The development of a bioassay expressing the human MRGPRX2 receptor for ex vivo drug discovery
Jan Tytgat A *
A *
A
Abstract
Successful discovery of potent and selective ligands for therapeutically relevant receptors is indispensable for future clinical applications to treat a wide array of diseases. The mas-related G protein-coupled receptor-X2 (MRGPRX2) is an important receptor that regulates mast cell activation and, thus, governs to a significant extent the human immune response. A number of ligands for MRGPRX2 have been identified including human cathelicidin (LL-37) and antimicrobial peptides from plants (PAMPS). Future drug discovery to identify MRGPRX2 specific ligands undoubtedly will benefit from a continued screening of small molecule and peptide libraries. Mast cells (MCs) are generally difficult and expensive to maintain in vitro and, therefore, are not economical to use for screening large numbers of molecules. Herein, we present the development of a bioassay expressing the human MRGPRX2 receptor, linked to inwardly rectifying potassium (GIRK1/2) channels that are opened upon binding of β-γ subunits of a Gi/o-type protein following activation of MRGPRX2. This communication presents a method-focused study demonstrating a simple, yet robust and inexpensive electrophysiological approach (voltage clamp) using oocytes from Xenopus laevis to perform high-throughput screening of libraries. Unexpectedly, this bioassay also supports the claim that the MRGPRX2 receptor can couple efficiently to Gi/o-type proteins, a pathway not yet described. Furthermore, the approach allows structure-function research using mutated MRGPRX2 genes and can study reciprocal cross talks between MRGPRX2 and other co-expressed membrane-bound proteins, such as voltage- or ligand-gated ion channels, mimicking in vivo conditions.
Keywords: drug discovery, electrophysiology, G protein-coupled receptor, mast cells, MRGPRX2, oocytes, potassium channel.
Introduction
Mast cells (MCs) are immune cells that can be found in the skin, the gastrointestinal and respiratory tracts. It is generally assumed that they are implicated in non-IgE-mediated pseudo-allergic reactions and inflammatory conditions, which include asthma or atopic dermatitis (for reviews, see Dziadowiec et al.1 and Quan et al.2). The mas-related G protein-coupled receptor-X2 (MRGPRX2) is a G protein-coupled receptor (GPCR) that was first reported to be expressed in MCs.3 MRGPRX2 has been shown to be activated by a wide range of endogenous and exogenous compounds, such as small cationic molecules and peptides that have amphipathic properties.4,5 Examples of endogenous ligands are the antimicrobial host defense peptide cathelicidin (LL-37) and the neuropeptide substance P (SP).6 By contrast, compound 48/80 (C48/80) is an exogenous ligand commonly used in receptor functional assays (also in our work7). To date, the exact role of MRGPRX2 in MCs remains to be clarified, despite numerous publications surmising that MRGPRX2 does play a pivotal role in pseudo-allergic reactions, neurogenic inflammation and inflammatory diseases, such as allergic contact dermatitis (ACD), chronic urticaria (CU), rosacea, rheumatoid arthritis, atopic dermatitis (AD), mastocytosis, ulcerative colitis and allergic asthma.8 In several of these pathologies, the usual treatment with classical antihistamines has been reported to be rather disappointing.9 As a consequence, the search for drugs acting on a different target, such as the MRGPRX2, receives much attention. We present here the development of a bioassay expressing the human MRGPRX2 receptor, linked to inwardly rectifying potassium (GIRK1/2) channels, based on a robust electrophysiological method. This assay allows for an ex vivo drug discovery screening, aimed at identifying and characterising novel MRGPRX2 ligands that could have therapeutic potential.
Results and discussion
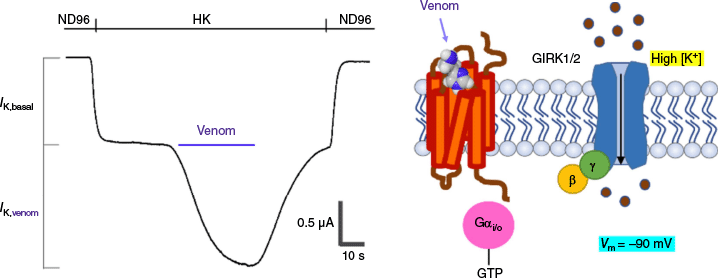
The methodological approach and readout we have used in our study is an ex vivo type of bioassay using oocytes of Xenopus laevis, heterologously co-expressing three proteins: MRGPRX2, GIRK1 and GIRK2 (Fig. 1). The latter two co-assemble to form a functional inwardly rectifying K channel.10 Inwardly rectifying K (Kir) channels allow K ions to move more easily into, rather than out of, the cell. They have diverse physiological functions depending on their type and location. In the presence of a high extracellular K concentration (HK), a robust inward GIRK1/2 current can be recorded reliably. This current is composed of a basal part (IK,basal) representing GIRK1/2 activity in the absence of activation of MRGPRX2. Upon activation of MRGPRX2, e.g., by screening a venom that is known to possess a pharmacophore of ligands with unknown targets, potentiation of an inward GIRK1/2 current component is observed (IK,venom). This results from the binding of β and γ subunits, which have been split off from the trimeric state of a Gi/o type protein, intracellularly after the conversion of GDP to GTP bound to the α subunit.
Methodological illustration of the action of a venom on MRGPRX2 (in orange), together with a representation of the GIRK1/2 channel (in blue). The Gi/o protein is shown in its activated form, whereby the α subunit is bound to GTP (in pink) and the β-γ subunits bound to GIRK1/2 (resp. in yellow–green). Binding of β-γ to GIRK1/2 opens the channel and generates, in high external potassium (HK), an inward flow of K current that can be measured at a holding potential of −70 mV.
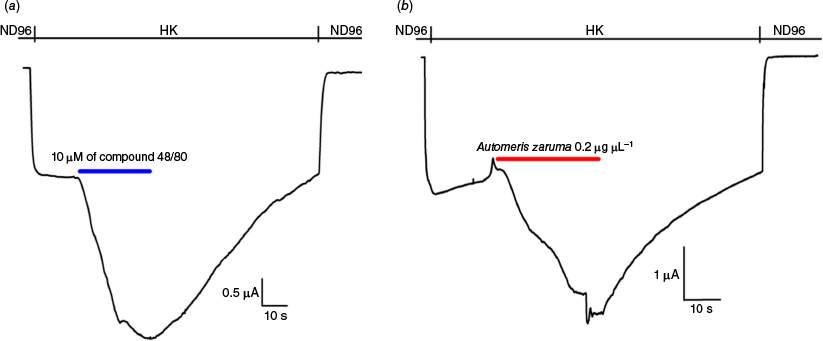
The immediate discomfort after dermal contact with the spines of caterpillars, such as Lonomia and Hylesia, suggests the presence of fast acting, bioactive compounds. Unfortunately, little is known about the mechanism of action causing the cutaneous effects. Given the assumed role of MRGPRX2 in different forms of dermatitis and pseudo-allergic reactions, we have investigated whether the venom of the caterpillar Automeris zaruma can activate MRGPRX2 and, as such, may explain (at least in part) the symptomatology following exposure to its spines. Since it is generally known that animal venoms contain a true pharmacophore of potent or selective bioactive substances, it is justified to use caterpillar venoms as a test and tool for our bioassay.11 Automeris zaruma venom was screened against MRGPRX2 and compared to the effect of the well-known mast cell degranulating compound 40/80.12 In both cases, a ligand-dependent potentiation of GIRK1/2 currents was observed, which was instant and reversible (Fig. 2). For validation purposes, we checked non-injected oocytes (n = 3) and oocytes injected with only GIRK1/2 (n > 6). Neither potentiation nor any current activation was observed under these conditions, eliminating the possible involvement of endogenous receptors or channels and ruling out a direct activation of GIRK1/2. The venom-dependent current (IK,venom) component amounted to 140 ± 10% (n = 3) of the basal current (IK,basal).
Representative traces, adapted from Seldeslachts et al.7 with permission, illustrating the potentiation of GIRK1/2 currents upon application of 10 μM of compound 40/80 (a, blue line; n = 6) and Automeris zaruma venom (b, red line; n = 6) in oocytes expressing MRGPRX2 and GIRK1/2.
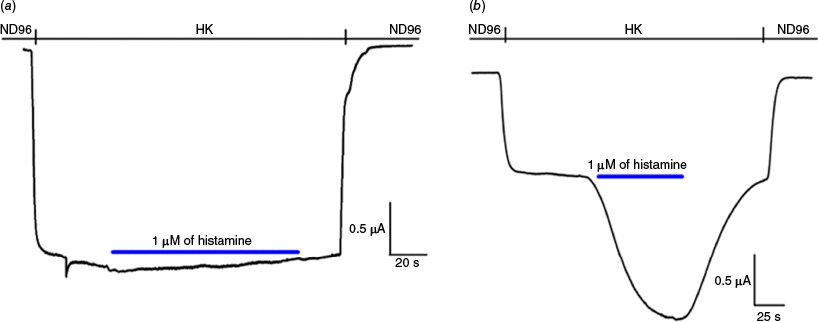
Under the general assumption that histamine is an important constituent of caterpillar venom,7 we next tested histamine on the MRGPRX2 and compared its effect on the histamine type 4 receptor (H4R)13 (Fig. 3). Interestingly, application of 1 µM of histamine did not evoke any response on the MRGRPX2, whereas it clearly potentiated H4R, as to be expected in the latter case.
Representative traces illustrating the effect of 1 μM of histamine (blue line): (a) no activation of GIRK1/2 currents in oocytes expressing MRGPRX2 and GIRK1/2 (n = 3); (b) clear potentiation of GIRK1/2 currents in oocytes expressing H4R and GIRK1/2 (n = 10).
Future experiments are needed to reveal which bioactive molecule(s) present in the venom of Automeris zaruma is (are) responsible for the observed activation of MRGPRX2. Undoubtedly, they will contribute to paving the way to find novel medications acting on this rather poorly characterised receptor.
Our method-focused study supports the claim that MRGPRX2 couples to Gi/o-type proteins, despite the absence of additional experimental evidence using other assays (e.g. G protein biosensors or bioluminescence resonance energy transfer (BRET)-based approaches). Though we dare to make this claim based on the following observations: (i) the oocyte system has proven to be an efficient and reliable system for the coupling of several types of GPCRs to Gi/o-type proteins, such as the opioid and cannabinoid receptors (resp., e.g. our previously published work14,15); (ii) in our paper by Seldeslachts et al.7 where we expressed GPCRs of the histamine class, type 2, 3 and 4 (H2R, H3R, and H4R), we incubated the oocytes with 2.5 ng of pertussis toxin (PTX) for 16 h. It is commonly known that PTX uncouples the α subunit of the Gi/o-driven inhibition of the adenylate cyclase (AC), which results in an elevation of the intracellular level of cAMP.16 At the same time, it has been shown that the β-γ subunits of the Gi/o-type protein interact directly at the level of membrane-bound GIRK1/2 channels, leading to opening of these K-selective pores. This interaction is PTX independent, indicating the exclusive role of the β-γ subunits of the Gi/o-type protein and the possibility to measure GIRK1/2 in a reliable manner.17 The final observation to support our claim is: (iii) when expressing the four different types of histamine receptors in oocytes, we observed that only the histamine-type 1 receptor (H1R) evoked a different kind of response, known as activation of Gq-type proteins.7 This particular manner of activation evokes in oocytes a typical calcium-dependent chloride current (known as CaCC) that is very different from GIRK1/2 current. It also confirms what is generally accepted and described in the literature, namely the coupling to Gq-type proteins with stimulation of phospholipase C and the production of inositol 1,4,5-trisphosphate (IP3), resulting in a transient release of Ca from endoplasmic reticulum (ER) stores with the activation of CaCC.18
Conclusion
The present communication demonstrates a method-based study using a simple, yet robust and inexpensive electrophysiological approach (voltage clamp) with oocytes from Xenopus laevis to perform high-throughput screening of libraries, allowing for the deorphanisation of the MRGPRX2 receptor. Our study also supports that the MRGPRX2 receptor can couple efficiently to Gi/o-type proteins in an ex vivo system using oocytes. Furthermore, the approach enables structure-function studies using mutated MRGPRX2 genes and allows for the involvement of reciprocal cross talks between MRGPRX2 and other co-expressed membrane-bound proteins, such as voltage- or ligand-gated ion channels, mimicking in vivo conditions.
Experimental
In vitro transcription and mRNA preparation
Plasmids containing the entire coding region of the following cDNA clones were used: the human mas-related G protein-coupled receptor-X2 (hMRGPRX2; NM_054030), the human histamine type 4 receptor (H4R; AB045370) and the mouse G protein-coupled inwardly rectifying potassium channels type 1 and 2 (resp. GIRK1 and GIRK2, also known under NM_008426 = Kir3.1 = KCNJ3; NM_001025584 = Kir3.2 = KCNJ6), following a protocol as previously described by Seldeslachts et al.19
Electrophysiological recordings
The isolation of Xenopus laevis oocytes was conducted as previously described.20 Mature female animals were purchased from Nasco (Fort Atkinson, WI, USA) and were housed in the Aquatic Facility (KU Leuven) in compliance with the regulations of the European Union (EU) concerning the welfare of laboratory animals as declared in Directive 2010/63/EU. The use of Xenopus laevis oocytes was approved by the Animal Ethics Committee of the KU Leuven with the license number P186/2019. Electrophysiological recordings were performed as previously described.19 In brief, the following two extracellular solutions were used: ND96 solution, composed of 96 mM of NaCl, 2 mM of KCl, 1.8 mM of CaCl2, 2 mM of MgCl2 and 5 mM of HEPES at pH 7.4; and high-potassium (HK) solution, composed of 96 mM of KCl, 2 mM of NaCl, 1 mM of MgCl2, 1.8 mM of CaCl2 and 5 mM of HEPES at pH 7.5.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgements
The author thanks Andrea Seldeslachts for experimental help and Dietrich Mebs for providing the venom of Automeris zaruma.
References
1 Dziadowiec A, Popiolek I, Kwitniewski M, Porebski G. Modulation of the Mas-related G protein-coupled receptor X2 (MRGPRX2) by xenobiotic compounds and its relevance to human diseases. J. Xenobiot 2024; 14: 380-403.
| Crossref | Google Scholar | PubMed |
2 Quan PL, Sabaté-Brescó M, Guo Y, Martín M, Gastaminza G. The multifaceted Mas-related G protein-coupled receptor member X2 in allergic diseases and beyond. Int J Mol Sci 2021; 22: 4421.
| Crossref | Google Scholar | PubMed |
3 Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 2006; 349: 1322-1328.
| Crossref | Google Scholar | PubMed |
4 Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008; 123: 398-410.
| Crossref | Google Scholar | PubMed |
5 Bakare OO, Gokul A, Fadaka AO, Wu R, Niekerk L-A, Barker AM, Keyster M, Klein A. Plant antimicrobial peptides (PAMPs): features, applications, production, expression and challenges. Molecules 2022; 27: 3703.
| Crossref | Google Scholar | PubMed |
6 Verjans E-T, Zels S, Luyten W, Landuyt B, Schoofs L. Molecular mechanisms of LL-37-induced receptor activation: an overview. Peptides 2016; 85: 16-26.
| Crossref | Google Scholar | PubMed |
7 Seldeslachts A, Peigneur S, Mebs D, Tytgat J. Unraveling the venom chemistry with evidence for histamine as key regulator in the envenomation by caterpillar Automeris zaruma. Front Immunol 2022; 13: 972442.
| Crossref | Google Scholar | PubMed |
8 Castells M, Madden M, Oskeritzian CA. Mast cells and Mas-related G protein-coupled receptor X2: itching for novel pathophysiological insights to clinical relevance. Curr Allergy Asthma Rep 2025; 25: 5.
| Crossref | Google Scholar | PubMed |
9 Facheris P, Jeffery J, Del Duca E, Guttman-Yassky E. The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment. Cell Mol Immunol 2023; 20: 448-474.
| Crossref | Google Scholar | PubMed |
10 Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun 1995; 208(3): 1166-1173.
| Crossref | Google Scholar | PubMed |
11 Bordon KCF, Cologna CT, Fornari-Baldo EC, Pinheiro-Júnior EL, Cerni FA, Amorim FG, Anjolette FAP, Cordeiro FA, Wiezel GA, Cardoso IA, Ferreira IG, de Oliveira IS, Boldrini-França J, Pucca MB, Baldo MA, Arantes EC. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol 2020; 11: 1132.
| Crossref | Google Scholar | PubMed |
12 Schemann M, Kugler EM, Buhner S, Eastwood C, Donovan J, Jiang W, Grundy D. The mast cell degranulator compound 48/80 directly activates Neurons. PLoS ONE 2012; 7(12): 52104.
| Crossref | Google Scholar | PubMed |
13 Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leucocytes. J Biol Chem 2000; 275(47): 36781-36786.
| Crossref | Google Scholar | PubMed |
14 Pil J, Tytgat J. The role of the hydrophylic Asn230 residue of the µ-opioid receptor in the potency of various opioid agonists. Br J Pharmacol 2001; 134: 496-506.
| Crossref | Google Scholar | PubMed |
15 An D, Peigneur S, Hendrickx LA, Tytgat J. Targeting cannabinoid receptors: current status and prospects of natural products. Int J Mol Sci 2020; 21: 5064.
| Crossref | Google Scholar | PubMed |
16 Inoue A, Raimondi F, Kadji FMN, Singh G, Kishi T, Uwamizu A, Ono Y, Shinjo Y, Ishida S, Arang N, Kawakami K, Gutkind S, Aoki J, Russell RB. Illuminating G-protein-coupling selectivity of GPCRs. Cell 2019; 177(7): 1933-1947.
| Crossref | Google Scholar | PubMed |
17 Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. Mapping the G βγ-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem 2003; 278(31): 29174-29183.
| Crossref | Google Scholar | PubMed |
18 Kuruma A, Hartzell C. Dynamics of calcium regulation of chloride currents in Xenopus oocytes. Am J Physiol – Cell Physiol 1999; 276: C161-C175.
| Crossref | Google Scholar | PubMed |
19 Seldeslachts A, Peigneur S, Tytgat J. Histamine receptors: ex vivo functional studies enabling the discovery of hits and pathways. Membranes 2023; 13: 897.
| Crossref | Google Scholar | PubMed |
20 Liman E, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992; 9(5): 861-871.
| Crossref | Google Scholar | PubMed |


