Use of the agricultural practice of pasture termination in reducing soil N2O emissions in high-rainfall cropping systems of south-eastern Australia
Oxana N. Belyaeva A E , Sally J. Officer A D , Roger D. Armstrong C , Rob H. Harris A , Ashley Wallace B , Debra L. Partington A , Kirsten Fogarty A and Andrew J. Phelan AA Department of Economic Development, Jobs Transport and Resources, Private Bag 105, Hamilton, Vic. 3300, Australia.
B Department of Economic Development, Jobs Transport and Resources, 110 Natimuk Road, Horsham, Vic. 3400, Australia.
C Department of Animal, Plant and Soil Sciences, La Trobe University, Bundoora, Vic. 3086, Australia.
D Deceased.
E Corresponding author. Email: oxana.belyaeva@ecodev.vic.gov.au
Soil Research 54(5) 585-597 https://doi.org/10.1071/SR15307
Submitted: 26 October 2015 Accepted: 17 March 2016 Published: 28 June 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Conversion of long-term pasture to cropping was investigated for its effects on nitrous oxide (N2O) emissions in a 2-year field experiment in the high-rainfall zone of south-western Victoria. Early termination (pasture terminated 6 months before sowing) followed by winter (ETw) and spring (ETs) crops and late termination (pasture terminated 1 month before sowing) followed by a winter crop (LTw) were compared with continuous, mown pasture (MP). Emissions of N2O were measured with an automated gas sampling and analysing system.
Emissions from MP were the lowest throughout the study, resulting in annual losses of 0.13 kg N2O-N ha–1 in the first and the second years of the experiment. N2O-N loss was 0.6 kg ha–1 from treatments without fallow in both years (LTw in 2013 and ETs in 2014). In the first year, annual losses from previous fallow in ETw and ETs plots were 7.1 and 3.6 kg N2O-N ha–1, respectively. Higher annual N2O losses from treatments with fallow periods continued in the second year of the study and were 2.0 and 1.3 kg N2O-N ha–1 from ETw and LTw treatments, respectively. High emissions were associated with N mineralisation and the accumulation of NO3-N in the soil during the extensive fallow period after early pasture termination or wheat harvest. Soil water content was a key factor influencing the temporal fluctuations in N2O emissions. Low emissions occurred when water-filled pore space was <30%, whereas high emissions occurred when it was >65%, suggesting that denitrification was the major source of N2O emission. Crop grain yield was not affected by the duration of fallow (and therefore timing of pasture termination) in the first year, but was lower (P < 0.05) in the treatment without fallow in the second year. Terminating pasture late rather than early, thus reducing the length of the fallow period, is a practical way of reducing N2O emissions from mixed pasture–cropping systems.
Additional keywords: fallow, nitrous oxide losses, pasture–crop system.
Introduction
Nitrous oxide (N2O) is a potent greenhouse gas, with an ozone-depleting potential ~300 times that of carbon dioxide (CO2) and 12 times that of methane (CH4) (Crutzen and Ehhalt 1997; IPCC 2007). Agriculture accounts for 71–87% of global N2O emissions (Houghton et al. 1995; Mosier et al. 1998), and within the agricultural sector, approximately one–third of N2O emissions are attributable to fluxes from soils (De Klein et al. 2006). Emission of N2O also represents a loss of valuable plant-available N from the soil. For these reasons, there is a growing interest in quantifying losses of N2O from agricultural soils and developing practical strategies for reducing N2O losses.
Emissions of N2O from soils are predominantly the result of autotrophic nitrification and dissimilatory denitrification. The key factors influencing the rate of nitrification are the availability of NH4+ in aerobic conditions (Schmidt 1982), whereas denitrification is favoured by simultaneous supply of NO3– and available C, and diminishing O2 supply (anoxic conditions) (Firestone and Davidson 1989). Agricultural management practices can have a large influence on N2O production, with greater emissions generally occurring under conditions of high availability of inorganic N, water and labile C (Dalal et al. 2003).
Most agricultural land in Australia is under natural and improved pasture, and even in areas where arable cropping dominates, rotations often involve intermittent pastures. Such rotations are used to control soilborne diseases and to improve soil fertility and soil organic matter status. Indeed, the use of legume-based pastures in rotation results in accumulation of soil N and reduces the need for fertiliser N inputs during the cropping phase (Whitehouse and Littler 1984). For example, Peoples and Baldock (2001) estimated that the input of fixed N from pastures could range from 2 to 284 kg N ha–1 across temperate and tropical Australian environments.
This research is focused on developing mitigation strategies for N2O emissions derived from soil organic matter following the termination of long-term grass–legume pasture in preparation for use in grain production in the high-rainfall zone (HRZ, >650 mm rainfall year–1) of south-eastern Australia. This region has seen an increasing move from livestock to grain cropping over the last 20 years, resulting from a long-term decline in wool prices and the potential for high grain yields in the region. This change in land use is likely to promote net N mineralisation and nitrification, causing accumulation of mineral N in the soil and creating the potential for increased gaseous losses of N, including N2O. The poor soil structure and high clay content of subsoils coupled with high winter rainfall in the region often result in waterlogging, providing conditions that lead to high losses through denitrification. Harris et al. (2013) showed that high rates of N2O (up to 457 g N2O-N ha–1 day–1) could be lost from these cropping systems.
However, information is limited regarding N2O emissions during the transition from pasture to cropping in the temperate zone of Australia (Dalal et al. 2003), and particularly in the in HRZ of south-western Victoria. There is, therefore, a need to quantify N2O emissions during and immediately after pasture termination. Management strategies then need to be formulated that minimise N2O emissions while maintaining crop productivity during the transition between pasture and cropping.
The objectives of this study were to compare the effect of several pasture-termination strategies on N2O emissions during transition from legume–grass pastures to annual cropping; and to assess the relation between soil mineral N, soil water content and temperature as they affect temporal N2O fluxes in the Victorian HRZ.
Materials and methods
Site description and soil properties
The experimental site was on the Department of Economic Development, Jobs, Transport and Resources research farm, ~9 km south of Hamilton, Victoria (–37.824226°S, 142.075882°E). The site is in the HRZ, with an average annual precipitation of 689 mm, 70% of which falls during winter (May–October). The annual evaporation rate is 1200 mm, mean monthly maximum temperature 25.9°C and mean monthly minimum temperature 4.2°C (Comm onwealth Bureau of Meteorology (http://www.bom.gov.au/climate). The experiment was located in a field with a history of long-term pasture (>50 years), mainly consisting of perennial grasses (Lolium perenne, Phalaris aquatica, Stellaria media) with a small proportion (<5%) of subterranean clover (Trifolium subterraneum). The soil was a Vertic, Sodic, Eutrophic, Brown Chromosol (Isbell 2002) characterised by an abrupt textural change, where clay content increases from 24% in the topsoil to 65% in the subsoil, and this restricts infiltration and drainage to the bottom part of the profile. The subsoil had a high sodium content (exchangeable sodium percentage 8–19%), further reducing drainage. Selected chemical properties of the soil are shown in Table 1. The site showed good pasture growth, although exchangeable aluminium (Al) contents tended to be high because of the low soil pH, and could limit the growth of species sensitive to high Al.

|
Experimental design and management
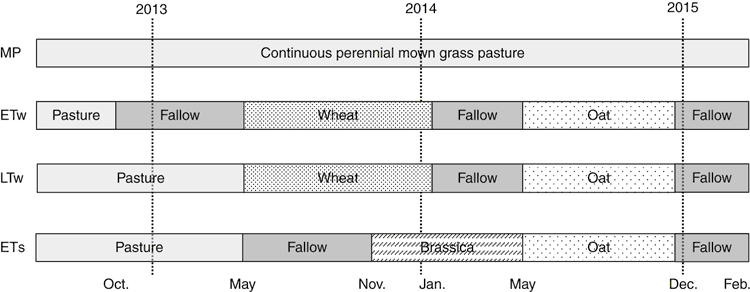
The experiment was conducted over two consecutive growing seasons, with measurements beginning on 1 March 2013 and finishing on 1 March 2015. There were four treatments arranged in a randomised block design with three replicates; each plot was 30 m long and 6 m wide. The sequence of treatments during the experiment is shown in Fig. 1.
Local farm practice was followed and two pasture termination strategies were used: early and late terminations. The ‘timing’ treatment refers to the length of delay in sowing. A management practice of early termination normally allows sowing of the subsequent crop 6 months after pasture termination. If the late termination is applied, a crop is sown within a shorter period from termination, usually 1 month. In our experiment, pasture was terminated either with glyphosate herbicide at 6 months before sowing (early termination), or by a combination of glyphosate application and cultivation to 10 cm depth at 2 weeks before sowing (late termination).
The research included two early-terminated treatments, started in November 2012 and April 2013, followed by winter and spring crops, respectively, whereas the later terminated treatments started in April 2013, followed by the winter crop. Continuous, mown pasture (MP) was used as a control. Winter wheat (Triticum aestivum, cv. Bolac) was sown on 10 May 2013 into early-terminated (treatment ETw) and late-terminated (treatment LTw) pasture and harvested on 22 January 2014. A spring crop of forage brassica (Brassica napus cv. Winfred) was sown into early-terminated (treatment ETs) pasture on 16 October 2014 and harvested on 7 May 2014 In the second year of study, all wheat and forage brassica plots were sown to oats (Avena sativa) cv. Wombat) on 14 May 2014 and harvested on 17 December 2014. All crops were direct-drilled to minimise soil disturbance. Phosphorous (P) and potassium (K) fertilisers were applied at seeding at 15 kg P ha–1 (as double superphosphate) in the first year of study and 15 kg P and 15 kg K ha–1 (as double superphosphate and potassium chloride) in the second year. Fertiliser application rates were representative of local farming practice and were made on the basis of soil chemical analysis.
Gas measurements
An automated system was used to collect and analyse gas samples for N2O and CO2 simultaneously. Moveable gas collection chambers (0.8 by 0.8 m wide by 0.5 m high) constructed from stainless steel and clear polycarbonate panels (with 0.5-m extensions added to increase chamber height as the crop grew) were installed in each plot. The top surface panel of these automatic chambers was divided into two lids that opened away from the top of the chamber to reduce rainfall interception. Chambers were clamped to open, stainless-steel base units (0.8 by 0.8 m wide by 0.15 m depth), with two base units installed in each plot, 0.5 m apart, so that chamber position could be alternated within each plot on a weekly basis to minimise micro-climatic artefacts. The chambers were pressure-vented during closure time with two fans (each 80 mm diameter) but they were programmed to be opened automatically if the temperature in the chamber exceeded 50°C, or if the site received >0.5 mm rain within 5 min (in order to maintain similar water contents under the chambers and on the rest of site).
The 12 automated gas chambers (one per plot) were integrated with an automated sampling system connected to a tuneable diode laser trace-gas analyser (TGA100; Campbell Scientific, Logan, UT, USA) and a non-dispersive infrared gas analyser (LI-840A; LI-COR Biosciences, Lincoln, NE, USA). The concentrations of CO2 and N2O in gas samples were measured by the following procedure. A block of chambers for the one replicate, each block of four chambers, was closed for 30 min and opened for 60 min. Chambers for each plot were sampled sequentially each 3 min over the 30-min closure period, with 10 measurements during that time (30 s each). This allowed 16 measurements of flux rates per day for each individual chamber and 48 values per treatment per day. The hourly N2O emissions were calculated from the slope of the liner increase in N2O concentration during the period of chamber closure. The same approach was used to determine night-time (measured from 21 : 30 to 05 : 30) CO2 hourly fluxes. Daily loses were calculated by averaging hourly day losses (n = 16) for each chamber over the 24-h period for N2O and over the 8-h night period for CO2 across the three replicate chambers. Flux values were tested for a significant linear trend based on a t-test. Seasonal cumulative fluxes for each plot were summed over the measurement year before averaging across the three replicates. The system design, calibration and calculation procedures were similar to those described by Officer et al. (2015). Because of equipment failure, emissions were not measured during the periods 6 June–22 July 2013, 25–29 October 2013, 1–10 March 2014 and 4–8 January 2015.
Temperature and soil water measurements
Soil temperature and moisture content were monitored continuously with Model 107 thermocouple probe Type T (Campbell Scientific, Logan, UT, USA) and ThetaProbe soil moisture probe Type ML2x (Delta-T Devices, Burwell, UK) at 5 cm depth on all plots, and also at 10 and 20 cm depth in the first replicate of each treatment.
Weather monitoring
A weather station was installed at the site, allowing measurements of climatic conditions. Solar radiation was measured at a height of 3 m using a 240-100 Fristschen type net radiometer (NovaLynx, Grass Valley, CA, USA). Air temperature and relative humidity were measured at the same height with a humidity–temperature probe (Rotronic HycroClip HC2-xx; Rotronic AG, Bassersdorf, Switzerland). Rainfall was measured with a CS700-L automated tipping bucket with a resolution 0.254 mm (Hydrological Services, Warwick Farm, NSW).
Soil and plant sampling and analysis
Profile soil samples were taken from all plots before sowing and anthesis following grain maturity of crops and at the completion of the study. Soil samples were collected at 10-cm increments to 40 cm depth, then at 20-cm increments to 100 cm depth at two locations per plot. Surface soil (0–10 cm) was sampled every 4 weeks at 15 random locations per plot. On all occasions, samples within each plot were bulked for each depth increment, and subsamples were analysed for soil mineral N and water-soluble C. Mineral N was extracted with 2 M KCl (1 : 10 ratio for 1 h) followed by colourimetric analysis of NH4-N and NO3-N by using a Lachat flow injection analyser (Hach, Loveland, CO, USA). Water-soluble organic C and N were measured in the second year of experiment via an aqueous extract (1 : 10 w/v ratio for 1 h; Zmora-Nahum et al. 2007), filtered through a 0.22-µm Millipore filter and analysed with a soluble C/N analyser (Shimadzu, Kyoto). Gravimetric soil water was determined in profile samples after drying subsamples at 105°C for at least 48 h. Bulk density was measured on naturally compacted samples (Haynes and Goh 1978). Volumetric water contents were calculated by multiplying the gravimetric soil water by the bulk density. Water-filled pore space was calculated by dividing the volumetric water content by the total porosity (Linn and Doran 1984).
Growth stages of grain crops were evaluated using the standardised reference Zadoks Growth Scale (Zadoks et al. 1974). Aboveground biomass of cereals was measured at mid-tillering (Z25), anthesis (Z65) and grain ripening (Z92) by cutting a 100-cm row of crop at four random locations per plot. At harvest, grain was separated from herbage, yield was measured, and a subsample of grain was retained for grain quality assessment. Samples of forage crops were collected from four random quadrats (0.63 m × 0.63 m) by cutting plants at the base, bulking the samples from each plot and oven drying at 60°C for 1 week before weighing. Dry matter (DM) cuts from pasture plots were taken during the growing season after ryegrass reached the 3-leaf stage. Forage brassica was mown three times during the growing season (February, April and May 2014). For all plant samples, two subsamples from each plot were analysed for total N content by dry (Dumas) automated high temperature combustion using a LECO analyser (LECO, St. Joseph, MI, USA).
Statistical analyses
Statistical analysis was conducted using Genstat 17th edition (VSN International, Hemel Hempstead, UK). Analysis of variance (ANOVA) and Fisher’s significance test were performed to detect the difference between treatment means with the level of significance at P = 0.05 for all data at each time. Repeated-measures ANOVA (with the Greenhouse-Geisser procedure) was applied to estimate the repeated-measurements of soil parameters and gas fluxes with time. No transformations were required to stabilise the variances of the values. Pearson correlation analysis was used to examine relationships between gas fluxes, soil and climatic parameters. Differences and the correlations were considered significant where P ≤ 0.05.
Results
Climatic conditions
Air and soil temperature fluctuations were typical of seasonal conditions in this region and are shown in Fig. 2a and Fig. 3c. Air temperature varied from –2.8°C (August 2014) to +41.8°C (January 2015) during the study. Temperature variations in the soil surface layer (0–5 cm) were less pronounced, ranging from 5.3°C in June 2013 to 29°C in February 2014 (Fig. 3c). In total, 779 mm of rain fell at the site in the first year of study, which was 90 mm higher than the long-term mean (Fig. 2b). By contrast, rainfall during the second year was 79 mm lower than the long-term mean. In both years, most precipitation (60–70%) occurred between May and October.

|
Variation in water-filled pore space (WFPS), mineral N and water-soluble C
Throughout the study, the WFPS was strongly associated with the rainfall events and generally did not differ (P ≥ 0.05) between treatments (Fig. 3c, treatment data combined). WFPS rose from May and reached a maximum by August 2013, followed by a relatively sharp decline, after which it remained low until autumn of the following year. The WFPS remained >60% for much longer during 2013 (157 days) than 2014 (59 days).
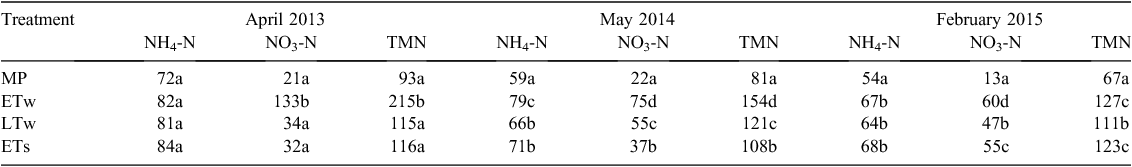
Temporal variations in NH4-N and NO3-N in the top 10 cm of soil are shown in Fig. 4. In all treatments, NH4-N content reached maximum values (45–67 kg N ha–1) in winter–early spring (Fig. 4a). Ammonium was the largest component of the extractable inorganic N pool in the mown pasture plots, whereas the content of NO3-N was lowest in this treatment and remained almost constant throughout the experiment, ranging between 1 and 14 kg ha–1 (Fig. 4a, b). By contrast, the NO3-N content fluctuated in the cropped treatments, ranging from 4.9 ± 3.1 to 67.1 ± 25.0 kg N ha–1. Highest NO3-N values were present in June–July.
Rapid accumulation of NO3-N during the 6-month summer fallow in 2013 in the ETw treatment resulted in accumulation of 215 kg N ha–1 of total mineral N in the top 100 cm of soil, and NO3-N comprised 64% of this (Table 2). However, NH4-N was the dominant form of mineral N in the soil profile in the other three treatments (mean 73% of total mineral N). A similar pattern of NO3-N accumulation occurred with 4 months of summer fallow in 2014 (Fig. 4b) in the ETw and LTw treatments. Based on duration of fallow and the amounts of N at the start and end of the fallow period, the average rates of increase of NO3-N concentration were 0.41 ± 0.05 and 0.32 ± 0.06 kg ha–1 day–1 for ETw and LTw treatments, respectively. Rapid accumulation of NO3-N was also evident during summer 2014–15 in the soil profile of all crop treatments (from oats maturity until the end of the experiment), with rates of NO3-N increase of 0.55 ± 0.07 kg ha–1 day–1 for ETw and ~0.38 ± 0.08 kg ha–1 day–1 for both LTw and ETs.
Water-soluble C concentrations in the topsoil in 2014 were predominantly higher (P ≤ 0.05) in the MP treatment (594–910 kg C ha–1) than in the three cropped treatments (Fig. 5) ranging from 400 to 810 kg C ha–1. WSC was lowest in January across all treatments when the soil was driest.
Temporal N2O fluctuations and night-time CO2
In both years, fluxes of N2O were low in the dry summer–early autumn months, but rose steadily with the onset of rainfall in May and greatly increased at the end of winter (July–September) (Fig. 3a). The greatest daily N2O losses occurred in winter–early spring following significant rainfall events, when WFPS was elevated to ≥65–85% in top 10 cm soil layer (Fig. 3a, c). A sharp decline in N2O emissions was observed when WFPS decreased to <60%. The greatest daily losses in 2013 were observed in August–September and were 365, 153, 22 and 4.4 g N ha–1 for ETw, ETs, LTw and MP, respectively. Daily fluxes in August 2014 were lower, being 68.3, 26.1, 53.1 and 1.7 g N ha–1 for ETw, ETs, LTw and MP, respectively.
Night-time CO2 fluxes during summer were low when soil was dry (Fig. 3b). These fluxes increased in both years in May after WFPS increased to >30% and remained elevated throughout winter and most of spring. A steady decline in soil respiration coincided with drying topsoils and WFPS dropping to <60%. Although temporal fluctuations in N2O and night-time CO2 emissions followed a similar broad trend, being greatest during winter and least in summer (Fig. 3a, b) across both 2013 and 2014, fluxes of CO2 occurred about 1 month before those for N2O (Fig. 3a, b).
Effect of timing pasture termination on N2O emissions
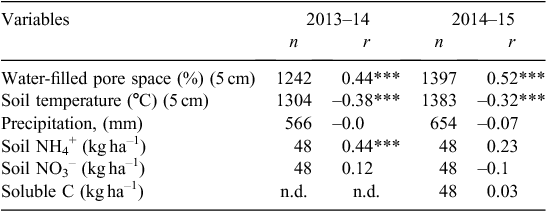
As shown in Table 3, N2O emissions (mean daily flux and cumulative flux) from all treatments where management included fallow were significantly higher in both years than where no fallow was imposed. In the first year, the total cumulative losses of N2O from the early-terminated treatments (ETw and ETs) were 7.1 and 3.6 kg N2O-N ha–1, whereas cumulative loss from the late-terminated plots (LTw) was only 0.56 kg N2O-N/ha. In the second year, the same trend was observed, and more N2O was emitted from treatments with a longer fallow period. Total emissions from treatments with a 4-month fallow (ETw and LTw) were 2.0 and 1.3 kg N2O-N ha–1, respectively, and only 0.56 kg N2O-N ha–1 was produced from the treatment with no fallow (ETs). The pulses of N2O emission were most strongly correlated with WFPS and not strongly correlated with soil temperature, mineral N or soluble C content (Table 4).
|
Night-time CO2 production was significantly higher (P ≤ 0.05) in the mown pasture treatment than in other treatments at the end of May–June 2013 and in June–July 2014. During June–September 2013, CO2 emissions were lowest from the ETs treatment.
Crop yields and timing of pasture termination
The grain yield of wheat was not significantly affected by timing of pasture termination in the first year (ETw = 5.53 t ha–1 and LTw = 5.49 t ha–1) (Table 3). In the second year, oat grain yield did not vary significantly (P > 0.05) between ETw (5.9 t ha–1) and LTw (6.9 t ha–1) treatments, but was significantly lower under ETs (4.7 t ha–1). This effect appeared 2 months after planting of oats and was evident throughout the 2014 growing season, resulting in reduced aboveground biomass of up to 40% at mid-till stage and up to 15% at anthesis (data not shown). In both years, the grain N content (protein) of wheat and oats did not differ significantly between treatments.
Discussion
Nitrous oxide emissions from continuous pastures
Emissions of N2O were extremely low throughout the experiment under mown pasture plots despite soils having a significantly high content of mineral N (Table 2). Daily fluxes generally ranged from 0.05 to 0.3 g N2O-N ha–1, resulting in cumulative annual losses of ~0.13 kg ha–1 in both years (Table 3). Our findings are comparable to those of other studies where cumulative losses from non-fertilised, non-grazed and/or extensively grazed pastures were low and normally within 1–2 kg N ha–1 year–1 (Griffith et al. 2002; van der Weerden et al. 1999). Much higher N2O emissions reaching 5–13 kg N2O-N ha–1 year–1 have been observed in intensively managed, grazed pasture systems where high N fertiliser applications are combined with high stocking rates (Eckard et al. 2010; Phillips et al. 2007).
The low losses of N2O are attributable to a low nitrification potential in pasture soils and/or high plant uptake of NO3-N (Neill et al. 1995). Strong competition for NH4-N from roots of perennial pasture species, which are active for most of the year, and microbial biomass in the rhizosphere can leave little NH4-N available for autotrophic nitrifiers, which are generally poor competitors with the heterotrophic biomass for NH4-N (Jones and Richards 1977). This can lead to a decline in the population of nitrifiers (Davidson et al. 1993). Furthermore, any NO3– produced is quickly used by the growing vegetation, lowering its concentration in the soil. Veldkamp et al. (1999), for instance, found that the concentration of NO3-N in soils decreased greatly with pasture age and that even under young pasture (3 years old), soil NH4-N significantly exceeded NO3-N content. Certainly, in our experiment, under a 25-year-old pasture (MP), NH4-N was consistently the dominant form of mineral N present. Because of the low NO3– concentrations (<10 mg kg–1), the potential for denitrification to occur was also low.
Effect of pasture termination on nitrous oxide emissions
In this experiment, the timing of pasture termination was critical for N2O production. Emissions were higher in both years from treatments that were terminated early, and N2O emissions from ETw were 12 times higher than from LTw. In 2014, a 4-month fallow period after wheat harvest in LTw and ETw resulted in 2.3–3.5-times higher emissions than from the treatment without fallow (ETs). Note that for treatments without fallow (LTw in 2013 and ETs in 2014), emissions were only 0.56 kg N2O-N ha–1 in both years. Pasture termination affected N2O emissions through subsequent accumulation of NO3– during the fallow period. Soil profile NO3– concentrations in treatments with fallow periods after pasture termination (or following crop harvest) were significantly (P < 0.05) higher than in treatments with no fallow. By contrast, NH4+ concentrations in these treatments were low and had similar values during the fallow, suggesting that nitrification was a dominant process. This flush of nitrification during fallow is attributable to decomposition of soil organic matter and plant residue after pasture termination or crop harvest (Peoples and Baldock 2001). Furthermore, where there is a fallow period, NO3-N accumulates in the soil, whereas when a crop is present the NO3– is taken up by the growing plants. In addition, nitrification might be dominant over immobilisation as a result of high soil temperatures over summer (Hoyle et al. 2006), Indeed, following pasture termination, a release of 70–150 kg mineral N ha–1, predominantly as NO3–, characteristically occurs (Fillery 2001). In our experiment, rates of NO3-N accumulation during summer fallows varied from 0.24 to 0.68 kg ha–1 day–1 with highest values from ETw plots in both years. In the HRZ of Victoria, precipitation exceeds evaporation during winter, and this, combined with the restricted drainage that is a characteristic of the duplex soils common in the region, creates conditions favourable for denitrification.
Thus, the high concentrations of NO3-N accumulated during fallow in the ETw act as a substrate for denitrifiers and may indicate the higher potential in the treatment with longer fallow when other conditions are present (e.g. high soil moisture, low plant uptake). Several studies have highlighted that excess NO3-N coupled with available C after a pasture phase can increase the risk of substantial N loss, including N2O by denitrification, especially if clay soils are subject to heavy rainfall or waterlogging (Avalakki et al. 1995; Pu et al. 1999). Nonetheless, few studies have estimated the extent of N2O losses after a pasture, with most experiments designed to minimise losses of N from fertiliser (Fillery 2001).
Reported rates of N2O emissions after pasture termination are conflicting and vary depending on soil type, precipitation, fertilisation rates and fallow duration. For example, MacDonald et al. (2011) observed total N2O fluxes of 17 kg N2O-N ha–1 from one year of chemical fallow when the year was particularly wet (1052 mm rainfall) in a high-rainfall environment in Canada. In fallow plots amended with organic manure in the same experiment, N2O fluxes reached 30 kg N2O-N ha–1. By contrast, limited fluxes of 0.68 kg N2O-N ha–1 were emitted from one year of fallow after termination of a native grass sod in a semi-arid area of Nebraska, USA (Kessavalou et al. 1998). Annual cumulative emissions were 0.08 kg N2O-N ha–1 from fallow plots in a fallow–wheat rotation in a Mediterranean climate in Spain (Tellez-Rio et al. 2015) and 0.24 kg N2O-N ha–1 in a semi-arid climate in North America (Dusenbury et al. 2008).
In addition to a fallow duration, the season during which termination occurred and subsequent management greatly affected N2O production, clearly demonstrating the importance of management in influencing N2O fluxes. Cumulative fluxes from ETs winter-fallow plots were nearly half those from ETw with summer fallow, 3.6 and 7.1 kg N2O-N ha–1 respectively. This lower cumulative flux may reflect the shorter period from ETs termination (30 April 2013) to the period of maximum fluxes (August–September 2013) compared with the full 6-month fallow under ETw and the associated reduction in NO3-N accumulation. After ETs was terminated in April 2013, mineral N content increased over the following several months (Fig. 4), but the increase was not sufficient to stimulate the same magnitude of loss as the summer-terminated ETw. This result is consistent with findings of Velthof and Oenema (1995), who reported a higher flux from unploughed, chemically terminated grassland during a dry (8.2 kg N2O-N ha–1) than a wet (5.8 kg N2O-N ha–1) period in The Netherlands. Herbicide application in ETs in April 2013 and two subsequent applications were only partially effective in removing pasture plants from this treatment, producing only an inhibition of plant growth rather than complete death. This may also help to explain the lower N2O production than in the ETw treatment.
Effect of soil environment (WFPS and temperature) on N2O production
Although the agronomic practice of pasture termination influenced N2O soil flux, the annual pattern of emissions was mostly determined by seasonal conditions (particularly rainfall and consequently WFPS). A prolonged stimulation of emissions in winter–spring coincided with soil rewetting and increasing WFPS (Fig. 3). The correlation between WFPS and N2O emissions was generally higher (P < 0.01) than between N2O and other environmental variables (Table 4) and was more significant during 2013 than 2014 (which had lower rainfall). Most of the N2O from experimental treatments was lost during relatively short timeframes in winter–spring. In the first year, up to 91% of annual averaged fluxes were emitted in the 3 months from 21 June 2013 to 10 November 2013, with a peak in September. In the second year, the period of high fluxes was shorter, from 1 August 2014 to 1 September 2014. In the present study, all days when high winter–spring emissions were recorded corresponded to WFPS values between 65% and 98% (146 and 54 days in the first and the second year, respectively).The flux peak coincided with a WFPS of 70–85% (101 and 30 days in the first and the second year, respectively) (Fig. 6).
Although WFPS not may be a completely reliable predictor of processes of nitrification and denitrification (Balaine and Clough 2013), this parameter is widely used for estimation of possible pathways of N2O production in soil (Dobbie et al. 1999). In this study, soil-water content ranged from 65% to 97% WFPS when most of the cumulative yearly emissions were recorded. The increased N2O production induced by lowering O2 availability in this WFPS range can be mediated by nitrifier denitrification of NO2– directly to N2O (one of the pathways of nitrification) or by heterotrophic denitrification of NO3– to N2O via nitric oxide (NO). Traditionally, denitrification is considered a major process driving N2O production, whereas losses from nitrification are not usually significant (Grundmann et al. 1995; Koops et al. 1997). Rowlings et al. (2015) observed an exponential increase in N2O production as WFPS increased from 60% to 82%, with the highest emissions recorded when WFPS was 82–84%, and concluded that most of the N2O loss resulted from denitrification. However, there is a growing body of evidence suggesting that nitrifier denitrification may be an important source of N2O production in soils if the WFPS ranges from 50% to 70% (Kool et al. 2011). For instance, Zhu et al. (2013) noted that nitrifier denitrification was the dominant pathway promoting 34–66% of N2O losses from application of ammonia fertiliser under low O2 status (≥0.5%), which is also conducive for heterotrophic denitrification. According to Bollmann and Conrad (1998), N2O derived from heterotrophic denitrification exceeded nitrification-derived N2O only when O2 was <0.1%.
The relative importance of nitrifier v. heterotrophic denitrification is difficult to assess because both can occur simultaneously over a wide range of O2 concentrations. However, it is believed that NO2– does not accumulate significantly in unfertilised soil (Venterea 2007). Thus, we do not think that the input of nitrifier denitrification can be substantial in our experiment, where N2O production was driven by mineralisation of soil N alone. Based on this and the high WFPS values recorded when most emissions occurred, we hypothesise that heterotrophic denitrification was the predominant process for production of N2O in our study. Verification of this hypothesis remains a subject for future research. A decline in NO3-N soil concentrations along with an increase in WFPS in our experiment might count as evidence for using this substrate for heterotrophic denitrification.
In our research, the highest concentration of WSC was found under the mown pasture. This is attributable to exudation of carbonaceous material into the pasture rhizosphere. In many experiments, rates of denitrification have been found highly correlated with available C (water-soluble or readily mineralisable C) (Drury et al. 1991; Patten et al. 1980). Nonetheless, N2O fluxes were lowest from the MP treatment, as already noted, and denitrification was restricted by low NO3– availability. The relatively high content of soluble carbon under the arable treatments (627–785 kg ha–1) during winter may have been important in allowing denitrification to proceed during this period. Furthermore, the high clay content of soil at the site would have favoured development of anoxic conditions necessary for denitrification (Velthof and Oenema 1995).
In both years, maximum night-time CO2 fluxes (used here as an indirect measure of soil microbial activity) occurred about 1 month before maximum N2O fluxes (Fig. 3a, b). That is, soil microbial activity was limited during dry periods and increased as WFPS increased. When WFPS became high (>65%), anoxic conditions are likely to have developed, so that denitrification was stimulated and N2O emissions were highest.
A decrease in N2O emissions was observed when the WFPS exceeded 86% (September 2013). Highly anaerobic conditions, at very high water contents approaching saturation, favour emissions of dinitrogen (N2) rather than N2O via denitrification, resulting in declining rates of N2O production at this time (Dalal et al. 2003). In 2013, a major decrease in N2O production was registered after September even though WFPS remained high and only gradually declined from 78% to 65% over the next 3 months. Because the crop absorbed most of the soil mineral N during this time (fully tillering–maturity), soil NO3-N rapidly decreased to 3–7 kg NO3-N at the end of the period. Thus, low NO3-N concentrations did not favour denitrification, resulting in low N2O emissions despite the high WFPS.
Cumulative N2O fluxes from cropped treatments were lower (P < 0.01) in 2014 than 2013 because of (i) reduced mineral N accumulation in the soil profile due to plant N uptake during 2013, and (ii) drier weather conditions in 2014 and a less prolonged period of high WFPS. Thus, conditions for denitrification were less favourable and accounted for the lower N2O fluxes in 2014.
Throughout this study, no strong correlations between N2O emissions and NH4-N and NO3-N were found and the relationship was especially weak with NO3-N (Table 4). This presumably reflects the fact that fluxes were driven by the water status of the soil. Several studies have reported a poor correlation between daily fluxes and mineral N (Barton et al. 2008; De Antoni Migliorati et al. 2014). Interestingly, during wet periods, the concentrations of NO3-N measured in the top 10 cm of soil were lowest when maximum N2O fluxes occurred (Fig. 4b). The highly mobile NO3– ion may have been leached deeper than 10 cm at this time. Such a transfer may have diminished the relationship between NO3-N concentration and N2O production in a wet winter–spring. Similarly, MacDonald et al. (2011) observed that N2O emissions from chemical fallow during the high-rainfall season better corresponded with changes in NO3-N at 30 cm depth than at 10 cm. During the hot and dry summer–autumn period, the effect of low WFPS is likely to override any effect of high substrate availability (mineral N) on N2O emissions, lowering the correlations in this period. Poor correlation could also be explained through the contributions of other N-transformation processes (e.g. nitrifier denitrification) and partial use of NO2– by microflora as a substrate for N2O production. A significant correlation has been found between cumulative N2O emissions and soil NO2– but not NO3– or NH4+ concentration in several other studies (Maharajan and Venterea 2013; Venterea and Rolston 2000).
Temperature was not correlated with seasonal N2O fluxes in this study (Table 4). Although the daily soil temperature was relatively low during most of the winter–spring period, ranging from 5°C to 12°C, N2O emissions were at their highest at this time (Fig. 3c). These temperatures are below the optimal range for both nitrification (25−35°C) and denitrification (20−40°C) reported by Bouwman et al. (2002). This suggests that the microbial population present in the studied soil (including denitrifying bacteria) is adapted to relatively low winter temperatures. High night-time CO2 emissions, which were observed over the winter–autumn period, also indicated that the heterotrophic microbial community was highly active during this period (Fig. 3b); such adaptation of microbial populations to various regional climatic zones has been described by other researchers (Keeney et al. 1979). Indeed, even under freezing conditions, biological activity and N2O emissions have been observed (Rõver et al. 1998). Moreover, studies that have found a strong relationship between increases in soil temperature and N2O were generally based on short-term seasonal measurements rather than yearly data (Smith et al. 1998; Dobbie et al. 1999). However, on an annual basis, the temperature effect might be dominated by other factors.
N2O mitigation strategies and crop yield
The mitigation strategy investigated here was designed to reduce accumulation of mineral N in the soil profile, and thus lower N2O emissions. This risks simultaneous reduction of crop yield because N is essential for crop growth. Nonetheless, the reduction in N2O emissions under the late termination occurred without a negative impact on grain yield in the first year. In the second year, however, a grain yield decrease was observed under the ETs treatment relative to ETw and LTw, even though both had a 4-month fallow in 2014 (Table 3). This reflects the fact that N mineralisation and N supply to the following crop are greatest in the first year after pasture termination and decrease progressively with time (Stephen 1982). Our results indicate that a shorter fallow period is an effective strategy for lowering N2O emissions. However, N availability should be monitored in the second year because crop production may decline. A side-dressing of fertiliser N may need to be applied. A potential benefit of longer fallow is higher soil water content for use by the following crop; however, this is not always evident in the HRZ because rainfall is usually adequate for crop growth.
Taking into account that higher N2O emissions were produced by longer fallows, late pasture termination is considered a suitable management practice for farmers in the HRZ to reduce N2O fluxes. In this experiment, accumulation of mineral N during fallow was mainly as NO3-N, and NO3– is known to be highly mobile and can be potentially lost through a variety of mechanisms. For example, the quantity of N2 produced during denitrification is generally expected to be much greater than that of N2O. The ratio of N2 : N2O can vary from 3 : 1 to 8 : 1 depending on soil and environmental conditions (Ryden et al. 1979; Rolston et al. 1982). Moreover, NO3– leaching is often considered the most important channel of N loss from the rooting zone of the first crop following legumes in rotations and can range from 40 to 100 kg N ha–1 year (Fillery 2001). Such losses represent an economic loss of N that could otherwise be used by a subsequent crop. In the HRZ of southern Australia, a large excess of rainfall over evapotranspiration for part of the year is commonly observed, favouring both denitrification and NO3– leaching. It is therefore likely that much of the mineral N released during pasture termination is lost rather than being used by following crops. Future studies should be carried out to estimate fully the scale of N loss that occurs in pasture–cropping systems in the HRZ and to devise methods to minimise such losses.
Conclusion
Emissions of N2O were the combined result of environmental variables and agronomic management. The main climatic factor affecting the pattern of N2O emissions was fluctuations in soil moisture caused by rainfall distribution. High N2O emissions were observed at a WFPS >65%, suggesting that denitrification was the dominant source of N2O. Greatest losses of N2O occurred where a prolonged fallow period followed pasture termination (and NO3-N accumulated in the soil). Applying late instead of early pasture termination (thus reducing the fallow period) can significantly decrease annual N2O emissions and minimise losses of N accumulated during the pasture phase in the Victorian high-rainfall cropping system. Results from this 2-year study suggest that famers should delay pasture termination because this strategy maximises the productivity benefits of biologically fixed N accumulated during the pasture phase to subsequent crops while minimising N2O emissions to the atmosphere.
Acknowledgements
We appreciate financial support from the Grain Research and Development Corporation and the federal Department of Agriculture, Fisheries and Forestry. We are truly grateful to Dr Duncan Robin Baigent for advice and excellent technical support.
References
Avalakki UK, Strong WM, Saffigna PG (1995) Measurement of gaseous emissions from denitrification of applied nitrogen-15. III. Field measurements. Australian Journal of Soil Research 33, 101–111.| Measurement of gaseous emissions from denitrification of applied nitrogen-15. III. Field measurements.Crossref | GoogleScholarGoogle Scholar |
Balaine N, Clough TJ (2013) Changes in relative gas diffusivity explain soil nitrous oxide flux dynamics. Soil Science Society of America Journal 77, 1496–1505.
| Changes in relative gas diffusivity explain soil nitrous oxide flux dynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsF2lurnM&md5=643a6315d899650a21bb3cb44c494a5cCAS |
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy VD (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Global Change Biology 14, 177–192.
Bollmann A, Conrad R (1998) Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Global Change Biology 4, 387–396.
| Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils.Crossref | GoogleScholarGoogle Scholar |
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilised fields: summary of available measurement data. Global Biogeochemistry Cycles 16, 1058
| Emissions of N2O and NO from fertilised fields: summary of available measurement data.Crossref | GoogleScholarGoogle Scholar |
Crutzen PJ, Ehhalt DH (1997) Effect of nitrogen fertilizers and combustion on the stratospheric ozone layer. Ambio 6, 112–117.
Dalal R, Wang W, Robertson P, Parton W (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
Davidson EA, Matson PA, Vitousek PM, Riley R, Dunkin K, Garciamendez G, Mass JM (1993) Process regulating soil emissions of NO and N2O in a seasonally dry tropical forest. Ecology 74, 130–139.
| Process regulating soil emissions of NO and N2O in a seasonally dry tropical forest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVymtro%3D&md5=2c6c50123054f1fe55c0d8565443cf38CAS |
De Antoni Migliorati M, Scheer C, Grace PR, Rowlings DW, Bell M, McGree J (2014) Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat-maize cropping system. Agriculture, Ecosystems & Environment 186, 33–43.
| Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat-maize cropping system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVCjtbo%3D&md5=0844cd1e106bc3431f689d097fcc0b36CAS |
De Klein C, Novoa RSA, Ogle S, Smith KA, Rochette P, Wirth TC, McConkey BG, Mosier A, Rypdal K (2006) N2O emissions from managed soils, and CO2 emissions from lime and uea application. In ‘IPCC Guidelines for national greenhouse gas inventories. Volume 4. Agriculture, forestry and other land use’. Ch. 11. (Intergovernmental Panel on Climate Change: Geneva) Available at http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_11_Ch11_N2O&CO2.pdf [verified 7 June 2016]
Dobbie KE, McTaggart IP, Smith KA (1999) Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables and mean emission factors. Journal of Geophysical Research 104, 26891–26899.
| Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables and mean emission factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXotFajsL0%3D&md5=bb68f8d2626177db5edeb4ee747bfc3eCAS |
Drury CF, McKeeney DJ, Findlay WI (1991) Relationship between denitrification, microbial biomass and indigenous soil properties. Soil Biology & Biochemistry 23, 751–755.
| Relationship between denitrification, microbial biomass and indigenous soil properties.Crossref | GoogleScholarGoogle Scholar |
Dusenbury MP, Engel RE, Miller PR, Lemke RL, Wallander R (2008) Nitrous oxide emissions from a Northern Great Plains soil as influenced by nitrogen management and cropping systems. Journal of Environmental Quality 37, 542–550.
| Nitrous oxide emissions from a Northern Great Plains soil as influenced by nitrogen management and cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVWktrY%3D&md5=d08a5f0d2088b8817f829988f93edcbfCAS | 18389938PubMed |
Eckard RJ, Grainger C, de Klein CAM (2010) Option for the abatement of methane and nitrous oxide from ruminant production: a review. Livestock Science 130, 47–56.
| Option for the abatement of methane and nitrous oxide from ruminant production: a review.Crossref | GoogleScholarGoogle Scholar |
Fillery IRP (2001) The fate of biologically fixed nitrogen in legume-based dryland farming systems: a review. Australian Journal of Experimental Agriculture 41, 361–381.
| The fate of biologically fixed nitrogen in legume-based dryland farming systems: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1Crtro%3D&md5=8f49c78c2ca27ba1a36468adefaba3f1CAS |
Firestone MK, Davidson EA (1989) Microbial basis of NO and N2O production and consumption in soil. In ‘Exchange of trace gases between terrestrial ecosystems and the atmosphere’. (Eds MO Andreae, DS Schimel) pp. 7–21. (John Wiley: Chichester, UK)
Griffith DWT, Leuning R, Denmead OT, Jamie IM (2002) Air-land exchanges of CO2, CH4 and N2O measured by FTIR spectrometry and micrometeorological techniques. Atmospheric Environment 36, 1833–1842.
| Air-land exchanges of CO2, CH4 and N2O measured by FTIR spectrometry and micrometeorological techniques.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivValtL0%3D&md5=265eae425474fc3f7fd0eeea4a1684efCAS |
Grundmann GL, Renault P, Rosso L, Bardin R (1995) Differential effects of soil water content and temperature on nitrification and aeration. Soil Science Society of America Journal 59, 1342–1349.
| Differential effects of soil water content and temperature on nitrification and aeration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXotFOgurg%3D&md5=e4674b2e9d9437aececf6c65ecd6a6bfCAS |
Harris RH, Officer SJ, Hill PA, Armstrong RD, Fogarty KM, Zollinger RP, Phelan AJ, Partington DL (2013) Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia? Nutrient Cycling in Agroecosystems 95, 269–285.
| Can nitrogen fertiliser and nitrification inhibitor management influence N2O losses from high rainfall cropping systems in South Eastern Australia?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotV2ru70%3D&md5=dfcb3a9185e64759c620914c4cfd4732CAS |
Haynes RJ, Goh KM (1978) Evaluation of potting media for commercial production of container-grown plants. IV. Physical properties of a range of peat-based media. New Zealand Journal of Agricultural Research 21, 449–456.
| Evaluation of potting media for commercial production of container-grown plants. IV. Physical properties of a range of peat-based media.Crossref | GoogleScholarGoogle Scholar |
Houghton JT, Meira FJG, Callander BA, Harris N, Kattenberg A, Maskell K (1995) ‘Climate change 1995. Contribution of Working Group I to the Second Assessment Report of the Intergovernmental Panel on Climate Change (IPCC).’ (Cambridge University Press: Cambridge, UK)
Hoyle FC, Murphy DV, Fillery IRP (2006) Temperature ad stubble management influences microbial CO2-C evolution and gross N transformation rates. Soil Biology & Biochemistry 38, 71–80.
| Temperature ad stubble management influences microbial CO2-C evolution and gross N transformation rates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlagsLbF&md5=46f46bbb7f089bb6e5f0e7b066e5b363CAS |
IPCC (2007) Agriculture. In ‘Climate change 2007. Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds B Metz, O Davidson, P Bosh, R Dave, L Meyer). (Cambridge University Press: Cambridge, UK and New York)
Isbell RF (2002) ‘The Australian Soil Classification.’ Revised edn. (CSIRO Publishing: Melbourne)
Jones JM, Richards BN (1977) Effect of deforestation on turnover of 15N-labelled nitrate and ammonium in relation to change in soil microflora. Soil Biology & Biochemistry 9, 383–392.
| Effect of deforestation on turnover of 15N-labelled nitrate and ammonium in relation to change in soil microflora.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXovValtQ%3D%3D&md5=df561ef4a04eb15953cdccdf23a0b8e5CAS |
Keeney DR, Fillery IR, Marx GP (1979) Effect of temperature on the gaseous nitrogen products of denitrification in a silt loam soil. Soil Science Society of America Journal 43, 1124–1128.
| Effect of temperature on the gaseous nitrogen products of denitrification in a silt loam soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhs1aksro%3D&md5=559a4107742d55aaee49915a7ae0a85dCAS |
Kessavalou A, Mosier AR, Doran WJ, Drijber RA, Lyon DJ, Heinemeyer O (1998) Fluxes of carbon dioxide, nitrous oxide and methane in grass sod and winter wheat-fallow tillage management. Journal of Environmental Quality 27, 1094–1104.
| Fluxes of carbon dioxide, nitrous oxide and methane in grass sod and winter wheat-fallow tillage management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtl2js7Y%3D&md5=44641a8b1758183ab9943ba3940b34bcCAS |
Kool DM, Dolfing J, Wrage N, Van Groenigen JM (2011) Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biology & Biochemistry 43, 174–178.
| Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVGrs7zL&md5=97daeefd5382538a4a238a5aa9e0eb95CAS |
Koops JG, van Beusichem ML, Oenema O (1997) Nitrous oxide production, its source and distribution in urine patches on grassland on peat soil. Plant and Soil 191, 57–65.
| Nitrous oxide production, its source and distribution in urine patches on grassland on peat soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXltVKjtb4%3D&md5=379426910fd3b4a5dc8f6b3f78a73d26CAS |
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Science Society American Journal 48, 1267–1272.
MacDonald DJ, Rochette P, Chantigny H, Angers DA, Royer I, Gasser MO (2011) Ploughing a poorly drained grassland reduced N2O emissions compared to chemical fallow. Soil & Tillage Research 111, 123–132.
| Ploughing a poorly drained grassland reduced N2O emissions compared to chemical fallow.Crossref | GoogleScholarGoogle Scholar |
Maharjan B, Venterea RT (2013) Nitrite intensity explains N management effects on N2O emissions in maize. Soil Biology & Biochemistry 66, 229–238.
| Nitrite intensity explains N management effects on N2O emissions in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVahsbvO&md5=5f09d40b4d42f85ad7ae902bae3636efCAS |
Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, van Cleemput O (1998) Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutrient Cycling in Agroecosystems 52, 225–248.
| Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnsVSmtLk%3D&md5=5ff8b6168c8281761fd7b99d2fdcd290CAS |
Neill C, Piccolo MC, Steudler PA, Melillo JM, Feigl DJ, Cerri CC (1995) Nitrogen dynamics in soils of forest and active pastures in the western Brazilian Amazon Basin. Soil Biology & Biochemistry 27, 1167–1175.
| Nitrogen dynamics in soils of forest and active pastures in the western Brazilian Amazon Basin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXntlGqsLc%3D&md5=ef0acfe6787ed3394361c198d1eddc2aCAS |
Officer SJ, Phillips F, Kearney G, Armstrong R, Graham J, Partington D (2015) Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models. Soil Research 53, 227–241.
| Response of soil nitrous oxide flux to nitrogen fertiliser application and legume rotation in a semi-arid climate, identified by smoothing spline models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosFGntL0%3D&md5=1f110bcfecd3b8de81e4f80e9df39fffCAS |
Patten DK, Bremner JM, Blackmer AM (1980) Effects of drying and air-dry storage of soils on their capacity for denitrification of nitrate. Soil Science Society of America Journal 44, 67–70.
| Effects of drying and air-dry storage of soils on their capacity for denitrification of nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhs1aku78%3D&md5=33e25a797b8e3634fbf7bd9483cd5fecCAS |
Peoples MB, Baldock JA (2001) Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legimes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems. Australian Journal of Experimental Agriculture 41, 327–346.
| Nitrogen dynamics of pastures: nitrogen fixation inputs, the impact of legimes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkt1Crtrw%3D&md5=e72421384b37dedfdfe26fb27436868bCAS |
Phillips FA, Leuning R, Baigent R, Kelly KB, Denmead OT (2007) Nitrous oxide measurements from an intensively managed irrigated pasture using micrometeorological techniques. Agricultural and Forest Meteorology 143, 92–105.
| Nitrous oxide measurements from an intensively managed irrigated pasture using micrometeorological techniques.Crossref | GoogleScholarGoogle Scholar |
Pu G, Saffigna PG, Strong WM (1999) Potential for denitrification in cereal soils of northern Australia after legume or grass pasture. Soil Biology & Biochemistry 31, 667–675.
| Potential for denitrification in cereal soils of northern Australia after legume or grass pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjs1Citr4%3D&md5=6d1f52aaffa23a2ab8869fea68f1c125CAS |
Rolston DE, Sharpley AN, Toy DW, Broadbent FE (1982) Field measurements of denitrification. III. Rates during irrigation cycles. Soil Science Society of America Journal 46, 289–296.
| Field measurements of denitrification. III. Rates during irrigation cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XktVGhsLY%3D&md5=2d09b1571adddaeaee31faf944733797CAS |
Rõver M, Heinemeyer O, Kaiser EA (1998) Microbial induced nitrous oxide emission from an arable soil during winter. Soil Biology & Biochemistry 30, 1859–1865.
| Microbial induced nitrous oxide emission from an arable soil during winter.Crossref | GoogleScholarGoogle Scholar |
Rowlings DW, Grace PR, Scheer C, Liu S (2015) Rainfall variability drives interannual variation in N2O emissions from a humid, subtropical pasture. The Science of the Total Environment 512–513, 8–18.
| Rainfall variability drives interannual variation in N2O emissions from a humid, subtropical pasture.Crossref | GoogleScholarGoogle Scholar | 25613765PubMed |
Ryden JC, Lund LJ, Letey J, Focht DD (1979) Direct measurement denitrification loss from soils. II. Development and application of field methods. Soil Science Society of America Journal 43, 110–118.
| Direct measurement denitrification loss from soils. II. Development and application of field methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXitValu7c%3D&md5=98c8aa29f2fe42a342f281abdf21565dCAS |
Schmidt EL (1982) Nitrification in soil. In ‘Nitrogen in agricultural soils’. (Ed. FJ Stevenson) pp. 253–288. (American Society of Agronomy: Madison, WI, USA)
Smith KA, Thomson PE, Clayton H, Mc Taggart IP, Conen F (1998) Effects of temperature, water content and nitrogen fertilization on emissions of nitrous oxide by soils. Atmospheric Environment 32, 3301–3309.
| Effects of temperature, water content and nitrogen fertilization on emissions of nitrous oxide by soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltFOgtr8%3D&md5=7b4aac75594953be653441d03d858ec0CAS |
Stephen RC (1982) Nitrogen fertilizers in cereal production. In ‘Nitrogen fertilizers in New Zealand agriculture’. (Ed. PB Lynch) pp. 77–93. (New Zealand Institute of Agricultural Science: Wellington, New Zealand)
Tellez-Rio A, Garcia-Marco S, Navas M, Lopez-Solanilla E, Tenorio JL, Vallejo A (2015) N2O and CH4 emissions from a fallow-what rotation with low N input in conservation and conventional tillage under a Mediterranean agroecosystem. The Science of the Total Environment 508, 85–94.
| N2O and CH4 emissions from a fallow-what rotation with low N input in conservation and conventional tillage under a Mediterranean agroecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvF2htrfI&md5=053e5f7d922f9c0e017b25a7dfd69279CAS | 25459752PubMed |
van der Weerden TJ, Sherlock RR, Williams PH, Cameron KC (1999) Nitrous oxide emissions and methane oxidation by soil following cultivation of two different leguminous pastures. Biology and Fertility of Soils 30, 52–60.
| Nitrous oxide emissions and methane oxidation by soil following cultivation of two different leguminous pastures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXntVCmsbc%3D&md5=77b41e21e9e8c8701aca092f3e05feb4CAS |
Veldkamp E, Davidson E, Erickson H, Keller M, Weitz A (1999) Soil nitrogen cycling and nitrogen oxide emissions along pasture chronosequence in the humid tropics of Costa Rica. Soil Biology & Biochemistry 31, 387–394.
| Soil nitrogen cycling and nitrogen oxide emissions along pasture chronosequence in the humid tropics of Costa Rica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVOrt78%3D&md5=3a8f2d7bbcc2bd2d4db8bd1ab08f4fb9CAS |
Velthof GL, Oenema O (1995) Nitrous oxide fluxes from grassland in the Netherlands: II. Effect of soil type, nitrogen fertilizer application and grazing. European Journal of Soil Science 46, 541–549.
| Nitrous oxide fluxes from grassland in the Netherlands: II. Effect of soil type, nitrogen fertilizer application and grazing.Crossref | GoogleScholarGoogle Scholar |
Venterea RT (2007) Nitrite-driven nitrous oxide production under aerobic soil conditions: kinetics and biochemical controls. Global Change Biology 13, 1798–1809.
| Nitrite-driven nitrous oxide production under aerobic soil conditions: kinetics and biochemical controls.Crossref | GoogleScholarGoogle Scholar |
Venterea RT, Rolston D (2000) Mechanistic modelling of nitrite accumulation and nitrogen oxide gas emissions during nitrification. Journal of Environmental Quality 29, 1741–1751.
| Mechanistic modelling of nitrite accumulation and nitrogen oxide gas emissions during nitrification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXosVGktrk%3D&md5=e2451a5efe687c152b556112f3c693faCAS |
Whitehouse MJ, Littler JW (1984) Effect of pasture on subsequent wheat crops on a black earth soil in the Darling Downs. Organic C, nitrogen and pH changes. Queensland Journal of Agriculture and Animal Sciences 41, 13–20.
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |
Zhu X, Burger M, Doane T, Horwath R (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proceedings of the National Academy of Sciences of the United States of America 110, 6328–6333.
| Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvVOltbk%3D&md5=e6595e76a6f8266c666327a9d5dbe70cCAS | 23576736PubMed |
Zmora-Nahum S, Nadar Y, Chen Y (2007) Physico-chemical properties of commercial compost varying in their source materials and country of origin. Soil Biology & Biochemistry 39, 1263–1276.
| Physico-chemical properties of commercial compost varying in their source materials and country of origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsVWju7c%3D&md5=a8da7dacb93e4bd50747054670018525CAS |