Comparative morphology and phylogeny of Nicotiana section Suaveolentes (Solanaceae) in Australia and the South Pacific
Claire E. Marks A , Ed Newbigin A and Pauline Y. Ladiges A BA School of Botany, The University of Melbourne, Vic. 3010, Australia.
B Corresponding author. Email: paulinel@unimelb.edu.au
Australian Systematic Botany 24(2) 61-86 https://doi.org/10.1071/SB11006
Submitted: 1 February 2011 Accepted: 25 May 2011 Published: 29 July 2011
Abstract
Molecular phylogenies alone have failed to resolve evolutionary relationships of Nicotiana L. section Suaveolentes Goodsp. (Solanaceae), a section largely comprising Australian endemics. Comparative morphology of Suaveolentes is illustrated and characters, together with the chromosome number, coded for phylogenetic analysis. Morphological characters included discrete characters of seeds and trichomes studied by scanning electron and light microscopy, and gap-coded quantitative measurements of flowers and vegetative organs. These data were analysed using both maximum parsimony and splits network, and compared to and combined with molecular analyses based on published nuclear and chloroplast-DNA sequences. Among morphological characters, there was a high level of homoplasy, possibly attributable to convergent evolution, hybridisation and introgression, and the underlying polyploid origin of the group. There was some conflict between the morphological and molecular datasets; however, overall there was a level of concordance that identified a phylogenetic sequence of taxa that reflected a reduction in the chromosome number. With N. africana as the functional outgroup, analyses showed N. fragrans (New Caledonia and Tongatapu) and N. fatuhivensis (Marquesas islands, formerly N. fragrans var. fatuhivensis) to be a basal lineage in the Australian and South Pacific clade of Suaveolentes. N. forsteri (eastern mainland Australia, Lord Howe Island and New Caledonia) is the sister taxon to all other Australian species of Suaveolentes, which form a well supported monophyletic group. Within this Australian endemic group, species with a chromosome count of n = 24 or 23 are early lineages. Two clades with a reduced chromosome number are the ‘N. simulans clade’ (all taxa with n = 20) and the ‘N. suaveolens clade’ (n = 19, 18, 16, or 15). The position of N. cavicola (n = 20 or 23) is equivocal, and it and several other species require further study. Some widespread taxa require greater sampling of populations to test for variation in morphology, DNA sequences and chromosome number for further elucidation of the evolutionary history of Suaveolentes.
Introduction
Nicotiana L. is best known for cultivated tobacco, N. tabacum L., and currently includes 86 species, many native to South America, divided into 13 sections (Knapp et al. 2004). Section Suaveolentes Goodsp. represents a significant radiation in Australia, including 27 native species and subspecies. One eastern Australian species, N. forsteri (synonymous with N. debneyi Domin, Marks 2010a) also occurs on Lord Howe Island and New Caledonia. Two additional taxa occur in the South Pacific, namely N. fragrans Hook. found on New Caledonia and Tongatapu and N. fatuhivensis F.Br. on the Marquesas Islands. There is a single species N. africana Merxm. that occurs in Namibia, Africa.
Nicotiana is sister to the Australian endemic tribe Anthocercidae (including Cyphanthera, Duboisia, Anthocercis and Symonanthus), together forming the subfamily Nicotianoideae, which is sister to the large subfamily Solanoideae including Solanum, Capsicum, Datura, Lycium, Nolana and many other genera (Olmstead et al. 1999, 2008; Garcia and Olmstead 2003; Martins and Barkman 2005).
Section Suaveolentes was first described by T. H. Goodspeed in 1945, and since that time, additional species have been described (e.g. see Purdie et al. 1982). Origin of the group has long been considered enigmatic (Goodspeed 1954), ambiguous (Symon 1991) and paradoxical (Chase et al. 2003). The section is monophyletic (Chase et al. 2003; Clarkson et al. 2004, 2010) and is retained in the current sectional classification (Knapp et al. 2004). The group has few distinctive morphological synapomorphies, although most plants are ephemeral, white-flowered herbs.
Suaveolentes is widespread in Australia, ranging from moist areas in eastern Australia to semi-arid and arid regions, being particularly species-rich in the central desert area. Some species have a restricted distribution and are of suggested conservation value, but are not listed as threatened. No pollinator has been documented in the field for any Australian Nicotiana, although the white, vespertine, strongly scented flowers suggest moth pollination. All Australian species display at least some incidences of cleistogamy, which may be induced by short-day conditions or influenced by temperature (Horton 1981). Aboriginal use of Nicotiana plants in central Australia is ongoing, mostly as ‘pituri’ or chewing tobacco and as a commodity for trade (Symon 2004).
The monoploid chromosome number (x) for Nicotiana is 12 (Olmstead and Palmer 1992; Olmstead and Sweere 1994; Olmstead et al. 1999) and a polyploid origin for section Suaveolentes is generally accepted (Knapp et al. 2004). As for several other clades within Nicotiana, section Suaveolentes appears to be the result of natural allopolyploidy, i.e. hybridisation between two different parents followed by genome doubling. Modern species have a chromosome number ranging from n = 24 to n = 15.
Determining the diploid progenitors of section Suaveolentes has been more problematic than for other allopolyploid sections of Nicotiana, with ancestors in the Alatae, Noctiflorae, N. sylvestris and Acuminatae all being suggested. Goodspeed (1954) suggested that the ancestors of Suaveolentes came from relatives of the Noctiflorae, Alatae and Acuminatae. By using GISH, Chase et al. (2003) supported this suggestion of an Alatae ancestor for the section. However, GISH did not reveal the other parental genome, possibly because of the great age of the polyploid event. An ancestor of N. sylvestris is frequently suggested as the maternal progenitor of Suaveolentes (Knapp et al. 2004; Kovarik et al. 2008; Leitch et al. 2008), a hypothesis supported by the phylogeny in Clarkson et al. (2010) who sequenced a fragment of the low-copy nuclear gene chloroplast-expressed glutamine synthetase (ncpGS). They found that all Suaveolentes taxa possessed two homeologous copies, each being derived from a different diploid progenitor. Previously, Clarkson et al. (2004) suggested an ancestor of section Noctiflorae to be the maternal progenitor, on the basis of a chloroplast phylogeny. The ncpGS phylogeny showed the paternal Suaveolentes gene copies to be sister to a clade including the diploid Trigonophyllae, although this relationship lacked bootstrap support (Clarkson et al. 2010). If this were true, then Suaveolentes would have the same progenitors as section Repandae, although it would be the result of a separate and probably older hybridisation event.
Several molecular phylogenies of Suaveolentes have been published (Aoki and Ito 2000; Chase et al. 2003; Clarkson et al. 2004, 2010), which generally have few well supported nodes and some conflicting patterns among different DNA datasets. Morphology, including anatomy, has not been explored in detail and has the potential to discover relationships within Suaveolentes. The present study documents both floral and vegetative characters for all recognised taxa, and uses a method of gap-coding to assess continuously variable characters. Results are combined with published molecular-DNA sequences.
Materials and methods
Plant material
Morphology was assessed primarily by using glasshouse-raised plants, supplemented with herbarium specimens when seed was unavailable. Seed sources and voucher specimens for each taxon are listed in Table 1. All taxa were determined according to Horton (1981) and Purdie et al. (1982), and 23% of seed supplied from seed banks was found to be misidentified.
Seeds were sterilised for 15 min in 1% available chlorine solution, prepared from 4 mL of sodium hypochlorite 12.5% w/v solution and two drops of Tween 20 as a wetting agent, made up to 50 mL with water. After sterilisation, seeds were rinsed three times in sterile water and placed in sealed Petri plates on two layers of filter paper. Two to four plates of each seed lot (~20–30 seeds per plate) were wetted with gibberellic acid solution comprising 10 mg gibberellic acid dissolved in 1 mL of 70% ethanol and made up to 50 mL with sterile water. Plates were sealed and kept in a growth cabinet set at 25°C, with 16 h of light and 8 h of dark per day. Plates were kept moist with gibberellic acid solution or sterile water. If plates became infected with fungi, germinating seeds were transferred to new sterile plates. Seedlings on sterile plates were transferred to pasteurised seedling mix in punnets when cotyledons were expanded. Seed lots that were known to be viable were sprinkled directly onto pasteurised seed-raising mix in punnets.
Plants remained in growth cabinets until they had developed several leaves; they were then transferred to the glasshouse and given Aquasol liquid fertiliser (Hortico, Sydney) at a rate of 4 g per 5 L per week. After several leaves had developed, 16 plants of each seed lot were repotted into single 10-cm-diameter pots containing sterilised potting mix and Osmocote slow release fertiliser (Scotts Australia, Sydney) at 50 g per 10 L of potting mix. When they were larger, eight plants of each seed lot were repotted into 15-cm-diameter pots filled with the same soil mixture. All plants were kept in a glasshouse at ~25°C and irrigated three times a day by using an automatic drip system. Supplemental light was provided during winter months.
Representative plants and seedlings were pressed for voucher herbarium specimens (MELU; Table 1). Wet collections (in 70% ethanol) were made of flowers, flower buds, fruits (not yet dehiscent capsules), leaves and stems for all seed lots. Morphological characters were based on direct observation and comparison with other studies, such as Goodspeed (1954).
Microscopy
For light microscopy, tissue preserved in ethanol was initially examined under a dissecting microscope. For a study of trichomes, plant tissue was sectioned by hand, mounted on slides and examined at ×400 and ×1000 magnification. For scanning electron microscopy (SEM), seeds were mounted directly onto stubs. Floral and vegetative plant parts were examined using the wet collections. These were dehydrated in each of five ethanol solutions (70%, 90%, 100%, 100%, 100%) for 7–24 h, and then critical-point dried. Samples were mounted on stubs and sputter-coated with gold in an Edwards Sputter Coater S150B for 1.5 min and viewed under a XL-30 Phillips Scanning Electron Microscope operated at 2.0 kV.
Character measurements
A total of 50 characters was scored, including binary, multistate and quantitative measurements (Table 2). Width, length and shape of cotyledons were recorded for 10 plants of each seed lot. Ten mature flowers of each seed lot were measured from several plants to average out any size variation within inflorescences or among plants from a seed lot. Twelve measurements were made by using calipers and a dissecting microscope, including corolla-limb diameter, floral-tube length, floral-tube width at end of calyx and at throat, calyx width at the widest point, calyx length, shortest and longest stamen lengths, shortest and longest stamen length free from tube wall and stigma width. Herbarium material was measured for taxa where seed was unavailable. Where several seed lots had been assigned to a taxon, measurements were combined. After comparison with herbarium material, it was clear that the flowers of N. heterantha grown in the glasshouse were all cleistogamous and much smaller than chasmogamous herbarium material, so only the herbarium material was used.
|
Seed shape and ornamentation were compared for most taxa by using SEM micrographs, and assigned coding classes. Seed length, width and depth were measured on the micrographs by using scale bars. Between 4 and 12 seeds of each seed lot were used for measurements; however, most often six seeds were used. Measurements were averaged and means graphed to determine gaps in the data for coding categories. Ratio characters were explored and revealed no additional patterns. Some taxa were measured and studied only under the light microscope but scored according to the categories assigned based on SEM.
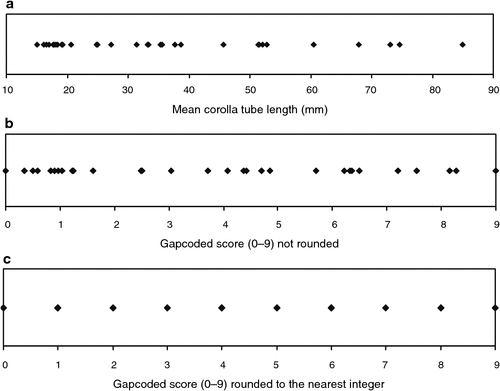
Fourteen variables were coded for cladistic analysis using the method of Thiele (1993). He argued that morphometric data can retrieve phylogenetic signal and he developed a method of gap-coding continuous variables known as gap-weighting. Garcia-Cruz and Sosa (2006) compared gap-weighting to other gap-coding methods and confirmed its utility in providing resolution of clades. Measurements here were log-transformed using log10(x+1) because of uneven variances and range standardised, with each character coded into 10 states and analysed as an ordered multistate. With 10 states and thus nine transformations possible between the states, the character was given a weight of 0.1111 so that it did not differentially dominate the analysis (Kitching et al. 1998). An example of gap-coding morphometric data is shown in Fig. 1.
|
Chromosome number
The chromosome number (n) for each taxon was scored as an ordered multistate character. Counts were recorded from the literature (Wheeler 1945; Goodspeed 1954; Burbidge 1960; Merxmüller and Buttler 1975; Williams 1975; Horton 1981; Symon 1984, 1998; Clarkson and Symon 1991; Symon and Kenneally 1994; Laskowska and Berbec 2003), and from root squashes of freshly grown material for 20 taxa, the latter providing new counts and checks of older records (Marks 2010b).
Phylogenetic analysis
The morphological dataset was analysed by maximum parsimony (heuristic search) using PAUP* 4.0b10 (Swofford 2003). The starting tree was found by stepwise addition and the addition sequence ‘closest’ was used. The branch-swapping algorithm tree-bisection-reconnection (TBR) and character-state optimisation using delayed transformation (DELTRAN) were implemented.
Outgroup choice
Preliminary analyses used N. alata (section Alatae), N. glauca (section Noctiflorae) and N. sylvestris (section Sylvestres) as outgroups. However, these taxa have diverged significantly, with many morphological differences among them. A more appropriate outgroup for rooting phylogenetic trees was the basal member of section Suaveolentes, N. africana. This was justified on the basis of published molecular studies that have shown N. africana to be the sister lineage to the rest of the Suaveolentes (Chase et al. 2003; Clarkson et al. 2004). It appears to have diverged after the hypothesised polyploid event that led to the evolution of the Suaveolentes. Use of this closer outgroup reduced problems of assessing the homology of morphological features. The discrimination ability of gap-coded characters was also improved because the more distantly related outgroup taxa had such different floral morphology that the differences within the Suaveolentes were compressed by range standardisation.
Neighbour-net splits network
A neighbour-net splits network was created with SplitsTree4 v4.10 (Huson 1998; Huson and Bryant 2006). The neighbour-net algorithm (Bryant and Moulton 2004) was used to calculate splits, which is a distance-based method for constructing phylogenetic networks by using the neighbour-joining algorithm of Saitou and Nei (1987). The equal-angle algorithm option was then used to compute a planar split network in which the distance, via edge lengths, between any two taxa corresponds to the weights of all splits separating the taxa (Huson 1998).
For this analysis, the morphological dataset was recoded into ordered or unordered binary characters for input. Gap-coded characters were recalculated into three states and recoded as ordered binary characters (00,01,11). This simplified the data, but because no weighting is possible in the program, using a greater number of states may cause the gap-coded characters to have a disproportionate influence on the analysis when compared with discrete characters. The ‘UncorrectedP’ option was used, which transforms characters to distances using Hamming distance (Hamming 1950).
Molecular data
Published DNA sequences for ITS, long and short copies of glutamine synthetase gene (ncpGS) and four chloroplast DNA regions (Aoki and Ito 2000; Chase et al. 2003; Garcia and Olmstead 2003; Clarkson et al. 2004, 2010) were downloaded from GenBank (National Centre for Biotechnology Information 2010) and aligned by eye, using Se-Al v2.0a11 (Rambaut 2002; the alignment is available from the authors). Inclusion of outgroups that were not from section Suaveolentes, such as N. sylvestris, resulted in areas of ambiguous alignment.
Data were analysed using maximum parsimony (PAUP* 4.0b10) as for the morphological data, with indels coded as separate characters. Support for nodes was assessed by bootstrap analysis (Felsenstein 1985) using a full heuristic search with 1000 pseudoreplicates.
The two South Pacific species were combined as a single terminal taxon in multi-region analyses; N. fragrans was available as a sequence only for matK and ITS, and N. fatuhivensis for ncpGS. On the basis of morphology, these species are likely to be sister taxa and, because the relationship of South Pacific plants are of biogeographic interest, it was important that the lineage be included. For the combined analysis of sequences and morphology, morphological characters scored for N. fatuhivensis were used for the combined N. fragrans–N. fatuhivensis taxon.
Results
The total morphological data matrix (see Table 3) consisted of 50 characters, including chromosome number as Character 50, scored for 29 taxa (some with missing values).

|
Seeds
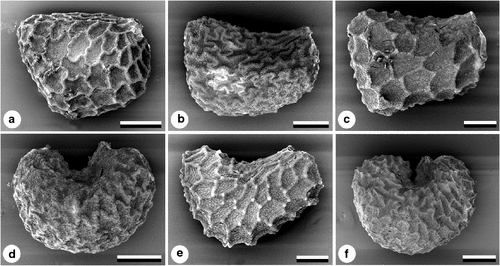
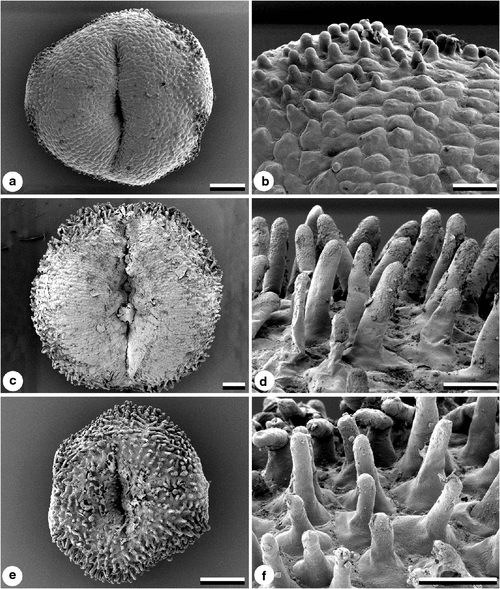
The shape of Suaveolentes seeds shows significant variation among taxa (Goodspeed 1954; Burbidge 1960; Horton 1981; Purdie et al. 1982; Bahadur and Farooqui 1986), and also within a taxon, partially caused by the position of the seed within the capsule (Gunn and Gaffney 1974). The following broad scoring categories (Character 1, Table 2) were used to encompass the variation within each taxon: trapezoid or acutely angled (Figs 2a–c); reniform (Figs 2d, e); and C-shaped (Fig. 2f). Hilum position and shape as potential characters proved not to be informative, being related to seed shape and very variable within taxa.
|
Reticulate seed ornamentation is common in the Solanaceae (Corner 1976; Edmonds 1983; Lester 1991; Zhang et al. 2005), and in Suaveolentes is formed by the raised anticlinal walls of testal cells, making a honeycombed pattern, often irregularly hexagonal. Cell walls of the testa were coded (Character 2) as either straight (Fig. 2c) or sinuous (Fig. 2a, b, d, f). In some taxa, the raised anticlinal walls have raised cell junctions (Character 3), which give the whole seed a knobbly appearance, as in N. simulans (Fig. 2e). No secondary sculpturing of walls or cuticle was observed. Seed colour differences, varying from brown to black, were too subtle for character coding.
Suaveolentes seed size covers much of the range seen in the genus as a whole. Nicotiana seed length varies from 0.4 to 1.3 mm (Goodspeed 1954) and Suaveolentes seed lengths are from 0.51 mm (N. benthamiana, mean 0.57 mm) to 1.26 mm (N. burbidgeae, mean 1.16 mm). Because the measurements formed a continuum without clear and unambiguous disjunctions, these data were gap-coded into 10 character states (Character 4). Seed width was also measured; however, because it was strongly correlated (r2 = 0.90, P < 0.001) with seed length, it was not included in the cladistic analysis. Seed thickness was not as highly correlated (r2 = 0.46) with seed length and was included (Character 5).
Corolla
The corolla in Nicotiana section Suaveolentes consists of a cylindrical floral tube, which broadens into a ‘corolla limb’ of five petals, or corolla lobes. The tube may be straight, broaden slightly towards the limb, or form a distinct ‘throat cup’ just before the corolla limb. Goodspeed (1954) distinguished a ‘tube proper’ and ‘throat cylinder’ (equivalent to the throat cup); however, this terminology is not used here because there is no distinguishable throat cup in many species of Suaveolentes. The corolla lobes vary in their apex shape and length.
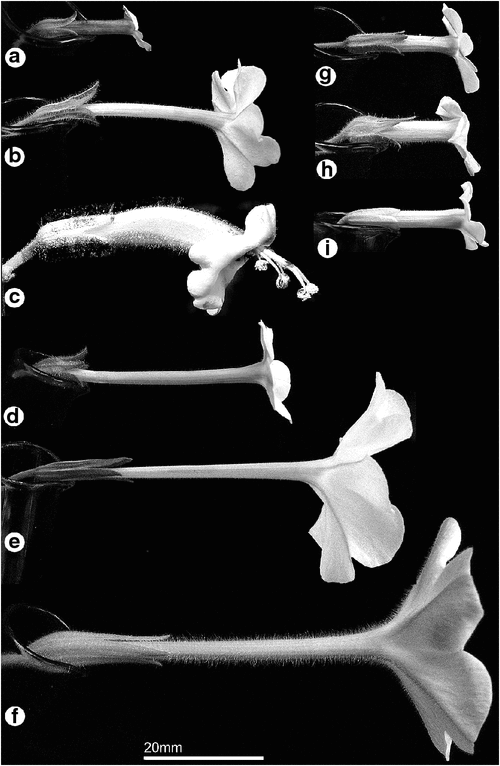
Most Suaveolentes flowers are slightly zygomorphic (Character 6), with the exception of N. africana, which is strongly zygomorphic (Fig. 3c). The corolla limb of this species is recurved and the stamens exserted, giving it a shape very different from all other flowers in the section (Fig. 3c). The zygomorphic symmetry in most other Suaveolentes taxa is more subtle, namely, the corolla limb is borne diagonally at full dilation (e.g. N. forsteri, Fig. 3a; N. amplexicaulis, Fig. 3h), the throat cup is slightly more pronounced on one side (e.g. N. truncata, Fig. 3i), or the floral tube is slightly longer on one side (e.g. N. occidentalis subsp. obliqua, Fig. 3b; N. rosulata subsp. ingulba, Fig. 3e).
|
The shape of the corolla lobes is a distinctive feature of Nicotiana flowers (Character 7). Figure 4 shows flowers arranged into four categories, namely, rounded, emarginate, mucronate and narrow lobes. Although there is variation within species, these categories summarise differences among them.

|
All taxa except N. africana have fold lines running down the centre of each corolla lobe, which is how the flowers close up. Horton (1981) and Purdie et al. (1982) noted that the corolla limb is usually closed in full sunlight and open in shade or after sunset; however, flowers were often observed open in sunlight in the glasshouse and the field. The corolla limb of some species, such as N. benthamiana (Fig. 4d), opens completely flat, whereas in others, such as N. burbidgeae (Fig. 4d), the limb is fuller and has a ruffled appearance when fully open. The most common corolla-lobe orientation (Character 8) is spreading perpendicular to the long axis of the corolla tube. N. africana is the only species with recurved corolla limb lobes. In some other species, including N. amplexicaulis (Fig. 4h), N. forsteri (Fig. 4a) and N. velutina (Fig. 4g), the upper lobes of the corolla are reflexed back towards the upper surface of the tube, with the often larger, lower lobes held diagonally.
White flowers are characteristic of the Suaveolentes (Goodspeed 1954; Knapp et al. 2004), although most flowers also have light shades of yellow, green and purple on the outer corolla-tube surface (which may indicate underlying spectral qualities important in pollination; not studied here). Most taxa have pure white corolla limb lobes on the ‘face’ side, but with a green–yellow vein running down the back of each corolla lobe. The corolla of N. africana is entirely light green–yellow (Character 9). Several taxa are green on the outside of the corolla tube (Character 10), which often becomes stronger towards the limb. Flowers in bud are often more strongly coloured and become paler by anthesis, and there is significant infraspecific colour variation. Six taxa have intermittent purple flushing of the corolla (Character 11), most often on the outer corolla tube or in bud. N. forsteri has a dark-purple corolla tube.
There are two main types of corolla-tube shape within the Suaveolentes (Character 12). Many species have straight corolla tubes, often, but not exclusively, with long tubes. In some species, such as N. maritima, the corolla tube is narrow within the calyx and widens beyond the calyx. Short, wide flowers are most likely to have this post-calyx swelling of the corolla tube. This characteristic was described by Horton (1981) in terms of the tube proper being distinct or not distinct from the throat cylinder.
Aestivation was documented extensively for corolla limb lobes by Goodspeed (1954). All Nicotiana species are conduplicate, with the divisions of the corolla each folded lengthwise (Goodspeed 1954). In Suaveolentes, these conduplications are arranged spirally, varying from a symmetric spiral to slightly or distinctly asymmetric spirals; however, there was insufficient variation for this character to be taxonomically useful.
Corolla diameter (Character 13) and corolla tube length (Character 14) are important in distinguishing taxa. Section Suaveolentes has an average floral-tube length ranging from 15.0 mm (N. rotundifolia) to 74.7 mm (N. fatuhivensis). The width of the corolla tube measured at the apex of the calyx (Character 15) is also included as a gap-coded measurement. The width of the corolla tube at the throat was compared with the corolla limb diameter. N. africana and N. wuttkei have a much larger throat width to limb diameter ratio (coded as States 8 and 9, respectively) than do other species.
Calyx
There is an interesting relationship between calyx shape and corolla-tube shape. Most taxa that have a calyx that is tightly appressed to the corolla tube (Character 17) also have a corolla tube that dilates just beyond the distal end of the calyx, e.g. N. velutina (Fig. 3g) and N. africana (Fig. 3c). Many taxa with the calyx not appressed to the corolla have a narrow, straight corolla tube, e.g. N. benthamiana (Fig. 3d).
In Suaveolentes, calyx consists of five sepals that are connate for at least half of their length and the free lobes are mostly uneven in size, although they can be all of the same length. Some taxa have lobe tips that are curled back from the corolla tube, whereas some lobe tips are extended forward (hence appearing straight). One unusual species, N. truncata, has very short, truncate corolla lobes (Character 18).
There are significant differences in the length of the calyx compared with the corolla tube (Character 19). The small-flowered N. rotundifolia has a calyx that is 65% of the length of the corolla tube, compared with N. fatuhivensis where the calyx is 19.5% of the length of the corolla tube. Linear regression shows a moderate relationship (r2 = 0.54) between corolla-tube length and relative length of calyx to corolla tube, where the shortest flowers have proportionally longer calyxes, and the longest flowers have proportionally shorter calyxes.
Calyx shape in section Suaveolentes was described by Purdie et al. (1982) as tubular to narrowly campanulate and there are significant differences in calyx shape among taxa. A group of species, including N. benthamiana, N. cavicola, N. burbidgeae and N. amplexicaulis, has rounded, cupulate or urceolate calyxes that are significantly wider than the corolla tube within. These four taxa all have flowers borne in leaf axils, arising from internodes or in leafy inflorescences, rather than borne in leafless inflorescences, as is more common in the section. Another common calyx shape is almost tubular and apressed to the corolla tube, sometimes with slightly flared lobe tips. This can be seen in N. occidentalis subsp. obliqua, N. megalosiphon, N. simulans and N. rosulata. N. sp. ‘Corunna’, N. goodspeedii and N. velutina. Other species have a similar but slightly more rounded elongated ovoid shape, such as in N. rotundifolia and N. gossei. A gap-coded ratio character was used to describe calyx shape (Character 20: calyx width : calyx length, Table 2), which varied along a continuum, with N. africana, the functional outgroup, having the highest ratio value.
Several calyx attributes were not able to be coded for cladistic analysis because they were too variable. It was observed that many species have a moderately prominent raised ridge running down the centre of each calyx lobe, which is very prominent in N. excelsior and almost indistinguishable in N. burbidgeae. Most calyxes are a mid-green colour, but occasionally with a purple tinge, or even with purple tips (e.g. N. rosulata subsp. rosulata). Thin, white membranes between the five green, fleshy lobes of the calyx are common (Purdie et al. 1982) and often become more prominent as the fruit expands owing to the developing seeds. In some species, such as N. gossei, the calyx splits as the capsule swells and matures. No member of the Suaveolentes has a split in the calyx at floral anthesis, which is present in some other Nicotiana species.
Androecium
In section Suaveolentes, the androecium consists of five stamens, four of which approximately reach the opening of the corolla tube, the fifth being shorter. Stamen attachment is variable. The shortest stamen may leave the corolla-tube wall at a strong angle, or may lie flat along the tube wall at the attachment point (Character 21). Both morphologies are common. The longest four stamens are straight in all species except N. africana, the longest stamens of which are kneed just above the stamen insertion point (Character 22). This stamen morphology is found in some other species of Nicotiana outside Suaveolentes, such as N. alata (section Alatae).
In most species of Suaveolentes, the longest four stamens are roughly equal in length or two stamens are a little shorter, although not as short as the fifth stamen. The terms ‘subequal’ and ‘subdidynamous’ have been used to describe this latter condition (Horton 1981; Purdie et al. 1982). However, the degree of subdidynamy varies significantly within species and was not coded as a character.
Only one taxon, N. africana, has obviously exserted stamens (Character 23). It is also unusual in having declinate stamens (Fig. 3c). All other Suaveolentes taxa have stamens arranged evenly in a ring around the corolla tube, although sometimes the anthers can be slightly declinate, especially in N. rosulata subsp. rosulata and N. simulans; however, they are not as strongly zygomorphic as in N. africana.
The proportion of the longest stamen that is free of the corolla-tube wall (Character 25) varies considerably. There is a large group of species with ratio values of 0.03–0.18, meaning that only 3–18% of the stamen length is free. N. goodspeedii has 27% of the longest stamen free, and N. forsteri 50%. The outgroup N. africana has 74% of the longest stamen free. The proportion of the shortest stamen that is free of the corolla-tube wall (Character 26) is extremely variable within Suaveolentes. N. simulans has the most attached, shortest stamens, with only 5% of the length free from the corolla-tube wall. The stamens of N. africana only shortly adhere to the corolla tube (with 79% of their length free).
The character ‘disparity of stamen length’ (Character 27) compares the length of the shortest stamen to the longest one. Within Suaveolentes, the shortest stamens are (71)78–82–(93)% of the length of the longest. Nicotiana sp. ‘Corunna’ (71%) and N. excelsior (73%) have the largest stamen-length disparity, and N. umbratica (93%) and N. fatuhivensis (93%) have the smallest.
Anthers are bilocular, dorsifixed, not cohering and dehiscent by longitudinal splits (Purdie et al. 1982). The slight variation in anther shape among species proved to be insufficient to code as a character. Three species have simple multicellular trichomes (bearing a simple gland at the tip and a slightly swollen basal cell) on the dorsal side of the anthers (Character 24). SEM revealed that N. africana has a textured anther surface, following cell outlines, with each cell rising to a point. The other extreme is N. burbidgeae where each raised cell is topped with a plate-like cap that is darkly pigmented under the light microscope. N. forsteri has an anther surface that is intermediate between these two, and N. gossei is a less regular version of N. africana. However, no characters could be scored across all taxa because of lack of material.
Gynoecium
The gynoecium of section Suaveolentes consists of an annular hypogynous disk (orange–yellow nectary), a bilocular ovary, a style and a green disc-shaped papillate stigma. The style is approximately equal in length to the floral tube, and may be slightly inserted or exserted. There is little stigma-shape variation, although there is much greater variation across the genus (Goodspeed 1954). Stigma width (Character 28), however, varies considerably in Suaveolentes, from a mean diameter of 0.63 mm (N. heterantha) to 2.05 mm (N. gossei).
Significant variation in stigma papillae (Character 29) was observed using SEM. Stigma papillae are not present in the centre of the disk of N. africana; however, there are small protruberances or short papillae around the edge of the disk (Fig. 5a, b). Other taxa, such as N. gossei, also have a smooth central disk, but with well developed finger-like papillae around the edge of the disk (Fig. 6c, d). In species such as N. maritima, the disk is completely covered with well developed papillae (Fig. 5e, f).
|

|
Cleistogamy is common in Suaveolentes, although it is not widespread in other sections of the genus. N. heterantha is facultatively cleistogamous (Symon and Kenneally 1994) and was the only species to develop cleistogamous flowers during the present glasshouse study; in fact, very few chasmogamous flowers were produced. It is likely that many species are facultatively cleistogamous to some degree and suggested triggers include short-day conditions (Burbidge 1960), increase in temperature (Horton 1981) or increasing plant age (Horton 1981). Cleistogamous flowers are usually much shorter than chasmogamous flowers, barely exceeding the calyx, and are usually also narrower. This feature, however, was not included as a character for the cladistic analysis.
Capsule
Persistence of the style and corolla on the maturing capsule (Character 30) was observed only in N. africana, although occasionally there is some persistence in N. simulans. N. heterantha has a unique capsule morphology; the capsule is slightly constricted at about a quarter of the length and the lowest quarter is thickened and hardened (Symon and Kenneally 1994). This has the effect that a few seeds are retained in the capsule after dehiscence (Character 31). N. monoschizocarpa also has a unique feature within the section; namely, the capsule dehisces by splitting in two, not four, as for the species in the rest of the section (Character 32). All Suaveolentes taxa have recurved capsule lobes.
Plant architecture
Most plants are soft-stemmed herbs; however, a woody caudex, associated with perennial plants (Harris and Harris 2001), develops in N. africana, N. fragrans and N. fatuhivensis (Character 33). The latter two species grow in crevices in coastal rocks and on vertical cliffs, and the caudex may aid anchorage to the substrate (Marks 2010a).
All Suaveolentes taxa have an upright habit, with the exception of N. heterantha, which has lax, wiry stems that creep along the ground (Character 34). Knapp et al. (2004) described Suaveolentes plants as rosette-forming herbs that occasionally lack a marked basal rosette. Plants with a basal rosette (Character 35) may also produce several large cauline leaves. Many taxa retain a clear basal rosette in the mature plant or form a rosette as a seedling that persists to first flowering. As more flowering stems grow, plants often develop more and larger cauline leaves and the original rosette leaves senesce. Species with this morphology include N. forsteri and N. wuttkei. Seven taxa never have a rosette past the seedling stage, and have only cauline leaves at the time of first flowering. A few others, such as N. heterantha and N. maritima, develop secondary shoots or rosettes of leaves at nodes along the stems as plants become mature. The basal rosette of N. africana is a little different from that of some other species, with leaf bases being slightly separated on the main stem and the oldest leaves dying off progressively, so that the rosette of living leaves is higher on the stem as the plant gets older. N. africana and N. fragrans are the two species where senescent leaves are retained on the plant (Character 36).
Some taxa have flowers borne on leafy stems, whereas others are borne in leafless inflorescences (Character 37). The three species that bear flowers on leafy stems, namely N. benthamiana, N. cavicola and N. burbidgeae, are those that do not form rosettes.
The number of stems is not a reliable character because many taxa are single-stemmed initially but develop new stems as the plant becomes older. Many taxa are arid-zone ephemerals, and the number of stems developed is affected by how long suitable moisture for growth persists. First-, second- and third-order branching within the inflorescence are all found in the section; however, they were not scored as characters because branching varies over the lifetime of a plant. The inflorescence of N. africana has very short internodes compared with those of other taxa. N. forsteri can also have short inflorescence internodes, although they tend to become longer with age.
Leaves
Leaf type within Suaveolentes is diverse and complicated by the stage of development and a level of plasticity, depending on environmental factors. All seedling leaves are similar and petiolate; most taxa have elliptical or ovate leaves, with the range of variation of shapes on one plant depending on leaf position and plant age. Plants growing in favourable conditions, such as those provided by a glasshouse environment, develop larger and broader leaves and more cauline leaves than plants grown under less favourable conditions (Burbidge 1960). Because of this variability in leaf size and shape, these characters were not scored.
Nicotiana excelsior and to a lesser degree N. monoschizocarpa have obviously decurrent leaf bases (Character 38). In mature comparable leaves, lamina tissue may extend along the petiole to form a wing. This feature (Character 39) may vary from broadly winged (e.g. N. amplexicaulis, N. cavicola, Fig. 6b, n) to only narrowly winged, with lamina tapering to base (e.g. N. rotundifolia, N. maritima, Fig. 6m, g). The mature leaves of N. benthamiana and N. burbidgeae are sessile (petiole absent, Fig. 6o, p). Six taxa have distinct auricules at the base of the petiole (Character 40). Many have some leaves with undulate margins; N. monoschizocarpa has a crinkled, rugose leaf surface (Character 41, Fig. 6f), which, combined with prominent white veins, makes the leaves distinctive within the group. N. fragrans and N. africana have leaves that appear slightly thicker and more fleshy than those in other taxa; however, this was not scored because the degree of leaf thickness appears to be a response to environmental conditions.
Cotyledons are small, being usually 3–5 mm wide and 4–7 mm long. They are generally bright green, narrowly ovate, widest near the base and with apex subacute–acute. They can be variable within a taxon and irregularly shaped or undulate. Plant size bears no relationship to cotyledon size, with N. africana having some of the largest leaves in the section, yet it has relatively small cotyledons. Cotyledon length (Character 42) and length : width ratio (Character 43) were gap-coded for cladistic analysis. Cotyledon length varied from N. rotundifolia, with a mean length of 4.1 mm, to N. suaveolens, with a mean length of 7.0 mm. Cotyledon length : width ratio was also variable. Three species, N. africana, N. forsteri and N. heterantha, had a ratio value close to 1.0, the cotyledons being as wide as they are long. A large group of taxa had ratios in the range 1.2–1.6, and a few had relatively narrow cotyledons, with ratio values 1.6–1.8.
Trichomes
There is a long history of using trichome types for describing and distinguishing taxa both across the family Solanaceae (e.g. in Solanum, Knapp 2001) and in Nicotiana (Goodspeed 1954). Although some types are common to all taxa within Suaveolentes, they are described and scored here for completeness. The greatest diversity of trichome types was found on the calyx.
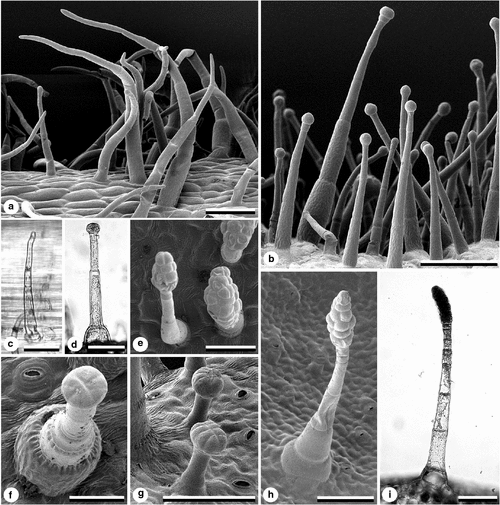
All Suaveolentes taxa and outgroups have eglandular trichomes (Horton 1981), equivalent to ‘Type A’ sensu Goodspeed (954), that are slender, uniseriate and usually multicellular (Character 44, Fig. 7a, c). Eglandular trichomes are found at least on the inner surface of the corolla where they tend to be long and tangled. They are also common on the outer surface of the corolla, base of staminal filament, and calyx (e.g. N. truncata, N. goodspeedii, N. sp. ‘Corunna’), leaves (e.g. N. suaveolens, N. rosulata subsp. ingulba, N. excelsior, N. sp. ‘Corunna’) and stem (e.g. N. maritima).
|
Trichomes with one simple gland cell at the apex (Character 45, Fig. 7b, d) are here referred to as glandular globular-headed trichomes (sensu Horton 1981), equivalent to ‘Type C’ sensu Goodspeed (1954). These trichomes have a stalk that is generally uniseriate and multicellular, with slender basal cells. They are common on most plant surfaces, including leaves, stems and calyces, and are found on all Suaveolentes taxa and outgroups.
Trichomes terminating in multiseriate and multicellular glands that form an ellipsoid head (Character 46) are the most distinctive trichome type (Fig. 7e, h, i). These glandular, ellipsoid-headed trichomes (Horton 1981) are equivalent to ‘Type C2’ trichomes of Goodspeed (1954). The glandular head consists of many cells and appears dark under the dissecting and light microscopes. The stalk is slender, multicellular and uniseriate. These trichomes are most dense on the calyx, and sometimes restricted to it, although in some taxa they also occur on the stem, leaf lamina, petiole and pedicel. Plants with ellipsoid-headed glandular trichomes are much more viscid than those without them (Burbidge 1960; Horton 1981). Goodspeed (1954) reported ellipsoid-headed glandular trichomes for N. benthamiana, which was supported by Burbidge (1960) but disputed by many other studies (Horton 1981; Purdie et al. 1982), including our study. The present study has confirmed that the following nine taxa in section Suaveolentes have ellipsoid-headed glandular trichomes: N. africana, N. cavicola, N. forsteri, N. fragrans, N. fatuhivensis, all three subspecies of N. occidentalis and N. umbratica; the other 20 taxa lack them. This trichome type is common in Nicotiana species outside of the Suaveolentes (Goodspeed 1954) and is thus probably plesiomorphic for Suaveolentes. Ellipsoid-headed glandular trichomes are found in the Australian solanaceous genus Anthocercis and to a lesser extent in other genera in the tribe Anthocercidae except Symonanthus (Haegi 1991). The character is thus plesiomorphic for Suaveolentes.
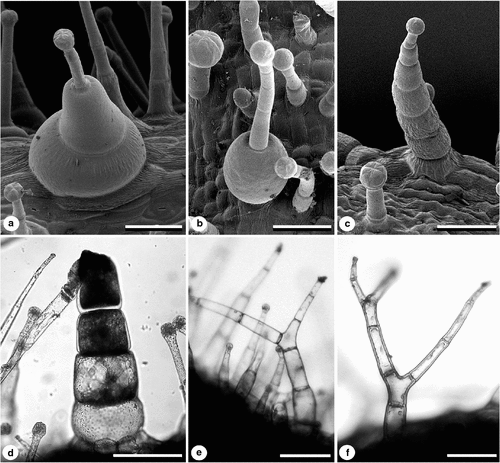
Hydathodes (sensu von Minden 1899 and ‘Type E’ trichomes of Goodspeed 1954) are small trichomes with a one- or two-celled stalk and a peltate disk of cells at the tip (Character 47, Fig. 7f, g). The disk usually consists of four cells, but can have six or eight (e.g. N. maritima, Fig. 8g). Occasionally, there are two or three layers of cells in the peltate head. Hydathodes are common on most plant tissues in all Suaveolentes taxa, although N. fatuhivensis was unable to be examined because of lack of material. Hydathodes are found in 28 species of Nicotiana outside Suaveolentes and are usually restricted to the leaves (Goodspeed 1954). Seithe (1979) identified hydathode-type trichomes in the genus Solanum, naming them ‘multicellular glands’, and Edmonds (1982) found them to be universally present in Solanum section Solanum.
|
Symon (1984) identified trichomes with two round, swollen basal cells in his description of N. burbidgeae. These trichomes typically have a small glandular head, a few slender stalk cells and one or two very large spherical or hemispherical basal cells (Fig. 8 a, b). Two distinct swollen basal cells are found abundantly in N. burbidgeae and are also present in N. truncata and N. gossei. However, some degree of basal-cell swelling is common in many taxa (Fig. 8c) and this trichome type was uninformative and not included in our analyses.
Dark specialised stalk cells (Character 48) of an unknown function can be observed under the light microscope (N. gossei, Fig. 8d). These dark cells have larger nuclei and visible cell contents and most often occur near the base of large, multicellular glandular trichomes; there can be several dark specialised cells in a single trichome. These trichomes were identified as ‘Type D’ by Goodspeed (1954) and were reported for just over half of all Nicotiana species. Dark specialised cells are present in the majority of Suaveolentes taxa, although they absent from at least seven taxa, e.g. N. goodspeedii. Merxmüller and Buttler (1975) found dark specialised cells to be absent from N. africana; however, the present study found many dark specialised cells in calyx trichomes of that species. Trichomes on the calyx of N. excelsior have cells that are darker, with more granulated contents than in other taxa.
Branched trichomes (Character 49) were not reported for any Suaveolentes taxa by Goodspeed (1954) or Horton (1981); however, they were documented for 19 Nicotiana species outside the Suaveolentes (Goodspeed 1954). However, Burbidge (1960) illustrated them for N. rotundifolia on the basis of field- and glasshouse-grown specimens. The present study found multicellular branched trichomes on the following four species of Suaveolentes: N. amplexicaulis, N. forsteri, N. rotundifolia and N. velutina (Fig. 8e, f). Only one branched trichome, with simple glandular apices, was seen on N. amplexicaulis, found on the outer corolla surface. N. rotundifolia has branched trichomes, with both simple glandular and eglandular tips on the outer surface of the corolla and on leaves, whereas N. velutina has branched trichomes, with simple glands only on the outer surface of the calyx although in reasonable quantity. Branched trichomes in these species are probably rare and not found on every individual. It is possible that additional Suaveolentes species have branched trichomes, but that they were not seen on the specimens examined here. Vestigial branching in the form of papillae or unicellular branches, corresponding to ‘Type B1’ of Goodspeed (1954), was observed for N. forsteri, N. rotundifolia and N. truncata on eglandular trichomes on the inner corolla surface (Character 49).
Assessing trichome density was problematic in Suaveolentes and not suitable for cladistic analysis. Horton (1981) found that the degree of pubescence varied considerably within the one species or subspecies, with occasional specimens in a usually densely pubescent species having glabrescent stems. Temporal factors also affect trichome density; older growth is usually more sparsely pubescent than recent growth (Horton 1981). Pubescence in Suaveolentes can be described as varying from dense, long and woolly, to short and velvety or sparse or even glabrous. This overall appearance can be useful for distinguishing or keying out species (e.g. N. maritima stem bases white- or grey-woolly, Purdie et al. 1982), but should not be coded for cladistic analysis because homology of trichome types cannot be assured.
Morphological phylogeny
For the phylogenetic analysis of the morphological dataset, including chromosome number, six taxa with missing data were excluded. These taxa were unable to be raised from seed and were scored from herbarium specimens. They included N. fatuhivensis (18 missing values), N. megalosiphon subsp. sessifolia (18 missing values), N. occidentalis subsp. occidentalis (13 missing values), N. occidentalis subsp. hesperis (9 missing values), N. umbratica (11 missing values) and N. wuttkei (13 missing values). N. fragrans was retained, even though it had 13 missing values, because it has been sequenced in the molecular datasets, and inclusion of at least one South Pacific taxon is important for biogeographical interpretation.
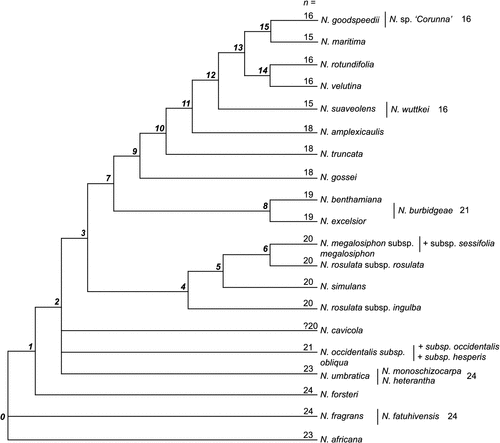
Parsimony analysis of 23 terminal taxa yielded one most parsimonious tree of 141.56 steps, with CI = 0.4364 and RI = 0.5734 (Fig. 9). The South Pacific species N. fragrans (Node 0) is sister to all Australian Suaveolentes. Early divergences (Nodes 1–4) are, in phyletic order, as follows: N. forsteri, N. heterantha, N. monoschizocarpa and N. occidentalis (as subsp. obliqua). Two clades diverge at Node 5. One of these clades (Node 7) includes N. rosulata (its two subspecies not forming a monophyletic group), N. simulans and N. megalosiphon. The other clade (Node 6) further diverges, showing N. excelsior, N. benthamiana, N. cavicola and N. burbidgeae related as a group (Node 10). N. gossei, N. truncata, N. amplexicaulis, N. velutina, sister taxa N. maritima and N. velutina, and the group of N. goodspeedii, N. rotundifolia and N. sp. ‘Corunna’ form a sister clade (Node 13).
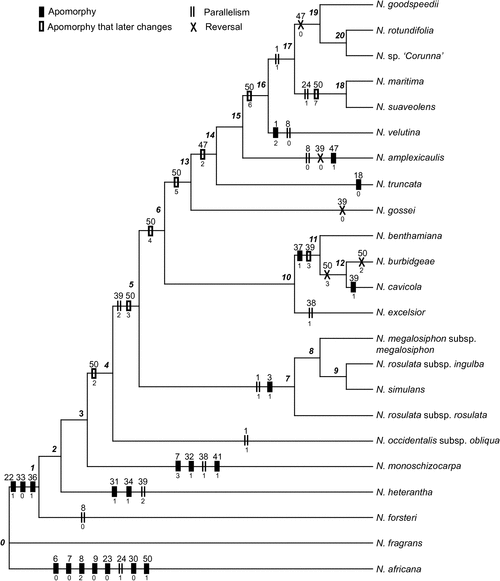
|
The CI value indicates a high level of homoplasy in the morphological dataset. All of the gap-coded characters have a CI index <0.500. Of the characters that have a CI index ≥0.500 (plotted on Fig. 9), eight are interpreted as autapomorphies for the outgroup N. africana and are associated with its distinctively different flowers (e.g. Characters 6–9, 23) and chromosome number n = 23 (Character 50). Three characters support the monophyly of the taxa at Node 1 (Fig. 9), excluding N. fragrans, which is basal. These characters are as follows: the longest four stamens straight (Character 22), plants lack a caudex (Character 33) and senescent leaves not retained on the plant (Character 36). The clade at Node 5 is characterised by leaves that have a petiole without wings (Character 39), but with two species (N. gossei and N. amplexicaulis) showing a reversal and two related taxa developing mature leaves that are sessile (N. benthamiana, N. burbidgeae). The clade of four taxa at Node 7 is characterised by reniform seeds (Character 1), with cells of the testa having distinctive raised junctions (Character 3). The species at Node 10 all have petals with mucronate lobes (Character 7). Node 14 is supported by hydathode trichomes (Character 47), with glands that have up to eight cells, except for N. amplexicaulis, which is unique in having six cells; also there is a reversal to the plesiomorphic condition of four cells at Node 19. Node 14 is also supported by branched hairs (Character 49); however, that character is homoplasious.
Chromosome number supports several nodes and infers a significant evolutionary trend of reduction. For internal, ancestral nodes, chromosome number is inferred as n = 21 (Node 4), n = 20 (Node 5), n = 19 (Node 6), n = 18 (Node 13), n = 16 (Node 16), and n = 15 (Node 18). However, the character optimisation of n = 19 at Node 6 and reversals from n = 19 to n = 20 and 21 for N. cavicola and N. burbidgeae, respectively, is only one possibility. An alternative optimisation for ancestral Node 6 is n = 20, with a loss of two chromosomes to n = 18 at Node 13. To see the effect of chromosome number on tree topology, the data were rerun without Character 50 (tree not shown); clades at Nodes 7 and 10 (as in Fig. 9) are retained whereas that at Node 14 is not supported.
The splits network, a distance-based phylogenetic method, constructed using simplified morphological data, is presented in Fig. 10. The network shows various interconnections among taxa, indicating a significant conflict among morphological characters, also indicated by the low CI value in the parsimony analysis. Nonetheless, groups of taxa in the network are similar to those in the cladogram (Fig. 9) and thus are reasonably robust. The long branch to the outgroup N. africana reflects the autapomorphic features of that species. N. fragrans and N. forsteri are closest to the outgroup, and N. heterantha, N. monoschizocarpa and occidentalis subsp. obliqua are the next to diverge. The three clades at Nodes 7, 10 and 13 in Fig. 9 are also evident in the splits network.
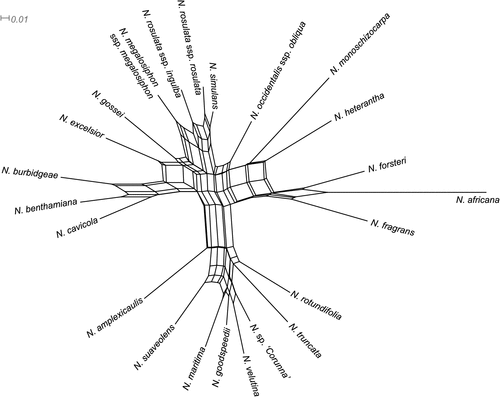
|
Molecular phylogenies
Of the published DNA sequences, those for the nuclear regions ITS (Chase et al. 2003) and ncpGS long copy and short copies (Clarkson et al. 2010) included the most taxa common to the morphological dataset. Parsimony analysis of these combined nuclear DNA sequences for 20 taxa resulted in 152 trees of length 4905, CI = 0.8312 and RI = 0.7089. The strict consensus tree resolved six nodes, with one additional node being resolved in the bootstrap tree that was collapsed in the consensus tree (Fig. 11).
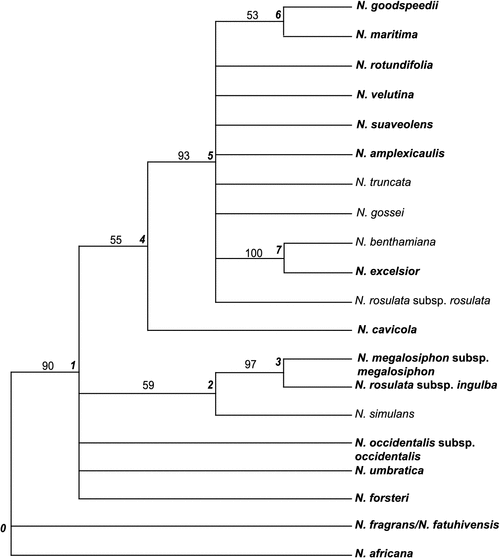
|
As in the morphological results, the South Pacific N. fragrans–N. fatuhivensis is sister to the Australian taxa (Node 1, 90% bootstrap support). Node 1 is a polytomy including N. forsteri, which is also in a basal position in the morphology cladogram (Fig. 9), N. umbratica (not able to be included in the morphology analysis but interestingly with a chromosome number n = 23 that supports its position here) and N. occidentalis, also shown as an early divergence in the morphology cladogram. The resolved clade at Node 2 supports the relationship on the basis of morphology of N. simulans, N. rosulata subsp. ingulba and N. megalosiphon. The nuclear DNA data suggest that N. cavicola is sister to the remaining Australian taxa (Node 5, 93% bootstrap support), a position different from the morphology result where it is placed in a clade with N. excelsior, N. benthamiana and N. burbidgeae, this last species not having been sequenced. The chromsome number for N. cavicola was scored as n = 20 on the basis of Williams (1975); however, it is noted that Burbidge (1960) recorded n = 23, which is more plesiomorphic among Suaveolentes. If correct, this would support the position of N. cavicola as shown in the molecular tree; these chromosome counts, including population variation, need further investigation. The sister relationship of N. benthamiana and N. excelsior on the basis of nuclear DNA (Node 7, 100% bootstrap value) is supported by their shared chromosome count of n = 19. Of the other taxa in the clade at Node 5, N. maritima (n = 15) appears to be related to N. goodspeedii (n = 16), although with low bootstrap support (53%), rather than to N. suaveolens (n = 15) as in the morphological analysis.
Chloroplast DNA sequences, including trnL intron, trnL–F spacer, trnS–G spacer and genes ndhF and matK (e.g. Clarkson et al. 2004), were available for a limited number of taxa, including only 13 ingroup taxa and the outgroup N. africana. Analysis of these data resulted in a cladogram (not shown) with eight resolved nodes, although only two had bootstrap support >90%. One node strongly supports the Australian clade of Suaveolentes (100% bootstrap support), with N. forsteri sister to it. The other well supported node is a clade of N. rosulata, N. rotundifolia and N. velutina (bootstrap 92%), the latter two species being closely related also in the nrDNA analysis. N. rotundifolia and N. velutina both have n = 16 chromosomes and share several morphological features that were homoplasious characters in the morphology analysis. These two species have branched trichomes, a basal rosette and a short corolla tube with deep-green colour.
Combined morphological and molecular data
The morphological data were combined with the nuclear DNA sequences for 20 taxa. The cpDNA sequences were not included because of the limited sampling of taxa and some obvious incongruence. Three taxa (N. heterantha, N. monoshizocarpa and N. burbidgeae) included in the morphology analysis were unable to be included in this combined analysis because of missing values in one or other of the molecular datasets.
Parsimony analysis of the combined morphological and nuclear DNA sequence data resulted in three trees, each of length 688.78, CI = 0.7395 and RI = 0.6194. Figure 12 shows those nodes in the strict consensus tree that received bootstrap support >50%. The combined data confirm the basal position of the South Pacific N. fragrans–fatuhivensis, and the sister relationship between N. forsteri (Node 1, bootstrap 81%), which occurs in Eastern Australia, Lord Howe Island and New Caledonia, and the remaining Australian taxa (at Node 2, bootstrap 69%). The consensus tree reflects the pattern of reduction in the chromosome number from n = 24 to n = 15–18 for the clade at Node 5. On the basis of chromosome count and morphology (Fig. 10), it is hypothesised that N. gossei (n = 18) is related to the clade at Node 5, even though its position is unresolved in the nuclear DNA and combined analyses.
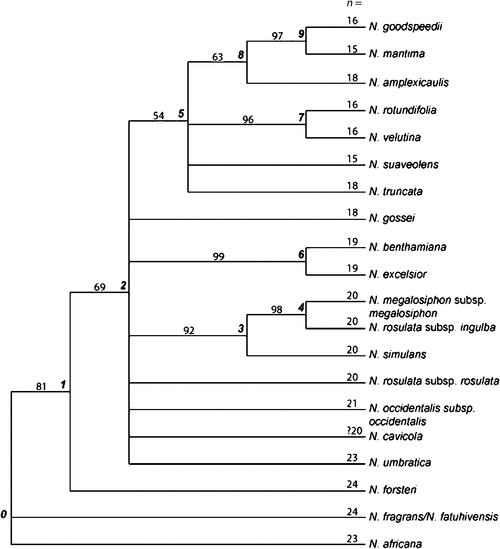
|
As an alternative method of combining data, nodes that received strong bootstrap support (>90%) from individual analyses of each of the ITS, ncpGS short, ncpGS long and cpDNA datasets were scored as 11 characters and added to a reduced morphological dataset. Taxa found in a supported clade in a particular molecular analysis were scored 1, those outside that node were scored 0, and missing taxa were scored as unknown (?). The eight most homoplasious morphological characters, each with a CI value <0.333 (Characters 5, 13, 14, 15, 16, 17, 20 and 42, seven of which were gap-coded quantitative measures), were not included in the analysis. Parsimony analysis of this dataset of 20 taxa × 54 characters resulted in four trees of length 116.4444, CI = 0.5143 and RI = 0.6285, and the strict consensus tree has 14 nodes resolved. One additional node (Node 1) was resolved in three of the four trees but was collapsed in the fourth; however, because that node also received support in the previous analyses it is included in Fig. 13, which represents a summary phylogeny. Among the Australian taxa, the major clade at Node 2 is supported by an unambiguous morphological character, loss of ellipsoid hairs (Character 46, CI = 1.00). Node 6 includes all those Australian taxa with n = 19 or fewer chromosomes, and Node 11 taxa with n = 16 or 15. Chromosome number (multistate Character 50) has a high CI of 0.778, with one parallel reduction from n = 16 to n = 15 in N. maritima and N. suaveolens. This analysis (Fig. 13) shows N. benthamiana as sister to N. excelsior (strongly supported by ITS), with N. cavicola outside the group in a more basal position at Node 2, similar to the results of the molecular analysis (e.g. Fig. 11) and in contrast to the results of the morphological analyses (Figs 9, 10).
|
Discussion
Gap-coding of morphological characters
Molecular phylogenetic analyses of Nicotiana have not resolved relationships among the taxa of section Suaveolentes. Comparative morphology has the potential to discover relationships; however, here it also posed a challenge because differences among taxa included morphometric characters in addition to discrete characters. The gap-coding method of Thiele (1993) proved useful in scoring such characters for cladistic analysis. Care was taken to avoid measurements that are correlated with a particular morphology; e.g. in the tubular flowers of Suaveolentes, stamen length, style length and corolla-tube length are all correlated (e.g. correlation between the corolla-tube length and the longest-stamen length, with r2 = 0.977). In such cases, one measurement character was included as a size indictor, and one ratio character as a shape indicator, ratios being especially useful when there is a range of shapes (Thiele 1993).
As also others have found (e.g. Goloboff et al. 2006), the inclusion of gap-coded characters increased the resolution of the morphological cladistic analysis but resulted in some hypothesised relationships that were incongruent with the molecular results. Several of the gap-coded morphometrics had low CI values.
Homoplasy
The CI value of 0.4364 for the parsimony analysis of the morphological dataset indicated a significant level of homoplasy, and the splits network method was useful for visualising this conflict. There was significantly more webbing in the Suaveolentes splits graph than in graphs for some other groups of organisms (Perrie et al. 2000; Lockhart and Cameron 2001), even for those with a large number of taxa (Wollscheid and Wägele 1999; Smith et al. 2004), although similar levels of ambiguity in the data were found in examples published by Bryant and Moulton (2004).
Homoplasy was particularly evident among characters associated with flower morphology (10 of the 14 gap-coded characters) and may be due in part to convergent evolution, with similar selection pressures being associated with pollinators. One exception was the presence of trichomes with ellipsoid-headed glands (Character 46). This trichome type is found in N. africana, N. fragrans, N. fatuhivensis, N. forsteri, N. umbratica, N. cavicola and all subspecies of N. occidentalis and is the plesiomorphic condition for the section. Trichomes with ellipsoid-headed glands have been ‘lost’ (at Node 6, Fig. 9; Node 4, Fig. 13) and in N. heterantha and N. monoschizocarpa. Other discrete characters go through at least two, often many more, transformations on the hypothesised phylogeny (Fig. 13). For example, the presence of a basal rosette of leaves (Character 35) is plesiomorphic and has been lost five times, namely, in N. umbratica, N. cavicola, in the N. benthamiana and N. excelsior clades and, independently, in N. amplexicaulis and N. suaveolens.
Such multiple ‘losses’ and ‘gains’ of morphological traits is not uncommon. In the Solanaceae, at a generic level, fruit evolution is homoplasious (Knapp 2002) and pollination syndromes are extremely homoplastic (Knapp 2010). The presence of different types of secondary compounds is also homoplasious across the family (Gemeinholzer and Wink 2001). In Solanum section Pteroidea, leaf complexity and the evolution of conical fruits are all labile and have been lost and gained multiple times in the section (Tepe and Bohs 2010).
Homoplasy may well be an indication of hybridisation among taxa with overlapping geographic distributions. As early as 1954, on the basis of crossing experiments, Goodspeed first hypothesised that repeated intrasectional hybridisation and introgression played a role in the evolution and speciation of Suaveolentes. The splits network highlighted morphological links among taxa, suggestive of hybridisation as an explanation for the homoplasy among them. Alternatively, some of the apparent homoplasy in Suaveolentes may be related to the underlying polyploidy of the group, where different gene copies may be expressed, affecting the phenotype. Kelly et al. (2010) showed a super networks analysis of diploid Nicotiana species also with significant conflict and reticulation, which was presumed to be due to the presence of intragenic recombination and the presence of species of homoploid hybrid origin in the group.
Summary phylogenetic pattern
Despite the above-mentioned issues, analysis of morphology, chromosome number and available nuclear-DNA sequences revealed a level of concordance among the datasets and phylogenetic relationships within section Suaveolentes. The analysis presented in Fig. 13 is used as a summary hypothesis of the relationships. The positions of species that were excluded because of missing sequence data are suggested on the basis of morphology and chromosome number (e.g. Figs 10, 11), as hypotheses for further testing.
Broader molecular studies of the genus Nicotiana support N. africana as sister to the rest of section Suaveolentes (ITS, Chase et al. 2003; cpDNA, Clarkson et al. 2004; nuclear glutamine synthetase, short copy but not the long copy, Clarkson et al. 2010). The character analysis here highlighted the morphological divergence between N. africana and the other members of the section. The history of the geographic disjunction between this species in Africa and its Australian–South Pacific relatives is intriguing. Explanations include the suggestion that an amphidiploid ancestor of Suaveolentes occurred in South America and dispersed to Africa and Australia separately about the same time (see Clarkson et al. 2004).
The analyses here support the position of N. fragrans–N. fatuhivensis as the sister taxon to the Australian and South Pacific group of Suaveolentes. N. fragrans (New Caledonia, Tongatapu) and N. fatuhivensis (Marquesas Islands) have never both been included in the same molecular analysis; however, given their morphological similarity (N. fatuhivensis is a former variety of N. fragrans), the two species are likely to be of the same lineage and sister taxa. Support was also found for the position of N. forsteri, from Eastern Australia, Lord Howe Island and New Caledonia, as the sister taxon to Australian endemic species, which are a monophyletic group. Whether there has been long-distance dispersal, facilitated by the small seeds of these plants, and in what directions (an Australian source or South Pacific source) are matters of speculation. As a widespread taxon, N. forsteri is a good candidate for a geographic population-level study.
Within the Australian endemic clade, on the basis of morphology and plesiomorphic chromosome number, early lineages include N. monoschizocarpa (n = 24) of northern Australia and N. heterantha (n = 24) of the Pilbara, Western Australia, although nuclear and chloroplast sequences for these species have yet to be obtained. Two other early lineages are N. umbratica (n = 23), also from the Pilbara, and N. occidentalis (n = 21), which includes three subspecies and ranges from the Pilbara (Western Australia) to central and southern Australia. The position of N. cavicola is equivocal among the different analyses. It is in a basal polytomy in the final analysis (Fig. 13), whereas it is associated with N. benthamiana, N. burbidgeae and N. excelsior (see below) on the basis of morphology (Figs 9, 10). The chromosome count for N. cavicola needs to be checked and the species variation studied across its geographic range; the chromosome count was coded here as n = 20, based on Williams (1975), but n = 23 was recorded in the earlier work of Burbidge (1960).
Further evolution within the Australian group shows a pattern of chromosome descending dysploidy, with the lowest chromosome numbers found in more derived clades. Chase et al. (2003) also showed Suaveolentes as a descending series, but as a result of repeat, independent reductions, i.e. homoplasy. Dysploidy is a common cytoevolutionary pattern among angiosperm families (Stace and James 1996; Shan et al. 2006) and has been reported for various Australian plant groups, particularly for soft-wooded and herbaceous taxa (James 1981).
Within Suaveolentes, two derived clades are identifiable. One is the ‘N. simulans clade’, including N. simulans, N. megalosiphon (represented here by subsp. megalosiphon) and N. rosulata subsp. ingulba, and N. rosulata subsp. rosulata, all with n = 20. However, the two subspecies of N. rosulata do not form a monophyletic group (as sister taxa) in any of the analyses presented here. They are divergent in floral size and shape, and the taxonomic reinstatement of N. ingulba J.M.Black as a separate species is here recommended.
The second larger clade is the ‘N. suaveolens clade’ of 10 or more species, with a chromosome number of n = 19, 18, 16 or 15, including N. benthamiana sister to N. excelsior, N. gossei, N. truncata, N. amplexicaulis, N. suaveolens, and a subclade of sister species N. goodspeedii and N. maritima, and sister species N. rotundifolia and N. velutina. Nicotiana sp. ‘Corunna’ (n = 16) is presumed to be related to the last two mentioned species. N. burbidgeae (n = 21) has yet to be included in any molecular studies, although it is possibly related to the ‘N. suaveolens clade’. It is a distinctive species with several autapomorphic features, and has mucronate corolla lobes and sessile broad leaves similar to N. benthamiana. Whether the narrow Queensland endemic N. wuttkei (chromosome number n = 14 according to Clarkson and Symon 1991, and n = 16 according to Laskowska and Berbec 2003) is part of the ‘N. suaveolens clade’ is as yet unknown; no molecular information is available for this species and lack of material precluded a full assessment of its morphology.
These hypothesised relationships within Nicotiana section Suaveolentes have the potential to reveal historic patterns of area differentiation within continental Australia, particularly, in the arid zone where Suaveolentes is most diverse. A biogeographic analysis is the subject of a subsequent paper (Ladiges et al. 2011).
Acknowledgements
C. E. M. acknowledges the receipt of an Australian Postgraduate Award, an Hansjörg Eichler Scientific Research Award from the Australian Systematic Botany Society Inc. to support field work, a student travel bursary from the Australian Biological Resources Study, and a School of Botany Foundation Travel Award together with a Melbourne Abroad Travel Scholarship to attend the PAA Solanaceae 2006 Conference (Madison, Wisconsin, USA). The following herbaria are acknowledged for providing access to collections: AD, BM, CANB, K, MEL, NT and NY. Seed was made available from the Australian Tropical Crops and Forages Collection, Biloela, Queensland, David Symon (AD), John Hamill (Monash University) and the Experimental Garden and Genebank of the Radboud University (Nijmegen, The Netherlands). David Albrecht (NT) and rangers at Watarrka National Park (Northern Territory, Australia) assisted with locating some field populations of Nicotiana. David Symon and Sandra Knapp also provided advice.
References
Aoki S, Ito M (2000) Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene. Plant Biology 2, 316–324.| Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksFSktLo%3D&md5=0fd5c7bef36b658832f6cfbb84581665CAS |
Bahadur B, Farooqui SM (1986) Seed and seed coat characters in Australian Nicotiana. In ‘Solanaceae, biology and systematics’. (Ed. WG D’Arcy). pp. 114–137. (Columbia University Press: New York)
Bryant D, Moulton V (2004) Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution 21, 255–265.
| Neighbor-net: an agglomerative method for the construction of phylogenetic networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFWmurs%3D&md5=dd0813df566e040213a036783afc353cCAS |
Burbidge NT (1960) The Australian species of Nicotiana L. (Solanaceae). Australian Journal of Botany 8, 342–378.
| The Australian species of Nicotiana L. (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Chase MW, Knapp S, Cox AV, Clarkson JJ, Butsko Y, Joseph J, Savolainen V, Parokenny AS (2003) Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Annals of Botany 92, 107–127.
| Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtVKqtL0%3D&md5=5242ec0f26cffab26e570447d2af4d4bCAS |
Clarkson JR, Symon DE (1991) Nicotiana wuttkei (Solanaceae): a new species from north-east Queensland with an unusual chromosome number. Austrobaileya 3, 389–392.
Clarkson JJ, Knapp S, Garcia VF, Olmstead RG, Leitch AR, Chase MW (2004) Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. Molecular Phylogenetics and Evolution 33, 75–90.
| Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFCrsb8%3D&md5=e3a77fa5ce985a363437c78e97e566d0CAS |
Clarkson JJ, Kelly LJ, Leitch AR, Knapp S, Chase MW (2010) Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids. Molecular Phylogenetics and Evolution 55, 99–112.
| Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXit1Gnt7k%3D&md5=1a99145634a7cded4e934c07ba40e45cCAS |
Corner EJH (1976) ‘The seeds of dicotyledons.’ (Cambridge University Press: Cambridge, UK)
Edmonds JM (1982) Epidermal hair morphology in Solanum L. section Solanum. Botanical Journal of the Linnean Society 85, 153–167.
Edmonds JM (1983) Seed coat structure and development in Solanum (Solanaceae). Botanical Journal of the Linnean Society 87, 229–246.
| Seed coat structure and development in Solanum (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791.
| Confidence limits on phylogenies: an approach using the bootstrap.Crossref | GoogleScholarGoogle Scholar |
Garcia VF, Olmstead RG (2003) Phylogenetics of the tribe Anthocercideae (Solanaceae) based on ndhF and trnL/F sequence data. Systematic Botany 28, 609–615.
Garcia-Cruz J, Sosa V (2006) Coding quantitative character data for phylogenetic analysis: a comparison of five methods. Systematic Botany 31, 302–309.
| Coding quantitative character data for phylogenetic analysis: a comparison of five methods.Crossref | GoogleScholarGoogle Scholar |
Gemeinholzer B, Wink M (2001) Solanaceae: occurrence of secondary compounds versus molecular phylogeny. In ‘Solanaceae V: advances in taxonomy and utilization’. (Eds RG van den Berg, GWM Barendse, GM van der Weerden, C Mariani) pp. 165–178. (Nijmegen University Press: Nijmegen, The Netherlands)
Goloboff PA, Mattoni CI, Quinteros AS (2006) Continuous characters analyzed as such. Cladistics 22, 589–601.
| Continuous characters analyzed as such.Crossref | GoogleScholarGoogle Scholar |
Goodspeed TH (1945) Studies in Nicotiana III. A taxonomic organization of the genus. University of California Publications in Botany 18, 335–343.
Goodspeed TH (1954) ‘The genus Nicotiana: origins, relationships and evolution of its species in the light of their distribution, morphology and cytokinetics.’ (Chronica Botanica Company: Waltham, MA)
Gunn CR, Gaffney FB (1974) Seed characteristics of 42 economically important species of Solanaceae in the United States. United States Department of Agriculture Technical Bulletin 1471, 1–32.
Haegi L (1991) Trichomes of Solanaceae tribe Anthocercidae. In ‘Solanaceae III: taxonomy, chemistry, evolution’. (Eds JG Hawkes, RN Lester, M Nee, N Estrada) pp. 181–195. (Royal Botanic Gardens Kew and Linnean Society of London: London)
Hamming RW (1950) Error detecting and error correcting codes. The Bell System Technical Journal 26, 147–160.
Harris JG, Harris MW (2001) ‘Plant identification terminology: an illustrated glossary.’ (Spring Lake Publishing: Spring Lake, UT)
Horton P (1981) A taxonomic revision of Nicotiana (Solanaceae) in Australia. Journal of the Adelaide Botanic Gardens 3, 1–56.
Huson DH (1998) SplitsTree: analysing and visualizing evolutionary data. Bioinformatics 14, 68–73.
| SplitsTree: analysing and visualizing evolutionary data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFygs70%3D&md5=9ba79031383b1e7ef5b7c4b425bd66ecCAS |
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23, 254–267.
| Application of phylogenetic networks in evolutionary studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntValsw%3D%3D&md5=9273bd0ef9f8697d1661c22911814306CAS |
James SH (1981) Cytoevolutionary patterns, genetic systems and the phytogeography of Australia. In ‘Ecological biogeography of Australia’. (Ed. A Keast) pp. 761–782. (W. Junk: The Hague, The Netherlands)
Kelly LJ, Leitch AR, Clarkson JJ, Hunter RB, Knapp S, Chase MW (2010) Intragenic recombination events and evidence for hybrid speciation in Nicotiana (Solanaceae). Molecular Biology and Evolution 27, 781–799.
| Intragenic recombination events and evidence for hybrid speciation in Nicotiana (Solanaceae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjslaltbo%3D&md5=4f432741935e5d57bfb351dae5ec425aCAS |
Kitching IJ, Forey PL, Humphries CJ, Williams DM (1998) ‘Cladistics: the theory and practice of parsimony analysis.’ (Oxford University Press: Oxford, UK)
Knapp S (2001) Is morphology dead in Solanum taxonomy? In ‘Solanaceae V: advances in taxonomy and utilization’. (Eds RG van den Berg, GWM Barendse, GM van der Weerden, C Mariani) (Nijmegen University Press: Nijmegen, The Netherlands)
Knapp S (2002) Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. Journal of Experimental Botany 53, 2001–2022.
| Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVGks7o%3D&md5=2af34ec82b7e84052e8f2c15720eb4d0CAS |
Knapp S (2010) On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365, 449–460.
| On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae.Crossref | GoogleScholarGoogle Scholar |
Knapp S, Chase MW, Clarkson JJ (2004) Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 53, 73–82.
| Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae).Crossref | GoogleScholarGoogle Scholar |
Kovarik A, Dadejova M, Lim KY, Chase MW, Clarkson JJ, Knapp S, Leitch AR (2008) Evolution of rDNA in Nicotiana allopolyploids: a potential link between rDNA homogenization and epigenetics. Annals of Botany 101, 815–823.
| Evolution of rDNA in Nicotiana allopolyploids: a potential link between rDNA homogenization and epigenetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVait7s%3D&md5=bda5c820af590232478f506ab6ea3619CAS |
Ladiges PY, Marks CE, Nelson G (2011) Biogeography of Nicotiana section Suaveolentes (Solanaceae) reveals geographic tracks in arid Australia. Journal of Biogeography 38,
| Biogeography of Nicotiana section Suaveolentes (Solanaceae) reveals geographic tracks in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Laskowska D, Berbec A (2003) Preliminary study of the newly discovered tobacco species Nicotiana wuttkei Clarkson et Symon. Genetic Resources and Crop Evolution 50, 835–839.
| Preliminary study of the newly discovered tobacco species Nicotiana wuttkei Clarkson et Symon.Crossref | GoogleScholarGoogle Scholar |
Leitch IJ, Hanson L, Lim KY, Kovarik A, Chase MW, Clarkson JJ, Leitch AR (2008) The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Annals of Botany 101, 805–814.
| The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3kvVKisg%3D%3D&md5=b358db367e8deaaa6a2c9e9da18256c7CAS |
Lester RN (1991) Evolutionary relationships of tomato, potato, pepino and wild species of Lycopersicon and Solanum. In ‘Solanaceae III: taxonomy, chemistry, evolution’. (Eds JG Hawkes, RN Lester, M Nee, N Estrada) pp. 283–301. (Royal Botanic Gardens Kew & Linnean Society of London: London)
Lockhart PJ, Cameron SA (2001) Trees for bees. Trends in Ecology & Evolution 16, 84–88.
| Trees for bees.Crossref | GoogleScholarGoogle Scholar |
Marks CE (2010a) Definition of South Pacific taxa of Nicotiana section Suaveolentes. Muelleria 28, 74–84.
Marks CE (2010b) The evolution of Nicotiana section Suaveolentes. PhD Thesis, The University of Melbourne.
Martins TR, Barkman TJ (2005) Reconstruction of Solanaceae phylogeny using the nuclear gene SAMT. Systematic Botany 30, 435–447.
| Reconstruction of Solanaceae phylogeny using the nuclear gene SAMT.Crossref | GoogleScholarGoogle Scholar |
Merxmüller H, Buttler KP (1975) Nicotiana in der Afrikanischen Namib – ein Planzengeographisches und phylogenetisches Ratsel. Mitteilungen (aus) der Botanischen Staatssammlung München 12, 91–104.
National Centre for Biotechnology Information (2010) ‘Genbank.’ (National Centre for Biotechnology Information, NCBI). Available at http://www.ncbi.nlm.nih.gov/nucleotide/ [accessed 1 March 2010].
Olmstead RG, Palmer JD (1992) A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. Annals of the Missouri Botanical Garden 79, 346–360.
| A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution.Crossref | GoogleScholarGoogle Scholar |
Olmstead RG, Sweere JA (1994) Combining data in phylogenetic systematics: an empirical approach using three molecular data sets in the Solanaceae. Systematic Biology 43, 467–481.
| Combining data in phylogenetic systematics: an empirical approach using three molecular data sets in the Solanaceae.Crossref | GoogleScholarGoogle Scholar |
Olmstead RG, Sweere JA, Spangler RE, Bohs L, Palmer JD (1999) Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. In ‘Solanaceae IV’. (Eds M Nee, DE Symon, RN Lester and JP Jessop) pp. 111–137. (Royal Botanic Gardens, Kew: London)
Olmstead RG, Bohs L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM (2008) A molecular phylogeny of the Solanaceae. Taxon 57, 1159–1181.
Perrie LR, Lockhart PJ, Brownsey PJ, Large MF (2000) Morphological and molecular concordance for the recognition of two species in the New Zealand Polystichum richardii (Hook.) J.Smith complex. Plant Systematics and Evolution 224, 97–107.
| Morphological and molecular concordance for the recognition of two species in the New Zealand Polystichum richardii (Hook.) J.Smith complex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosFyht7g%3D&md5=a3dfb80890dc27c93b06ded53f10c63fCAS |
Purdie RW, Symon DE, Haegi L (1982) Solanaceae. In ‘Flora of Australia’. (Australian Government Publishing Service: Canberra)
Rambaut A (2002) ‘Se-Al v2.0a11.’ (University of Oxford: Oxford, UK)
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstruction of phylogenetic trees. Molecular Biology and Evolution 4, 406–425.
Seithe A (1979) Hair types as taxonomic characters in Solanum. In ‘The biology and taxonomy of the Solanaceae’. (Eds JG Hawkes, RN Lester, AD Skelding) pp. 307–319. (Academic Press: London)
Shan F, Yan G, Plummer JA (2006) Basic chromosome number in Boronia (Rutaceae) – competing hypotheses examined. Australian Journal of Botany 54, 681–689.
| Basic chromosome number in Boronia (Rutaceae) – competing hypotheses examined.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVyqt77F&md5=5ece75c0b146b703697fe43b7d0b0857CAS |
Smith VS, Page RDM, Johnson KP (2004) Data incongruence and the problem of avian louse phylogeny. Zoologica Scripta 33, 239–259.
| Data incongruence and the problem of avian louse phylogeny.Crossref | GoogleScholarGoogle Scholar |
Stace HM, James SH (1996) Another perspective on cytoevolution in Lobelioideae (Campanulaceae). American Journal of Botany 83, 1356–1364.
| Another perspective on cytoevolution in Lobelioideae (Campanulaceae).Crossref | GoogleScholarGoogle Scholar |
Swofford DL (2003) ‘PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.’ (Sinauer Associates: Sunderland, MA)
Symon DE (1984) A new species of Nicotiana (Solanaceae) from Dalhousie Springs, South Australia. Journal of the Adelaide Botanic Gardens 7, 117–121.
Symon DE (1991) Gondwanan elements of the Solanaceae. In ‘Solanaceae III: taxonomy, chemistry, evolution’. (Eds JG Hawkes, RN Lester, M Nee, N Estrada) pp. 139–150. (Royal Botanic Gardens Kew & Linnean Society of London: London)
Symon DE (1998) A new Nicotiana (Solanceae) from near Coober Pedy, South Australia. Journal of the Adelaide Botanic Gardens 18, 1–4.
Symon DE (2004) Nicotiana in Australia. Australian Biologist 17, 63–68.
Symon DE, Kenneally KF (1994) A new species of Nicotiana (Solanaceae) from near Broome, Western Australia. Nuytsia 9, 421–425.
Tepe EJ, Bohs L (2010) A molecular phylogeny of Solanum sect. Pteroidea (Solanaceae) and utility of COSII markers in resolving relationships among closely related species. Taxon 59, 733–743.
Thiele K (1993) The Holy Grail of the perfect character: the cladistic treatment of morphometric data. Cladistics 9, 275–304.
| The Holy Grail of the perfect character: the cladistic treatment of morphometric data.Crossref | GoogleScholarGoogle Scholar |
von Minden MD (1899) Beiträge zur anatomischen und physiologischen Kenntnis Wasser-secernierender Organe. Bibliotheca Botanica 9, 1–76.
Wheeler HM (1945) A contribution to the cytology of the Australia–South Pacific species of Nicotiana. Proceedings of the National Academy of Sciences, USA 31, 177–185.
| A contribution to the cytology of the Australia–South Pacific species of Nicotiana.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zhvFKitg%3D%3D&md5=8f9479f967f49229b7e55a8b9982adb4CAS |
Williams E (1975) A new chromosome number in the Australian species Nicotiana cavicola L. (Burbidge). New Zealand Journal of Botany 13, 811–812.
Wollscheid E, Wägele H (1999) Initial results on the molecular phylogeny of the Nudibranchia (Gastropoda, Opisthobranchia) based on 18S rDNA data. Molecular Phylogenetics and Evolution 13, 215–226.
| Initial results on the molecular phylogeny of the Nudibranchia (Gastropoda, Opisthobranchia) based on 18S rDNA data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXotFOhuro%3D&md5=b6cbdbe1b1810121d78cf0374700ec29CAS |
Zhang Z-Y, Yang D-Z, Lu A-M, Knapp S (2005) Seed morphology of the tribe Hyoscyameae (Solanaceae). Taxon 54, 71–83.
| Seed morphology of the tribe Hyoscyameae (Solanaceae).Crossref | GoogleScholarGoogle Scholar |