Morphological analysis and a resolution of the Ryparosa javanica species complex (Achariaceae) from Malesian and Australian tropical rainforests
Bruce L. Webber A B and Ian E. Woodrow AA School of Botany, The University of Melbourne, Vic. 3010, Australia.
B Current address: School of Biological Sciences, University of East Anglia, Norwich Research Park, Norwich, Norfolk NR4 7TJ, UK. Corresponding author. Email: b.webber@bigfoot.com
Australian Systematic Botany 19(6) 541-569 https://doi.org/10.1071/SB06001
Submitted: 20 January 2006 Accepted: 5 September 2006 Published: 14 December 2006
Abstract
A study of morphological variation in Ryparosa javanica (Blume) Kurz ex Koord. & Valeton sensu lato (Achariaceae; Flacourtiaceae pro parte) was undertaken after distinct differences were observed between Australian and Bornean populations. The confusing taxonomic history of R. javanica is first summarised. Phenetic techniques of agglomerative classification and ordination were used to analyse herbarium and field-collected specimens. Distinct groupings based on vegetative characters were supported by reproductive traits, plant architecture, ant–plant associations and geographical discontinuities. This work demonstrates that the current circumscription of R. javanica is a complex of at least nine species: R. javanica sensu stricto now confined to Sumatra, Java and Bali; three species that warrant reinstatement: R. amplifolia (K.Sch.) Mildbr. from New Guinea, R. kurzii King from the Andaman and Nicobar Islands and R. wrayi King from southern Myanmar and Thailand, the Malay Peninsula and northern Sumatra; and five new species described as R. maculata B.L.Webber from eastern New Guinea, R. anterides B.L.Webber from eastern Borneo, R. milleri B.L.Webber from New Guinea, R. maycockii B.L.Webber from western Borneo and R. kurrangii B.L.Webber from northern Australia. A key to the species and commonly mistaken taxa is provided.
Introduction
Ryparosa Blume is a small genus of ~19 tropical rainforest tree species placed within the relatively homogeneous tribe Pangieae according to all recent treatments (Hutchinson 1967; Lemke 1988; Takhtajan 1997; Chase et al. 2002). Pangieae have recently been included in Achariaceae sens. lat. (Chase et al. 2002) and are distributed largely in the Austro-Malesian region (Sleumer 1954a), with the only representatives of the tribe from outside this region being the monotypic genus Chiangiodendron T.Wendt (Mexico and Central America; Wendt 1988; Sosa et al. 2003) and Kiggelaria L. (southern Africa; Dahlgren and van Wyk 1986). Ryparosa has its greatest diversity in Borneo and is distributed from Myanmar throughout south-east Asia and New Guinea, with the rainforests of northern Australia forming the south-eastern limits.
Achariaceae sensu Chase et al. (2002) are united by the presence of cyanogenic glycosides, wood with mostly solitary pores and no tracheids, more petals than sepals (without any specific relative orientation of parts) and typically linear anthers, and differ from the Salicaceae sensu Chase et al. (2002) in their lack of a disk and salicoid leaf dentation. Chase et al. (2002) emphasised that Flacourtiaceae, in which Ryparosa was included, had a reputation as being confusing and largely unrecognisable in the field; specimens of Flacourtiaceae are frequently confused with those of Euphorbiaceae (Sleumer 1954a) and are known to have no single character to distinguish them from other families (Lemke 1988). Of the 30 genera previously in Flacourtiaceae incorporated into the revised Achariaceae, 14 are monospecific and Ryparosa is the most speciose genus after Hydnocarpus Gaertn., a south-east Asian taxon of ~40 species. Ryparosa is similar to other Pangieae in having small, unisexual flowers, sepals and petals in separate whorls distinguishable from each other, and petals with a fleshy scale adaxially (Hutchinson 1967; Chase et al. 2002). As a genus, Ryparosa is distinguished by its multi-flowered racemes (perhaps with the exception of R. porcata P.F.Stevens; Jarvie and Stevens 1998), a lack of stipules and bifurcate T-shaped hairs (often with one branch strongly reduced).
Ryparosa was first found in Australia in the mid-1960s in the Cape Tribulation region in far north Queensland. Based on the descriptions of Sleumer (1954a), the specimens collected were assigned to R. javanica (BPM Hyland pers. comm.). This meant that the geographical distribution of Ryparosa javanica sensu Sleumer (1954a) ranged from southern Myanmar and the Andaman Islands, throughout the Malesian islands and as far east as New Guinea and northern Australia. No other species of Ryparosa is known from such a wide region, R. kunstleri (Malay Peninsula, Sumatra), R. hullettii King (Borneo, Malay Peninsula), R. acuminata Merr. (Borneo, Malay Peninsula, Thailand) and R. caesia (Java, Sumatra) being the only other species with relatively widespread distributions (Sleumer 1954a, 1985; for some of these species this is most likely because of incorrect determinations, BL Webber unpubl. data).
Sleumer’s 1954 study (Sleumer 1954a) remains the most recent generic revision of Ryparosa and only one new species, R. porcata from Borneo, has been described in the last 50 years (Jarvie and Stevens 1998). More recently, our interest in the wide geographical range of R. javanica was rekindled during fieldwork on the island of Borneo in 2000. Several localities at which R. javanica populations were supposed to occur in Brunei Darussalam and the Malaysian state of Sabah were visited, but the plants there differed from those growing in Australia. A preliminary examination of herbarium specimens determined as R. javanica sens. lat. indicated that species delimitation in Ryparosa was unsatisfactory and based on unsuitable (i.e. poorly described and incorrectly interpreted) characters. Therefore, the objective of this study is to combine a field-based knowledge of vegetative and reproductive characteristics of Ryparosa javanica sens. lat. with those acquired from preserved herbarium specimens, and to delimit and describe species in a way that accurately reflects patterns of morphological variation. Since Ryparosa individuals in the field generally lack much in the way of reproductive structures, a strong emphasis will be placed on understanding vegetative differences within the species complex.
Historical taxonomy of Ryparosa javanica sens. lat
Ryparosa javanica (Blume) Kurz ex Koord. & Valeton was originally described as Bergsmia javanica Blume, based on specimens collected in 1821 in the Bantam region of West Java by Kuhl and van Hasselt (Blume 1848). Kurz (1873) later recognised the similarity between Blume’s Bergsmia and the genus Ryparosa, with the sole taxon at the time, Ryparosa caesia Blume (placed in the Euphorbiaceae), described by Blume in 1825. Apparently by inadvertence, in the preface of his Flora Javae, Blume (1828) referred to the genus Ryparosa as Ryparia, adding more confusion to the already muddled situation. Subsequently, the family alliance of Bergsmia and Ryparosa (and Ryparia) became uncertain, as Bentham and Hooker (1867) listed Bergsmia in the Euphorbiaceae but later excluded Ryparosa from the same family, as a genus of dubious affinity, making no suggestion as to its most appropriate taxonomic position (Bentham and Hooker 1880). At around the same time, Kurz (1873) merged Bergsmia and Ryparosa into the genus Ryparia, and was the first person to include the genus in the tribe Pangieae in the Bixaceae (Kurz 1873, 1876, 1877; King 1890). In 1890, King and colleagues (King 1890; Brühl and King 1896) cleared up the confusion between Ryparia and Ryparosa, using the latter (and original) name in his description of new species from the Malay peninsula.
All Ryparosa specimens from the Andaman and Nicobar islands were originally determined by Kurz as Ryparia caesia (Kurz 1876, 1877); however, King (1890) thought that Kurz was mistaken, and described these specimens as R. kurzii. In 1900, Koorders and Valeton (1900a) redefined Ryparosa javanica in relation to two other species of Ryparosa – R. caesia and Ryparosa longipedunculata Boerl. However, Koorders and Valeton expressed a lack of confidence in the diagnostic characters they used to distinguish these species, and shortly afterwards they merged R. javanica and R. longipedunculata on account of the type specimen for Ryparia javanica being somewhat ‘abnormal’ (Koorders and Valeton 1900b). Koorders and Valeton (1900b) also merged Ryparosa kurzii King and Ryparosa kunstleri King with R. javanica without any explanation.
The next substantial treatment of the genus (now ascribed to the Flacourtiaceae) was between 1919 and 1925 by van Slooten (1919, 1925), who conducted an extensive revision of the Flacourtiaceae of the Dutch East Indies. In this work, van Slooten resurrected R. kunstleri and concluded that the decision of Koorders and Valeton to combine R. kurzii with R. javanica was most likely incorrect (van Slooten 1919).
By the first half of the 19th century, some 24 species of Ryparosa had been described, but there had been no synthesis of the entire genus. As part of the Flora Malesiana series, Sleumer (1954a) produced a detailed revision of the genus between 1953 and 1958. During this revision, Sleumer described Ryparosa baccaureoides Sleumer and R. kostermansii Sleumer (Sleumer 1954b), from Bornean material that had been identified as R. javanica. Going against the recommendations of van Slooten (1919), Sleumer combined R. kurzii with R. javanica, but left R. kunstleri as a distinct species. Sleumer also decided to merge Ryparosa wrayi King, Ryparosa amplifolia (K.Sch.) Mildbr. and R. caesia from Dutch New-Guinea (van Slooten 1924; based on one specimen) with R. javanica, despite these species having several distinct differences from R. javanica sens. str. For example, Sleumer recognised that R. amplifolia had nearly twice the number of leaf secondary veins to R. javanica (Sleumer 1954a), yet failed to mention that descriptions of the former species had consistently noted swollen stems with ant domatia and a dense indumentum on the abaxial leaf surface (Schumann and Lauterbach 1901; Gilg 1918; Mildbraed 1928).
Ryparosa wrayi was first described by King (1890) and the species was retained by van Slooten in his revision of the genus (van Slooten 1919, 1925). Gertrudia amplifolia K. Sch., of the monotypic Gertrudia described by Schumann and Lauterbach (1901), was originally placed in the Flacourtiaceae–Pangieae–Hydnocarpinae, close to Trichadenia because it had ‘single ovules on the placenta’. Gilg (1918) questioned this alignment of G. amplifolia, based on an examination of flowers that were subsequently shown to belong to Scleromelum aurantiacum K. Schum. & Lauterb. (= Scleropyrum aurantiacum Pilg.), hardly surprisingly concluding that Gertrudia belonged neither to Flacourtiaceae nor Euphorbiaceae. Mildbraed (1928) recognised that Gilg’s (1918) description was based on a mixed collection and combined Gertrudia with Ryparosa, as Ryparosa amplifolia (K. Sch.) Mildbr., based on the presence of bifid hairs (unique to Ryparosa in the Flacourtiaceae), an irregularly rupturing calyx and petals with a hairy scale adaxially. At the same time he described Ryparosa calotricha Mildbr. (Mildbraed 1928).
Sleumer (1954a) created a rather broad taxon description to accommodate all the species he merged with R. javanica. He described R. javanica sens. lat. as being a dioecious tree between 15 and 30 m in height, with small flowers on racemes from cauline tubercles, leaf axils or defoliate branchlets, and leaf blades between 120 and 450 mm long with a glabrous or hairy abaxial surface and between five and nine secondary vein pairs. Unfortunately, to those unfamiliar with Ryparosa, it created the situation where almost any specimen could be keyed out to R. javanica. Furthermore, the only two images of R. javanica (tree III-F-15 from the Bogor Botanic gardens) included in his account in the Flora Malesiana were not only uninformative in terms of characters used to delineate the species, but the figure legends were also incorrect.
It seems as if Sleumer’s decision to unite R. javanica, R. kurzii R. wrayi and R. amplifolia was primarily on account of the staminal column being pilose in the upper half in staminate flowers, in contrast to the glabrous staminal columns described for other Ryparosa species (Sleumer 1954a). He described the indumentum on the vegetative plant parts as being caducous and placed a low emphasis on the importance of the inflorescence position on a tree, although he noted that the characters used for specific distinctions in Ryparosa were difficult. Moreover, Sleumer chose not to describe four apparently new species because the specimens available were inadequate, and instead determined these specimens as R. javanica.
Materials and methods
Collections
Material was borrowed from 15 herbaria (BM, BO, BRI, CANB, K, KEP, L, LAE, MEL, MELU, NSW, NY, QRS, SING, UPNG; herbarium abbreviations following Holmgren et al. 1990) and comprised 304 specimens currently determined as Ryparosa javanica sens. lat., 19 undetermined Ryparosa and 15 specimens of other Ryparosa species. These specimens were collected between 1821 and 2003 throughout south-east Asia from southern Myanmar and the Andaman Islands, throughout the Indo-Malesian islands, New Guinea and northern Australia. Many old specimens are in a very poor condition and most of specimens have few flowers or fruits. Most specimens had also been seen by Sleumer in his revision of Malesian Flacourtiaceae in the 1950s (Sleumer 1954a). Eight recently collected specimens that had not been seen by Sleumer and that were clearly not Ryparosa (five sheets determined as R. javanica, three sheets determined as Ryparosa sp.), were excluded to eliminate the coding of unnecessary characters. However, two specimens from L that were very unlikely to be Ryparosa, but had been clearly determined as R. javanica by Sleumer in 1974 (Balakrishnan & Bhargava, 3465; Nair, 3639), were included.
Specimen mapping
To determine accurately distributions, geographical information for collection locations was collated. Because many specimens had been collected in the first half of last century, very few specimens had latitude and longitude information, and many had poorly written or limited location data. Geographical coordinates for such specimens were collected from associated maps and gazetteers (e.g. Australian Army Royal Australian Survey Corps 1942–1945; United States Army 1943–1946; Royal Army of Great Britain Royal Engineers 1969; Rosenberg 1996–2004; Spot Domain 2000–2005), compared against expedition schedules, itineraries and collecting locality details for early south-east Asian taxonomists (e.g. Koorders-Schumacher 1913; Van Steenis 1958; Van Steenis-Kruseman 1958) and cross-referenced against Google Earth software (Google Inc. 2005).
Morphometric analysis
A list of 68 vegetative and reproductive characters was assembled to code the specimens. These characters were based on observations made during six years of fieldwork on Ryparosa, the early works of van Slooten (1919, 1925) and the revision of Sleumer (1954a). Several characters used in earlier revisions were deemed inappropriate and excluded, based on field knowledge of the genus and an initial inspection of the material. First, all characters based on specimen colour were confounded by the wide range in specimen age and preservation techniques. Second, stem lenticels are easily confused as food-body scars, eliminating lenticels as a useful character. Food-body production in Ryparosa has recently been discovered (Webber 2005) and the ant–plant associations of the genus (including specialised stem domatia, food bodies and hemipteran trophobionts) will be described in detail elsewhere (Webber et al. 2006, 2007). Third, several characters have been observed to change during leaf expansion and toughening (Webber 2005), so only specimens with mature foliage were measured. Lastly, reproductive characters were significantly under-represented relative to all others owing to dimorphous flowers and the distinctive reproductive phenology (e.g. widespread flowering only every 2–3 years, staminate and pistillate racemes temporally separated on individual trees; Webber 2005) of Ryparosa. Hairs on the fruit epicarp surface were commonly abraded on older specimens and seemed to show strong ontogenetic variation. Standardising the considerable developmental variation in reproductive characters was difficult, and while this was helped by stratification (based on raceme maturity), it only served to further reduce the number of specimens with precisely defined reproductive characters in common.
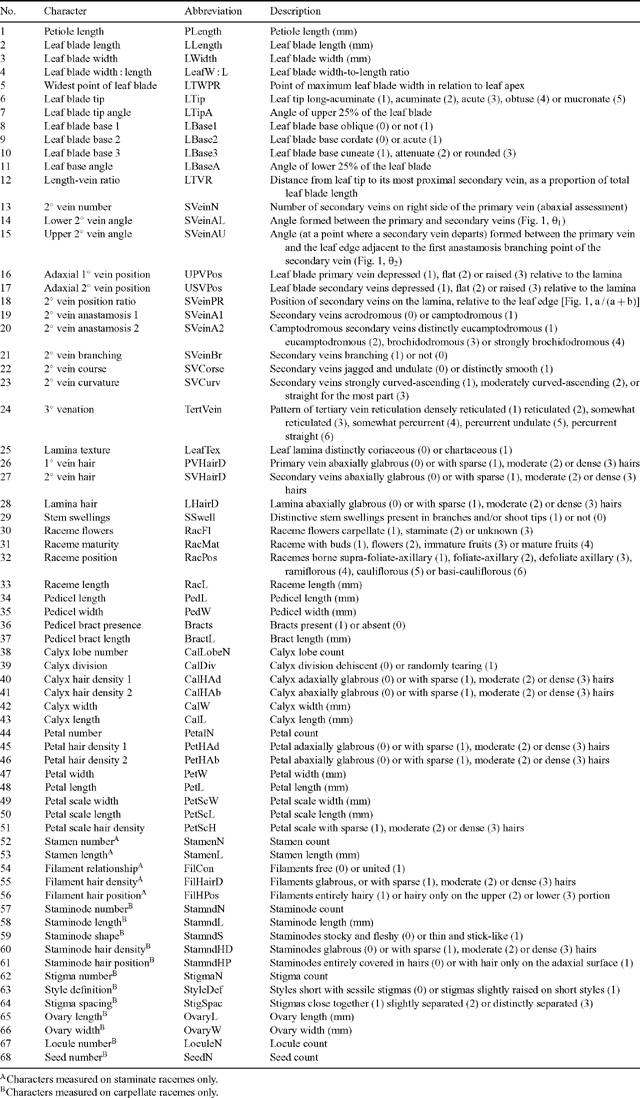
The final character list contained 65 individual characters and three derived ratios (Table 1). These were composed of 25 continuous quantitative, eight discrete quantitative and 35 discrete qualitative characters. All characters were expressed quantitatively where reasonably possible, to avoid misrepresenting the potential range of variation (Stevens 1991; Thiele 1993). Terminology and character definitions follow that of Radford et al. (1974) and Hickey (1979). Leaf venation characters (14–24, Table 1; after Hickey 1979) and hair density characters (26–28, Table 1) were recorded at a point closest to one-third of the distance from the leaf base. However, these characters were consistent and held for the entire lamina surface, with the exception of the angles of the most apical and basal secondary veins. All measurements were measured in laboratory conditions using a dissecting microscope (magnification 20×) to score hair and food-body characters. The mean value for up to three measurements of each character was recorded for each specimen (less when the specimen did not provide enough material).
|
Phenetic analyses
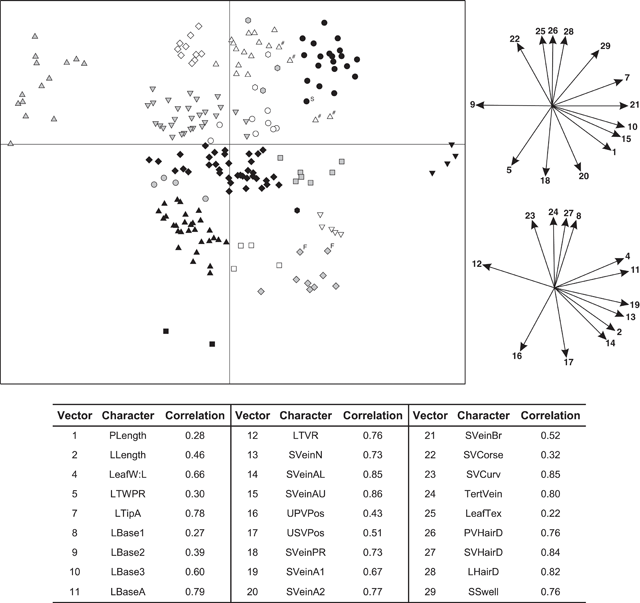
The final data matrix used in phenetic analyses included 206 specimens (R. javanica sens. lat., n = 182; other Ryparosa species, n = 14; undetermined Ryparosa, n = 10) measured for 27 characters (characters 1, 2, 4, 5, 6 to 29; Table 1). Leaf width (character 3) was not included as a character as it was highly correlated with leaf length (Pearson correlation 0.77). Moreover, the two ratios leaf width : length (character 4; Table 1) and leaf widest point (character 5; Table 1) gave a better indication of overall leaf shape. Leaf tip shape (character 6; Table 1) was also excluded owing to missing data on many specimens and difficulties in accurately describing the observed variation. Given the extremely limited amount of data that could be collected from the available specimens for reproductive characters, it was decided to remove these characters (characters 30–68; Table 1) from any phenetic analyses and, rather, present this data as supplementary information. The PATN software package (Belbin 1995) and the Gower metric distance measure (Gower 1971) were used to construct a dissimilarity matrix for subsequent cluster and ordination analyses. The Gower metric is a range-standardised coefficient of similarity (Sneath and Sokal 1973), and is applicable to a combination of both continuous and discrete characters, as in this dataset. Both cluster and ordination analyses were performed with the PATN software package (Belbin 1995). The flexible unweighted pair group method of averaging (UPGMA; Sokal and Michener 1958) was used to produce a hierarchical, agglomerative classification (β = –0.25; Sneath and Sokal 1973). Ordinations of the dataset were produced in two and three dimensions using the non-metric multidimensional scaling technique (NMDS; Shepard 1962; Kruskal 1964a, 1964b; Faith et al. 1987). Five ordination analyses were performed using random starting configurations and the ordination with the lowest stress value (i.e. the best ‘fit’) after 100 iterations was used. Correlations of characters with the ordinations were examined by plotting directions of best fit of the character vectors. Correlation coefficients of vectors with the ordinations were calculated to provide an estimation of the fit of each vector to the observed pattern (Belbin 1995).
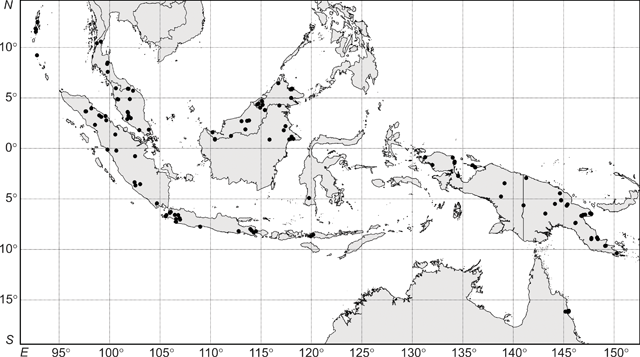
The interpretation of these phenetic analyses, including designation of groups and clusters, even if somewhat arbitrary, was informed by a detailed appraisal of the available data and how they fit with (1) current species descriptions and those of previously recognised species; (2) observed geographical and morphometric discontinuities in the specimens at hand; and (3) any other supporting data not included in the morphometric analyses (i.e. reproductive data). Thus, the 18-group level was chosen as that at which groups would be recognised in the UPGMA analysis (Fig. 3). For these reasons, also, interpreting the data at any other level created groupings that did not support the clusters apparent in the ordination (Fig. 4).
|
|
|
|
Results
Vegetative characters of Ryparosa javanica sensu lato
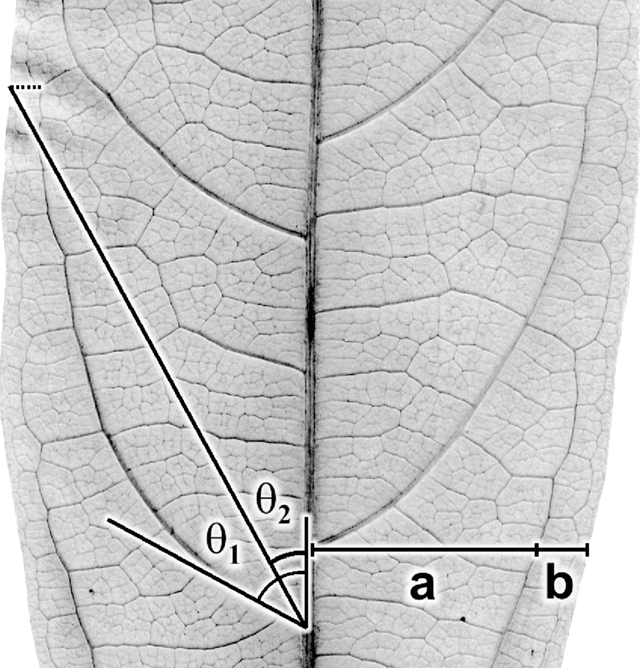
The geographical range (Andaman Islands to northern Australia) of Ryparosa javanica sens. lat. specimens included in this study covered the entire species distribution described by Sleumer (1954a; Andaman Islands to New Guinea; Fig. 2), over an altitudinal gradient of 5–1450 m, and included trees between 1.2 and 40 m in height that varied considerably in many vegetative attributes (Accessory Publication, Appendix 1; available online at the website of Australian Systematic Botany). Thus, the leaf lamina ranged from being entirely glabrous to densely strigose on the abaxial surface, from 86 to 373 mm in length, and in having between 2 and 11 secondary veins. Venation was acrodromous to camptodromous, with a range of eucamptodromous to clearly brochidodromous types. Leaf blade shape ranged from ovate to distinctly obovate, and lamina bases were oblique, cordate or rounded.
Phenetic classification
An agglomerative classification based on 206 herbarium specimens scored for 27 vegetative characters had a stress level of 0.0147 (merged at a dissimilarity of 0.1; Fig. 3). Specimens were examined at the 18-group level (dissimilarity = 0.25) and the difference between groups was significant (P < 0.001). Distinct differences were observed between groups for many measured characters (Appendix 1). Ordination analyses on the same dissimilarity matrix in two dimensions showed distinct (occupying a contiguous area of morphometric space) clustering of specimens (Fig. 4); adding a third dimension resulted in little improvement in the stress values (data not shown). Character vector correlations for the ordinations ranged from 0.22 to 0.86 (Fig. 4). There was no obvious trend between the magnitude of correlation and the type of character for the 27 characters included in analysis (Fig. 4). The clustering of groups in the ordination analysis (Fig. 4) closely corresponded to those groupings generated in the cluster analysis (Fig. 3).
In the UPGMA analysis, there were four distinct clusters of groups – Groups 1–6, Groups 7–9, Groups 10–16 and Groups 17 and 18 (Fig. 3). Group 1, referred to as R. javanica sens. str. contained 37 specimens and included the type specimens for Bergsmia javanica and Ryparosa longipedunculata (Fig. 3). In the ordination analysis, these specimens formed a distinct cluster (Fig. 4). These specimens covered a geographic range from Bali through Java to Sumatra. Group 1 was the most morphologically variable of the groups; leaf blades varied considerably (mean ± 1 s.e., range) in length (241 ± 9.6, 111–317 mm) and width (80 ± 3.3, 37–115 mm), but were elliptic with an attenuate base. Leaf venation was consistently eucamptodromous with six (rarely five or seven) curved-ascending veins, while hairs on the abaxial blade were sparse, particularly away from the veins (Appendix 1).
Group 2 from the cluster analysis, referred to as R. kurzii sens. str., was situated next to, but distinct from, Group 1 in the ordination analysis (Figs 3, 4). Group 2 included seven specimens; all specimens were originally identified as R. kurzii and collected from the Andaman and Nicobar Islands. The leaf blades were ovate-elliptic with a base angle of 69–78°. Like R. javanica sens. str., R. kurzii sens. str. had curved-ascending, eucamptodromous secondary venation with sparse hairs on the abaxial blade; however, the number of secondary vein pairs was generally six or seven and sometimes eight (Appendix 1).
In the cluster analysis, Group 3 included four specimens from New Guinea; two determined as R. calotricha, the other two as Ryparosa sp. (Fig. 3). In the ordination analysis, two of these specimens clustered as outliers to specimens corresponding to Group 8, while the other two were aligned near specimens corresponding to Group 7 (hashed data points; Fig. 4). Group 8 from the cluster analysis consisted of 15 specimens; one determined as R. calotricha and nine determined as R. aff. javanica, which also formed a group in the ordination analysis (Figs 3, 4). These specimens were collected in New Guinea, with the exception of one specimen from Sumatra. Group 7 in the cluster analysis consisted of 20 specimens; three determined as R. amplifolia sens. str. (including the type specimen for Gertrudia amplifolia) from New Guinea and the sole specimen of Ryparosa collected from Sulawesi. Also aligning with Group 8 in the ordination were the four specimens from Group 4 in the cluster analysis (Fig. 4). Collected in the 1980s, three of these specimens were from Borneo and determined as ‘R. cf. javanica (closest to New Guinea forms)’, while the fourth specimen was from Sumatra.
Taking into account both cluster and ordination analyses, as well as the condition of the specimens, the following taxon groupings are proposed for the specimens described above. Groups 3 and 8 (with the exception of the Sumatran specimen) from the cluster analysis will be referred to here as R. calotricha. Both groups had very similar vegetative characteristics, differing only in leaf blade width : length (0.46 ± 0.014 for Group 3 compared with 0.35 ± 0.007 for Group 8; mean ± 1 s.e.) and absence of shoot tip stem swellings (in Group 3; Appendix 1). Leaf blades were elliptic-obovate and generally 155–200 mm in length with (6–)7–8(–9) secondary vein pairs. Venation was eucamptodromous and secondary venation straight for the most part, with percurrent tertiary venation, and the entire abaxial surface of the leaf blades was densely covered in short adpressed hairs, particularly so on the primary and secondary veins. Moderately swollen hollow stems, sometimes with small round entry holes, were found on some R. calotricha specimens (Appendix 1).
Group 7 (R. amplifolia sens. str.; containing the type specimen for G. amplifolia) from the cluster analysis was characterised by large, distinctly obovate leaf blades, up to 370 mm in length. Specimens generally had acute-mucronate leaf tips and (7–)8–9 pairs of secondary veins. Distinctive hollow stem swellings with small round entry holes were commonly found at shoot tips and in branches (Appendix 1). Similar to R. calotricha specimens, the abaxial leaf blade had a dense indumentum of short adpressed hairs.
Vegetatively, the three Bornean specimens from Group 4 were very similar to specimens classed as R. calotricha; the most notable difference was that the primary vein was depressed relative to the adaxial leaf blade. Stem swellings were not present in any of these specimens, although non-swollen portions of the stem appeared hollow and had round entry holes. These specimens will be referred to as R. sp. nov. aff. calotricha, as given the limited information available, it would be premature to recognise them as a new taxon.
The two Sumatran specimens from Groups 4 and 8 were very similar in vegetative appearance, and may have clustered in separate groups on account of only one specimen (Laumonier & Torquebiau, TFB 4245) having swollen shoot tips, the other (Burley & Tukirin et al., 1223) not. Leaf blades were again, somewhat shorter than average (150–200mm) with between six and eight pairs of secondary veins (Appendix 1). The specimens were most clearly defined by their prominent tertiary venation, which was straight percurrent and distinctly perpendicular to the primary vein. This is a character unique to these specimens and was not scored in this study. Based on a detailed analysis of material not included in the phenetic analyses, these Sumatran specimens compare closely to specimens identified as R. multinervosa Slooten, and will here be referred to as such.
Group 5 from the cluster analysis consisted of eight specimens from New Guinea, two determined as R. javanica, the rest as Ryparosa sp. In the ordination analysis, these specimens clustered adjacent to, and somewhat overlapping with, specimens that formed Group 6 (see below) in the cluster analysis (Fig. 4). Group 5 specimens had relatively small (108–172 mm long) obovate leaf blades with five or six secondary vein pairs. Short adpressed hairs on the abaxial leaf blade were moderately dense on the primary and secondary veins and somewhat sparse on the lamina (Appendix 1). Group 5 specimens were distinctly different from all other Ryparosa specimens, yet uniform as a group, and will be referred to as R. taxon A.
Group 6 from the cluster analysis consisted of 29 specimens from Myanmar, Thailand, the Malay Peninsula and northern Sumatra (Fig. 3). This group included the type specimen for R. wrayi and nine specimens originally determined as R. wrayi, and will therefore be referred to here as R. wrayi sens str. In the ordination analysis, this group of specimens formed a cluster adjacent to, but distinct from, R. javanica sens. str. specimens, and overlapped slightly with R. taxon A specimens from New Guinea (Fig. 4). Vegetatively, R. wrayi sens. str. had elliptic leaf blades (177–336 mm long) with five (rarely four or six) widely spaced secondary vein pairs (the most apical rather distant from the leaf tip) and a moderate covering of short adpressed hairs abaxially. Three specimens were observed to have hollow stems with small round entrance holes; however, these were not associated with specific stem swellings (Appendix 1).
Ten specimens from southern Borneo formed Group 9 in the cluster analysis (Fig. 3) and a discrete group in the ordination analysis (Fig. 4). These included specimens determined as R. javanica (n = 5), R. aff. javanica (n = 3) and R. cf. javanica vel sp. nov. (n = 2). Specimens were characterised by generally smaller (165 ± 10.7 mm long; mean ± 1 s.e.) elliptic leaf blades, with a moderate covering of short adpressed hairs abaxially. Secondary veins were straight for the most part and consisted of six (rarely five or seven) pairs, the most apical rather distant from the leaf tip. Shoot tips and stems were commonly swollen and hollow with small round entry holes (Appendix 1). Given the lack of association with known Ryparosa taxa and the uniformity of Group 9, these specimens will be referred to as R. taxon B.
Group 10 of the cluster analysis, referred to as R. baccaureoides, was composed of four specimens from Borneo, none of which had been examined by Sleumer, and one specimen determined as R. baccaureoides. These specimens were loosely grouped in the ordination analysis (Fig. 4), but distinct from other clusters. A comparison of these specimens with high-resolution scans of specimens from K supported the link to R. baccaureoides. Leaf blades from these specimens were distinctly coriaceous, with eight brochidodromous secondary vein pairs and a sparse scattering of short adpressed hairs abaxially (Appendix 1).
The two specimens that formed Group 11 in the cluster analysis (Fig. 3) were distinct in the ordination space from all other specimens (Fig. 4). One specimen was originally determined as Hydnocarpus castanea Hook.f. & Thomson (Achariaceae; Flacourtiaceae pro parte), and the distinctly oblique leaf base with ovate-lanceolate leaves indicated that this species was not Ryparosa. Leaves were entirely glabrous and had 8–10 pairs of secondary veins that were curved-ascending but distinctly undulate (Appendix 1). A comparison of these specimens to others not included in the phenetic analyses, confirmed that these specimens were Drypetes longifolia Pax & K.Hoffm. (Putranjivaceae, formerly Euphorbiaceae).
Five specimens collected in New Guinea, all determined as R. cf. javanica, formed Group 12 in the cluster analysis (Fig. 3) and a discrete group in the ordination analysis (Fig. 4). These specimens had leaves with very short petioles (10–11 mm), a distinctly rounded leaf blade base, seven or eight pairs of secondary vein that are distinctly undulate and sometimes branching, and a sparse scattering of short adpressed hairs on the abaxial primary and secondary veins (Appendix 1). These specimens will be referred to as R. taxon C, given their lack of association with known Ryparosa taxa and the uniformity of the group in analyses.
Group 13 in the cluster analysis comprised nine specimens, seven from Borneo (five determined as R. aff. javanica and one as R. aff. glauca) and two from Flores (determined as R. javanica). In the ordination this group of specimens was loosely clustered, with those specimens from Flores (having slightly smaller leaf blades than the Bornean specimens) as outliers on one side and a specimen in poor condition from Brunei as an outlier towards R. baccaureoides (Fig. 4). Leaves of this group were entirely glabrous, had 8–10(–11) pairs of brochidodromous secondary veins that were strongly curved-ascending and diverging at a wide angle to the primary vein (58–74°; Appendix 1). Given the strong similarity to vegetative characteristics of specimens of R. glauca Ridl. not included in the phenetic analyses, Group 13 will be referred to as R. glauca.
Group 14 in the cluster analysis consisted of four very distinct specimens from central Borneo (Fig. 3). These specimens separated distinctly in the ordination space (Fig. 4) and had leaves with a shallowly cordate blade base (a character not previously recorded for Ryparosa) and seven or eight (rarely nine) brochidodromous secondary vein pairs. The leaf abaxial surface had a dense covering of short adpressed bifid hairs, and smaller branches were commonly hollow with minor swellings (Appendix 1). Given the unique characters seen in these specimens and a lack of association with other known Ryparosa taxa, these specimens will be referred to as R. taxon D.
Group 15 in the cluster analysis was composed of four specimens from Borneo (currently determined as R. javanica) and one specimen from New Guinea (determined as R. sp. nov. aff. javanica). The four Bornean specimens were loosely clustered adjacent to R. javanica sens. str. in the ordination analysis, while the specimen from New Guinea was somewhat isolated in the ordination space (Fig. 4), but closest to specimens identified as R. taxon C. The four Bornean specimens were characterised by rather thin leaves with (5–)6 pairs of reasonably straight-ascending secondary veins. Hairs on the abaxial leaf blade were moderately dense on the primary and secondary veins and somewhat sparse on the lamina (Appendix 1). A comparison of the Bornean specimens to others not included in the phenetic analyses confirmed that these specimens were vegetatively similar to R. acuminata, and will be referred to as such here. The sole specimen from New Guinea was in quite poor condition, and due to its uncertain affinities with other Ryparosa taxa and a lack of similar specimens for comparison, will be referred to as R. sp. nov. aff. acuminata.
Group 16 of the cluster analysis comprised 29 specimens from northern Australia, all currently determined as R. javanica (Fig. 3), which clustered as a discrete group in the ordination analysis (Fig. 4). Specimens from Group 16 had narrow leaves (W : L 0.21–0.29) with seven or eight (rarely nine) pairs of secondary veins that were brochidodromous and curved-ascending. Hairs on the abaxial leaf blade were moderate in density on the primary and secondary veins and sparse on the lamina (Appendix 1). Given their lack of association with other known Ryparosa taxa, and the uniformity of specimens within the group, these specimens will be referred to as R. taxon E.
Groups 17 and 18 in the cluster analysis separated at a very low level from the other 16 groups (Fig. 3), and formed an isolated cluster in the ordination analysis (Fig. 4). Vegetatively, the only distinct difference between both groups was the branching of leaf secondary veins in Group 17 specimens and a slightly higher hair density on the abaxial leaf blade (Appendix 1). Specimens from both groups had imperfect, suprabasal, acrodromous venation, small leaf blades 80–160 mm long, short petioles 10–21 mm long and two or three (rarely four) secondary vein pairs (Appendix 1). Group 17 included the type specimen for R. kostermansii, and seven specimens currently identified as R. kostermansii were divided between both groups; therefore, both groups will be referred to here as such.
Reproductive morphology
In the following section the final groupings of specimens described above will be referred to as ‘taxa’. Reproductive material from specimens that formed the basis for this study was severely limited in many aspects. Only three specimens had racemes of both staminate and pistillate flowers, and several specimen labels specifically describe the plant collected as being dioecious. Rather than conducting morphometric analyses on what would be a very small and rather uninformative dataset, we decided to present the available data in tabulated form (Accessory Publication, Appendix 2; available online at the website of Australian Systematic Botany). This information was gathered from 39 reproductive characters and arranged based on the taxa ultimately derived from the phenetic analyses. The description of fruit epicarp characteristics was not included in the final dataset, due to distinct developmental variation observed across all Ryparosa taxa: immature fruits were covered in a dense indumentum of adpressed hairs, but were generally glabrous when mature.
The reproductive features of Drypetes longifolia specimens were distinctly different from those of Ryparosa specimens, with fasciculate inflorescences arising from small ramiflorous tubercles. Fruits from D. longifolia had much longer pedicels (13–19 mm), two locules, and slightly stalked stigmas in the shape of a bow tie.
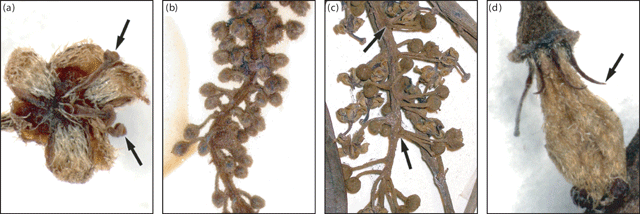
Some of the 39 reproductive characters measured were constant in all Ryparosa taxa (Appendix 2). The calyx ruptured irregularly into three (sometimes two or four) segments, often remaining incompletely divided until the flower was fully open. Staminate and carpellate flowers had a flattened scale, densely covered in long hairs, adaxial in each petal. Staminate flowers, for the most part, had filaments united in a column with extrose anthers that undergo dehiscence along a vertical slit. The one exception to this was two specimens identified as R. kostermansii, which had three free filaments (Fig. 5a). All carpellate flowers had strongly reduced staminodes, almost always without antherode formation. There were, however, several unusual flowers on the racemes of two specimens of R. taxon E, which had partially formed, but apparently sterile anthers.
|
Several distinct differences in reproductive traits were observed between Ryparosa taxa. Racemes were found in a range of positions from axillary to arising from large tubercles on the main trunk. Ryparosa acuminata, R. kostermansii, R. amplifolia, R. taxon A and R. taxon C most commonly had racemes in the axils of leaves, while R. javanica sens. str., R. calotricha, R. kurzii, R. wrayi and R. taxa B and D usually had racemes arising from defoliate axils. While several taxa had ramiflorous racemes from small tubercles, this was most common in R. glauca. Cauliflorous racemes arising from tubercles on the main trunk were observed in only three species: the lower portion of the main trunk was the predominant raceme position for R. baccaureoides and R. taxon E, while some R. glauca specimens also had cauliflorous racemes (Appendix 2).
Raceme length was highly variable between specimens within each Ryparosa taxon and also differed between staminate and carpellate racemes. Maximum staminate raceme length exceeded 300 mm in R. javanica sens. str., R. acuminata, R. wrayi and R. taxon E, while for R. baccaureoides, R. calotricha, R. glauca, R. kostermansii and R. kurzii it was between 150 and 300 mm. In R. taxa A and B, maximum staminate raceme length did not exceed 150 mm (Appendix 2). In general, carpellate racemes were shorter than staminate racemes, particularly when bearing fruits. Only two species had carpellate racemes exceeding 300 mm (R. wrayi and R. taxon E); in most they were less than 150 mm long. Indumentum on the raceme and abaxial calyx was generally adpressed puberulent and somewhat sparse. The exceptions to this were R. acuminata, which had a densely hirsute indumentum (Fig. 5b), and R. taxon D, in which the indumentum was densely pilose. Both R. calotricha and R. amplifolia had racemes in which the flower pedicels appear to be clumped in whorls on the raceme (Fig. 5c), a character particularly obvious at the bud stage (Appendix 2).
Perianth parts of carpellate flowers tended to be larger than those of staminate flowers, while overall flower dimensions of R. kostermansii specimens were generally smaller than average and those of R. taxon E specimens were generally larger than average (Appendix 2). Ryparosa glauca and R. wrayi had distinctively long pedicels (6.8 mm and 7.0 mm respectively) in carpellate flowers. Hair density on the filament tubes of staminate flowers showed some variation within species and even between different flowers on the same raceme (Appendix 2). Filaments of R. acuminata and R. kostermansii were consistently glabrous, while those of R. javanica sens. str., R. glauca, R. kurzii and R. taxon B were sparsely villous. The filaments of R. baccaureoides (sparsely villous in the upper portion), R. calotricha (entirely glabrous or sparsely villous in the upper portion) and R. taxon E (sparsely villous or only in the upper portion) were more or less intermediate.
The staminodes of carpellate flowers varied in length, stature and density of hair coverage (Appendix 2). Staminodes were either thin filiform (R. javanica sens. str., R. calotricha, R. kurzii, R. wrayi, R. taxa A and B) or fleshy and stocky (R. glauca, R. amplifolia, R. taxa C and E, and R. javanica sens. str. specimens from Bali). Staminodes of R. kurzii were longer than average (1.1–1.3 mm) and had a distinctively pointed tip (Fig. 5d). Most species groupings had staminodes that were pilose on the adaxial surface and glabrous on the abaxial surface. However, R. calotricha, R. taxa A and E (sometimes entirely glabrous), together with R. glauca and R. taxon C (entirely pilose) were somewhat intermediate.
Fruits of Ryparosa specimens studied commonly contained two seeds in one locule, occasionally divided by a false septum (Appendix 2). Ryparosa javanica sens. str., R. kostermansii and R. taxon E sometimes had fruits containing one seed, while R. calotricha and R. taxon A had fruits with three seeds. Stigmas were generally sessile and situated close together on flowers and fruits across all Ryparosa species groups. However, R. wrayi had stigmas that were slightly stalked, and R. multinervosa had stigmas distinctly separated on immature fruits.
Other supporting characters
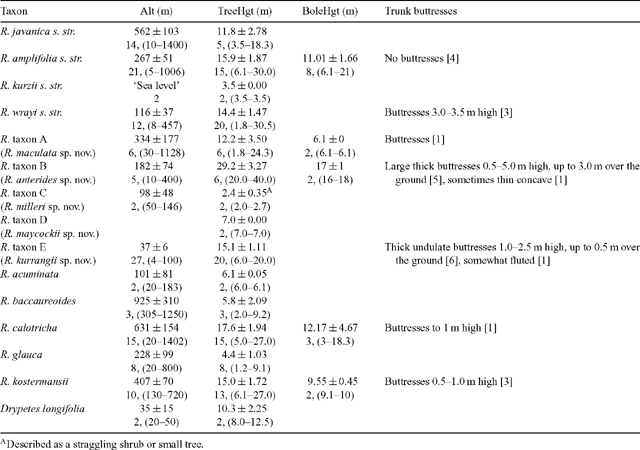
Information, largely absent from older specimens, was collated from labels according to taxa defined from the phenetic analyses, and there were some obvious differences between species groupings (Table 2). Many of the taxa were collected over a considerable altitudinal gradient, R. javanica sens. str., R. baccaureoides, R. calotricha, R. amplifolia and R. taxon A all being collected below 100 m and also above 1000 m (with the exception of R. baccaureoides; Table 2). R. javanica, R. calotricha and R. kostermansii were collected more commonly above 400 m, while R. amplifolia, R. wrayi and R. taxa A, B and E were more commonly collected below 400 m.
|
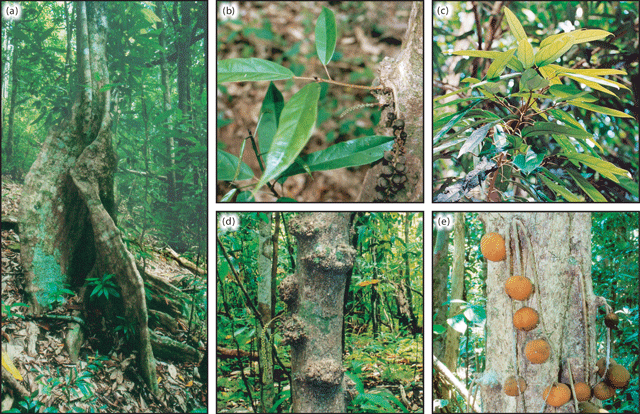
Tree height varied considerably between species groupings, from large trees of 40 m in height to straggling trees of 2 m (Table 2). Ryparosa taxon B specimens were distinctly larger than any other species, having an average height of 29 m (maximum of 40 m). Five other species, R. calotricha, R. kostermansii, R. amplifolia, R. wrayi and R. taxon A, exceeded 20 m in height, although on average, specimens of these species were between 15 and 20 m tall. Ryparosa acuminata, R. baccaureoides, R. glauca, R. kurzii and R. taxon D specimens were not recorded as being more than 10 m tall, and R. taxon C was up to only 3 m tall. The only species that was frequently described as having a distinct bole (between 6–21 m long) was R. amplifolia, while buttresses were observed in R. calotricha, R. kostermansii, R. wrayi and R. taxa A, B and E (Table 2; Fig. 9). Buttressing was prominent in a large proportion of specimens of R. taxa B and E, extending up to 5 m high and 3 m over the ground in the former. Distinctive stem swellings in shoot tips or young stems, commonly hollow with small circular entry holes, were present in R. amplifolia, R. calotricha, and R. taxon B. In R. amplifolia, shoot tip swellings and entry holes were usually associated with slit-like prostomata. The extent of ant–plant associations in Ryparosa appear to be a critical part of understanding taxonomic groupings in the genus and will be discussed in detail elsewhere (Webber et al. 2006, 2007).
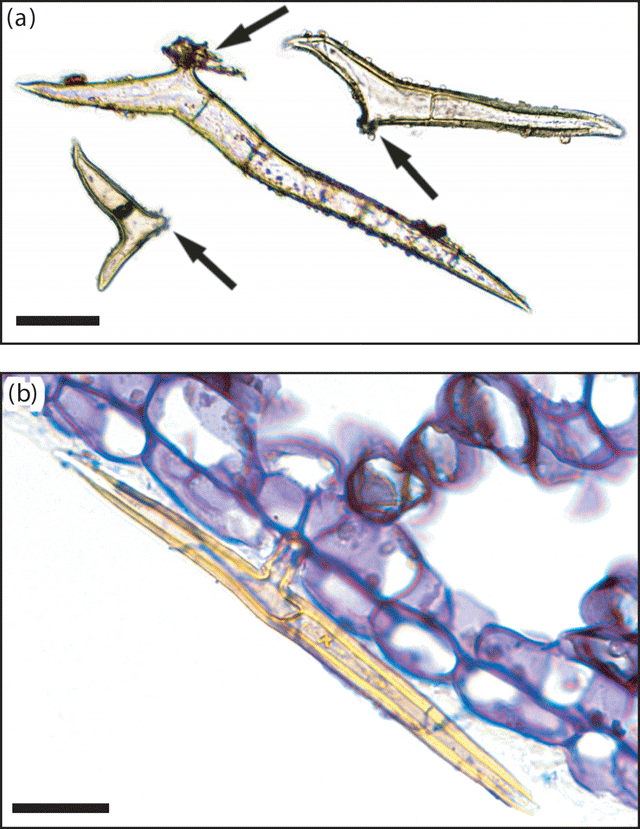
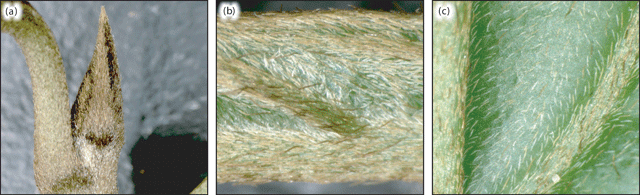
Multicellular, bifurcate hairs were observed on representative specimens from all taxa defined by the phenetic analyses, with the exception of Drypetes longifolia (Fig. 6a). In situ, these T-shaped hairs were generally adpressed, with one arm distinctly longer than the other (Fig. 6b). Variation in the magnitude of difference between hair arm lengths differed both within specimens and between taxa. In all Ryparosa specimens, hairs on the abaxial leaf blade were very short, up to 0.7 mm in length on the primary and secondary veins and ~0.2–0.3 mm on the lamina. Hairs were generally visible to the naked eye only when present at a high density, for example, on young shoot tips and unexpanded leaves or on the abaxial leaf blades of R. amplifolia, R. calotricha, and R. taxon D (Fig. 7). In all other taxa, a hand lens of suitable magnification was required to see the hairs.
|
|
Geographical distribution
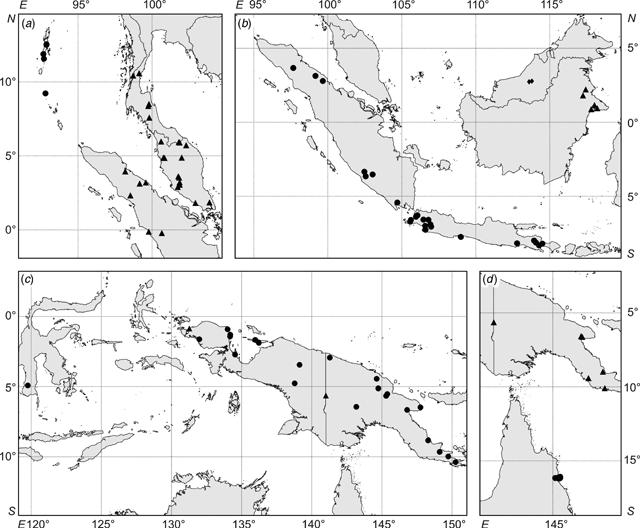
The distributions of taxa recognised in the phenetic analyses were geographically circumscribed, incorporating data from additional specimens not used in the phenetic analyses (because they were so poor but nevertheless could be identified to species; R. amplifolia, n = 8; R. calotricha, n = 2; R. glauca, n = 2; R. javanica sens. str., n = 7; R. wrayi, n = 3). Importantly, this added data did not extend the distribution range for any taxon. Overall, distinct geographical discontinuities were noted among taxa (Fig. 8).
|
|
Ryparosa wrayi is distributed predominantly on the Malay Peninsula and also southern Thailand and Myanmar (Fig. 8a). In northern Sumatra it overlaps with the northern limits of R. javanica sens. str., which has a distribution range restricted to Sumatra, Java and Bali (Fig. 8b). The two R. multinervosa specimens were both from central Sumatra, while R. kurzii is found only in the Andaman and Nicobar Islands (Fig. 8a). The island of Borneo, particularly Sabah, contains the highest diversity of species groupings. Ryparosa acuminata, R. baccaureoides, R. glauca and R. kostermansii collection locations span Sabah, and most distributions extend south into Sarawak, Brunei and Kalimantan. Ryparosa taxon B (Kalimantan), along with R. taxon D and R. sp. nov. aff. calotricha (Sarawak), are also restricted to Borneo and the only Bornean species to extend its range beyond the island is R. glauca, with specimens from Flores clustering in the same species group as the Bornean specimens (Fig. 8b). The only specimens collected in Australia are those identified as R. taxon E, while R. taxon A and R. sp. nov. aff. acuminata are restricted to eastern New Guinea (Fig. 8d). R. amplifolia, R. calotricha and R. taxon C extend throughout New Guinea; the former is also known from a single specimen from southern Sulawesi (Fig. 8c).
Discussion
Morphological variation and character suitability
As a genus, Ryparosa is little studied, poorly known in the field, and has only rarely appeared in the wider scientific literature since it was first described nearly 200 years ago. Taxon delimitation within Ryparosa has previously been based largely on a haphazard mix of vegetative qualities and reproductive characters, when they were known. Indeed, staminate or carpellate flowers of many species of Ryparosa are still unknown, yet new species have been described taking these limitations into account (e.g. Ridley 1936; Sleumer 1954b; Jarvie and Stevens 1998). Herbarium specimens of R. javanica sens. lat. are often in poor condition with brief or non-existent label notes and a distinct lack of informative reproductive material. This has not only made correct determinations harder for collectors, but has led to the situation where the genus is widely documented as being dioecious (e.g. Sleumer 1954a; Hutchinson 1967; Renner and Ricklefs 1995; Jarvie and Stevens 1998). However, observing tagged individuals in Australian Ryparosa populations (R. taxon E), made it possible to follow the reproductive biology of individual trees over several seasons, revealing that at least Ryparosa taxon E is monoecious (Webber 2005).
In the only monograph on Ryparosa, Sleumer (1954a) used the presence of hairs on the staminal column and a glabrous berry to distinguish R. javanica from other species within the genus. On several specimens, he stated ‘filaments pilose’ with his determination, and seemed to overlook other significant characters in assigning these specimens to R. javanica. Although he stated that hairs seemed to be caducous, he emphasised leaf blade hair density in the species key, but then in individual descriptions gave a wide range of possible densities for some species (Sleumer 1954a). He used the position of inflorescences in the first lead of the key, but expressed uncertainty about the robustness of this character. Vegetative features, such as the relative prominence of the leaf primary vein on the adaxial surface and leaf petiole and blade length were also used at times throughout the generic key.
Based on field observations on Australian Ryparosa and on herbarium specimens, the importance of Sleumer’s (1954a) primary defining characters was generally supported. The relative prominence of the leaf primary vein on the adaxial surface, as well as indumentum density on the abaxial leaf blade was consistent between taxon groupings and supported by observations of fresh material. In the original species descriptions of taxa ascribed to Ryparosa javanica sens. lat., many authors make a point of describing the density of bifid hairs on the abaxial surface of the leaf lamina and note that the leaves appear glabrous unless examined under magnification (e.g. Mildbraed 1928; King 1890). Indeed, the original description of R. amplifolia described the leaves as being glabrous (Schumann and Lauterbach 1901), but Mildbraed (1928) recognised that R. amplifolia (and R. calotricha) had quite dense, adpressed bifid hairs on the abaxial leaf surface. Likewise, van Slooten (1924) recognised the distinct hairiness of the abaxial leaf surface in a Ryparosa specimen from New Guinea (Branderhorst, 327), and as a result determined it to be R. caesia, before Sleumer recognised its affinity to R. amplifolia sens. str., and therefore determined it to be R. javanica.
We found that inflorescence position, overall organ dimensions, the number of secondary vein pairs and leaf blade shape were more consistent within species groupings than Sleumer had previously outlined (Sleumer 1954a). For example, R. baccaureoides and R. taxon E produce racemes from large tubercles on the lower portion of the main trunk (e.g. Fig. 9) whereas R. javanica sens. str. does not appear to produce trunk tubercles (BL Webber unpubl. data; Dakkus 1957; Astuti et al. 2001) and has axillary or fasciculate racemes on small branches. However, racemes on many specimens were detached and had no notes on their position on the tree, which compounded the poor records of this trait. Furthermore, some taxa with large leaves (e.g. R. amplifolia) or racemes (e.g. R. javanica, R. taxon E) are likely to be represented in herbaria by an abundance of specimens with smaller features than might have been observed in the field. While several more recent specimen labels indicated this limitation, this will appear to reduce the differences between similar species. For example, smaller R. amplifolia leaves can appear superficially similar to ordinary-sized leaves of R. calotricha. In respect to raceme length, maximum length appears to be the best measure.
In contrast, other characters that were emphasised in Sleumer’s key and other previous work (e.g. van Slooten 1925) now appear to be somewhat erroneous. For example, R. scortechinii and R. javanica have been defined as having an entirely glabrous berry (van Slooten 1919, 1925), which is not true for immature fruits – the indumentum of the fruit epicarp had just been lost by fruit maturity. A better understanding of food-body morphology (Webber et al. 2006, 2007) has reduced the majority of fruit epicarp variation to developmental differences rather than taxon differences. Indeed, although Sleumer (1954a) used lenticels on the branchlets as a defining character separating R. fasciculata and R. glauca, these distinctive marks (concentrated on young shoots, abaxial leaf surfaces and fruit epicarps) are now known to be the scars remaining after ‘Type B’ food body (sensu Webber et al. 2006) removal; they are present in all Ryparosa species (Webber et al. 2007). Now that a better understanding of plant–ant symbioses in Ryparosa is emerging, the inclusion of useful characters associated with ant–plant interactions in taxon delimitation in the future is possible (e.g. Janzen 1974; McKey 2000). These include traits such as food-body scars (at a generic level), and at a species level, distinctive stem swellings and prostomata seen on younger branches of certain taxa. Notably, D. longifolia is known to have stem domatia and is colonised by the same ant taxa (Cladomyrma petalae Agosti and C. nudidorsalis Agosti, Moog & Maschwitz) as R. fasciculata (Agosti et al. 1999; Moog et al. 2003). This emphasises the importance of considering myrmecophilic features alongside a suite of other characters in species recognition and delimitation.
Other questionable fruit characters are those described from dried material. Fruits are often described as having little pulp and being quite angular (e.g. Brühl and King 1896; King 1890; Sleumer 1954a). By observing changes in pericarp structure during fruit drying, it was clear that the fleshiness of these fruits is difficult to discern from observations of dry specimens (a point also recognised by Sleumer 1954a), and that the angularity of dried fruits is not necessarily representative of fresh specimens. There is also considerable conjecture on the number of locules present in Ryparosa fruits, with one locule (Koorders and Valeton 1900a; Chase et al. 2002) and occasionally two locules (Kurz 1873; van Slooten 1919) reported. It will be important to conduct a more detailed study of flowers at anthesis and fruit development to clarify these disagreements. In some Ryparosa taxa, the staminal column is consistently glabrous; however, in those species with filament hairs, noticeable variation in hair presence was observed (even within flowers on the same raceme). Therefore, the use of this character as a taxon-defining trait, particularly in relation to the position of hairs (on the upper or lower part of the staminal column), should be treated with caution.
This study has also revealed new characters that have not been used specifically in the past, that are useful in defining taxa within Ryparosa. First, patterns of secondary and tertiary venation were distinctly variable between, but consistent within, species (Appendix 1). Patterns of secondary vein anastamosis varied consistently between species and when combined with tertiary venation patterns and the density of abaxial leaf hairs, provide a quick way of discriminating between taxa with otherwise similar vegetative features. Previous authors have alluded to different venation patterns between Ryparosa species (e.g. Mildbraed 1928; Sleumer 1954b). However, the recent description of R. porcata (Jarvie and Stevens 1998) is the only study to specifically refer to secondary venation patterns as a defining character. Second, while the majority of morphometric flower characters were not consistently different between species, the length and shape of carpellate flower staminodes may be a useful character for species delimitation. Two forms of staminode shape were observed: fleshy and stocky, and thin and filiform, the latter appearing to be distinctly longer and more pointed in R. kurzii than in other species. Unlike the extensive reduction in size of petal scales in dried specimens, this variation in staminode form does not seem to be an artefact of specimen preservation or time since collection, as staminode type appeared constant within taxa. Staminode characters were used by Kurz (1873) to highlight the differences between R. caesia and R. javanica (stipitate and subulate staminodes respectively), although Koorders and Valeton (1900b) point out that filiform (not subulate) staminodes were observed on the R. javanica sens. str. material they examined.
Species delimitation
Sleumer’s work for the 1954 revision of Flacourtiaceae for the Flora Malesiana was conducted on dried herbarium material, and his only chance for field observations on the genus would have been after this monograph was completed, during trips to south-east Asia and New Guinea between 1961 and 1963 (Sleumer 1980). He would have had little opportunity to examine fresh material, or material at known stages of maturity, and so would have not been familiar with developmental variation in reproductive and vegetative tissue. Sleumer’s decision to unite R. kurzii, R. wrayi and R. amplifolia with R. javanica created a species complex that not only geographically spanned almost the entire known distribution for the genus, but also morphometrically spanned observed differences in many of the other Ryparosa species. There is no doubt that this created confusion for subsequent collectors, and specimens collected since 1954 have commonly been determined as R. javanica when they were actually other Ryparosa species.
Phenetic analyses of variation within Ryparosa javanica sens. lat. have allowed us to identify distinct differences in vegetative morphology between numerous groupings of specimens (Appendix 1; Figs 3, 4). While these groupings were not as clearly defined as perhaps may be expected from phenetic analyses of more variable species or data sets that also incorporate reproductive data, they were supported by differences in reproductive characters, tree architecture, and geographical distributions (Appendix 2; Table 2; Fig. 8). In summary, Ryparosa species are not readily identifiable by spot characters, but rather each species is distinguished (and defined) by a ‘character suite’ – a particular combination of characters and / or character states unique to that species (for examples, see bold text in species descriptions). Within Ryparosa, there appears to be (based on vegetative characters) only a few characters with widely varying states. Therefore, characters with only very narrowly variable states must be included in phenetic analyses to differentiate and improve the resolution between groups. Although similarities among states for characters used in phenetic analyses will inevitably draw groups together, differences in a few characters and character states will keep them distinct. Across all the species treated here, the range of characters and character states comprising the ‘character suite’ varies considerably and between any two species the overlap might be great or small. This situation is found also in other ‘flacourtiaceous’ genera (S Zmarzty pers. comm.).
Ryparosa javanica sensu lato
The three Ryparosa taxa included by Sleumer (1954a) in R. javanica sens. lat. (R. amplifolia, R. kurzii and R. wrayi) are recognisable as distinct from R. javanica sens. str. by vegetative characters. Superimposing reproductive traits, habit and geographical data onto these groupings further enhance delimitations between these taxa. Interestingly, unpublished notes made by Sleumer during the 1980s for a revision of the genus indicated that he intended to resurrect these three species (HO Sleumer unpubl. data); however, he died before this was finished. These three taxa warrant recognition at the species level, as originally described, and are separable by a combination of morphological and geographical discontinuities.
As a result, the circumscription of Ryparosa javanica sens. str. includes distinctly less variation. Ryparosa javanica has leaves with eucamptodromous venation, five or six (rarely seven) pairs of secondary veins and very sparse hairs on the abaxial blade, particularly so on the lamina. Flower racemes are, for the most part, defoliate-axillary and the staminodes of carpellate flowers are thin-filiform in shape. Ryparosa amplifolia is most noticeably different from R. javanica and more similar to R. calotricha than to any other species of Ryparosa (see below). Its large, obovate, leaf blades with a dense abaxial indumentum and up to nine pairs of secondary veins contrasts distinctly with R. javanica sens. str. Likewise, R. wrayi differs from R. javanica sens. str. by having a moderately dense indumentum on the abaxial leaf blade and generally fewer pairs of secondary veins, the most apical being quite distant from the leaf apex. Identification of the invertebrate(s) responsible for entry holes in the stems of R. wrayi warrants further investigation in light of the discoveries about ant–plant interactions in other species of the genus. Reproductively, R. amplifolia differs from R. javanica sens. str. in having short racemes with bunched pedicels, while R. wrayi appears to have carpellate flowers on rather long pedicels. Lastly, R. kurzii is characterised by generally ovate leaf blades and long subulate-filiform staminodes to 1.3 mm in length. The staminodes of Ryparosa javanica sens. str. were consistently around 0.7 mm in length (this study), or considerably shorter (0.3 mm; Koorders and Valeton 1900b; described from one numbered tree, 1120α, from Palaboeanratoe, Java). It is anticipated that further differences will become apparent with a better documentation of reproductive traits from fresh material. For example, Brühl and King (1896) depict considerable differences in fruit shapes between R. wrayi (obconic) and R. kurzii (globose), while R. wrayi is consistently listed as having only one seed per fruit, compared with two seeds in R. javanica sens. str. (King 1890; Ridley 1922; van Slooten 1925).
New Ryparosa taxa
In addition to those species previously assigned by Sleumer to R. javanica sens. lat., several other groupings within Ryparosa were recognisable. Based on morphological and geographical discontinuities and differences in reproductive characters, five of these new taxa deserve specific status. Ryparosa taxon A, here described as Ryparosa maculata sp. nov., differs from R. javanica sens. str. by its small obovate leaf blades with moderately dense hairs on the abaxial lamina, and fruits with two or three seeds. R. taxon B, here described as Ryparosa anterides sp. nov., is consistently taller than any other known Ryparosa species and has prominent buttressing. It is distinctly different from R. javanica sens. str. and is more similar to Ryparosa micromera Slooten, from Sumatra, than to any other species. Although R. micromera has similarly dense hairs on the abaxial leaf surface, it has smaller leaves (60–120 mm long) and shorter racemes (rarely longer than 60 mm) than R. anterides, and leaves that appear crowded on the branches. Ryparosa anterides is readily distinguished by its eucamptodromous venation, secondary veins that are straight for the most part and diverge at a narrow angle from the primary vein, and moderately dense indumentum on the abaxial blade. The species also has small round entry holes in hollow stems and prominent shoot tip swellings.
R. taxon C, here described as Ryparosa milleri sp. nov., is noted as a small tree or straggling shrub by collectors and is recognised from R. javanica sens. str. by its broadly rounded leaf base, short petioles and leaves with brochidodromous secondary veins that are curved-ascending but distinctly undulate. Secondary veins in all other Ryparosa taxa examined had no undulations (i.e. distinctly smooth) and varied from strongly curved-ascending (e.g. R. glauca) to straight for the most part (e.g. R. calotricha). Specimens from Borneo, identified as R. taxon D and here described as Ryparosa maycockii sp. nov. were unique in that they had leaf blades with a cordate base, a character not previously documented in Ryparosa. While this character was not consistent on all leaves on all specimens, it was present in the majority of leaves and appears to be unique to the species. Other distinctive characters include dense hairs on the abaxial leaf blade and flower racemes, seven or eight (rarely nine) pairs of brochidodromous secondary veins, and hollow young stems. Lastly, Ryparosa taxon E is here described as Ryparosa kurrangii sp. nov. and is distinguished from R. javanica sens. str. by its long, thin leaf blades, 7–9 pairs of brochidodromous secondary veins and random-reticulate tertiary venation. This new species compares most closely to R. acuminata, from which it differs in respect to leaf shape, raceme position (predominantly basi-cauline compared with foliate-axillary in R. acuminata) and indumentum density on racemes (the calyx is distinctly hirsute abaxially in R. acuminata rather than adpressed puberulent).
The two unassigned taxa from Borneo (R. sp. nov. aff. calotricha) and New Guinea (R. sp. nov. aff. acuminata) were not designated as new species due to limited replication and the poor condition of specimens. Future work on these taxa (listed in other species examined) may elucidate their status, and more work is also needed to clarify the identify of the two specimens from Flores and the single specimen from Sulawesi, tentatively identified in this study as R. cf. glauca and R. cf. amplifolia, respectively.
Existing taxa
Specimens of existing Ryparosa species incorrectly determined as R. javanica sens. lat. were also identified by the phenetic classification (Fig. 3); of the 182 specimens originally determined as R. javanica, 18% were incorrectly determined at the species level and belonged to six other Ryparosa species. Ryparosa acuminata specimens are characterised by their brochidodromous venation, primary venation flat on the adaxial leaf surface, and distinctly hirsute racemes, particularly at the bud stage. Hirsute racemes have previously been used as trait defining R. acuminata (e.g. van Slooten 1925), and are indeed characteristic of the species. Two other species from Borneo, R. baccaureoides and R. glauca, are characterised by having randomly reticulate tertiary venation and distinctly brochidodromous venation; the latter species has secondary veins diverging at a wide angle from the primary vein. Secondary vein pairs are more numerous than R. javanica sens. str. (~8 and 8–11, respectively, compared with 5–7) and flowering is generally from branches or cauline. Ryparosa baccaureoides and R. glauca are distinguished from each other most clearly by the complete lack of indumentum on the leaves of R. glauca and the distinctively coriaceous lamina of R. baccaureoides. Specimens of another species, R. multinervosa are distinguished by their tertiary venation, which is straight percurrent and distinctly perpendicular to the primary vein, and with stigmas separated on immature fruits.
Specimens of Ryparosa calotricha (New Guinea) are easily distinguished from R. javanica sens. str. by their obovate leaves and dense hairs on the abaxial leaf lamina. However, the similarities between R. calotricha and R. amplifolia sens. str. seem to be obscured by collections of R. amplifolia sens. str. apparently specifically collected with foliage small enough to fit on herbarium sheets. Ryparosa calotricha and R. amplifolia both had swollen shoot tips (although domatia were far more common in R. amplifolia), buds bunched on racemes and leaves with strong percurrent tertiary venation, 7–9 secondary vein pairs and hairs dense on the abaxial blade. The two species differ in respect to leaf size and the distinctly obovate leaf blades with broadly acute-mucronate tips of R. amplifolia sens. str. Lastly, specimens of R. kostermansii are easily recognised by their small leaves and flowers1, and leaf blades with a dense indumentum abaxially, acrodromous venation and two or three secondary vein pairs.
Lastly, two specimens from the Andaman Islands were identified as Drypetes longifolia and are immediately separated from Ryparosa taxa by their oblique leaf base, 2-locular fruits and bow-tie shaped stigmas. Confusion between specimens from the Euphorbiaceae sens. lat. (until recently, Drypetes longifolia was assigned to the Euphorbiaceae, but recent work has seen the genus moved to the Putranjivaceae; Savolainen et al. 2000) and the Flacourtiaceae is common (e.g. Sleumer 1954a), and D. longifolia specimens from the Andaman Islands have also frequently been erroneously ascribed to Hydnocarpus castanea (e.g. Rao and Chakrabarty 1985; Chakrabarty and Gangopadhyay 1992).
Geographical distributions reconsidered
By resurrecting the three taxa that Sleumer (1954a) combined into R. javanica sens. lat., recognising five new species, and assigning erroneously attributed specimens to their correct species, the geographical range of R. javanica sens. str. is reduced quite considerably (Fig. 8); it is now restricted to the southern half of Sumatra, Java and Bali. Within the Ryparosa javanica complex, R. kurzii and R. amplifolia are endemic to the Andaman and Nicobar Islands and New Guinea, respectively, while the distribution of R. wrayi overlaps with R. javanica sens. str. in northern Sumatra. Likewise, the distributions of R. anterides and R. maycockii overlap in Borneo, and of R. maculata and R. milleri in New Guinea. This sympatry in newly recognised taxa provides supporting evidence for the existence of these entities. Interestingly, the Australian taxon, R. kurrangii, is not only the sole representative of the genus known from Australia, but one of only two species of Achariaceae recorded from Australia (the other being the monotypic Baileyoxylon lanceolatum C.T.White; Sleumer 1954a; Chase et al. 2002). When specimens from the other six Ryparosa species were correctly determined, no collections were from regions outside their previously documented species distribution. It appears that Ryparosa species often reflect geographically circumscribed as well as morphologically distinct entities. Moreover, in both species that had specimens that were geographic outliers (R. glauca and R. amplifolia), these isolated specimens were also outliers in the morphometric analyses.
Conclusions
It is clear that considerable confusion exists in species delimitations within Ryparosa, and the groupings of certain taxa in Sleumer’s (1954a) revision did little to improve the situation. It seems that past species descriptions have not taken into account important identifying characters of the genus, for the primary reason that Ryparosa remains little studied and poorly known from field observations. By using a field-based knowledge of the genus to select meaningful characters, along with relevant characters from the previous generic revision (Sleumer 1954a), it was possible to delineate distinctive groupings within Ryparosa javanica sens. lat. In addition to re-defining R. javanica sens. str., we found that three previously described species should be reinstated and five new taxa deserve recognition at a species level (see species key). Collections of more reproductive material will no doubt improve the resolution of these and new species groupings within Ryparosa, and will be a useful supplement for constructing a more detailed species key. In summary, it is clear that a new generic revision of Ryparosa is required, combining field observations of live specimens with analyses of dried and wet herbarium collections.
A dichotomous key for species in the Ryparosa javanica sens. lat. complex based on vegetative morphology.
Quantitative data are presented as an interquartile range followed by the minimum and maximum values (if distinctly different) in parentheses
Taxonomic treatment
Place name descriptors were spelled as by the collector (based on best possible interpretations of handwriting on the specimen label), with extra large-scale information (e.g. districts, regencies) and current versions of place names added where necessary (in square brackets) to clarify locations. Dutch and German place name descriptors were changed to their equivalent English version where possible (e.g. Seroei to Serui); however, Malay descriptors (e.g. bukit, gunung) have been retained when used on the original labels. In agreement with the policy of this journal, Australian specimen citations have been deliberately abbreviated for conservation management purposes. Bold text in the descriptions indicates a character trait of importance for the particular species.
Unless otherwise mentioned, for all species described in this treatment plants are most likely monoecious (confirmed for R. kurrangii sp. nov.); young twigs and shoot tips are tomentose when young, glabrescent with Type B food-body scars; leaves are borne spirally on the stem, leaf margins are entire (Fig. 9c), the primary vein is distinctly prominent abaxially, the petiole is thickened at both ends, and prominently kneed at the apex; stipules are absent; hairs (sometimes only visible under magnification) are T-shaped bifurcate, and with one branch often reduced, multicellular (Fig. 6); inflorescences are racemes; the calyx splits irregularly and is glabrous adaxially, petals have a basal scale adaxially that is densely villous, the ovary is superior, stigmas are close together both in flower and fruit, and bracts are generally absent from fruit racemes.
Ryparosa javanica (Blume) Kurz ex Koord. & Valeton, Bijdr. Booms. 5: 11 (1900), l.c. 6: 185 (1900)
Tree up to 18 m tall. Outer bark rough, grey-brown, inner bark pink; wood straw yellow. Leaf blades elliptic to ovate-elliptic, (110–)190–290(–320) × (40–)65–95(–115) mm, acute at the apex, (46–)52–58°, sometimes with a short acuminate tip, attenuate at the base, (52–)57–64(–69)°, texture chartaceous, surface glabrous adaxially, abaxially with sparse hairs on the primary (and secondary) veins, lamina glabrous (with sparse hairs), primary vein sunken adaxially, secondary veins eucamptodromous, five or six pairs, slightly raised adaxially and prominent abaxially, diverging at (27–)40–51(–61)° from the primary vein, curved-ascending towards the margin, tertiary venation subpercurrent; petiole (22–)30–39(–50) mm long. Staminate inflorescences to 325 mm long, single from foliate or defoliate axils (solitary or in fascicles of 2 or 3 on branchlets); bracts c. 0.5 mm long; pedicels 1.8–3.7 × 0.3–0.5 mm; calyx splitting into two or three lobes, lobes 2.2–2.6 mm long, abaxially sparse adpressed-puberulent; petals 5, 2.7–3.1 × 1.1–1.3 mm, adaxially glabrous, abaxially densely tomentose; scale 1.1–1.4 × 0.5–0.7 mm; filaments five, united, villous; stamens 2.3–3.6 mm long. Carpellate inflorescences to 185 mm long, single from foliate or defoliate axils (solitary or in fascicles of 2 or 3 on branchlets); bracts 0.4–0.5 mm long; pedicels (2.7–)3.9–5.0 × 0.3–0.5 mm; calyx splitting into two or three lobes, lobes 2.3–3.8 mm long, abaxially sparse adpressed puberulent; petals four or five, 3.0–4.4 × 1.2–2.0 mm, glabrous adaxially; abaxially densely tomentose, scale 1.1–1.5 × 0.7–1.0 mm; staminodes 4 or 5, 0.4–0.9 mm long, thin filiform (somewhat fleshy in Bali specimen), pilose adaxially; ovary ellipsoid (obovoid); stigmas 2 or 3, sessile. Fruit racemes to 180 mm long; pedicels 3.3–6.2 × 0.6–1.4 mm. Fruits globose, 1-locular, 1 or 2-seeded; stigmas 2 or 3, sessile.
Notes
Likely syntypes for Bergsmia javanica Blume (Kuhl & van Hasselt, s.n.) are currently held by Leiden (L0351538, L0351539, L0351540, L0351541, L0351542). Ryparosa longipedunculata Boerl. was originally described from three living trees (III-F-15, IX-A-32 and IX-A-34) in the Bogor Botanic Gardens, which were grown from seed collected in Sumatra (Boerlage 1899). The lectotype specimen for R. longipedunculata (L0011217) was taken from the tree III-F-15; however, as no date was recorded and the tree died only in 1979, it is possible that the lectotype specimen may have been collected after the species description (e.g. another specimen from III-F-15 [L0351617] was collected on 07.xi.1953). Other designated types (incorrectly indicated by det. slips placed on specimens by H.O. Sleumer; L0011216, L0011218) are from another tree (III-F-15a), which was grown from seed collected from the same maternal plant as III-F-15, but is a separate individual. In their description of R. longipedunculata, Koorders & Valeton (1900a) cite a synonymy with R. caesia var. macrostachya, the description of which is attributed to ‘Kurz.! msc.’ with a specimen lodged in ‘Herb. Bog.’. However, no evidence of the specimen or of a published description of R. caesia var. macrostachya could be found. In western Java, S.H. Koorders collected from permanently tagged trees and some of his ‘specimen numbers’ refer to individual trees and not true collection numbers (e.g. 2728β above; van Slooten 1925). The drawing of Bergsmia javanica in Blume’s description (Tab. 178C, Fig. 2; Blume 1848) depicts the racemes of R. javanica far too large relative to the foliage on which they are positioned, while Sleumer (1954a) incorrectly labels the staminate racemes of R. javanica (figs 18, 19) as carpellate racemes.
Distribution and ecology
Distributed through southern Sumatra, Java and Bali (Fig. 8b). In contrast to previous reports, not known from Borneo, the Malay Peninsula or east of Bali. Rainforest habitats up to 500 m, occasionally up to 1400 m, frequently found near streams. Flowering: May–Nov; fruiting: Jul–Jan.
Vernacular
JAVA: Bantam region: Langit, Kisijung (referring to the scent of the bark; i.e. almond, cyanide); Preanger region: Ki manjěti, Ki měngati, Hoeroe gading, Hoeroe tangkalak; Banyumas region: Langit, Wera, Selemati; Besoeki region: Hendog, Boeroe merak.
Selected specimens (60 specimens examined)
SUMATRA: Aceh Province: [Aceh Tenggara Regency], Gunung Leuser Nature Reserves, Ketambe Research Station and vicinity, Alas River valley (c. 35 km NNW of Kutacane), [03°40′ N, 97°40′ E], de Wilde, W.J.J.O. & de Wilde-Duyfjes, B.E.E., 18547, 10.vii.1979 (L). Benkulu Province: Redjang [Rejang Lebong Regency], Konak, 2 km from Kepahiang, [03°40′ S, 102°34′ E], Boschproefstation, bb 15438, 1.iii.1931 (L). Sumatera Selatan Province: [Musi Rawas Regency], Tandjong Ring [cf. Tandjoengning, now Tanjungaing, on the Sungai Saling, 03°32′ S, 103°02′ E], Forbes, H.O., 2720, 1881 (BM, L). Sumatera Utara Province: Asahan [now Simalungun Regency], Silo Maradja [cf. Silomalela], in the vicinity of Taloen Djoring, [c. 03°08′ N, 99°10′ E], si Toroes, Rahmat (with Hamel, C.), 49, 1.xii.1927 (SING). JAVA: Central Java: Banjoermas [Banyumas] Region, Nusa Kambangan [Island], near Tjilatjap [Cilacap, 07°45′ S, 108°59′ E], Koorders, S.H., 24628β (1339c), 21.ix.1896 (L). East Java: Besuki [Besoeki Region], Gunung Raung, south slope above village Gunungsari, [08°08′ S, 114°05′ E], Jacobs, M., 4821, 17.v.1957 (K, L, SING); Banyumas Prov. [unlikely], Pasoeroean [Region], Tangkill Evidergebergte [cf. Tangkil, 08°11′ S, 112°48′ E], Koorders, S.H., 30291β (1581x), 27.vi.1896 (L). West Java: [Bantam Region], foot of Mt Hondje, [06°43′ S, 105°34′ E], Kostermans, 19312, 29.xii.1961 (L); Bantam Region, Gunung Karang, [06°17′ S, 106°03′ E], Koorders, S.H., 2763β, 12.vi.1892 (L); Preanger Region, Palaboeanratoe [Pelabuhan Ratu, 07°00′ S, 106°35′ E], Koorders, S.H., 33066β (1120a), 23.iii.1899 (L); Preanger Region, Takoka [now Takokak, 07°03′ S, 106°59′ E], Koorders, S.H., 25650β (2433a), 27.x.1896 (L). BALI: [Bakungan Mountains], Gunung Kelatakan, [08°12′ S, 114°30′ E], Sarij (Maier, R. E. P.), 110, 19.vii.1918 (BO, L).
Ryparosa amplifolia (K. Sch.) Mildbr., Notizbl. Berl.-Dahlem 10:339 (1928)
Tree 14–22(–30) m tall; bole 8–12 m. Outer bark rough, grey-brown, wood straw yellow. Stem swellings at shoot tips and younger internodes commonly hollow with ant domatia. Leaf blades distinctly obovate, (140–)235–310(–370) × (70–)95–125(–145) mm, rounded at the apex, 71–74(–78)°, generally with a short mucronate tip, cuneate to attenuate at the base, (67–)72–77(–81)°; texture chartaceous, surface glabrous adaxially, abaxially with dense hairs on the primary and secondary veins, subdense on the lamina, food-body scars distinct, primary vein sunken adaxially, secondary veins eucamptodromous, (7–) 8 or 9 pairs, slightly raised adaxially and prominent abaxially, diverging at (36–)43–48(–55)° from the primary vein, reasonably straight ascending towards the margin, tertiary venation undulate, percurrent; petiole 28–38(–50) mm long. Staminate inflorescences single from foliate or defoliate axils, or in fascicles of up to 4 from small tubercles on branchlets; buds clumped in distinct bunches on raceme peduncle; mature flowers not seen. Carpellate inflorescences to 75 mm long, single from foliate or defoliate axils, or in fascicles of up to 4 from small tubercles on branchlets; buds clumped in distinct bunches on raceme peduncle; bracts c. 0.5 mm long; pedicels 2.4 × 0.7 mm; calyx splitting into three lobes, lobes c. 4.1 mm long, sub-dense adpressed puberulent abaxially; petals 5; c. 4.7 mm × 1.9 mm, glabrous adaxially, abaxially densely tomentose; scale 2.5 × 1.2 mm; staminodes 0.9 mm long, somewhat fleshy, pilose adaxially; ovary ellipsoid; stigmas 2, sessile. Fruit racemes to 85 mm long; pedicels (2.8–)5.8–7.5 × (0.9–)1.3–2.3 mm. Fruits globose; stigmas 2, sessile.
Notes
The original type specimen, Lauterbach, 2848 (lodged at B), appears to have been destroyed during World War II (Zepernick 1978, R Vogt pers. comm.). At some point in his studies, Sleumer created ‘kleptotypes’ from the B holotype, which are now lodged at Leiden (consisting of one small deformed leaf; L0011215) and Wroclaw (consisting of leaf, raceme and fruit fragments). This is all that remains of the original type specimen and it does not allow for the precise application of the taxon name. The only paratype listed by Mildbraed (1928; Schlechter, 17599) is also in poor condition and is unidentifiable; therefore, an epitype has been here designated.
Distribution and ecology
Distributed through Indonesian New Guinea and Papua New Guinea (Fig. 8c). The correct identity of the single specimen from Sulawesi (Meijer, 10788) is uncertain at this stage. Rainforest habitats up to 300 m, occasionally much higher, often found near rivers and streams. Consistently associated with stem-nesting ants (perhaps Camponotus sp.; Webber et al. 2007). Flowering: Mar–Oct; fruiting: May–Dec.
Vernacular
NEW GUINEA: Sorong Regency, West New Guinea: Tokahee (Tehid); Manokwari Regency, West New Guinea: Bendaang (Hattam), Aidia (Wandammen), Wobbrijka (Mamikiong), Sagoboeatemol (Colonial Dutch); Madang District, Papua New Guinea: Abai (Bilia), Okabidelo (Amele), Papoia (Dumpu), Mangu (Faita); Northern District, PNG: Sanga, Siditoteh (Orokaiva, Mumuni).
Selected specimens (51 specimens examined)
SULAWESI: SW peninsula, NE of Makassar, within 54–60 km on the road, [04°55′ S, 119°45′ E], Meijer, W., 10788, 4.vii.1976 (L). NEW GUINEA: Papua New Guinea: Madang District, lower slopes of Bismark Range, Ramu Valley, c. 5 miles SE of Faita airstrip, [05°40′ S, 145°18′ E], Saunders, J.C., 380, 1.vii.1955 (L); [Madang District], Kaiser Wilhelmsland [NE New Guinea], woods at Djamu [Ramu river, c. 04°27′ S, 144°38′ E], Schlechter, F.R.R., 17599, 24.iv.1909 (L); Milne Bay District, Sag Alotau Subprovince, north slopes of Pini Range, [10°23′ S, 150°16′ E], Gideon, O., LAE 76985, 4.iii.1984 (CANB, L, LAE); West Sepik [now Sandaun] District, Vanimo Subdistrict, Ossima, [02°56′ S, 141°17′ E], Streimann, H. & Kairo, A., NGF 39271, 30.i.1969 (L); [Southern Highlands District], 20 km SSW of Kutubu, near Waro airstrip, [06°27′ S, 143°11′ E], Jacobs, M., 9159, 12.x.1973 (L, LAE). Western New Guinea: [Jaya Wijaya Regency], Sabang [Lorentz River, 04°46′ S, 138°47′ E], Branderhorst, B., 327, 2.iv.1908 (L); Hollandia [Jayapura Regency], Bernhard bivak [Bernhardkamp, on the Idenburg River, 03°27′ S, 139°08′ E], Neth. Ind. For. Service, bb 25751, 8.viii.1938 (L); [Manokwari Regency], Wandammen Peninsula, Wondiwoi Mountains, [02°43′ S, 134°32′ E], Schram, F.A.W., BW 13455, 14.iii.1962 (L, LAE); [Yapen Waropen Regency], Eil Japen [Yapen Island], Seroei [Serui, 01°52′ S, 136°13′ E], Neth. Ind. For. Service, bb 30610, 16.ix.1939 (L, SING); [Manokwari Regency], Warmare valley (c. 20 km SW of Manokwari), [00°55′ S, 134°00′ E], Moll, O., BW 2481, 4.iv.1962 (L).
Ryparosa kurzii King, J. As. Soc. Beng. 59:125 (1890)
Typus: Andaman Islands, South Andaman Island, Mt Harriet, [11°43′ N, 92°44′ E], Kurz, S., s.n., 2.ii.1875 (lectotype, here designated: K [K000186455]).
A rather small tree up to 7 m tall. Outer bark rough, grey-brown, inner bark pink; wood straw yellow. Leaf blades distinctly ovate-elliptic, 200–260 × 95–110 mm, acute at the apex, (58–)62–65°, attenuate to rounded at the base, 69–78(–88)°, texture chartaceous, surface glabrous adaxially, abaxially with sparse hairs on the primary and secondary veins and lamina (lamina glabrous), food-body scars distinct, primary vein sunken adaxially, secondary veins distinctly eucamptodromous, 6 or 7 (rarely 8) pairs, slightly raised adaxially and prominent abaxially, diverging at 46–55° from the primary vein, curved-ascending towards the margin, tertiary venation somewhat reticulated; petiole 23–28(–33) mm long. Staminate inflorescences to 185 mm long, single from foliate or defoliate axils; bracts 0.3–0.5 mm long; pedicels 1.8–2.8 × 0.3–0.4 mm; calyx splitting into three lobes, lobes 2.2–2.6 mm long, abaxially strigose; petals 5, 2.7–3.1 × 0.8–1.0 mm, glabrous adaxially, abaxially densely tomentose; scale 1.0–1.4 × 0.5–0.7 mm; filaments 5, united, villous; stamens 2.8–2.9 mm long. Carpellate inflorescences to 120 mm long, single from defoliate axils (foliate axils), or solitary on branchlets; bracts c. 0.5 mm long; pedicels 4.2–4.5 × 0.4–0.5 mm; calyx splitting into three lobes, lobes c. 3.0 mm long, abaxially strigose; petals 5, 3.3 × 1.2 mm, glabrous adaxially, abaxially densely tomentose, scale 1.6 × 0.6 mm; staminodes distinctly elongated, 1.1–1.3 mm long, thin filiform with a pointed tip, pilose adaxially; ovary ellipsoid-obovoid; stigmas 2, sessile. Fruit racemes to 110 mm long; pedicels long, 9.3 × 0.7 mm. Fruits globose; stigmas 2, sessile.
Distribution and ecology
Distribution restricted to the Andaman and Nicobar Islands (Fig. 8a). Mixed rainforest habitats at low altitude, noted as being rare by Nair in 1974 (e.g. Nair, N.G., 1632). Flowering: Mar–Aug; fruiting: May–Nov.
Selected specimens (7 specimens examined)
ANDAMAN ISLANDS: Andaman Islands, Parkinson, C.E., 336, 18.ii.1915 (K). Middle Andaman Island: Bom Lung Ta [Bomlungta, 12°30′ N, 92°52′ E], Parkinson, C.E., 1191, 14.iv.1916 (BM). South Andaman Island: Dhani Khari, [11°33′ N, 92°41′ E], Dr King’s Collector, s.n., 17.iii.1894 (BM). NICOBAR ISLANDS: North Nicobar Island: Car Nicobar Island, Lapathy District, [Lapate, 09°13′ N, 92°48′ E], Nair, N.G., 1632, 14.vi.1974 (L).
Ryparosa wrayi King, J. As. Soc. Beng. 59: 126 (1890)
Typus: No location listed [likely to be Malaysia, Peninsular Malaysia, Perak, Taiping region, c. 04°51′ N, 100°51′ E], Wray, L. Jr., 3190, no date (lectotype, here designated: K [K000186444]; isolectotypes: BM [BM624231], CAL [33781]).
Tree 13–15(–30) m tall; buttressed to 3.0 m. Young twigs rarely with hollow stem internodes and round entry holes. Leaf blades elliptic, (175–)200–255(–335) × (45–)55–80(–120) mm, acute at the apex, (42–)47–53°, attenuate at the base, (44–)54–65(–76)°, texture chartaceous, surface glabrous adaxially, abaxially with hairs moderately dense, distinct food-body scars, primary vein sunken adaxially, secondary veins distinctly eucamptodromous, (4–)5 or 6 pairs, the most apical distinctly distant from the leaf tip, raised adaxially and prominent abaxially, diverging at (34–)39–48° from the primary vein, moderately curved-ascending towards the margin, tertiary venation percurrent; petiole (20–)26–35(–41) mm long. Staminate inflorescences to 550 mm long, solitary from foliate or defoliate axils, or on branchlets; mature flowers not seen. Carpellate inflorescences to 305 mm long, solitary from foliate or defoliate axils, or on branchlets; bracts c. 0.5 mm long; pedicels elongated c. 7.0 × 0.6 mm; calyx sparse adpressed puberulent abaxially; petal glabrous adaxially, abaxially dense adpressed puberulent; staminodes 5, thin filiform, pilose adaxially; ovary ellipsoid-obovoid; stigmas 2 (3), somewhat sessile. Fruit racemes to 270 mm long; pedicels 5.5–6.5 × 1.1–1.6 mm. Fruits globose, 1-locular, 2-seeded; stigmas 2 (3), slightly stalked or rarely sessile.
Notes
Specimens from the Malay Peninsula are frequently misidentified as R. caesia, most likely due to the hairs on the abaxial leaf lamina. Ryparosa wrayi is occasionally associated with stem-nesting ants, although entry holes into hollow internodes do not seem to be associated with swollen stems (Webber et al. 2007).
Distribution and ecology
Distributed from southern Thailand and Myanmar through the Malay Peninsula, also in northern Sumatra (Fig. 8a). Primary rainforest habitats up to 200 m, occasionally up to 500 m, often found near streams. Flowering: Mar–Oct; fruiting: May–Jan.
Vernacular
THAILAND: Trang Province: Kluai; SUMATRA: Sumatera Utara Province: Tjingkuang, Badja.
Selected specimens (36 specimens examined)
MYANMAR: Tenasserim [now Tanintharyi] Division: Hui Sai Kao, [c. 10°26′ N, 98°44′ E], Kerr, A.F.G., 21650, 1.vi.1932 (K). PENINSULAR THAILAND: Chumphaun [Chumphon] Province: Tha Sae District, Ta San [cf. Tha Sae, 10°35′ N, 99°08′ E], Kerr, A.F.G., 16252, 22.xii.1925 (BM, L). Nakhon Si Thammarat Province: Lansagah [probably Phrom Khiri] District, Khao Luang NP, Prom Loke falls, [08°30′ N, 99°47′ E], Maxwell, J.F., 87–191, 24.ii.1987 (L). Narathiwat Province: Waeng, [05°54′ N, 101°52′ E], Phusomsaeng, S., 41198, 1.v.1968 (L). Trang Province: Na Yong District, Khao Chong F.R., [07°35′ N, 99°49′ E], Phusomsaeng, S. & Pinnin, S., 315, 10.xii.1969 (K, L). PENINSULAR MALAYSIA: Johore: Kota Tinggi, Medang, Panti FR, No. 72, (01°51′ N, 103°53′ E), Baker, F.G.A., 93640, 8.vii.1961 (KEP). Kelantan: [Tanah Merah], Ulu Sat FR, [05°43′ N, 102°19′ E], Suppiah, T., KEP 104565, 16.vi.1968 (L). Perak: Larut, (04°53′ N, 100°47′ E), Dr King’s Collector, 6642, ix.1884 (BM, L). Selangor: Kajang, Sungai Lalang FR, Sungai Seningoh river valley, [03°03′ N, 101°53′ E], Symington, C.J., 22668, 6.iii.1930 (SING); Ulu Langat, Bukit Tangkol [Bukit Tunggul], [02°54′ N, 101°45′ E], Gadoh anak Unbai (for Millard, A.H.), KL 2181, 2.x.1960 (KEP). SUMATRA: Sumatera Barat Province: Pasaman Regency, Batang Mandiangin, [00°07′ S, 99°47′ E], Laumonier, Y., TFB 3528, 21.iii.1982 (L). Sumatera Utara Province: East Coast, Longkat [Lankat] Regency, Telaga Said, [03°57′ N, 98°12′ E], Boschproefstation, bb 9376, 10.xi.1925 (L).
Ryparosa maculata B.L.Webber, sp. nov.
A R. javanica (Blume) Kurz ex Koord. & Valeton in laminis minoribus (125–160 mm longis) obovatis indumento paginis abaxialibus praeditis, et fructibus 2–3-seminibus differt.
Typus: New Guinea, [Papua New Guinea], Northern District, S slopes of Hydrographers Range, Managalase area, near Siurane [cf. Siurani] village, [08°59′ S, 148°23′ E], Pullen, R., 5479, 28.vii.1964 (holotype: LAE [75752]; isotype: L [L0351604]).
Tree up to 24 m tall (butressed). Outer bark rough, light brown-grey, wood straw yellow. Leaf blades obovate, (110–)125–160(–170) × (40–)45–65(–70) mm, acute-acuminate at the apex, 59–64°, with a well defined acuminate tip to 15 mm, attenuate at the base, 60–65°, texture subcoriaceous, surface glabrous adaxially, abaxially with hairs moderately dense on the primary and secondary veins, sparse on the lamina, food-body scars, distinctive circular pellucid spots in the lamina abaxially, primary vein sunken adaxially, secondary veins eucamptodromous, 5 or 6 pairs, slightly raised adaxially and prominent abaxially, diverging at 38–43° from the primary vein, curved-ascending towards the margin, tertiary venation percurrent undulate; petiole (9–)17–23 mm long. Staminate inflorescences to 120 mm long, solitary from foliate or defoliate axils; bracts 0.5–0.6 mm long; pedicels c. 0.5–1.3 × 0.4 mm; calyx lobes c. 2.2 mm long, abaxially sparse adpressed puberulent; petals four or five, 2.0–2.3 × 0.9–1.1 mm, glabrous adaxially, abaxially densely tomentose, scale c. 1.0 × 0.4–0.6 mm; filaments four or five, united, villous (glabrous); stamens 1.9–2.7 mm long. Carpellate inflorescences to 35 mm long, solitary from foliate or defoliate axils; bracts c. 0.5 mm long; pedicels c. 1.2 × 0.5 mm; calyx splitting into three lobes, lobes c. 2.3 mm long, abaxially sparse adpressed puberulent; petals c. 3.1 × 1.9 mm, glabrous adaxially, abaxially densely tomentose, scale c. 1.1 × 0.7 mm; staminodes 5, c. 1.1 mm long, thin filiform, pilose adaxially or entirely glabrous; ovary ellipsoid (obovoid); stigmas two, sessile. Fruit racemes to 65 mm long; pedicels stocky, c. 1.8 × 1.6 mm. Fruits globose, 1 locule, 2 or 3 seeds; stigmas 2, sessile.
Distribution and ecology
The species is only known only from the rainforests of eastern New Guinea (Papua New Guinea; Fig. 8d) where it has been collected at altitudes from 30 to 1100 m. Flowering known from Feb to Sep and fruiting from Aug to Dec.
Etymology
From the Latin, maculatus, spotted, referring to the conspicuous circular pellucid spots in the abaxial leaf lamina.
Additional specimens examined
NEW GUINEA: Papua New Guinea: Central District, c. 15 km NE of Cape Rodney, Mori River, [10°06′ S, 148°31′ E], Pullen, R., 8189, 1.ix.1969 (LAE); Central District, Ilolo, [09°25′ S, 147°25′ E], Streimann, H. & Kairo, A., NGF 26185, 3.ii.1966 (LAE); Morobe District, ~7 miles N of Lae, between Busu and Butibum rivers, [06°38′ S, 146°59′ E], Hartley, Thomas G., TGH 11443, 20.iii.1963 (CANB, LAE); Morobe District, Lae, end of Sunkwep Logging Rd, [06°35′ S, 146°55′ E], Katik, P. & Steven, F., NGF 46786, 23.vii.1971 (CANB, L, LAE); Western District [Kiunga Subdistrict], near Ingembit village, [05°39′ S, 141°01′ E], Henty, E.E., Ridsdale & Galore, NGF 31863, 10.vi.1967 (LAE).
Ryparosa anterides B.L.Webber, sp. nov.
A R. micromera Slooten in statura (ad 40 m), trunco anteridibus praedito, nervis eucamptodromis pro parte maxima rectis a costa angulis angustis divergentibus, et caulibus tumoribus myrmecophilis distinctis ornatis, differt.
Typus: East Borneo [East Kalimantan], East Kutei, Sangkulirang Subdivision, Sungai Susuk region, [00°57′ N, 118°13′ E], Kostermans, A.J.G.H., 5481, 27.vi.1951 (holotype: L [L0351551 & L0351552]; isotypes: BM [BM756086], CAL, K, NY, SING [SING0039112])
A large tree up to 25–35(–40) m tall; bole fluted, 16–18 m; large thick buttresses up to 5.0 m high, 3.0 m over the ground. Outer bark smooth, light-brown; wood pale brownish-yellow. Distinctive stem swellings at shoot tips and young stem internodes commonly hollow with circular entry holes formed by ants. Leaf blades elliptic, 135–195(–215) × 40–50(–55) mm, acute at the apex, (39–)43–46°, without a sharply acuminate tip, attenuate at the base, 54–60(–64)°, texture chartaceous, surface glabrous adaxially, abaxially with hairs moderately dense, distinct food-body scars, primary vein sunken adaxially, secondary veins distinctly eucamptodromous, 5 or 6 (–7) pairs, slightly raised adaxially and prominent abaxially, diverging at (27–)30–38(–41)° from the primary vein, rather straight-ascending at a distance from the margin, tertiary venation undulate percurrent; petiole (20–)26–30(–35) mm long. Staminate inflorescences to 120 mm long, solitary from foliate or defoliate axils; bracts c. 0.6 mm long; pedicels c. 1.7 × 0.3 mm; calyx splitting into two or three lobes, lobes c. 2.2 mm long, abaxially sparse adpressed puberulent; petals five, c. 2.1 × 0.9 mm, glabrous adaxially, abaxially densely tomentose, scale c. 0.6 × 0.3 mm; filaments 5, united, villous; stamens c. 2.5 mm long. Carpellate inflorescences solitary from foliate or defoliate axils; bracts c. 0.5 mm long; pedicels c. 4.5 × 0.4 mm, calyx splitting into two or three lobes, lobes 2.4–2.6 mm long, abaxially sparse adpressed puberulent; petals 4 or 5, c. 3.2–3.7 × 1.3 mm, glabrous adaxially, abaxially densely tomentose, scale 1.6–2.3 × 1.0–1.1 mm; staminodes 5 (4), 0.7–0.8 mm long, thin filiform, pilose adaxially; ovary ellipsoid; stigmas 2, sessile. Fruit racemes to 125 mm long; pedicels 5.0–6.0 × 1.4–1.9 mm. Fruits globose; stigmas 2, sessile.
Notes
The mature fruits of R. anterides are said by Kostermans (Kostermans, 13720) to be brown and edible (mature fruits are not known from the vegetatively similar R. micromera).
Distribution and ecology
Known only from the rainforests of east Kalimantan (Fig. 8b), sometimes on sand or loam soil containing limestone rocks and in wet or periodically inundated areas; noted as uncommon to rare by Kostermans in the 1950s (e.g. Kostermans, A., 13440). Ryparosa anterides is consistently associated with stem-nesting ants (identity unknown). Habitat altitude 10–400 m. Flowering: May–Oct; fruiting: Jul–Nov.
Vernacular
BORNEO: Berau Region, Kalimantan: Rukun (Bassap).
Etymology
The epithet is a noun in apposition, from the Latin, anterides, buttresses, referring to the distinctive large buttresses formed by this taxon.
Selected specimens (15 specimens examined)
BORNEO: East Kalimantan: Berouw [Berau] Region, foot of Mt Ilas Bungaan, [02°13′ N, 117°26′ E], Kostermans, A., 13720, 8.ix.1957 (K, L); Berau Region, Sungai Kelai, base of Mt Njapa, [01°48′ N, 117°16′ E], Kostermans, A., 21216, 11.x.1963 (L); East Kutei, S of Sangkulirang, Gunung Sekrat, [00°53′ N, 117°49′ E], Kostermans, A, 5923, 28.vii.1951 (BM, L, SING); [East Kutei], Sangkulirang District, north of Sangkulirang, Mt Medadam [Gunung Madadem, 01°09′ N, 118°02′ E], Kostermans, A., 13424, 9.viii.1957 (K, L, LAE, SING).
Ryparosa milleri B.L.Webber, sp. nov.
A R. javanica (Blume) Kurz ex Koord. & Valeton foliis in basin late rotundatis, petiolis brevibus (12–14 mm longis), nervis foliorum brochidodromis curvati-ascendentibus undulatis differt.
Typus: Netherlands New Guinea [West New Guinea, West Irian Jaya Province], Sorong District, hills S of Radio Sorong, [00°52′ S, 131°16′ E], van Royen, P., 3332, 7.iv.1954 (holotype: L [L0351582]; isotypes: BRI [BRI376716], LAE [20341], SING [SING0039128]).
Small tree (straggling shrub) up to 3.0 m tall. Outer bark brown, wood straw yellow. Leaf blades distinctly oblong-elliptic, 170–205(–215) × 60–75 mm, acuminate at the apex, 52–57°, generally with a well defined tip to 15 mm, rounded at the base, 74–79°, texture chartaceous, surface glabrous adaxially, abaxially with sparse hairs on the primary vein (secondary veins), lamina glabrous, food-body scars; primary vein sunken adaxially, secondary veins brochidodromous (undulate), seven or eight pairs, slightly raised adaxially and prominent abaxially, diverging at 51–57° from the primary vein, curved-ascending close to the leaf margin, tertiary venation reticulated; petiole short, 12–14 mm long. Staminate inflorescences not known. Carpellate inflorescences to 100 mm long, solitary from foliate, somewhat supra-foliate or defoliate axils; bracts 0.5–0.6 mm long; pedicels 1.9–2.2 × 0.3–0.4 mm; calyx splitting into two or three lobes, lobes 1.7–1.9 mm long, sparse adpressed puberulent abaxially; petals 4 or 5, 2.1–2.7 × 1.1–1.5 mm, glabrous adaxially, abaxially densely tomentose, scale c. 0.8 × 0.6–0.7 mm; staminodes 0.2–0.3 mm long, thick fleshy, pilose on both sides; ovary ellipsoid; stigmas 2, sessile. Fruit racemes to 65 mm long; pedicels c. 2.0 × 1.6 mm. Fruits globose, stigmas 2, sessile.
Distribution and ecology
Ryparosa milleri is known from two widely-separated locations in New Guinea (Fig. 8c). Primary rainforest habitats sometimes on swampy ground up to 150 m. A flowering specimen was collected in July and a specimen with immature fruit in April.
Etymology
The specific epithet honours Dr J.R.M. (Roy) Miller O.B.E., pioneering surgeon in Kenya, grandfather and source of inspiration to the first author throughout the course of his studies.
Additional specimens examined
NEW GUINEA: Papua New Guinea: Western District [Kiunga Subdistrict], near Ingembit village, [05°39′ S, 141°01′ E], Henty, E.E., Ridsdale & Galore, NGF 31883, 12.vi.1967 (L, LAE).
Ryparosa maycockii B.L.Webber, sp. nov.
A R. javanica (Blume) Kurz ex Koord. & Valeton in laminis basin leviter cordatis, paginis abaxialibus racemisque indumento denso praeditis, laminis nervis 7–8 paribus brochidodromis valde curvato-ascendentinus et undulatis, et petalis adaxialibus indumento subdenso praeditis, differt.
Typus: [Borneo, Malaysia, Sarawak], 7th Division, Ulu Sungai Belaga, [02°46′ N, 113°49′ E], Othman et al., S 43823, 3.xi.1981 (holotype: K; isotypes: KEP [K56075], L [L0351547], SAN?, A?).
Tree up to 7 m tall. Leaf blades elliptic, 230–280 × 80–120 mm, acuminate at the apex, 52–65° (acuminate for up to 20 mm), cordate to a depth of 2–3 mm at the base, 70–80°, texture chartaceous, adpressed hairs scattered on the primary vein adaxially, otherwise glabrous, abaxially with hairs dense on the primary and secondary veins, subdense on the lamina, food-body scars, primary vein level with the lamina adaxially, secondary veins distinctly brochidodromous, 7–8(–9) pairs, raised adaxially and prominent abaxially, diverging at 52–57° from the primary vein, strongly curved-ascending towards the margin, tertiary venation random reticulate; petiole 48–55 mm long. Staminate inflorescences not known. Carpellate inflorescences to 90 mm long, solitary from foliate or defoliate axils; bracts c. 0.4 mm long; pedicels c. 1.0 × 0.7 mm; calyx splitting into three or four lobes, lobes c. 2.9 mm long, densely pilose abaxially; petals 5, c. 3.3 mm long, moderately puberulent adaxially, abaxially densely tomentose, scale small relative to the petal; staminodes pilose adaxially; ovary ellipsoid; stigmas 2, sessile. Fruit racemes to 125 mm long; pedicels 2.8–3.0 × 1.7–2.5 mm. Fruits globose, stigmas 2, sessile.
Notes
Although a complete description of reproductive material is lacking for this taxon, it agrees with Ryparosa owing to the presence of racemose flowers, an irregularly splitting calyx, a lack of stipules and bifurcate hairs on the leaves and stems. The presence of hairs on the adaxial petals of carpellate flowers (staminate flowers are unknown) and the shallowly cordate base of leaf blades appear to be unique in the genus.
Distribution and ecology
Ryparosa maycockii is known from two locations in mixed Dipterocarp rainforest in the Sungai Belaga region of Sarawak, Borneo (Fig. 8b). A flowering specimen was collected in October and specimens with immature fruits in October and November.
Etymology
The specific epithet honours Dr Colin Maycock, a plant ecologist and colleague with considerable knowledge of the Bornean rainforests.
Additional specimens examined
BORNEO: Sarawak: 7th Division [actually 4th Division], Belaga, Ulu Sungai Semawat, [02°43′ N, 113°38′ E], Othman et al., S 43553, 17.x.1981 (KEP, L).
Ryparosa kurrangii B.L.Webber, sp. nov.
A R. acuminato Merr. foliis longis (240–280 mm), tenuibus (58–73 mm), nervis binatis 7–9 paribus curvato-ascendentibus, racemis e tubercularis grandis in trunco principali ortis, et racemis inflorescentiarum juvenium sine indumento denso, differt.
Understorey tree 15–20(–23) m tall, larger specimens with fluted trunks and undulate buttresses up to 2.5 m high, 0.5 m over the ground (Fig. 9a), some branching near the ground from large distinctive tubercles (Fig. 9b). Outer bark rough, grey-brown, inner bark bright pink; wood straw yellow. Leaf blades elliptic, (210–)240–280(–320) × (55–)60–75(–85) mm, acuminate at the apex, (35–)39–43(–54)°, with a well defined tip to 17 mm, attenuate at the base, (41–)47–51(–56)°, texture chartaceous, surface glabrous adaxially, abaxially with hairs moderately dense on the primary vein, sparse on the secondary veins and abaxial lamina (rarely glabrous on the abaxial lamina), distinct food-body scars, primary vein somewhat sunken or level with the lamina adaxially, secondary veins brochidodromous, 7 or 8 (rarely 9) pairs, raised adaxially and prominent abaxially, diverging at 47–56(–63)° from the primary vein, curved-ascending close to the leaf margin, tertiary venation reticulated; petiole (34–)40–50(–60) mm long. Staminate inflorescences to 300–400 mm long, in fascicles of up to 5 (solitary), cauliflorous from large tubercles on the lower main trunk (Fig. 9d, e; rarely from smaller branches); unopened flower buds globose; bracts 0.5–0.9 mm long; pedicels 1.3–2.3 × 0.3–0.5 mm; calyx splitting into 3 or 4 (2) lobes, lobes 2.3–4.0 mm long, sparse adpressed puberulent abaxially; petals (4–)5, 3.1–4.2 × 1.0–2.1 mm, glabrous adaxially, abaxially densely tomentose; scale fleshy (when fresh), 0.8–1.4 × 0.5–1.0 mm (dried); filaments (4–)5, united, sparsely villous beneath the anthers or entirely villous; stamens 3.1–3.9 mm long, extrorse anther dehiscence. Carpellate inflorescences to 250–350(–570) mm long, generally shorter than the staminate inflorescences, in fascicles of up to 5 (solitary), cauliflorous from large tubercles on the lower main trunk (very rarely from smaller branches); unopened flower buds pyriform; bracts 0.5–0.8 mm long; pedicels (0.8–)2.2–4.7 × 0.4–0.7 mm; calyx splitting into (2–)3(–4) lobes, lobes 3.0–4.0 mm long, sparse adpressed puberulent abaxially; petals (4–)5, 3.3–4.8 × 1.4–2.5 mm, glabrous adaxially, abaxially densely tomentose, scale fleshy (when fresh), 1.0–2.2 × 0.6–1.3 mm (dried); staminodes (4–)5, 0.3–0.7 mm long, fleshy stocky, pilose adaxially or entirely glabrous; ovary ellipsoid (obovoid); stigmas 2(–3), sessile. Fruit racemes to 260 mm long with extensive secondary thickening of racemes and pedicels. Fruits ellipsoid-oblate to globose drupes, 30–60 mm diameter when ripe, indehiscent, epicarp c. 1 mm thick, mesocarp fleshy, dark green when immature changing to bright yellow-orange when ripe, 1 locule; stigmas 2(–3), lateral in 1-seeded fruits, terminal in 2-seeded fruits, sessile. Seeds (1–)2, flattened-globose (2-seeded) or globose (1-seeded), 20–30 × 15–20 mm, containing copious endosperm surrounded by a thin, dark chartaceous layer and a protective woody layer (c. 0.6 mm thick with no lines of dehiscence). Germination cryptocotylar epigeal with fleshy haustorial cotyledons and a significant endosperm reserve.
Notes
Further collections (lodged at MELU) from several permanently tagged trees (including the tree from which the type specimen was collected) have both staminate and carpellate racemes and also fruits, so documenting the monoecious nature of R. kurrangii.
Distribution and ecology
Ryparosa kurrangii is restricted to a small strip of coastal rainforest between the Alexandra range and Cape Tribulation in the Daintree World Heritage area of northern Queensland, Australia (Fig. 8d). Typically a sub-canopy understorey tree, R. kurrangii is found at low altitudes (only once observed at an altitude above 240 m) in lowland tropical rainforests (Type 1a, 2a; sensu Tracey 1982), where it consistently grows near creeks and ephemeral streams. Young leaves flush in distinctive bursts during the late dry and early wet seasons (Oct–Dec). Peak flowering is during Jun–Sep (staminate and carpellate flowering times are generally separated by 3–4 weeks on individual plants), while fruiting is concentrated in Oct–Dec. Flowers are strongly scented (sickly sweet with overtones of wet-dog and body odour) with heavy nocturnal nectar production. Flower pollination is most likely carried out by small, unspecialised invertebrates (Webber 2005). Extant seed dispersal vectors are most likely restricted to scatter-hoarding animals (short distances) and the cassowary (between populations and over long distances; Webber and Woodrow 2004). The species is highly cyanogenic, with cyanogenic glycosides detected in all plant tissues (Webber 2005). Petiolar and leaf lamina tissue sections were found to contain crystal idioblasts with druse crystals (Webber 2005).
Conservation status
A significant proportion of the remaining R. kurrangii populations are on private land, outside the boundaries of protected reserves. Continued settlement and development in the Daintree region is threatening current populations and introduced animals are having a detrimental effect on potential seed vectors, which, in turn, has a negative effect on population recruitment (Webber 2005). Based on detailed knowledge of the distribution and age structure of current populations, the number of individuals in reserved areas and on freehold land, and known processes threatening the most flourishing of the remaining populations (Webber and Woodrow 2004; Webber 2005), an upgraded conservation code of vulnerable 2VCi (Briggs and Leigh 1996) is strongly recommended for this species.
Indigenous use
Kuku Yalangi elders said that the fruit of R. kurrangii were eaten during hunting and fishing trips in the lowlands region (B Walker, R Harrigan, pers. comm.). Fruits were gathered and taken back to more permanent camps in the nearby uplands region (e.g. Thornton Peak, Roaring Meg Creek), but the seeds did not grow at these camps when the Yalangi tried to establish trees for use as an uplands food source (R Harrigan pers. comm.). Flowers are reported as being used as a water infusion for ‘clearing the head’ (A Small pers. comm.). Flower racemes were soaked in hot water when heavy with nectar, and the resulting steam from the solution was inhaled deeply.
Etymology
From the local Kuku Yalangi dialect, kurrangi (cassowary), referring to the traditional name for a prominent coastal feature of the restricted taxon distribution (Cape Tribulation), as well as the apparent mutualism between R. kurrangii and cassowaries (Webber and Woodrow 2004).
Selected specimens (45 specimens examined)
AUSTRALIA: Queensland, Cook District: [Daintree lowlands region], Arsenic Creek, [16°10′ S, 145°25′ E], Stocker, G.C., 1617, 2.xi.1977 (QRS); [Daintree lowlands region], Cooper Creek, [16°10′ S, 145°24′ E], Nicholson, D.I., AFO 5078, 1.x.1980 (QRS); Daintree lowlands region, Mt Sorrow Valley, [description abbreviated, 16°07′ S, 145°27′ E], Webber, B.L., BW 008, 14.vii.2002 (MELU); Daintree NP, Mt Hutchinson [description abbreviated, 16°12′ S, 145°25′ E], Webber, B.L. & Curtis, A.S.O., BW 018, 12.iv.2003 (MELU); [Daintree NP], Oliver Creek, c. 1 km upstream from Cape Tribulation road, [16°08′ S, 145°26′ E], Hind, P. & Healey, D., KHILL 2080, 8.viii.1986 (NSW); [Daintree Region], Noah Creek, [16°10′ S, 145°25′ E], Hyland, B., 1080 RFK, 11.x.1967 (BRI).
Other specimens examined (selection only)
Ryparosa sp. nov. aff. acuminata
NEW GUINEA: Papua New Guinea: Morobe District, Sattelberg, Yuinzaing [Yunzain, 06°25′ S, 147°37′ E], Clemens, M.S., 6461, 21.vi.1937 (K, L, NY).
Ryparosa sp. nov. aff. calotricha
BORNEO: Sarawak: Matang (proposed NP), SW slope of Gunong Bawang, [01°35′ N, 110°10′ E], Burley, J.S. & Lee, Bernard, 317, 1.x.1987 (L, SING). West Kalimantan: G. Bentuang area, 150 km NE of Pontiank, 5–10km N of Masa village, [00°52′ N, 110°26′ E], Burley, J.S. & Tukirin et al., 2303, 6.vi.1989 (K).
Ryparosa acuminata Merr.
BORNEO: East Kalimantan: North Borneo Conomisi, Bukit Sungai Toelit [Tulit, near Tenampak, 04°15′ N, 116°49′ E], Amdjah (with van Genderen Stort, Capt. P.), 705, ix.1912 (BO, K). Sabah: North-East Borneo, Sandakan Bay, southern part of Nunukun [Nunuyan] Island, [05°55′ N, 118°07′ E], Kostermans, A., 9205, 1.i.1954 (BO, BRI, K, SING); Papar District, Simpangan., [06°27′ N, 116°43′ E], Burgess, P.F., SAN 28428, 28.v.1962 (K).
Ryparosa baccaureoides Sleumer
BORNEO: Sabah: Mt Kinabalu FR, [Naradaw area], intersection of Ulu Liwagu and Ulu Mesilau, [05°58′ N, 116°37′ E], Chew, W.L., Corner, E.J.H. & Stainton, A., RSNB 2926, 10.ix.1961 (CANB). Sarawak: Kuching District, Matang, Ulu China, [01°36′ N, 110°12′ E], Ashton, P.S., S 16699, 22.xi.1962 (MEL); 4th Division, Bario, route to Batu Lawi, [03°48′ N, 115°23′ E], Awa, Dayang et al., S 50477, 1.viii.1985 (KEP, L).
Ryparosa calotricha Mildbr.
NEW GUINEA: Papua New Guinea: Morobe District, Oomsis (near Lae), [06°41′ S, 146°48′ E], White, K.J., NGF 10484, 5.iii.1959 (BRI, CANB, L, LAE, SING); Milne Bay District, Rabaraba Subdistrict, junction of Ugat and Mayu rivers, near Mayu 1, [09°39′ S, 149°10′ E], Streimann, H. & Katik, P., NGF 28904, 15.vii.1972 (BO, L, LAE); [Northern District, Owen Stanley Range], Isuarava, [09°00′ S, 147°44′ E], Carr, C.E., CEC 15169, 4.ii.1936 (K, L, NY, SING). Western New Guinea: [West Irian Jaya Province] Sorong Regency, Sausapor, [00°32′ S, 132°06′ E], Versteegh, Chr., BW 4617, 19.x.1956 (CANB).
Ryparosa glauca Ridl.
BORNEO: Brunei: Temburong, Bukit Biang, [04°41′ N, 115°06′ E], Forman, L.L., LLF 924, 17.x.1989 (K). Sarawak: 4th Division, Gunong Mulu NP, Ulu Sungai Berar, [04°02′ N, 114°55′ E], Chai, Paul, S 39626, 11.i.1978 (KEP); Tau Range, Sungei Mayeng, [02°42′ N, 113°05′ E], Purseglove, J.W., P 5331, 4.vi.1956 (NY, SING). LESSER SUNDA ISLANDS: Flores: Manggarai Regency, [Laboehanbadjo District], Naga, [08°42′ S, 119°56′ E], Schmutz, Fr. E., 4135, 6.v.1978 (L); Manggarai Regency, Palie [cf. Reo District, Palit, c. 08°32′ S, 120°11′ E], Schmutz, Fr. E., 4818, 3.iii.1981 (L).
Ryparosa kostermansii Sleumer
BORNEO: East Kalimantan: Peak of Balikpapan, (Gunung Beratus), Beoul, [01°02′ N, 116°20′ E], Kostermans, A., 7564, 16.vii.1952 (L). West Kalimantan: [Bukit Raya NP], Bukit Raya, [00°45′ S, 112°47′ E], Nooteboom, H.P., 4886, 6.ii.1983 (L). Sabah: Lahad Datu, Kennedy Bay, Takun (Section 42), [05°00′ N, 118°00′ E], Chai, Nuin, SAN 26079, 6.ix.1961 (BO). Sarawak: 1st Division, Lundu District, Gunung Pueh, [01°48′ N, 109°41′ E], Mamit, James et al., S 34417, 14.vi.1974 (L); 5th Division, Limbang, path to Bukit Pagon, [04°18′ N, 115°09′ E], Awa, Dayang & Lee, B., S 47569, 29.vii.1984 (L).
Ryparosa multinervosa Slooten
SUMATRA: Sumatra, Riau Province: [Indragiri Hulu Regency], Tigapuluh Mountains, 5 km W of Talanglakat on Rengat-Jambi Rd, Bukit Karampal area, [00°46′ S, 102°32′ E], Burley, J.S. & Tukirin et al., 1223, 6.xi.1988 (L); North of Kampar Regency (not far from North Sumatra), River Mahato Hulu (kiri) area, [01°23′ N, 100°34′ E], Laumonier, Y. & Torquebiau, E., TFB 4245, 13.iv.1983 (L).
Drypetes longifolia Pax & K.Hoffm.
ANDAMAN ISLANDS: South Andaman Island: Wrightmyo, [11°47′ N, 92°43′ E], Balakrishnan, N.P. & Bhargava, N., 3465, 14.iii.1976 (L); Dhani Khari, [11°33′ N, 92°41′ E], Nair, N.G., 3639, 27.iii.1976 (L).
Acknowledgments
We acknowledge Bennett Walker and Ronnie Harrigan and the elders of the Kuku Yalangi people for passing on their local knowledge of Australian Ryparosa, Alan Curtis for his tireless assistance in the field, Sue Zmarzty for providing expert advice and access to K specimens, and an anonymous reviewer for providing detailed feedback on earlier versions of this manuscript. Pauline Ladiges, Nicole Middleton, Bryan Mole, Sita Ariati, Julisasi Hadiah, Rismita Sari, Sunil Srivastava and Pieter Baas were a great help in providing either methodological advice or background information and Neville Walsh assisted with nomenclature recommendations and Latin translations. Niels Klazenga, Juergen Kellerman, Bernardo Foth and Anton Cozijnsen helped with translating the many early taxonomic papers, while Brendan Whyte was generous with his time and patience in helping find obscure locations throughout south-east Asia. BLW was a recipient of an Australian Postgraduate Award PhD Scholarship. Field sampling was conducted under Scientific Purposes permits issued by the Queensland Department of Environment and Heritage (F1/000233/00/SAA, WISP01144103, WITK01149403) and with the full permission and support of private landholders when collected on freehold land.
Agosti D,
Moog J, Maschwitz U
(1999) Revision of the Oriental plant–ant genus Cladomyrma. American Museum Novitates 3283, 1–24.

Brühl P, King G
(1896) A century of new and rare Indian plants. Annals of the Royal Botanic Garden, Calcutta
, 71–170.

Chakrabarty T, Gangopadhyay M
(1992) The Flacourtiaceae of Andaman–Nicobar islands. Journal of Economic and Taxonomic Botany 16, 715–722.

Chase MW,
Zmarzty S,
Lledo MD,
Wurdack KJ,
Swensen SM, Fay MF
(2002) When in doubt, put it in Flacourtiaceae: a molecular phylogenetic analysis based on plastid rbcL DNA sequences. Kew Bulletin 57, 141–181.

Dahlgren RMT, van Wyk AE
(1986) Structures and relationships of families endemic to or centered in southern Africa. Monographs in Systematic Botany from the Missouri Botanical Garden 25, 1–94.

Faith DP,
Minchin PR, Belbin L
(1987) Compositional dissimilarity as a robust measure of ecological distance: a theoretical model and computer simulations. Vegetatio 69, 57–68.
| Crossref | GoogleScholarGoogle Scholar |

Gilg E
(1918) Die bis jetzl aus Neu-Guinea bekannt gewordenen Flacourtiaceen. Botanische Jahrbucher fur Systematik, Pflanzengeschichte und Pflanzengeographie 55, 273–294.

Gower JC
(1971) A general coefficient of similarity and some of its properties. Biometrics 27, 857–871.
| Crossref | GoogleScholarGoogle Scholar |

Jarvie JK, Stevens PF
(1998) New species and notes on Violaceae and Flacourtiaceae from Indo-Malesia. Harvard Papers in Botany 3, 253–262.

King G
(1890) Materials for a flora of the Malayan Peninsula. Journal of the Asiatic Society of Bengal, Calcutta 59, 113–206.

Koorders SH, Valeton T
(1900a) Bijdrage No. 5 tot de Kennis der Boomsoorten op Java. Additamenta ad cognitionem Florae arboreae Javanicae. Mededeelingen uit ’sLands Plantentuin 33, 1–464.

Koorders SH, Valeton T
(1900b) Bijdrage No. 6 tot de Kennis der Boomsoorten op Java. Additamenta ad cognitionem Florae arboreae Javanicae. Mededeelingen uit ’s Lands Plantentuin 40, 1–193.

Kruskal JB
(1964a) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29, 1–27.
| Crossref | GoogleScholarGoogle Scholar |

Kruskal JB
(1964b) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29, 115–129.
| Crossref | GoogleScholarGoogle Scholar |

Kurz S
(1873) On the affinity and position of Ryparia, Bl. Journal of Botany 11, 233–234.

Kurz S
(1876) A sketch of the vegetation of the Nicobar Islands. Journal of the Asiatic Society of Bengal, Calcutta XLV, 105–164.

Lemke DE
(1988) A synopsis of Flacourtiaceae. Aliso 12, 29–43.

McKey D
(2000) Leonardoxa africana (Leguminosae: Caesalpinioideae): a complex of mostly allopatric subspecies. Adansonia 22, 71–109.

Mildbraed J
(1928) Gertrudia K. Schum. = Ryparosa Bl. Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem 10, 338–340.

Rao MKV, Chakrabarty T
(1985) Drypetes longifolia (Euphorbiaceae) in Andamans. Journal of Economic and Taxonomic Botany 6, 445.

Renner SS, Ricklefs RE
(1995) Dioecy and its correlates in the flowering plants. American Journal of Botany 82, 596–606.
| Crossref | GoogleScholarGoogle Scholar |

Ridley HN
(1936) Notes on Malay Flacourtiaceae. Journal of Botany 74, 221–225.

Savolainen V,
Fay MF,
Albach DC,
Backlund A,
van der Bank M,
Cameron KM,
Johnson SA,
Lledó MD,
Pintaud JC,
Powell M,
Sheahan MC,
Soltis DE,
Soltis PS,
Weston P,
Whitten WM,
Wurdack KJ, Chase MW
(2000) Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequence. Kew Bulletin 55, 257–309.

Shepard RN
(1962) The analysis of proximities: multidimensional scaling with an unknown distance function. Psychometrika 27, 125–140.
| Crossref | GoogleScholarGoogle Scholar |

Sleumer HO
(1954b) Florae Malesianae Precursores. VII. New Malaysian Flacourtiaceae. Blumea 7, 484–497.

Sleumer HO
(1985) The Flacourtiaceae of Thailand. Blumea 30, 217–250.

van Slooten DF
(1924) Flacourtiaceae. Nova Guinea 14, 190–194.

van Slooten DF
(1925) Contributions à l’étude de la Flore des Indes Néerlandaises—the Flacourtiaceae of the Dutch East Indies. Bulletin du Jardin Botanique Buitenzorg. Series III 7, 291–421.

Sokal RR, Michener CD
(1958) A statistical method for evaluating systematic relationships. University of Kansas Science Bulletin 38, 1409–1438.

Sosa V,
Chase MW, Barcenas C
(2003) Chiangiodendron (Achariaceae): an example of the Laurasian flora of tropical forests of Central America. Taxon 52, 519–524.

Stevens PF
(1991) Character states, morphological variation, and phylogenetic analysis: a review. Systematic Botany 16, 553–583.
| Crossref | GoogleScholarGoogle Scholar |

Thiele K
(1993) The holy grail of the perfect character: the cladistic treatment of morphometric data. Cladistics 9, 275–304.
| Crossref | GoogleScholarGoogle Scholar |

Webber BL, Woodrow IE
(2004) Cassowary frugivory, seed defleshing and fruit fly infestation influence the transition from seed to seedling in the rare Australian rainforest tree, Ryparosa sp. nov. 1 (Achariaceae). Functional Plant Biology 31, 505–516.
| Crossref | GoogleScholarGoogle Scholar |

Webber BL,
Abaloz BJ, Woodrow IE
(2006) Myrmecophilic food body production in the understorey tree, Ryparosa kurrangii (Achariaceae), a rare Australian rainforest taxon. New Phytologist in press ,

Webber BL,
Moog J,
Curtis ASO, Woodrow IE
(2007) The diversity of ant-plant interactions in the rainforest understorey tree, Ryparosa (Achariaceae): food bodies, domatia, prostomata and hemipteran trophobionts. Botanical Journal of the Linnean Society in press ,

Wendt T
(1988) Chiangiodendron (Flacourtiaceae: Pangieae), a new genus from southeastern Mexico representing a new tribe for the New World flora. Systematic Botany 13, 435–441.
| Crossref | GoogleScholarGoogle Scholar |

Zepernick B
(1978) Typen und Typoide der Flacourtiaceae im Generalherbar des Botanischen Museums Berlin-Dahlem. Willdenowia 8, 409–424.

1 The observation of free stamens in staminate R. kostermansii flowers is not the first time this has been reported in the genus (see R. caesia, van Slooten 1919, 1925; R. hirsuta, Sleumer 1954a).


 ; 151), R. baccaureoides (□; 10), R. calotricha (
; 151), R. baccaureoides (□; 10), R. calotricha ( ; 3, 81), R. glauca (
; 3, 81), R. glauca ( ; 13), R. kostermansii (
; 13), R. kostermansii ( ; 17, 18), R. multinervosa (
; 17, 18), R. multinervosa ( ; 41, 82), R. javanica s. str. (◆; 1), R. amplifolia s. str. (●; 7), R. kurzii s. str. (
; 41, 82), R. javanica s. str. (◆; 1), R. amplifolia s. str. (●; 7), R. kurzii s. str. ( ; 2), R. wrayi s. str. (
; 2), R. wrayi s. str. ( ; 6), R. taxon A (R. maculata sp. nov.; ○; 5), R. taxon B (R. anterides sp. nov.; ◇; 9), R. taxon C (R. milleri sp. nov.; ; 12), R. taxon D (R. maycockii sp. nov.; ▼; 14), R. taxon E (R. kurrangii sp. nov.; ▲; 16), R. sp. nov. aff. acuminata (
; 6), R. taxon A (R. maculata sp. nov.; ○; 5), R. taxon B (R. anterides sp. nov.; ◇; 9), R. taxon C (R. milleri sp. nov.; ; 12), R. taxon D (R. maycockii sp. nov.; ▼; 14), R. taxon E (R. kurrangii sp. nov.; ▲; 16), R. sp. nov. aff. acuminata ( ; 152) and R. sp. nov. aff. calotricha (
; 152) and R. sp. nov. aff. calotricha ( ; 42). Numbers refer to specimen groups.
; 42). Numbers refer to specimen groups.