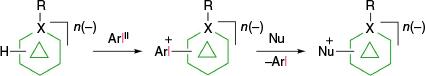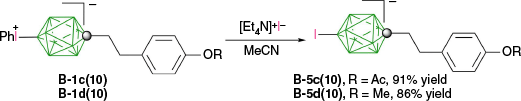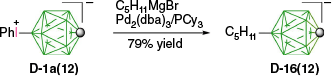closo-Borate aryliodonium zwitterions: convenient intermediates for functional closo-borane derivatives and molecular materials
Piotr Kaszyński A B C *
A B C *
A
B
C

Piotr Kaszyński is a professor of chemistry at the Centre of Molecular and Macromolecular Studies of the Polish Academy of Sciences and University of Łódź in Poland. He received his MSc degree from Warsaw Polytechnic in Poland in 1985, PhD degrees from the University of Texas at Austin in 1991 and the University of Łodź in 2007. He was a postdoctoral fellow at Caltech before joining Vanderbilt University in Nashville. In 2015, he moved the bulk of his research program to Polish Academy of Sciences and University of Łódź, while maintaining ties with Middle Tennessee State University in USA. He has published over 200 original papers, and several book chapters and reviews. |
Abstract
Zwitterionic aryliodonium derivatives of closo-borates are attractive intermediates in regioselective functionalization of anionic boron clusters through nucleophilic substitution with halides, and N-, O- and C-centered reagents. They are easily available in high yields by highly regioselective direct aryliodination of the closo-borate anions with ArIII species, typically PhI(OAc)2 and undergo four types of transformations: substitution through a rearrangement of a 10-I-3 species, dissociation to boronium ylides and trapping with the solvent, single electron transfer and homolysis of the 9-I-2 species to B–I products, and iodoaryl slippage to cage arylation byproducts. These zwitterions exhibit moderate and well-balanced thermal stability, which, in most cases, is sufficient for their isolation and synthetic applications. It depends on the closo-borane, position of the aryliodonium substituent, the presence of other substituents and finally the nature of the aryl group. The two-step process, aryliodination and substitution, can be regarded as a regioselective activation of the B–H bond for nucleophilic substitution. This review summarizes the formation, structure, stability and reactions of zwitterionic derivatives of 10- and 12-vertex closo-borate anions accumulated in the literature in the past decade.
Keywords: closo-borates, carboranes, functionalization, functional group transformations, hypervalent iodine, iodonium zwitterions, regioselectivity, synthesis.
Introduction
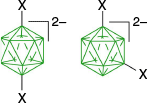
closo-Boranes, such as A–H in Fig. 1, belong to an extensive family of inorganic boron hydrides with three-dimensional molecular structures characterized by three-center two-electron bonds.1 They generally exhibit high chemical, electrochemical and thermal stabilities and are regarded as sigma-aromatic species.2 Their electronic structures, the symmetry and energy of MOs, allow them to interact with π substituents with the strength dependent on the cluster and substitution position.3–5 For these reasons closo-boranes, almost exclusively the 10- and 12-vertex clusters (Fig. 1), have been explored as components of photonic materials,3,6–8 liquid crystals,9 anisotropic electrolytes10,11 and pharmacophores,12 among others.13–16 One of the key issues in such applications is the regioselectivity of closo-borane substitution and functionalization to tailor specific steric and electronic properties of their derivatives.
Selected 10- and 12-vertex closo-boranes and COSAN (E). Each vertex corresponds to a B–H fragment and the CH groups are marked by closed circles.

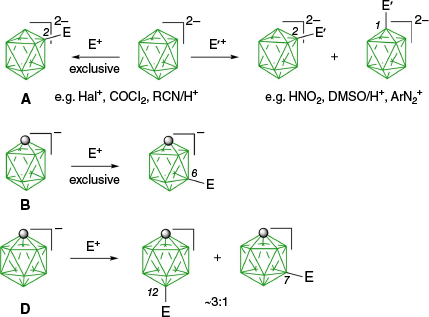
The most common method for the introduction of substituents to closo-boranes is the reaction with electrophiles.17 The reactivity of the electrophile and regioselectivity of such reactions depend on the cluster size and the overall electric charge. In general, reactivity towards electrophiles increases with the increasing negative charge, and 10-vertex species are more reactive than their 12-vertex analogues. This general trend is related to the level of the HOMO in the clusters. In the 12-vertex mono-anion, [closo-1-CB11H12]− (D),18 the electrophilic attack of E+ takes place mainly on the antipodal position leading to the dominant B(12) substitution product (~75%; Fig. 2). The preparation of specific derivatives of the icosahedral dianion [closo-B12H12]2− (C) is complicated by selectivity of the mono- v. di-substitution and separation of isomers of the latter. As a consequence, there are fairly few derivatives of the dodecaborate C.19 By contrast, electrophilic substitution with E+ in 10-vertex anions, [closo-B10H10]2− (A)20 and [closo-1-CB9H10]− (B),21 usually leads to the equatorial derivatives (Fig. 2), not the apical regioisomers, which are the most interesting for steric and electronic reasons. Thus, for dianion A, there are very few electrophiles, E′+, which give the apical product (B(1)-substituted) typically in a mixture with the equatorial isomer (B(2)-substituted). On the other hand, the [closo-1-CB9H10]− (B) anion reacts with electrophiles, giving exclusively the equatorial (B(6)-substituted) derivatives (Fig. 2). This regioselectivity of substitution changed dramatically with the use of ArIII reagents as electrophiles.
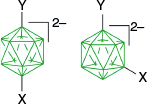
Summary of electrophilic substitution of three closo-boranes. The partial cage numbering system is shown.
Diaryliodonium salts22,23 undergo nucleophilic displacement of an ArI fragment and for this reason they have been recognized as synthetically useful intermediates. It was also demonstrated that aryliodonium salts derived from 12-vertex carboranes24 F–H undergo substitution reactions serving, in many cases, as useful intermediates to functional carborane derivatives.25–27
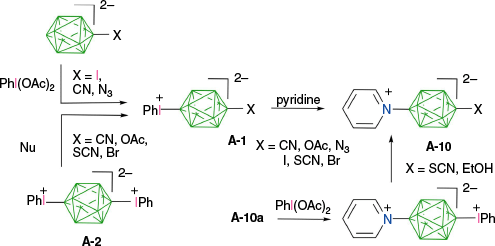
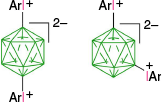
A decade ago, it was demonstrated that anionic closo-boranes A–E easily form aryliodonium zwitterions (rather than salts), which opened up new opportunities in functionalization of these anions through a two-step process: substitution with an aryliodonium group followed by reaction with a nucleophile (Fig. 3). The first step, electrophilic substitution of the closo-borane with the ArI+ group, is highly selective and can be regarded as activation of a specific B–H bond towards nucleophilic substitution. In addition to the unprecedented high regioselectivity of the aryliodination process, the resulting zwitterions are electrically neutral, although highly polar species, which can be purified using standard methods, e.g. chromatography. This review concentrates on zwitterionic aryliodonium derivatives of closo-borate anions: their formation, structural data, stability and use in synthesis of functional derivatives. Finally, several examplary applications of these zwitterions in synthesis of molecular materials are provided.
Formation of aryliodonium zwitterions
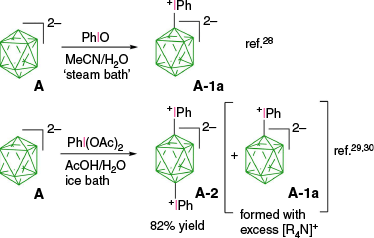
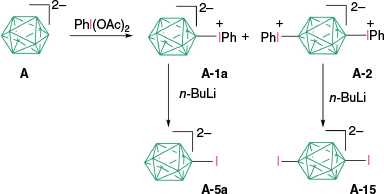
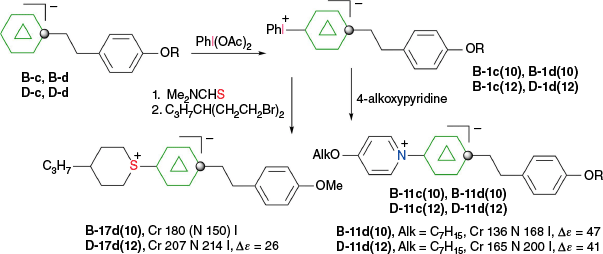
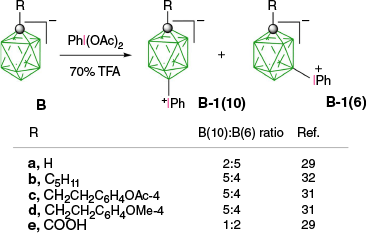
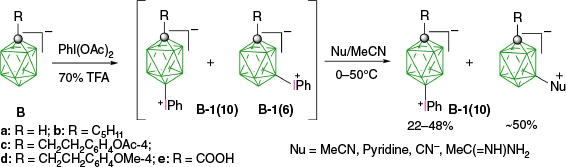
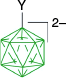
The first reported aryliodonium zwitterion of closo-borane, A-1a, was obtained in 15% yield by treatment of [closo-B10H10]2− anion (A) with PhIO in hot aqueous solutions (Scheme 1).28 Experiments conducted five decades later demonstrated that, while the original procedure was irreproducible, treatment of A with PhI(OAc)2 in aqueous AcOH at 0°C gives a high yield of bis-zwitterion A-2 as the only product.29 It is the dominant product even with 1 eq of PhI(OAc)2. The efficient preparation of A-1a, up to 65% yield, requires the presence of large amounts of [R4N]+ to precipitate anion A-1a from the reaction mixture, preventing it from further substitution (Scheme 1).7,30 The observed exclusive formation of the apical products renders PhIII reagents the most selective for electrophilic substitution of anion A. This reaction was expanded to other anionic closo-borates B–E, demonstrating again high yields and the highest degree of regioselectivity for any electrophilic substitution reactions in these clusters. Most importantly, reactions of [closo-1-CB9H10]− (B) and its C(1) derivatives with PhI(OAc)2 gave, for the first time, products of electrophilic substitution at the B(10) position, opening up access to a facile functionalization of this important site. The ratio of the B(10) v. B(6) phenyliodination products depends on the nature of the C(1) substituent and is the highest, ~5:4, for R = alkyl31,32 (B-b, B-c, B-d, Scheme 2), while for R = COOH (B-e) is 1:2 and the lowest for the parent anion B (2:5). The [closo-1-CB11H12]− anion (D) also gave the highest to date ratio of B(12) to B(7) isomers (>7:1),29,31 whereas COSAN (E) yielded the B(8) substitution product exclusively, which is typical for this anion.33,34
Phenyliodination of C(1)-substituted derivatives of anion B. Ratio of regioisomers in phenyliodination of [closo-1-CB9H9-1-R]− as determined by 1H NMR of the crude products.29,31,32 In the labels, the capital letter defines the type of the cluster, the numeral is the derivative, the lower-case letter is the additional substituent and the numeral in parentheses is the substitution position.
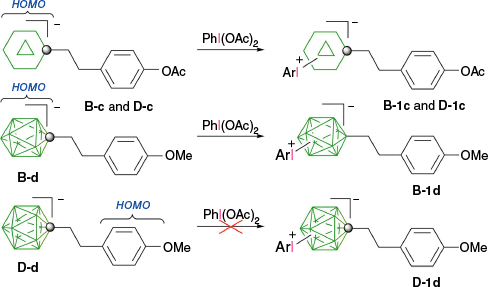
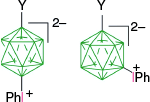
The higher reactivity of the [closo-1-CB9H10]− anion (B) as compared to the [closo-1-CB11H12]− anion (D) towards IIII reagents affects the chemoselectivity of the phenyliodination process in some derivatives. Thus, while reactions of B-c and D-c containing the acetoxyphenyl group proceeds, giving the expected mixtures of respectively regioisomers B-1c and D-1c, only the methoxy analog of B-1c, compound B-1d, gave a similar result (Scheme 3).31 By contrast, the 12-vertex derivative D-d formed only intractable products. This result was explained by the difference in localization of the HOMO: it is localized on the {closo-CB9} cluster in both derivatives B-c and B-d and on {closo-CB11} only in D-c. In the methoxy derivative D-d, the HOMO is now localized on the electron rich benzene ring, which becomes the reactive site with IIII species giving rise to intractable products.31
Phenyliodination of derivatives B-c, D-c, B-d and D-d. The HOMO is localized as indicated.31
The aryliodination of closo-borates A–E is performed typically with PhI(OAc)2 in aqueous acid (AcOH or CF3COOH) and the products are conveniently isolated by filtration.29 For substituted clusters, especially derivatives of the most reactive [closo-B10H10]2− anion (A), reactions were successfully performed in MeCN solutions.8,29 The presence of halide anions, such as Br− from the [R4N]+ salts, even in small amounts in the reaction medium, must be avoided since they undergo oxidation with IIII species and halogenate the cluster. In several cases, 4-MeOC6H4I(OAc)2 instead of PhI(OAc)2 was employed to improve solubility of the products, increase their yields and enhance stability of the zwitterions, mainly those derived from the [closo-B12H12]2− anion (C).29,35,36 These and other aryliodination reactions of closo-boranes are discussed later.
Purification and separation methods of isomeric zwitterions depend on the cluster. For example, B(12)/B(7) regioisomeric mixtures formed by [closo-1-CB11H12]− (D) and its derivatives are conveniently separated by column chromatography, taking advantage of the different polarities of both isomers.29,31 Similarly, mixtures of mono- (A-1a) and bis-iodonium (A-2) zwitterions of [closo-B10H10]2− (A) are separated by chromatography. This technique is ineffective for the separation of regioisomers of [closo-1-CB9H9-1-R]− (B). Instead, they were separated kinetically, benefiting from much higher reactivity (lower thermal stability) of the B(6) isomers B-1(6) than their B(10) analogues B-1(10) towards nucleophiles.29,31,32 Products of aryliodination of the [closo-B12H12]− dianion (C) and its derivatives are among the least thermally stable zwitterions of closo-boranes. Therefore, crude mixtures of zwitterions are often reacted with the desired nucleophile, and the separation of regioisomers, 1,7 and 1,12, is performed on the final products.36
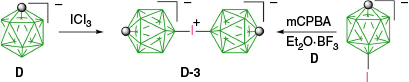
For the completeness of this report, the formation of other iodonium derivatives of carboranes needs to be mentioned. Thus, reaction of [closo-CB11H12]− (D) with ICl3 gave the [closo-CB11H11-12–I–12′-CB11H11-closo]− zwitterion (D-3) in 51% yield (Scheme 4).37 Alternatively D-3 was obtained in 82% yield by oxidation of [closo-CB11H11-12-I]− with mCPBA followed by reaction with [closo-CB11H12]− (D) in the presence of BF3·Et2O.37 This method, involving oxidation of iodine(I) to iodine(III) followed by reaction with an arene, was used earlier for the preparation of aryliodonium derivatives of carboranes F–H. The preparation of these cations and their reactions with nucleophiles were reviewed previously.25–27 In this case, the net result is activation of the iodine atom in carboranes towards substitution, whereas in the case of closo-borane anions, it is activation of a hydrogen atom.
The structure of aryliodonium zwitterions
So far, there have been 10 XRD structures of aryliodonium derivatives of closo-borates (Tables 1 and 2), in addition to aryliodonium carboranes.38 Analysis of the experimental data indicates that the iodine has a trigonal bipyramidal geometry with the most bulky closo-borane substituent in the equatorial position and the aryl in the axial position. This is also true for the structure of phenyl(9-o-carboranyl)iodonium iodide.38
| AA | A-1aB | A-2C | A-1eD | A-1fD | A-1gE | BF | B-1d(10)G | ||
|---|---|---|---|---|---|---|---|---|---|
| X= | H | H | PhI+ | COOH | CNMe+ | c-ON | H | R | |
| Y= | H | PhI+ | PhI+ | PhI+ | PhI+ | PhI+ | H | PhI+ | |
| Z= | B | B | B | B | B | B | C | C | |
| X–Z(1) | – | – | 2.178(7) | 1.582(4) | 1.54(1) | 1.474(3) | 0.97 | 1.517(3) | |
| Z(1)–B(2)avg | 1.701(3) | 1.69(1) | 1.67(1) | 1.696(1) | 1.685(14) | 1.686(14) | 1.597(4) | 1.608(2) | |
| Z(1)⋯B(2–5)H | 1.100 | 1.089 | 1.029 | 1.084 | 1.049 | 1.061 | 0.933 | 0.942 | |
| B(2)–B(3)avg | 1.835(9) | 1.83(2) | 1.87(1) | 1.845(10) | 1.865(9) | 1.85(2) | 1.834(2) | 1.842(5) | |
| B(2)–B(6)avg | 1.813(6) | 1.81(2) | 1.81(1) | 1.810(8) | 1.81(1) | 1.81(4) | 1.805(4) | 1.809(4) | |
| B(6)–B(7)avg | 1.835(9) | 1.86(2) | 1.87(1) | 1.865(9) | 1.86(1) | 1.872(8) | 1.839(6) | 1.864(8) | |
| B(10)⋯B(6–9)H | 1.100 | 1.043 | 1.029 | 1.039 | 1.021 | 1.027 | 1.090 | 1.033 | |
| B(10)–B(9) | 1.701(3) | 1.68(3) | 1.67(1) | 1.678(5) | 1.666(8) | 1.66(2) | 1.697(3) | 1.675(2) | |
| B(10)–I | – | 2.19(1) | 2.178(7) | 2.179(3) | 2.190(6) | 2.189(3) | – | 2.178(2) | |
| Z(1)⋯B(10) | 3.717 | 3.65(2) | 3.56(1) | 3.629(4) | 3.57(1) | 3.593(4) | 3.529 | 3.482(3) | |
| I–Ph | – | 2.16(1) | 2.102(5) | 2.116(3) | 2.121(5) | 2.113(3) | – | 2.114(2) | |
| B–Z(1)–Xavg | 130.3(12) | 130 | 128(2) | 129.7(8) | 128(2) | 105.3(2)I | – | 125.9(4) | |
| B–B(10)–Yavg | 130.3(12) | 129(1) | 128(2) | 128(3) | 127.8(5) | 128(3) | – | 128.1(2) | |
| B(10)–I–Ph | – | 100.1(4) | 98.3(8) | 101.1(1) | 95.9(2) | 100.1(1) | – | 97.82(8) |
All distances are in Ångstroms and angles in degrees. Except for distances that are unique in each molecule, all parameters are average (avg) values and the esd refers to the distribution of individual values.
| DA | D-1a(12)B | D-1b(12)B,C | D-1d(12)D | D-3E | ||
|---|---|---|---|---|---|---|
| X= | H | H | C5H11 | R | H | |
| Y= | H | PhI+ | PhI+ | PhI+ | {CB11}I | |
| C(1)–X | – | – | 1.537(3) | 1.532(3) | – | |
| C(1)–B(2)avg | 1.706(5) | 1.703(3) | 1.715(8) | 1.718(6) | 1.711(4) | |
| C(1)⋯B(2–6)F | 0.789 | 0.779 | 0.815(6) | 0.811 | 0.792(4) | |
| B(2)–B(3)avg | 1.778(3) | 1.781(3) | 1.774(6) | 1.780(3) | 1.782(4) | |
| B(2)–B(7)avg | 1.775(4) | 1.771(5) | 1.770(6) | 1.773(4) | 1.776(3) | |
| B(7)–B(8)avg | 1.786(4) | 1.800(4) | 1.800(4) | 1.802(4) | 1.801(4) | |
| B(7)–B(12) | 1.785(4) | 1.763(7) | 1.756(3) | 1.754(4) | 1.763(4) | |
| B(12)⋯B(7–11)F | 0.936 | 0.874 | 0.860(5) | 0.854 | 0.874(2) | |
| B(12)–Y | – | 2.226(3) | 2.234(11) | 2.233(2) | 2.230(6) | |
| B–C(1)–Xavg | 117.6(1) | 117.2(2) | 118(2) | 118(3) | 117.6(1) | |
| B–B(12)–Yavg | 121.7(1) | 120(1) | 119(1) | 119(2) | 120(4) | |
| C(1)⋯B(12) | 3.233(3) | 3.152(5) | 3.175(5) | 3.168(3) | 3.171(2) | |
| B(12)–I–Ph | – | 99.9(1) | 97.1(3) | 101.39(9) | 109.68(5)G | |
| I–Ph | – | 2.116(2) | 2.107(5) | 2.112(2) | – |
All distances are in Ångstroms and angles in degrees. Except for distances that are unique in each molecule, all parameters are average (avg) values and the esd refers to the distribution of individual values.
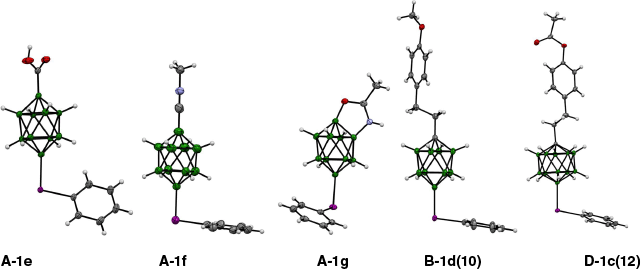
The B–I bond length depends on the cluster. In the 10-vertex zwitterions, the average B(10)–I distance is 2.184(6) Å (Table 1), whereas in 12-vertex analogues the B(12)–I distance is ~0.05 Å longer (average. 2.231(4) Å; Table 2). This difference is related to the coordination number of the bonded boron atom: five-coordinated in the former and six-coordinated in the latter derivatives. The average I–Ph distance is 2.113(6) Å in both series of compounds, excluding the anomalously long C–I bond (2.16(1) Å) found in A-2, which is presumably affected by the low quality of the data.40 The B–I–C angle is ~99(2)°, with the lowest value measured for the CN-Me derivative A-1f (95.9(2)°,41 Fig. 4). This iodine-centered angle is the largest in derivative D-3 (109.68(5)°)37 due to steric reasons. Five examples of iodonium zwitterions, carboxylic acid A-1e,41 nitrilium A-1f,41 ring fused zwitterion A-1g42 and phenethyl derivatives B-1d(10) and D-1c(12),31 are shown in Fig. 4.
Examples of phenyliodonium zwitterions: [closo-B10H8-1-COOH-10-IPh]− (A-1e),41 [closo-B10H8-1-CNMe-10-IPh] (A-1f),41 {closo-B10}-fused diboraoxazoline A-1g,42 [closo-1-CB9H8-1-R-10-IPh] (B-1d(10))31 and [closo-1-CB11H10-1-R-12-IPh] (D-1c(12)).31 Structures are generated from Open Access CIF files. Ellipsoids are drawn at 50% probability.
The PhI+ substituent exerts a significant effect on the molecular structure of the cage. Thus, substitution of the PhI+ group at the apical boron atom lowers the height of the adjacent pyramid (base formed by the equatorial boron atoms) by 0.06 Å and by 0.01 Å in the antipodal pyramid in compounds derived from all three anions, A, B and D (Tables 1 and 2). This shortening of the distance is due to the strong electron withdrawing character of the PhI+ substituent, which is the second to most electron withdrawing after the N2+ group (σp = 1.91).45 A correlation of the C(1)–H 1H NMR chemical shifts v. Hammett parameters σp in a series of B(10)-substituted derivatives of B anion, [closo-1-CB9H9-10-Y]−, allowed an estimate of the σp value for the PhI+ group as 1.5(1).32,46 The chemical shift observed in zwitterions of B exhibits an excellent correlation with the height of the tetragonal pyramid in a series of symmetric 1,10-disubstituted derivatives of the [closo-B10H10]2− cluster (A).40 The substituent effects on the molecular structure appear to be cumulative.
Mechanistic aspects of reactions with nucleophiles
Aryliodonium zwitterions of closo-borane anions undergo four types of reactions, as identified to date. Typically, the most desired transformation, a replacement of the ArI group with a nucleophile, takes place according to path a or path b (Fig. 5). The former mechanism is common in hypervalent iodine derivatives and was discussed in detail by Grushin in the context of iodonium derivatives of carboranes F–H.25,27 It involves coordination of a nucleophile, Nu, to the 8-I-2 iodine center and formation of the 10-I-3 intermediate, in which the tricoordinated iodine atom has 10 valence electrons. This intermediate rearranges through pseudorotation and reductive elimination of the substitution product (path a). Alternatively, the substitution can follow a dissociative mechanism (path b), in which a highly reactive boronium ylide is formed and subsequently trapped with the available nucleophile, typically the solvent. The preference for the mechanism (path a v. path b in Fig. 5) depends on the thermodynamic stability of the zwitterions (vide infra), the type of the nucleophile, its ability to form the 10-I-3 intermediate and temperature. Thus, it is believed that the dissociative mechanism is important for zwitterions with low thermal stability (derivatives of [closo-B12H12]2−, 6-substituted derivatives of [closo-1-CB9H10]− and COSAN) in weakly nucleophilic solvents, such as MeCN and thian.29 Experimental data obtained thus far indicate that in the nucleophilic substitution process, all aryliodonium zwitterions of closo-borane anions A–D react with elimination of ArI and formation of the B-substituted products.
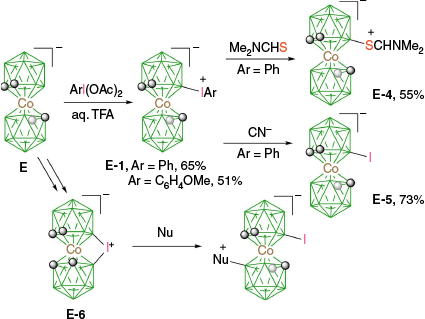
There are only two examples of transformations of aryliodonium zwitterions of COSAN. Thus, thermal reaction of E-1 with excess Me2NCHS leads to the protected mercaptan E-4, but when reacted with the CN− anion, only iodide E-5 was obtained (Scheme 5), presumably through the single electron transfer (SET) mechanism (path d, Fig. 5).29 In addition, the inner iodonium zwitterion E-647 has been demonstrated to undergo a number of reactions with nucleophiles, giving products of interest for biomedical applications.34,48 It has been postulated that this reaction proceeds through the breaking of the B–I bond and formation of the boronium ylide (analogous to path b in Fig. 5).
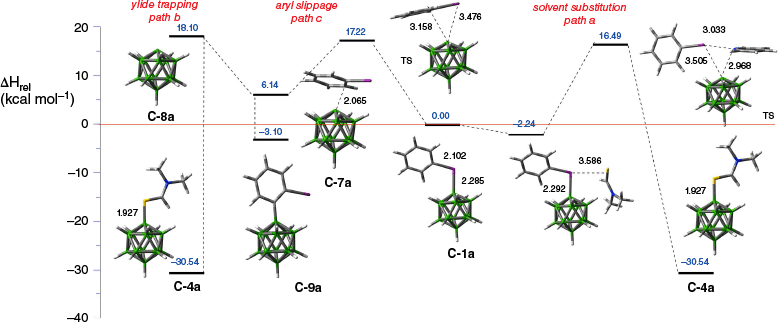
A process competing with nucleophilic substitution is arylation of the closo-borane cage, which involves transfer of the boron cage from the iodine center to the carbon atom of the arene ring (path c, Fig. 5). Products of this process, B-arylated boron cages, have been observed in reactions of derivatives of the [closo-B12H12]2− anion (C).35 All three reaction pathways, nucleophilic substitution, formation of boronium ylides and aryl slippage, have been modeled for mono-zwitterions [closo-B10H9-1-IPh]− (A-1a) and [closo-B12H11-1-IPh]− (C-1a) using DFT methods.35 Results for the latter species in reaction with Me2NCHS, shown in Fig. 6, demonstrate that solvation of zwitterion C-1a by Me2NCHS is exothermic by 2.24 kcal mol−1. The solvate undergoes rearrangement with the activation enthalpy ΔH‡ = 18.73 kcal mol−1 to yield the substitution product C-4a. Alternatively, the zwitterion undergoes thermal rearrangement with the activation enthalpy ΔH‡ = 17.22 kcal mol−1 to yield C-7a, a π complex of the boronium ylide C-8a with PhI. Upon proton transfer to the solvent, C-7a yields the B-arylated product C-9a with a modest overall exotherm of ΔH = −3.10 kcal mol−1. The π complex C-7a can dissociate to form the free ylide C-8a with an endotherm of ΔH = 11.96 kcal mol−1, which is trapped with the solvent giving rise to the substitution product C-4a. Analysis of the DFT data demonstrates that activation energy for aryl slippage (path c, Fig. 5) is lower by 1.51 kcal mol−1 than that for substitution (path a), which suggests it is the preferred reaction pathway. These computational results are consistent with experimental observations for the formation of significant amounts of the arylated and ylide trapping products.35
Three pathways of transformation of C-1a. Enthalpy, ΔH, relative to the zwitterion C-1a (red line) for each step was calculated using the M06-2x/6-31+G(2d,p)//M06-2x/6-31G(2d,p) method in Me2NCHS dielectric medium (the PCM model) and is given in kilocalories per mole (blue numbers). Equilibrium geometry structures have indicated key distances in Ångstroms (black numbers). Data from Tokarz et al.35
Similar calculations for other zwitterions indicate that both processes, substitution and aryl slippage, typically have comparable activation energies (S. Ciastek-Iskrzycka et al., unpubl. data). Experimental observations also indicate that the substitution process (path a, Fig. 5) is more favorable for [closo-B10H10]2− (A) and [closo-B12H12]2− (C) anions containing electron withdrawing substituents or analogous carbaboranes B and D.
The [closo-CB11H11-12–I–12′-CB11H11-closo]− zwitterion (D-3) was reported to be stable under nucleophilic substitution conditions, presumably due to steric reasons and inability to achieve a thermodynamically favorable TS for reductive elimination.37
Finally, the fourth type of transformation of closo-borate zwitterions involves a single electron transfer and formation of 9-I-2 intermediates, which undergo a rapid homolysis and formation of B-iodo derivatives and aryl radicals (path d, Fig. 5). Particularly effective electron donors in this context are organolithium and Grignard reagents, which provide convenient and clean access to iodo-closo-boranes29–31,49 (vide infra). The exclusive formation of iodo-closo-borates was also observed in the case of a reaction between the CN− anion and COSAN zwitterion E-1, presumably through SET. Such a process could also be responsible for the formation of ionic byproducts in reactions of [closo-B10H10]2− zwitterions with some nucleophiles.7 Similar transformations of arylhalonium derivatives of carboranes F–H were reviewed by Grushin.27
Stability of aryliodonium zwitterions
Aryliodonium zwitterions of closo-borane anions have moderate, well-balanced thermal stability, which, in most cases, is sufficient for their isolation and synthetic applications. The stability depends on the closo-borane, position of the aryliodonium substituent, the presence of other substituents and finally, the nature of the aryl group.
Observations revealed that the stability of the zwitterions follows the order: [closo-B12H12]2− (C) < [3,3′-Co(1,2C2B9H11)2]− (E) <[closo-1-CB11H12]− (D) <[closo-1-CB9H10]− (B) <[closo-B10H10]2− (A). Consequently, they undergo practical transformations in the temperature range −10 to 70°C. Compound [closo-B12H11-IPh]− (C-1a) exhibits a particularly low stability, decomposing in solutions during preparation at 0°C.35 Therefore, crude C-1a was isolated by precipitation from the reaction mixture immediately upon formation. Subsequent kinetic measurements of the resulting crude zwitterion gave the rate of decomposition k = 3.30(4) × 10−4 s−1 at 0°C in CD3CN, which gives the half-life of 25 min at this temperature. A slower decomposition rate of k = 1.960(1) × 10−4 s−1 was measured for the anisyl analogue, [closo-B12H11-(IC6H4OMe-4)]−, under the same conditions.35 Analysis of the decomposition products revealed the presence of arylated {closo-B12} cage and products from the trapping of the boronium ylide [closo-B12H11]− (C-8a) with the solvent (CD3CN). This suggests that the dominant reactivity is along paths b and c (Fig. 5). In reactions with nucleophiles (pyridine and Me2NCHS) the methoxyphenyliodonium zwitterion was more effective and higher yields of the desired products, e.g. C-10, were obtained at low temperatures (−10°C) than with the [closo-B12H11-IPh]− analogue (C-1a, Scheme 6).35 Another study demonstrated that complete decomposition of [closo-B10H9-1-IPh]− (A-1a) was observed after 16 h in MeCN at 55–60°C.7
Synthesis and transformations of aryliodonium zwitterions derived from [closo-B12H12]2− (C).35,36
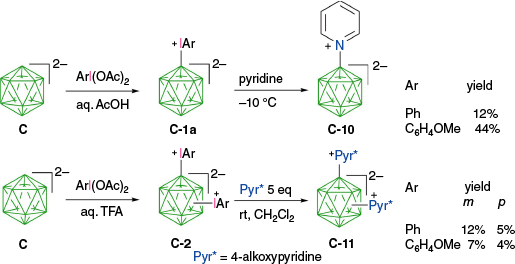
Double substitution of the [closo-B12H12]2− anion with ArI+ groups gives more thermally stable bis-zwitterions. Although [closo-B12H10-(IPh)2] (C-2) was poorly soluble, the dianisyl analogue, [closo-B12H11-(IC6H4OMe-4)2], could be separated into 1,7 (meta) and 1,12 (para) isomers by column chromatography.29 In contrast to reactions of C-1a, similar yields of the double substitution products with 4-alkoxypyridines, C-11, were obtained for bis-zwitterions C-2 [closo-B12H10-(IPh)2] and [closo-B12H10-(IC6H4OMe-4)2] (Scheme 6).36 The presence of another onium group on the [closo-B12H12]2− cluster, such as Me2S+ in C-1h, also significantly stabilizes the aryliodonium zwitterions, allowing for separation of the meta (1,7) and para (1,12) isomers by chromatography.29,36 A similar trend in stability was observed for derivatives of anion A: the presence of an electron withdrawing substituent (e.g. A-2) stabilizes zwitterions A-1 and facilitates clean substitution reactions, whereas electron donating groups (e.g. alkoxyphenyl8 and alkyl, see R. Jakubowski, K. Ogunmola et al., unpubl. data) make the zwitterions unstable to isolation and purification.
Playing an even greater role in the stabilization of aryliodonium zwitterions, is the replacement of a BH fragment with the CH group in the 12-vertex boron cluster. Thus, the [closo-1-CB11H11-12-IPh] (D-1a(12)) and also its B(7) isomer (D-1a(7)) decompose significantly slower than [closo-B12H10-1-SMe2-12-IPh] (C-1h(12)). Quantitative kinetic studies of the decomposition of selected zwitterions were conducted in DMF-d7 and the results will be reported elsewhere (S. Ciastek-Iskrzycka et al., unpubl. data). The experimental activation energies were well reproduced with DFT calculations. It is interesting to note that, although phenyliodination of [closo-1-CB11H12]− and [closo-1-CB11H11-1-alkyl]− worked well, giving isolable stable zwitterions, the analogous reaction of carboxylic acid [closo-1-CB11H11-1-COOH]− (D-e) failed to give the expected zwitterion D-1e (J. Pecyna and P. Kaszyński, unpubl. data). Instead, only PhI and some unidentified products of oxidized boron clusters were found. This can be explained by the fast dissociation of the transient zwitterion D-1e under the reaction conditions to the corresponding boronium ylide D-8e and trapping with the aqueous solvent.
Although the position of the aryliodonium group (1,7 v. 1,12) has little effect on the zwitterion stability in 12-vertex anions, for derivatives of [closo-1-CB9H10]−, the B(6) zwitterion [closo-1-CB9H9-6-IPh] (B-1a(6)) is significantly less stable than its B(10) isomer [closo-1-CB9H9-10-IPh] (B-1a(10)). This difference in stability was exploited in the separation of the two isomers by kinetic methods, since column chromatography was ineffective. For instance, a crude mixture of regioisomers of phenyliodonium zwitterions derived from [closo-1-CB9H9-1-R]− was first reacted with a nucleophile Nu, such as pyridine, CN− or MeC(=NH)NH2,31,32 at temperatures ranging from 0 to 50°C in MeCN solutions, or simply thermolyzed in MeCN. The resulting mixture of [closo-1-CB9H8-1-R-6-Nu] and [closo-1-CB9H8-1-R-10-IPh] was separated by chromatography (Scheme 7). DFT calculations demonstrated that, although the activation energy, ΔG‡298, for the transformation of [closo-1-CB9H9-6-IPh] (B-1a(6)) to the B(6) substituted products is 19.0 (for Nu = pyridine) and 15.2 (for Nu═CN−) kcal mol−1, the analogous transformations of the B(10) isomer, [closo-1-CB9H9-10-IPh] (B-1a(10)), to the B(10) products is higher by 3.4 and 5.7 kcal mol−1 respectively.32 This difference in activation energies is sufficient for effective kinetic separation of the two isomers.
Phenyliodination of [closo-1-CB9H9-1-R]− and kinetic resolution of isomeric products.29,31,32
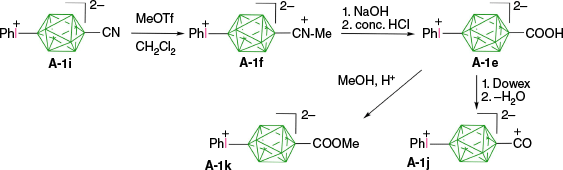
Aryliodonium zwitterions of anions A and D exhibit thermal and chemical stabilities that permit functional group interconversion without affecting the iodine center. Thus, nitrile [closo-B10H8-1-CN-10-IPh]− (A-1i) smoothly undergoes N-methylation with CF3SO3Me and the resulting nitrilium group in A-1f hydrolyzes under basic and then acidic conditions to yield carboxylic acid A-1e in a high overall yield of 54–59% (Scheme 8).41 The ammonium counterion in A-1e was replaced with hydronium, and the acid [closo-B10H8-1-COOH-10-IPh]− (A-1e) was dehydrated to carbonyl derivative A-1j. Treatment of acid A-1e with acidic MeOH gave ester A-1k (Scheme 8).41
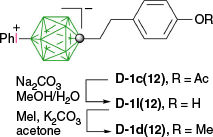
The acetate D-1c(12) was hydrolyzed under basic conditions and the resulting phenol D-1l(12) was methylated with MeI in the presence of the PhI+ functionality to give the methoxy derivative D-1d(12) in 90% overall yield (Scheme 9).31
Applications of aryliodonium zwitterions in synthesis
There are two types of synthetically useful reactions of the zwitterions: nucleophilic displacement reactions leading to B-substituted closo-boranes (path a and path b, Fig. 5) and reduction of the zwitterions yielding B-iodo derivatives (path d). Some examples are discussed below, whereas all reactions involving aryliodonium zwitterions of anions A–E reported to date are listed in Table 3.
 | |||
|---|---|---|---|
| Anion | Aryliodonium derivative percentage yield and references | Substitution product percentage yield and references | |
 |  |  | |
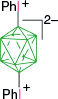
| A | A-1a, 46%29 | A-5, X = I, 90%7,30,49 | |
| 60–65%30 | A-10, X = [C5H5N]+, 74%,29 50%7 | ||
| 50%7 | A-12, X = BuNH2+, BnNH2+, 42%50 and A-13, X = [R(BuNH)C = NH]+, [R(BnNH)C = NH]+, ~45%50 | ||
| A-i, X = CN, 71%29 50%7 | |||
| A-m, X = N3, 95%7 | |||
| X = [4-CNC5H4N]+, 53%7 | |||
| X = [4-EtOOCC5H4N]+, 67%7 | |||
| X = [4-MeC5H4N]+, 63%7 | |||
| X = [4-MeOC5H4N]+, 50%7 | |||
| X = C4H4N2+, 25, 61%51 | |||
 | 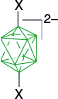 | ||
| A-2, 82%29 | A-11, X = [4-MeOC5H4N]+, 57%29 | ||
| 78%52 | A-11, X = [4-C7H15OC5H4N]+, 79%29 | ||
| A-11, X = [4-ROC5H4N]+, 55–60%36,53 | |||
| A-15, X = I, 90–95%30,49 | |||
| X = [MeC(NH2)NH]+, 61%54 | |||
| X = CN, 62–67%40 | |||
| X = [Me2NCHO]+, 72–80%40 | |||
| X = [Me2NCHS]+, 88%52 | |||
| X = [C5H5N]+, 40%7 | |||
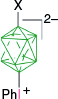 | |||
| X = AcO, 60%29 and 45%7 | |||
| A-1i, X = CN, 53–60%,7 47–50%51 | |||
| X = SCN, 16%7 | |||
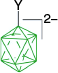 | 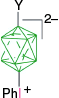 | 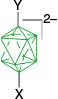 | |
| Y = [4-C5H11C7H12N]+, 59%29 | Y = [4-C5H11C7H12N]+, X = [4-C7H15OC5H4N]+, 93%29 | ||
| A-1m, Y = N3, 66–78%7 | Y = N3, X = [C5H5N]+, 83%7 | ||
| Y = I, 91%7 | Y = I, X = [C5H5N]+, 46%7 | ||
| Y = [OC4H8NH]+, 30% from A through A-1a7 | Y = [OC4H8NH]+, X = [C5H5N]+, 76%7 | ||
| Y = [C5H5N]+, 69%7 | Y = [C5H5N]+, X = [EtHO]+, 69%7 | ||
| Y = [C5H5N]+, X = CN, 60%7 | |||
| Y = [4-CNC5H4N]+, 48–52%7 | Y = [4-CNC5H4N]+, X = EtO, 42%7 | ||
| Y = butyl, 45%A | Y = butyl, X = CN, 30%A | ||
| Y = octyl, 54%A | Y = octyl, X = [4-C7H15OC5H4N]+, 72%A | ||
| A-1o, Y = C12H25OC6H4, not isolated.8 | A-19, Y = C12H25OC6H4, X = [Me2NCHO]+, 35–43% overall8 | ||
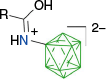 | 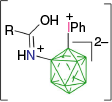 | 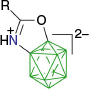 | |
| A-n | postulated as transient42 | A-g, ~85% with 1.5 eq PhI(OAc)255 | |
 | |||
| A-1g, 82% with 3 eq PhI(OAc)242 | |||
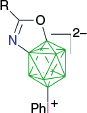
| From A-2 | 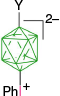 |  | |
| Y = AcO7,29 | A-10, Y = OAc, X = [C5H5N]+, 74%7 | ||
| Y = SCN7 | Y = SCN, X = [C5H5N]+, 78%7 | ||
| Y = Br7 | Y = Br, X = [C5H5N]+, 75%7 | ||
| Y = [OC4H8NH]+7 | Y = [OC4H8NH]+, X = [C5H5N]+, 76%7 | ||
| A-1i, Y = CN7,51 | A-10i, Y = CN, X = [C5H5N]+7 | ||
| A-22i, Y = CN, X = [C4H4N2]+, 50–78%51 | |||
| A-22i, Y = CN, X = [NC5H4-4-C5H4N]+, 50%51 | |||
| From A-1i | A-1e, R = COOH | A-10e, R = COOH, X = [C5H5N]+, 68%41 | |
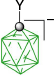 | 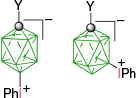 | 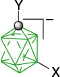 | |
| B-1a, Y = H, ratio: 2:5, 93%29 | From kinetic separation | ||
| B-10a(6), Y = H, X = [C5H5N]+, 54%32 | |||
| Y = H, X = [MeC(=NH)NH2]+, 54%32 | |||
| B-1b, Y = C5H11, ratio, 1:132 | Y = C5H11, X = CN, 42%32 | ||
| Y = C5H11, X = [4-C5H11C5H4N]+, 31%B | |||
| B-11b(6), Y = C5H11, X = [4-R′OC5H4N]+, 38–43%B | |||
| B-1c, Y = C2H4C6H4-4-OAc, ratio: 5:431 | B-10c(6), Y = C2H4C6H4-4-OAc, X = [C5H5N]+, 27%31 | ||
| B-1d, C2H4C6H4-4-OMe, ratio: 5:431 | B-10d(6), Y = C2H4C6H4-4-OMe, X = [C5H5N]+, 39%31 | ||
| B-11d(6), Y = C2H4C6H4-4-OMe, X = [4-C5H11OC5H4N]+, 38%B | |||
| B-1e, Y = COOH, ratio 1:2, 95%29 | Y = COOH, X = NH3+, 54%29 | ||
| Y = COOC6H4-4-C3H7B | Y = COOC6H4-4-C3H7, X = [4-C5H11OC5H4N]+, 47%B | ||
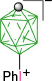 | 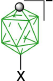 | ||
| B-1a(10), 22–30% from kinetic separation32 | B-4a(10), X = [Me2NCHS]+, 87%32 | ||
| B-10a(10), X = [C5H5N]+, 93%32 | |||
| X = [MeC(=NH)NH2]+, 89%32 | |||
| X = CN, 96%32 | |||
| X = PhCOO, 91%32 | |||
| X = N3, 95%32 | |||
 | 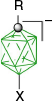 | ||
| B-1b(10), R = C5H11, 48% from kinetic separation32 | B-11b(10), R = C5H11, X = [4-C7H15OC5H4N]+, 95%32 | ||
| B-5b(10), R = C5H11, X = I, 97%32 | |||
| B-1c(10), R = C2H4C6H4-4-OAc 30% from kinetic separation31 | B-5c(10), R = C2H4C6H4-4-OAc, and B-5d(10), C2H4C6H4-4-OMe, X = I, ~90%31 | ||
| B-11c(10), R = C2H4C6H4-4-OAc, and B-11d(10), C2H4C6H4-4-OMe, X = [4-R′OC5H4N]+, ~88%56 | |||
| B-1d(10), R = C2H4C6H4-4-OMe, 41% from kinetic separation31 | B-4d(10), R = C2H4C6H4-4-OMe, X = [Me2NCHS]+, 98%56 | ||
| B-1e(10), R = COOH, 27% from kinetic separation29 | B-11e(10), R = COOH, X = [4-C7H15OC5H4N]+, 90%29 | ||
| R = COOMe, X = (CH2)5S+, 81%29 | |||
 | 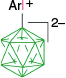 | 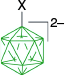 | |
| C | C-1a, Ar = Ph, 4-MeOC6H4, 74–95%35 | C-4a, X = [Me2NCHS]+, 25–27%35 | |
| C-1a, Ar = Ph, 75%,57 88%58 | C-10a, X = [C5H5N]+, 41–44%35 | ||
| X = [NH2OH]+, 79%57 | |||
| X = [NH2CH2COOEt]+, 70%58 | |||
| X = [NH2CHBnCOOEt]+, 75%58 | |||
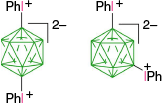 |  | ||
| C-2, 69%29 | C-11, X = [4-ROC5H4N]+, ~5 and ~10% overall yield based on C.36 | ||
 |  | ||
| Ar = 4-MeOC6H4, 51% | C-11, X = [4-ROC5H4N]+, ~4 and ~7% overall yield based on C.36 | ||
| C-2(12), 12% and C-2(7), 34%29 | |||
 |  |  | |
| C-1h, Y = +SMe2, ratio: 1:3, 77%29 | C-11h, Y = +SMe2, X = [4-MeOC5H4N]+, overall 10 and 30%29 | ||
| Y = [4-C5H11C5H9S]+, 67%36 | Y = [4-C5H11C5H9S]+, X = [4-ROC5H4N]+, overall yield 5 and 12%36 | ||
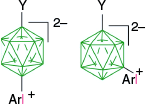 | |||
| C-1h, Y = +SMe2, Ar = 4-MeOC6H4 | |||
| C-1h(12), 13%, C-1h(7), 39%29 | |||
| Y = [4-C5H11C5H9S]+, Ar = 4-MeOC6H4, 5 and 11%36 | |||
 |  |  | |
| C-n | C-1n(2), postulated as transient59 | C-g, overall 60–100%59 | |
 |  |  | |
| D | D-1a(12), 81%29 | D-4a(12), X = [Me2NCHS]+, 71%29 | |
| D-5a(12), X = I, 83%29 | |||
| D-11a(12), X = [4-MeOC5H4N]+, 87%29 | |||
| X = (CH2)5S+, 30%29 | |||
| X = CN, 65%29 | |||
| D-16a(12), X = C5H11, 79%60 | |||
| X = C10H21, 73%60 | |||
 | |||
| D-1a(7), 11%29 | |||
| isolated from mixtures with D-1a(12) | |||
 |  | 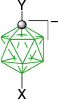 | |
| D-1b(12), Y = C5H11, 81%29 | D-4b(12), Y = C5H11, X = [Me2NCHS]+, 78%60 | ||
| D-11b(12), Y = C5H11, X = [4-MeOC5H4N]+, 61%29 | |||
| Y = C10H21, 55%60 | Y = C10H21, X = [Me2NCHS]+, 79%60 | ||
| D-1c(12), Y = C2H4C6H4-4-OAc, 70%31 | D-5c(12), Y = C2H4C6H4-4-OAc, X = I, ~70%31 | ||
| D-11c(12), Y = C2H4C6H4-4-OAc, X = [4-ROC5H4N]+, ~88%56 | |||
| D-1d(12), Y = C2H4C6H4-4-OMe, 90% from D-1c(12)31 | D-5d(12), Y = C2H4C6H4-4-OMe, X = I, ~70%31 | ||
| D-11d(12), Y = C2H4C6H4-4-OMe, X = [4-ROC5H4N]+, ~88%56 | |||
| D-4d(12), Y = C2H4C6H4-4-OMe, X = [Me2NCHS]+, 90%56 | |||
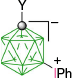 | 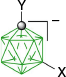 | ||
| D-1b(7), Y = C5H11, 8%29 Y = C10H21, 9%60 | D-11c(7), Y = C2H4C6H4-4-OAc, X = [4-C5H11OC5H4N]+, 87%B | ||
| D-1c(7), Y = C2H4C6H4-4-OAc, 7%31 separated from mixtures with D-1(12) | |||
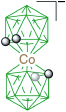 |  |  | |
| E | E-1a, Ar = Ph, 65%29 | E-4, X = [Me2NCHS]+, 55%29 | |
| E-1a, Ar = MeOPh, 51%29 | E-5, X = I, 73%29 | ||
Simple nucleophilic displacement reactions
Nucleophiles reacted with aryliodonium zwitterions of closo-boranes thus far can be grouped by the type of the substituting atom:
Small liquid nucleophiles, such as pyridine, DMF,8,40 Me2NCHS,29,32,35,52,56,60 thian29 and MeCN29,32,50 (see also J. Pecyna and P. Kaszyński, unpubl. data), are used as solvents and their excess is removed by evaporation or vacuum distillation. In other cases, the substitution is performed in MeCN or THF58 solutions with up to five times the molar ratio of the nucleophile. The former method of the substitution works well for both the substitutive and dissociative mechanisms (paths a and b), whereas the second method is best suited for path a. In the case of reactions of the zwitterions with aliphatic amines in an organic nitrile (e.g. MeCN), products of both mechanistic pathways were observed in comparable proportions.50 Thus, zwitterion A-1a in reaction with an amine in RCN gave the expected product A-12 and N-substituted amidine A-13 (Scheme 10). Although the former can be formed according to the substitutive mechanism (path a, Fig. 5), the amidine A-13 requires dissociation of A-1a to boronium ylide A-8a (path c). The ylide is trapped with the solvent yielding a transient product A-14, which is subsequently reacted with the amine present in the solution.50
Analysis of results obtained for reactions of [closo-B10H9-1-IPh]− (A-1a) demonstrates that nucleophile reactivity follows the order N3− > CN− > Br− > SCN− > AcO− ≫ morpholine.7 It was noted that only the reaction of the N3− anion gave a single product, [closo-B10H9-1-N3]2− (A-m), isolated in 95% yield, whereas all others were accompanied by by-products (Scheme 11). For instance, a reaction of A-1a with the CN− anion was slower than that with N3− and the expected nitrile A-i was isolated in 50–55% yield from a mixture. The mixtures contained side products resulting from independent decomposition of the zwitterion in MeCN at 55–60°C. Other nucleophiles, SCN−, AcO−, morpholine, Br− and I−, gave mixtures of products in which the desired compound was either a minor component or was not found at all.7 Much cleaner and faster reactions with nucleophiles with fewer byproducts were observed for bis-zwittterion A-2, which was ascribed to higher electrophilicity of the iodine center exerted by the second strongly electron withdrawing PhI+ group in the antipodal position. This different rate of reactivity of the bis-zwitterion v. mono-zwitterion was exploited in sequential selective hetero-disubstitution reactions of anion A (Scheme 11).7 Thus, bis-zwittterion A-2 was reacted with 1 eq of the nucleophile and the resulting purified product A-1 was treated with pyridine, giving A-10. Some of the reactions were conducted as one-pot processes without purification of the intermediate A-1. Considering the important effect of an onium group in the antipodal position on the yield of the substitution process, the synthesis of A-10 was redesigned. Thus, [closo-B10H9-1-NC5H5]− (A-10a) was treated with PhI(OAc)2 in MeCN giving [closo-B10H8-1-NC5H5-10-IPh] in 69% yield, which was reacted with [Et4N]+SCN− or thermolyzed in EtOH (100°C) giving the corresponding substitution products A-10 in 55 and 69% yield respectively (Scheme 11).7
Similar trends in reactivity were observed in reactions of [closo-1-CB9H9-10-IPh] (B-1a(10)) with nucleophiles, among which amines did not perform well. Thus, the reaction of B-1a(10) with l-leucine benzyl ester, glycine benzyl ester or (R)-1-phenylethylamine in MeCN (40°C up to 24 h) did not give the desired products. In the first two cases, the iodonium zwitterion was recovered, whereas in the reaction with the amine, the substrate underwent extensive decomposition.32 The amines may react with the zwitterions through the SET mechanism (path d, Fig. 5) giving rise to the 9-I-2 intermediate, which undergoes dissociation, yielding the B-iodide, although this hypothesis was not verified.
Many products of reactions of iodonium zwitterions 1 and 2 with nucleophiles were the desired final compounds. By contrast, reactions of the zwitterions with DMF,8,40 Me2NCHS,29,32,35,52,56,60 MeCN29,32,50 (see also J. Pecyna and P. Kaszyński, unpubl. data) and acetamidine32,54,56 led to derivatives with masked OH, SH and NH2 groups, which were used in subsequent transformations. Some of these processes are shown below.
Cyclization reactions
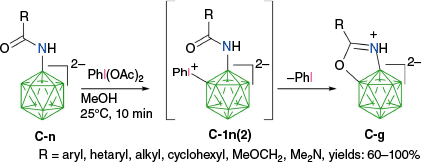
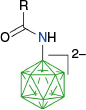
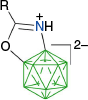
The activation of the B–H bond in closo-borates by PhI(OAc)2 was recently used in an interesting carbonyl-directed oxidative cyclization of amides C-n derived from [closo-B12H11-1-NH2]2− (Scheme 12) and efficient formation of ring-fused diboraoxazolines C-g.59 According to the postulated mechanism,61 the carbonyl directs the phenyliodination to the B(2) position and formation of intermediate C-1n(2), which then undergoes intramolecular nucleophilic displacement of PhI and formation of C-g, presumably through the dissociative mechanism (path b).
This method was later used for directed oxidative cyclization of amides A-n in the presence of PhI(OAc)2 and the preparation of diboraoxazolines A-g in yields 83–87% (Scheme 13).55 Cyclization conducted with excess PhI(OAc)2 leads to tri-substituted derivatives A-1g isolated in ~82% yield.42
Preparation B-iodo derivatives
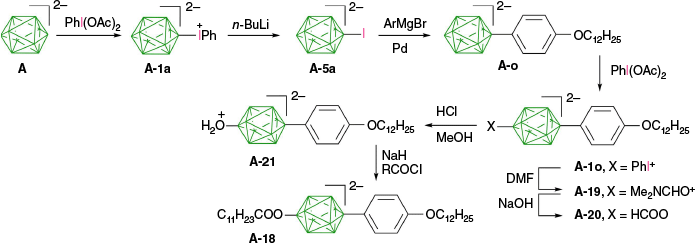
Over four decades ago, it was demonstrated that polarographic reduction or photolysis of zwitterion A-1a leads to [closo-B10H9-1-I]2− (A-5a),62 which cannot be obtained with direct iodination methods. More recently, it was discovered that treatment of A-1a and A-2 with n-BuLi in THF solutions leads to clean formation of the corresponding iodides A-5a and A-15 (Scheme 14).30
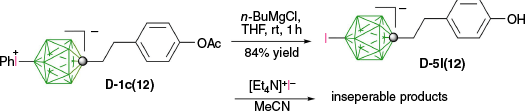
Grignard reagents also serve as effective electron donors, and [closo-1-CB11H11-12-I]− (D-5a(12)) was cleanly obtained in 83% isolated yields by treatment of [closo-1-CB11H11-12-IPh] (D-1a(12)) with C6H13MgBr.29 Similarly, treatment of acetate D-1c(12) with n-BuMgBr removed both the phenyl and acetate groups, giving iodo phenol D-5l(12) isolated in 84% yield, whereas the use of n-BuLi or MeLi gave D-5l(12) with ‘various amounts of byproducts’31 (Scheme 15).
The dephenylation of [closo-1-CB9H8-1-C5H11-10-IPh] (B-1b(10)) with n-BuLi gave the desired iodide B-5b(10) contaminated (7%) with [closo-1-CB9H8-1-C5H11-10-Ph]− derivative.32 Clean iodide B-5b(10) was obtained in 97% yield, when B-1b(10) was reacted with [Et4N]+I− in MeCN. This method was successfully used for obtaining iodides B-5c(10) and B-5d(10) in ~90% of isolated yields (Scheme 16).31 Interestingly, with the same method D-1c(12) gave a mixture of inseparable products instead of the expected D-5c(12). It is not clear whether the formal dephenylation of these zwitterions with I− proceeds according to path a with the nucleophilic displacement, or through path d with the SET from I−.
The obtained B-iodo derivatives were subsequently used for Pd-catalyzed cross-coupling reactions with alkyl (R. Jakubowski, K. Ogunmola et al., unpubl. data) and aryl8,30,31 Grignard reagents. It was also demonstrated that the isolation of the intermediate iodide in such reactions can be avoided and the iodonium zwitterion can be directly used for coupling reactions. Thus, treatment of D-1a(12) with excess pentylmagnesium bromide in the presence of Pd2(dba)3/PCy3 cleanly gave the coupling product [closo-1-CB11H11-12-C5H11]− (D-16(12)) in 79% overall yield, presumably through the transient iodide D-5a(12) (Scheme 17).60
Molecular materials
The high efficiency and regioselectivity of B–H activation in closo-borane anions towards nucleophilic substitution through aryliodonium zwitterions has been used in the synthesis of several types of molecular materials, which include ionic liquid crystals,8,10 highly polar liquid crystals,56 photonic materials,7 and ditopic51 and other41 ligands. Selected examples are provided below.
The phenethyl zwitterions 1c and 1d were developed as key intermediates for polar liquid crystals for LCD applications.56 Thus, phenyliodonium B-1c(10) and D-1c(12) were reacted with neat 4-alkoxypyridines, giving pyridinium derivatives B-11c(10) and D-11d(12) in ~88% yield (Scheme 18). Similarly, a reaction with neat Me2NCHS gave crude protected mercaptans B-4d(10) and D-4d(12), which under basic hydrolytic conditions, were cycloalkylated to the sulfonium derivatives B-17d(10) and D-17d(12) in ~75% overall yields.
The pyridinium derivatives exhibit nematic phases with clearing temperatures over 150°C.56 Dielectric analysis of low concentration solutions in a nematic liquid crystal host gave the extrapolated dielectric anisotropy, Δε, 26 for sulfonium D-17d(12) and over 40 for pyridinium analogues 11d (Scheme 18).
The full selectivity of phenyliodination at the apical positions of dianion A has been used to prepare a series of ionic liquid crystals exhibiting intermolecular charge transfer with the energy in the visible range.8 The preparation of one such derivative A-18 is shown in Scheme 19. The iodide A-5a, prepared from A-1a,30 was arylated in the presence of a Pd-catalyst. The resulting derivative A-o (88% yield) was phenyliodinated, giving an unstable derivative A-1o, which, without further purification, was thermolyzed in DMF, leading to the protected hydroxy derivative A-19 isolated in 35–43% yield. Treatment of this DMF adduct with NaOH gave the formate A-20 in 93% yield. Exposure of the formate to dilute HCl in MeOH yielded the protonated hydroxy derivative A-21, which, without purification, was treated with C11H23COCl in the presence of NaH, giving the final ester A-18 in 34–40% overall yield. Ion pairs of the dianion A-18 with 4-substituted pyridinium cation exhibited smectic behavior and intermolecular charge transfer bands in the range of 414–530 nm, depending on the substituent.8
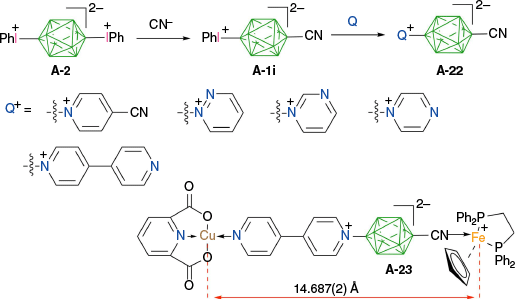
Finally, a new class of photo-active heteroditopic ligands A-22 containing strongly binding CN and weakly binding azine groups was prepared, taking advantage of selective mono-substitution of bis-phenyliodonium derivative A-2 (Scheme 20).51 Thus, treatment of A-2 with 1.1 eq of [Et4N]CN in MeCN gave nitrile A-1i in 47–50% yield, which was reacted with 5 eq of 4-cyanopyridine, diazine (pyrazine, pyridazine, pyrimidine) or bipyridyl, furnishing derivatives A-22 in ~55% yield. One of the products was converted to a heterobimetallic complex A-23 (Scheme 20) in which the two metal centers are 14.687(2) Å apart.
Summary and outlook
The past decade has witnessed an increase in the use of selective B–H activation in closo-borate clusters towards nucleophilic substitution with ArIII reagents. The intermediate iodonium zwitterions of closo-borates have been reacted with a range of nucleophiles, leading to the efficient formation of B–C, B–N, B–O, B–S and B–Hal bonds. In addition, one electron reduction of the zwitterions leads to the clean formation of iodoboranes, which are valuable intermediates in Pd-catalyzed cross-coupling reactions. The original simple bimolecular nucleophilic substitution process in aryliodonium zwitterions was recently expanded to intramolecular processes, which lead to regio-controlled cyclizations and provide access to new classes of heterocyclic compounds. These recent developments further demonstrate the great potential of such aryliodonium zwitterions for the formation of more complex compounds and new types of (poly)functional derivatives of closo-borates that are attractive for bio- and materials-related research.
Data availability
Data sharing is not applicable as no new data were generated or analyzed during this study.
Declaration of funding
The author thanks the National Science Foundation (DMR1207585 and DMR1611250), Narodowe Centrum Nauki (2015/17/B/ST5/02801) and Foundation for Polish Science (TEAM 3/2016) for supporting research leading to many results described in this review.
References
2 Poater J, Solà M, Viñas C, Teixidor F. π Aromaticity and three‐dimensional aromaticity: two sides of the same coin? Angew Chem Int Ed 2014; 53: 12191-12195.
| Crossref | Google Scholar | PubMed |
3 Jakubowski R, Januszko A, Tilford RW, Radziszewski GJ, Pietrzak A, Young VG, Jr, Kaszyński P. Photophysical behavior of self-organizing derivatives of 10- and 12-vertex p-carboranes, and their bicyclo[2.2.2]octane and benzene analogues. Chem Eur J 2023; 29: e202203948.
| Crossref | Google Scholar | PubMed |
4 Kaszyński P, Pakhomov S, Young VG, Jr. Investigations of electronic interactions between closo-boranes and triple-bonded substituents. Collect Czech Chem Commun 2002; 67: 1061-1083.
| Crossref | Google Scholar |
5 Pakhomov S, Kaszyński P, Young VG, Jr. 10-Vertex closo-boranes as potential π linkers for electronic materials. Inorg Chem 2000; 39: 2243-2245.
| Crossref | Google Scholar | PubMed |
6 Núñez R, Tarrés M, Ferrer-Ugalde A, de Biani FF, Teixidor F. Electrochemistry and photoluminescence of icosahedral carboranes, boranes, metallacarboranes, and their derivatives. Chem Rev 2016; 116: 14307-14378.
| Crossref | Google Scholar | PubMed |
7 Kapuściński S, Abdulmojeed MB, Schafer TE, Pietrzak A, Hietsoi O, Friedli AC, Kaszyński P. Photonic materials derived from the [closo-B10H10]2– anion: Tuning photophysical properties in [closo-B10H8-1-X-10-(4-Y-NC5H4)]–. Inorg Chem Front 2021; 8: 1066-1082.
| Crossref | Google Scholar |
8 Jacob L, Rzeszotarska E, Koyioni M, Jakubowski R, Pociecha D, Pietrzak A, Kaszyński P. Tunable intermolecular charge transfer in ionic liquid crystalline derivatives of the [closo-B10H10]2- anion. Chem Mater 2022; 34: 6476-6491.
| Crossref | Google Scholar |
9 Kaszyński P. closo-Boranes as structural elements for liquid crystals. In: Hosmane NS, Eagling R, editors. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 3: Boron in Materials Chemistry; 2018. pp. 57–114. World Scientific. doi:10.1142/9781786344663_0002
10 Jacob L, Niedzicki L, Jakubowski R, Pociecha D, Kaszyński P. Lithium salt of a pro-mesogenic [closo-CB11H12]– derivative: anisotropic Li+ ion transport in liquid crystalline electrolytes. Dalton Trans 2024; 53: 10293-10302.
| Crossref | Google Scholar | PubMed |
11 Guschlbauer J, Niedzicki L, Jacob L, Rzeszotarska E, Pociecha D, Kaszyński P. Liquid crystalline electrolytes derived from the 1,12-disubstituted [closo-CB11H12]– anion. J Mol Liq 2023; 377: 121525.
| Crossref | Google Scholar |
12 Grams RJ, Santos WL, Scorei IR, Abad-García A, Rosenblum CA, Bita A, Cerecetto H, Viñas C, Soriano-Ursúa MA. The rise of boron-containing compounds: advancements in synthesis, medicinal chemistry, and emerging pharmacology. Chem Rev 2024; 124: 2441-2511.
| Crossref | Google Scholar | PubMed |
13 Hosmane NS, Eagling R, editors. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 1: Boron in Organometallic Chemistry. 2018. World Scientific. doi:10.1142/q0130-vol1
14 Hosmane NS, Eagling R, editors. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 2: Boron in Catalysis. 2018. World Scientific. doi:10.1142/q0130-vol2
15 Hosmane NS, Eagling R, editors. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 3: Boron in Materials Chemistry. 2018. World Scientific. doi:10.1142/q0130-vol3
16 Hosmane NS, Eagling R, editors. Handbook of Boron Science: With Applications in Organometallics, Catalysis, Materials and Medicine. Vol. 4: Boron in Medicine. 2018. World Scientific. doi:10.1142/q0130-vol4
17 Olid D, Núñez R, Viñas C, Teixidor F. Methods to produce B–C, B–P, B–N and B–S bonds in boron clusters. Chem Soc Rev 2013; 42: 3318-3336.
| Crossref | Google Scholar | PubMed |
18 Kanazawa J, Kitazawa Y, Uchiyama M. Recent progress in the synthesis of the monocarba-closo-dodecaborate(–) anions. Chem Eur J 2019; 25: 9123-9132.
| Crossref | Google Scholar | PubMed |
19 Sivaev IB, Bregadze VI, Sjöberg S. Chemistry of closo-dodecaborate anion [B12H12]2-: a review. Collect Czech Chem Commun 2002; 67: 679-727.
| Crossref | Google Scholar |
20 Sivaev IB, Prikaznov AV, Naoufal D. Fifty years of the closo-decaborate anion chemistry. Collect Czech Chem Commun 2010; 75: 1149-1199.
| Crossref | Google Scholar |
21 Shmal’ko AV, Sivaev IB. Chemistry of carba-closo-decaborate anions [CB9H10]– (Review). Russ J Inorg Chem 2019; 64: 1726-1749.
| Crossref | Google Scholar |
22 Merritt EA, Olofsson B. Diaryliodonium salts: a journey from obscurity to fame. Angew Chem Int Ed 2009; 48: 9052-9070.
| Crossref | Google Scholar | PubMed |
23 Yusubov MS, Maskaev AV, Zhdankin VV. Iodonium salts in organic synthesis. ARKIVOC 2011; 2011: 370-409.
| Crossref | Google Scholar |
25 Grushin VV, Demkina II, Tolstaya TP. Unified mechanistic analysis of polar reactions of diaryliodonium salts. J Chem Soc Perkin Trans 2 1992; 1992(4): 505-511.
| Crossref | Google Scholar |
26 Grushin VV, Shcherbina TM, Tolstaya TP. The reactions of phenyl(B-carboranyl)iodonium salts with nucleophiles. J Organomet Chem 1985; 292: 105-117.
| Crossref | Google Scholar |
27 Grushin VV. Carboranylhalonium ions: From striking reactivity to a unified mechanistic analysis of polar reactions of diarylhalonium compounds. Acc Chem Res 1992; 25: 529-536.
| Crossref | Google Scholar |
28 Miller HC, Hertler WR, Muetterties EL, Knoth WH, Miller NE. Chemistry of boranes. XXV. Synthesis and chemistry of base derivatives of B10H102– and B12H122–. Inorg Chem 1965; 4: 1216-1221.
| Crossref | Google Scholar |
29 Kaszyński P, Ringstrand B. Functionalization of closo-borates via iodonium zwitterions. Angew Chem Int Ed 2015; 54: 6576-6581.
| Crossref | Google Scholar | PubMed |
30 Rzeszotarska E, Novozhilova I, Kaszyński P. Convenient synthesis of [closo-B10H9-1-I]2– and [closo-B10H8-1,10-I2]2– anions. Inorg Chem 2017; 56: 14351-14356.
| Crossref | Google Scholar | PubMed |
31 Jakubowski R, Pietrzak A, Friedli AC, Kaszyński P. C(1)-Phenethyl derivatives of [closo-1-CB11H12]– and [closo-1-CB9H10]– anions: difunctional building blocks for molecular materials. Chem Eur J 2020; 26: 17481-17494.
| Crossref | Google Scholar | PubMed |
32 Żurawiński R, Jakubowski R, Domagała S, Kaszyński P, Woźniak K. Regioselective functionalization of the [closo-1-CB9H10]– anion through iodonium zwitterions. Inorg Chem 2018; 57: 10442-10456.
| Crossref | Google Scholar | PubMed |
33 Sivaev IB, Bregadze VI. Chemistry of cobalt bis(carbolides). A review. Collect Czech Chem Commun 1999; 64: 783-805.
| Crossref | Google Scholar |
34 Dash BP, Satapathy R, Swain BR, Mahanta CS, Jena BB, Hosmane NS. Cobalt bis(dicarbollide) anion and its derivatives. J Organomet Chem 2017; 849–850: 170-194.
| Crossref | Google Scholar |
35 Tokarz P, Kaszyński P, Domagała S, Woźniak K. The [closo-B12H11-1-IAr]– zwitterion as a precursor to monosubstituted derivatives of [closo-B12H12]2–. J Organomet Chem 2015; 798: 70-79.
| Crossref | Google Scholar |
36 Ali MO, Lasseter JC, Żurawiński R, Pietrzak A, Pecyna J, Wojciechowski J, Friedli AC, Pociecha D, Kaszyński P. Thermal and photophysical properties of highly quadrupolar liquid-crystalline derivatives of the [closo-B12H12]2- anion. Chem Eur J 2019; 25: 2616-2630.
| Crossref | Google Scholar | PubMed |
37 Kitazawa Y, Watanabe M, Masumoto Y, Otsuka M, Miyamoto K, Muranaka A, Hashizume D, Takita R, Uchiyama M. “Dumbbell”- and “clackers”-shaped dimeric derivatives of monocarba-closo-dodecaborate. Angew Chem Int Ed 2018; 57: 1501-1504.
| Crossref | Google Scholar | PubMed |
38 Ionov VM, Subbotin MY, Grushin VV, Tolstaya TP, Lisichina IN, Aslanov LA. Molecular and crystallographic structures of phenyl-9-o-carboranyliodonium iodide. J Struct Chem 1984; 24: 638-641.
| Crossref | Google Scholar |
39 Mebs S, Kalinowski R, Grabowsky S, Förster D, Kickbusch R, Justus E, Morgenroth W, Paulmann C, Luger P, Gabel D, Lentz D. Real-space indicators for chemical bonding. Experimental and theoretical electron density studies of four deltahedral boranes. Inorg Chem 2011; 50: 90-103.
| Crossref | Google Scholar | PubMed |
40 Jacob L, Rzeszotarska E, Pietrzak A, Young VG, Jr, Kaszyński P. Synthesis, structural analysis, and functional group interconversion in the [closo-B10H8-1,10-X2]2– (X = CN, OCRNMe2+, OCOR, and [OH2]+) derivatives. Eur J Inorg Chem 2020; 3083-3093.
| Crossref | Google Scholar |
41 Jakubowski R, Kapuściński S, Hietsoi O, Friedli AC, Kaszyński P. [closo-B10H8-10-PhI-1-COOH]– anion: an intermediate for functional anionic carboxylate ligands. Inorg Chem 2024; 63: 13831-13834.
| Crossref | Google Scholar | PubMed |
42 Voinova VV, Selivanov NA, Bykov AY, Kubasov AS, Zhdanov AP, Zhizhin KY, Kuznetsov NT. Synthesis and structure of trisubstituted closo-decaborane [B10H7(1-IPh)(6(7),10-NHOCCH3)]: specifics of interaction between the [2-B10H9NH=C(OH)CH3]– ion and PhI(OAc)2. Russ J Inorg Chem 2023; 68: 678-783.
| Crossref | Google Scholar |
43 McArthur SG, Jay R, Geng L, Guo J, Lavallo V. Below the 12-vertex: 10-vertex carborane anions as non-corrosive, halide free, electrolytes for rechargeable Mg batteries. Chem Commun 2017; 53: 4453-4456.
| Crossref | Google Scholar | PubMed |
44 Kimata H, Sumitani R, Mochida T. Phase transitions and crystal structures of ionic plastic crystals comprising quaternary ammonium cations and carborane anion. Chem Lett 2019; 48: 859-862.
| Crossref | Google Scholar |
45 Hansch C, Leo A, Taft RW. Survey of Hammet substituent constants and resonance and field parameters. Chem Rev 1991; 91: 165-195.
| Crossref | Google Scholar |
46 Pietrzak A, Carr MJ, Kaszyński P. Substituent effects on the [closo-1-CB9H10]– anion geometry: experimental and DFT studies. CrystEngComm 2023; 25: 3790-3798.
| Crossref | Google Scholar |
47 Plešek J, Štibr B, Heřmanek SA. [8,8′-μ-I-3-Co(1,2-C2B9H10)2] Metallacarborane complex with an iodonium bridge. Evidence for a bromonium analog. Collect Czech Chem Commun 1984; 49: 1492-1496.
| Crossref | Google Scholar |
48 Bregadze VI, Kosenko ID, Lobanova I, Starikova A, Godovikov ZA, Sivaev IA, IB. C–H Bond activation of arenes by [8,8′-μ-I-3,3′-Co(1,2-C2B9H10)2] in the presence of sterically hindered Lewis bases. Organometallics 2010; 29: 5366-5372.
| Crossref | Google Scholar |
49 Li S, Qiu P, Kang J, Ma Y, Zhang Y, Yan Y, Jensen TR, Guo Y, Zhang J, Chen X. Iodine-substituted lithium/sodium closo-decaborates: syntheses, characterization, and solid-state ionic conductivity. ACS Appl Mater Interfaces 2021; 13: 17554-17564.
| Crossref | Google Scholar | PubMed |
50 Bil’bulyan AA, Nelyubin AV, Selivanov NA, Bykov AY, Klyukin IN, Zhdanov AP, Zhizhin KY, Kuznetsov NT. New method for synthesis of substituted 1-amidine-closo-decaborates [1-B10H9NH=C(R1)NHR2] (R1 = Me, iPr, Ph; R2 = nBu, Bn). Russ J Inorg Chem 2023; 68: 1511-1515.
| Crossref | Google Scholar |
51 Jakubowski R, Abdulmojeed MB, Hietsoi O, Friedli AC, Kaszyński P. [closo-B10H8‐1-CN-10-Azinium]– anions: photoactive heteroditopic ligands for metal complexes. Inorg Chem 2024; 63: 17774-17784.
| Crossref | Google Scholar | PubMed |
52 Golubev AV, Baltovskaya DV, Kubasov AS, Bykov AY, Zhizhin KY, Kuznetsov NT. Synthesis of 1,10-disulfanyl-closo-decaborate anion and its disulfonium tetraacetylamide derivative. Russ J Inorg Chem 2024; 69: 649-658.
| Crossref | Google Scholar |
53 Ali MO, Pociecha D, Wojciechowski J, Novozhilova I, Friedli AC, Kaszyński P. Highly quadrupolar derivatives of the [closo-B10H10]2- anion: investigation of liquid crystalline polymorphism in an homologous series of 1,10-bis(4-alkoxypyridinium) zwitterions. J Organomet Chem 2018; 865: 226-233.
| Crossref | Google Scholar |
54 Hietsoi O, Kapuściński S, Friedli AC, Kaszyński P. [closo-B10H8-1,10-(NHC(=NH2)Me)2]: a rare zwitterionic amidinium derivative. J Mol Struct 2023; 1284: 135324.
| Crossref | Google Scholar |
55 Voinova VV, Selivanov NA, Plyushchenko IV, Vokuev MF, Bykov AY, Klyukin IN, Novikov AS, Zhdanov AP, Grigoriev MS, Rodin IA, Zhizhin KY, Kuznetsov NT. Fused 1,2-diboraoxazoles based on closo-decaborate anion–novel members of diboroheterocycle class. Molecules 2021; 26: 248.
| Crossref | Google Scholar | PubMed |
56 Jakubowski R, Pecyna J, Ali MO, Pietrzak A, Friedli AC, Kaszyński P. Polar derivatives of [closo-1-CB9H10]- and [closo-1-CB11H12]– anions as high Δε additives to a nematic host: a comparison of the CH2CH2 and COO linking groups. Dalton Trans 2021; 50: 3671-3681.
| Crossref | Google Scholar | PubMed |
57 Nelyubin AV, Selivanov NA, Bykov AY, Klyukin IN, Novikov AS, Zhdanov AP, Zhizhin KY, Kuznetsov NT. N-Borylated hydroxylamines [B12H11NH2OH]– as a novel type of substituted derivative of the closo-dodecaborate anion. Russ J Inorg Chem 2020; 65: 795-799.
| Crossref | Google Scholar |
58 Burdenkova AV, Zhdanov AP, Klyukin IN, Selivanov NA, Bykov AY, Zhizhin KY, Kuznetsov NT. Synthesis of derivatives of closo-dodecaborate anion based on amino acid esters. Russ J Inorg Chem 2021; 66: 1616-1620.
| Crossref | Google Scholar |
59 Sun Y, Zhang J, Zhang Y, Liu J, van der Veen S, Duttwyler S. The closo-dodecaborate dianion fused with oxazoles provides 3D diboraheterocycles with selective antimicrobial activity. Chem Eur J 2018; 24: 10364-10371.
| Crossref | Google Scholar | PubMed |
60 Pecyna J, Żurawiński R, Kaszyński P, Pociecha D, Zagórski P, Pakhomov S. Polar liquid crystals derived from sulfonium zwitterions of the [closo-1-CB11H12]– anion. Eur J Inorg Chem 2016; 2923-2931.
| Crossref | Google Scholar |
61 Zhu TC, Xing YY, Sun Y, Duttwyler S, Hong X. Directed B–H functionalization of the closo-dodecaborate cluster via concerted iodination–deprotonation: reaction mechanism and origins of regioselectivity. Org Chem Front 2020; 7: 3648-3655.
| Crossref | Google Scholar |
62 Middaugh RL. Photolytic and electrolytic reduction of iodobenzenenonahydro-closo-decaborate(1-) ion, an anionic analog of diphenyliodonium ion, and preparation of 1-iodononahydro-closo-decaborate(2-) ion. Inorg Chem 1974; 13: 744-745.
| Crossref | Google Scholar |
 Piotr Kaszyński is a professor of chemistry at the Centre of Molecular and Macromolecular Studies of the Polish Academy of Sciences and University of Łódź in Poland. He received his MSc degree from Warsaw Polytechnic in Poland in 1985, PhD degrees from the University of Texas at Austin in 1991 and the University of Łodź in 2007. He was a postdoctoral fellow at Caltech before joining Vanderbilt University in Nashville. In 2015, he moved the bulk of his research program to Polish Academy of Sciences and University of Łódź, while maintaining ties with Middle Tennessee State University in USA. He has published over 200 original papers, and several book chapters and reviews. |