Australian Journal of Chemistry
Volume 78
Number 8 2025
Undergraduate students should appreciate that chemical understanding is not static; what we teach is our ‘best guess’ to explain observables. Textbooks frequently conflate (and often incorrectly describe) the concept of the nucleus, inferred by Rutherford from Marsden’s ‘gold foil’ experiment, with the model of the nucleus, containing protons and neutrons, despite nearly 25 years between these advances. We argue that an opportunity is lost in the teaching of chemistry, to illustrate to students the incremental progress in chemistry and that the ‘wrong’ explanation can be very useful. (Image credit: © Sidney Harris, originally appearing in American Scientist in 1977, reproduced with permission.)
This mini-review highlights tactics to engineer MCoTI-II, a cyclic cystine-knot peptide natural product, into next-generation therapeutics and diagnostics. Through both rational modification and genetically encoded selection platforms, variants of the MCoTI-II cyclotide have been developed for a diverse range of potential biomedical applications. (Image credit: Sven Ullrich.)
This article belongs to the collection: 70th Birthday tribute to Prof. David Craik.
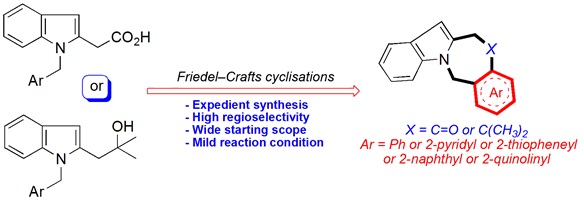
Indole fused N-heterocycles form the structural core of a large number of natural products and pharmaceuticals. These properties make them exciting targets for synthetic chemists, with a wide range of synthetic methodologies reported. However, these often have a number of drawbacks, including cost, scalability and low yields. Herein, we explore an efficient construction of benzo-, pyrido-, thieno-, naphtho-fused azepinoindolones and indolo-azepinoquinolinones from readily available starting materials through Lewis and Brønsted acid mediated Friedel–Crafts reactions. (Image credit: Hassan A. K. Abd El-Aal.)
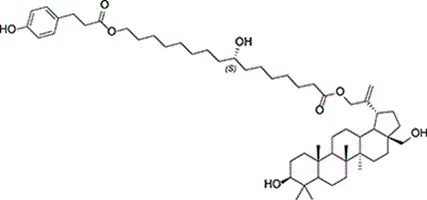
The south-west of Western Australia is a biodiversity hotspot, where collaborations between local communities and scientists play a pivotal role in the study of native plants. The leaf resin of Dodonaea caespitosa, growing in Nowanup, contained only two compounds, one of which was an unusual bioconjugate comprising a triterpene, a cutin monomer and a p-hydroxycinnamate. This natural product was the first isolated to date and will help our understanding of the biology and ecology of this plant. (Image credit: Andrew M. Bloomfield.)