Of a different feather: two new species of featherheads from the Ptilotus macrocephalus (Amaranthaceae) complex
Timothy A. Hammer A C , Robert W. Davis B and Kevin R. Thiele
A C , Robert W. Davis B and Kevin R. Thiele  A
A
A School of Biological Sciences, Faculty of Science, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Western Australian Herbarium, Science and Conservation, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia.
C Corresponding author. Email: timothy.hammer@research.uwa.edu.au
Australian Systematic Botany 32(1) 61-70 https://doi.org/10.1071/SB18065
Submitted: 30 October 2018 Accepted: 8 April 2019 Published: 8 May 2019
Abstract
Ptilotus macrocephalus (R.Br.) Poir. (Amaranthaceae), commonly known as a featherhead, is a widespread and common species in many parts of Australia. In the present study, we assess morphological variation in P. macrocephalus throughout its geographic range and provide evidence for the recognition of two new species, namely, P. psilorhachis T.Hammer & R.W.Davis and P. xerophilus T.Hammer & R.W.Davis. Geospatial analyses indicated that these new species are partitioned ecologically and geographically.
Additional keywords: Australia, biogeography, ecology, taxonomy.
Introduction
Ptilotus R.Br. comprises ~120 species, all of which are native to mainland Australia, with one species (P. conicus R.Br.) also occurring on the Lesser Sunda Islands and the Aru Islands, and one (P. spathulatus (R.Br.) Poir.) occurring in Tasmania. Species diversity is highest in arid and semi-arid regions, particularly in Western Australia (Hammer et al. 2015, 2018a).
Ptilotus macrocephalus (R.Br.) Poir., known by several common names, including featherheads and green mulla mulla, is a widespread species found throughout mainland Australia. It was one of six species first described by Brown (1810) in Trichinium R.Br. (as T. macrocephalum R.Br.), on the basis of a specimen from Victoria. Poiret (1816) synonymised Trichinium under Ptilotus, making the new combination P. macrocephalus (R.Br.) Poir. Moquin-Tandon (1849) rejected Poiret’s synonymy and retained Trichinium and Ptilotus as two distinct genera but misapplied the name T. macrocephalum to specimens of P. exaltatus Nees (e.g. A. Cunningham 202 at MEL; see also Hammer et al. 2018b). Confusion between T. macrocephalum and P. exaltatus led Moquin-Tandon to describe T. pachocephalum Moq., also on the basis of a specimen from Victoria, and T. angustifolium Moq. from the Hunter River, New South Wales. Further confusion was created when Lindley (in Mitchell 1848) misapplied the name T. fusiforme R.Br. to specimens of T. macrocephalum.
Von Mueller (1868) followed Poiret (1816) by including Trichinium within Ptilotus. He recognised T. macrocephalum sensu Moquin-Tandon (1849) as a synonym of his own concept of P. nobilis (Lindl.) F.Muell., and considered T. angustifolium, T. pachocephalum and T. fusiforme sensu Lindley (in Mitchell 1848) to be conspecific (including them under P. pachocephalus (Moq.) F.Muell.). Bentham (1870) recognised the similarity of Ptilotus and Trichinium, but, nevertheless, maintained them as separate genera and listed Mueller’s P. pachocephalus as a synonym of T. macrocephalum. Black (1948) synonymised Trichinium under Ptilotus, reinstating the name P. macrocephalus, and this view has been followed by subsequent taxonomists. The phylogeny presented in Hammer et al. (2015) showed that all of Brown’s original Trichinium species are nested within Ptilotus. Ptilotus macrocephalus is placed in an informal ‘Clade D’ and is closely related to P. polystachyus (Gaudich.) F.Muell., P. giganteus (A.Cunn. ex Moq.) R.W.Davis & R.Butcher and related species.
Bean (2008) noted that P. macrocephalus occurs across a wide range of environments, from arid regions of Western Australia and western Queensland to high-rainfall areas near the coast in eastern Australia, and is morphologically variable. He recorded that sepals (termed ‘tepals’ by Bean) are longest in plants from north-eastern Queensland, nearly as long in near-coastal areas of Queensland and New South Wales, and shortest in Western Australia; he considered this variation to be clinal. In the present study, we prefer to continue the use of the term ‘sepals’ to the previously used ‘tepals’ (Hammer 2018; Hammer et al. 2018a), because recent studies have clarified the calycine origin of the uniseriate perianth of the Caryophyalles (e.g. Ronse de Craene 2013), the corolla having been lost, and following the change in terminology for other genera in Amaranthaceae (e.g. Vrijdaghs et al. 2014; Borsch et al. 2018).
The present study critically examines morphological variation in P. macrocephalus across its range and shows that there is strong morphological evidence for the recognition of three distinct geographically and ecologically partitioned taxa.
Materials and methods
Morphological examinations
In total, 676 fertile specimens from the National Herbarium of Victoria (MEL), the National Herbarium of New South Wales (NSW), the Queensland Herbarium (BRI), the State Herbarium of South Australia (AD) and the Western Australian Herbarium (PERTH) were examined, including material from throughout the geographic range of P. macrocephalus. Specimens from the Northern Territory Herbarium (DNA) were not accessed, but an adequate amount of material collected from the Northern Territory was available from other herbaria. Important morphological characters were identified, and specimens categorised into three distinct morphotypes without a priori consideration of geography or habitat.
Geospatial analyses
Collection localities of all specimens examined were obtained from the Australasian Virtual Herbarium (http://avh.chah.org.au/, accessed 15 April 2019). Records with a georeferenced precision >10 km were discarded, leaving n = 427 samples (Table S1, available as Supplementary material to this paper). A geospatial dataset of 40 Bioclim climate variables from the CliMond dataset (Kriticos et al. 2012, 2014; see https://www.climond.org, accessed 15 April 2019) for each included locality was constructed using the Point Sampling Tool in QGIS (ver. 3.3, Open Source Geospatial Foundation Project, see http://qgis.osgeo.org, accessed 15 April 2019). Given the broadly allopatric and very wide ranges of the three morphotypes, climate variables were considered to be suitable for modelling their distributions. Maximum-entropy modelling (Maxent, ver. 3.4.1, see https://biodiversityinformatics.amnh.org/open_source/maxent/, accessed 1 May 2019; Phillips et al. 2004; Phillips and Dudík 2008) analyses were used to assess ecological and geographic partitioning between the three morphotypes (see also Hammer et al. 2018b). No feature selection was conducted on the data. Multiple occurrence records of each morphotype from any given 10- × 10-km cell were ignored. Bootstrapping was performed for each model with 10 replications and 25% of records were randomly withheld for model testing. All other settings were left as default. Model accuracy was assessed using the area under curve (AUC) statistic (Fielding and Bell 1997), and jackknife tests were used to assess variable importance. Taxon distribution maps were created using QGIS.
A principal-component analysis (PCA) was conducted on the geospatial dataset using the R statistical platform (ver. 3.5.1, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/, accessed 15 April 2019) in RStudio (ver. 1.1.456, RStudio, Inc., Boston, MA, USA, see https://www.rstudio.com/, accessed 15 April 2019), to determine whether the morphotypes occupied discrete environmental envelopes. Standardisation of the variables was implemented. The PCA was visualised using the R packages ggplot2 (see https://ggplot2.tidyverse.org/, accessed 15 April 2019; Wickham 2016) and ggfortify (see https://github.com/sinhrks/ggfortify, accessed 15 April 2019; Tang et al. 2016).
Results
Morphological examinations
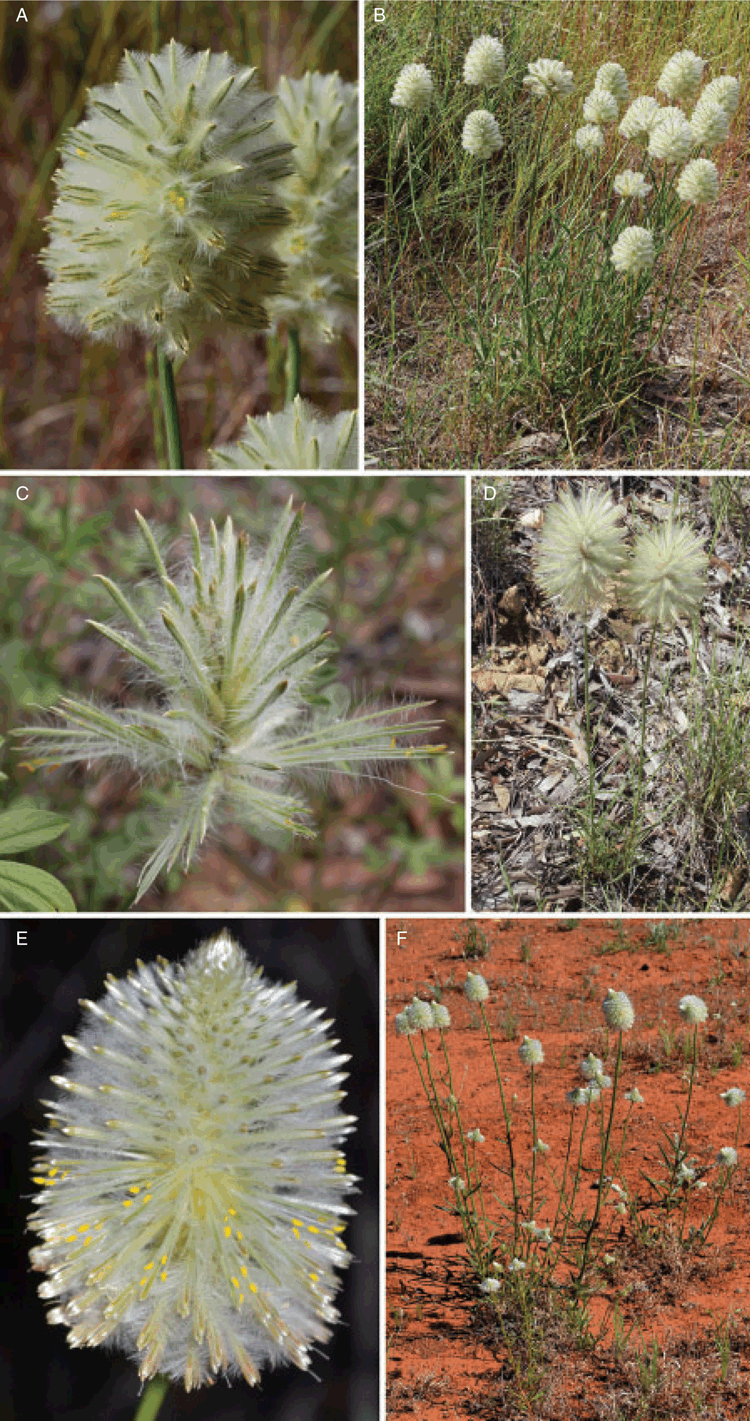
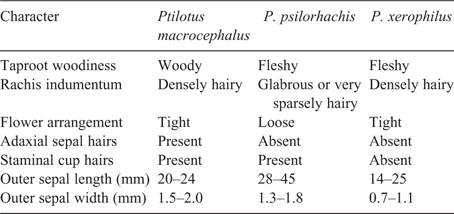
All specimens of Ptilotus macrocephalus sens. lat. (i.e. as currently circumscribed) examined could be reliably segregated into one of three morphotypes (Fig. 1), one of which included the type specimens of T. macrocephalum, T. angustifolium and T. pachocephalum. The morphotypes are distributed in (1) south-eastern Australia, from far south-eastern South Australia through Victoria and into eastern New South Wales, (2) arid parts of central and western Australia, closely associated with the Eremaean botanical province, and (3) eastern Queensland from west of Brisbane to near Cairns. The morphotypes are, hereafter, referred to as (1) P. macrocephalus, (2) P. xerophilus and (3) P. psilorhachis (see Taxonomy section below). Distinguishing morphological characters are summarised in Table 1.
|
Plants of P. macrocephalus are perennial herbs with a woody, sometimes branching, taproot; many specimens appear to be long-lived (Fig. 1B) on the basis of scars and dead branches from previous seasons being retained on the crown. By contrast, the other two species have fleshy taproots and seem to be mainly annuals but may persist for several seasons given favourable conditions (Fig. 1D, F). Field observations of P. xerophilus in Western Australia, the Northern Territory and northern South Australia (T. A. Hammer, pers. obs.) and of P. psilorhachis near Cairns, Queensland (P. J. H. Hurter, pers. comm.) confirm their primarily annual habit.
Inflorescences of P. psilorhachis are markedly different from those of P. macrocephalus and P. xerophilus. A key feature of P. psilorhachis is a glabrous or very sparsely hairy rachis (i.e. a few scattered hairs may be present). This is in distinct contrast to the densely villous or tomentose indumentum that obscures the surface of the rachis in the other two taxa. Flowers on pressed specimens of P. psilorhachis are also loosely arranged, with the rachis being visible between the flowers (Fig. 1C), whereas flowers in P. macrocephalus and P. xerophilus are more tightly arranged, with the overlapping bracts and bracteoles obscuring the rachis (Fig. 1A, E).
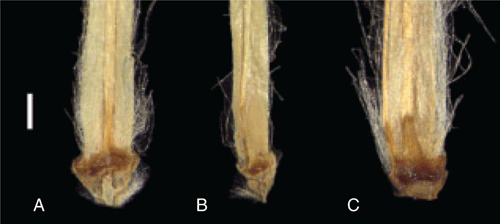
Differences in sepal indumentum between species of Ptilotus are often informative (e.g. Hammer 2018; Hammer et al. 2018b). The indumentum on the abaxial surface of the sepals differs among the three taxa (Fig. 2). The hairs in P. macrocephalus and P. psilorhachis are long, spreading and verticillate for almost the entire length of the sepal (Fig. 2B, F), except for glabrous apices. In P. xerophilus, the hairs are short, appressed and simple in the lower half of the sepal (some specimens are almost glabrous), with only the upper half having long, spreading, verticillate hairs below the glabrous apices (Fig. 2C). These variations in abaxial sepal indumentum give the inflorescences a different appearance overall (Fig. 1). The indumentum within the flowers also varies among the three taxa (Fig. 3). In P. macrocephalus, the inner sepals are adaxially villous and the apex of the staminal cup has simple hairs (Fig. 3A). In P. xerophilus, the adaxial surface of the inner sepals and staminal cup are glabrous (Fig. 3B). In P. psilorhachis, the inner sepals are adaxially glabrous and the apex of the staminal cup has simple hairs (Fig. 3C).

|
|
As Bean (2008) noted, there are clear differences in sepal length within P. macrocephalus sens. lat (Fig. 2). The outer sepals of P. psilorhachis are noticeably longer (28–45 mm) than those of P. macrocephalus sens. str. (20–24 mm) and P. xerophilus (14–25 mm). Bean (2008) suggested that sepal length was clinal in P. macrocephalus sens. lat., but we found no evidence of clinal change in sepal length in our concepts of P. macrocephalus sens. str. or P. xerophilus. There is a general clinal trend in sepal length in P. psilorhachis (with long sepals predominantly at the northern end of the range and shorter sepals in the south); however, we found that there were exceptions at both ends of the geographic range. In addition to the differences in sepal length, the outer sepals of P. xerophilus are narrower (0.7–1.1 mm wide) than those of P. macrocephalus (1.5–2.0 mm) and P. psilorhachis (1.3–1.8 mm).
Geospatial analyses
Ptilotus macrocephalus was represented in the geospatial dataset for the Maxent modelling by 130 specimens, P. psilorhachis by 35 specimens and P. xerophilus by 262 specimens.
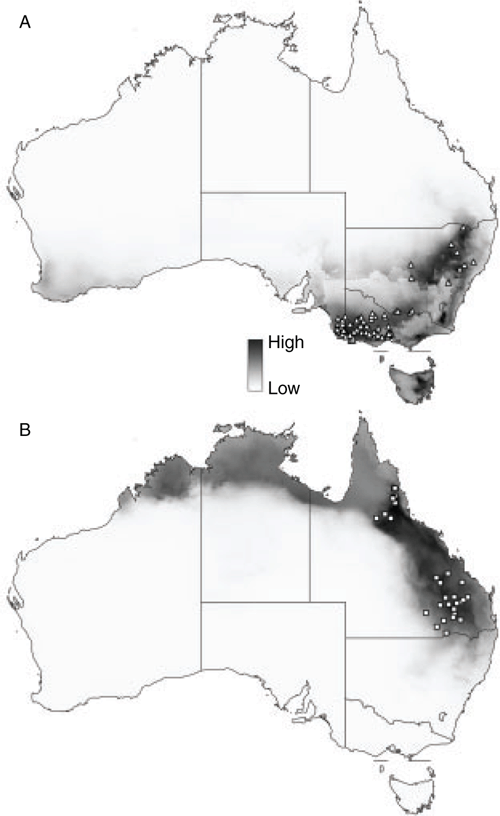
The distribution model for P. macrocephalus largely fits its known distribution (Fig. 4A), with areas in the south-east of mainland Australia and in Tasmania fitting well with the climatic conditions modelled for the species. The model returned an average AUC of 0.899 with a standard deviation of 0.008. Variables contributing most to the model were Bio27 (radiation of coldest quarter; 34.8%) and Bio28 (annual mean moisture index; 29.5%). The highest probability areas were in eastern Victoria and eastern to central New South Wales; an apparent disjunction, on the basis of known specimens, between southern Victoria and the Northern and Central Tablelands of New South Wales appears to not be a sampling artefact, with reduced probabilities of occurrence in the gap. A specimen from Toowoomba in southern Queensland (BRI AQ0178569) was determined as P. macrocephalus in the present study, but it was not included in the geospatial analysis because of the georeferenced precision being >10 km.
|
The model for P. psilorhachis predicted its occurrence through much of eastern Queensland, with lower probabilities on Cape York Peninsula, extending west across the northern end of the Northern Territory (the ‘Top End’) to the Kimberley region of Western Australia (Fig. 4B). The model returned an average AUC of 0.976 with a standard deviation of 0.004. Variables contributing most to the model were Bio18 (precipitation of warmest quarter; 48.0%) and Bio28 (21.8%). There is an apparent gap between the occurrences of P. psilorhachis in northern Queensland inland of Cairns and southern Queensland, which may be due to under-collecting or perhaps indicates that the available habitats there are not suitable for the species. The distribution models of P. macrocephalus and P. psilorhachis overlap around the border of northern New South Wales and southern Queensland, but no specimens of P. psilorhachis are known from New South Wales.
The model for P. xerophilus predicted its occurrence accurately throughout central and western Australia, excluding the far north, south and east (Fig. 5A). The model returned an average AUC of 0.791 with a standard deviation of 0.007. Variables that contributed most to the model were Bio21 (highest weekly radiation; 37.1%), Bio28 (22.9%) and Bio27 (12.4%). Narrow distributional gaps in northern South Australia and the south-eastern Northern Territory may be real on the basis of lower probabilities of modelled occurrence.
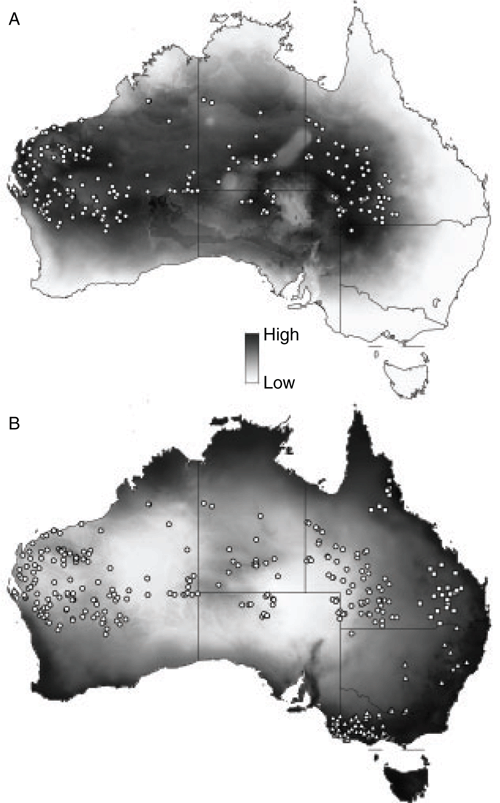
|
The species distribution models for each taxon were geographically allopatric or parapatric (Fig. 4, 5), with very little overlap at the margins of each. Across all three models, the variable Bio28 (annual mean moisture index) was an important predictor in driving the distribution of the taxa (Fig. 5B).
The PCA of the entire dataset shows that the three taxa, originally separated by differences in morphology, occupy discrete climatic envelopes (Fig. 6). Ptilotus psilorhachis occupies the largest climatic space of the three taxa, with specimens from southern Queensland and northern New South Wales being resolved disjunctly from those in northern Queensland and adjacent to a few specimens of P. macrocephalus from northern New South Wales. These latter specimens are also climatically disjunct from the main area of distribution of P. macrocephalus in Victoria.

|
Discussion
Geospatial analyses
Maxent modelling and the PCA ordination on the basis of environmental parameters showed that the three morphologically defined taxa are also ecologically partitioned, with the partitioning matching their distributions.
Because few specimens of P. macrocephalus from New South Wales were available for the geospatial analysis, the climatic space occupied by that species may be more continuous than is apparent in the current PCA (see Fig. 6), although the Maxent model showed that these occurrences in northern New South Wales and those from Victoria may be separated by unsuitable environments on the Southern Tablelands and South-western Slopes. An apparent environmental disjunction between northern and southern populations of P. psilorhachis may be caused by under-collecting or by a lack of suitable habitat in these areas (Fig. 4B).
The distribution of P. xerophilus is disjunct from the other two taxa, and it is more clearly different, in ecology, from the other two species, which occur in noticeably higher-rainfall regions of Australia. Despite its wider geographic distribution, it occupied the smallest environmental envelope on the PCA (Fig. 6), further suggesting that the geographically narrow disjunctions in its modelled distribution in northern South Australia and the southern Northern Territory are likely to be real.
Phylogenetic relationships
A single specimen of P. xerophilus was included (as P. macrocephalus sens. lat.) in the phylogeny of Hammer et al. (2015), where it formed a clade with P. beckerianus (F.Muell.) F.Muell. ex J.M.Black, P. distans (R.Br.) Poir., P. capensis (Benl) A.R.Bean, P. fusiformis (R.Br.) Poir., P. giganteus and P. polystachyus.
Relationships among some of these species remain unresolved or poorly supported, owing to a lack of sequence variation within the markers used for the phylogeny, perhaps indicating recent speciation within the group (Hammer et al. 2015). Taxonomic patterns within this group are complex, as evidenced by several new species being recognised recently, on the basis of discrete but subtle segregation of morphological characters (e.g. P. capensis separated from P. distans by Bean 2008 and P. giganteus from P. polystachyus by Davis and Butcher 2010).
Because P. macrocephalus sens. str. and P. psilorhachis were not included in these phylogenies, their relationships to the other species in the clade are uncertain. The three taxa in P. macrocephalus sens. lat. recognised here may not comprise a monophyletic group.
Ptilotus distans, P. capensis and P. giganteus are distributed in the monsoonal tropics of northern Australia in the Top End of the Northern Territory, Cape York Peninsula to just south of Cairns, and the Kimberley region of Western Australia respectively. These areas all fall within the predicted area of distribution of P. psilorhachis. It is possible that all these species comprise a single Northern Australian radiation from a common ancestor adapted to the monsoon tropics.
Ptilotus beckerianus is a rare endemic in South Australia, occurring in the southern part of the Eyre Peninsula and on Kangaroo Island, west of and disjunct from the western edge of the range of P. macrocephalus sens. str. (i.e. occurrences in the Naracoorte Coastal Plain IBRA bioregion). Both species are perennial herbs with woody taproots (Black 1948). Ptilotus beckerianus is perhaps most morphologically similar to P. macrocephalus sens. str. within this clade and may be closely related, perhaps indicating a small southern radiation from a temperate-adapted ancestor. The species that are the sister group to the subclade that includes all these species (i.e. P. clementii (Farmar) Benl, P. gardneri Benl, P. polystachyus) all occur in northern arid or semi-arid areas (the last with a wide distribution, including temperate southern and monsoon-tropical northern Australia). It is, thus, possible that the clade has diversified by recent peripatric or parapatric speciation from an arid-adapted common ancestor, with radiation into new tropical and temperate niches.
Taxonomic implications
The present study has demonstrated that, as currently circumscribed, P. macrocephalus sens. lat. includes three distinct taxa that are partitioned morphologically, geographically and ecologically. We consider the morphological differences among these species to be significant, and the ecological differences between these taxa add validation to the concept that they are distinctly evolving metapopulation lineages, as recognised by the general-lineage species concept (de Queiroz 2007). We, therefore, regard that these taxa are most appropriately recognised at species rank. Accordingly, we formally name and describe here the new species P. psilorhachis T.Hammer & R.W.Davis and P. xerophilus T.Hammer & R.W.Davis, and recircumscribe P. macrocephalus in the narrow sense as discussed above.
Taxonomy
Ptilotus macrocephalus (R.Br.) Poir., Lam., Encycl. Suppl. 4: 620 (1816)
Type: Port Phillip [Victoria], s. dat., Anon. s.n. (syn: BM 000895593, image! https://plants.jstor.org/stable/10.5555/al.ap.specimen.bm000895593; syn: GB 0047020, image! https://plants.jstor.org/stable/10.5555/al.ap.specimen.gb-0047020).
Type: New South Wales, the upper parts of Hunter River, 1825, A.Cunningham s.n. (syn: K 000356810, image! https://plants.jstor.org/stable/10.5555/al.ap.specimen.k000356810; syn: P 00609981, image! https://plants.jstor.org/stable/10.5555/al.ap.specimen.p00609981; syn: G-DC (microfiche), fide Bean 2008).
Type: Port Phillip [Victoria], 20 Mar. 1840, R.C.Gunn s.n. (syn: P 00609982, image! https://plants.jstor.org/stable/10.5555/al.ap.specimen.p00609982).
Erect perennial herbs with a woody, sometimes branching, taproot, 30–50 cm high. Stems terete, ribbed, glabrous or glabrescent towards stem apex; hairs crisped-nodose or subverticillate. Basal leaves not seen. Cauline leaves linear-lanceolate to narrowly oblanceolate, sessile, 30–120 mm long, 2–14 mm wide, glabrous or glabrescent with sparse, verticillate hairs; margins entire, often undulate. Inflorescences spiciform, terminal, cylindrical, creamish-green (rarely with a pinkish flush), 30–90 mm long, 45–60 mm wide; rachis densely tomentose or sometimes villous; flowers tightly arranged on rachis; apex rounded or truncate. Bracts narrowly ovate to ovate, 7.5–11 mm long, 3.0–4.5 mm wide, transparent, glabrous or with verticillate hairs along the conspicuous, white or pale brown midrib; apex mucronate, the mucro 1.0–1.2 mm long; margins entire. Bracteoles broadly ovate to obovate, 6.5–9.8 mm long, 3.2–5 mm wide, transparent, glabrous; midrib conspicuous, white; apex shortly mucronate, the mucro 0.1–0.2 mm long; margins serrate. Outer sepals lanceolate, 20–24 mm long, 1.5–2.0 mm wide, creamish-green (rarely with a pale pink flush towards apex), adaxially villous, abaxially villous with spreading verticillate hairs almost throughout; apex acute, glabrous. Inner sepals lanceolate, 19–23 mm long, 0.8–1.2 mm wide, with colour and indumentum as for outer. Fertile stamens 4; filaments cream, 15–19 mm long, unequal, filiform; anthers yellow, 1.2–2.0 mm long, 0.4–1.0 mm wide. Staminode 1, 0.5–3.5 mm long. Staminal cup symmetrical, unlobed, 0.4–0.5 mm long, strongly adnate to sepal tube, apically covered with simple hairs. Ovary obconical, 0.8–1.3 mm long, 1.0–1.8 mm wide, apically covered with simple hairs; stipe 1.0–2.0 mm long. Style sigmoidal, 17–22 mm long, cream, excentrically fixed on the ovary, verticillate-hairy on the lower half. Stigma capitate. Seed smooth, pale brown, ~2.7 mm long, ~1.5 mm wide. (Fig. 1A, B, 2A, B, 3A.)
Distribution and habitat
Ptilotus macrocephalus occurs from south-eastern South Australia, through central and northern Victoria, into central to eastern New South Wales, and just over the border into south-eastern Queensland (Fig. 4A). On the basis of herbarium label data, the species commonly inhabits open grasslands dominated by Austrostipa spp. and Themeda triandra on brown or reddish loamy soils derived from basalt in the south of its range. In the north of the range, it is recorded on north-facing hill slopes or on flats in open eucalyptus woodlands with stony, red or brown sandy or clayey loam soils.
Conservation status
Common in South Australia and Victoria; known occurrences in New South Wales are somewhat sporadic, many being older collections, indicating that it may be restricted in occurrence and uncommon in that state. The species may be rare in Queensland, occurring only near the border with New South Wales; therefore, its conservation status should be assessed.
Notes
The epithet of Trichinium pachocephalum deviates from the usual construction (‘pachycephalum’), as it derives from the Greek noun pachos (thickness) rather than the more usual adjective pachys (thick). Although this construction is not strictly grammatically correct, it is valid under Art. 60.10 of the ICN (Turland et al. 2018) and is not a correctable orthographic error.
Selected specimens examined
QUEENSLAND. Toowoomba, C.H.Hartmann s.n. (BRI AQ0178569). SOUTH AUSTRALIA. Near Donovan’s Landing, B.Copley 1807 (AD); Desert Camp Conservation Park, D.J.Duval & M.K.Jones 275A (AD); Mary Seymour Conservation Park, H.P.Vonow 2906 (AD). NEW SOUTH WALES. Carters Road, Pigna Barney, N.Cobcroft s.n. (NSW 441772); 8.2 miles [~13.2 km] W of Sandy Hollow on the Mudgee Road, R.G.Coveny 2451 (NSW); alongside Carters Road E of Boonara, J.R.Hosking 2125 (NSW); Goulburn River Valley, ~5 km SW of Baerami, T.A.James, W.Bishop & S.V.Goodwin 660 (NSW); Narrabri, J.H.Maiden s.n. (NSW 29487); near Lake Urana, W.E.Mulham W975 (NSW). VICTORIA. Hawkes Road, C.W.Ahrens 40 (MEL); Hands Road, C.W.Ahrens 43 (MEL); Oliver’s Lake Flora and Fauna Reserve, A.C.Beauglehole 86470 (AD); Grampian Mountains, D.Keane s.n. (AD 96947051); Werribee, A.Morrison 1467 (PERTH); 1.8 km upstream from Donovan, R.D.Pearce 395 (AD); Anglesea, V.Stajsic 99 (MEL); Willaura–Wickliffe road, N.G.Walsh & Z.Smith 5698 (MEL).
Ptilotus psilorhachis T.Hammer & R.W.Davis, sp. nov.
Type: Queensland, Emu Creek Station, 8.5 km NNE of Petford, 20 Jan. 2002, P.I.Forster, R.Booth & R.Jensen PIF 28204 (holo: BRI AQ555919!; iso: AD 142716!, MEL 2291403A!).
Erect annual or short-lived perennial herbs with fleshy taproot, 40–60 cm high. Stems terete, ribbed, glabrous or glabrescent towards stem apex; hairs crisped-nodose or subverticillate. Basal leaves not seen. Cauline leaves linear-lanceolate to narrowly oblanceolate, sessile, 20–120 mm long, 1–6 mm wide, glabrescent or with sparse, verticillate hairs; margins undulating or entire. Inflorescences spiciform, terminal, cylindrical, creamish-green (rarely with a pinkish flush), 40–100 mm long, 60–90 mm wide; rachis glabrous or very sparsely hairy; flowers loosely arranged on rachis; apex rounded or truncate. Bracts narrowly ovate to ovate, 9–12 mm long, 2.5–4 mm wide, transparent, glabrous or with verticillate hairs along midrib; midrib conspicuous, white or pale brown; apex mucronate, the mucro ~1 mm long; margins entire. Bracteoles obovate, 7–11.5 mm long, 2.5–5 mm wide, transparent, glabrous; midrib conspicuous, white; apex shortly mucronate, the mucro 0.1–0.2 mm long; margin serrate. Outer sepals lanceolate, 28–45 mm long, 1.3–1.8 mm wide, creamish-green or rarely with pale pink flush towards apex, adaxially villous, abaxially villous with spreading verticillate hairs; apex acute, glabrous. Inner sepals lanceolate, 27–42 mm long, 0.6–1.0 mm wide, creamish-green or rarely with pale pink flush towards apex, adaxially glabrous, abaxially villous with spreading verticillate hairs; apex acute, glabrous. Fertile stamens 4; filaments cream, 20–36 mm long, unequal, filiform; anthers yellow, 2.0–3.0 mm long, 0.5–1.0 mm wide. Staminode 1, 0.4–6 mm long. Staminal cup symmetrical, not lobed, 0.3–0.5 mm long, strongly adnate to sepal tube, the apex covered with simple hairs. Ovary obconical, 1.2–2.2 mm long, 1.4–2.0 mm wide, glabrous; stipe ~1 mm long. Style sigmoidal, 25–40 mm long, cream, excentrically fixed on the ovary, with verticillate hairs on lower half. Stigma capitate. Seed smooth, pale brown, 3.3–3.5 mm long, 1.9–2.0 mm wide. (Fig. 1C, D, 2E, F, 3C.)
Distribution and habitat
Ptilotus psilorhachis is distributed in eastern Queensland from west of Cairns south to west of Brisbane (Fig. 4B), including the IBRA subregions of Einasleigh Uplands, Brigalow Belt North and Brigalow Belt South. On the basis of herbarium label data, the species commonly inhabits open eucalypt woodlands, commonly dominated by Eucalyptus populnea, E. crebra or E. leptophleba, on flats with brown or red sandy or clayey loam soil, sometimes with a gravelly surface.
Etymology
From the Greek psilos (naked) and rhakhis (a spine or rachis), referring to the conspicuous and glabrous or very sparsely hairy rachis characteristic of the species.
Conservation status
Ptilotus psilorhachis is common across its range and is not considered to be of conservation concern.
Selected specimens examined
QUEENSLAND. 3 km along Pearlinga road W of Mundubbera, A.R.Bean 11936 (BRI); 65 km from Mitchell on road to Bollon, A.R.Bean 24397 (BRI); 16.6 km along Roche Creek Road E of Wandoan, A.R.Bean 29487 (BRI); Gayndah Dirnbir, E.W.Bick s.n. (BRI AQ0178578); ‘Potters Flats’, ~52 km N of Yuleba, C.Eddie CPE2472 (BRI); ~1.5 km by road E of Dimbulah, K.R.McDonald KRM4927 (BRI); 40 miles [~64.4 km] S of Ayr on W bank of Burdekin River, H.C.Seton 5 (BRI); Lakeland Downs near Condamine, K.M.Stephens, C.Thrupp & A.Daniel s.n. (BRI AQ0855297); 4 km from Petford along road to Irvinebank, I.R.Telford & R.J.Rudd 11249 (BRI); Nangram Station, Condamine Highway, ~10 miles [~16 km] NE of Condamine, W.G.Trapnell s.n. (BRI AQ0178565); 4 km S of Mount Garnet on the Gunnawarra Road, B.S.Wannan 3998 (BRI); Old coal road between Gladstone and Biloela, M.Worthington 1676 (BRI).
Ptilotus xerophilus T.Hammer & R.W.Davis, sp. nov.
Type: Western Australia, 700 m south along Paynes Find–Yalgoo road from junction of Geraldton–Mount Magnet road, 26 Aug. 2018, R.Davis & T.Hammer RD12901 (holo: PERTH!; iso [to be distributed]: BRI!, CANB!)
Erect annual herbs with fleshy taproot, 20–120 cm high. Stems terete, ribbed, glabrous or glabrescent towards stem apex; hairs crisped-nodose or subverticillate. Basal leaves not seen. Cauline leaves linear-lanceolate to narrowly oblanceolate, sessile, 10–130 mm long, 2–8 mm wide, glabrescent or with sparse verticillate hairs; margins undulating or entire. Inflorescences spiciform, terminal, cylindrical, creamish-green or rarely with pinkish flush, 30–70 mm long, 32–50 mm wide, sweetly scented at night; rachis densely tomentose or sometimes villous; flowers tightly arranged on rachis; apex acute, rounded or truncate. Bracts ovate to broadly ovate, 6–10 mm long, 2.5–4.5 mm wide, transparent, glabrous or with verticillate hairs along midrib; midrib conspicuous, white or pale brown; apex mucronate, the mucro 0.5–0.6 mm long; margins entire. Bracteoles obovate, 5–9 mm long, 2–4 mm wide, transparent, glabrous; midrib conspicuous, white; apex shortly mucronate, the mucro ∼0.1 mm long; margins serrate. Outer sepals linear to lanceolate, 14–25 mm long, 0.7–1.1 mm wide, creamish-green or rarely with pale pink flush towards apex, adaxially glabrous, abaxially villous with spreading verticillate hairs in the upper half, the indumentum sparse, short and appressed in lower half to one third; apex acute, glabrous. Inner sepals linear to lanceolate, 13–25 mm long, 0.5–0.8 mm wide, creamish-green or rarely with pale pink flush towards apex, adaxially glabrous, abaxially villous with spreading verticillate hairs, the indumentum sparse, short and appressed in lower half to one third; apex acute, glabrous. Fertile stamens 4; filaments cream, 11–20 mm long, unequal, filiform; anthers yellow, 1.3–2.0 mm long, 0.5–0.6 mm wide. Staminode 1, 0.5–4 mm long. Staminal cup symmetrical, not lobed, 0.2–0.4 mm long, strongly adnate to sepal tube, glabrous. Ovary obconical, 0.8–1.5 mm long, 1–1.5 mm wide, glabrous or with a row of simple hairs apically; stipe ~1 mm long. Style sigmoidal, 12–22 mm long, cream, excentrically fixed on the ovary, with verticillate hairs on lower half. Stigma capitate. Seed smooth, pale brown, 1.8–2 mm long, 1.0–1.1 mm wide. (Fig. 1E, F, 2C, D, 3B.)
Distribution and habitat
Occurs throughout arid regions of Western Australia, Northern Territory and in northern South Australia, western Queensland and north-western New South Wales (Fig. 5A), typically on plains with clayey or loamy soils and often in open mulga shrublands.
Etymology
From the Greek xeros (dry) and philia (love), referring to the distribution of this species in lower-rainfall regions of Australia than that of the other two species in the P. macrocephalus complex, which occur in higher-rainfall regions of eastern Australia (see Fig. 5B).
Conservation status
The species is common in Western Australia, Northern Territory, South Australia and Queensland, but may be uncommon or rare in north-western New South Wales, with only a few specimens recorded from the north-western corner of the state, where its status should be assessed.
Notes
This species often occurs en masse, particularly in Western Australia. We consistently observed this species being visited by night-flying moths (e.g. in the family Erebidae) in Western Australia. Floral observations were also made in daylight, and no insect visitation was seen. The flowers of this species are conspicuously sweetly scented at night. The closely related species P. polystachyus, which is also green-flowered and night-scented, was also observed to be visited by moths at night. We suspect P. xerophilus and the related green-flowering species to be primarily pollinated by nocturnal moths (see also Hammer et al. 2018b).
Selected specimens examined
WESTERN AUSTRALIA. Eastern Hamersley Range, N.Casson & E.M.Mattiske MCPL1002 (PERTH); 186 km S of Carnarvon on North West Coastal Highway, R.Davis 10995 (PERTH); on Rio Tinto Rail Access Road, ~18 km N of of Nanutarra–Munjina road, T.Hammer & S.Dillon TH30 (PERTH); Woolgorong Station Homestead Gate, T.Hammer & R.Davis TH61 (PERTH); 80 km N of Mullewa, T.Hammer & R.Davis TH65 (PERTH). NORTHERN TERRITORY. Ruby Gorge, Hale River, 112 km ENE of Alice Springs, A.C.Beauglehole 20724 (AD); 11.6 miles [~18.7 km] NE of Frewena, G.M.Chippendale 7346 (PERTH); Mount Olga, W side at Docker River road junction, N.N.Donner 4408 (AD); ~5 km SE of Alice Springs, J.Z.Weber 893 (AD). SOUTH AUSTRALIA. Cordillo Downs, J.Bates 47179 (AD); Arckaringa Station, P.J.Lang BSOP-441 (AD); on the Indulkana Range Plateau, P.J.Lang & P.D.Canty BS23–29163 (AD); ~16 km W of Arckaringa Homestead along road to Evelyn Downs, T.S.Te, D.J.Duval & D.E.Murfet 1021 (AD). QUEENSLAND. 7.2 km E of Stonehenge, A.R.Bean 22437 (BRI); 40 km E of Quilpie towards Charleville, A.R.Bean 29976 (BRI); Colwell Mackinlay, D.M.Collings s.n. (BRI AQ0178574); 67 km WNW of Mount Isa and 6 km N of Mingera, P.L.Harris 281 (BRI); Idalia National Park, Hobbs Tank, C.Morgan cm11 (BRI); ~11 km E of Scott’s Tank, Diamantina National Park, M.Mostert MM256 (BRI); Well Flat, 2 km S of Wathopa Homestead, J.L.Silcock JLS1522 (BRI). NEW SOUTH WALES. 5 miles [~8 km] W of Tibooburra, G.M.Cunningham 1164 (NSW).
Key to species of the P. macrocephalus group|
1 Inflorescence rachis glabrous or with a few sparse hairs present |
|
2 Annual or short-lived perennial herb with fleshy taproot; outer sepal <1.2 mm wide; abaxial sepal surface with short appressed hairs on bottom half; hairs absent on adaxial sepal surface; staminal cup hairs absent |
Conflicts of interest
Kevin R. Thiele is an Associate Editor for Australian Systematic Botany. Despite this relationship, he did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making process for their manuscripts. The authors declare that they have no further conflicts of interest.
Declaration of funding
T. A. Hammer acknowledges the support of a Forrest Research Foundation PhD scholarship and University Postgraduate Award (UWA).
Acknowledgements
The authors thank the Directors and staff of the cited herbaria for access to their collections and for loaning material. P. Johan H. Hurter is thanked for early discussions and observations made concerning these taxa. Paul Macintyre is thanked for advice regarding the geospatial component. Graham Goods, Maree Goods, Jill Newland and Roger Fryer are thanked for permission to use their photographs. The authors acknowledge the reviewers for their helpful comments.
References
Bean AR (2008) A synopsis of Ptilotus (Amaranthaceae) in eastern Australia. Telopea 12, 227–250.| A synopsis of Ptilotus (Amaranthaceae) in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bentham G (1870) ‘Flora australiensis: a Description of the Plants of the Australian Territory. Vol. 5.’ (L. Reeve & Co: London, UK) https://doi.org/
Black JM (1948) Amaranthaceae. In ‘Flora of South Australia. Part 2’, 2nd edn. pp. 323–332. (Government Printer: Adelaide, SA, Australia)
Borsch T, Flores-Olvera H, Zumaya S, Müller K (2018) Pollen characters and DNA sequence data converge on a monophyletic genus Iresine (Amaranthaceae, Caryophyllales) and help to elucidate its species diversity. Taxon 67, 944–976.
| Pollen characters and DNA sequence data converge on a monophyletic genus Iresine (Amaranthaceae, Caryophyllales) and help to elucidate its species diversity.Crossref | GoogleScholarGoogle Scholar |
Brown R (1810) Trichinium. In ‘Prodromus: florae Novae Hollandiae et Insulae Van Diemen’. pp. 414–415. (R. Taylor et Socii: London, UK) https://doi.org/
Davis RW, Butcher R (2010) Re-evaluation of Ptilotus polystachyus sens. lat. (Amaranthaceae) and creation of the new combination Ptilotus giganteus. Nuytsia 20, 217–227.
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar | 18027281PubMed |
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation 24, 38–49.
| A review of methods for the assessment of prediction errors in conservation presence/absence models.Crossref | GoogleScholarGoogle Scholar |
Hammer TA (2018) The Ptilotus murrayi species group: synonymisation of P. petiolatus under P. murrayi and description of the new Western Australian species P. unguiculatus (Amaranthaceae). Swainsona 31, 93–100.
Hammer TA, Davis RW, Thiele KR (2015) A molecular framework phylogeny for Ptilotus (Amaranthaceae): evidence for the rapid diversification of an arid Australian genus. Taxon 64, 272–285.
| A molecular framework phylogeny for Ptilotus (Amaranthaceae): evidence for the rapid diversification of an arid Australian genus.Crossref | GoogleScholarGoogle Scholar |
Hammer TA, Davis RW, Thiele KR (2018a) A key to Ptilotus (Amaranthaceae) in Western Australia. Nuytsia 29, 217–227.
Hammer TA, Macintyre PD, Nge FJ, Davis RW, Mucina L, Thiele KR (2018b) The noble and the exalted: a multidisciplinary approach to resolving a taxonomic controversy within Ptilotus (Amaranthaceae). Australian Systematic Botany 31, 262–280.
| The noble and the exalted: a multidisciplinary approach to resolving a taxonomic controversy within Ptilotus (Amaranthaceae).Crossref | GoogleScholarGoogle Scholar |
Kriticos DJ, Webber BL, Leriche A, Ota N, Macadam I, Bathols J, Scott JK (2012) CliMond: global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods in Ecology and Evolution 3, 53–64.
| CliMond: global high-resolution historical and future scenario climate surfaces for bioclimatic modelling.Crossref | GoogleScholarGoogle Scholar |
Kriticos DJ, Jarošik V, Ota N (2014) Extending the suite of Bioclim variables: a proposed registry system and case study using principal components analysis. Methods in Ecology and Evolution 5, 956–960.
| Extending the suite of Bioclim variables: a proposed registry system and case study using principal components analysis.Crossref | GoogleScholarGoogle Scholar |
Mitchell T (1848) ‘Journal of an expedition into the interior of tropical Australia, in search of a route from Sydney to the Gulf of Carpentaria.’ (Longman, Brown, Green and Longmans: London, UK)
Moquin-Tandon A (1849) Amaranthaceae. In ‘Prodromus systematis naturalis regni vegetabilis, vol. 13’. (Ed. ALPP de Candolle) pp. 231–241. (Masson: Paris, France) https://doi.org/
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175.
| Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation.Crossref | GoogleScholarGoogle Scholar |
Phillips SJ, Dudík M, Schapire RE (2004) A maximum entropy approach to species distribution modeling. In ‘Proceedings of the Twenty-First International Conference on Machine Learning’, 4–8 July 2004, Banff, AB, Canada. pp. 655–662. (Association for Computing Machinery: New York, NY, USA)
Poiret JLM (1816) Ptilotus. In ‘Encyclopédie méthodique: Botanique. Suppl. 4’. (Ed. JB Lamarck) pp. 619–620. (Agasse: Paris, France) https://doi.org/
Ronse de Craene LP (2013) Reevaluation of the perianth and androecium in Caryophyllales: implications for flower evolution. Plant Systematics and Evolution 299, 1599–1636.
| Reevaluation of the perianth and androecium in Caryophyllales: implications for flower evolution.Crossref | GoogleScholarGoogle Scholar |
Tang Y, Horikoshi M, Li W (2016) ggfortify: unified interface to visualize statistical result of popular R packages. The R Journal 8, 474–489.
| ggfortify: unified interface to visualize statistical result of popular R packages.Crossref | GoogleScholarGoogle Scholar |
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (Eds) (2018) ‘International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code)’, adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile, vol. 159. (Koeltz Botanical Books: Glashütten, Germany) https://doi.org/
von Mueller FJH (1868) ‘Fragmenta Phytographiae Australiae, Vol. 6.’ (Government Printer: Melbourne, Vic., Australia) https://doi.org/
Vrijdaghs A, Flores-Olvera H, Smets E (2014) Enigmatic floral structures in Alternanthera, Iresine, and Tidestromia (Gomphrenoideae, Amaranthaceae): a developmental homology assessment. Plant Ecology and Evolution 147, 49–66.
| Enigmatic floral structures in Alternanthera, Iresine, and Tidestromia (Gomphrenoideae, Amaranthaceae): a developmental homology assessment.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2016) ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag: New York, NY, USA)