285.
| Size at birth and adult fat mass in twin sheep are determined in early gestation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383pt1Ojsg%3D%3D&md5=642fe1de16b82be6a1ed1609e63efa1aCAS | 22183720PubMed |
Harris, A., and Seckl, J. (2011). Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 59, 279–289.
| Glucocorticoids, prenatal stress and the programming of disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1WisLw%3D&md5=1ec991efd5b01c9fdd2c0831d049af8eCAS | 20591431PubMed |
Henry, C., Kabbaj, M., Simon, H., Lemoal, M., and Maccari, S. (1994). Prenatal stress increases the hypothalamo–pituitary–adrenal axis response in young and adult-rats. J. Neuroendocrinol. 6, 341–345.
| Prenatal stress increases the hypothalamo–pituitary–adrenal axis response in young and adult-rats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FgsVGntg%3D%3D&md5=025d895efd66c285ba07728b37340d1cCAS | 7920600PubMed |
Herbison, A. E., Robinson, J. E., and Skinner, D. C. (1993). Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 57, 751–759.
| Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlsFagsbo%3D&md5=b84b88bffc08e68d4362a845ed1bd291CAS | 8367037PubMed |
Hernandez, C. E., Matthews, L. R., Oliver, M. H., Bloomfield, F. H., and Harding, J. E. (2010). Effects of sex, litter size and periconceptional ewe nutrition on offspring behavioural and physiological response to isolation. Physiol. Behav. 101, 588–594.
| Effects of sex, litter size and periconceptional ewe nutrition on offspring behavioural and physiological response to isolation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtl2nsLrI&md5=caac91654b49030baa676c968f6f8827CAS | 20826171PubMed |
Heyman, Y., Chavatte-Palmer, P., Berthelot, V., Fromentin, G., Hocquette, J. F., Martignat, L., and Renard, J. P. (2007). Assessing the quality of products from cloned cattle: an integrative approach. Theriogenology 67, 134–141.
| Assessing the quality of products from cloned cattle: an integrative approach.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28jisVWgsQ%3D%3D&md5=86af4a48f0fec29973b14ecb8542c265CAS | 17092550PubMed |
Hild, S., Clark, C. C. A., Dwyer, C. M., Murrell, J. C., Mendl, M., and Zanella, A. J. (2011). Ewes are more attentive to their offspring experiencing pain but not stress. Appl. Anim. Behav. Sci. 132, 114–120.
| Ewes are more attentive to their offspring experiencing pain but not stress.Crossref | GoogleScholarGoogle Scholar |
Hill, J., Winger, Q., Jones, K., Keller, D., King, W. A., and Westhusin, M. (1999). The effect of donor cell serum starvation and oocyte activation compounds on the development of somatic cell cloned embryos. Cloning 1, 201–208.
| The effect of donor cell serum starvation and oocyte activation compounds on the development of somatic cell cloned embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlvFektLg%3D&md5=be68f6c6f178d5b9562df08d6f1f6f2cCAS | 16218820PubMed |
Hinde, K., Carpenter, A. J., Clay, J. S., and Bradford, B. J. (2014). Holsteins favour heifers, not bulls: biased milk production programmed during pregnancy as function of fetal sex. PLoS One 9, e86169.
| Holsteins favour heifers, not bulls: biased milk production programmed during pregnancy as function of fetal sex.Crossref | GoogleScholarGoogle Scholar | 24498270PubMed |
Hofmann, J. P., Denner, P., Nussbaum-Krammer, C., Kuhn, P.-H., Suhre, M. H., Scheibel, T., Lichtenthaler, S. F., Schätzl, H. M., Bano, D., and Vorberg, I. M. (2013). Cell-to-cell propagation of infectious cytosolic protein aggregates. Proc. Natl Acad. Sci. USA 110, 5951–5956.
| Cell-to-cell propagation of infectious cytosolic protein aggregates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsFWmsL4%3D&md5=be7befc70c9df21a4c36a549519d7e04CAS | 23509289PubMed |
Hon, G. C., Rajagopal, N., Shen, Y., McCleary, D. F., Yue, F., Dang, M. D., and Ren, B. (2013). Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat. Genet. 45, 1198–1206.
| Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlGltL7P&md5=e4b42863d36748b2161dc181d9e98e91CAS | 23995138PubMed |
Hori, N., Nagai, M., Hirayama, M., Hirai, T., Matsuda, K., Hayashi, M., Tanaka, T., Ozawa, T., and Horike, S. (2010). Aberrant CpG methylation of the imprinting control region KvDMR1 detected in assisted reproductive technology-produced calves and pathogenesis of large offspring syndrome. Anim. Reprod. Sci. 122, 303–312.
| Aberrant CpG methylation of the imprinting control region KvDMR1 detected in assisted reproductive technology-produced calves and pathogenesis of large offspring syndrome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFahs7vP&md5=dc15d286ec6df060a14354ba053145deCAS | 21035970PubMed |
Horvath, T. L., and Bruning, J. C. (2006). Developmental programming of the hypothalamus: a matter of fat. Nat. Med. 12, 52–53.
| Developmental programming of the hypothalamus: a matter of fat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Kgsg%3D%3D&md5=ec5d2f12355106acac650a9079cad45cCAS | 16397567PubMed |
Houghton, P. L., Lemenager, R. P., Horstman, L. A., Hendrix, K. S., and Moss, G. E. (1990). Effects of body-composition, prepartum and postpartum energy-level and early weaning on reproductive-performance of beef-cows and preweaning calf gain. J. Anim. Sci. 68, 1438–1446.
| 1:STN:280:DyaK3czgslGqsg%3D%3D&md5=976a908dba4c5751843a96cbad1c70c9CAS | 2365654PubMed |
Hu, S., Ni, W., Sai, W., Zi, H., Qiao, J., Wang, P., Sheng, J., and Chen, C. (2013a). Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep. PLoS One 8, e58521.
| Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltFyqt7c%3D&md5=0ee9402e9f4b57845a8fc2670c4132abCAS | 23526994PubMed |
Hu, Y., Xu, H., Li, Z., Zheng, X., Jia, X., Nie, Q., and Zhang, X. (2013b). Comparison of the genome-wide DNA methylation profiles between fast-growing and slow-growing broilers. PLoS One 8, e56411.
| Comparison of the genome-wide DNA methylation profiles between fast-growing and slow-growing broilers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjsFSitb0%3D&md5=df8adf106299dc80909dd1733f1da161CAS | 23441189PubMed |
Hyldig, S. M., Croxall, N., Contreras, D. A., Thomsen, P. D., and Alberio, R. (2011). Epigenetic reprogramming in the porcine germ line. BMC Dev. Biol. 11, 11.
| Epigenetic reprogramming in the porcine germ line.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtVyltro%3D&md5=4de816b262389e33cf77971ac2b88050CAS | 21352525PubMed |
Ichiyanagi, T., Ichiyanagi, K., Miyake, M., and Sasaki, H. (2013). Accumulation and loss of asymmetric non-CpG methylation during male germ-cell development. Nucleic Acids Res. 41, 738–745.
| Accumulation and loss of asymmetric non-CpG methylation during male germ-cell development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFyju78%3D&md5=f3abb14aa18c9f5a160f0cf04c3cdb47CAS | 23180759PubMed |
International Embryo Transfer Society (IETS) (2012) ‘Manual of the International Embryo Transfer Society.’ (IETS: Savoy, IL.)
Illingworth, R., Kerr, A., Desousa, D., Jorgensen, H., Ellis, P., Stalker, J., Jackson, D., Clee, C., Plumb, R., Rogers, J., Humphray, S., Cox, T., Langford, C., and Bird, A. (2008). A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol. 6, e22.
| A novel CpG island set identifies tissue-specific methylation at developmental gene loci.Crossref | GoogleScholarGoogle Scholar | 18232738PubMed |
Iqbal, K., Jin, S. G., Pfeifer, G. P., and Szabo, P. E. (2011). Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl Acad. Sci. USA 108, 3642–3647.
| Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFKqs7Y%3D&md5=79c7434a6329e8080b2c5309bf1fdf9dCAS | 21321204PubMed |
Ironside, J. W. (2012). Variant Creutzfeldt–Jakob disease: an update. Folia Neuropathol. 50, 50–56.
| 1:CAS:528:DC%2BC38XoslejtL4%3D&md5=93776c92ae92df94650e9923d37adfb0CAS | 22505363PubMed |
Jablonka, E. (1994). Inheritance systems and the evolution of new levels of individuality. J. Theor. Biol. 170, 301–309.
| Inheritance systems and the evolution of new levels of individuality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FovFemuw%3D%3D&md5=c68f126c778d0e5b6ab88329d4561d20CAS | 7996858PubMed |
Jaquiery, A. L., Oliver, M. H., Honeyfield-Ross, M., Harding, J. E., and Bloomfield, F. H. (2012). Periconceptional undernutrition in sheep affects adult phenotype only in males. J. Nutr. Metab. 123, 610.
Jarvis, S., Moinard, C., Robson, S. K., Baxter, E., Ormandy, E., Douglas, A. J., Seckl, J. R., Russell, J. A., and Lawrence, A. B. (2006). Programming the offspring of the pig by prenatal social stress: neuroendocrine activity and behaviour. Horm. Behav. 49, 68–80.
| Programming the offspring of the pig by prenatal social stress: neuroendocrine activity and behaviour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlCrsbfL&md5=8349410bf25fb91515a8d10779884c75CAS | 15961089PubMed |
Jiang, Y. L., Li, N., Plastow, G., Liu, Z. L., Hu, X. X., and Wu, C. X. (2002a). Identification of three SNPs in the porcine myostatin gene (MSTN). Anim. Biotechnol. 13, 173–178.
| Identification of three SNPs in the porcine myostatin gene (MSTN).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xnt1Wlsbk%3D&md5=25caaa0228814bd91a78cfd53c5d2d2fCAS | 12212941PubMed |
Jiang, Y. L., Li, N., Fan, X. Z., Xiao, L. R., Xiang, R. L., Hu, X. X., Du, L. X., and Wu, C. X. (2002b). Associations of T → A mutation in the promoter region of myostatin gene with birth weight in Yorkshire pigs. Asian Australas. J. Anim. Sci. 15, 1543–1545.
| Associations of T → A mutation in the promoter region of myostatin gene with birth weight in Yorkshire pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsV2mtLo%3D&md5=67fa25e662d89d5ebcee540c2698b9c2CAS |
Jirtle, R. L., and Skinner, M. K. (2007). Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 8, 253–262.
| Environmental epigenomics and disease susceptibility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXivVGktbY%3D&md5=94cd18b89708ffa628fee0a88c12a56bCAS | 17363974PubMed |
Jurie, C., Picard, B., Heyman, Y., Cassar-Malek, I., Chavatte-Palmer, P., Richard, C., and Hocquette, J. F. (2009). Comparison of cloned and non-cloned Holstein heifers in muscle contractile and metabolic characteristics. Animal 3, 244–250.
| Comparison of cloned and non-cloned Holstein heifers in muscle contractile and metabolic characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVSns7vE&md5=b01c2d3f59c0549b8d81904fcca33feaCAS | 22444227PubMed |
Kanitz, E., Otten, W., Tuchscherer, M., and Manteuffel, G. (2003). Effects of prenatal stress on corticosteroid receptors and monoamine concentrations in limbic areas of suckling piglets (Sus scrofa) at different ages. J. Vet. Med. A Physiol. Pathol. Clin. Med. 50, 132–139.
| Effects of prenatal stress on corticosteroid receptors and monoamine concentrations in limbic areas of suckling piglets (Sus scrofa) at different ages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXksFOlsbo%3D&md5=c3b727154e1e8a5ebb29d621fb710e33CAS | 12757550PubMed |
Kaplan, J. L., Shi, H. N., and Walker, W. A. (2011). The role of microbes in developmental immunologic programming. Pediatr. Res. 69, 465–472.
| The role of microbes in developmental immunologic programming.Crossref | GoogleScholarGoogle Scholar | 21364495PubMed |
Katari, S., Turan, N., Bibikova, M., Erinle, O., Chalian, R., Foster, M., Gaughan, J. P., Coutifaris, C., and Sapienza, C. (2009). DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum. Mol. Genet. 18, 3769–3778.
| DNA methylation and gene expression differences in children conceived in vitro or in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhurfE&md5=b9429215b3c0eb853ee39a7187ae0668CAS | 19605411PubMed |
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L., and Gordon, J. I. (2011). Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336.
| Human nutrition, the gut microbiome and the immune system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsFOqsb4%3D&md5=11991b48831e70ba71bbb60c82527e17CAS | 21677749PubMed |
Keady, T. W. J., and Hanrahan, J. P. (2009). Effects of shearing at housing, grass silage feed value and extended grazing herbage allowance on ewe and subsequent lamb performance. Animal 3, 143–151.
| Effects of shearing at housing, grass silage feed value and extended grazing herbage allowance on ewe and subsequent lamb performance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVSns7rP&md5=4fea43788f8048a35d87dcff75f4f1c0CAS |
Kelly, J. M., Kleemann, D. O., and Walker, S. K. (2005). The effect of nutrition during pregnancy on the in vitro production of embryos from resulting lambs. Theriogenology 63, 2020–2031.
| The effect of nutrition during pregnancy on the in vitro production of embryos from resulting lambs.Crossref | GoogleScholarGoogle Scholar | 15823357PubMed |
Kelsey, G., and Bartolomei, M. S. (2012). Imprinted genes… and the number is? PLoS Genet. 8, e1002601.
| Imprinted genes… and the number is?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsFalur4%3D&md5=73f25188229bec0acf29966600126570CAS | 22479197PubMed |
Khanal, P., Husted, S. V., Axel, A. M., Johnsen, L., Pedersen, K. L., Mortensen, M. S., Kongsted, A. H., and Nielsen, M. O. (2014). Late gestation over- and undernutrition predispose for visceral adiposity in response to a post-natal obesogenic diet, but with differential impacts on glucose-insulin adaptations during fasting in lambs. Acta Physiol. (Oxf.) 210, 110–126.
| Late gestation over- and undernutrition predispose for visceral adiposity in response to a post-natal obesogenic diet, but with differential impacts on glucose-insulin adaptations during fasting in lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFalurvL&md5=f2773389e317853fddd985cabc690817CAS | 23746217PubMed |
Khanal, P., Axel, A. M., Kongsted, A. H., Husted, S. V., Johnsen, L., Pandey, D., Pedersen, K. L., Birtwistle, M., Markussen, B., Kadarmideen, H. N., and Nielsen, M. O. (2015). Late gestation under- and overnutrition have differential impacts when combined with a post-natal obesogenic diet on glucose-lactate-insulin adaptations during metabolic challenges in adolescent sheep. Acta Physiol. (Oxf.) 213, 519–536.
| Late gestation under- and overnutrition have differential impacts when combined with a post-natal obesogenic diet on glucose-lactate-insulin adaptations during metabolic challenges in adolescent sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXlvFCntA%3D%3D&md5=2aaadd9dbe6652b34a22c7855821e763CAS | 25204637PubMed |
Knight, C. H., and Sorensen, A. (2001). Windows in early mammary development: critical or not? Reproduction 122, 337–345.
| Windows in early mammary development: critical or not?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntVGksLs%3D&md5=4511383420188385128dcd3bc2a3c5a6CAS | 11597300PubMed |
Kobayashi, S., Isotani, A., Mise, N., Yamamoto, M., Fujihara, Y., Kaseda, K., Nakanishi, T., Ikawa, M., Hamada, H., Abe, K., and Okabe, M. (2006). Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr. Biol. 16, 166–172.
| Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xosl2mtw%3D%3D&md5=3d45f96e25d6d8dd93e720126608a98fCAS | 16431368PubMed |
Kobayashi, H., Sakurai, T., Imai, M., Takahashi, N., Fukuda, A., Yayoi, O., Sato, S., Nakabayashi, K., Hata, K., Sotomaru, Y., Suzuki, Y., and Kono, T. (2012). Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet. 8, e1002440.
| Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVyitrg%3D&md5=f9aca4af45c15f4c82c99dcda19197efCAS | 22242016PubMed |
Koohmaraie, M., Shackelford, S. D., Wheeler, T. L., Lonergan, S. M., and Doumit, M. E. (1995). A muscle hypertrophy condition in lamb (callipyge): characterization of effects on muscle growth and meat quality traits. J. Anim. Sci. 73, 3596–3607.
| 1:CAS:528:DyaK28XptFWiug%3D%3D&md5=8736f19aa458e4d0e663e4d877ff18d0CAS | 8655433PubMed |
Korosi, A., Naninck, E. F. G., Oomen, C. A., Schouten, M., Krugers, H., Fitzsimons, C., and Lucassen, P. J. (2012). Early-life stress mediated modulation of adult neurogenesis and behavior. Behav. Brain Res. 227, 400–409.
| Early-life stress mediated modulation of adult neurogenesis and behavior.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC387mtlKmuw%3D%3D&md5=b1151a9e0609783aaef98fdf19684cdfCAS | 21821065PubMed |
Kotsampasi, B., Chadio, S., Papadomichelakis, G., Deligeorgis, S., Kalogiannis, D., Menegatos, I., and Zervas, G. (2009). Effects of maternal undernutrition on the hypothalamic–pituitary–gonadal axis function in female sheep offspring. Reprod. Domest. Anim. 44, 677–684.
| Effects of maternal undernutrition on the hypothalamic–pituitary–gonadal axis function in female sheep offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVaiurbM&md5=d9c70435ff3e2d1413f498a2e2eeea54CAS | 19642222PubMed |
Kranendonk, G., Hopster, H., Fillerup, M., Ekkel, E. D., Mulder, E. J. H., Wiegant, V. M., and Taverne, M. A. M. (2006a). Lower birth weight and attenuated adrenocortical response to ACTH in offspring from sows that orally received cortisol during gestation. Domest. Anim. Endocrinol. 30, 218–238.
| Lower birth weight and attenuated adrenocortical response to ACTH in offspring from sows that orally received cortisol during gestation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVartL4%3D&md5=955a0dab04dbb556bdfc7228ad8a273fCAS | 16107308PubMed |
Kranendonk, G., Hopster, H., Fillerup, M., Ekkel, E. D., Mulder, E. J. H., and Taverne, M. A. M. (2006b). Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender difference in their offspring. Horm. Behav. 49, 663–672.
| Cortisol administration to pregnant sows affects novelty-induced locomotion, aggressive behaviour, and blunts gender difference in their offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksVCqurY%3D&md5=31ec171949b3612a250f37094806188bCAS | 16488416PubMed |
Kraugerud, M., Aleksandersen, M., Nyengaard, J. R., Ostby, G. C., Gutleb, A. C., Dahl, E., Berg, V., Farstad, W., Schweder, T., Skaare, J. U., and Ropstad, E. (2012). In utero and lactational exposure to PCB 118 and PCB 153 alter ovarian follicular dynamics and GnRH-induced luteinizing hormone secretion in female lambs. Environ. Toxicol. 27, 623–634.
| In utero and lactational exposure to PCB 118 and PCB 153 alter ovarian follicular dynamics and GnRH-induced luteinizing hormone secretion in female lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFalsbvI&md5=144a47867c7a4cd1a328eb97c7de5dcaCAS | 21344607PubMed |
Laible, G., Brophy, B., Knighton, D., and Wells, D. N. (2007). Compositional analysis of dairy products derived from clones and cloned transgenic cattle. Theriogenology 67, 166–177.
| Compositional analysis of dairy products derived from clones and cloned transgenic cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht12rsbrP&md5=58f415b0951f9e5b46b1e250eb9d72faCAS | 17052749PubMed |
Langlands, J., Donald, G., and Paull, D. (1984). Effects of different stocking intensities in early life on the productivity of Merino ewes grazed as adults at two stocking rates. 2. Reproductive performance. Aust. J. Exp. Agric. 24, 47–56.
| Effects of different stocking intensities in early life on the productivity of Merino ewes grazed as adults at two stocking rates. 2. Reproductive performance.Crossref | GoogleScholarGoogle Scholar |
Langley-Evans, S. C. (2013). Fetal programming of CVD and renal disease: animal models and mechanistic considerations. Proc. Nutr. Soc. 72, 317–325.
| Fetal programming of CVD and renal disease: animal models and mechanistic considerations.Crossref | GoogleScholarGoogle Scholar | 23312451PubMed |
Lauri, A., Lazzari, G., Galli, C., Lagutina, I., Genzini, E., Braga, F., Mariani, P., and Williams, J. L. (2013). Assessment of MDA efficiency for genotyping using cloned embryo biopsies. Genomics 101, 24–29.
| Assessment of MDA efficiency for genotyping using cloned embryo biopsies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWnu73E&md5=80f87136d0155ad4380a8e1f406caa89CAS | 22982297PubMed |
Lay, D. C., Randel, R. D., Friend, T. H., Jenkins, O. C., Neuendorff, D. A., Bushong, D. M., Lanier, E. K., and Bjorge, M. K. (1997). Effects of prenatal stress on suckling calves. J. Anim. Sci. 75, 3143–3151.
| 1:CAS:528:DyaK2sXotVWgt7w%3D&md5=1db94ce7648db4809bc846a5ef24c33cCAS | 9419987PubMed |
Le Dividich, J., Rooke, J. A., and Herpin, P. (2005). Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 143, 469–485.
| Nutritional and immunological importance of colostrum for the new-born pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvFGmug%3D%3D&md5=f74f79b3c22295928f9ff4026f3f4635CAS |
Lea, R. G., Andrade, L. P., Rae, M. T., Hannah, L. T., Kyle, C. E., Murray, J. F., Rhind, S. M., and Miller, D. W. (2006). Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction 131, 113–124.
| Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsFegsrg%3D&md5=ee3d59198c85791ae2814284f9f2f5dcCAS | 16388015PubMed |
Lee, S.-J. (2004). Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61–86.
| Regulation of muscle mass by myostatin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVaqu7nF&md5=9f2178726d5c7a316cb0ada4f1820361CAS | 15473835PubMed |
Lehman, M. N., Robinson, J. E., Karsch, F. J., and Silverman, A. J. (1986). Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J. Comp. Neurol. 244, 19–35.
| Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL287ktV2ktw%3D%3D&md5=b952fa62fd36245ef21898bf79f67f92CAS | 3512631PubMed |
Lepikhov, K., Zakhartchenko, V., Hao, R., Yang, F., Wrenzycki, C., Niemann, H., Wolf, E., and Walter, J. (2008). Evidence for conserved DNA and histone H3 methylation reprogramming in mouse, bovine and rabbit zygotes. Epigenetics Chromatin 1, 8.
| Evidence for conserved DNA and histone H3 methylation reprogramming in mouse, bovine and rabbit zygotes.Crossref | GoogleScholarGoogle Scholar | 19014417PubMed |
Li, Y., and O’Neill, C. (2012). Persistence of cytosine methylation of DNA following fertilisation in the mouse. PLoS One 7, e30687.
| Persistence of cytosine methylation of DNA following fertilisation in the mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xitlaqsb8%3D&md5=e7377e5aa508665b1b796cc05b21b835CAS | 22292019PubMed |
Li, E., Bestor, T. H., and Jaenisch, R. (1992). Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926.
| Targeted mutation of the DNA methyltransferase gene results in embryonic lethality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVGgsr0%3D&md5=78e220d181b54234250ed26f6520f037CAS | 1606615PubMed |
Li, L., Wang, L., Xu, X., Lou, H., Le, F., Li, L., Sheng, J., Huang, H., and Jin, F. (2011). Genome-wide DNA methylation patterns in IVF-conceived mice and their progeny: a putative model for ART-conceived humans. Reprod. Toxicol. 32, 98–105.
| Genome-wide DNA methylation patterns in IVF-conceived mice and their progeny: a putative model for ART-conceived humans.Crossref | GoogleScholarGoogle Scholar | 21672625PubMed |
Lister, R., Mukamel, E. A., Nery, J. R., Urich, M., Puddifoot, C. A., Johnson, N. D., Lucero, J., Huang, Y., Dwork, A. J., Schultz, M. D., Yu, M., Tonti-Filippini, J., Heyn, H., Hu, S., Wu, J. C., Rao, A., Esteller, M., He, C., Haghighi, F. G., Sejnowski, T. J., Behrens, M. M., and Ecker, J. R. (2013). Global epigenomic reconfiguration during mammalian brain development. Science 341, 1237905.
| Global epigenomic reconfiguration during mammalian brain development.Crossref | GoogleScholarGoogle Scholar | 23828890PubMed |
Liu, J., and Jia, G. (2014). Methylation modifications in eukaryotic messenger RNA. J. Genet. Genomics 41, 21–33.
| Methylation modifications in eukaryotic messenger RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVWgtbvP&md5=9bd5f4c944f3929f21f09803d6761132CAS | 24480744PubMed |
Liu, W., Shan, T., Yang, X., Liang, S., Zhang, P., Liu, Y., Liu, X., and Kuang, S. (2013). A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci. 126, 3527–3532.
| A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFelu7fJ&md5=0b5ec6fd751c92fd2e14b2c6dec1f894CAS | 23781029PubMed |
Long, N. M., George, L. A., Uthlaut, A. B., Smith, D. T., Nijland, M. J., Nathanielsz, P. W., and Ford, S. P. (2010a). Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J. Anim. Sci. 88, 3546–3553.
| Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGlsL7J&md5=b24a27c4da1d52eb4dc9fd9a9851a2aeCAS | 20622177PubMed |
Long, N. M., Nijland, M. J., Nathanielsz, P. W., and Ford, S. P. (2010b). The effect of early to mid-gestational nutrient restriction on female offspring fertility and hypothalamic–pituitary–adrenal axis response to stress. J. Anim. Sci. 88, 2029–2037.
| The effect of early to mid-gestational nutrient restriction on female offspring fertility and hypothalamic–pituitary–adrenal axis response to stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVeqt7Y%3D&md5=ae86fc5b7f69a29bc5cbe49e60c84decCAS | 20190172PubMed |
Louey, S., Cock, M. L., and Harding, R. (2005). Long term consequences of low birthweight on postnatal growth, adiposity and brain weight at maturity in sheep. J. Reprod. Dev. 51, 59–68.
| Long term consequences of low birthweight on postnatal growth, adiposity and brain weight at maturity in sheep.Crossref | GoogleScholarGoogle Scholar | 15750297PubMed |
Luther, J., Aitken, R., Milne, J., Matsuzaki, M., Reynolds, L., Redmer, D., and Wallace, J. (2007). Maternal and fetal growth, body composition, endocrinology, and metabolic status in undernourished adolescent sheep. Biol. Reprod. 77, 343–350.
| Maternal and fetal growth, body composition, endocrinology, and metabolic status in undernourished adolescent sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Ontr4%3D&md5=7e89d64e03f6b67089503156d59cb690CAS | 17475926PubMed |
Maalouf, W. E., Alberio, R., and Campbell, K. H. (2008). Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos. Epigenetics 3, 199–209.
| Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos.Crossref | GoogleScholarGoogle Scholar | 18698155PubMed |
Magee, D. A., Sikora, K. M., Berkowicz, E. W., Berry, D. P., Howard, D. J., Mullen, M. P., Evans, R. D., Spillane, C., and MacHugh, D. E. (2010). DNA sequence polymorphisms in a panel of eight candidate bovine imprinted genes and their association with performance traits in Irish Holstein–Friesian cattle. BMC Genet. 11, 93.
| DNA sequence polymorphisms in a panel of eight candidate bovine imprinted genes and their association with performance traits in Irish Holstein–Friesian cattle.Crossref | GoogleScholarGoogle Scholar | 20942903PubMed |
Mahoney, M. M., and Padmanabhan, V. (2010). Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol. Appl. Pharmacol. 247, 98–104.
| Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsFyju70%3D&md5=eba1965ba409884322a1872ead8151ffCAS | 20621667PubMed |
Maltin, C. A., Delday, M. I., Sinclair, K. D., Steven, J., and Sneddon, A. A. (2001). Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction 122, 359–374.
| Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntVGksLk%3D&md5=1fe987d63f01da2cfa2fd9ace03a6f8aCAS | 11597302PubMed |
Marsit, C. J., Maccani, M. A., Padbury, J. F., and Lester, B. M. (2015). Placental 11β hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioural outcome. PLoS One 7, 33794.
| Placental 11β hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioural outcome.Crossref | GoogleScholarGoogle Scholar |
Martin, J. L., Cupp, A. S., Rasby, R. J., Hall, Z. C., and Funston, R. N. (2007). Utilization of dried distillers grains for developing beef heifers. J. Anim. Sci. 85, 2298–2303.
| Utilization of dried distillers grains for developing beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVGlsLY%3D&md5=ad1379b3b3d5d981f82215283dd04625CAS | 17526658PubMed |
Macias, H., and Hink, L. (2012). Mammary gland development. WIREs Dev. Biol. 1, 533–557.
| Mammary gland development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Sqs77I&md5=e172bf587bf0f15055017d3298ccdc84CAS |
Maxfield, E. K., Sinclair, K. D., Broadbent, P. J., McEvoy, T. G., Robinson, J. J., and Maltin, C. A. (1998). Short-term culture of ovine embryos modifies fetal myogenesis. Am. J. Physiol. 274, E1121–E1123.
| 1:CAS:528:DyaK1cXktVCrs78%3D&md5=4baac49ce2f051ee8bdd9ff1839d35d2CAS | 9611165PubMed |
McCann, M. A., Goode, L., Harvey, R. W., Caruolo, E. V., and Mann, D. L. (1989). Effects of rapid weight gain to puberty on reproduction, mammary development and lactation of ewe lambs. Theriogenology 32, 55–68.
| Effects of rapid weight gain to puberty on reproduction, mammary development and lactation of ewe lambs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvFCitA%3D%3D&md5=2f48cb91b2ad57d3704ceaa57e033d8cCAS | 16726652PubMed |
McEvoy, T. G., Sinclair, K. D., Broadbent, P. J., Goodhand, K. L., and Robinson, J. J. (1998). Post-natal growth and development of Simmental calves derived from in vivo or in vitro embryos. Reprod. Fertil. Dev. 10, 459–464.
| Post-natal growth and development of Simmental calves derived from in vivo or in vitro embryos.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FltlGqtA%3D%3D&md5=496fcab22a7574de23b8a6f07bb65017CAS | 10588375PubMed |
McGee, M., Drennan, M. J., and Caffrey, P. J. (2006). Effect of age and nutrient restriction pre partum on beef suckler cow serum immunoglobulin concentrations, colostrum yield, composition and immunoglobulin concentration and immune status of their progeny. Ir. J. Agric. Food Res. 45, 157–171.
McMillen, I. C., and Robinson, J. S. (2005). Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633.
| Developmental origins of the metabolic syndrome: prediction, plasticity, and programming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjt12lsLw%3D&md5=e616c9456d7ab6c1ffa2d34a7ed40eb1CAS | 15788706PubMed |
McNatty, K. P., Smith, P., Hudson, N. L., Heath, D. A., Tisdall, D. J., O, W. S., and Braw-Tal, R. (1995). Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. J. Reprod. Fertil. Suppl. 49, 123–135.
| 1:STN:280:DyaK2MzkvV2qug%3D%3D&md5=bdf651fa24e60d3d637fa2f65222f6aaCAS | 7623307PubMed |
McPherron, A. C., and Lee, S. J. (1997). Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl Acad. Sci. USA 94, 12 457–12 461.
| Double muscling in cattle due to mutations in the myostatin gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXns1ylsbk%3D&md5=65984576db5d4e3a5c84f6ceca66b021CAS |
Merlot, E., Quesnel, H., and Prunier, A. (2013). Prenatal stress, immunity and neonatal health in farm animal species. Animal 7, 2016–2025.
| Prenatal stress, immunity and neonatal health in farm animal species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sfmsl2jsQ%3D%3D&md5=0cea128270e772312a415814bce29ebcCAS | 23915487PubMed |
Mesquita, A. R., Wegerich, Y., Patchev, A. V., Oliveira, M., Leão, P., Sousa, N., and Almeida, O. F. X. (2009). Glucocorticoids and neuro- and behavioural development. Semin. Fetal Neonatal Med. 14, 130–135.
| Glucocorticoids and neuro- and behavioural development.Crossref | GoogleScholarGoogle Scholar | 19084485PubMed |
Micke, G. C., Sullivan, T. M., Gatford, K. L., Owens, J. A., and Perry, V. E. A. (2010a). Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 121, 208–217.
| Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsrrE&md5=13118cfec67173e08866e3a3aefead72CAS | 20591585PubMed |
Micke, G. C., Sullivan, T. M., Rolls, P. J., Hasell, B., Greer, R. M., Norman, S. T., and Perry, V. E. A. (2010b). Dystocia in 3-year-old beef heifers; relationship to maternal nutrient intake during early- and mid-gestation, pelvic area and hormonal indicators of placental function. Anim. Reprod. Sci. 118, 163–170.
| Dystocia in 3-year-old beef heifers; relationship to maternal nutrient intake during early- and mid-gestation, pelvic area and hormonal indicators of placental function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGltbg%3D&md5=1bab6e7c1e273c0cb0cd7456d9e73a4cCAS | 19762178PubMed |
Micke, G. C., Sullivan, T. M., Soares Magalhaes, R. J., Rolls, P. J., Norman, S. T., and Perry, V. E. (2010c). Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim. Reprod. Sci. 117, 1–10.
| Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlyjt7vO&md5=07f1d00228169d580dac4b9d63766155CAS | 19394770PubMed |
Micke, G. C., Sullivan, T. M., McMillen, I. C., Gentili, S., and Perry, V. E. A. (2011a). Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R. Mol. Cell. Endocrinol. 332, 234–241.
| Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1WqtLfL&md5=dc9a15e0bb0379cbbc68a7b1da3e77b2CAS | 21056085PubMed |
Micke, G. C., Sullivan, T. M., McMillen, I. C., Gentili, S., and Perry, V. E. A. (2011b). Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots. Reproduction 141, 697–706.
| Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFCis78%3D&md5=1f98fc3a86e3a6836e0a1e3fe54b9c40CAS | 21310814PubMed |
Micke, G. C., Sullivan, T. M., Kennaway, D. J., Hernandez-Medrano, J., and Perry, V. E. A. (2015). Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny. Theriogenology 83, 604–615.
| Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFKmsb7F&md5=dbc71d9ab6939ce2be5d0df1b8812316CAS | 25492373PubMed |
Miranda, A., Ramos-Ibeas, P., Pericuesta, E., Ramirez, M. A., and Gutierrez-Adan, A. (2013). The role of prion protein in stem cell regulation. Reproduction 146, R91–R99.
| The role of prion protein in stem cell regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVaiu77M&md5=00d78a1becf5c32055ce4e5ac372d5d1CAS | 23740082PubMed |
Mok, G. F., and Sweetman, D. (2011). Many routes to the same destination: lessons from skeletal muscle development. Reproduction 141, 301–312.
| Many routes to the same destination: lessons from skeletal muscle development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkt1Crtro%3D&md5=c47c873105446b0c9b7c0440fb780869CAS | 21183656PubMed |
Morgan, H. D., Sutherland, H. G., Martin, D. I., and Whitelaw, E. (1999). Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 23, 314–318.
| Epigenetic inheritance at the agouti locus in the mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnt1Gns7w%3D&md5=c048c474369a9c83e93daf14bf9ca35fCAS | 10545949PubMed |
Morgan, H. D., Santos, F., Green, K., Dean, W., and Reik, W. (2005). Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14, R47–R58.
| Epigenetic reprogramming in mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjsFeksb4%3D&md5=0982f8cee6b62c29af98cb0b981b4bc6CAS | 15809273PubMed |
Morgavi, D. P., Forano, E., Martin, C., and Newbold, C. J. (2010). Microbial ecosystem and methanogenesis in ruminants. Animal 4, 1024–1036.
| Microbial ecosystem and methanogenesis in ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVajtr0%3D&md5=423789f5201f93b566b99bf40a0e6e2cCAS | 22444607PubMed |
Morris, S. T., McCutcheon, S. N., and Revell, D. K. (2000). Birth weight responses to shearing ewes in early to mid gestation. Anim. Sci. 70, 363–369.
Morrison, A. G., Callanan, J. J., Evans, N. P., Aldridge, T. C., and Sweeney, T. (2003). Effects of endocrine disrupting compounds on the pathology and oestrogen receptor alpha and beta distribution in the uterus and cervix of ewe lambs. Domest. Anim. Endocrinol. 25, 329–343.
| Effects of endocrine disrupting compounds on the pathology and oestrogen receptor alpha and beta distribution in the uterus and cervix of ewe lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlymsr0%3D&md5=4df1d71037eafd3199641341e5d022dfCAS | 14652134PubMed |
Mossa, F., Walsh, S. W., Butler, S. T., Berry, D. P., Carter, F., Lonergan, P., Smith, G. W., Ireland, J. J., and Evans, A. C. (2012). Low numbers of ovarian follicles >/=3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 95, 2355–2361.
| Low numbers of ovarian follicles >/=3 mm in diameter are associated with low fertility in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFygtLo%3D&md5=b8760125f735f2663f09b42764bb8452CAS | 22541464PubMed |
Mossa, F., Carter, F., Walsh, S. W., Kenny, D. A., Smith, G. W., Ireland, J. L., Hildebrandt, T. B., Lonergan, P., Ireland, J. J., and Evans, A. C. (2013). Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88, 92.
| Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring.Crossref | GoogleScholarGoogle Scholar | 23426432PubMed |
Mousavi, K., Zare, H., Dell’orso, S., Grontved, L., Gutierrez-Cruz, G., Derfoul, A., Hager, G. L., and Sartorelli, V. (2013). eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 51, 606–617.
| eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlGqsrrE&md5=6fa4d1dfc66f1acf32baec3b26918984CAS | 23993744PubMed |
Muhlhausler, B. S., and Ong, Z. Y. (2011). The fetal origins of obesity: early origins of altered food intake. Endocr. Metab. Immune Disord. Drug Targets 11, 189–197.
| The fetal origins of obesity: early origins of altered food intake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOksLnO&md5=8299488cd7af6ba5f13ec57db74083beCAS | 21831032PubMed |
Mühlhäusler, B. S., Adam, C. L., Marrocco, E. M., Findlay, P. A., Roberts, C. T., McFarlane, J. R., Kauter, K. G., and McMillen, I. C. (2005). Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J. Physiol. 565, 185–195.
| Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation.Crossref | GoogleScholarGoogle Scholar | 15661821PubMed |
Muhlhausler, B. S., Adam, C. L., Findlay, P. A., Duffield, J. A., and McMillen, I. C. (2006). Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 20, 1257–1259.
| Increased maternal nutrition alters development of the appetite-regulating network in the brain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvFelt7Y%3D&md5=c876b97e73bbf1fcef6410e2944497ffCAS | 16684802PubMed |
Muñoz, C., Carson, A. F., Mccoy, M. A., Dawson, L. E. R., O’Connell, N. E., and Gordon, A. W. (2009). Effect of plane of nutrition of 1- and 2-year-old ewes in early and mid-pregnancy on ewe reproduction and offspring performance up to weaning. Animal 3, 657–669.
| Effect of plane of nutrition of 1- and 2-year-old ewes in early and mid-pregnancy on ewe reproduction and offspring performance up to weaning.Crossref | GoogleScholarGoogle Scholar | 22444443PubMed |
Murdoch, W. J., Van Kirk, E. A., Vonnahme, K. A., and Ford, S. P. (2003). Ovarian responses to undernutrition in pregnant ewes, USA. Reprod. Biol. Endocrinol. 1, 6.
| Ovarian responses to undernutrition in pregnant ewes, USA.Crossref | GoogleScholarGoogle Scholar | 12646075PubMed |
Mychasiuk, R., Gibb, R., and Kolb, B. (2011). Prenatal stress produces sexually dimorphic and regionally specific changes in gene expression in hippocampus and frontal cortex of developing rat offspring. Dev. Neurosci. 33, 531–538.
| Prenatal stress produces sexually dimorphic and regionally specific changes in gene expression in hippocampus and frontal cortex of developing rat offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xkt1Cntr0%3D&md5=fcf82352b9415421310138ad8cf1caccCAS | 22286693PubMed |
Mychasiuk, R., Harker, A., Ilnytskyy, S., and Gibb, R. (2013). Paternal stress prior to conception alters DNA methylation and behaviour of developing rat offspring. Neuroscience 241, 100–105.
| Paternal stress prior to conception alters DNA methylation and behaviour of developing rat offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvF2gsLo%3D&md5=987e75b5cfe7b732f0f76a7bfcad744aCAS | 23531434PubMed |
Nakamura, T., Liu, Y. J., Nakashima, H., Umehara, H., Inoue, K., Matoba, S., Tachibana, M., Ogura, A., Shinkai, Y., and Nakano, T. (2012). PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 486, 415–419.
| 1:CAS:528:DC%2BC38XovFyrtro%3D&md5=637ad143958af36c18e17d0fd0e6afb8CAS | 22722204PubMed |
Neugebauer, N., Räder, I., Schild, H. J., Zimmer, D., and Reinsch, N. (2010). Evidence for parent-of-origin effects on genetic variability of beef traits. J. Anim. Sci. 88, 523–532.
| Evidence for parent-of-origin effects on genetic variability of beef traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktVOqtrY%3D&md5=d88637e7f11d0e8241dca48688b6728dCAS | 19854988PubMed |
Norman, H. D., and Walsh, M. K. (2004). Performance of dairy cattle clones and evaluation of their milk composition. Cloning Stem Cells 6, 157–164.
| Performance of dairy cattle clones and evaluation of their milk composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFeltLc%3D&md5=36eedab63623cebc4a2eea051af27bfbCAS | 15268790PubMed |
Oliver, V. F., Miles, H. L., Cutfield, W. S., Hofman, P. L., Ludgate, J. L., and Morison, I. M. (2012). Defects in imprinting and genome-wide DNA methylation are not common in the in vitro fertilization population. Fertil Steril 97, 147–153.e7.
| Defects in imprinting and genome-wide DNA methylation are not common in the in vitro fertilization population.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht12rtg%3D%3D&md5=38d6824df7504347584edcc3972e9387CAS | 22112648PubMed |
Ooi, S. K., Wolf, D., Hartung, O., Agarwal, S., Daley, G. Q., Goff, S. P., and Bestor, T. H. (2010). Dynamic instability of genomic methylation patterns in pluripotent stem cells. Epigenetics Chromatin 3, 17.
| Dynamic instability of genomic methylation patterns in pluripotent stem cells.Crossref | GoogleScholarGoogle Scholar | 20868487PubMed |
Otten, W., Kanitz, E., Tuchscherer, M., Puppe, B., and Nurnberg, G. (2007). Repeated administrations of adrenocorticotropic hormone during gestation in gilts: effects on growth, behaviour and immune responses of their piglets. Livest. Sci. 106, 261–270.
| Repeated administrations of adrenocorticotropic hormone during gestation in gilts: effects on growth, behaviour and immune responses of their piglets.Crossref | GoogleScholarGoogle Scholar |
Otten, W., Kanitz, E., Couret, D., Veissier, I., Prunier, A., and Merlot, E. (2010). Maternal social stress during late pregnancy aggects hypothalamic–pituitary–adrenal function and brain neurotransmitter systems in pig offspring. Domest. Anim. Endocrinol. 38, 146–156.
| Maternal social stress during late pregnancy aggects hypothalamic–pituitary–adrenal function and brain neurotransmitter systems in pig offspring.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFGkt78%3D&md5=255251341fa2258d2f8cfede53691b8bCAS | 19879712PubMed |
Otten, W., Kanitz, E., and Tuchscherer, M. (2015). The impact of prenatal stress on offspring development in pigs. J. Agric. Sci. 153, 907–919.
| The impact of prenatal stress on offspring development in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXptVSjsb0%3D&md5=4497fb0f268a2854049aedc3c09c8493CAS |
Padmanabhan, N., Jia, D., Geary-Joo, C., Wu, X., Ferguson-Smith, A. C., Fung, E., Bieda, M. C., Snyder, F. F., Gravel, R. A., Cross, J. C., and Watson, E. D. (2013). Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell 155, 81–93.
| Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFemsLvO&md5=3217352b85519e86732c74dc44eb2227CAS | 24074862PubMed |
Palacios, D., Summerbell, D., Rigby, P. W., and Boyes, J. (2010). Interplay between DNA methylation and transcription factor availability: implications for developmental activation of the mouse Myogenin gene. Mol. Cell. Biol. 30, 3805–3815.
| Interplay between DNA methylation and transcription factor availability: implications for developmental activation of the mouse Myogenin gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVOlsLvL&md5=f691f976ac7776240712daa3b5da2aaaCAS | 20498275PubMed |
Park, C. S., Erickson, G. M., Choi, Y. J., and Marx, G. D. (1987). Effect of compensatory growth on regulation of growth and lactation: response of dairy heifers to a stair-step growth pattern. J. Anim. Sci. 64, 1751–1758.
| 1:CAS:528:DyaL2sXksFOrt7k%3D&md5=3aff7504d0cb45009d89644c370e27f1CAS | 3597190PubMed |
Parr, R. A., Williams, A. H., Campbell, I. P., Witcombe, G. F., and Roberts, A. M. (1986). Low nutrition of ewes in early pregnancy and the residual effect on the offspring. J. Agric. Sci. 106, 81–87.
| Low nutrition of ewes in early pregnancy and the residual effect on the offspring.Crossref | GoogleScholarGoogle Scholar |
Paten, A. M., Kenyon, P. R., Lopez-Villalobos, N., Peterson, S. W., Jenkinson, C. M. C., Pain, S. J., and Blair, H. T. (2013). Maternal nutrition during lactation and mod-to-late pregnancy: comparative effects on milk production of twin-born ewe progeny during their first lactation. J. Anim. Sci. 91, 676–684.
| Maternal nutrition during lactation and mod-to-late pregnancy: comparative effects on milk production of twin-born ewe progeny during their first lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVKnurc%3D&md5=9b41080cf73bdab72da91f746cbdeaa3CAS | 23230109PubMed |
Patterson, D. C., Steen, R. W. J., and Kilpatrick, D. J. (1993). A comparison of growth, food efficiency and carcass characteristics of single and twin beef calves derived by embryo transfer. Anim. Sci. 57, 81–89.
Paul, C., Rhind, S. M., Kyle, C. E., Scott, H., McKinnell, C., and Sharpe, R. M. (2005). Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge. Environ. Health Perspect. 113, 1580–1587.
| Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge.Crossref | GoogleScholarGoogle Scholar | 16263515PubMed |
Peralta, O. A., Huckle, W. R., and Eyestone, W. H. (2011). Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation 81, 68–77.
| Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFagsL7J&md5=284407c7223ba6fe1f04cd8d6d76e017CAS | 20926176PubMed |
Peralta, O. A., Huckle, W. R., and Eyestone, W. H. (2012). Developmental expression of the cellular prion protein (PrP(C)) in bovine embryos. Mol. Reprod. Dev. 79, 488–498.
| Developmental expression of the cellular prion protein (PrP(C)) in bovine embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpvVektL0%3D&md5=9843f1a632b5781d128e1966fdd1ea21CAS | 22674901PubMed |
Peters, A. H., O’Carroll, D., Scherthan, H., Mechtler, K., Sauer, S., Schofer, C., Weipoltshammer, K., Pagani, M., Lachner, M., Kohlmaier, A., Opravil, S., Doyle, M., Sibilia, M., and Jenuwein, T. (2001). Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337.
| Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosVKhsb4%3D&md5=a4a02ad7e383e0d779b914bc7953d0f4CAS | 11701123PubMed |
Pocar, P., Perazzoli, F., Luciano, A. M., and Gandolfi, F. (2001a). In vitro reproductive toxicity of polychlorinated biphenyls: effects on oocyte maturation and developmental competence in cattle. Mol. Reprod. Dev. 58, 411–416.
| In vitro reproductive toxicity of polychlorinated biphenyls: effects on oocyte maturation and developmental competence in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhs1Siu7c%3D&md5=ad73a2f83c51fd307c827b3821e117b1CAS | 11241777PubMed |
Pocar, P., Brevini, T. A., Perazzoli, F., Cillo, F., Modina, S., and Gandolfi, F. (2001b). Cellular and molecular mechanisms mediating the effects of polychlorinated biphenyls on oocyte developmental competence in cattle. Mol. Reprod. Dev. 60, 535–541.
| Cellular and molecular mechanisms mediating the effects of polychlorinated biphenyls on oocyte developmental competence in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosl2qt7g%3D&md5=c9569fa76fd2169d7fa5fca6c5f54fcbCAS | 11746964PubMed |
Popp, C., Dean, W., Feng, S., Cokus, S. J., Andrews, S., Pellegrini, M., Jacobsen, S. E., and Reik, W. (2010). Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463, 1101–1105.
| Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitl2gs7Y%3D&md5=6475413046d805441cd03a9315e9505bCAS | 20098412PubMed |
Posfai, E., Kunzmann, R., Brochard, V., Salvaing, J., Cabuy, E., Roloff, T. C., Liu, Z., Tardat, M., van Lohuizen, M., Vidal, M., Beaujean, N., and Peters, A. H. (2012). Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev. 26, 920–932.
| Polycomb function during oogenesis is required for mouse embryonic development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmslOgt78%3D&md5=d65166576a4223f7a89b1e2d03dd9379CAS | 22499591PubMed |
Quesnel, H., Boulot, S., Serriere, S., Venturi, E., and Martinat-Botté, F. (2010). Post-insemination level of feeding does not influence embryonic survival and growth in highly prolific gilts. Anim. Reprod. Sci. 120, 120–124.
| Post-insemination level of feeding does not influence embryonic survival and growth in highly prolific gilts.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3czktVOkug%3D%3D&md5=6bb12e1fd08f7dd09189c583aac08503CAS | 20434856PubMed |
Rae, M. T., Palassio, S., Kyle, C. E., Brooks, A. N., Lea, R. G., Miller, D. W., and Rhind, S. M. (2001). Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction 122, 915–922.
| Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhvVWisw%3D%3D&md5=233b1d8abea184e0a3dce728524124dcCAS | 11732987PubMed |
Rae, M. T., Kyle, C. E., Miller, D. W., Hammond, A. J., Brooks, A. N., and Rhind, S. M. (2002a). The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim. Reprod. Sci. 72, 63–71.
| The effects of undernutrition, in utero, on reproductive function in adult male and female sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltFahsLw%3D&md5=05a267ce9d685a9c5afc127a8db071a9CAS | 12106966PubMed |
Rae, M. T., Rhind, S. M., Fowler, P. A., Miller, D. W., Kyle, C. E., and Brooks, A. N. (2002b). Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses. Reproduction 124, 33–39.
| Effect of maternal undernutrition on fetal testicular steroidogenesis during the CNS androgen-responsive period in male sheep fetuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtFaks7s%3D&md5=ebc9b8af68cb6b805d6daf39de7fc94fCAS | 12090916PubMed |
Ramsahoye, B. H., Biniszkiewicz, D., Lyko, F., Clark, V., Bird, A. P., and Jaenisch, R. (2000). Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl Acad. Sci. USA 97, 5237–5242.
| Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsVWntLs%3D&md5=c26ef496162e2e3f10d7e434271557e7CAS | 10805783PubMed |
Rehfeldt, C., Pas, M. F. W. T., Wimmers, K., Brameld, J. M., Nissen, P. M., Berri, C., Valente, L. M. P., Power, D. M., Picard, B., Stickland, N. C., and Oksbjerg, N. (2011a). Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. II – Genetic factors related to animal performance and advances in methodology. Animal 5, 718–730.
| Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. II – Genetic factors related to animal performance and advances in methodology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFenu7k%3D&md5=dded05d808b9f676af13f112211be0edCAS | 22439994PubMed |
Rehfeldt, C., Pas, M. F. W. T., Wimmers, K., Brameld, J. M., Nissen, P. M., Berri, C., Valente, L. M. P., Power, D. M., Picard, B., Stickland, N. C., and Oksbjerg, N. (2011b). Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. I. Regulation of myogenesis and environmental impact. Animal 5, 703–717.
| Advances in research on the prenatal development of skeletal muscle in animals in relation to the quality of muscle-based food. I. Regulation of myogenesis and environmental impact.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFenu70%3D&md5=e7d282d9c3740e26fe2f2e5aa58f432eCAS | 22439993PubMed |
Renfree, M. B., Suzuki, S., and Kaneko-Ishino, T. (2013). The origin and evolution of genomic imprinting and viviparity in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120151.
| The origin and evolution of genomic imprinting and viviparity in mammals.Crossref | GoogleScholarGoogle Scholar | 23166401PubMed |
Rhind, S. M. (2005). Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod. Domest. Anim. 40, 282–290.
| Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsVartrk%3D&md5=39bdeb7430d41cebc02f8786963fe308CAS | 16008758PubMed |
Rhind, S. M., Elston, D. A., Jones, J. R., Rees, M. E., McMillen, S. R., and Gunn, R. G. (1998). Effects of restriction of growth and development of Brecon Cheviot ewe lambs on subsequent lifetime reproductive performance. Small Rumin. Res. 30, 121–126.
| Effects of restriction of growth and development of Brecon Cheviot ewe lambs on subsequent lifetime reproductive performance.Crossref | GoogleScholarGoogle Scholar |
Rhind, S. M., Rae, M. T., and Brooks, A. N. (2003). Environmental influences on the fetus and neonate – timing, mechanisms of action and effects on subsequent adult function. Domest. Anim. Endocrinol. 25, 3–11.
| Environmental influences on the fetus and neonate – timing, mechanisms of action and effects on subsequent adult function.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3svit1Ojsg%3D%3D&md5=f97f5c37c855044affbb337e00185bbeCAS | 12963095PubMed |
Rhind, S. M., Kyle, C. E., Mackie, C., McDonald, L., Zhang, Z., Duff, E. I., Bellingham, M., Amezaga, M. R., Mandon-Pepin, B., Loup, B., Cotinot, C., Evans, N. P., Sharp, R. M., and Fowler, P. A. (2010). Maternal and fetal tissue accumulation of selected endocrine disrupting compounds (EDCs) following exposure to sewage sludge-treated pastures before or after conception. J. Environ. Monit. 12, 1582–1593.
| Maternal and fetal tissue accumulation of selected endocrine disrupting compounds (EDCs) following exposure to sewage sludge-treated pastures before or after conception.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVSgtb0%3D&md5=9d16ad285694808f74c9c6831e7fff12CAS | 20676422PubMed |
Rhind, S. M., Kyle, C. E., Kerr, C., Osprey, M., and Zhang, Z. L. (2011). Effect of duration of exposure to sewage sludge-treated pastures on liver tissue accumulation of persistent endocrine disrupting compounds (EDCs) in sheep. Sci. Total Environ. 409, 3850–3856.
| Effect of duration of exposure to sewage sludge-treated pastures on liver tissue accumulation of persistent endocrine disrupting compounds (EDCs) in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVGktbjE&md5=196b56ddcbbd3a7fa1984ea1a855a380CAS | 21767868PubMed |
Riggs, A. D., Martienssen, R. A., and Russo, V. E. A. (1996). ‘Epigenetic Mechanisms of Gene Regulation.’ (Ed. V. E. A. Russo et al. ) pp. 1–4. (Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York.)
Rivera, O. E., Varayoud, J., Rodriguez, H. A., Munoz-de-Toro, M., and Luque, E. H. (2011). Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 32, 304–312.
| Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtl2htbfJ&md5=55f44c6b7b3e5fda285060a77248bef4CAS | 21722727PubMed |
Rivera, O. E., Varayoud, J., Rodriguez, H. A., Santamaria, C. G., Bosquiazzo, V. L., Osti, M., Belmonte, N. M., Munoz-de-Toro, M., and Luque, E. H. (2015). Neonatal exposure to xenoestrogens impairs the ovarian response to gonadotropin treatment in lambs. Reproduction 149, 645–655.
| Neonatal exposure to xenoestrogens impairs the ovarian response to gonadotropin treatment in lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFamtb7L&md5=f21ace458dd08fd8a59411011e09fddcCAS | 25778539PubMed |
Robinson, G. W., Karpf, A. B. C., and Kratochwil, K. (1999). Regulation of mammary gland development by tissue interaction. J. Mammary Gland Biol. Neoplasia 4, 9–19.
| Regulation of mammary gland development by tissue interaction.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3jsFejuw%3D%3D&md5=cbd96f0b3aed817aaace6571aa061801CAS | 10219903PubMed |
Robinson, D. L., Cafe, L. M., and Greenwood, P. L. (2013). Meat Science and Muscle Biology Symposium: developmental programming in cattle: consequences for growth, efficiency, carcass, muscle, and beef quality characteristics. J. Anim. Sci. 91, 1428–1442.
| Meat Science and Muscle Biology Symposium: developmental programming in cattle: consequences for growth, efficiency, carcass, muscle, and beef quality characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmsFKnu7s%3D&md5=e521be7c4f2506ee261f676d0853db6cCAS | 23230118PubMed |
Rodgers, B. D., and Garikipati, D. K. (2008). Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr. Rev. 29, 513–534.
| Clinical, agricultural, and evolutionary biology of myostatin: a comparative review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVKnsrvJ&md5=086096118bef0ffe7baa3a4b7f05d908CAS | 18591260PubMed |
Rooke, I. A., Dwyer, C. M., and Ashworth, C. J. (2008). The potential for improving physiological, behavioural and immunological responses in the neonatal lamb by trace element and vitamin supplementation of the ewe. Animal 2, 514–524.
| The potential for improving physiological, behavioural and immunological responses in the neonatal lamb by trace element and vitamin supplementation of the ewe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvVKnsLY%3D&md5=53286087703031ce0060a9f50dbc49efCAS |
Rooke, J. A., Houdijk, J. G. M., McIlvaney, K., Ashworth, C. J., and Dwyer, C. M. (2010). Differential effects of maternal undernutrition between Days 1 and 90 of pregnancy on ewe and lamb performance and lamb parasitism in hill or lowland breeds. J. Anim. Sci. 88, 3833–3842.
| Differential effects of maternal undernutrition between Days 1 and 90 of pregnancy on ewe and lamb performance and lamb parasitism in hill or lowland breeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGnsrrK&md5=f6a7fdc8f6d63c3320e175c9b311502fCAS | 20675602PubMed |
Rooke, J. A., Arnott, G., Dwyer, C. M., and Rutherford, K. M. D. (2015). The importance of the gestation period for welfare of lambs: maternal stressors and lamb vigour and well-being. J. Agric. Sci. 153, 497–519.
| The importance of the gestation period for welfare of lambs: maternal stressors and lamb vigour and well-being.Crossref | GoogleScholarGoogle Scholar |
Rosenberg, E., and Zilber-Rosenberg, I. (2011). Symbiosis and development: the hologenome concept. Birth Defects Res. C Embryo Today 93, 56–66.
| Symbiosis and development: the hologenome concept.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFGiurc%3D&md5=7bcdb56355af428115f4f14486fb9625CAS | 21425442PubMed |
Ross, E. M., Moate, P. J., Marett, L. C., Cocks, B. G., and Hayes, B. J. (2013). Metagenomic predictions: from microbiome to complex health and environmental phenotypes in humans and cattle. PLoS One 8, e73056.
| Metagenomic predictions: from microbiome to complex health and environmental phenotypes in humans and cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVCrsrnE&md5=c3f73f37772f59c38a307c1211d30e95CAS | 24023808PubMed |
Roussel, S., Hemsworth, P. H., Boissy, A., and Duvaux-Ponter, C. (2004). Effects of repeated stress during pregnancy in ewes on the behavioural and physiological responses to stressful events and birth weight of their offspring. Appl. Anim. Behav. Sci. 85, 259–276.
| Effects of repeated stress during pregnancy in ewes on the behavioural and physiological responses to stressful events and birth weight of their offspring.Crossref | GoogleScholarGoogle Scholar |
Roussel, S., Hemsworth, P. H., Leruste, H., White, C., Duvaux-Ponter, C., Nowak, R., and Boissy, A. (2006). Repeated transport and isolation during pregnancy in ewes: effects on the reactivity to humans and to their offspring after lambing. Appl. Anim. Behav. Sci. 97, 172–189.
| Repeated transport and isolation during pregnancy in ewes: effects on the reactivity to humans and to their offspring after lambing.Crossref | GoogleScholarGoogle Scholar |
Roussel-Huchette, S., Hemsworth, P. H., Boissy, A., and Duvaux-Ponter, C. (2008). Repeated transport and isolation during pregnancy in ewes: differential effects on emotional reactivity and weight of their offspring. Appl. Anim. Behav. Sci. 109, 275–291.
| Repeated transport and isolation during pregnancy in ewes: differential effects on emotional reactivity and weight of their offspring.Crossref | GoogleScholarGoogle Scholar |
Rudenko, L., and Matheson, J. C. (2007). The US FDA and animal cloning: risk and regulatory approach. Theriogenology 67, 198–206.
| The US FDA and animal cloning: risk and regulatory approach.Crossref | GoogleScholarGoogle Scholar | 17055042PubMed |
Rudenko, L., Matheson, J. C., and Sundlof, S. F. (2007). Animal cloning and the FDA – the risk assessment paradigm under public scrutiny. Nat. Biotechnol. 25, 39–43.
| Animal cloning and the FDA – the risk assessment paradigm under public scrutiny.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1GlsA%3D%3D&md5=da0d0913ee6c65bd7eb29a5b9068c536CAS | 17211392PubMed |
Rutherford, K. M., Robson, S. K., Donald, R. D., Jarvis, S., Sandercock, D. A., Scott, E. M., Nolan, A. M., and Lawrence, A. B. (2009). Pre-natal stress amplifies the immediate behavioural responses to acute pain in piglets. Biol. Lett. 5, 452–454.
| Pre-natal stress amplifies the immediate behavioural responses to acute pain in piglets.Crossref | GoogleScholarGoogle Scholar | 19411272PubMed |
Rutherford, K., Donald, R., Arnott, G., Rooke, J., Dixon, L., Mehers, J., Turnbull, J., and Lawrence, A. (2012). Farm animal welfare: assessing risks attributable to the prenatal environment. Anim. Welf. 21, 419–429.
| Farm animal welfare: assessing risks attributable to the prenatal environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFOntL%2FE&md5=58370c99fd0bfa09f270362510f58e69CAS |
Rutherford, K. M. D., Baxter, E. M., D’Eath, R. B., Turner, S. P., Arnott, G., Roehe, R., Ask, B., Sandøe, P., Moustsen, V. A., Thorup, F., Edwards, S. A., Berg, P., and Lawrence, A. B. (2013). The welfare implications of large litter size in the domestic pig I: biological factors. Anim. Welf. 22, 199–218.
| The welfare implications of large litter size in the domestic pig I: biological factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnt1ajuro%3D&md5=91d667b6bf1322b7adb54bcd6084b374CAS |
Rutherford, K. M. D., Piastowska, A., Donald, R. D., Robson, S. K., Ison, S. H., Brunton, P. J., Russell, J. A., and Lawrence, A. B. (2014). Prenatal stress produces anxiety prone female offspring and impaired maternal behaviour in the domestic pig. Physiol. Behav. 129, 255–264.
| Prenatal stress produces anxiety prone female offspring and impaired maternal behaviour in the domestic pig.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmvVWmsrY%3D&md5=bf5d7b812f3f21f9cf063a0066f6f3c0CAS |
Sabin, L. R., Delas, M. J., and Hannon, G. J. (2013). Dogma derailed: the many influences of RNA on the genome. Mol. Cell 49, 783–794.
| Dogma derailed: the many influences of RNA on the genome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktVOlsrw%3D&md5=cedf021afe6c4b53bae5c80b4c07e532CAS | 23473599PubMed |
Saitou, M., and Yamaji, M. (2012). Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 4, a008375.
| Primordial germ cells in mice.Crossref | GoogleScholarGoogle Scholar | 23125014PubMed |
Salloum, R. H., Rubin, J. P., and Marra, K. G. (2013). The role of steroids in mesenchymal stem cell differentiation: molecular and clinical perspectives. Horm. Mol. Biol. Clin. Investig. 14, 3–14.
| The role of steroids in mesenchymal stem cell differentiation: molecular and clinical perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVektbc%3D&md5=35e05e8cf942ae93d9750ec8065cc3c2CAS | 25436715PubMed |
Santos, F., Hyslop, L., Stojkovic, P., Leary, C., Murdoch, A., Reik, W., Stojkovic, M., Herbert, M., and Dean, W. (2010). Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum. Reprod. 25, 2387–2395.
| Evaluation of epigenetic marks in human embryos derived from IVF and ICSI.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGru77P&md5=11c4f428437df004ec1f20636d638106CAS | 20634187PubMed |
Savabieasfahani, M., Kannan, K., Astapova, O., Evans, N. P., and Padmanabhan, V. (2006). Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 147, 5956–5966.
| Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1yqtrrN&md5=908be9d377df0a5f9a6974f4b19d9b3bCAS | 16946013PubMed |
Schiaffino, S., and Mammucari, C. (2011). Regulation of skeletal muscle growth by the IGF1–Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1, 4.
| Regulation of skeletal muscle growth by the IGF1–Akt/PKB pathway: insights from genetic models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFShurk%3D&md5=6a2ecb38ed30930a568f4079ee93382fCAS | 21798082PubMed |
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J., and Baskin, D. G. (2000). Central nervous system control of food intake. Nature 404, 661–671.
| 1:CAS:528:DC%2BD3cXis1Grur4%3D&md5=abcdebe7204d862a8a05e7851cb01ef3CAS | 10766253PubMed |
Seale, P., Bjork, B., Yang, W., Kajimura, S., Chin, S., Kuang, S., Scime, A., Devarakonda, S., Conroe, H. M., Erdjument-Bromage, H., Tempst, P., Rudnicki, M. A., Beier, D. R., and Spiegelman, B. M. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967.
| PRDM16 controls a brown fat/skeletal muscle switch.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVWns7%2FN&md5=c9cdef098b3691abf44a71cb4ea5e26eCAS | 18719582PubMed |
Sébert, S. P., Hyatt, M. A., Chan, L. L., Patel, N., Bell, R. C., Keisler, D., Stephenson, T., Budge, H., Symonds, M. E., and Gardner, D. S. (2009). Maternal nutrient restriction between early and midgestation and its impact upon appetite regulation after juvenile obesity. Endocrinology 150, 634–641.
| Maternal nutrient restriction between early and midgestation and its impact upon appetite regulation after juvenile obesity.Crossref | GoogleScholarGoogle Scholar | 18818297PubMed |
Seisenberger, S., Peat, J. R., and Reik, W. (2013). Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells. Curr. Opin. Cell Biol. 25, 281–288.
| Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkt1KrsLk%3D&md5=fbc1433723a930923a7c242e8f47ad65CAS | 23510682PubMed |
Sharifabadi, H. R., Zamiri, M. J., Rowghani, E., and Bottje, W. G. (2012). Relationship between the activity of mitochondrial respiratory chain complexes and feed efficiency in fat-tailed Ghezel lambs. J. Anim. Sci. 90, 1807–1815.
| Relationship between the activity of mitochondrial respiratory chain complexes and feed efficiency in fat-tailed Ghezel lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsFWgsb4%3D&md5=142f94b9e076f0598b5a0b7c10483702CAS | 22147474PubMed |
Sharpe, R. M., McKinnell, C., Kivlin, C., and Fisher, J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784.
| Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltFelt7c%3D&md5=10cf4b0261e962d361c84957f26be906CAS | 12773099PubMed |
Shin, J., Velleman, S. G., Latshaw, J. D., Wick, M. P., Suh, Y., and Lee, K. (2009). The ontogeny of delta-like protein 1 messenger ribonucleic acid expression during muscle development and regeneration: comparison of broiler and leghorn chickens. Poult. Sci. 88, 1427–1437.
| The ontogeny of delta-like protein 1 messenger ribonucleic acid expression during muscle development and regeneration: comparison of broiler and leghorn chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Omtb0%3D&md5=de2ceb4d8e98526dbf6f26612b56658eCAS | 19531714PubMed |
Shirane, K., Toh, H., Kobayashi, H., Miura, F., Chiba, H., Ito, T., Kono, T., and Sasaki, H. (2013). Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases. PLoS Genet. 9, e1003439.
| Mouse oocyte methylomes at base resolution reveal genome-wide accumulation of non-CpG methylation and role of DNA methyltransferases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnt1ejurw%3D&md5=7141afa7bdeb0ea15d72faf4491f4775CAS | 23637617PubMed |
Sibbald, A. M., and Davidson, G. C. (1998). The effect of nutrition during early life on voluntary food intake by lambs between weaning and 2 years of age. Anim. Sci. 66, 697–703.
| The effect of nutrition during early life on voluntary food intake by lambs between weaning and 2 years of age.Crossref | GoogleScholarGoogle Scholar |
Siklenka, K., Erkek, S., Godmann, M., Lambrot, R., McGraw, S., Lafleur, C., Cohen, T., Xia, J., Suderman, M., Hallett, M., Trasler, J., Peters, A. H., and Kimmins, S. (2015). Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350, aab2006.
| Disruption of histone methylation in developing sperm impairs offspring health transgenerationally.Crossref | GoogleScholarGoogle Scholar | 26449473PubMed |
Sinclair, K. D., Broadbent, P. J., and Dolman, D. F. (1995). In vitro produced embryos as a means of achieving pregnancy and improving productivity in beef cows. Anim. Sci. 60, 55–64.
| In vitro produced embryos as a means of achieving pregnancy and improving productivity in beef cows.Crossref | GoogleScholarGoogle Scholar |
Sinclair, K. D., Young, L. E., Wilmut, I., and McEvoy, T. G. (2000). In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum. Reprod. 15, 68–86.
| In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men.Crossref | GoogleScholarGoogle Scholar | 11263539PubMed |
Sinclair, K. D., Allegrucci, C., Singh, R., Gardner, D. S., Sebastian, S., Bispham, J., Thurston, A., Huntley, J. F., Rees, W. D., Maloney, C. A., Lea, R. G., Craigon, J., McEvoy, T. G., and Young, L. E. (2007). DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl Acad. Sci. USA 104, 19 351–19 356.
| DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVOjug%3D%3D&md5=75bba51e8598b1ca3ed0d168ef755b4cCAS |
Skinner, M. K., Manikkam, M., and Guerrero-Bosagna, C. (2010). Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222.
| Epigenetic transgenerational actions of environmental factors in disease etiology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVejtLc%3D&md5=42dfc0362c4e143155e65b513bd50548CAS | 20074974PubMed |
Smallwood, S. A., and Kelsey, G. (2012). De novo DNA methylation: a germ cell perspective. Trends Genet. 28, 33–42.
| De novo DNA methylation: a germ cell perspective.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvV2msA%3D%3D&md5=44574d749151e47986ba566827c0afb3CAS | 22019337PubMed |
Smit, M. N., Spencer, J. D., Almeida, F. R. C. L., Patterson, J. L., Chiarini-Garcia, H., Dyck, M. K., and Foxcroft, G. R. (2013). Consequences of a low litter birth weight phenotype for postnatal lean growth performance and neonatal testicular morphology in the pig. Animal 7, 1681–1689.
| Consequences of a low litter birth weight phenotype for postnatal lean growth performance and neonatal testicular morphology in the pig.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjnslyrtw%3D%3D&md5=9af1db00edb6f0eddbd8352f8dc2ad04CAS | 23822933PubMed |
Smith, J. A., Lewis, A. M., Wiener, P., and Williams, J. L. (2000). Genetic variation in the bovine myostatin gene in UK beef cattle: allele frequencies and haplotype analysis in the South Devon. Anim. Genet. 5, 306–309.
| Genetic variation in the bovine myostatin gene in UK beef cattle: allele frequencies and haplotype analysis in the South Devon.Crossref | GoogleScholarGoogle Scholar |
Smith, L. C., Suzuki, J., Goff, A. K., Filion, F., Therrien, J., Murphy, B. D., Kohan-Ghadr, H. R., Lefebvre, R., Brisville, A. C., Buczinski, S., Fecteau, G., Perecin, F., and Meirelles, F. V. (2012). Developmental and epigenetic anomalies in cloned cattle. Reprod. Domest. Anim. 47, 107–114.
| Developmental and epigenetic anomalies in cloned cattle.Crossref | GoogleScholarGoogle Scholar | 22827358PubMed |
Steegers-Theunissen, R. P., Twigt, J., Pestinger, V., and Sinclair, K. D. (2013). The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum. Reprod. Update 19, 640–655.
| The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1GqurnM&md5=ec97771a71178d4c3ac06376edb63de9CAS | 23959022PubMed |
Stevens, A., Begum, G., Cook, A., Connor, K., Rumball, C., Oliver, M., Challis, J., Bloomfield, F., and White, A. (2010). Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151, 3652–3664.
| Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Oju77J&md5=5caa46473163365bb0404088424e769eCAS | 20573728PubMed |
Sullivan, T. M., Micke, G. C., Greer, R. M., Irving-Rodgers, H. F., Rodgers, R. J., and Perry, V. E. A. (2009). Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves. Reprod. Fertil. Dev. 21, 773–784.
| Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVOhu7Y%3D&md5=c685abd4b12a720747ac82ee77c7e914CAS | 19567220PubMed |
Sullivan, T. M., Micke, G. C., Greer, R. M., and Perry, V. E. (2010). Dietary manipulation of Bos indicus × heifers during gestation affects the prepubertal reproductive development of their bull calves. Anim. Reprod. Sci. 118, 131–139.
| Dietary manipulation of Bos indicus × heifers during gestation affects the prepubertal reproductive development of their bull calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGltbo%3D&md5=7bf3a413b3a1cfb6b2acecb3c515f382CAS | 19671489PubMed |
Suteevun-Phermthai, T., Curchoe, C. L., Evans, A. C., Boland, E., Rizos, D., Fair, T., Duffy, P., Sung, L. Y., Du, F., Chaubal, S., Xu, J., Wechayant, T., Yang, X., Lonergan, P., Parnpai, R., and Tian, X. C. (2009). Allelic switching of the imprinted IGF2R gene in cloned bovine fetuses and calves. Anim. Reprod. Sci. 116, 19–27.
| Allelic switching of the imprinted IGF2R gene in cloned bovine fetuses and calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKkt7jM&md5=b3b7e0459c1fe4fe7c1cc667666320c6CAS | 19217227PubMed |
Swali, A., and Wathes, D. C. (2006). Influence of the dam and sire on size at birth and subsequent growth, milk production and fertility in dairy heifers. Theriogenology 66, 1173–1184.
| Influence of the dam and sire on size at birth and subsequent growth, milk production and fertility in dairy heifers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28vps1yitA%3D%3D&md5=56e197d52db82210a11de727e1907485CAS | 16647111PubMed |
Swatland, H. J., and Cassens, R. G. (1972). Muscle growth: the problem of muscle fibers with an intrafascicular termination. J. Anim. Sci. 35, 336–344.
| 1:STN:280:DyaE383ms1Khsg%3D%3D&md5=71adf21e3cbe73357eb9280815e3e748CAS | 4115636PubMed |
Sweeney, T., Nicol, L., Roche, J. F., and Brooks, A. N. (2000). Maternal exposure to octylphenol suppresses ovine fetal follicle-stimulating hormone secretion, testis size, and Sertoli cell number. Endocrinology 141, 2667–2673.
| 1:CAS:528:DC%2BD3cXlt1Wmu70%3D&md5=4823169c6fc92cf566e750a3dd12ae3eCAS | 10875272PubMed |
Sweeney, T., Fox, J., Robertson, L., Kelly, G., Duffy, P., Lonergan, P., O’Doherty, J., Roche, J. F., and Evans, N. P. (2007). Postnatal exposure to octylphenol decreases semen quality in the adult ram. Theriogenology 67, 1068–1075.
| Postnatal exposure to octylphenol decreases semen quality in the adult ram.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitFOnsrs%3D&md5=5e6ff1d1f47912c3c612bba41cb89b44CAS | 17284332PubMed |
Sweetman, D. (2012). The myogenic regulatory factors: critical determinants of muscle identity in development, growth and regeneration. In ‘Skeletal Muscle: From Myogenesis to Clinical Relations’. (Ed. J. Cseri.) pp. 31–48. (Intech.: Rijeka, Croatia.)
Symonds, M. E., Bryant, M. J., Shepherd, D. A., and Lomax, M. A. (1988). Glucose metabolism in shorn and unshorn pregnant sheep. Br. J. Nutr. 60, 249–263.
| Glucose metabolism in shorn and unshorn pregnant sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXls1OrsLo%3D&md5=22743408b49c831f4dbed340afc49212CAS | 3143399PubMed |
Takahashi, S., and Yoshihiko, I. (2004). Evaluation of meat products from cloned cattle: biological and biochemical properties. Cloning Stem Cells 6, 165–171.
| Evaluation of meat products from cloned cattle: biological and biochemical properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFeltb4%3D&md5=fbdee5c317b482f9dfe29ff6ee88ad85CAS | 15268791PubMed |
Tang, W.-Y., Newbold, R., Mardilovich, K., Jefferson, W., Cheng, R. Y. S., Medvedovic, M., and Ho, S.-M. (2008). Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology 149, 5922–5931.
| Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVGltrzP&md5=e0abd80be568f7109658ac7ee3988e47CAS | 18669593PubMed |
Tao, S., Monteiro, A. P. A., Thompson, I. M., Hayen, M. J., and Dahl, G. E. (2012). Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 95, 7128–7136.
| Effect of late-gestation maternal heat stress on growth and immune function of dairy calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12gu7vF&md5=e9dd39b81d8996fc05ca406a19088884CAS | 23021751PubMed |
Tellam, R. L., Cockett, N. E., Vuocolo, T., and Bidwell, C. A. (2012). Genes contributing to genetic variation of muscling in sheep. Front. Genet. 3, 164.
| Genes contributing to genetic variation of muscling in sheep.Crossref | GoogleScholarGoogle Scholar | 22952470PubMed |
Thayer, K., Heindel, J., Bucher, J., and Gallo, M. (2012). Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ. Health Perspect. 120, 779–789.
| Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVOjtrbN&md5=2ef353a13fe4be5c497bec6bd3bba335CAS | 22296744PubMed |
Thurston, A., Taylor, J., Gardner, J., Sinclair, K. D., and Young, L. E. (2008). Monoallelic expression of nine imprinted genes in the sheep embryo occurs after the blastocyst stage. Reproduction 135, 29–40.
| Monoallelic expression of nine imprinted genes in the sheep embryo occurs after the blastocyst stage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhs1CksL0%3D&md5=0fe2a04c59dd34bcfe13e2a93504b98aCAS | 18159081PubMed |
Tiemann, U., Pohland, R., and Schneider, F. (1996). Influence of organochlorine pesticides on physiological potency of cultured granulosa cells from bovine preovulatory follicles. Theriogenology 46, 253–265.
| Influence of organochlorine pesticides on physiological potency of cultured granulosa cells from bovine preovulatory follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsVShsLs%3D&md5=1b7914da82f310b12f4d70379647d97bCAS | 16727895PubMed |
Tomé, D., Dubarry, M., and Fromentin, G. (2004). Nutritional value of milk and meat products derived from cloning. Cloning Stem Cells 6, 172–177.
| Nutritional value of milk and meat products derived from cloning.Crossref | GoogleScholarGoogle Scholar | 15268792PubMed |
Tomizawa, S., Nowacka-Woszuk, J., and Kelsey, G. (2012). DNA methylation establishment during oocyte growth: mechanisms and significance. Int. J. Dev. Biol. 56, 867–875.
| DNA methylation establishment during oocyte growth: mechanisms and significance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvVyqsLY%3D&md5=184a63c79ef7bf989fdc67a1b58c2557CAS | 23417409PubMed |
Tsumagari, K., Baribault, C., Terragni, J., Varley, K. E., Gertz, J., Pradhan, S., Badoo, M., Crain, C. M., Song, L., Crawford, G. E., Myers, R. M., Lacey, M., and Ehrlich, M. (2013). Early de novo DNA methylation and prolonged demethylation in the muscle lineage. Epigenetics 8, 317–332.
| Early de novo DNA methylation and prolonged demethylation in the muscle lineage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVantLw%3D&md5=b07c120b0b2f8464e72c62c83ccf1ad6CAS | 23417056PubMed |
Tuchscherer, M., Kanitz, E., Otten, W., and Tuchscherer, A. (2002). Effects of prenatal stress on cellular and humoral immune response in neonatal pigs. Vet. Immunol. Immunopathol. 86, 195–203.
| Effects of prenatal stress on cellular and humoral immune response in neonatal pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsVSqtb0%3D&md5=591ab6336600bb8b96458aa2924e17ddCAS | 12007885PubMed |
Tuchscherer, M., Otten, W., Kanitz, E., Grabner, M., Tuchscherer, A., Bellmann, O., Rehfeldt, C., and Metges, C. (2012). Effects of inadequate maternal dietary protein: carbohydrate ratios during pregnancy on offspring immunity in pigs. BMC Vet. Res. 8, 232.
| Effects of inadequate maternal dietary protein: carbohydrate ratios during pregnancy on offspring immunity in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVOltb0%3D&md5=95c853da83d5e21c0cab9899d8af4c97CAS | 23190629PubMed |
Valasek, P., Theis, S., DeLaurier, A., Hinits, Y., Luke, G. N., Otto, A. M., Minchin, J., He, L., Christ, B., Brooks, G., Sang, H., Evans, D. J., Logan, M., Huang, R., and Patel, K. (2011). Cellular and molecular investigations into the development of the pectoral girdle. Dev. Biol. 357, 108–116.
| Cellular and molecular investigations into the development of the pectoral girdle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWrs7rP&md5=ab991ee31b5f0d349b3f31ab6c3e5955CAS | 21741963PubMed |
Vallet, J. L., McNeel, A. K., Johnson, G., and Bazer, F. W. (2013). Triennial Reproduction Symposium: limitations in uterine and conceptus physiology that lead to fetal losses. J. Anim. Sci. 91, 3030–3040.
| Triennial Reproduction Symposium: limitations in uterine and conceptus physiology that lead to fetal losses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFChsL3N&md5=a4e82971d7fd69853853596058771041CAS | 23798512PubMed |
van der Linden, D. S., Kenyon, P. R., Blair, H. T., Lopez-Vilalobos, N., Jenkinson, C. M. C., Peterson, S. W., and Mackenzie, D. D. S. (2009). Effects of ewe size and nutrition on fetal mammary gland development and lactational performance of offspring at their first lactation. J. Anim. Sci. 87, 3944–3954.
| Effects of ewe size and nutrition on fetal mammary gland development and lactational performance of offspring at their first lactation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVOhs7bO&md5=c169466ae68a8b174b9847d5938da160CAS | 19684261PubMed |
van Wagtendonk-de Leeuw, A. M., Mullaart, E., de Roos, A. P., Merton, J. S., den Daas, J. H., Kemp, B., and de Ruigh, L. (2000). Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring. Theriogenology 53, 575–597.
| Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7pvFahtA%3D%3D&md5=f6a087098732d6e3a5b6aae8cb499b79CAS | 10735051PubMed |
Veiga-Lopez, A., Luense, L. J., Christenson, L. K., and Padmanabhan, V. (2013). Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884.
| Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntFWks70%3D&md5=2ef17829a4033b5c106aabb8a921f08eCAS | 23525218PubMed |
Vilette, Y., and Theriez, M. (1981). Influence of birth weight on lamb performance. I. Level of feed intake and growth. Ann. Zootech. 30, 151–168.
Villette, Y., and Theriez, M. (1983). Milk intake in lambs suckled by their dams during the first week of life. Ann. Zootech. 30, 169–182.
| Milk intake in lambs suckled by their dams during the first week of life.Crossref | GoogleScholarGoogle Scholar |
Vincent, I. C., Williams, H. L., and Hill, R. (1985). The influence of a low-nutrient intake after mating on gestation and perinatal survival of lambs. Br. Vet. J. 141, 611–617.
| The influence of a low-nutrient intake after mating on gestation and perinatal survival of lambs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL28%2Flt1Grsw%3D%3D&md5=feabfe95a4fca74c02572615a3ee31a5CAS | 4063783PubMed |
Vonnahme, K. A., Wilson, M. E., Foxcroft, G. R., and Ford, S. P. (2002). Impacts of conceptus survival in a commercial swine herd. J. Anim. Sci. 84, 2316–2337.
Wagner, K. D., Wagner, N., Ghanbarian, H., Grandjean, V., Gounon, P., Cuzin, F., and Rassoulzadegan, M. (2008). RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev. Cell 14, 962–969.
| RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVCrsbc%3D&md5=4abaa53ee39da3acb394086c751cf82eCAS | 18539123PubMed |
Walker, S. K., Hartwich, K. M., and Seamark, R. F. (1996). The production of unusually large offspring following embryo manipulation: concepts and challenges. Theriogenology 45, 111–120.
| The production of unusually large offspring following embryo manipulation: concepts and challenges.Crossref | GoogleScholarGoogle Scholar |
Wallace, C. (1953). Observations on mammary development in calves and lambs. J. Agric. Sci. 43, 413–421.
| Observations on mammary development in calves and lambs.Crossref | GoogleScholarGoogle Scholar |
Wallace, J. M. (2011). Adaptive maternal, placental and fetal responses to nutritional extremes in the pregnant adolescent: lessons from sheep. In ‘Reproduction and Adaptation’. (Eds C. G. N. Mascie-Taylor and L. Rosetta.) pp. 112–127. (Cambridge University Press.)
Wallace, J. M., Milne, J. S., Green, L. R., and Aitken, R. P. (2011a). Postnatal hypothalamic-pituitary-adrenal function in sheep is influenced by age and sex, but not by prenatal growth restriction. Reprod. Fertil. Dev. 23, 275–284.
| Postnatal hypothalamic-pituitary-adrenal function in sheep is influenced by age and sex, but not by prenatal growth restriction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFeqsA%3D%3D&md5=47b37ef1c47cf6b9d610410f15a82992CAS | 21211460PubMed |
Wallace, J. M., Aitken, R. P., Milne, J. S., Bake, T., and Adam, C. L. (2011b). Growth, body composition and metabolism in neonatal and adolescent life stages in low birth weight offspring. Proc. Nutr. Soc. 70, OCE1.
| Growth, body composition and metabolism in neonatal and adolescent life stages in low birth weight offspring.Crossref | GoogleScholarGoogle Scholar |
Wallace, J. M., Milne, J. S., Aitken, R. P., and Adam, C. L. (2014a). Impact of embryo donor adiposity, birthweight and gender on early postnatal growth, glucose metabolism and body composition in the young lamb. Reprod. Fertil. Dev. 26, 665–681.
| Impact of embryo donor adiposity, birthweight and gender on early postnatal growth, glucose metabolism and body composition in the young lamb.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVentb3K&md5=2f5ff6a0055992f18ec17d4e5deea09dCAS | 23714163PubMed |
Wallace, J. M., Milne, J. S., Aitken, R. P., and Adam, C. L. (2014b). Influence of birthweight and gender on lipid status and adipose tissue gene expression in lambs. J. Mol. Endocrinol. 53, 131–144.
| Influence of birthweight and gender on lipid status and adipose tissue gene expression in lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1enu7nL&md5=d9e631447b7fa121d569f6a3f42a84deCAS | 24928206PubMed |
Wallace, J. M., Milne, J. S., Aitken, R. P., Redmer, D. A., Reynolds, L. P., Luther, J. S., Horgan, G. W., and Adam, C. L. (2015). Undernutrition and stage of gestation influence fetal adipose tissue gene expression. J. Mol. Endocrinol. 54, 263–275.
| Undernutrition and stage of gestation influence fetal adipose tissue gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsFKjtLnE&md5=1e4dfda69c2dc7c89a137461069f3098CAS | 25917833PubMed |
Warnes, K. E., Morris, M. J., Symonds, M. E., Phillips, I. D., Clarke, I. J., Owens, J. A., and McMillen, I. C. (1998). Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J. Neuroendocrinol. 10, 51–57.
| Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtFOiu7k%3D&md5=3b9c36bada280b044842856424ad79d3CAS | 9510058PubMed |
Warrington, B. G., Byers, F. M., Schelling, G. T., Forrest, D. W., Baker, J. F., and Greene, L. W. (1988). Gestation nutrition, tissue exchange and maintenance requirements of heifers. J. Anim. Sci. 66, 774–782.
| 1:STN:280:DyaL1c3ktlGnsQ%3D%3D&md5=fdf04723ef48cf415a014801e9a4ada6CAS | 3378933PubMed |
Watanabe, S., and Nagai, T. (2008). Health status and productive performance of somatic cell cloned cattle and their offspring produced in Japan. J. Reprod. Dev. 54, 6–17.
| Health status and productive performance of somatic cell cloned cattle and their offspring produced in Japan.Crossref | GoogleScholarGoogle Scholar | 18319570PubMed |
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M., and Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854.
| Epigenetic programming by maternal behavior.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtVamt7c%3D&md5=6563ea224e434bf3117919fe39a741ceCAS | 15220929PubMed |
Wei, Y., Yang, C. R., Wei, Y. P., Zhao, Z. A., Hou, Y., Schatten, H., and Sun, Q. Y. (2014). Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl Acad. Sci. USA 111, 1873–1878.
| Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitFyjs7c%3D&md5=fb27a6013aad55a49cc21e625103c268CAS | 24449870PubMed |
White, P. J., Windsor, P. A., Dhand, N. K., and Toribio, J. A. L. M. L. (2010b). Risk factors for congenital chondrodystrophy of unknown origin in beef cattle herds in south-eastern Australia. Prev. Vet. Med. 96, 36–48.
| Risk factors for congenital chondrodystrophy of unknown origin in beef cattle herds in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 20638972PubMed |
Wijchers, P. J., and Festenstein, R. J. (2011). Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 27, 132–140.
| Epigenetic regulation of autosomal gene expression by sex chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvV2murg%3D&md5=a1ee1cd8dbfd7589674fb79fa713399bCAS | 21334089PubMed |
Wilkins, J. F., Fry, R. C., Hearnshaw, H., Cafe, L. M., and Greenwood, P. L. (2006). Ovarian activity in heifers at 30 months of age following high or low growth in utero and from birth to weaning. In ‘Australian Society of Animal Production 26th Biennial Conference’. (Eds K. Cocker, N. Adams and D. Lindsay.) (Australian Society of Animal Production: Perth.)
Wilson, S. J., Mcewan, J. C., Sheard, P. W., and Harris, A. J. (1992). Early stages of myogenesis in a large mammal: formation of successive generations of myotubes in sheep tibialis cranialis muscle. J. Muscle Res. Cell Motil. 13, 534–550.
| Early stages of myogenesis in a large mammal: formation of successive generations of myotubes in sheep tibialis cranialis muscle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s%2FpsVegtA%3D%3D&md5=3eb52edd84560ed773cd355b681e1013CAS | 1460082PubMed |
Wossidlo, M., Arand, J., Sebastiano, V., Lepikhov, K., Boiani, M., Reinhardt, R., Scholer, H., and Walter, J. (2010). Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 29, 1877–1888.
| Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsVWjsLY%3D&md5=9e18d6f6f3a38a3ff3338d8f1b2b7f78CAS | 20442707PubMed |
Wrathall, A. E., Holyoak, G. R., Parsonson, I. M., and Simmons, H. A. (2008). Risks of transmitting ruminant spongiform encephalopathies (prion diseases) by semen and embryo transfer techniques. Theriogenology 70, 725–745.
| Risks of transmitting ruminant spongiform encephalopathies (prion diseases) by semen and embryo transfer techniques.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1crjtVyqsQ%3D%3D&md5=07150a421afcec3d165bf42089af4f3fCAS | 18586320PubMed |
Wright, C., Evans, A. C., Evans, N. P., Duffy, P., Fox, J., Boland, M. P., Roche, J. F., and Sweeney, T. (2002). Effect of maternal exposure to the environmental estrogen, octylphenol, during fetal and/or postnatal life on onset of puberty, endocrine status, and ovarian follicular dynamics in ewe lambs. Biol. Reprod. 67, 1734–1740.
| Effect of maternal exposure to the environmental estrogen, octylphenol, during fetal and/or postnatal life on onset of puberty, endocrine status, and ovarian follicular dynamics in ewe lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptVels7Y%3D&md5=157f0f841d5b79ce5f3db87196ffb64dCAS | 12444047PubMed |
Wu, G., Bazer, F. W., Wallace, J. M., and Spencer, T. E. (2006). Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84, 2316–2337.
| Board-invited review: intrauterine growth retardation: implications for the animal sciences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFGktLs%3D&md5=b593f5476e5a12e92c091576679dd13eCAS | 16908634PubMed |
Wu, J., Boström, P., Sparks Lauren, M., Ye, L., Choi Jang, H., Giang, A.-H., Khandekar, M., Virtanen Kirsi, A., Nuutila, P., Schaart, G., Huang, K., Tu, H., van Marken Lichtenbelt Wouter, D., Hoeks, J., Enerbäck, S., Schrauwen, P., and Spiegelman Bruce, M. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376.
| Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVekur3I&md5=c4ca93fc0f9ae575c0e6863be7427dc3CAS | 22796012PubMed |
Young, L. E., Sinclair, K. D., and Wilmut, I. (1998). Large offspring syndrome in cattle and sheep. Rev. Reprod. 3, 155–163.
| Large offspring syndrome in cattle and sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntlaltL8%3D&md5=47d38389154db7dca4d8ad6cda8d16f6CAS | 9829550PubMed |
Young, L. E., Fernandes, K., McEvoy, T. G., Butterwith, S. C., Gutierrez, C. G., Carolan, C., Broadbent, P. J., Robinson, J. J., Wilmut, I., and Sinclair, K. D. (2001). Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 27, 153–154.
| Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtFGktL8%3D&md5=eb2f860a259dfd94c8b31fde3d21914aCAS | 11175780PubMed |
Young, L. E., Schnieke, A. E., McCreath, K. J., Wieckowski, S., Konfortova, G., Fernandes, K., Ptak, G., Kind, A. J., Wilmut, I., Loi, P., and Feil, R. (2003). Conservation of IGF2–H19 and IGF2R imprinting in sheep: effects of somatic cell nuclear transfer. Mech. Dev. 120, 1433–1442.
| Conservation of IGF2–H19 and IGF2R imprinting in sheep: effects of somatic cell nuclear transfer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlyns7Y%3D&md5=e3f81b71582f35ffd412cc7e502cce5eCAS | 14654216PubMed |
Yuan, S., Oliver, D., Schuster, A., Zheng, H., and Yan, W. (2015). Breeding scheme and maternal small RNAs affect the efficiency of transgenerational inheritance of a paramutation in mice. Sci. Rep. 5, 9266.
| Breeding scheme and maternal small RNAs affect the efficiency of transgenerational inheritance of a paramutation in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosVKnt74%3D&md5=46d994e9e378c191e6e68553e0a07911CAS | 25783852PubMed |
Zuena, A. R., Mairesse, J., Casolini, P., Cinque, C., Alema, G. S., Morley-Fletcher, S., Chiodi, V., Spagnoli, L. G., Gradini, R., Catalani, A., Nicoletti, F., and Maccari, S. (2008). Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One 3, e2170.
| Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats.Crossref | GoogleScholarGoogle Scholar | 18478112PubMed |


