Non-linear response of soil N2O emissions to nitrogen fertiliser in a cotton–fallow rotation in sub-tropical Australia
Clemens Scheer A B , David W. Rowlings A and Peter R. Grace AA Institute for Future Environments, Queensland University of Technology, Brisbane, Qld 4000, Australia.
B Corresponding author. Email: clemens.scheer@qut.edu.au
Soil Research 54(5) 494-499 https://doi.org/10.1071/SR14328
Submitted: 20 November 2014 Accepted: 8 October 2015 Published: 6 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Nitrogen (N) fertiliser is a major source of atmospheric nitrous oxide (N2O), and over recent years there has been growing evidence for a non-linear, exponential relationship between N fertiliser application rate and N2O emissions. However, there is still a high level of uncertainty around the relationship of N fertiliser rate and N2O emissions for many cropping systems. We conducted year-round measurements of N2O emission and lint yield in four N-rate treatments (0, 90, 180 and 270 kg N ha–1) in a cotton–fallow rotation on a black vertosol in Australia. We observed a non-linear exponential response of N2O emissions to increasing N fertiliser rates with cumulative annual N2O emissions of 0.55, 0.67, 1.07 and 1.89 kg N ha–1 for the four respective N fertiliser rates, but no N response to yield occurred above 180 kg N ha–1. The annual N2O emission factors induced by N fertiliser were 0.13, 0.29 and 0.50% for the 90, 180 and 270 kg N ha–1 treatments respectively, significantly lower than the IPCC Tier 1 default value of 1.0%. This nonlinear response suggests that an exponential N2O emissions model may be more appropriate for estimating emission of N2O from soils cultivated to cotton in Australia. It also demonstrates that improved agricultural N-management practices can be adopted in cotton to substantially reduce N2O emissions without affecting yield.
Introduction
Nitrous oxide (N2O) contributes to the greenhouse effect due to its high global warming potential (nearly 300 times greater than carbon dioxide over a 100-year horizon) and is also the largest ozone depleting substance of the 21st century (IPCC 2007; Ravishankara et al. 2009). Increased use of nitrogen (N) fertiliser and animal manure are the main sources of atmospheric N2O. Globally, croplands are responsible for 66% of total anthropogenic N2O emissions and these emissions are predicted to double by 2050 (Davidson and Kanter 2014). In soils, N2O is mainly produced by the microbial processes of autotrophic nitrification (oxidation of ammonium to nitrate) and heterotrophic denitrification (reduction of nitrate to N2O and ultimately N2). The magnitude of these emissions is strongly affected by soil conditions and agricultural management, in particular the addition of N fertiliser (Butterbach-Bahl et al. 2013).
Total added N fertiliser is the most important predictor of N2O emissions from cropping land, and early studies showed a linear relationship between N input and direct N2O emission from the soil (Bouwman 1996). Consequently, the IPCC adopted a linear relationship for its Tier 1 emission factor (EF) methodology (IPCC 1997, 2006). This EF approach assumes that a fixed amount of N added as fertiliser is transformed and emitted as N2O, and is currently still being used for most national greenhouse gas (GHG) inventories (IPCC 2006). However, the relationship between N fertiliser rate and N2O emission is complex and few studies have measured N2O emissions under more than two N fertiliser rates to establish clear functional relationships. Consequently, there is still a high degree of uncertainty in the relationship between N fertiliser rate and N2O emission for many cropping systems.
In recent years there has been growing evidence for a non-linear, exponential relationship from a range of cropping systems, particularly for fertiliser rates that greatly exceed crop requirements (Hoben et al. 2011; Kim et al. 2013; McSwiney and Robertson 2005). In a recent global meta-analysis of 78 different studies, Shcherbak et al. (2014) found a general trend of exponentially increasing N2O emissions as N fertiliser rates increased above crop N demand for the majority of crop types examined. However, such data do not currently exist for cotton cropping systems. A non-linear response of N2O emissions to N fertiliser additions means that N2O emission factors are not constant and will depend on N input rates. A clear understanding of this relationship is crucial for both N2O inventory estimates and the design of effective mitigation strategies.
Cotton production provides an ideal opportunity to investigate the relationship between N2O emissions and N fertiliser inputs due to high N fertiliser (up to 400 kg N ha–1) and irrigation inputs, and significant emissions of N2O from irrigated cotton fields have been reported (Scheer et al. 2013). In Australia, cotton is grown on almost 600 000 ha in the inland regions of northern New South Wales and southern Queensland. However, there are still limited data available on the effect of fertiliser N rate on N2O emissions and to date no emission factors have been reported for a cotton–fallow rotation in Australia over a full year. Consequently, this field-based study measured N2O emissions from a typical cotton–fallow rotation with four N fertiliser rates over one year in southeast Queensland, Australia. The two main objectives of the study were: (i) to investigate N2O emissions (including EFs) and lint yield from a cotton–fallow rotation in response to increasing N fertiliser rate; and (ii) test the assumption that N2O emissions increase linearly in response to N fertiliser rates.
Material and methods
Study site
The field experiment was conducted during the 2010/11 cotton season at the Agri-Science Queensland, Department of Employment, Economic Development and Innovation (DEEDI) Kingsthorpe Research Station. The station is located in the Darling Downs region ~140 km west of Brisbane (27°31ʹS, 151°47ʹE, 431 m above mean sea level). Prior to the present study, the field site was used for an irrigation study under a cotton–wheat rotation for which crop residues were removed from the plots after harvest (Scheer et al. 2012, 2013). The field site included an overhead sprinkler irrigation system and so there were no ridges or furrows. The region has a sub-tropical climate with an average annual precipitation of 630 mm (1990–2010) (Commonwealth Bureau of Meteorology, www.bom.gov.au/climate) with most rainfall during October–March in the summer crop growing season. The soil at the site is classified as a haplic, self-mulching, black vertosol using the Australian Soil Classification (Isbell 2002). It has a heavy clay texture (76% clay) in the 1.5-m root zone profile, with a distinct change in soil colour from brownish black (10YR22) in the top 90 cm to dark brown (7.5YR33) deeper in the profile. The soil is formed in an alluvial fan of basalt rock origin, slowly permeable, with a surface slope of ~0.5%. Physical and chemical characteristics of the soil profile are shown in Table 1.

|
Experimental design
The fertiliser response trial was set up as a randomised complete block design. Each block was replicated three times, with four N fertiliser rates in each block. Each experimental plot was 13 m wide × 20 m in length, with the cotton crop (Gossypium hirsutum L. cv. Bollgard® II) planted in a north–south orientation. A buffer zone (4 m) was planted between plots and an access track (4 m) was located at the centre of the research area. Cotton was planted on 5 November 2007 at a density of ~17 seeds m–1, a depth of 4 cm and a row spacing of 1 m. The aim was to get an established stand of 11–12 plants m–1, which is the recommended density for Bollgard® II. Weeds were controlled by a combination of manual chipping and a chemical control (1 kg ha–1 of glyphosate). Only N fertiliser was applied at sowing and evenly broadcasted by hand to each replicate plot to prevent possible inaccuracy in N2O measurements due to uneven fertiliser distribution.
The N fertiliser treatments were
-
Zero nitrogen fertiliser (0N) – i.e. no added fertiliser;
-
90 kg N ha–1 (90N) – 90 kg N ha–1 urea basal application at planting (4 November 2010);
-
180 kg N ha–1 (180N) – 90 kg N ha–1 urea basal application at planting (4 November 2010) and 90 kg N ha–1 urea in two side dressings (5 January and 3 March 2011);
-
270 kg N ha–1 (270N) – 90 kg N ha–1 urea basal application at planting (4 November 2010) and 180 kg N ha–1 urea in two side dressings (5 January and 3 March 2011).
The plots could be irrigated individually with bore water using a hand-shifted sprinkler and partial-circle sprinkler heads to avoid irrigating adjacent plots. However, during this study, growing season rainfall exceeding the typical water demand (600–800 mm) of cotton and irrigation did not occur.
N2O flux measurement
The N2O fluxes were measured over an entire year, including the cotton cropping season from 5 November 2010 to 9 June 2011 and the following fallow phase from 9 June to 15 November 2011. Emissions were measured using the closed chamber technique using quality criteria as outlined by de Klein and Harvey (2013) and Parkin and Venterea (2010). This method uses a gas-tight chamber which encloses soil for a given interval. The chamber consists of a frame inserted a few centimetres into the soil and a lid that is fixed to the frame throughout the sampling period. Chamber enclosure is achieved by a sealed gasket at the lower edge of the lid. We used cylindrical PVC-chambers with an inner diameter of 22.5 cm and a height of 20 cm that were randomly inserted between the plant rows (which were 1 m apart) in each plot (i.e. the measurements did not account for potential N2O emissions directly from the cotton plants). The volume of each chamber was ~0.008 m3 and the cross-sectional area was 0.04 m2. Fluxes were measured by collecting air samples from the chamber head space. Of headspace air, 20 mL was drawn through a septum into gas-tight 20-mL polypropylene syringes at 0, 30 and 60 min after the soil was covered and inserted into evacuated vials (Exetainers®). Chamber temperature was monitored during the measurement using an electronic temperature sensor. The gas samples were then analysed for N2O using a gas chromatograph (Shimadzu GC-2014, Kyoto, Japan) equipped with an electron capture detector.
The N2O flux rates were measured from three replicated chambers per experimental plot (n = 9 per treatment) to minimise the error associated with the spatial variability of N2O emissions over the cotton growing period. The number of chambers was decreased to one replicated chamber within each treatment per experimental plot (n = 3 per treatment) over the fallow period when only small fluxes (<5.0 g N2O-N ha–1 day–1) were observed in all treatments. Sampling frequency was optimised according to the recommendations of Reeves and Wang (2015). Specifically, measurements were conducted three times a week immediately after fertilisation followed by heavy rainfall events and weekly over the remaining period, which was expected to provide a highly accurate estimate (±10% error) compared with measurements with a sub-daily temporal resolution (Reeves and Wang 2015). Fluxes were measured once on each sampling day during 0900–1100 hours, which has been shown to best approximate the daily mean N2O flux (Reeves and Wang 2015).
Ancillary measurements
An EnviroStation (ICT International Pty Ltd, Armidale, NSW, Australia) electronic weather station was installed at the research site to measure local weather variables. The station recorded both daily and hourly values of solar radiation, air temperature (maximum, minimum and average), relative humidity, wind speed and rainfall. Volumetric soil water content was measured in the surface soil (0–10 cm) in each experimental plot at each gas sampling using a hand-held MP406 standing wave soil moisture probe (ICT International Pty Ltd) that was calibrated for the soil at the research site. Water-filled pore space (WFPS) was calculated by dividing volumetric water content by total porosity. Total porosity was calculated as [1 – (bulk density/particle density)] × 100%] using measured soil bulk density data (arithmetic means of four samples) and an assumed particle density of 2.65 g cm–3 (Barton et al. 2008). Additionally, at the beginning of the growing season, bulk soil samples were taken from each plot by combining 5–10 soil cores (0–10 cm depth) and analysed for soil texture, total carbon (C%) and total nitrogen (N%). Seed cotton and lint yield were determined in each plot by harvesting a 2.5 m length of two cotton rows outside the chamber area by hand and the seed cotton was ginned.
Statistical analysis and calculations
The N2O emissions were calculated from the linear increase of the gas concentration at each sampling time (0, 30 and 60 min during the time of chamber closure), adjusted for area and volume of the chamber and corrected for chamber temperature and air pressure as described by Scheer et al. (2014). The coefficient of determination (R2) for the linear regression was calculated and used as a quality check for the measurement. For R2 < 0.9 (R2 < 0.7 for small flux rates <5.0 g N2O-N ha–1 day–1) the measurement was rejected and not used in subsequent analyses.
Annual N2O emissions from each plot were calculated by integrating hourly losses with time. Days where fluxes were not measured were filled by linear interpolation across missing days. Emission factors of the N fertiliser applied to the soil were calculated using:
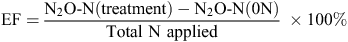
where EF is the percentage of the total fertiliser N applied that was emitted as N2O-N, N2O-N is the total N2O over one year (kg N ha–1 year–1) for a N fertiliser rate and total N applied is the amount of N fertiliser applied (kg N ha–1 year–1). Effects of treatment on total emissions were assessed by two way analysis of variance, which estimated variability due to experimental block and treatments. The null hypothesis significance test for treatment was conducted using an F-ratio test. Treatment effects on average total emissions were compared statistically by comparison with a least significant difference calculated at 5% critical value. Statistical analysis was undertaken using SPSS 16.0 (SPSS Inc., USA).
Results
Seasonal variability of environmental and soil conditions
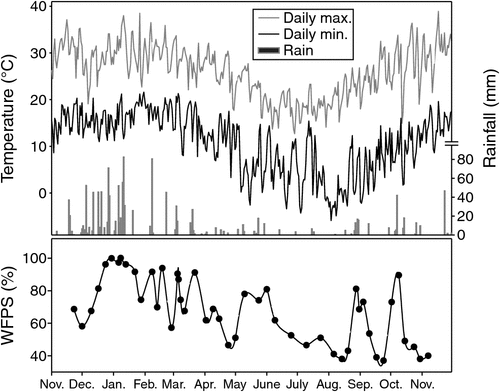
Over the one-year observation period, 1260 mm of rainfall was recorded at the study site. This rainfall was double the long-term annual precipitation (630 mm). The study site received exceptionally high rainfall over a two-month period from mid-November 2010 to mid-January 2011. Over 700 mm of rainfall was recorded during this two-month period, including several heavy rainfall events exceeding 50 mm on a single day (Fig. 1). Over 1000 mm of rainfall was recorded during the entire cotton cropping period, exceeding the typical water demand (600–800 mm) of cotton at the experimental site. Consequently there was no irrigation over the 2010/11cotton cropping period. The mean air temperature during the study period was 18.4°C; maximum hourly air temperature (38.9°C) was recorded in November and minimum hourly air temperature (–5.9°C) in August 2011 (Fig. 1).
|
Soil WFPS in the surface soil (0–10 cm) varied over the year in response to rainfall. Soil WFPS was high over the cotton growing season (57–100%), due to frequent rainfall at the onset of the study and the high water holding capacity of the clay; whereas WFPS was significantly lower (38–91%) over the fallow period (Fig. 1). The lowest calculated WFPS (38%) was in August 2011 after an extended dry period.
Influence of N fertiliser rates on N2O emissions and cotton yield
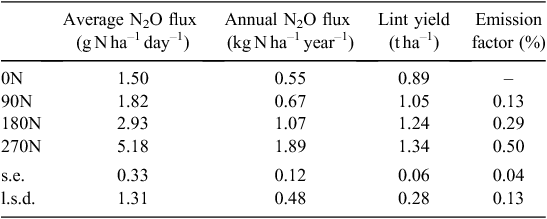
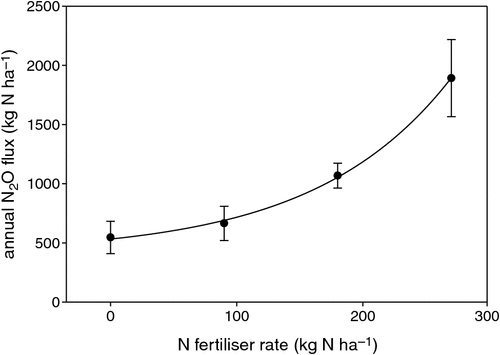
Average N2O flux (over 1 year) was 5.2, 2.9, 1.8 and 1.5 g N2O-N ha–1 day–1 in the 270N, 180N, 90N and 0N treatments respectively, corresponding to cumulative annual emissions of 1.89, 1.07, 0.67 and 0.55 kg of N emitted as N2O (Table 2). There was a clear non-linear response of N2O emissions to N fertiliser rates, which was best described by a non-linear exponential growth function (R2 = 0.99, Fig. 2).
|
|
There was no significant difference in annual N2O emissions from 0N and 90N treatments, but there was a significant increase with increasing fertiliser rate for 180N and 270N treatments. EFs were 0.13, 0.29 and 0.50% of the total amount of mineral N applied to the plots for the 90N, 180N and 270N treatments respectively (Table 2).
There was a significant effect of N fertiliser rate on average lint yield. Lint yield was highest in the 270N with 1.34 t ha–1 and was not significantly different to the 1.24 t ha–1 of the 180N treatment. There was also no significant difference in lint yield between the 0N (0.89 t ha–1) and the 90N (1.05 t ha–1) treatments, but average yield was significantly lower in the 0N than the 180N and 270N treatments (Table 2). Lint percentage of the seed cotton was ~43% and did not vary with N fertiliser rate. These yields were lower than the average cotton yield in Australia in the 2010/11 season (1.5 t ha–1) (CRDC 2014), but within the range of yields reported from previous cotton trials at the same site (Payero 2010; Scheer et al. 2013).
Seasonal variability of N2O emissions
The N2O emissions showed typical high temporal and spatial variability as frequently reported for soil N2O fluxes (Fig. 3). Seasonal N2O fluxes during the cotton cropping period showed a high degree of spatial variability, with the coefficients of variation across replicate chambers (n = 9) in the range of 20–53%. The majority of N2O fluxes occurred over the cotton growing period when there was a clear influence of fertilisation events on N2O emissions. Highest emissions occurred after heavy rainfall following fertilisation and there was a significant effect of the N fertiliser rate on the magnitude of the flux. For example, the first N2O peak occurred following the first rainfall after planting on 18 November, reaching 18.2 g N2O-N ha–1 day–1 in the 90N, 180N and 270N treatments and was significantly lower (7.1 g N2O-N ha–1 day–1) in the 0N treatment. In the 0N and 90N treatments, for which no additional fertiliser was applied after planting, there were no further N2O emission peaks; however, in the 180N and 270N treatments, two additional emission peaks occurred after the application of N fertiliser and subsequent rainfall in January and March 2011. Overall highest emissions were observed on 13 January and reached 72 g N2O-N ha–1 day–1 in the 270N treatment after the application of 90 kg N urea ha–1 on 5 January was followed by heavy rainfall events (50 mm on 6 January and 78 mm on 11 January). The magnitude of this ‘emission pulse’ was significantly lower (28.1 g N2O-N ha–1 day–1) in the 180N treatment, for which only 45 kg N urea ha–1 was applied on 5 January. From April onwards there were no significant differences in N2O emissions between the treatments and only small fluxes (<5.0 g N2O-N ha–1 day–1) were observed in all treatments.
Discussion
Annual N2O emissions (0.55–1.9 kg N2O-N ha–1) were in good agreement with values reported from other cotton systems in Australia (Macdonald et al. 2015; Scheer et al. 2013), but at the lower end of emissions from cotton farming systems in other countries. In northern China, Liu et al. (2010) observed annual emissions of 2.6 kg-N ha–1 year–1 from irrigated cotton fertilised with 66 kg N ha–1 and Scheer et al. (2008) reported seasonal emissions of 0.9–6.5 kg N ha–1 from a range of different irrigated cotton systems in Uzbekistan (fertiliser rates of 162.5–250 kg N ha–1). These results confirm that the N2O emission potential from cotton on vertosols in Australia is generally low, most likely due to limited availability of labile carbon in the soil and the neutral to alkaline soil pH, which restricts denitrification activity and increases the N2/N2O emissions ratio (Scheer et al. 2012).
This study showed a non-linear exponential response of N2O emissions to N fertiliser rates that may be typical for fertilised cotton systems on black vertosols in Australia. In our experiment, high rates of N fertiliser led to increased emissions of N2O with no significant effect on yield. We observed a clear non-linear response of N2O emissions to N fertiliser rates. There was no significant difference in cumulative N2O emissions between the 0N and 90N treatments, indicating that soil microbes responsible for N2O production had limited access to N. The N2O fluxes in the 180N and 270N treatments increased by 60 and 184% respectively, compared with the 90N treatment. Previous studies reported both linear and non-linear responses of cumulative N2O emissions to N fertiliser rates (Hoben et al. 2011; Lebender et al. 2014; Liu et al. 2012; Ma et al. 2010; McSwiney and Robertson 2005). However, recent metadata analyses suggest a non-linear response of direct N2O emissions to increased N additions (Kim et al. 2013; Shcherbak et al. 2014). It is not entirely clear what drives the relationship of N2O emissions to N input in different agro-ecosystems. In theory, a linear response is expected in N-limited systems where N2O emission is primarily controlled by the competition of plants vs microbes for available N. A non-linear exponential response is expected as soon as N fertiliser application exceeds plant demand, and then small increases in N fertiliser rates will result in disproportionally higher N2O fluxes at higher N application rates. Once N addition increases beyond the capacity of soil microbes to take up and utilise N, the rate of increase for N2O production would slow and finally reach a steady state (Kim et al. 2013).
In this study, N2O emissions were mainly controlled by the combined impact of fertilisation and rainfall. Highest emissions occurred after heavy rainfall following fertilisation and there was a significant effect of the N fertiliser rate on the magnitude of the flux. This is consistent with previous studies on N2O emissions from cotton where highest emissions following irrigation or rainfall immediately after fertiliser N application have been reported (Liu et al. 2010; Scheer et al. 2008, 2013). Consequently, the non-linear increase in N2O emissions was mainly caused by highly elevated emissions after the side dressing of N fertiliser in the 180N and 270N treatments at the beginning of January and beginning of March 2011 that were followed by heavy rainfall. Applying double the N rate in the 270N treatment (90 kg N ha–1) in January increased the emission pulse by almost four-fold compared with the 180N treatment (45 kg N ha–1). The strong increase in N2O emissions with the higher N fertiliser rate shows that at that stage the conditions were such that N supply greatly exceeded crop demand and other factors such as soil moisture or soil temperature were not limiting. This indicates that a large amount of unused N was available for soil microbes responsible for N2O production, and resulted in a higher proportion of the applied N fertiliser being lost as N2O to the environment. There was no significant difference in lint yield between the 180N and 270N treatments, suggesting an optimal fertiliser range of 180–270 kg N ha–1 for maximum yield in the investigated cotton system. It should be noted that this was not a N-response trial to determine optimum N rates, which would require more N rates; however, the overall trend is in good agreement with previous results from Australian cotton systems where an optimal economic N rate of 200 kg N ha–1 has been reported (Macdonald et al. 2015; Rochester 2012). Above the optimal fertiliser rate, N2O emissions will increase exponentially with no significant effect on yield.
The annual N2O EFs, induced by N fertiliser in the present study, were 0.13, 0.29 and 0.50% for the 90N, 180N and 270N treatments respectively (Table 2). These are lower than the IPCC default value used from global inventories (1% of N applied (IPCC 2006)) but in reasonable agreement with the EF for irrigated cotton (0.5%) used by the Australian Government for their national GHG Inventory report (ANGA 2010). It is also at the lower end of EFs reported for other irrigated cotton systems with 0.12–4.0% (Liu et al. 2010; Macdonald et al. 2015; Scheer et al. 2008). This highlights the need for differentiated EFs that take the non-linear response to N fertiliser rates into account to reliably estimate emissions from different agricultural systems. More data is required to assess N2O emissions as a function of added N for other intensively fertilised systems in Australia.
Conclusion
This study demonstrated a non-linear exponential response of N2O emissions to N fertiliser rates that may be typical for fertilised cotton systems on black vertosols in Australia. Corresponding EFs increased from 0.13 to 0.50% when N fertiliser rates increased from 90 to 270 kg N ha–1, but there was no significant increase in yield between the 180 and 270 kg N ha–1 treatments. The study confirmed that an optimised fertiliser strategy can be adopted in cotton to substantially reduce N2O emissions without affecting yield potential, corroborating previous studies in cotton systems. More studies on the effect of N fertiliser on N2O emissions are required to develop N2O response curves for other intensively fertilised systems in Australia. This study highlights the potential to reduce N losses to the environment by improved agricultural N-management practices.
Acknowledgement
This research was undertaken as part of the National Agricultural Nitrous Oxide Research Program funded by the Australian Department of Agriculture.
References
ANGA (2010) ‘Australian national greenhouse accounts, vol. 1.’ (Department of Climate Change and Energy Efficiency: Canberra, Australia)Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate Global Change Biology 14, 177–192.
| Nitrous oxide emissions from a cropped soil in a semi-arid climateCrossref | GoogleScholarGoogle Scholar |
Bouwman A (1996) Direct emission of nitrous oxide from agricultural soils. Nutrient Cycling in Agroecosystems 46, 53–70.
| Direct emission of nitrous oxide from agricultural soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXotlCiuw%3D%3D&md5=5f648d45683c40a80fb498f46f7cde7fCAS |
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368, 20130122.
| Nitrous oxide emissions from soils: how well do we understand the processes and their controls?Crossref | GoogleScholarGoogle Scholar | 23713120PubMed |
Cotton Research and Development Coorporation (CRDC) (2014) CRDC Annual Report 2013–2014, CRDC, Narrabri, NSW, Australia
Davidson EA, Kanter D (2014) Inventories and scenarios of nitrous oxide emissions Environmental Research Letters 9, 105012.
| Inventories and scenarios of nitrous oxide emissionsCrossref | GoogleScholarGoogle Scholar |
de Klein C, Harvey M (2013) ‘Nitrous oxide chamber methodology guidelines.’ (Ministry for Primary Industries: Wellington, UK)
Hoben J, Gehl R, Millar N, Grace P, Robertson G (2011) Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on‐farm corn crops of the US Midwest. Global Change Biology 17, 1140–1152.
| Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on‐farm corn crops of the US Midwest.Crossref | GoogleScholarGoogle Scholar |
IPCC (1997) ‘Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories.’ (IPCC: Paris, France)
IPCC (2006) ‘Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme.’ (IGES: Japan)
IPCC (2007 ) ‘Climatic change 2007: the physical science basis. Summary for policymakers.’ (IPCC: Geneva, Switzerland)
Isbell RF (2002) ‘The Australian soil classification. Vol. 4.’ Australian soil and land survey handbook. (CSIRO Publishing: Melbourne)
Kim D-G, Hernandez-Ramirez G, Giltrap D (2013) Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis. Agriculture, Ecosystems & Environment 168, 53–65.
| Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVyhs7k%3D&md5=3f8a4f707d6fb031dbf8da0b9d13c498CAS |
Lebender U, Senbayram M, Lammel J, Kuhlmann H (2014) Impact of mineral N fertilizer application rates on N2O emissions from arable soils under winter wheat. Nutrient Cycling in Agroecosystems 100, 111–120.
| Impact of mineral N fertilizer application rates on N2O emissions from arable soils under winter wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlarsrnP&md5=85773e4cb6ad7b98362fc085229176b8CAS |
Liu C, Zheng X, Zhou Z, Han S, Wang Y, Wang K, Liang W, Li M, Chen D, Yan Z (2010) Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China. Plant and Soil 332, 123–134.
| Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntlGhurs%3D&md5=d357942aa991335af83f7c83a0abdfeeCAS |
Liu C, Wang K, Zheng X (2012) Responses of N2O and CH4 fluxes to fertilizer nitrogen addition rates in an irrigated wheat-maize cropping system in northern China. Biogeosciences 9, 839–850.
| Responses of N2O and CH4 fluxes to fertilizer nitrogen addition rates in an irrigated wheat-maize cropping system in northern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovVWjs78%3D&md5=14605cddc56da8ce5336c96c02668be8CAS |
Ma BL, Wu TY, Tremblay N, Deen W, Morrison MJ, McLaughlin NB, Gregorich EG, Stewart G (2010) Nitrous oxide fluxes from corn fields: on‐farm assessment of the amount and timing of nitrogen fertilizer. Global Change Biology 16, 156–170.
| Nitrous oxide fluxes from corn fields: on‐farm assessment of the amount and timing of nitrogen fertilizer.Crossref | GoogleScholarGoogle Scholar |
Macdonald BC, Rochester IJ, Nadelko A (2015) High yielding cotton produced without excessive nitrous oxide emissions. Agronomy Journal 107, 1673–1681.
| High yielding cotton produced without excessive nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xlt1Wksbc%3D&md5=7e5a00040fd5c2f276c7dc4fcd68cbf1CAS |
McSwiney CP, Robertson GP (2005) Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Global Change Biology 11, 1712–1719.
| Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system.Crossref | GoogleScholarGoogle Scholar |
Parkin TB, Venterea RT (2010) ‘USDA-ARS GRACEnet project protocols, chapter 3. Chamber-based trace gas flux measurements sampling protocols.’ pp. 1–39. (Beltsville, MD, USA).
Payero J (2010) Maximising profitability with limited water in cotton farming systems. Available at http://www.insidecotton.com/xmlui/handle/1/247 [verified 15 June 2016]
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125.
| Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtF2hs7jF&md5=d412beb03e751c999a0044beb213d53fCAS | 19713491PubMed |
Reeves S, Wang W (2015) Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system. The Science of the Total Environment 530–531, 219–226.
| Optimum sampling time and frequency for measuring N2O emissions from a rain-fed cereal cropping system.Crossref | GoogleScholarGoogle Scholar | 26046430PubMed |
Rochester IJ (2012) Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton. Field Crops Research 127, 140–145.
| Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Scheer C, Wassmann R, Klenzler K, Lbragimov N, Eschanov R (2008) Nitrous oxide emissions from fertilized irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices. Soil Biology & Biochemistry 40, 290–301.
| Nitrous oxide emissions from fertilized irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlajs7bN&md5=632f84bcfab0a6dead24d4cc75a89447CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant and Soil 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia Soil Biology & Biochemistry 77, 243–251.
| Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical AustraliaCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht12ntL3M&md5=ee89302220febfea18060a4a6d021cc2CAS |
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proceedings of the National Academy of Sciences of the United States of America 111, 9199–9204.
| Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpsVamurY%3D&md5=8382fab4f0e01c7b581e0fdf91fd058aCAS | 24927583PubMed |



