Effects of amendment of different biochars on soil carbon mineralisation and sequestration
Lei Ouyang A , Liuqian Yu A and Renduo Zhang A BA Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology, School of Environmental Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, China.
B Corresponding author. Email: zhangrd@mail.sysu.edu.cn
Soil Research 52(1) 46-54 https://doi.org/10.1071/SR13186
Submitted: 25 June 2013 Accepted: 2 September 2013 Published: 17 January 2014
Abstract
The aim of this study was to determine the impact of addition of different biochars on soil carbon mineralisation and sequestration. Different biochars were produced from two types of feedstock, fresh dairy manure and pine tree woodchip, each of which was pyrolysed at 300, 500, and 700°C. Each biochar was mixed at 5% (w/w) with a forest loamy soil and the mixture was incubated at 25°C for 180 days, during which soil physicochemical properties and soil carbon mineralisation were measured. Results showed that the biochar addition increased soil carbon mineralisation at the early stage (within the first 15 days) because biochar brought available organic carbon to the soil and changed associated soil properties, such increasing soil pH and microbial activity. The largest increase in soil carbon mineralisation at the beginning of incubation was induced by the dairy manure biochar pyrolysed at 300°C. Soil carbon mineralisation was enhanced more significantly by the dairy manure biochars than by the woodchip biochars, and the enhancement effect decreased with increasing pyrolysis temperature. Although the biochar addition induced increased soil carbon mineralisation at the beginning of the incubation, soil carbon mineralisation rates decreased sharply within a short time (within 15 days) and then remained very low afterwards. Carbon mineralisation kinetic modelling indicated that the stable organic matter in biochars could be sequestrated in soil for a long time and resulted in high levels of carbon sequestration, especially for the woodchip biochars pyrolysed from higher temperatures.
Additional keywords: biochar, carbon sequestration, feedstock, pyrolysis temperature, soil carbon mineralisation, soil physicochemical properties.
Introduction
Soil plays a key role in the global carbon (C) cycle. Soil is the most important terrestrial C sink that releases carbon dioxide (CO2) into the atmosphere. There is much interest in biochar applications to soils as a means of improving soil quality and sequestering C in soils (Lehmann et al. 2006; Lehmann 2007; Laird 2008). Biochar is the product of biomass through pyrolysis. Several studies have shown the potential role of biochar in climate change mitigation by offsetting C emission associated with the burning of fossil fuels (Lehmann 2007; Laird 2008).
Soil C mineralisation is the CO2 efflux produced from soil metabolic processes, which are influenced by many soil properties. The amount and availability of soil organic C has a large impact on the atmospheric CO2 concentration because soil organic C can be decomposed and eventually returned to the atmosphere (Lal et al. 2007). The decomposition rate of soil organic C is determined by various soil physicochemical factors. For instance, Blagodatskaya and Kuzyakov (2008) reported that the amount of primed CO2 emission increased with increasing soil pH. Warnock et al. (2007) proposed that alteration of soil properties, including surface area, pH, and C/N ratio, was partly responsible for changes of soil microbial biomass and activity, and could consequently influence soil carbon mineralisation.
Biochar addition has been shown to have important effects on soil physicochemical properties, including improvement of soil structure, increase in soil pH, and enhancement of soil aeration and water retention capacity (Wardle et al. 2008; Downie et al. 2009; Laird et al. 2010). Nonetheless, results about C mineralisation in soils following biochar addition are not consistent in the literature. Novak et al. (2010) reported that biochar addition did not significantly change soil C mineralisation, possibly attributable to the highly condensed aromatic structure that was physically resistant to degradation. Zimmerman et al. (2011) observed that biochar produced at low temperatures stimulated C mineralisation due to decomposition of labile components of biochar over a short term, whereas biochar produced at high temperatures suppressed C mineralisation. Luo et al. (2011) found that biochars produced with different pyrolysis temperatures consistently promoted soil C mineralisation. Besides the differences in soils and biochars used in these studies, the diverse results should also be related to the accompanying changes in soil physicochemical properties following biochar applications (Sohi et al. 2010).
The importance of biochar as a C sequestration agent requires full understanding of its properties and the mechanisms controlling its activity in soils (Abdullah et al. 2010; Spokas 2010). For example, the volatile matter content in biochar should be a simple indicator to evaluate the stability of biochar in soils (Zimmerman 2010). The C/N ratio in biochar can change the ratio of fungi to bacteria in soils and hence affect the turnover of soil organic matter (Helfrich et al. 2008). All of these biochar properties are largely dependent on the feedstock and pyrolysis condition for biochar production. As shown in the literature, biochars made from wood have higher C/N values than those made from grass (Krull et al. 2009). Higher pyrolysis temperatures result in lower volatile matter content and higher C/N ratio in biochar (Braadbaart et al. 2004; Downie et al. 2009; Krull et al. 2009).
To better understand the change in soil C mineralisation and sequestration following biochar additions, it is necessary to quantify the characteristics of different biochars and the effects of biochars on soil physicochemical properties associated with soil C cycling. The objectives of this study were to investigate the characteristics of biochars made with different feedstocks and pyrolysis temperatures, and to study effects of the different biochars on soil physicochemical properties (e.g. soil pH, soil organic C, soil dissolved C) related to soil C mineralisation and sequestration. It was hypothesised that treatments with biochars made with different feedstocks and pyrolysis temperatures affected soil physicochemical properties as well as soil C mineralisation and sequestration differently.
Materials and methods
Production of biochar
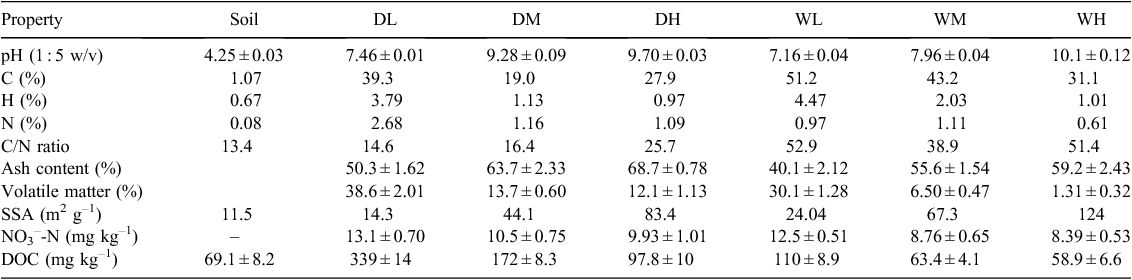
Two different types of biomass feedstock (fresh dairy manure and pine tree woodchip) were used for biochar production following the procedure of Yu et al. (2013). Briefly, after air-drying and crushing, each of the feedstocks was filled into crucibles sealed with lids to prevent oxygen from entering, and then pyrolysed in a muffle furnace, heating at a rate of 10°C min–1 and holding for 1 h at 300, 500, or 700°C. Biochars produced from dairy manure at the low (300°C), medium (500°C), and high (700°C) temperatures were denoted DL, DM, and DH, respectively, whereas biochars produced from woodchip were denoted WL, WM, and WH, respectively. After cooling, each biochar was passed through a 2-mm sieve. Biochar characteristics were examined as follows. Structural characteristics of biochars (e.g. distributions of pores and particles) were observed from scanning electron microscopy (SEM) (Peng et al. 2011). Surface organic functional groups presented on the biochars were determined by Fourier transform infrared spectroscopy (FTIR) (Nicolet iS10; Thermo Fisher Scientific Inc., Waltham, MA, USA) (Luo et al. 2011). Biochar ash content (w/w in %) was determined by dry combustion at 760°C in air for 6 h using a laboratory muffle furnace (Novak et al. 2009). Volatile matter was estimated from weight loss of biochar after combustion at 900°C for 6 min in a ceramic crucible (Zimmerman et al. 2011). Specific surface area (SSA) of biochar was evaluated using the Brunauer, Emmett, and Teller (BET) nitrogen (N) adsorption technique (Brunauer et al. 1938). Concentrations of elemental C, hydrogen (H), and N were determined with an elemental analyser (Vario EL; Elementar Analysensysteme GmbH, Hanau, Germany) (Baneschi et al. 2013), from which C/N ratios were estimated. Biochar pH was measured using a pH probe with a 1 : 2.5 (w/w) suspension of biochar in deionised water (Luo et al. 2011). Water-extractable concentrations of NO3– were measured with an ion chromatograph (DX-600; Dionex Corp., Sunnyvale, CA, USA) following Yu et al. (2013). The initial dissolved organic C (DOC) of biochars was determined with the method of Bruun et al. (2012). Briefly, 5 g of biochar was suspended in 25 mL 0.01 m CaCl2, which was shaken on a low speed reciprocal shaker for 1 h. The supernatant was filtered through a micro-filter (0.45 mm). The extract was analysed for DOC on a total organic C (TOC) analyser (TOC-V CSH; Shimadzu Corp., Kyoto, Japan).
Soil collection and experimental design
Soil samples (0–10 cm depth) were collected in the Dinghushan Nature Reserve (23°09′21″–23°11′30″N, 112°30′39″–112°33′41″E), Guangdong Province in southern China. After air-drying, the soil samples were sieved with a 2-mm sieve and thoroughly homogenised. The soil was a forest loamy soil with 40% sand, 35% silt, and 25% clay, and with a bulk density of 1.22 g cm–3. Total C, H, and N, and initial DOC of the soil were measured using the methods mentioned above.
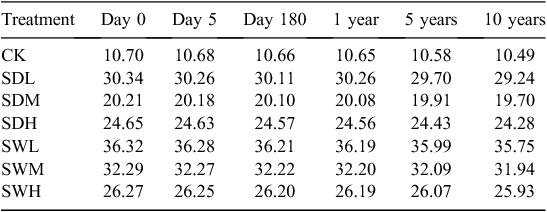
An incubation experiment was conducted at 25°C for 180 days as follows. Each of the six types of biochar was mixed into 120 g of the air-dried soil sample based on a rate of 5% (w : w on an air-dried weight basis), and soil without biochar addition was used as the control. The seven treatments were denoted with S (for soil) followed by the biochar treatment acronym, and CK (the control). The initial C contents for CK, SDL, SDM, SDH, SWL, SWM, and SWH were 10.7, 30.3, 20.2, 24.7, 36.3, 32.3, and 26.3 mg C g–1 soil, respectively. The bulk density of the mixture of soil and biochar was calculated as follows (Adams 1973):

where ρb is the bulk density of the mixture of soil and biochar (g cm–3), x is the percentage by weight of biochar, ρ1 is the bulk density of biochar (g cm–3), and ρ2 is the bulk density of soil (g cm–3). A glass cylinder was filled with biochar particles. The filled volume and mass of biochar in the cylinder were used to calculate the biochar bulk density (Pastor-Villegas et al. 2006). Bulk densities were 1.22, 0.60, 0.60, 0.59, 0.29, 0.41, and 0.38 g cm–3 for the soil and biochars DL, DM, DH, WL, WM, and WH, respectively. Based on Eqn 1, the initial bulk densities of the soil–biochar mixtures were 1.16, 1.16, 1.16, 1.05, 1.11, and 1.10 g cm–3 for SDL, SDM, SDH, SWL, SWM, and SWH, respectively. Each of the mixtures of soil and biochar or the soil alone was packed into a 500-mL pot based on the bulk densities above. The pot was covered with a piece of plastic sheet to prevent moisture loss. A few small holes were pricked on the plastic sheet to keep atmosphere pressure inside the bottle. For each pot, deionised water was added to retain a volumetric water content of 80% of field water capacity (the water content at suction of 330 cm) determined based on the soil-water retention curve (Ouyang and Zhang 2013). During the incubation period, the soil water content was adjusted weekly based on weight loss. Twenty-four replicates (pots) were prepared for each treatment.
Chemical analyses
Soil organic C (SOC), DOC, microbial biomass C (MBC), pH, and C/N ratio were measured on days 0, 5, 15, 30, 50, 80, 120, and 180 of the incubation experiment. These measurements were conducted using three destructive samples from the replicates on each sampling date. The SOC of the mixtures was measured by oxidation with potassium dichromate (Peng et al. 2011), DOC was measured using the method of Bruun et al. (2012), and MBC was determined using the fumigation–extraction method, in which soil K2SO4 extract was analysed using the TOC analyser (Brookes et al. 1985). Sample pH and C/N ratio were measured using the methods mentioned above.
Soil C mineralisation measurements were conducted every day from days 0 to 15, every 3 days from days 16 to 60, and every 10 days from day 61 to the end of incubation, using the method of Tang et al. (2011). At each measurement day, the initial concentration of CO2 within the headspace of each incubation pot was measured with an infrared ray gas analyzer (IRGA). Then the pot was airproofed. After 2 hours, the concentration of CO2 within the headspace of the pot was measured with IRGA. The CO2 emission rate was calculated using the following relationship:
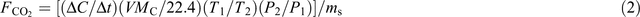
where FCO2 is the CO2 emission rate (μg C s–1 g–1 soil), ΔC/Δt is the CO2 concentration gradient calculated with the two measurements at each measurement day (μg g–1 soil s–1), V is the headspace volume (L), MC is the C molecule weight (g mol–1), T1 is the temperature in the normal state (273 K), T1 is the air temperature in the headspace (K), P1 is the air pressure in the normal state (1.013 × 105 Pa), P2 is the air pressure in the headspace (Pa), and ms is the dry mass of total incubated soil in the pot (g). Carbon mineralisation kinetics was fitted with a two-pool model as follows (Cayuela et al. 2010):

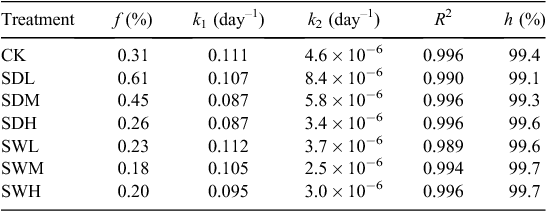
where Cr is the C fraction remained in the soil (%), f is the C fraction of the active or fast turnover pool (%), k1 is the decomposition rate constant for the active pool (day–1), k2 is the rate constant for the slow turnover pool (day–1), and t is the incubation time (days). The humification coefficient (h) is defined as the fraction of organic matter remaining in the soil after 1 year and is calculated with Eqn 3 by setting t = 365 days (Bradbury et al. 1993). The humification coefficient gives an estimation of the stable organic matter remaining in the soil and it is used as an indicator of C sequestration potential (Cayuela et al. 2010).
Statistical analyses
All data were analysed using the SPSS software package (SPSS Inc. 2003). Significant differences among different treatments and sampling dates were tested by the one-way analysis of variance (ANOVA), in which the post-hoc test of least significant difference (l.s.d.) was used. The two-way ANOVA was used to identify the primary factors (feedstock and pyrolysis temperature) on C mineralisation. Differences between the values were considered to be statistically significant at P = 0.05. The coefficient of determination (R2) was used to determine the accuracy of regressions.
Results
Biochar properties
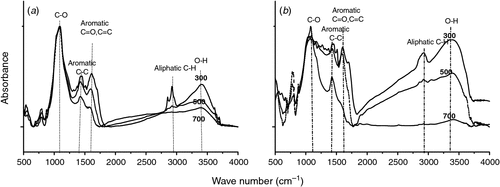
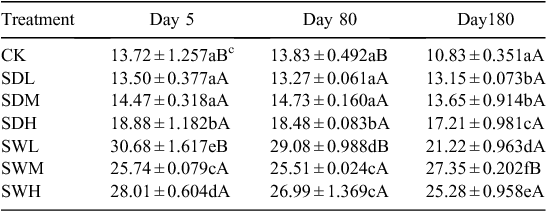
The measured physicochemical properties of the biochars and soil are listed in Table 1. The pH values of biochars were significantly higher than that of the soil (P < 0.05). The woodchip biochars had higher C/N ratio and specific surface area than the dairy manure biochars. The volatile component of the biochars decreased with increasing pyrolysis temperature, and the dairy manure biochars contained a higher volatile component than the woodchip biochars. Under the same pyrolysis temperature, the concentrations of available N were significantly higher in the dairy manure biochars than in the woodchip biochars (P < 0.05). Most of the initial DOC values were significantly higher in the biochars, especially in the dairy biochars produced at lower temperatures, than in the soil (P < 0.05). Higher pyrolysis temperatures resulted in greater surface areas, higher pH values and ash contents, and lower available N contents (Table 1). Scanning electron microscopy of the biochars showed that biochar particles became smaller as the pyrolysis temperatures increased (Ouyang and Zhang 2013). Graphs of FTIR are presented for the dairy manure biochars (Fig. 1a) and the woodchip biochars (Fig. 1b) produced under temperatures from 300 to 700°C. The spectrums included several adsorption bands. The band around 800–1600 cm–1 was associated with aromatic C-H, C=C, C=O stretching, 2900 cm–1 with aliphatic C-H stretching, and 3400 cm–1 with O-H stretching, respectively. As the charring temperature increased, adsorption intensities of biochar at the bands 3400 cm–1 and 2900 cm–1 decreased, indicating a reduction of O-H and aliphatic C-H bonds, whereas the adsorption at the band 1400 cm–1 was intensified, indicating an increase of aromatic C, especially for the woodchip biochars (Peng et al. 2011).
|
Carbon mineralisation
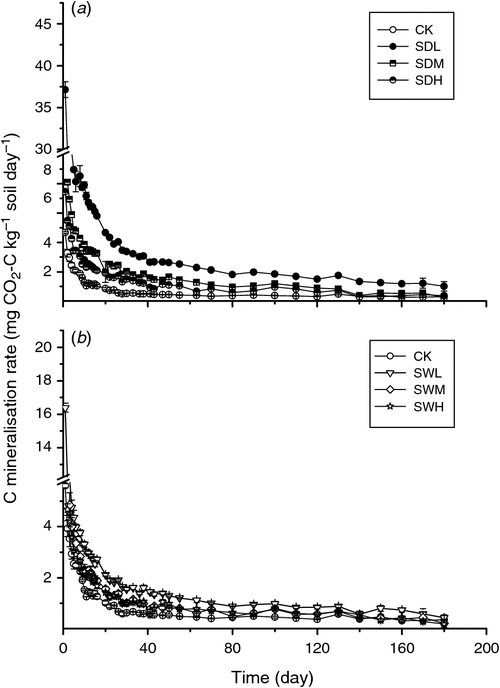
Throughout the incubation, maximum mineralisation rates were observed for all seven treatments on day 1, with the SDL treatment showing the highest mineralisation rate (Fig. 2). For the same feedstock, the biochar-induced increases in soil mineralisation at the early incubation stage were SDL > SDM > SDH (increase by 5.41, 2.69, and 1.92 times over CK, respectively), and SWL > SWM > SWH (increase by 2.58, 1.78, and 1.61 times over CK, respectively). At the later incubation stage (after about day 15), except for SDL, the differences of soil C mineralisation rates among the six treatments were not significant (P > 0.05). Soil cumulative CO2 emissions were significantly different among the seven treatments, ranging from 41.31 to 224.4 mg CO2-C kg–1 soil day–1 at the end of the incubation (P < 0.05) (Fig. 3). Soil cumulative CO2 emissions were significantly different between treatments with the dairy manure biochars and the woodchip biochars produced with the same pyrolysis temperature (P < 0.05). In all biochar treatments, the cumulative C losses in the soil were positively correlated with the volatile matter contents in the biochars (R2 = 0.75). The two-way ANOVA analysis showed that partial η2 values for feedstock v. C mineralisation and pyrolysis temperature v. C mineralisation were 0.985 and 0.993, respectively, indicating that the pyrolysis temperature and the feedstock were equally important in affecting C mineralisation. The temporal distributions of C mineralisation rates in all treatments were similar (Figs 2 and 3).
|
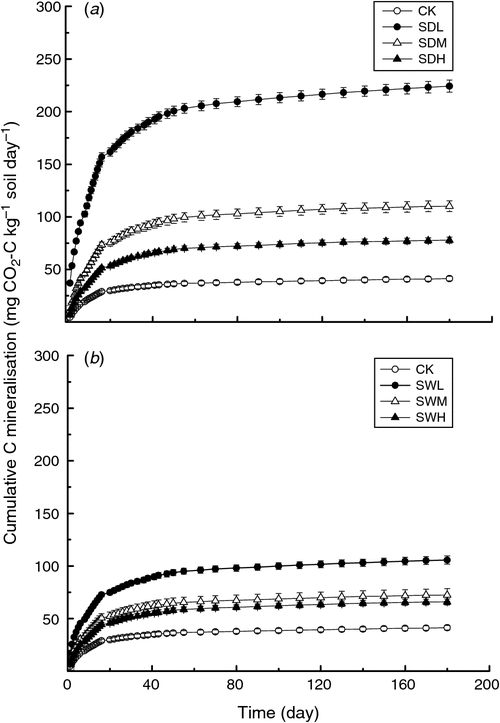
|
The C decay patterns in soil after the application of different biochars were fitted with the two-pool model. As shown in Table 2, values of the C fraction of the active or fast turnover pool (f) for the seven treatments ranged from 0.18% to 0.61%, whereas values of the humification coefficient (h) were >99%. Values of the decomposition rate constant for the active pool (k1) in all treatments were ~0.1 day–1, whereas values of the rate constant for the slow turnover pool (k2) were as low as 2.5 × 10−6 to 8.4 × 10−6 day–1. The values of f and k2 in all biochar-treated soils, except SDL and SDM, were lower than those of CK. After the 180-day incubation, all biochar treatments led to >99% of C sequestrated in the mixtures, and the h values and the remaining C amount were higher in the treatments with the woodchip biochars than with dairy manure biochars (Table 2). Based on the two-pool model simulation results (Table 3), the amounts of sequestrated C in biochar-treated soils will increase by 1.88–3.28 times compared with the sole soil in 10 years.
|
|
Soil organic C, and dissolved organic C
The biochar application systematically increased SOC, although the increases varied with the different biochars (Table 4). Biochars produced under the lower pyrolysis temperature (300°C) increased the SOC more markedly than biochars produced at the higher pyrolysis temperatures (500 and 700°C). The SOC values of the treatments with the woodchip biochars were significantly higher than those with the dairy manure biochars produced with the same pyrolysis temperature (P < 0.05).
As shown in Fig. 4, the DOC values were significantly higher (P < 0.05) in most of biochar-treated soils than in the control. The DOC values in the biochar treatments decreased as pyrolysis temperatures increased. With the same pyrolysis temperature, the dairy manure biochars led to a larger increase in soil DOC than the woodchip biochars. The temporal changes of DOC, which decreased with time within the whole incubation, were similar for all treatments.
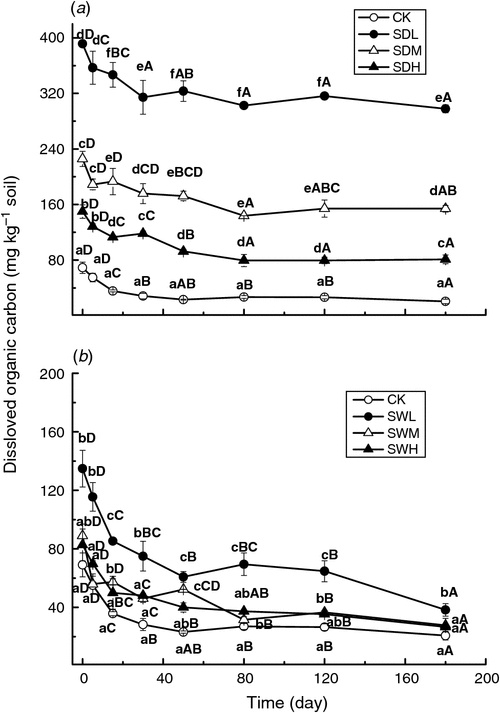
|
For the different sampling dates, the C mineralisation rates of the seven treatments were positively correlated with the corresponding DOC values, with R2 values ranging from 0.707 to 0.953. The slopes of the linear relationships decreased from 0.089 on day 0 to 0.0024 on day 180. For the samplings on days 0, 5, and 30, the C mineralisation rates and the MBC values of the seven treatments also showed a positive relationship, with R2 values of 0.936, 0.726, and 0.775, respectively. However, R2 values of the linear regressions between the C mineralisation rates and the MBC values for days 80, 120, and 180 were 0.428, 0.107, and 0.034, respectively.
Soil pH and C/N
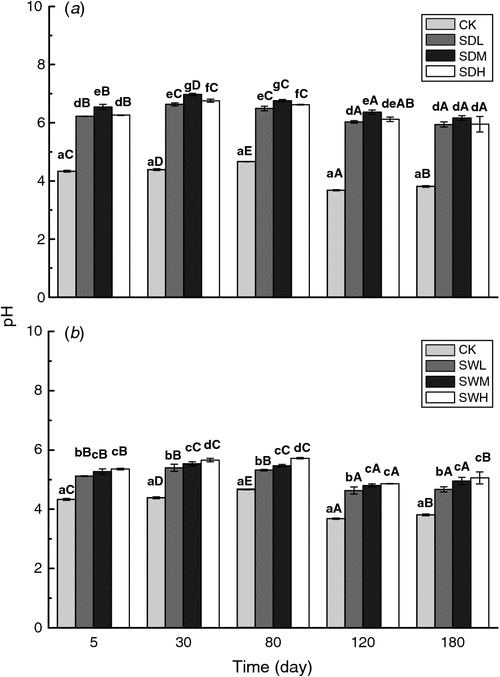
Soil pH values significantly increased with the addition of biochar (Fig. 5). On average, the different biochar treatments resulted in an increase of 0.79–2.21 pH units. On the sampling dates, SDM and SWH resulted in the largest increases in pH among the dairy manure biochars and the woodchip biochars, respectively. The dairy manure biochars had greater effect in increasing soil pH than the woodchip biochars. After 120 days of incubation, pH values decreased significantly with time in all treatments relative to the start of the experiment.
|
Most of the biochar treatments increased the C/N ratio of the mixtures, with the extent of increase related to the feedstock and the pyrolysis temperature for biochars (Table 5). The C/N ratios were significantly higher in the woodchip biochar treatments than in the dairy manure biochar treatments (P < 0.05). The C/N values in the dairy manure biochar treatments increased with the pyrolysis temperature, whereas in the woodchip biochar treatments, C/N ratio in SWL (300°C) was the highest at the early incubation stage.
|
Discussion
Similar to other studies (Hamer et al. 2004; Hilscher et al. 2009), all of the biochars examined here stimulated soil C mineralisation, especially at the early incubation stage. The increase in soil C mineralisation was a result of stimulation of microbial activity through the addition of labile C and nutrients within biochars (Hamer et al. 2004). The volatile matter and available nutrients in the biochars (Table 1) could be a good source for microorganisms, resulting in an increase in SOC and DOC in all biochar-treated soils, and consequently enhancing the microbial biomass. In this study, biochar application increased the SOC content, possibly because biochar contained high C content and could protect organic C from utilisation (Glaser et al. 2002; Cayuela et al. 2010). In addition, the woodchip biochars were mostly composed of highly condensed, aromatic structures that were physically resistant to degradation. Although the application of woodchip biochars resulted in higher SOC values, the dairy manure biochar treatments contained higher DOC content, which could provide a more labile source of energy and nutrients for soil microorganisms (Bowen 2006). The linear relationships between the C mineralisation rates and DOC values of the seven treatments indicated that the enhanced C mineralisation was partly attributed to increased DOC values following biochar additions. This may explain the higher C mineralisation rates in the soil treated with the dairy manure biochars. The different contents of labile C in the seven treatments were also reflected from their distinct microbial responses. As reported by Ouyang and Zhang (2013), the MBC was significantly higher in the biochar treatments than in the control, which showed the stimulating effects of biochars on soil microbial activities. Similar results have been reported in the literature, mainly related to the availability of an easily decomposable, non-aromatic fraction of biochar (Steiner et al. 2008; Kolb et al. 2009; Kuzyakov et al. 2009; Novak et al. 2010). The biochars pyrolysed at lower temperatures induced a greater increase in C mineralisation than the biochars pyrolysed at higher temperatures, primarily because the biochars produced at lower temperatures contained more of the incompletely pyrolysed fraction of the feedstock material (Bruun et al. 2011). For instance, biochars pyrolysed at higher temperatures have less impact on soil MBC and DOC during the incubation. Further, the different chemical properties and physical structures of biochars, which were largely determined by feedstock and pyrolysis temperatures, significantly influenced short-term biochar-C loss. As shown in Fig. 1, more aromatic C-C bonds in biochars pyrolysed at higher temperatures indicated greater chemical recalcitrance and stability of the biochars, whereas more aliphatic C-H bonds in biochars pyrolysed at lower temperatures indicated more useable substrate in the biochars (Bruun et al. 2008; Yuan et al. 2011). The dairy manure biochars decayed faster than the woodchip biochars, which might be due to the lower C/N ratios and higher available-N contents in the dairy manure biochars (Helfrich et al. 2008).
The increase in soil pH with biochar application also affected C mineralisation. Increases in soil pH can stimulate CO2 emission, and the effect is more pronounced in low pH soils (Blagodatskaya and Kuzyakov 2008; Luo et al. 2011). With relatively low pH in our soil (4.25), the significantly increased pH values in the mixtures after applying biochar should partially contribute to the increased CO2 emission. Another factor contributing to the increased CO2 emission in the biochar-amended soil might be that the biochar enhanced mineralisation of native organic matter in the soil (Wardle et al. 2008). Soil C/N ratio and pH can change the microbial population and activity (Helfrich et al. 2008). The short-term microbial responses might also be related to the increased solubility of organic matter with the increase in soil pH (Curtin et al. 1998). The increased pH values in the original low pH soil could also enhance the availability of nutrients and consequently increase soil microbial biomass (Atkinson et al. 2010; Warnock et al. 2010). At the early stage of incubation, the increase in soil pH after biochar addition might benefit r-strategists, which are more dominant in higher pH soils (Bååth and Anderson 2003) and can rapidly utilise liable components (Fig. 5). At the later stage of incubation, rates of soil C mineralisation were not significantly different among the treatments. A possible reason for the result is that the labile composition of biochars was almost mineralised and the soil organic matter might be adsorbed to biochars, either within biochar pores or onto external biochar surfaces (Zimmerman et al. 2011). Sobek et al. (2009) showed that biochars could adsorb organic matter and protect it from being utilised. In this study, the lower soil mineralisation rates in the treatments with biochars produced at higher temperatures than at lower temperatures might be partly attributable to their higher surface areas with greater adsorption affinity for natural organic matter (Kasozi et al. 2010).
Although biochar addition induced significant short-term CO2 emission from the soil, rates of soil C mineralisation decreased sharply within a short period and then remained at stably low values with time. Most of the C (>99%) from the biochar addition remained in the soil for a long time, indicating that biochar has high C-sequestration potential. The low degradability of C from biochar has been reported in the literature (Kuzyakov et al. 2009; Major et al. 2010) and is strongly promoted for C-sequestration aims (Lehmann 2007; Laird et al. 2009). The small rate constant values for the slow turnover pool (in the order of 10−6 day–1) indicate that the increased C resulting from biochar application will stay in the soil for long time. The higher refractory C in biochar should also account for the C-sequestration potential of biochar. As suggested by Cayuela et al. (2010), biochar with more refractory C and higher C/N ratio had higher C sequestration potential. Compared with the dairy manure biochar treatments, the woodchip biochar treatments resulted in lower rates of C mineralisation, and higher values of SOC, C/N ratio, and humification coefficient, suggesting that the woodchip biochars should have higher C-sequestration potential. Although the increase of pyrolysis temperature resulted in a decrease in C content in biochar, the treatments with biochars produced at higher temperatures showed higher C-sequestration ability, as indicated by the lower rate constant for the slow turnover pool and higher humification coefficient.
Conclusions
This study investigated the effects of biochars produced from dairy manure and woodchip under different temperatures on soil C turnover. The biochar application induced a rapid increase in soil C mineralisation during the early incubation period (within the first 15 days), primarily because of the labile C fraction in the biochars. However, rates of soil C mineralisation decreased sharply after a short period and then remained at stably low values with time. Soil physicochemical changes with the biochar addition, including increases in SOC, DOC, MBC, and pH values, also affected soil C mineralisation and sequestration. The feedstock and pyrolysis temperature largely determined the physicochemical properties of biochars and, hence, had a significant impact on effects of the biochars on soil C turnover and associated soil properties. The dairy manure biochars produced at lower temperature, which contained more labile fraction and relatively easily degradable structure, resulted in the largest increase in soil C mineralisation and the highest microbial biomass. Although significant C losses from soils treated with biochars were observed, the incorporation of biochar increased total SOC and compensated for the C losses in the soil. The high humification coefficients and low rate constants for the slow turnover pool in the biochar-treated soils indicated the biochar’s C-sequestration potential. Meanwhile, >99% of biochar-induced C remained as stable organic C in the soil after 180 days, further demonstrating the ability of biochar in sequestering C. Biochar produced from woodchip and at higher pyrolysis temperature showed higher C sequestration potential. The information from this study should be valuable for the utilisation of biochar for soil C sequestration.
Acknowledgements
This study was partly supported by grants from the Chinese National Natural Science Foundation (Nos 51039007 and 51179212) and Doctoral Fund of Ministry of Education of China (No. 20120171110040).
References
Abdullah H, Mediaswanti AK, Wu H (2010) Biochar as a fuel: 2. Significant differences in fuel quality and ash properties of biochars from various biomass components of Mallee trees. Energy & Fuels 24, 1972–1979.| Biochar as a fuel: 2. Significant differences in fuel quality and ash properties of biochars from various biomass components of Mallee trees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1ansrs%3D&md5=e2d52e0c808f978109f7aacbed975a46CAS |
Adams WA (1973) The effect of organic matter on the bulk and true densities of some uncultivated podzoilc soils. Journal of Soil Science 24, 10–17.
| The effect of organic matter on the bulk and true densities of some uncultivated podzoilc soils.Crossref | GoogleScholarGoogle Scholar |
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant and Soil 337, 1–18.
| Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVWmtb3J&md5=41c67e956c94c52fbd96c13d98ae67e7CAS |
Bååth E, Anderson T-H (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biology & Biochemistry 35, 955–963.
| Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques.Crossref | GoogleScholarGoogle Scholar |
Baneschi I, Dallai L, Giazzi G, Guidi M, Krotz L (2013) A method for the definition of the carbon oxidation number in the gases dissolved in waters and the redox variations using an elemental analyser (FlashEA 1112). Preliminary data from a stratified lake. Journal of Geochemical Exploration 124, 14–21.
| A method for the definition of the carbon oxidation number in the gases dissolved in waters and the redox variations using an elemental analyser (FlashEA 1112). Preliminary data from a stratified lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhslaku7%2FN&md5=8d1512f738a26444c4cb118e83e399a5CAS |
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biology and Fertility of Soils 45, 115–131.
| Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review.Crossref | GoogleScholarGoogle Scholar |
Bowen S (2006) Biologically-relevant characteristics of dissolved organic carbon (DOC) from soil. PhD Thesis, School of Biological and Environmental Sciences, University of Stirling, Stirling, UK.
Braadbaart F, Boon JJ, Veld H, David P, Van Bergen PF (2004) Laboratory simulations of the transformation of the peas as a result of heat treatment: Changes of the physical and chemical properties. Journal of Archaeological Science 31, 821–833.
| Laboratory simulations of the transformation of the peas as a result of heat treatment: Changes of the physical and chemical properties.Crossref | GoogleScholarGoogle Scholar |
Bradbury NJ, Whitmore AP, Hart PBS, Jenkinson DS (1993) Modelling the fate of nitrogen in crop and soil in the years following application of 15N-labeled fertilizer to winter wheat. The Journal of Agricultural Science 121, 363–379.
| Modelling the fate of nitrogen in crop and soil in the years following application of 15N-labeled fertilizer to winter wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhs1altbw%3D&md5=1a9b697f784dc01956b393f1d9705982CAS |
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry 17, 837–842.
| Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XhvFSgug%3D%3D&md5=f6c6ccaff6eb87cb83866980559b9b82CAS |
Brunauer S, Emmett PH, Teller J (1938) Adsorption of gases in multi molecular layers. Journal of the American Chemical Society 60, 309–319.
| Adsorption of gases in multi molecular layers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA1cXivFaruw%3D%3D&md5=1536ba83d6f4bdc385086a721ec8edafCAS |
Bruun S, Jensen E, Jensen L (2008) Microbial mineralization and assimilation of black carbon: dependency on degree of thermal alteration. Organic Geochemistry 39, 839–845.
| Microbial mineralization and assimilation of black carbon: dependency on degree of thermal alteration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsFegsrg%3D&md5=796b2ed81cf56daf1cc5d20cd047c6deCAS |
Bruun EW, Hauggaard-Nielsen H, Norazana I, Egsgaard H, Ambus P, Jensen PA, Dam-Johansen K (2011) Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass and Bioenergy 35, 1182–1189.
| Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1ehur4%3D&md5=139e57781f954c11ae5f476d926e66adCAS |
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biology & Biochemistry 46, 73–79.
| Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosFCltg%3D%3D&md5=ac82db99ce3d541962cdaf10195d1a94CAS |
Cayuela ML, Oenema O, Kuikmanw PJ, Bakkerz RR, van Groenigen JW (2010) Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. Global Change Biology Bioenergy 2, 201–213.
Curtin D, Campbell CA, Jalil A (1998) Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biology & Biochemistry 30, 57–64.
| Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtVantQ%3D%3D&md5=71d2d42fa7dd1bb0c770954fc6cdb39bCAS |
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In ‘Biochar for environmental management: Science and technology’. (Eds J Lehmann, S Joseph) pp. 13–29. (Earthscan: London)
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biology and Fertility of Soils 35, 219–230.
| Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xkt1Wmsrc%3D&md5=d18ae5235ba5353425a5a810075f94c4CAS |
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralization. Organic Geochemistry 35, 823–830.
| Interactive priming of black carbon and glucose mineralization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksFKju7k%3D&md5=259dfb3f810a1f421cc8228173ce4c09CAS |
Helfrich M, Ludwig B, Potthoff M, Flessa H (2008) Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen. Soil Biology & Biochemistry 40, 1823–1835.
| Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1eksLw%3D&md5=f8e7d108ebc672e96f0cf577c9ef5089CAS |
Hilscher A, Heister K, Siewert C, Knicker H (2009) Mineralization and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Organic Geochemistry 40, 332–342.
| Mineralization and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitFCisLo%3D&md5=b96c4ece5203f0df4e4b9c8f2cef5729CAS |
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environmental Science & Technology 44, 6189–6195.
| Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsV2msLY%3D&md5=d75b61032b6383c6f4f5ea993607e63eCAS |
Kolb S, Fermanich K, Dornbush M (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Science Society of America Journal 73, 1173–1181.
| Effect of charcoal quantity on microbial biomass and activity in temperate soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos1Ggs7s%3D&md5=29b79a6d9dcfac31e2af878185fb04a7CAS |
Krull ES, Baldock JA, Skjemstad JO, Smernik RJ (2009) Characteristics of biochar: organo-chemical properties. In ‘Biochar for environmental management: Science and technology’. (Eds J Lehmann, S Joseph) pp. 53–65. (Earthscan: London)
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biology & Biochemistry 41, 210–219.
| Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotV2isg%3D%3D&md5=0577ae6e3f0d372d7ad21b4ed838ba83CAS |
Laird DA (2008) The charcoal vision: a win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agronomy Journal 100, 178–181.
| The charcoal vision: a win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality.Crossref | GoogleScholarGoogle Scholar |
Laird DA, Brown RC, Amonette JE, Lehmann J (2009) Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels, Bioproducts and Biorefining 3, 547–562.
| Review of the pyrolysis platform for coproducing bio-oil and biochar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFegtLvE&md5=57605e40dcd1e747865d2f67d06b8ad6CAS |
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158, 443–449.
| Impact of biochar amendments on the quality of a typical Midwestern agricultural soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyjsLfO&md5=6609b7399c6f2c53ea85d90a6fb5549bCAS |
Lal R, Follett R, Stewart B, Kimble J (2007) Soil carbon sequestration to mitigate climate change and advance food security. Soil Science 172, 943–956.
| Soil carbon sequestration to mitigate climate change and advance food security.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVelsLjJ&md5=7d15aca6793057d30aaccd8d6f5ab586CAS |
Lehmann J (2007) Bioenergy in the black carbon. Frontiers in Ecology and the Environment 5, 381–387.
| Bioenergy in the black carbon.Crossref | GoogleScholarGoogle Scholar |
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitigation and Adaptation Strategies for Global Change 11, 395–419.
| Bio-char sequestration in terrestrial ecosystems—a review.Crossref | GoogleScholarGoogle Scholar |
Luo Y, Durenkamp M, Nobili MD, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralization of biochar following its incorporation to soils of different pH. Soil Biology & Biochemistry 43, 2304–2314.
| Short term soil priming effects and the mineralization of biochar following its incorporation to soils of different pH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtF2rsLnM&md5=966475778cb91cae547831c8b47c71efCAS |
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil applied black carbon: downward migration, leaching and soil respiration. Global Change Biology 16, 1366–1379.
| Fate of soil applied black carbon: downward migration, leaching and soil respiration.Crossref | GoogleScholarGoogle Scholar |
Novak JM, Busscher WJ, Laird DL, Ahmedna M, Watts DW, Niandou MAS (2009) Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Science 174, 105–112.
| Impact of biochar amendment on fertility of a southeastern coastal plain soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvVSkt78%3D&md5=e6875745e12a1d7289fb9627b92651f3CAS |
Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS (2010) Short-term CO2 mineralization after additions of biochar and switch grass to a Typic Kandiudult. Geoderma 154, 281–288.
| Short-term CO2 mineralization after additions of biochar and switch grass to a Typic Kandiudult.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1WltLfI&md5=6e4a19b9b1baceecf95380c0fd65adbbCAS |
Ouyang L, Zhang R (2013) Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. Journal of Soils and Sediments
| Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties.Crossref | GoogleScholarGoogle Scholar |
Pastor-Villegas J, Pastor-Valle JF, Meneses-Rodriguez JM, Garcia M (2006) Study of commercial wood charcoals for the preparation of carbon absorbents. Journal of Analytical and Applied Pyrolysis 76, 103–108.
| Study of commercial wood charcoals for the preparation of carbon absorbents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVSjtLw%3D&md5=e3bd7fec72b69acee91178c2c135e8caCAS |
Peng X, Ye L, Wang C, Zhou H, Sun B (2011) Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil & Tillage Research 112, 159–166.
| Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China.Crossref | GoogleScholarGoogle Scholar |
Sobek A, Stamm N, Bucheli TD (2009) Sorption of phenyl urea herbicides to black carbon. Environmental Science & Technology 43, 8147–8152.
| Sorption of phenyl urea herbicides to black carbon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFKktL3F&md5=3e7ca4023ee92b18a994fff2d7f07dffCAS |
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Advances in Agronomy 105, 47–82.
| A review of biochar and its use and function in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtlGitbo%3D&md5=aace8ad37c202bd7ba7298cc8703498eCAS |
Spokas KA (2010) Review of the stability of biochar in soils: predictability of O : C molar ratios. Carbon Management 1, 289–303.
| Review of the stability of biochar in soils: predictability of O : C molar ratios.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGrs7fO&md5=cf51875711291abcc0486dd2ddaf37e9CAS |
Steiner C, Das K, Garcia M, Forster B, Zech W (2008) Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 51, 359–366.
| Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol.Crossref | GoogleScholarGoogle Scholar |
Tang J, Mo Y, Zhang J, Zhang R (2011) Influence of biological aggregating agents associated with microbial population on soil aggregate stability. Applied Soil Ecology 47, 153–159.
| Influence of biological aggregating agents associated with microbial population on soil aggregate stability.Crossref | GoogleScholarGoogle Scholar |
Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320, 629
| Fire-derived charcoal causes loss of forest humus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFyju78%3D&md5=e57ac922f2efe785babb993b2a0aa691CAS | 18451294PubMed |
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant and Soil 300, 9–20.
| Mycorrhizal responses to biochar in soil—concepts and mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1Cgt7zI&md5=f1c7c7e5a4dedbd9bf5e60bc323c891aCAS |
Warnock DD, Mummey DL, McBride B, Major J, Lehmann J, Rillig MC (2010) Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Applied Soil Ecology 46, 450–456.
| Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments.Crossref | GoogleScholarGoogle Scholar |
Yu L, Tang J, Zhang R, Wu Q, Gong M (2013) Effects of biochar application on soil methane emission at different soil moisture levels. Biology and Fertility of Soils 49, 119–128.
| Effects of biochar application on soil methane emission at different soil moisture levels.Crossref | GoogleScholarGoogle Scholar |
Yuan J, Xu R, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technology 102, 3488–3497.
| The forms of alkalis in the biochar produced from crop residues at different temperatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXks1GitQ%3D%3D&md5=e96411c403c61ed95dacaf6d0ce0418fCAS | 21112777PubMed |
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environmental Science & Technology 44, 1295–1301.
| Abiotic and microbial oxidation of laboratory-produced black carbon (biochar).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslWgug%3D%3D&md5=4e1ff972fef83d1c777b406ea581255eCAS |
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soil. Soil Biology & Biochemistry 43, 1169–1179.
| Positive and negative carbon mineralization priming effects among a variety of biochar-amended soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvFagt7c%3D&md5=95770e605ca77f1f59053c82dbf933d2CAS |