Inheritance and covariation of specialised metabolites among cannabis chemotypes
Matthew T. Welling A # * , Myrna A. Deseo
A # * , Myrna A. Deseo  A # * , Laura Steel A , Gayathree I. Senevirathne
A # * , Laura Steel A , Gayathree I. Senevirathne  A , Kim L. Johnson A , Anthony R. Gendall A , Monika S. Doblin A § and Antony Bacic A §
A , Kim L. Johnson A , Anthony R. Gendall A , Monika S. Doblin A § and Antony Bacic A §
A
# Co-first authors.
§ Co-senior authors.
Handling Editor: John Wade
Abstract
The major phytocannabinoid bioactives produced by Cannabis sativa L. (cannabis) are Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), with many minor phytocannabinoids (PCs) also thought to contribute to the pharmacological efficacy. Cannabis typically segregates into three main chemical phenotypes (chemotypes) based on their Δ9-THC/CBD ratio, a highly heritable trait determined by the segregation of two closely related berberine bridge-like enzymes that perform stereoselective oxidative cyclisation on the geranyl moiety of cannabigeroid intermediates. Apart from a small number of metabolome-wide association studies, few attempts have been made to either understand metabolite coupling among Δ9-THC/CBD chemotypes or to examine the inheritance of alternative biomarkers that could be used to discriminate chemotype. Here, we examined the metabolomes of 108 F2 segregants derived from a cross between a Δ9-THC-predominant chemotype I and a CBD-predominant chemotype III plant. Although segregation of the Δ9-THC/CBD ratio followed Mendelian genetics expectations, covariation in the inheritance of minor PCs, including cannabichromene (CBC)-types, indicated changes in cannabinoid synthase product specificity among chemotypes. In addition, several non-PC related metabolites were identified that may serve as potential biomarkers for chemotype prediction. These data have important implications for the pre-breeding and selection of cannabis chemovars and highlight the need to adopt metabolic engineering strategies to optimise PC production.
Keywords: cannabichromene, cannabidiol, Cannabis sativa L., liquid chromatography, mass spectrometry, phytocannabinoids, untargeted metabolomics, Δ9-tetrahydrocannabinol.
Introduction
Cannabis sativa L. (cannabis) is a monotypic and phenotypically diverse annual herb of the Cannabaceae family.1 Plants are wind pollinated (anemophilous) and mostly dioecious, meaning that unisexual male staminate and female pistillate flowers occur on separate plants.2 As such, the diploid genomes (2n = 20) of cannabis, which consist of nine autosomes and a pair of sex chromosomes (X and Y), are highly heterozygous and unusually diverse for a single species.3 Domestication has spanned many thousands of years and plants are now cultivated globally.4 The stems and seeds are used for industrial applications (fibre and food), whereas the pistillate flowers are used for recreational and medicinal end uses.5
The bioactivity of cannabis flowers is largely attributed to a class of isoprenylated resorcinyl polyketides known as phytocannabinoids (PCs). These are synthesised from an activated fatty acid starter unit (e.g. hexanoyl-CoA) and a methylerythritol phosphate (MEP)-derived geranyl diphosphate (GPP) within specialised structures called glandular trichomes, predominantly found on the leaves (calyx) surrounding pistils.6,7 More than 160 PCs have been identified, and these can be grouped into nine major subclasses and types based on the topological arrangement of the geranyl moiety.8 Many of these subclasses show cannabimimetic-like properties, such as cannabichromene (CBC), which modulates the G protein-coupled receptor, cannabinoid receptor 2 (CB2), and various transient receptor potential (TRP) channels of the mammalian endocannabinoid system.8–10 Extensive research has been conducted on the pharmacology of Δ9-tetrahydrocannabinol (Δ9-THC)- and cannabidiol (CBD)-types, which vary structurally at the pyran B-ring – open for CBD-types and closed for Δ9-THC-types. Δ9-THC and CBD are the active pharmaceutical ingredients of Dronabinol and Epidiolex, which are Federal Drug Administration (FDA)- and Therapeutic Goods Administration (TGA)-approved prescription medicines, with the latter recently gaining approval as an adjunct therapy for rare forms of childhood-onset epileptic encephalopathies.11,12 In addition to their use as single compounds, the dried flowers are also consumed for a variety of ailments, including chronic pain relief.13
Δ9-THC and CBD are produced in plants in their carboxylated (acidic) forms, Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and cannabidiolic acid (CBDA), from a common precursor cannabigerolic acid (CBGA). Stereoselective oxidative prototropic cyclisation of the linear geranyl moiety of CBGA is catalysed by Δ9-THCA synthase (THCAS) and CBDA synthase (CBDAS) enzymes, respectively producing Δ9-THCA and CBDA, as well as H2O2, a by-product, in a molar ratio of 1:1 (Fig. 1).14,15 The structural and functional properties of these covalently flavinylated oxidases are remarkably similar. They have a similar amino acid chain length (544 v. 545 aa), mass (~75 kDa) and number of asparagine N-linked glycosylation sites, but their chemical reactions differ at the proton transfer step.16 Both catalyse hydride transfer from the benzylic geranyl methylene to flavin adenine dinucleotide (FAD). The resulting cation is then quenched by the terminal double bond, and the reaction can then be terminated by either allylic deprotonation (CBDAS) or by attack of an ortho phenolic hydroxy group (THCAS).15,16 The two enzymes differ only in the way the cyclisation cascade is terminated (deprotonation v. intramolecular oxygen trapping). Subtle differences in the active site architecture that alter the orientation of the geranyl moiety are thought to be responsible for this specificity. However, using recombinantly expressed THCAS and CBDAS to study CBGA conversion suggests that the mechanism of the oxidation and the termination steps are not strictly controlled. Consistent with the formation of conditional isomers or stereoisomers, up to eight products were formed that had the same mass and MSn fragmentation as their primary product, including cannabichromenic acid (CBCA), which is formed by oxidative pericyclic cyclisation.17
Schematic representation of the conversion of cannabigeroids (CBG(V)A) by Δ9-tetrahydrocannabinolic acid synthase (THCAS; EC:1.21.3.7) and cannabidiolic acid synthase (CBDAS; EC:1.21.3.8). The red coloured section of the chemical structure represents the resorcinol moiety, and the blue represents the isoprenoid moiety.
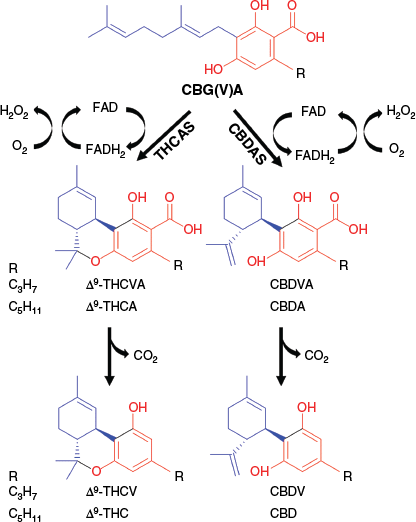
Cannabis plants are demarcated based on the levels of the intoxicant psychoactive Δ9-THC, but the regulation varies as set by legislation and by end-use (e.g. industrial hemp fibre or hemp seed v. medicinal or recreational).5 Furthermore, five main chemotypes are acknowledged, although most plants can be readily classified based on the ratio of Δ9-THC and CBD.18–21 These ratios are stable throughout the life cycle of the plant and resistant to environmental influences.22,23 Chemotype I plants, which are typically associated with drug-type varieties, are predominant in Δ9-THC; chemotype II plants have an intermediate Δ9-THC/CBD ratio, whereas chemotype III plants are predominant in CBD and are typically found among industrial fibre and seed varieties.5 The less common chemotype IV lacks functioning PC synthases and therefore accumulates high levels of cannabigerol (CBG)-type PCs,19 and the even rarer chemotype V plants produce only trace amounts of PCs.20
Inheritance patterns of the Δ9-THC/CBD ratio are consistent with a simple Mendelian model comprising a single B locus, whereby codominant BT and BD alleles encode for THCAS and CBDAS respectively. Crossing of chemotype I (BTBT) and III (BDBD) plants produces F1 progeny with an intermediate chemotype II (BTBD), of which selfing results in a 1:2:1 (chemotype I, II, III) segregation ratio in the F2 progeny.18,24,25 The genomic organisation of PC synthase genes is more complex, with multiple repeat tandem arrays of THCAS and CBDAS gene paralogs clustered within a highly repetitive pericentromeric region of chromosome 7.3 Confusingly, and in contrast to a monogenic model, THCAS and CBDAS arrays do not map to the exact location on chromosome 7 (>1 cM).3,24 Regardless, only a single full-length copy of THCAS and CBDAS is expressed in chemotype I or III plants respectively.3,24 As such, although the PC synthases appear non-allelic and may have no alternative allele at their respective locus (hemizygotic), chemotypes are determined by the inheritance of a single gene copy of THCAS (chemotype I) and CBDAS (chemotype III) or both in the case of the intermediate chemotype II plants.
Several attempts have been made to either categorise or differentiate chemotypes using an alternative to Δ9-THC/CBD ratio. Chemotypic screens of 21 chemotype I–III plants found leaf shape and resistance/susceptibility against Botrytis cinerea to be connected with the Δ9-THC/CBD ratio,26 whereas low copy number variation of Golovinomyces chicoracearum (powdery mildew) resistance genes were associated with chemotype I in a cohort of 40 cannabis varieties.26,27 Analysis of 50 accessions associated cannabiripsolic acid with CBD-predominant chemotype III plants, whereas tetrahydrocannabutolic acid and tetrahydrocannabiphorolic acid were associated with Δ9-THC-predominant chemotype I plants.21 Large-scale screens involving up to 157 accessions showed a strong positive correlation between CBC and CBD content among chemotype II and III plants, but not between CBC and Δ9-THC among chemotype I plants.28 In addition to changes in the frequency of minor PCs, various flavonoids, sesquiterpenoids and monoterpenoids have been strongly correlated with chemotype I and III plants,29 indicating that the metabolic fingerprints of chemotypes may extend beyond changes in Δ9-THC/CBD composition. As is the case for genome-wide association studies, metabolome-wide screens are susceptible to spurious associations arising from systematic differences in the study population, which can inflate false positive error.30 In this study, we performed a genetic cross between a Δ9-THC- and CBD-predominant plant and assessed PC and metabolomic variation among F1 and F2 segregants to better understand metabolite coupling among chemotypes and identify robust biomarkers to differentiate chemotype I and III plants.
Results and discussion
Assignment of chemical phenotype (chemotype) to parental and filial plants
In order to understand metabolite coupling among the major Δ9-THC/CBD chemotypes, a genetically female Δ9-THC-type (chemotype I; P1) plant and a genetically female CBD-type (chemotype III; P2) plant were crossed by silver thiosulfate (STS)-induced staminate flowering. The dried pistillate inflorescences of the resulting filial F1 plants, as well as inflorescences from F2 plants cultivated from seeds from a selfed F1 plant, were examined by ultra-high-performance liquid chromatography–electrospray ionisation–high-resolution mass spectrometry (UHPLC-ESI-HRMS) using data-dependent MS2 acquisition (DDA). The use of a feminised bi-parental population allowed us to score the metabolomes of unisexual female inflorescences for both parents. This circumvented the use of either a male or hermaphroditic paternal parent, which produce morphologically and chemically distinct staminate inflorescences.2
To accurately assign chemotypes to the parental and filial plants, 19 PCs were quantitatively determined by comparison with certified reference standards, and the results are summarised in Table 1 and Supplementary Table S1. Of the 19 PCs, 10 were the acid forms Δ9-tetrahydrocannabinolic acid A (Δ9-THCAA, 1, commonly referred to as Δ9-THCA), CBDA (2), CBCA (3), CBGA (4), cannabinolic acid (CBNA, 5), Δ9-tetrahydrocannabivarinic acid (Δ9-THCVA, 6), cannabidivarinic acid (CBDVA, 7), cannabichromevarinic acid (CBCVA, 8), cannabigerovarinic acid (CBGVA, 9) and cannabivarinic acid (CBVA, 10); and nine were the decarboxylated analogues Δ9-THC (11), CBD (12), CBC (13), cannabigerol (CBG, 14), cannabinol (CBN, 15), Δ9-tetrahydrocannabivarin (Δ9-THCV, 16), cannabidivarin (CBDV, 17), cannabigerovarin (CBGV, 18) and cannabivarin (CBV, 19). This allowed us to examine both the segregation of the major wild-type pentyl- (e.g. Δ9-THCAA, Δ9-THC, CBDA and CBD) and propyl- (Δ9-THCVA, Δ9-THCV, CBDVA and CBDV), Δ9-THC(V)- and CBD(V)-type PCs associated with the activity of THCAS and CBDAS,31 as well as to quantify, for the first time, the co-segregation of pentyl and propyl side chain homologues of CBC(V)-type (e.g. CBCA, CBC and CBCVA) and CBG(V)-type (e.g. CBGA, CBG, CBGVA and CBGV) PCs among parental and filial populations.
| Phyto-cannabinoids | Retention time (min) | Concentration (mmol/100 g) | ||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Average | Median | |||
| Δ9-THCAA (1) | 14.7 | 0.0800 | 16.9534 | 3.2119 | 1.9541 | |
| CBDA (2) | 11.8 | 0.0104 | 10.4997 | 4.0100 | 4.2892 | |
| CBCA (3) | 15.0 | 0.0665 | 0.8975 | 0.3924 | 0.3762 | |
| CBGA (4) | 12.1 | 0.0007 | 0.1544 | 0.0381 | 0.0270 | |
| CBNA (5) | 14.0 | 0.0002 | 0.0200 | 0.0031 | 0.0019 | |
| Δ9-THCVA (6) | 13.1 | 0.0237 | 3.9632 | 0.8717 | 0.7354 | |
| CBDVA (7) | 10.8 | 0.0050 | 3.3960 | 1.0685 | 1.1103 | |
| CBCVA (8) | 13.6 | 0.2042 | 2.2867 | 1.0141 | 0.9585 | |
| CBGVA (9) | 11.1 | 0.0004 | 0.0661 | 0.0162 | 0.0123 | |
| CBVA (10) | 12.4 | 0.0013 | 0.1310 | 0.0255 | 0.0168 | |
| Δ9-THC (11) | 13.9 | 0.0098 | 0.8496 | 0.1581 | 0.1256 | |
| CBD (12) | 12.1 | 0.0001 | 0.3924 | 0.1050 | 0.0999 | |
| CBC (13) | 14.5 | 0.0019 | 0.0550 | 0.0171 | 0.0151 | |
| CBG (14) | 12.1 | 0.0008 | 0.1972 | 0.0404 | 0.0297 | |
| CBN (15) | 13.3 | 0.00002 | 0.0014 | 0.0003 | 0.0002 | |
| Δ9-THCV (16) | 11.0 | 0.0035 | 0.3327 | 0.0891 | 0.0723 | |
| CBDV (16) | 12.3 | 0.0001 | 0.1249 | 0.0348 | 0.0295 | |
| CBGV (18) | 11.0 | 0.0011 | 0.2339 | 0.0413 | 0.0268 | |
| CBV (19) | 11.7 | 0.0001 | 0.0062 | 0.0012 | 0.0009 | |
Phyto-cannabinoids: Δ9-THCAA (1), Δ9-Tetrahydrocannabinolic acid A, commonly referred to as Δ9-tetrahydrocannabinolic acid (Δ9-THCA); CBDA (2), Cannabidiolic acid; CBCA (3), cannabichromenic acid; CBGA (4), cannabigerolic acid; CBNA (5), cannabinolic acid; Δ9-THCVA (6), Δ9-tetrahydrocannabivarinic acid; CBDVA (7), cannabidivarinic acid; CBCVA (8), cannabichromevarinic acid; CBGVA (9), cannabigerovarinic acid; CBVA (10), cannabivarinic acid; Δ9-THC (11), Δ9-tetrahydrocannabinol; CBD (12), cannabidiol; CBC (13), cannabichromene; CBG (14), cannabigerol; CBN (15), cannabinol; Δ9-THCV (16), Δ9-tetrahydrocannabivarin; CBDV (17), cannabidivarin; CBGV (18), cannabigerovarin; CBV (19), cannabivarin.
Acidic PCs are decarboxylated non-enzymatically following exposure to light and heat.32 To simplify comparisons between the PC species, inflorescence dry weight concentrations (mmol/100 g) of the acidic and decarboxylated PCs were combined and expressed as a total (e.g. the addition of Δ9-THCA and Δ9-THC is equal to Δ9-THCtot). This was applied to all pentyl and propyl PC species except for CBCVA, as the decarboxylated standard for this PC (CBCV) was not available at the time of analysis.
Parental chemotypes are consistent with chemotype I and III
Although the absolute PC content within cannabis inflorescences is a polygenic trait and subject to environmental stimuli, the relative proportions of Δ9-THC-/CBD-type PCs is under strict genetic control and dependent on the inheritance of discrete loci encoding for THCAS and CBDAS (Fig. 1).3 Analysis of the PC profiles of the unisexual pistillate inflorescences of clonally propagated parentals grown alongside 10 F1 clones is consistent with this model. The chemotype I parent P1 was predominant in Δ9-THCtot and its propyl C3 homologue Δ9-THCVtot (Fig. 2a), of which THCAS catalyses both homologues from either pentyl CBGA or propyl CBGVA substrates respectively (Fig. 1).31 For P1, these two compounds made up 92% of the 19 PCs assessed, with Δ9-THCtot as the predominant PC at 57%. Conversely, the chemotype III parent P2 was predominant in only CBDtot (86% total PCs) formed by CBDAS catalysis of CBGA (Fig. 1), with >95% of PCs having pentyl, not propyl, side chains, as evidenced by the low proportion of CBDVtot in this chemotype (Fig. 2a).
Composition of phytocannabinoids (PCs) among the parentals (P1, P2) and filial generations: F1 (n = 10) and F2 (n = 108). (a) Interleaved scatter plot of PC proportions. (b) Violin plot of F2 PC concentrations (concentrations expressed in millimoles per 100 g, dry matter basis). The subscript ‘tot’ refers to the sum of the PC acid form and its respective decarboxylated form.
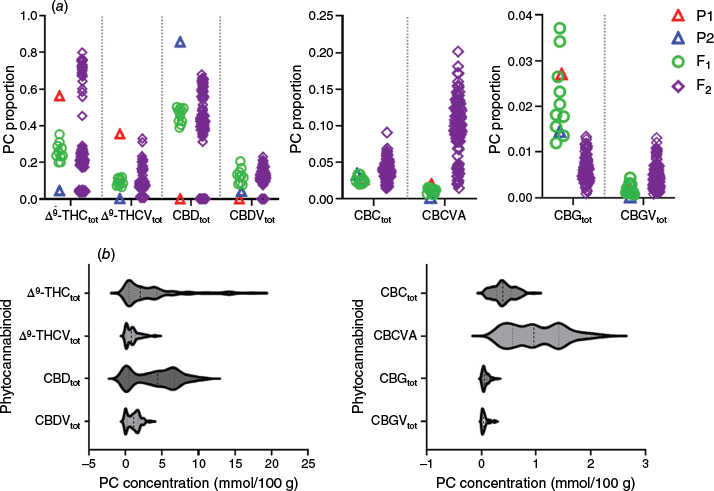
F1 hybrid progeny have PC values intermediate to the parental plants
Analogous with a co-dominant model of inheritance for THCAS and CBDAS, the pentyl Δ9-THCtot and CBDtot values were uniformly intermediate as compared with P1 and P2, and, as expected, are consistent with chemotype II (Fig. 2a). A similar trend among the F1 plants was observed for the other propyl and pentyl PC species, except for CBDVtot, which exceeded levels observed for both parentals (Fig. 2a). Transgressive segregation of CBDVtot, together with the intermediate F1 Δ9-THCVtot values, is interpreted as a sign of homozygosity at the An locus for P1 and P2. This multi-locus complex is hypothesised to govern pentyl and propyl PC proportions,33 and elevated levels of CBDVtot among F1 hybrids are consistent with the inheritance of alleles from P1 that form CBGVA, as well as CBDAS from P2 that allows catalysis of CBGVA, forming CBDVA (Fig. 1).
Next, we selfed a single female F1 plant by silver thiosulfate (STS) application to generate a segregating F2 population, of which 108 plants were grown from seed within an environmentally controlled glasshouse. The mature 6-week-old inflorescences of the F2 progeny were then harvested, dried and subjected to UHPLC-ESI-HRMS analysis (see Experimental). As was observed for the parents and F1 progeny, Δ9-THCtot and CBDtot were the predominant PCs (Fig. 2b), of which the acid forms made up the majority and ranged from 0.08 to 16.95 mmol/100 g for Δ9-THCAA and 0.01–10.50 mmol per 100 g for CBDA. Overall, the median concentrations of the acid PCs were in the order of CBDA > Δ9-THCAA > CBDVA, CBCVA > THCVA > CBCA ≫ CBGA > CBVA, CBGVA > CBNA (Table 1 and Supplementary Table S1).
Minor PC composition among F2 segregants deviates from the parentals
Although the absolute inflorescence PC contents between parental/F1 clones and seed-propagated F2 individuals are not directly comparable (see Experimental), the relative PC proportions between these populations indicated subtle changes in the abundance of the minor PCs (Fig. 2a). This was evident for the propyl CBCVA and the pentyl CBCtot, which were both elevated in the F2 relative to the parents (P1 and P2) and F1. Among the F2, the average propyl CBCVA content was >2-fold that of the pentyl CBCtot content (1.0 v. 0.41 mmol per 100 g) (Fig. 2b), despite showing the reverse trend in previous generations (e.g. the pentyl CBCtot proportion was higher in P1, P2 and F1) (Fig. 2a). Differences were also observed for the PC intermediate propyl- and pentyl- CBG-types, with F2 proportions for CBGVtot and CBGtot higher and lower, compared with the parentals and F1 respectively (Fig. 2a).
F2 progeny segregate into three chemotypes
As expected, three distinct Δ9-THC-/CBD-type chemotypes were observed among F2 plants. Chemotypes were demarcated by plotting Δ9-THCtot and CBDtot contents (dry matter basis), and these were in agreement with the initial chemotype classifications of the parents and F1 (Fig. 3a). Three chemotypes co-segregating with Δ9-THCtot and CBDtot contents were also distinguishable by comparison of propyl PCs Δ9-THCVtot and CBDVtot contents (Fig. 3a). This confirmed that THCAS and CBDAS can accept CBG-type PCs with either pentyl or propyl alkyl side chains, and that changes in alkyl length occur prior to the oxidative cyclisation step performed by PC synthases. Chemotype I Δ9-THC(V)-predominant F2 plants had log(Δ9-THCtot/CBDtot) ≥ 2.0, whereas chemotype III CBD(V)-predominant F2 plants had log(Δ9-THCtot/CBDtot) ≤ −1.0 (Fig. 3b). For chemotype II plants, log(Δ9-THCtot/CBDtot) values were ~−0.2, and the F2 progeny was not significantly different from the 1:2:1 Mendelian expectation of chemotype I:II:III (25:51:32 F2 plants, χ2 = 0.59, 2 d.f. (P = 0.746)).
F2 chemotypic distribution patterns. (a) Scatterplot of propyl and pentyl Δ9-THC(V)-type and CBD(V)-type contents (concentrations expressed in millimoles per 100 g, dry matter basis). Black arrows indicate PC contents of the F1 hybrid individual, which was selfed to produce the F2 population. (b) Histogram of THC(V)-type, CBD(V)-type and propyl/pentyl PC ratios. Numerals within parentheses indicate chemotype. The subscript ‘tot’ refers to the sum of the PC acid form and its respective decarboxylated form.
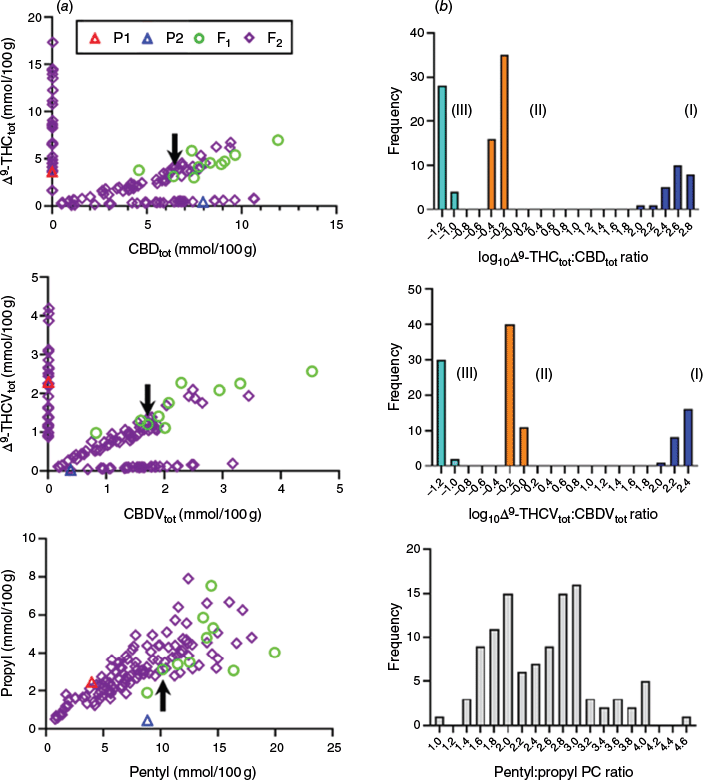
Pentyl:propyl PCs deviate from Mendelian expectation
Surprisingly, the pentyl (Δ9-THCtot + CBDtot + CBGtot + CBCtot) and propyl (Δ9-THCVtot + CBDVtot + CBGVtot + CBCVA) PC contents were skewed towards the high propyl PC parent P1, for both F1 and F2 generations, whereas the recovery of the low propyl–high pentyl contents of P2 was not observed among the 108 F2 segregants (Fig. 3a). Moreover, the ratio of pentyl:propyl PC contents showed a continuous distribution not inconsistent with a Gaussian (normal) distribution (D’Agostino–Pearson K2 normality test = 3.891, P = 0.1429) (Fig. 3b). Taken together, these observations indicate a di- or oligo- genic mode of inheritance for pentyl:propyl PC composition and dominance at either one or more An loci.
CBC-type and CBG-type PCs associate with chemotypes
To understand covariation of PC classes associated with PC synthase activity, we calculated the Spearman r correlation coefficient of 108 F2 plants using the proportional data of PCs that act as precursors (CBGA-CBG, CBGVA-CBGV), as well as products of THCAS (Δ9-THCA, Δ9-THC, Δ9-THCVA and Δ9-THCV), CBDAS (CBDA, CDA, CBDVA and CBDV) and oxidative pericyclic cyclisation (CBCA, CBC and CBCVA) (Fig. 4a, Supplementary Table S2). Concurrent with the co-segregation of Δ9-THCtot/CBDtot and Δ9-THCVtot/CBDVtot ratios among chemotypes I, II and III, strong positive correlations were observed for propyl/pentyl PC homologues of Δ9-THC(A) and Δ9-THCV(A) (Spearman r ≥ 0.72, P < 0.001), as well as for CBD(A) and CBDV(A) (Spearman r ≥ 0.62, P < 0.001) (Fig. 4b, Supplementary Table S2).
Covariation of PC proportions among F2 progeny (n = 108). (a) Heatmap of Spearman r correlation coefficient. Colour indicates correlation coefficient: blue indicates negative correlation and red indicates positive correlation. (b) Comparison of CBCA and CBCVA proportions among chemotypes I, II and III. (c) Comparison of CBGA and CBGVA proportions among chemotypes I, II and III.
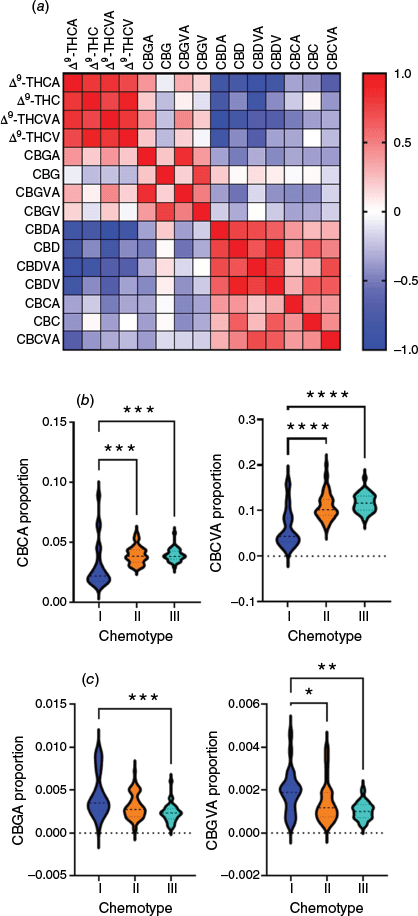
Unexpectedly, patterns of covariation were broadly identified with individual CBC(V)-type PCs, with r indicating a positive trend for individual CBC(V)/CBD(V)-type PCs and a negative or inverse trend for individual CBC(V)/Δ9-THC(V)-type PCs (Fig. 4a). This was most obvious for CBCVA/CBDVA (Spearman r 0.71, P = 7.13 × 10−18) and CBCVA/Δ9-THCA (Spearman r −0.68, P = 1.48 × 10−5) (Fig. 4a), which may indicate changes in the product specificity of CBDAS as compared with THCAS. To examine this, we compared the proportions of CBCVA and CBCA among chemotypes and observed a ~2-fold increase in CBC(V)A among chemotypes II and III, as compared with THCAS-associated chemotype I plants (Kruskal–Wallis one-way, P < 0.0001, Dunn’s test, P < 0.05) (Fig. 4b).
Unlike the inheritance of THCAS and CBDAS, a fixed non-allelic C locus is proposed for CBCA synthase (CBCAS), a third oxidoreductase isolated from the young leaves of cannabis that catalyses the oxidative pericyclic cyclisation of CBG(V)A, forming CBC(V)A.34,35 While CBC(V)A maxima and presumably CBCAS activity are reached at the juvenile stages of plant development, in vitro enzyme assays using recombinant THCAS and CBDAS indicate that both of these enzymes are capable of forming small amounts of CBCA, with product specificity towards CBCA being pH-dependant (>pH 6 and <6 for THCAS and CBDAS respectively).17 Changes in the product specificity of THCAS and CBDAS within the microenvironment of the trichome secretory cavity, where this reaction takes place,36 could partially account for the lower proportions of CBC-type PCs among the mature flowers of chemotype I THCAS expressing plants.
Discrete patterns of covariation were also observed for the acid forms of CBG(V)-type PCs, with r indicating a positive trend for CBG(V)A-/Δ9-THC(V)A-types and a negative or inverse trend for CBG(V)A-/CBD(V)A-types (Fig. 4a). This could potentially indicate a reduction in the enzymatic efficiency of THCAS, leading to an excess of CBG(V)A among THCAS-only expressing chemotype I plants, which is consistent with the reported lower catalytic efficiency of THCAS (kcat/Km = 1382 M−1 s−1) as compared with CBDAS (1492 M−1 s−1).35 Comparisons between Δ9-THC- and CBD-type chemotypes confirmed this trend, with small but measurable increases in CBG(V)A proportions in chemotype I plants as compared with chemotypes II and III (Kruskal–Wallis one-way, P < 0.005, Dunn’s test, P < 0.05) (Fig. 4c).
Untargeted metabolomic analysis
Deconvolution of the raw MS data was carried out on the F2 dataset using the software Compound Discoverer (CD, ver. 3.3, ThermoFisher Scientific, see https://www.thermofisher.com/au/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/compound-discoverer-software.html). Out of the 24,166 compounds detected, 10,366 were putatively annotated, which represented 42.89% of the total compounds (Supplementary Table S3). The complexity of the metabolite composition of cannabis is a challenge for correctly identifying the compounds present in the absence of a reference standard. Since we quantitatively analysed 19 PCs with reference standards, we were able to compare the MS2 spectral information and retention time to identify the correct PCs. Despite the high-scoring matches with databases, the annotation of multiple compounds assigned to the same compound name but with different retention times clearly shows the shortcomings in metabolomics studies, especially for a non-model plant such as Cannabis. Wishart et al.37 reported 6172 chemical entries in the freely available online database (Cannabis Compound Database, CCDB, see https://cannabisdatabase.ca/)37 and this database was also used for compound identification.
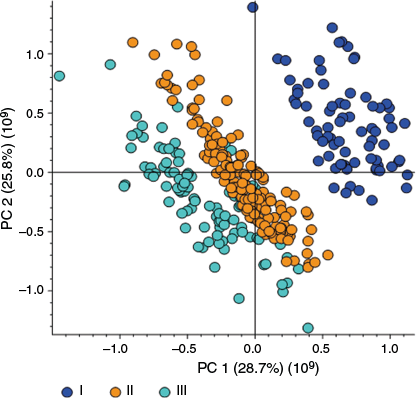
Principal component analysis (PCA) of the 24,166 detected compounds showed the separation of the three chemotypes in the scores plot of principal component 1 (PC1) v. principal component 2 (PC2), wherein 28.7 and 25.8% respectively accounted for the variability in the dataset (Fig. 5 and Supplementary Fig. S1). The PCA scores plot demonstrated a similar pattern to the classification shown in Fig. 3b. Samples classified as chemotype I (Δ9-THCA-dominant) clustered separately from chemotypes II and III samples. Chemotype II is closely related to chemotype III, which is CBDA-dominant, and the PCA scores plot showed the closeness of these two groupings. From the scores plot, five samples and the respective replicates (×3) classified as chemotype III were observed to cluster with chemotype II samples (Fig. 5). The effect of the 15 sample points was initially tested by excluding them in the analysis, but their exclusion did not affect the number of compounds in the list and thus, they were included in the analysis. The proximity of the grouping of chemotype II to chemotype III suggests a similarity in the biosynthetic origin of the metabolites in the two chemotypes. The PCA scores plot clearly shows differentiation among the samples between chemotype I and chemotypes II and III, and the two groupings that showed distinct separation (chemotypes I and III) were selected for further analysis in this study.
Principal component analysis (PCA scores plot of PC1 v. PC2) of 24,166 metabolites from flower tissues analysed by UHPLC-ESI-HRMS in positive ion mode. Samples were from an F2 cannabis population (n = 108).
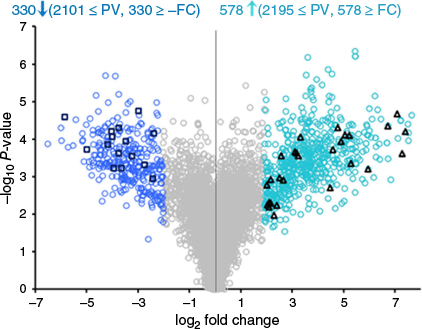
Differential analysis of chemotypes III and I (III v. I) indicated that 330 metabolites were significantly decreased or downregulated (P ≤ 0.05, log2 fold ≤ −2.0) and 578 metabolites were significantly increased or upregulated (P ≤ 0.05, log2 fold ≥ 2.0) (Fig. 6, Supplementary Tables S4 and S5 respectively). Of the 19 PCs that were analysed quantitatively, Δ9-THCAA (1), Δ9-THC (11), THCVA (6), CBNA (5) and CBVA (10) were significantly downregulated, and CBDA (2), CBD (12), CBDVA (7) and CBDV (7) were significantly upregulated. This result was not surprising since chemotype I is Δ9-THCA-dominant and chemotype III is CBDA-dominant. The downregulation of THCVA (and THCV) and upregulation of CBDVA (and CBDV) supported the homology in the biosynthesis of the (pentyl) C5 alkyl (THCA, CBDA) with the (propyl) C3 alkyl (THCVA, CBDVA) PCs.8
Differential analysis of compounds in chemotype III v. chemotype I. Samples were from an F2 cannabis population (n = 108). Blue circles and black squares represent the significantly downregulated compounds in chemotype I (P ≤ 0.05 and log2 fold change ≤ −2), and the teal circles and black triangles represent the significantly upregulated compounds in chemotype III (P ≤ 0.05 and log2 fold change ≥ 2). The black squares and black triangles respectively represent the significantly downregulated and significantly upregulated compounds that were putatively annotated (Tables 2 and 3).
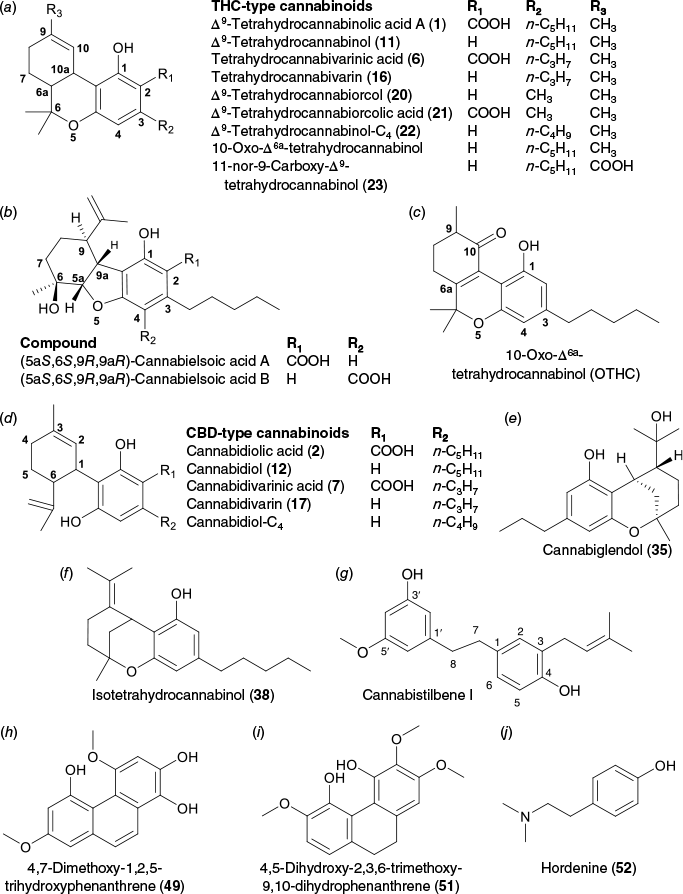
A mass search of the significantly downregulated and upregulated metabolites was carried out using the Cannabis Compound Database (CCDB, https://cannabisdatabase.ca/),37 which provided greater confidence in their putative annotation since the database contains information on metabolites found in Cannabis. After consensus evaluation of the CD and CCDB results (Supplementary Appendix S1), there were 15 significantly downregulated and 28 significantly upregulated metabolites with putative annotations as summarised in Tables 2 and 3 respectively. In addition to the five downregulated PCs (1, 5, 6, 11 and 16) and four upregulated PCs (2, 7, 12 and 17) that were quantitatively analysed, 20 other metabolites were putatively identified as PCs. These were Δ9-tetrahydrocannabiorcol (20) and its acid form Δ9-tetrahydrocannabiorcolic acid (21), Δ9-tetrahydrocannabinol-C4 (22), 11-nor-9-carboxy-tetrahydrocannabinol isomer (23), cannabielsoic acid isomers (25, 27 and 30), two isomers of 10-oxo-Δ6a-tetrahydrocannabinol (28 and 29), three CBDA isomers (33, 42 and 50), cannabiglendol-C3 (35), two isomers of cannabidiol-C4 (36 and 45), isotetrahydrocannabinol (38), CBCV isomer (39), CBCVA isomer (40) and two CBNA isomers (44 and 48). Compounds 20, 21, 22, 23, 25, 27, 28 and 29 were downregulated. Compounds 30, 33, 35, 36, 38, 39, 40, 42, 44, 45, 48 and 50 were upregulated.
| Compound name | Retention time (min) | Formula | Monoisotopic mass | [M + H]+ m/z | CCDB compound ID | |
|---|---|---|---|---|---|---|
| Δ9-Tetrahydrocannabinolic acid A (1) | 14.6 | C22H30O4 | 358.2144 | 359.2218 | CDB000016 | |
| Cannabinolic acid (5) | 14.0 | C22H26O4 | 354.1831 | 355.1906 | CDB000028 | |
| Tetrahydrocannabivarinic acid (6) | 13.1 | C20H26O4 | 330.1831 | 331.1904 | CDB000020 | |
| Δ9-Tetrahydrocannabinol (11) | 13.9 | C21H30O2 | 314.2246 | 315.2320 | CDB000001 | |
| Tetrahydrocannabivarin (16) | 12.2 | C19H26O2 | 286.1933 | 287.2006 | CDB000021 | |
| Δ9-Tetrahydrocannabiorcol (20) | 11.0 | C17H22O2 | 258.1620 | 259.1693 | CDB000416 | |
| Δ9-Tetrahydrocannabiorcolic acid (21) | 11.7 | C18H22O4 | 302.1518 | 303.1591 | CDB000415 | |
| Δ9-Tetrahydrocannabinol-C4 (22) | 13.1 | C20H28O2 | 300.2089 | 301.2163 | CDB000019 | |
| 11-nor-9-Carboxy-Δ9-tetrahydrocannabinol isomer (23) | 13.9 | C21H28O4 | 344.1988 | 345.2062 | CDB006353 | |
| Gibberellin A37 (24) | 11.2 | C20H26O5 | 346.1780 | 347.1855 | CDB005240 | |
| Cannabielsoic acid isomer (25) | 12.6 | C22H30O5 | 374.2093 | 375.2168 | CDB000423 | |
| Cannabistilbene I isomer (26) | 13.1 | C20H24O3 | 312.1725 | 313.1799 | CDB000477 | |
| Cannabielsoic acid isomer (27) | 12.4 | C22H30O5 | 374.2093 | 375.2167 | CDB000423 | |
| 10-Oxo-Δ6a-tetrahydrocannabinol isomer (28) | 14.0 | C21H28O3 | 328.2038 | 329.2113 | CDB000037 | |
| 10-Oxo-Δ6a-tetrahydrocannabinol isomer (29) | 14.6 | C21H28O3 | 328.2038 | 329.2112 | CDB000037 |
CCDB compound IDs are available in the Cannabis Compound Database (see https://cannabisdatabase.ca/).37
| Compound name | Retention time (min) | Formula | Monoisotopic mass | [M + H]+ m/z | CCDB compound ID | |
|---|---|---|---|---|---|---|
| Cannabidiolic acid (2) | 11.7 | C22H30O4 | 358.2144 | 359.2217 | CDB000010 | |
| Cannabidivarinic acid (7) | 10.8 | C20H26O4 | 330.1831 | 331.1904 | CDB000014 | |
| Cannabidiol (12) | 12.1 | C21H30O2 | 314.2246 | 315.2319 | CDB000002 | |
| Cannabidivarin (17) | 11.0 | C19H26O2 | 286.1933 | 287.2006 | CDB000015 | |
| Cannabielsoic acid isomer (30) | 9.6 | C22H30O5 | 374.2093 | 375.2166 | CDB000424 | |
| Cannabistilbene I isomer (31) | 10.8 | C20H24O3 | 312.1725 | 313.1798 | CDB000477 | |
| Gibberellin A44 (32) | 9.1 | C20H26O5 | 346.1780 | 347.1853 | CDB005051 | |
| Cannabidiolic acid isomer (33) | 10.2 | C22H30O4 | 358.2144 | 359.2217 | CDB000010 | |
| Gibberellin A53 (34) | 9.6 | C20H28O5 | 348.1937 | 349.2010 | CDB005241 | |
| Cannabiglendol (35) | 9.9 | C19H28O3 | 304.2038 | 305.2111 | CDB000446 | |
| Cannabidiol-C4 (36) | 11.5 | C20H28O2 | 300.2089 | 301.2162 | CDB000013 | |
| Cannabistilbene I isomer (37) | 9.8 | C20H24O3 | 312.1725 | 313.1798 | CDB000477 | |
| Isotetrahyrocannabinol (38) | 13.6 | C21H30O2 | 314.2246 | 315.2319 | CDB005834 | |
| Cannabichromevarin isomer (39) | 12.0 | C19H26O2 | 286.1933 | 287.2006 | CDB000009 | |
| Cannabichromevarinic acid isomer (40) | 12.8 | C20H26O4 | 330.1831 | 331.1904 | CDB000008 | |
| Cannabistilbene I isomer (41) | 11.7 | C20H24O3 | 312.1725 | 313.1798 | CDB000477 | |
| Cannabidiolic acid isomer (42) | 14.3 | C22H30O4 | 358.2144 | 359.2217 | CDB000023 | |
| Phenylacetaldehyde (43) | 3.3 | C8H8O | 120.0575 | 121.0648 | CDB004975 | |
| Cannabinolic acid isomer (44) | 9.3 | C22H26O4 | 354.1831 | 355.1904 | CDB000028 | |
| Cannabidiol-C4 (45) | 12.8 | C20H28O2 | 300.2089 | 301.2162 | CDB000013 | |
| 3,4-Dihydroxybenzaldehyde (46) | 6.0 | C7H6O3 | 138.0317 | 139.0390 | CDB006366 | |
| Epicatechin (47) | 6.0 | C15H14O6 | 290.0790 | 291.0863 | CDB004928 | |
| Cannabinolic acid isomer (48) | 10.7 | C22H26O4 | 354.1831 | 355.1904 | CDB000028 | |
| 4,7-Dimethoxy-1,2,5-trihydroxyphenanthrene (49) | 8.9 | C16H14O5 | 286.0841 | 287.0914 | CDB000748 | |
| Cannabidiolic acid isomer (50) | 13.0 | C22H30O4 | 358.2144 | 359.2217 | CDB000010 | |
| 4,5-Dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (51) | 9.4 | C17H18O5 | 302.1154 | 303.1227 | CDB000749 | |
| Hordenine (52) | 1.4 | C10H15NO | 165.1154 | 166.1226 | CDB000053 |
CCDB compound IDs are available in the Cannabis Compound Database (see https://cannabisdatabase.ca/).37
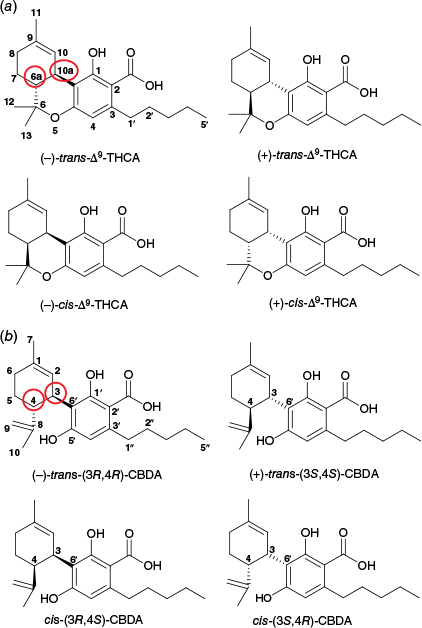
The downregulated metabolites Δ9-tetrahydrocannabiorcol (20, Δ9-THC-C1, C17H22O2, [M + H]+ m/z 259.1693), Δ9-tetrahydrocannabiorcolic acid (21, Δ9-THCA-C1, C18H22O4, [M + H]+ m/z 303.1591) and Δ9-tetrahydrocannabinol-C4 (22, Δ9-THC-C4, C20H28O2, [M + H]+ m/z 301.2163) belong to the Δ9-THC type of cannabinoids38; hence, it was not surprising that these compounds were also downregulated. Compounds 20 and 21 are homologues of Δ9-THC and Δ9-THCA respectively, with a methyl substituent at the C-3 position instead of a pentyl (Fig. 7a). Compound 22 is also a homologue of Δ9-THC that has a butyl substituent at the C-3 position (Fig. 7a). Compound 23 (C21H28O4, [M + H]+ m/z/345.2062) was annotated as 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH, Fig. 7a). This compound has been reported as a breakdown product of Δ9-THC in the human body after cannabis consumption,39 but this is the first report from the plant. Thus, compound 23 was presumed to be an isomer of 11-nor-9-carboxy-tetrahydrocannabinol. It is important to note that the major THC-type compound in Cannabis is (−)-trans-Δ9-THCAA (1), shortened to Δ9-THCA throughout this manuscript. There are four stereoisomers of Δ9-THCAA (and subsequently Δ9-THC)40,41 as shown in Fig. 8a, and these stereoisomers could be present in the plant as minor constituents.
Chemical structures of downregulated and upregulated compounds in cannabis. (a) THC-type cannabinoids. (b) Cannabielsoic acid (CBEA)-type compounds. (c) Oxo-cannabinoid compound. (d) CBD-type cannabinoids. (e) Iso-hydroxy cannabinoid compound with propyl side chain. (f) Iso-cannabinoid compound with pentyl side chain. (g) Stilbene-type compound. (h, i) Phenanthrene-type compounds. (j) Phenethylamine alkaloid compound.
Chemical structures of the stereoisomers of (a) Δ9-THCAA (1),40,41 and (b) CBDA (2),47 showing the configuration of the stereocentres (circled in red).
Compounds 25 and 27, which eluted at respectively 11.0 and 11.7 min, were both annotated as cannabielsoic acid (CBEA-C5, C22H30O5, [M + H]+ m/z 375.2167) and belong to the CBE-type cannabinoids.38 There are two isomers of CBEA-C5 reported, CBEA-C5 A and CBEA-C5 B, that are differentiated by the position of the carboxyl substituent at C-2 in the former and at C-4 in the latter (Fig. 7b). There was doubt whether these compounds are natural products since these can be formed by either oxidation or pyrolysis of CBDA,38,42,43 but cannabielsoic acids A and B and their respective methyl esters were isolated from hashish44 and recently, Parveen et al.45 reported the identification of CBEA-C5 isomers from a hemp extract. Compounds 28 and 29, which eluted at respectively 14.0 and 14.7 min, both had a molecular formula of C21H28O3 ([M + H]+ m/z 329.2112) and were annotated as 10-oxo-Δ6a-tetrahydrocannabinol (OTHC), which was reported from a cannabis extract by Friedrich-Fiechtl and Spiteller.46 OTHC is an oxidised derivative of Δ6-THC that has a ketone group at the C-10 position (Fig. 7c).37
In addition to the upregulated CBDA (2), there were three metabolites, 33, 42 and 50, with the same molecular formula as CBDA (C20H28O5, [M + H]+ m/z 359.2219) and with similar MS profiles that eluted at respectively 10.2, 13.0 and 14.3 min (Table 3). These three metabolites were presumed to be CBDA isomers. Similar to Δ9-THCA, CBDA has four stereoisomers (Fig. 8b) and the dominant form in the plant is the (−)-trans-CBDA (2).47
Two metabolites, 36 and 45, with a molecular formula of C20H28O2 ([M + H]+ m/z 301.2163) and retention times of respectively 11.5 and 12.8 min, were putatively annotated as cannabidiol-C4 (CBD-C4, Fig. 7d) and would most probably be isomers of each other. Compound 35 had a molecular formula of C19H28O3 ([M + H]+ m/z 305.2113) and was annotated as cannabiglendol (Fig. 7e). Compound 38, which eluted at 13.6 min and had a molecular formula of C19H28O3 ([M + H]+ m/z 315.2320), was annotated as isotetrahydrocannabinol or Δ4-iso-tetrahydrocannabinol (Fig. 7f). Compound 39, with retention time of 12.0 min and a molecular formula of C19H26O2 ([M + H]+ m/z 287.2005), was annotated as cannabichromevarin (CBCV). However, this did not match the 13.0 min retention time of the reference standard in our in-house database and thus was assigned to be a CBCV isomer (Table 3). Compound 40, with a molecular formula of C20H26O4 ([M + H]+ m/z 331.1905) and retention time of 12.8 min, was initially annotated as CBCVA, but from our quantitative analysis, CBCVA (8) eluted at 13.6 min. The similarity of the MS profiles of these two compounds suggested that compound 40 is a CBCVA isomer. Compound 42 had the same molecular formula as the downregulated compounds 28 and 29 and was thus annotated to be another OTHC isomer. Compounds 44 and 48 that eluted at respectively 9.3 and 10.7 min had the same molecular formula and similar MS profile as CBNA (5) and were deemed to be CBNA isomers.
Several non-PC metabolites were putatively identified. Compound 26, with retention time of 13.1 min, had a molecular formula of C20H24O3 ([M + H]+ m/z 313.1799) and was annotated as cannabistilbene I (Fig. 7g), which is one of the 12 known dihydrostilbenes in cannabis.48 Three upregulated metabolites, 31, 37 and 41 with retention times of respectively 10.8, 9.8 and 11.7 min, were annotated similarly, and mass spectral information suggested these four compounds to be cannabistilbene I isomers.
A downregulated metabolite at 11.2 min had a molecular formula of C20H26O5 ([M + H]+ m/z 347.1855) (Table 2) and was putatively annotated as a gibberellin that could either be one of the isomers gibberellin A37 or gibberellin A44. Similarly, an upregulated metabolite, compound 32, that eluted at 9.1 min had the same mass and was also putatively annotated either as gibberellin A37 or gibberellin A44 (Table 3). Another upregulated metabolite, compound 34, with retention time of 9.6 min and molecular formula of C20H28O5 ([M + H]+ m/z 349.2011), was annotated as gibberellin A53 (Table 3). Gibberellins (GAs) are plant hormones that play various roles in plant growth and development49 and they have been reported to affect inflorescence development in cannabis.50,51
Compound 49, which eluted at 8.9 min, was putatively annotated as 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene (Fig. 7h) with a molecular formula of C16H14O5 ([M + H]+ m/z 287.0915), and compound 51, eluting at 9.4 min, was annotated as 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (Fig. 7i) with a molecular formula of C17H18O5 ([M + H]+ m/z 303.1228) (Table 3). Compound 52, which eluted at 1.4 min with [M + H]+ m/z 166.1226 indicating that it was a nitrogen-bearing metabolite, was annotated as the phenethylamine alkaloid hordenine (Fig. 7j) with a molecular formula of C10H15NO (Table 3, Supplementary Table S5). A compound that eluted at 3.30 min (43) with molecular formula of C8H8O ([M + H]+ m/z 121.0645) was annotated as phenylacetaldehyde (Table 3, Supplementary Table S5), which is commonly found in plants.52
Two closely eluting metabolites at 6.0 min were deconvoluted with [M + H]+ m/z 139.0389 and [M + H]+ m/z 291.0864 for compounds 46 and 47 respectively (Table 3, Supplementary Table S4). Compound 46 was putatively annotated as protocatechuic aldehyde (or 3,4-dihydroxybenzaldehyde) with amolecular formula of C8H8O, and compound 47 was annotated as epicatechin, which is commonly found in plants, including cannabis.53,54
Among the annotated metabolites, the main differentiation between the two chemotypes I and III are the major PCs Δ9-THCA/THC and CBDA/CBD and their suite of THC-type cannabinoids and CBD-type cannabinoids respectively. However, it is interesting to note that among the upregulated metabolites (chemotypes III v. I), eight compounds were non-PCs and included two phenanthrenes, two phenolics, two aldehydes, one phenyl ketone and one alkaloid (Table 3). This suggested that there could be non-PC biomarkers that can be used to differentiate cannabis chemotypes.
Conclusion
While much of the chemical variability among chemotypes can be explained by changes in the proportions of Δ9-THC-/CBD-type PCs, we were able to demonstrate metabolite coupling and co-variation of minor PCs with chemotypes I and III. These observations are not insignificant for the breeding of cannabis chemovars, as the pharmacological effects of cannabis, when consumed as a whole plant extract or crude herbal preparation, are ultimately determined by the sum of all PCs that are produced. Despite the limited range of PC synthase heterogeneity reported across the extant gene pool,55 the engineering of PC synthases, using techniques such as site-directed mutagenesis, may offer novel opportunities to improve product specificity and enzymatic efficiency, and therefore the efficacy of cannabis-based medicines.17 The assessment of chemotypic segregation among Δ9-THC/CBD filial populations also allowed us to identify putative biomarkers, which, when validated across a broader representation of germplasm, may allow for the discrimination of chemotypes without prior knowledge of the PC constituents of plants.
Experimental
All research activities, including the procurement of germplasm and cultivation of medicinal cannabis, were carried out under relevant Australian Federal and State Government licenses, permits and authorisations.
Genetic materials and cultivation of medicinal cannabis
Cannabis sativa L. (industrial hemp) parental seed accessions were as described by Welling et al.2,22 A single female genotype (P1; MW6-15) predominant in Δ9-THCA and Δ9-THCVA was crossed with a single female genotype (P2; MW12-3) predominant in CBDA. Biparental female crosses and the generation of feminised F1 hybrid and F2 populations were achieved by means of silver thiosulfate (STS)-induced staminate flowering.56 Three successive treatments of 3 mM of STS on newly forming pistillate inflorescences of a female clone of MW12-3 at 0, 7 and 14 days post-induction to short-day photoperiod (12 h of light/12 h of dark) allowed development of fertile male staminate flowers, from which viable pollen was collected and manually applied to a single female clone of MW6-15. One of two feminised clones derived from a single MW6-15 × MW12-3 F1 hybrid seed was masculinised as above, and the pollen was manually applied to the second F1 clone, allowing for the selfing of a single F1 genotype and generation of a feminised F2 population.
Parental and F1 plants were cultivated from cuttings in a controlled environment room as described by Welling et al.2,57 with clonal material derived from nodal cuttings of parental and F1 mother plants using methods described by Jost et al.58
F2 plants were grown from seed. The seeds were sterilised by agitation in 70% (v/v) ethanol (2 min) and 1% (v/v) sodium hypochlorite (10 min), then incubated in the dark in 1% (v/v) hydrogen peroxide at room temperature for 24 h. Seeds were sown as above in Rotterdam 70:30 coco-perlite soil media (Rotterdam Gardening, Netherlands). Seedlings were transplanted first into 500-mL pots (12 days post-sowing) and then into 4.5-L pots (4 weeks post-sowing) containing Rotterdam 70:30 coco-perlite soil media. Plants were grown in a climate-controlled glasshouse equipped with blackout screen and Oreon Grow Light 3.0 LED lighting (12 h of light at 28°C/12 h of dark at 22°C with 55% humidity), with an average intensity of ~250 µmol m−2 s−1 (400–900 µmol m−2 s−1 as measured at the apical inflorescence). Nutrient regimes followed those described by Welling et al.57 Plants were grown for 6 weeks after terminal flowering, defined as the differentiation of the shoot apex into a terminal flower,59 and floral samples from the top 40 cm of each individual plant were harvested.
Reagents and standards
All cannabinoid certified reference materials were purchased from Novachem Pty Ltd (Melbourne, Vic., Australia) as distributor for Cerilliant Corporation (Round Rock, TX, USA). Reagent and analytical standard preparation were as reported previously.2
UHPLC-ESI-HRMS analysis
Floral samples from the F2 plants (n = 108) were air-dried under forced ventilation at room temperature for 14 days (~78–80% moisture loss), then dried in a Thermo Scientific Heratherm gravity convection oven at 40°C for 72 h. Sample preparation and UHPLC-ESI-HRMS analysis were as reported previously2 with minor modifications. Briefly, analysis was carried out on a ThermoFisher Vanquish Flex UHPLC system with solvent degasser, quaternary pump, temperature-controlled sampler/auto injector and column compartment, and photodiode array detector (PDA) coupled to an Orbitrap IDX Tribrid high-resolution mass spectrometer (ThermoFisher Scientific Inc., Waltham, MA, USA). Chromatographic separation was performed using a Phenomenex Kinetex C18 column, 1.7 μm, 150 mm × 2.1 mm (Phenomenex Australia Pty Ltd, Lane Cove, Sydney, NSW, Australia). Mobile phase A was water with 0.1% (v/v) formic acid and mobile phase B was acetonitrile with 0.1% (v/v) formic acid, and the following gradient was used: 10% B, 0–2 min; 10–40% B, 2–3 min; 40% B, 3–5 min; 40–80% B, 5–6 min; 80% B, 6–9 min; 80–90% B, 9–11 min; 90–100% B, 11–12 min; 100% B, 12–15 min; 100–10% B, 15–16 min; and 10% B from 16–20 min.
The mass spectrometer was operated using a HESI interface in positive mode. For single and tandem mass spectrometry (MS and MS2), the orbitrap was the mass analyser used. Pierce FlexMix calibration solution was used to calibrate the mass spectrometer prior to data acquisition, and the internal mass calibrant fluoranthene (Easy-IC) was activated for real-time mass calibration during data acquisition. Xcalibur software (ver. 4.6, ThermoFisher Scientific Inc., see https://www.thermofisher.com/au/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/lc-ms-data-acquisition-software.html) was used for data acquisition.
Data processing and statistical analysis
Full MS data were processed using Trace Finder (ver. 5.1, ThermoFisher Scientific Inc., Waltham, MA, USA) for quantitative analysis. Peak identity was determined by a comparison of retention time and MS spectra with reference standards and integration of extracted ion peaks. The concentration of each analyte was determined by interpolation from standard calibration curves generated in MS Excel and results were expressed as average concentration ± standard deviation. Results were plotted using GraphPad Prism (ver. 10.5, GraphPad Software, Boston, MA, USA, see https://www.graphpad.com/features).
The high-resolution accurate mass (HRAM) data were further processed using CD software with the workflow set up for untargeted metabolomics with statistics and detection of unknown compounds using databases. Libraries used were mzCloud (see https://www.mzcloud.org/), ChemSpider (see https://www.chemspider.com/), BioCyc (see https://biocyc.org/) and Metabolika pathways, which is a module within CD for pathway analysis. There were 24,166 compounds detected, grouped by molecular weight and retention time across all the 108 sample extracts analysed, after applying a filter set that excluded peaks in the first and last 0.5 min of the chromatogram and a maximum compound peak area of less than 50,000 (Supplementary Table S2). Within the CD software, the observed m/z values and predicted elemental composition were matched with the online spectral library mzCloud and databases ChemSpider, BioCyc and Metabolika pathways, and the annotation made use of the algorithm ‘mzLogic’ within CD. There were several instances of one annotation for several compounds wherein one compound name was putatively annotated to multiple entries in the list that was misleading and thus required a lot of manual interrogation. For example, there were 67 compounds in the list putatively annotated as CBDA (2) with some annotations based on high scores from spectral library match from mzCloud. This inferred the presence of multiple isomers for CBDA and there were other metabolites in the list that suggested several compounds with molecular isomers.
Differential analysis between chemotype III and chemotype I samples was also carried out in CD software. The significantly downregulated and upregulated metabolites were filtered to only those that had MS2 data, and the list was reduced to 114 metabolites (34.5% of downregulated) and 98 metabolites (17% of upregulated) respectively. A mass search (m/z values) for metabolites with MS2 data was carried out using the Cannabis Compound Database (CCDB, see https://cannabisdatabase.ca/).37 The putative annotations from CD were compared with the results from CCDB and although there were some compounds that had the same annotation between the two results, there were more annotation hits from the CCDB for compounds that have been reported in cannabis.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
The authors acknowledge the generous support of the Australian Research Council (ARC) Linkage, Infrastructure, Equipment and Facilities grant scheme (LE200100117, to A. Bacic), the Ian Potter Foundation (grant number 31110299, to A. Bacic) and La Trobe University infrastructure funds for contributing to the purchase of the mass spectrometers in the La Trobe University Proteomics and Metabolomics Research Platform. This research was funded by the ARC through the Industrial Transformation Research Hub for Medicinal Agriculture (IH180100006, to A. Bacic, M. S. Doblin, K. L. Johnson and A. R. Gendall) and a Linkage Program grant (LP160101317, to A. Bacic and M. S. Doblin), with La Trobe University and business partners including Cannoperations Pty Ltd. L. Steel and G. I. Senevirathne are funded through the ARC Research Hub for Medicinal Agriculture Graduate Research Scholarship and the La Trobe University Full Fee Remission PhD Research Scholarship.
References
1 Small E, Cronquist A. A practical and natural taxonomy for Cannabis. Taxon 1976; 25(4): 405-435.
| Crossref | Google Scholar |
2 Welling MT, Deseo MA, Bacic A, Doblin MS. Untargeted metabolomic analyses reveal chemical complexity of dioecious Cannabis flowers. Aust J Chem 2021; 74(6): 463-479.
| Crossref | Google Scholar |
3 Grassa CJ, Weiblen GD, Wenger JP, Dabney C, Poplawski SG, Timothy Motley S, Michael TP, Schwartz CJ. A new Cannabis genome assembly associates elevated cannabidiol (CBD) with hemp introgressed into marijuana. New Phytol 2021; 230(4): 1665-1679.
| Crossref | Google Scholar | PubMed |
4 Bradshaw RHW, Coxon P, Greig JRA, Hall AR. New fossil evidence for the past cultivation and processing of hemp (Cannabis sativa L.) in eastern England. New Phytol 1981; 89(3): 503-510.
| Crossref | Google Scholar |
5 Staginnus C, Zörntlein S, de Meijer E. A PCR marker linked to a THCA synthase polymorphism is a reliable tool to discriminate potentially THC-rich plants of Cannabis sativa L. J Forensic Sci 2014; 59(4): 919-926.
| Crossref | Google Scholar | PubMed |
6 Livingston SJ, Rensing KH, Page JE, Samuels AL. A polarized supercell produces specialized metabolites in cannabis trichomes. Curr Biol 2022; 32(18): 4040-4047.e4.
| Crossref | Google Scholar | PubMed |
7 Stout JM, Boubakir Z, Ambrose SJ, Purves RW, Page JE. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J 2012; 71(3): 353-365.
| Crossref | Google Scholar | PubMed |
8 Welling MT, Deseo MA, Bacic A, Doblin MS. Biosynthetic origins of unusual cannabimimetic phytocannabinoids in Cannabis sativa L: a review. Phytochemistry 2022; 201: 113282.
| Crossref | Google Scholar | PubMed |
9 Zagzoog A, Mohamed KA, Kim HJJ, Kim ED, Frank CS, Black T, Jadhav PD, Holbrook LA, Laprairie RB. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci Rep 2020; 10(1): 20405.
| Crossref | Google Scholar | PubMed |
10 Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, de Novellis V, Di Marzo V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol 2011; 162(3): 584-596.
| Crossref | Google Scholar | PubMed |
11 Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study Group. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376(21): 2011-2020.
| Crossref | Google Scholar | PubMed |
12 Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995; 10(2): 89-97.
| Crossref | Google Scholar | PubMed |
13 Moreno-Sanz G, Madiedo A, Lynskey M, Brown MRD. “Flower power”: controlled inhalation of THC-predominant Cannabis flos improves health-related quality of life and symptoms of chronic pain and anxiety in eligible UK patients. Biomedicines 2022; 10(10): 2576.
| Crossref | Google Scholar | PubMed |
14 Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Wada Y, Shoyama Y, Taura F. The gene controlling marijuana psychoactivity: molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J Biol Chem 2004; 279(38): 39767-39774.
| Crossref | Google Scholar | PubMed |
15 Taura F, Sirikantaramas S, Shoyama Y, Yoshikai K, Shoyama Y, Morimoto S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett 2007; 581(16): 2929-2934.
| Crossref | Google Scholar | PubMed |
16 Shoyama Y, Tamada T, Kurihara K, Takeuchi A, Taura F, Arai S, Blaber M, Shoyama Y, Morimoto S, Kuroki R. Structure and function of ∆1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of Cannabis sativa. J Mol Biol 2012; 423(1): 96-105.
| Crossref | Google Scholar | PubMed |
17 Zirpel B, Kayser O, Stehle F. Elucidation of structure-function relationship of THCA and CBDA synthase from Cannabis sativa L. J Biotechnol 2018; 284: 17-26.
| Crossref | Google Scholar | PubMed |
18 de Meijer EP, Bagatta M, Carboni A, Crucitti P, Moliterni VM, Ranalli P, Mandolino G. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003; 163(1): 335-346.
| Crossref | Google Scholar | PubMed |
19 de Meijer EPM, Hammond KM. The inheritance of chemical phenotype in Cannabis sativa L. (II): cannabigerol predominant plants. Euphytica 2005; 145(1): 189-198.
| Crossref | Google Scholar |
20 de Meijer EPM, Hammond KM, Sutton A. The inheritance of chemical phenotype in Cannabis sativa L. (IV): cannabinoid-free plants. Euphytica 2009; 168(1): 95-112.
| Crossref | Google Scholar |
21 Cerrato A, Citti C, Cannazza G, Capriotti AL, Cavaliere C, Grassi G, Marini F, Montone CM, Paris R, Piovesana S, Laganà A. Phytocannabinomics: untargeted metabolomics as a tool for Cannabis chemovar differentiation. Talanta 2021; 230: 122313.
| Crossref | Google Scholar | PubMed |
22 Welling MT, Liu L, Raymond CA, Ansari O, King GJ. Developmental plasticity of the major alkyl cannabinoid chemotypes in a diverse Cannabis genetic resource collection. Front Plant Sci 2018; 9: 1510.
| Crossref | Google Scholar | PubMed |
23 Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, Etxebarria N, Usobiaga A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod 2016; 79(2): 324-331.
| Crossref | Google Scholar | PubMed |
24 Weiblen GD, Wenger JP, Craft KJ, ElSohly MA, Mehmedic Z, Treiber EL, Marks MD. Gene duplication and divergence affecting drug content in Cannabis sativa. New Phytol 2015; 208(4): 1241-1250.
| Crossref | Google Scholar | PubMed |
25 Welling MT, Liu L, Raymond CA, Kretzschmar T, Ansari O, King GJ. Complex patterns of cannabinoid alkyl side-chain inheritance in Cannabis. Sci Rep 2019; 9(1): 11421.
| Crossref | Google Scholar | PubMed |
26 Jin D, Henry P, Shan J, Chen J. Identification of phenotypic characteristics in three chemotype categories in the genus Cannabis. Hortic Sci 2021; 56(4): 481-490.
| Crossref | Google Scholar |
27 McKernan KJ, Helbert Y, Kane LT, Ebling H, Zhang L, Liu B, Eaton Z, McLaughlin S, Kingan S, Baybayan P, Concepcion G, Jordan M, Riva A, Barbazuk W, Harkins T. Sequence and annotation of 42 cannabis genomes reveals extensive copy number variation in cannabinoid synthesis and pathogen resistance genes. BioRxiv 2020; 2020: 2020.01.03.894428 [Preprint, published 5 January 2020].
| Crossref | Google Scholar |
28 Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot 2004; 91(6): 966-975.
| Crossref | Google Scholar | PubMed |
29 Jin D, Henry P, Shan J, Chen J. Identification of chemotypic markers in three chemotype categories of Cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark, and roots. Front Plant Sci 2021; 12: 699530.
| Crossref | Google Scholar | PubMed |
30 Chadeau-Hyam M, Ebbels TMD, Brown IJ, Chan Q, Stamler J, Huang CC, Daviglus ML, Ueshima H, Zhao L, Holmes E, Nicholson JK, Elliott P, De Iorio M. Metabolic profiling and the metabolome-wide association study: significance level for biomarker identification. J Proteome Res 2010; 9(9): 4620-4627.
| Crossref | Google Scholar | PubMed |
31 Luo X, Reiter MA, d’Espaux L, Wong J, Denby CM, Lechner A, Zhang Y, Grzybowski AT, Harth S, Lin W, Lee H, Yu C, Shin J, Deng K, Benites VT, Wang G, Baidoo EEK, Chen Y, Dev I, Petzold CJ, Keasling JD. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019; 567(7746): 123-126.
| Crossref | Google Scholar | PubMed |
32 Dussy FE, Hamberg C, Luginbühl M, Schwerzmann T, Briellmann TA. Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis products. Forensic Sci Int 2005; 149(1): 3-10.
| Crossref | Google Scholar | PubMed |
33 de Meijer EPM, Hammond KM. The inheritance of chemical phenotype in Cannabis sativa L. (V): regulation of the propyl-/pentyl cannabinoid ratio, completion of a genetic model. Euphytica 2016; 210(2): 291-307.
| Crossref | Google Scholar |
34 de Meijer EPM, Hammond KM, Micheler M. The inheritance of chemical phenotype in Cannabis sativa L. (III): variation in cannabichromene proportion. Euphytica 2009; 165(2): 293-311.
| Crossref | Google Scholar |
35 Morimoto S, Komatsu K, Taura F, Shoyama Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998; 49(6): 1525-1529.
| Crossref | Google Scholar | PubMed |
36 Rodziewicz P, Loroch S, Marczak Ł, Sickmann A, Kayser O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci 2019; 284: 108-116.
| Crossref | Google Scholar | PubMed |
37 Wishart DS, Hiebert-Giesbrecht M, Inchehborouni G, Cao X, Guo AC, LeVatte MA, Torres-Calzada C, Gautam V, Johnson M, Liigand J, Wang F, Zahraei S, Bhumireddy S, Wang Y, Zheng J, Mandal R, Dyck JRB. Chemical composition of commercial cannabis. J Agric Food Chem 2024; 72(25): 14099-14113.
| Crossref | Google Scholar | PubMed |
38 ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci 2005; 78(5): 539-548.
| Crossref | Google Scholar | PubMed |
39 Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA. Δ9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem 2009; 55(12): 2180-2189.
| Crossref | Google Scholar | PubMed |
40 Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep 2016; 33(12): 1357-1392.
| Crossref | Google Scholar | PubMed |
41 Tolomeo F, Russo F, Kaczorova D, Vandelli MA, Biagini G, Laganà A, Capriotti AL, Paris R, Fulvio F, Carbone L, Perrone E, Gigli G, Cannazza G, Citti C. Cis-Δ9-tetrahydrocannabinolic acid occurrence in Cannabis sativa L. J Pharm Biomed Anal 2022; 219: 114958.
| Crossref | Google Scholar | PubMed |
42 Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod 1980; 43(2): 169-234.
| Crossref | Google Scholar | PubMed |
43 Seo C, Jeong M, Lee S, Kim EJ, Rho S, Cho M, Lee YS, Hong J. Thermal decarboxylation of acidic cannabinoids in Cannabis species: identification of transformed cannabinoids by UHPLC-Q/TOF–MS. J Anal Sci Technol 2022; 13(1): 42.
| Crossref | Google Scholar |
44 Shani A, Mechoulam R. Cannabielsoic acids: Isolation and synthesis by a novel oxidative cyclization. Tetrahedron 1974; 30(15): 2437-2446.
| Crossref | Google Scholar |
45 Parveen I, Allen NR, Wonfor RE, Al-Fadhli AA, Forde-Thomas JE, Giles JL, Walton RT, Threadgill MD, Nash DM. Characterisation of components of an extract of hemp and preliminary assessment of anti-inflammatory activity in an ex vivo model of bovine endometritis. S Afr J Bot 2025; 180: 254-264.
| Crossref | Google Scholar |
46 Friedrich-Fiechtl J, Spiteller G. Neue cannabinoide – 1. Tetrahedron 1975; 31(6): 479-487 [In German with abstract in German and English].
| Crossref | Google Scholar |
47 Rao VK, Lewis-Bakker MM, Wasilewski E, Clarke HA, Kotra LP. Stereoisomers of cannabidiols and their pharmacological activities – a potentially novel direction for cannabinoids. Biorg Med Chem 2025; 117: 118019.
| Crossref | Google Scholar |
48 Radwan MM, Chandra S, Gul S, ElSohly MA. Cannabinoids, phenolics, terpenes and alkaloids of Cannabis. Molecules 2021; 26(9): 2774.
| Crossref | Google Scholar | PubMed |
49 Castro-Camba R, Sánchez C, Vidal N, Vielba JM. Plant development and crop yield: the role of gibberellins. Plants (Basel) 2022; 11(19): 2650.
| Crossref | Google Scholar | PubMed |
50 Alter H, Sade Y, Sood A, Carmeli-Weissberg M, Shaya F, Kamenetsky-Goldstein R, Bernstein N, Spitzer-Rimon B. Inflorescence development in female cannabis plants is mediated by photoperiod and gibberellin. Hortic Res 2024; 11(11): 245.
| Crossref | Google Scholar | PubMed |
51 Mansouri H, Asrar Z, Mehrabani M. Effects of gibberellic acid on primary terpenoids and delta-tetrahydrocannabinol in Cannabis sativa at flowering stage. J Integr Plant Biol 2009; 51(6): 553-561.
| Crossref | Google Scholar | PubMed |
52 Günther J, Halitschke R, Gershenzon J, Burow M. Heterologous expression of PtAAS1 reveals the metabolic potential of the common plant metabolite phenylacetaldehyde for auxin synthesis in planta. Physiol Plant 2023; 175(6): e14078.
| Crossref | Google Scholar | PubMed |
53 Izzo L, Castaldo L, Narváez A, Graziani G, Gaspari A, Rodríguez-Carrasco Y, Ritieni A. Analysis of phenolic compounds in commercial Cannabis sativa L. inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020; 25(3): 631.
| Crossref | Google Scholar | PubMed |
54 Curtasu MV, Pallesen BE, No̷rskov NP. Quantitative distribution of polyphenolic compounds during plant development in five varieties of organic hemp (Cannabis sativa L.). J Agric Food Chem 2025; 73(26): 16359-16369.
| Crossref | Google Scholar | PubMed |
55 Lynch RC, Padgitt-Cobb LK, Garfinkel AR, Knaus BJ, Hartwick NT, Allsing N, Aylward A, Bentz PC, Carey SB, Mamerto A, Kitony JK, Colt K, Murray ER, Duong T, Chen HI, Trippe A, Harkess A, Crawford S, Vining K, Michael TP. Domesticated cannabinoid synthases amid a wild mosaic cannabis pangenome. Nature 2025; 643: 1001-1010.
| Crossref | Google Scholar | PubMed |
56 Mohan Ram HY, Sett R. Induction of fertile male flowers in genetically female Cannabis sativa plants by silver nitrate and silver thiosulphate anionic complex. Theor Appl Genet 1982; 62(4): 369-375.
| Crossref | Google Scholar | PubMed |
57 Welling MT, Deseo MA, O’Brien M, Clifton J, Bacic A, Doblin MS. Metabolomic analysis of methyl jasmonate treatment on phytocannabinoid production in Cannabis sativa. Front Plant Sci 2023; 14: 1110144.
| Crossref | Google Scholar | PubMed |
58 Jost R, Berkowitz O, Pegg A, Hurgobin B, Tamiru-Oli M, Welling MT, Deseo MA, Noorda H, Brugliera F, Lewsey MG, Doblin MS, Bacic A, Whelan J. Sink strength, nutrient allocation, cannabinoid yield, and associated transcript profiles vary in two drug-type Cannabis chemovars. J Exp Bot 2025; 76(1): 152-174.
| Crossref | Google Scholar | PubMed |
59 Steel L, Welling M, Ristevski N, Johnson K, Gendall A. Comparative genomics of flowering behavior in Cannabis sativa. Front Plant Sci 2023; 14: 1227898.
| Crossref | Google Scholar | PubMed |


