The impact of fungicides with contrasting toxicities to rhizobia on the growth and nodulation of pulses in southern Australia
J. R. Rathjen A * , M. H. Ryder A , T. V. Lai A , I. T. Riley A , J. Brand B and M. D. Denton
A * , M. H. Ryder A , T. V. Lai A , I. T. Riley A , J. Brand B and M. D. Denton  A
A
A
B
Abstract
Pulse crops generally need inoculation with compatible rhizobia to optimise nodulation and nitrogen (N) fixation; however, seed-applied fungicide, often applied in chickpea, may compromise rhizobial survival.
The fungicide P-Pickel T (PPT; thiram and thiabendazole) is highly toxic to rhizobia and nodulation, but this has not been validated outside a controlled environment. Other fungicides containing metalaxyl with low toxicity to rhizobia could be used under certain disease environments to improve root health and nodulation.
Field experiments were conducted to provide evidence of the toxic effect of thiram-based fungicides in chickpea, and to test whether the fungicides Apron XL, Uniform and Vibrance applied on-seed and in-furrow could improve nodulation of chickpea and lentil. These experiments were conducted in four environments over 3 years throughout southern Australia.
Different inoculant types and inoculation methods showed that PPT application with peat inoculant markedly reduced nodulation in both lower (<300 mm) to medium (>400 mm) rainfall sites, where N fixation was lowered by up to 20% (chickpea N derived from N fixation, %Ndfa) and by 60% (mg N fixed/plant). Granular inoculant improved nodulation compared with using a seed-applied inoculant. Nodulation was increased by nearly 50% at two sites with Apron XL, Uniform and Vibrance.
PPT should not be used in contact with rhizobial inoculants in chickpea and farmers should be encouraged to separate the fungicide by using an in-furrow inoculant.
Fungicides with low to no toxicity to rhizobia may promote nodulation and provide an alternative fungicide for use on pulses.
Keywords: fungicides, grain legumes, inoculation, N fixation, nodulation, pulses, rhizobia, seed treatments.
Introduction
Pulses are an increasingly important rotational crops in intensive cropping systems, providing disease and weed breaks, and biologically fixed nitrogen accumulation in the soil, which all benefit the subsequent crop. The rhizobial symbiosis of pulses can contribute, on average, 40–200 kg/ha of nitrogen in a growing season, provided they are effectively nodulated (Evans et al. 2001; Anglade et al. 2015). To ensure effective nodulation, growers usually need to apply rhizobial inoculants at sowing that are formulated to promote survival of rhizobia and compatible symbioses.
It is also a common practice for farmers to apply fungicides to pulse seed before sowing, so as to prevent fungal infections of young seedlings. In Australia, P-Pickel T (a combination of thiram and thiabendazole) is mostly used in pulses to prevent ascochyta blight (Didymella pinodes), botrytis grey mould (Botrytis cinerea) and seedling root rots (Pythium spp. and Fusarium spp.) and is recommended for chickpea and other pulses (GRDC 2017). Chickpea crops throughout the world are particularly susceptible to ascochyta blight, especially in the winter cropping areas of southern Australia that typically have a longer growing season. Thiram-based fungicides such as P-Pickel T applied to the seed coat are an effective control of these fungal diseases. Multiple studies have shown that fungicides that contain thiram are toxic to rhizobia, with a decline in rhizobial survival and nodulation in both the laboratory and greenhouse (Isoi and Yoshida 1988; Kyei-Boahen et al. 2001; Rathjen et al. 2020). Both in vitro and on seed toxicity tests of the commercial product P-Pickel T (active ingredients 360 g/L thiram (dimethylcarbamothioylsulfanyl N,N-dimethylcarbamodithioate) and 200 g/L thiabendazole (2-(4-thiazolyl)benzimidazole)) were performed in a previous study (Rathjen et al. 2020). Thiram was determined to be the toxic ingredient in P-Pickel T, with the thiabendazole component found to be non-toxic (Rathjen et al. 2020). The exposure time between coating the seed with fungicide and inoculant has also been shown to have a significant effect on rhizobial survival and nodulation, with a marked reduction in rhizobial survival within 8 h of contact (Graham et al. 1980). In general, toxicity effects on rhizobial survival leading to reduced nodulation have been performed under mono-axenic conditions in the greenhouse, and may not always be indicative of rhizobial survival and nodulation in a competitive soil environment (Rathjen et al. 2020). Of the few studies focused on chickpea in the field (Kyei-Boahen et al. 2001; Gaind et al. 2007), results have often been conflicting and some nodulation responses are obscured by high rhizobial backgrounds (see e.g. Kutcher et al. 2002).
To reduce the toxic effects of P-Pickel T on rhizobia survival and nodulation, separating rhizobia from direct contact with the PPT fungicide may be effective. This can be achieved with the use of different inoculant formulations such as granules or a liquid inoculant applied in the sowing furrow, below seed in the sowing procedure (Denton et al. 2017). Although one study showed a decrease in nodulation in bean plants coated with thiram following both seed and granular inoculation (Graham et al. 1980), other studies found no toxic effect of seed-applied thiram when using granular inoculant with soybean (Rennie and Dubetz 1984; Cardillo et al. 2019). Overall, there has been little investigation into the effect of thiram-containing P-Pickel T on nodulation and plant growth traits of chickpea as well as investigation into the use of thiram-based fungicides in field trials over multiple environments and seasons, and the potential benefits of separation of the inoculant from the fungicide have not been verified.
Other fungicides that contain alternative active ingredients, such as metalaxyl, that can be applied as a seed-based fungicide or a liquid in-furrow treatment, can control other crop root diseases such as Pythium and Phytophthora. Studies to determine the impact of metalaxyl on nodulation when applied on the seed with the inoculant in chickpea, pea, lentil and faba bean in field environments has shown little effect (Rennie et al. 1985; Kyei-Boahen et al. 2001). However, in vitro studies to determine the effect metalaxyl-containing fungicides on rhizobial survival have concluded a negative effect when exposed together for 4 h or more on chickpea seed prior to sowing (Kyei-Boahen et al. 2001; Panwar et al. 2015). In another study, no detrimental effect was found on nodulation and there was a positive effect on root growth in the presence of root pathogens (Edmisten et al. 1988), which indicates inconsistencies and scarcity of information on the effect of metalaxyl on rhizobia survival in the literature. It is possible that in combination with a high compatible background soil rhizobial population, these metalaxyl-containing fungicides may promote nodulation through controlling root disease. Pythium root rot removes root hairs (Lawrence and Harvey 2006), which are required for early stages of rhizobial symbiosis. Although not registered for use with chickpeas in many countries, these fungicides have the potential to improve root growth and health, thereby improving nodulation; however, the direct effect of these fungicides on rhizobial survival and nodulation is still ambiguous.
The aims of this investigation were to determine the effect of P-Pickel T on nodulation, growth, yield and nitrogen (N) fixation of chickpeas in multiple field environments and years across southern Australia. Many studies have verified the toxicity of thiram-based fungicides to rhizobia, but there have been varied conclusions on the overall effect of fungicides in the field, including their effects on different inoculant types (Revellin et al. 1993; Kyei-Boahen et al. 2001; Kutcher et al. 2002; Aamil et al. 2004). Clarifying the toxicity of P-Pickel T in the field will enable farmers to make informed decisions regarding inoculation and fungicide application. There is a requirement for rigorous investigation into the effect of P-Pickel T on nodulation and plant growth and yield in field environments, to improve and provide reliable information for farmers. A further aim was to investigate whether improving root health through the application of non-toxic fungicides, compared with P-Pickel T, may improve nodulation and plant growth of chickpea and lentil in soils with a high compatible rhizobial background and potential root disease inoculum. Lentil was included because it is a rapidly expanding crop in southern Australia and, although susceptible to common root fungal pathogens, very little work has been undertaken on this topic. These series of experiments are a novel approach to the use of fungicides with rhizobial inoculant, and may provide methods to safely use fungicides with rhizobial inoculants.
Materials and methods
Effect of P-Pickel T on rhizobial survival, nodulation and N fixation in chickpea
Field sites were selected across South Australia (SA) and Victoria (Vic) to cover a wide range of pulse-growing environments. The experimental sites ranged in soil type, pH and rainfall in environments classified as high rainfall to low rainfall and clay loam, sandy loam or loamy sand (Table 1). Enviroments were classified as warm or cool temperate (Table 1).
| Site | Coordinates | Soil type | Soil texture | pH (H2O) | Growing season (Apr–Oct) rainfall (average annual rainfall) (mm) | Environment | Year | Chickpea cultivar | Sowing date | Nodulation assessment date | Treatments | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mallala | 34°24′S, 138°30′ E | Sodosol | Clay loam | 7.3 | 220 (400) | Medium rainfall, warm temperate | 2019 | Genesis 509 | 16 May | 13 Sep | (1) Peat ± PPT on seed, inoculation conducted 0 h and 24 h before sowing (2) Freeze-dried ± PPT on seed, inoculation conducted 0 h and 24 h before sowing (3) Granules ± PPT on seed | |
| Gymbowen | 36°43′S, 141°33′E | Grey Vertosol | Clay loam | 5.6 | 305 (487) | High rainfall, cool temperate | 2019 | PBA Striker | 30 Apr | 5 Aug | (1) Peat inoculant (2) Granular inoculant (3) Uniform in furrow (4) PPT on seed (5) Uniform in furrow + PPT | |
| Ouyen | 35°23′S, 142°26′E | Calcarosol | Sandy loam | 6.4 | 237 (265) | Low rainfall, warm temperate | 2020 | PBA Striker | 15 May | 5 Aug | (1) Freeze-dried ± PPT on seed (2) Granular inoculant (3) Peat ± PPT on seed | |
| Angas Valley | 34°45′S, 139°18′E | Calcarosol | Loamy sand | 6.1 | 216 (280) | Low rainfall, warm temperate | 2020 | PBA Hattrick | 27 May | 7 Sep | (1) Freeze-dried liquid in furrow ± PPT on seed (2) Peat liquid in furrow ± PPT on seed (3) Peat ± PPT on seed |
The sites in SA and Vic were pre-screened for low background of chickpea-nodulating rhizobia (Mesorhizobium spp.) and had no recent history of chickpea production. To confirm the background rhizobial population, soil samples (0–10 cm) were collected about 2 months before planting and used for growing chickpeas in the greenhouse. Chickpea plants were grown for 4 weeks and assessed for nodulation. Only soils that yielded zero or few (1–3 small nodules per plant located distally) nodules on chickpea plants in tests were included as sites in this study. Experiments were conducted at the four identified suitable sites across 2 years (2019–2020) in Angas Valley and Mallala, SA, and Gymbowen and Ouyen, Vic (Table 1). All sites have a Mediterranean-type climate with winter-dominant rainfall. Sowing was conducted after the opening rains for the growing season, into moist soil. At all sites, chickpea was sown in plots at an estimated plant density of 35 plants/m2. The plots were 12 m × 1.8 m with 23 cm row spacing (six rows), except at Mallala, which had plots of 9 m × 1.5 m with 25 cm row spacing (six rows).
The seeds were coated with the recommended rate of P-Pickel T (360 g/L thiram plus 200 g/L thiabendazole, 120 g active ingredient per 100 kg of seed). First, the product was diluted 10-fold in reverse osmosis (RO) water, then 10 mL/kg seed was added to the seeds in a zip-lock bag and the bag was shaken vigorously to fully coat the seed (as indicated by the green dye in the product). The seeds were then subdivided for inoculation treatments and weighed into envelopes for sowing.
The seeds used in each experiment were inoculated with either commercial peat (CC1192, New-Edge Microbials, or BASF Nodulaid, Southbank, Vic, Australia) or freeze-dried (New-Edge Microbials EasyRhiz Legume Inoculant CC1192, Albury, NSW, Australia) formulations according to the manufacturer’s instructions. The inoculants were applied immediately before sowing, or within 24 h (Mallala site only). Other methods of inoculation included granular inoculant (Nodulator) supplied by BASF (Southbank, Vic, Australia), which was mixed with the seed at commercial rate before sowing (Mallala and Gymbowen) and as a liquid in-furrow (Angas Valley).
Within 3 h after sowing or within 24 h for the ‘24 h incubation’ treatments (Mallala site), rhizobial numbers on the inoculated and PPT-treated or untreated seeds were estimated by dilution plating. Seed samples (20 seeds) from the inoculated treatments were placed in sterile water and agitated. The suspensions were then diluted in a 10-fold series with sterile water, and three 20-μL drops of required dilutions were plated on yeast mannitol agar. Plates were incubated for 3 days at 28°C. Seed rhizobial populations are shown in Table 2.
| Inoculant | PPT | Time before sowing (h) | Population estimate (log10 CFU/seed) | |
|---|---|---|---|---|
| Peat | – | 0 | 4.8 | |
| Peat | + | 0 | 5.0 | |
| Peat | – | 24 | 3.5 | |
| Peat | + | 24 | n.d. | |
| Freeze-dried | – | 0 | n.d. | |
| Freeze-dried | + | 0 | n.d. | |
| Freeze-dried | – | 24 | n.d. | |
| Freeze-dried | + | 24 | n.d. |
Samples were processed within 3 h of sowing for 0 h and at 24 h of incubation for the 24 h time of sowing.
PPT, P-Pickel T; CFU, colony-forming units; n.d., not detected (below detection limit).
The experiment at Mallala in 2019 was set up as a completely randomised design with three replicates. The treatment structure was a nested factorial, as follows: (1) with and without P-Pickel T seed coating, (2) with and without inoculation, and (3) three combinations of inoculant type (peat formulation from New-Edge Microbials and Nodulator granules from BASF). Time of inoculant application was 0 and 24 h before sowing, with the latter having a 24 h delay between coating and sowing. Seeds inoculated 24 h before sowing were kept at room temperature and protected from light. Granules were added to the seeds in envelopes and mixed before sowing at the recommended rate equivalent to 3.9 kg/ha.
The experiment at Gymbowen 2019 was a split-plot design with three replicates. The fungicide Uniform (Syngenta Australia, not registered for chickpea or lentil), active ingredients 322 g/L azoxystrobin (methyl (2E)-2-(2-{[6-(2-cyanophenoxy)pyrimidin-4-yl]oxy}phenyl)−3-methoxyprop-2-enoate) and 124 g/L metalaxyl-M, was included as an alternative fungicide to compare with P-Pickel T. Uniform was applied as a liquid in-furrow at 400 mL/ha. The treatment structure was a nested factorial of (1) fungicide seed coatings or none, P-Pickel T, Uniform (in furrow) and a combination of both fungicides, (2) with and without peat inoculation, and (3) inoculant type (Nodulaid peat formulation from BASF and Nodulator granules from BASF). Granules were added to the seeds in an envelope just before sowing at the recommended rate equivalent to 4.6 kg/ha.
The experiment at Ouyen in 2020 was a completely randomised block design with three replicates. The treatment structure was a nested factorial of (1) with and without P-Pickel T, (2) with and without inoculation, and (3) inoculant type (peat and freeze-dried formulations from New-Edge Microbials and Nodulator granules from BASF).
The experiment at Angas Valley 2020 was a completely randomised design with three replicates. The treatment structure was a nested factorial of (1) with and without P-Pickel T, (2) with and without inoculation, (3) inoculant type (peat and freeze-dried formulations from New-Edge Microbials), and (4) inoculant application method (seed coating and liquid in-furrow at 100 L/ha). For the liquid in-furrow peat inoculation, 22 g of peat in a nylon stocking was immersed in 10 L of RO water and infused with some agitation for 2 h before application. For freeze-dried liquid in-furrow inoculation, protecting agent (as supplied by New-Edge Microbials) was suspended in 1.5 L RO water, and 60 mg of freeze-dried rhizobia formulation was added. The rhizobial populations in the in-furrow peat and freeze-dried suspension were 7.1 and 9.1 log10 CFU/mL respectively, determined by the method given above.
Nodulation assessment was conducted approximately 12 weeks after sowing. Plants and roots with soil were dug up in groups of three across the central four rows of each plot, approximately 1 m apart, with a total of 12 plants being collected from each plot. Soil was gently shaken from the roots, which were then washed clean for nodule rating assessment. Nodule rating for chickpea and lentil was conducted using a 0–5 scale based on several parameters, paying particular attention to the number, size, position, and distribution of nodules on the crown and lateral roots (Corbin et al. 1977).
Shoot dry weight (SDW) assessment was conducted at peak biomass (Table 1). Above-ground plant shoot matter was taken as described above and dried at 60°C for 48 h before weighing.
At maturity, plots were harvested with a small plot harvester and grain yield (t/ha) was recorded.
To estimate the amount of N fixed by inoculation treatments at the Mallala and Gymbowen sites in 2019, the 15N natural abundance method was applied (Unkovich et al. 2008). Canola plants were used as non-fixing reference plants and seeded in a transect across the first row of plots. The canola plants were harvested at the same time as the legume biomass samples. Dried plant shoot samples were finely ground and analysed for δ15NAir (%) and total shoot N (μg/g) (Stable Isotope Facility, University of California, Davis, CA, USA). Chickpea N derived from N fixation (%Ndfa) was calculated by comparing the δ15N in inoculated plants (δ15N legume) with that of non-nodulating canola reference plants (δ15N canola). The following formula was applied to calculate the %Ndfa of chickpeas inoculated with rhizobial strains:
where the B value (−2.1) is the δ15N of chickpea plants that are fully dependent on N2 fixation and sampled at the same growth stage as were the field-grown plants.
The amount of N2 fixed per ha were calculated from estimates of legume %Ndfa, legume SDW and %N, by using following formulae:
Field trials assessing the effect on nodulation of chickpea and lentil of fungicides non-toxic to rhizobia that are used to reduce the impact of root disease
The in vitro toxicity of fungicides to rhizobial strains Mesorhzobium ciceri CC1192 (chickpea) and Rhizobium leguminosarum bv viciae WSM1455 (pea/bean/lentil) were tested before designing field experiments. The three fungicides used were: (1) Apron (Apron XL, Syngenta Australia), active ingredient metalaxyl-M (methyl N-(2,6-dimethylphenyl)-N-(methoxyacetyl)alaninate); (2) Uniform (Syngenta Australia, not registered for chickpea or lentil), active ingredients 322 g/L azoxystrobin (methyl (2E)−2-(2-{[6-(2-cyanophenoxy)pyrimidin-4-yl]oxy}phenyl)-3-methoxyprop-2-enoate) and 124 g/L metalaxyl-M; and (3) Vibrance (Syngenta Australia, not registered for chickpea or lentil), with active ingredients 66.2 g/L difenoconazole (1-((2-(2-chloro-4-(4-chlorophenoxy)phenyl)-4-methyl-1,3-dioxolan-2-yl)methyl)-1H-1,2,4-triazole), 16.5 g/L metalaxyl-M and 13.8 g/L sedaxane (N-[2-([1,1′-bi(cyclopropan)]-2-yl)phenyl]-3-(difluoromethyl)-1-methyl-1 H-pyrazole-4-carboxamide). Uniform and Vibrance were added to filter discs at 0.1, 0.15, 0.3 and 0.9 μg/disc, with 0.3 μg being equivalent to the commercial rate that would be applied, on average, to a single seed. Apron was applied to filter discs at 4, 12, 40 and 120 μg/disc, with 40 μg being equivalent to the commercial rate that would be applied, on average, to a single seed.
Surface-sterilised chickpea and lentil seeds were coated with Apron XL (350 g/L Metalaxyl-A with label recommendation of 75 mL of the product per 100 kg seed) by first diluting 1 in 10 with RO water, then applying 1 mL to 100 g of seeds in a zip-lock bag and shaking to fully coat the seeds. Fungicide-coated seeds were allowed to air dry before applying any rhizobial inoculants. The other fungicides (Vibrance and Uniform) were applied by injecting in-furrow at sowing at a rate of 400 mL/ha. These fungicides are not registered for chickpea or lentil in Australia and were applied at the rate recommended for barley and wheat. For treatments where P-Pickel T or rhizobia inoculation were included, chickpea and lentil seeds were coated as described in the section above (Seed treatments and inoculation).
Sites at Blyth, SA (2020), Ouyen, Vic (2020), Nhill, Vic (2020) and Hart, SA (2021), were selected with a previous history of chickpea and lentil crops to ensure a compatible soil rhizobial background (Table 3). Seeds were coated with Apron, whereas fungicides Uniform and Vibrance were applied in-furrow (as described in the previous section). P-Pickel T was used as a seed treatment at the Ouyen and Hart field sites. Treatments were replicated three times in a completely randomised design. All treatments were inoculated with the relevant rhizobial peat inoculant. Chickpea (cv. Hattrick) was sown in plots 12 m × 1.8 m with 22.8 cm row spacing (six rows) and an estimated plant density of 35 plants/m2 and lentil was sown in the same size plots with an estimated plant density of 120 plants/m2.
| Site | Location coordinates | Year | Soil type | pH (H2O) | Crop and cultivar(s) | Sowing date | Nodulation assessment date | |
|---|---|---|---|---|---|---|---|---|
| Blyth | 33°56′20″S, 138°28′24″E | 2020 | Chromosol | 7.9 | Chickpea cv. PBA Hattrick | 28 May | 14 Sep | |
| Ouyen | 35°23′14″S, 142°26′07″E | 2020 | Calcarosol | 6.4 | Chickpea cv. PBA Striker | 15 May | 5 Aug | |
| Nhill | 36°25′17″S, 141°29′32″E | 2020 | Tenosol | 8.4 | Lentil cv. Hallmark | 15 May | 5 Aug | |
| Hart | 33°45′21″S, 138°24′54″E | 2021 | Calcarosol | 8.3 | Chickpea cv. PBA Slasher Lentil cv. Hurricane | 11 Jun | 8 Sep |
Composite soil samples from the Blyth and Hart field sites were collected and analysed for the presence and level of root pathogens, including Pythium (Clades f and i), by using the Predicta B DNA-based soil testing service at South Australian Research and Development Institute (SARDI). Samples of root material dried at 40°C for 24 h, which were representative of some treatments in trials at Blyth and Ouyen in 2020, and Hart in 2021, were analysed using the Predicta B Research test panel specifically designed for Pulse Research Root Testing (SARDI). The DNA extracted from these roots was analysed for the presence of common pulse root pathogens found in South Australia: https://pir.sa.gov.au/research/services/molecular_diagnostics/predicta_research/test_panel_pulse_research.
Soil collected from the Blyth field site contained Pythium (Clades f and i) detected at 12 pg DNA/g, and soil from the Hart site contained Didymella pinoides at 46 pg DNA/g, Pythium (Clade f) at 13 pg DNA/g and a trace of Macrophomina phaseolina.
Nodulation (13 and 15 weeks after sowing) and shoot dry-weight assessment (at peak biomass) were measured as described in the section above (Nodulation and plant growth assessment) At crop maturity, plots were harvested with a small plot harvester to determine grain yield (Mg/ha).
Statistical analysis
Statistical analysis was performed in R by using least-squares ANOVA in the ‘Stats’ package (ver. 4.4.2, www.R-project.org, R Core Team 2024). For the P Pickel T and inoculation experiments at Mallala, Ouyen and Gymbowen (excluding Angas Valley), nested linear models were fitted with inoculation (with/without) and inoculant (type) nested individually and factorially within fungicide (with/without PPT), with blocking being applied. These linear models were applied to all metrics (viz. nodule rating, grain yield, shoot dry weight, %Ndfa or total N fixed) as measured at Mallala, Gymbowen, Angas Valley and Ouyen, except for N metrics at Gymbowen, because these data were not collected for all treatment combinations at that site. For the Uniform, Vibrance and Apron fungicide experiments, nested linear models were also used with fungicide (type) nested within fungicide treatment (with/without), with no blocking at any site. These models were applied to all metrics (viz. nodule rating, nodule number per plant, grain yield or shoot dry weight). For both datasets, treatment comparisons were made only for combinations within significance levels by using R package ‘agricolae’ (ver. 1.3.7, https://cran.r-project.org/web/packages/agricolae/index.html; De Mendiburu 2023). The R package was also used for visualisation of mean responses as applicable to the design of the experiment at each site: Dataset 1, fungicide treatments (represented by coloured symbols) divided into strata as overall, inoculation (with/without) and inoculant (type); and Dataset 2, fungicide treatments (represented by coloured symbols) divided in to strata as treated (with/without) and fungicide (type). Group means are given as a horizontal bar (as applicable) and significance for strata is included above the y-axis.
Results
The effect of P-Pickel T on nodulation, plant biomass, grain yield and N fixation of inoculated chickpea in the field
In general, P-Pickel T reduced nodulation (measured on a 0–5 scale of nodule rating) of seed-inoculated chickpea plants across the tested environments and cultivars, compared with plants grown from seed without fungicide coating (Figs 1a, 2a and 3a). At the Mallala, Ouyen and Angas Valley trial sites, the average nodule rating per plant was below the adequate level (rating of 2, i.e. a low number of nodules on the crown of the plant, Farquharson et al. 2022) (Figs 1a, 3a and 4a). At Angas Valley, the nodulation was so low in the plants from uncoated seeds (no P Pickel T) that no toxic effect of P-Pickel T was evident (Fig. 4a). Plants grown at Gymbowen had the highest nodule rating of all the sites, but the level was still moderately low with mean values reaching a rating of 3 (out of a maximum 5) in plants inoculated with peat and no P-Pickel T (Fig. 2a).
Effect of seed-applied fungicide P-Pickel T (PPT), compared with an uninoculated control, on (a) chickpea nodule rating, (b) shoot dry weight, (c) yield, (d) %Ndfa and (e) amount of nitrogen fixed in 2019 at Mallala, South Australia, Australia. Seeds were coated with P-Pickel T and then inoculated with freeze-dried (FD) or peat slurry inoculant and sown immediately (0 h) or left for 24 h before sowing. Granular inoculant was applied with the seed in-furrow. For each measure, the nested strata in the analysis of variance model are presented, separated by dotted vertical lines with significance (n.s., not significant; *P < 0.05; **P < 0.01; and ***P < 0.001) indicated for main effects (F, fungicide; I, inoculation; and IT, inoculant type) and their interactions. The dashed horizontal line, the overall mean, included for ease of comparison across strata.
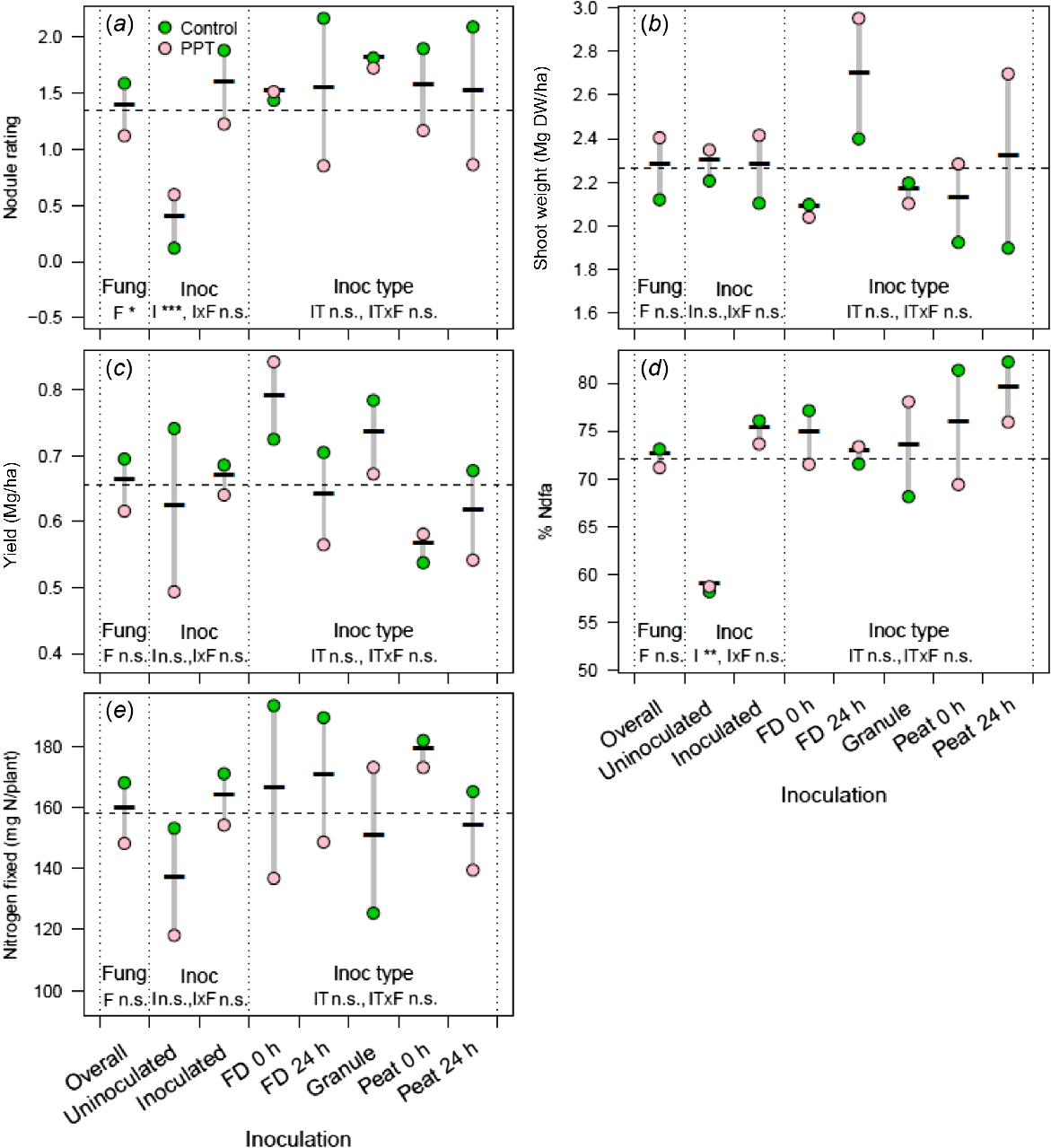
Effect of seed- and furrow-applied fungicides P-Pickel T (PPT) and Uniform (Uni) respectively, alone or in combination, compared with an uninoculated control, on (a) chickpea nodule rating, (b) shoot dry weight, (c) yield, (d) amount of nitrogen fixed and (e) %Ndfa in 2019 at Gymbowen, Victoria, Australia. Seeds were coated with P-Pickel T and then inoculated with peat slurry and sown immediately. Granular inoculant and Uniform were applied in-furrow. Offset graphical points are presented for clarity to avoid overlain points. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines with significance (n.s., not significant; **P < 0.01; and ***P < 0.001) indicated for main effects (F, fungicide; I, inoculation; and IT, inoculant type) and their interactions. Data for c, d and e were collected only for peat-inoculated treatments and are consequently presented for a simplified statistical model. The dashed horizontal line, the overall mean, is included for ease of comparison across strata.

Effect of seed-applied fungicide P-Pickel T (PPT) on (a) chickpea nodule rating, (b) shoot dry weight, and (c) yield in 2020 at Ouyen, Victoria, Australia. Seeds were coated with P-Pickel T and then inoculated with freeze-dried (FD) or peat slurry and sown immediately. Granular inoculant was applied in-furrow. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines with significance (n.s., not significant; *P < 0.05; and **P < 0.01) indicated for main effects (F, fungicide; I, inoculation; and IT, inoculant type) and their interactions. The dashed horizontal line, the overall mean, is included for ease of comparison across strata.
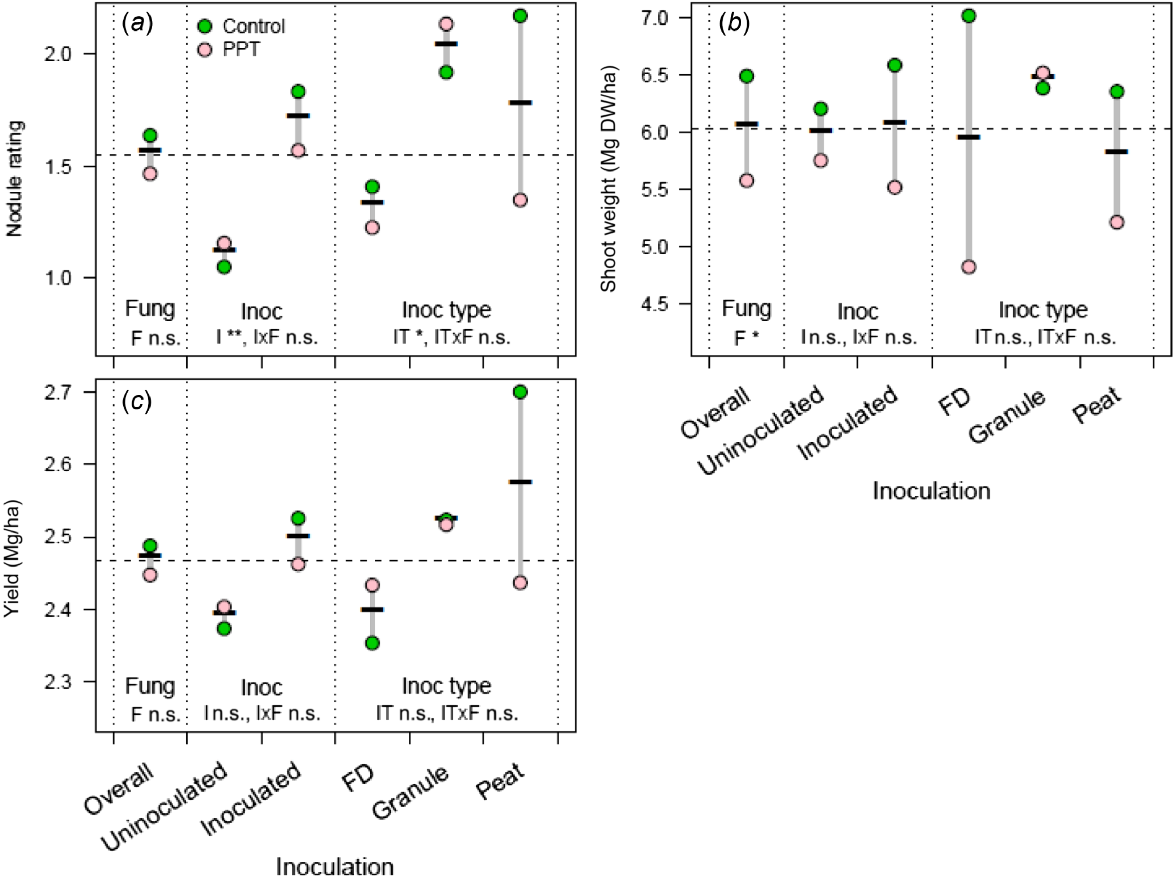
Effect of seed-applied fungicide P-Pickel T (PPT), compared with an uninoculated control, on (a) chickpea nodule rating, (b) shoot dry weight, and (c) and yield in 2020 at Angas Valley, South Australia, Australia. Seeds were coated with P-Pickel T, followed by a peat inoculant slurry and sown immediately or inoculated in-furrow with a liquid freeze-dried (FD) or peat inoculant. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines with significance (n.s., not significant; *P < 0.05) indicated for main effects (F, fungicide; I, inoculation; and IT, inoculant type) and their interactions. The dashed horizontal line, the overall mean, is included for ease of comparison across strata.
At the Mallala site, there was a lower nodule rating in plants with seeds coated with PPT and then inoculated (Fig. 1a). Although P-Pickel T decreased chickpea nodulation across all inoculant types, there was no statistical difference in the interactive effect of nodule rating with inoculation type × fungicide, or inoculation type on its own (Fig. 1a). There was also no difference in shoot dry weight with any of the inoculation or fungicide treatments, but the shoot biomass was low compared with other sites (Fig. 1b). Nitrogen fixation expressed as %Ndfa and mg N fixed/plant, was not different among any of the treatments (Fig. 1d, e). Although not statistically significant, N fixation was negatively affected by fungicide in plants that were inoculated with freeze-dried formulation and those seeds sown 24 h after inoculation with peat, but this did not occur with granular inoculant (Fig. 1d, e). There was no decrease in N fixation in plants from seeds coated with fungicide and inoculated either with granules or sown immediately after inoculation with peat. There were also no differences in the yield among treatments at the Mallala site (Fig. 1c).
The experiment at Gymbowen investigated the singular and interactive effects of P-Pickel T and Uniform fungicide on seeds inoculated with peat formulation. P-Pickel T reduced chickpea nodule rating, but the same detrimental effect was not observed with Uniform (Fig. 2a). There was a decrease in nodulation when both fungicides were coated together onto the seed before inoculation, but this was not as great as the negative effect of P-Pickel T on its own (Fig. 2a). There was no interaction between fungicide and inoculation, but there was a decrease in the nodule rating of plants coated with P-Pickel T compared with Uniform. There was a similar observation in plants inoculated with peat over P-Pickel T, which showed reduced nodulation compared with the other treatments. The plants inoculated with peat had a higher average nodule rating than did the plants with the granular inoculant (Fig. 2a). Yield was higher in plants with seeds coated with P-Pickel T, where both the singular and combined coating of P-Pickel T and Uniform improved yield when the seeds were also coated with peat inoculant (Fig. 2b). Coating seeds with P Pickel T reduced chickpea N fixation (%Ndfa and mg N/plant) compared with plants treated with Uniform or no fungicide (Fig. 2d, e). Plants with seeds coated with P-Pickel T had less than 50%Ndfa (Fig. 2d). When the inoculant was separated from P-Pickel T either with granular or liquid in-furrow inoculant, the results were mixed. The granular inoculant reduced the toxic effect, but the nodulation was not equivalent to the level of peat with no fungicide (Fig. 2a). For all measurements excluding shoot dry weight, (nodule rating, yield, N fixation), the peat inoculant was better, even though the nodulation was affected more by the toxicity of P-Pickel T (Fig. 2a–e).
The nodule rating of chickpea plants grown at Ouyen (2020) differed only between ±inoculation and inoculant type. There was no effect of P-Pickel T on nodule rating, which was moderately low (Fig. 3a). Shoot weight was negatively affected by P-Pickel T, but there was no difference between the treatments in seed yield (Fig. 3b, c).
At Angas Valley (2019), there was a decrease in nodule rating associated with the application of P-Pickel T (Fig. 4a). There was no difference in either shoot weight or yield with any treatments at this site (Fig. 4b, c). Angas Valley was a very dry site in 2019 (Table 1), with harsh environmental conditions, which is reflected in the low nodule rating, shoot dry weight and yield.
Promotion of nodulation with in vitro non-toxic fungicides in a high soil rhizobial background with root disease potential
The growth of strain CC1192 (chickpea) was not affected by Apron, Uniform or Vibrance, even at the highest concentrations (0.9 μg/disc Apron and 40 μg/disc Uniform/Vibrance). The in vitro growth of WSM1455 (pea/bean/lentil) was also not affected by these fungicides at the tested concentrations.
Testing of dried roots of lentil and chickpea for the presence and level of root pathogens by using DNA abundance showed low incidence of common pulse root diseases in plants grown at Blyth, Hart and Ouyen. Pythium (Clade f) was detected at very low concentrations in chickpea roots sampled at Hart and Blyth. The plants treated with P-Pickel T, Uniform and no fungicide at Ouyen had a high yield of DNA associated with Pythium (Clade f) (12,000–15,000 pg DNA/sample), whereas the concentration in plants treated with Apron was about 93% lower at 365 pg DNA/sample. Didymella pinoides was found in low amounts in all sites (<1000 pg DNA/sample). No significant concentrations of Pythium DNA were found on roots of plants grown at either Blyth or Hart experimental sites.
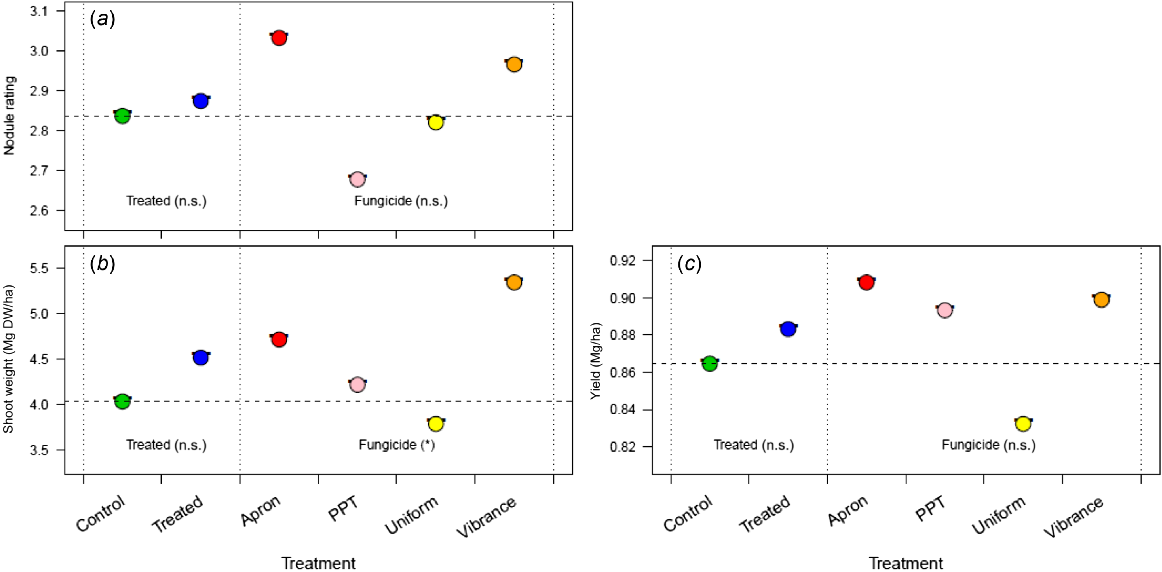
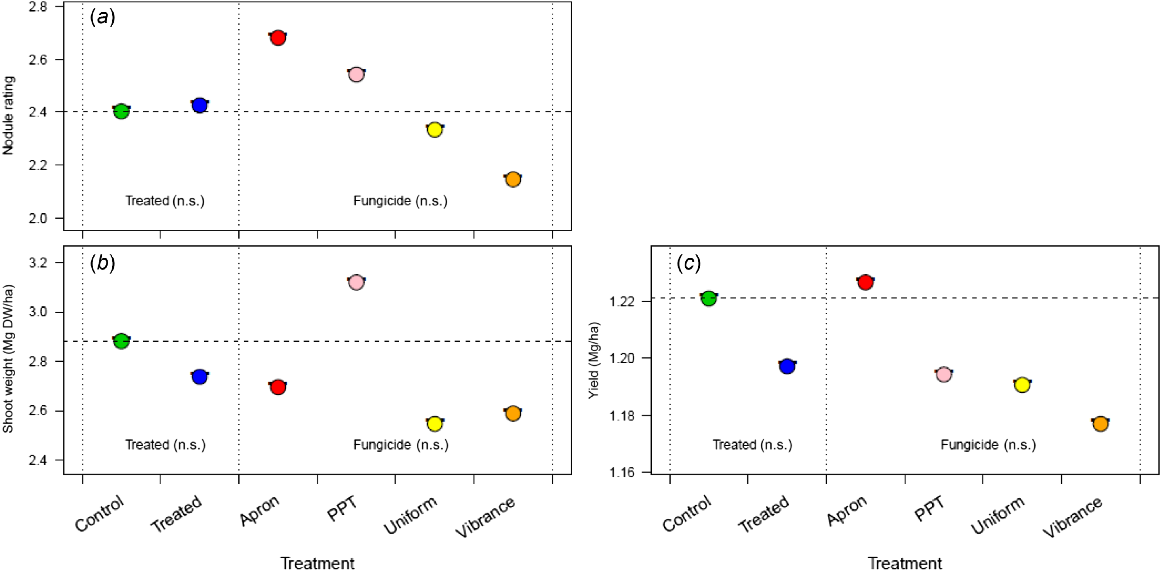
Field trials were conducted across southeastern Australia at sites near Blyth, Hart (SA), Ouyen and Nhill (Vic) for chickpea and lentil. At Blyth, treatment with the fungicides Apron and Vibrance enhanced chickpea nodule rating compared to the control (Fig. 5a); Uniform fungicide was not beneficial. There was no difference between treatments in yield or nodule number per plant (Fig. 5b, c), but shoot weight was highest in the Apron treatment (Fig. 5d).
Effect of the three fungicides (Apron, Vibrance and Uniform) compared with an uninoculated control, on (a) chickpea nodule rating, (b) nodule number, (c) shoot dry weight, and (d) yield in 2021 at Blyth, South Australia, Australia, with only P Pickel T being known to be toxic to rhizobia. Apron was seed-applied, and Vibrance and Uniform were applied in-furrow, and these fungicides are considered non-toxic to rhizobia. All seeds were inoculated with a peat inoculant slurry. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines, with significance (n.s., not significant; *P < 0.05); the Treated stratum includes the control and the combined effect of fungicide application, and the Fungicide stratum includes the individual effects of the fungicide nested within Treated. The dashed horizontal line, the control mean, is included for ease of comparison across strata.

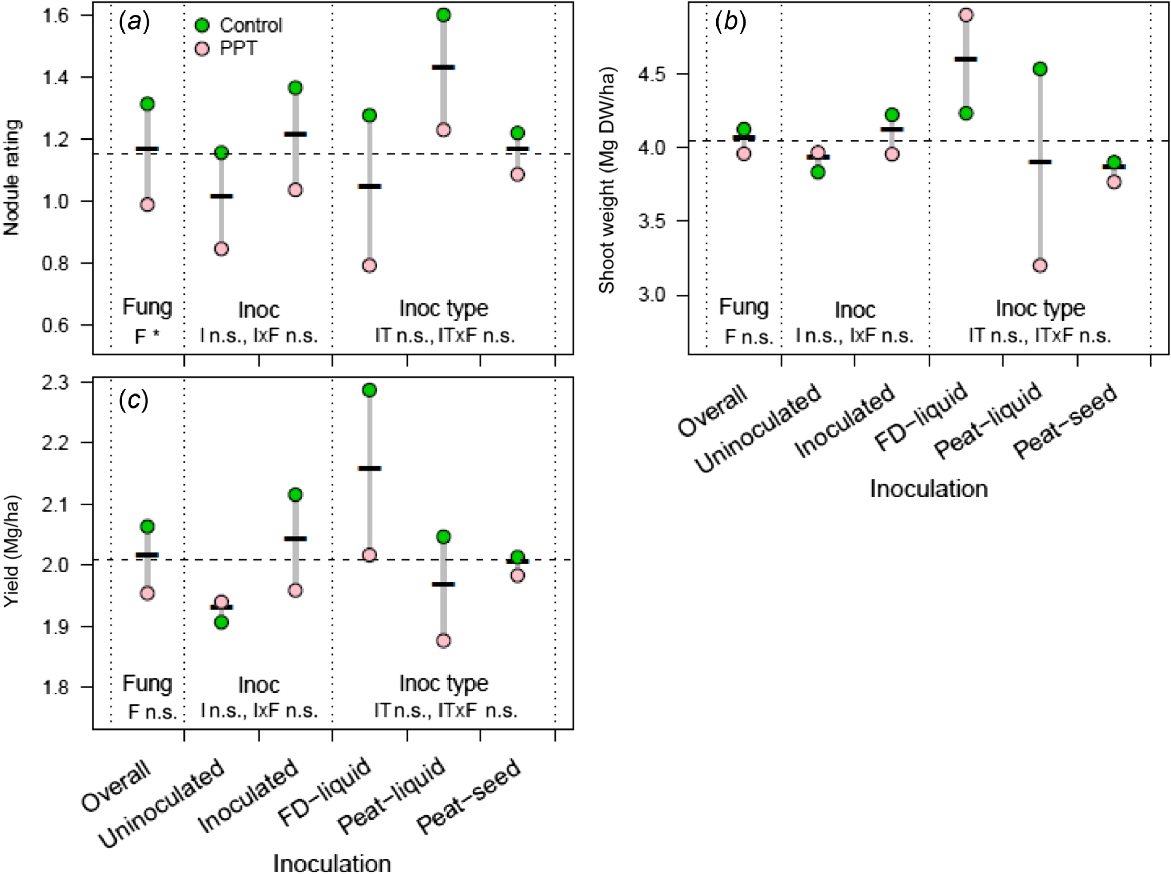
At Hart (SA), there was no difference between the control and fungicide-treated chickpea plants in nodule rating and yield (Fig. 6a, b), but the shoot weight of plants treated with Apron and Vibrance was higher than that of the untreated plants (Fig. 6c). The treatment of lentil with Apron, Uniform and Vibrance did not result in any differences in the measured plant parameters from the negative (no fungicide) control (Fig. 7). The incidence of Pythium (Clade f) found in the plants treated with P-Pickel T, Uniform and no fungicide was high compared with plants treated with Apron, but this did not affect nodulation or plant growth traits.
Effect of four fungicides (Apron, P-Pickel T, Vibrance and Uniform) on (a) chickpea nodule rating, (b) shoot dry weight, and (c) yield in 2021 at Hart, South Australia, Australia. Apron and P-Pickel T were seed-applied and Vibrance and Uniform were applied in-furrow. Of these fungicides, only P-Pickel T is known to be toxic to rhizobia. All seeds were inoculated with a peat inoculant slurry. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines, with significance (n.s., not significant; *P < 0.05); the Treated stratum includes the control and the combined effect of fungicide application, and the Fungicide stratum includes the individual effects of the fungicide nested within Treated. The dashed horizontal line, the control mean, is included for ease of comparison across strata.
Effect of four fungicides (Apron, P-Pickel T, Vibrance and Uniform) on (a) lentil nodule rating, (b) shoot dry weight, and (c) yield in 2021 at Hart, South Australia, Australia. Apron and P-Pickel T were seed-applied and Vibrance and Uniform were applied in-furrow. Of these fungicides, only P-Pickel T is known to be toxic to rhizobia. All seeds were inoculated with a peat inoculant slurry. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines, with all non-significant (n.s.) at this site, but included for comparison with related figures; the Treated stratum includes the control and the combined effect of fungicide application, and the Fungicide stratum includes the individual effects of the fungicide nested within Treated. The dashed horizontal line, the control mean, is included for ease of comparison across strata.
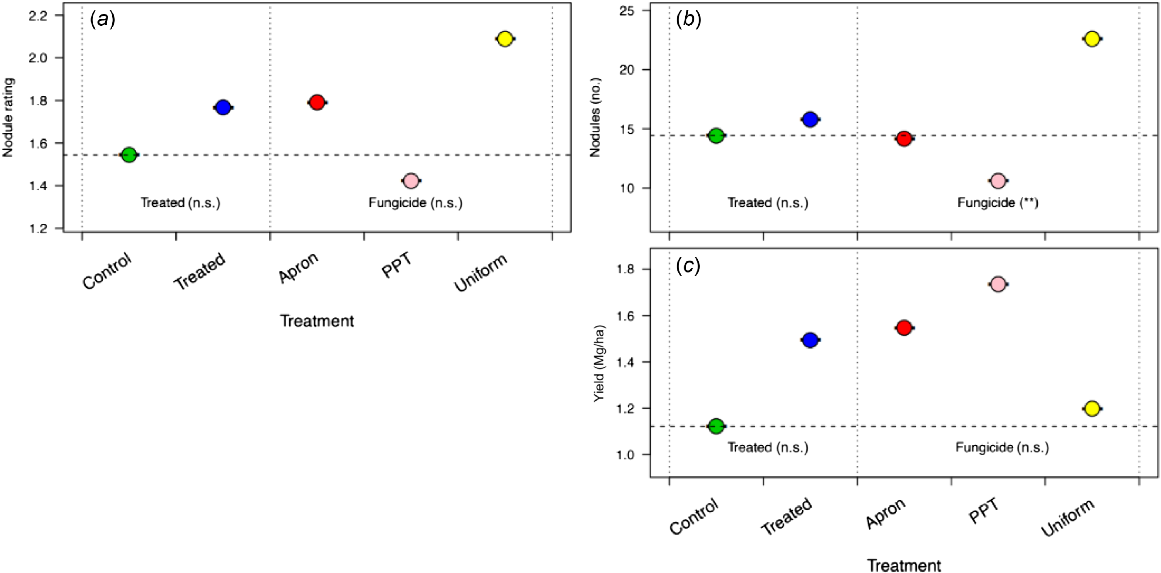
The chickpea plants treated with Uniform at Ouyen (Vic) had more nodules than did the plants with the other fungicide treatments, whereas P-Pickel T-treated plants had a low nodule number compared with the negative control (Fig. 8b). There was no difference among the fungicide treatments in nodule rating or yield (Fig. 8a, c).
Effect of three fungicides (Apron, P-Pickel T and Uniform) on (a) lentil nodule rating, (b) nodule number, and (c) yield in 2020 at Ouyen, Victoria, Australia. Apron and P-Pickel T were seed-applied and Vibrance and Uniform were applied in-furrow. Of these fungicides, only P-Pickel T is known to be toxic to rhizobia. All seeds were inoculated with a peat inoculant slurry. For each measure, the nested strata in the ANOVA model are presented, separated by dotted vertical lines, with significance (n.s., not significant; **P < 0.01); the Treated stratum includes the control and the combined effect of fungicide application, and the Fungicide stratum includes the individual effects of the fungicide nested within Treated. The dashed horizontal line, the control mean, is included for ease of comparison across strata.
Discussion
Effect of toxic thiram-based fungicides on chickpea growth and development
The results presented in this study show that the thiram-based fungicide P-Pickel T is toxic to rhizobial survival on the seed of chickpea, and has a detrimental effect on nodulation in the field. Farmers are recommended to coat chickpea seed with P-Pickel T in Australia to protect against seedling infection by Didymella rabiei (ascochyta blight), which is a serious problem in chickpea when sown as a winter crop. Ascochyta blight can cause complete crop failure in chickpea, and seed-borne transmission is a major mode of infection around the world (Pande et al. 2005). In Australia, Ascochyta blight fungicides, both as seed dressing and foliar, have added importance because of limited genetic resistance (Fanning et al. 2022). There have been many other investigations into the toxicity of seed-applied fungicides globally, but this study is the first to provide evidence of a consistent reduction in nodulation in the field over a variety of environments and years.
It has been documented that thiram is toxic to the growth of different rhizobia genera in vitro, for example Bradyrhizobium (Odeyemi and Alexander 1977), Ensifer (Osman et al. 2012) and Mesorhizobium (Rathjen et al. 2020). Rhizobia are killed by thiram when exposed as a pure culture, but there is some disagreement among studies that have investigated the interaction with inoculation and nodulation, with some concluding a negative effect (Revellin et al. 1993) and some no effect (Kutcher et al. 2002) on nodulation of soybeans and field peas respectively. For chickpea, there have been similar discrepancies in reports of the effect of thiram on nodulation and N fixation, which may have been dependent on the level of disease at the field study sites (Kyei-Boahen et al. 2001; Aamil et al. 2004), or, in many cases, sowing into soil with a high background rhizobial population, which reduces measurable impacts of the fungicide on the inoculant (Kutcher et al. 2002). These studies are further compounded by inconsistencies in the amount of active ingredient of thiram and variations among countries in commercially recommended rates of fungicide application. For example, the commercial recommendation in Australia for P-Pickel T is 0.72 g/kg seed, whereas Canadian formulations recommend 0.5 g/kg seed (Matus et al. 2003). Increasing the amount of active ingredient of thiram per seed is associated with greater toxicity and detrimental impacts on nodulation (Aamil et al. 2004; Rathjen et al. 2020). There is little argument about the toxicity of thiram to bacteria, particularly rhizobia, and using Australian commercial recommendations on seed coating, our study reconfirmed this toxic impact on nodulation of chickpea in the field.
Impact of P-Pickel T on nodulation and plant growth of chickpea according to inoculant type
The type of inoculant formulation and the application method used may also affect nodulation and N fixation when exposing rhizobia to a thiram-based fungicide. Peat-based inoculants are considered to be protective to rhizobia by improving desiccation and stress tolerance (Atieno et al. 2018), and, in this study, also peat formulation appeared to reduce fungicide toxicity compared with a freeze-dried formulation of rhizobia when both were applied directly to the seed, if sowing occurs well within 24 h after inoculation. Although peat can protect rhizobia from desiccation on the seed (Casteriano et al. 2013), this protective effect does not extend to toxic compounds present on the seed surface and direct inoculation of the seed bed by using granules is a safer option (Deaker et al. 2004). Our research showed that using a granular inoculant that separates the rhizobia from the seed-applied fungicide can reduce the effect of thiram, as was observed at the Mallala site in 2019 and the Gymbowen site in 2020. Despite the perceived benefits of using the granular inoculant to enable physical separation from the toxic fungicide, nodulation of chickpea plants was lower than that with seeds coated with the peat inoculant at Gymbowen (2020), regardless of any interaction with seeds coated with P-Pickel T. Separation of seed-applied fungicide from inoculant through liquid inoculation of rhizobia in the planting furrow at Angas Valley (2020) did not reduce the impact of P-Pickel T, but nodulation of the control plants without fungicide was comparable to or better than for the plants grown from seed coated with peat inoculant. Therefore, although physical separation of the inoculant and fungicide has advantages, this may not always result in complete amelioration of the reduced nodulation caused by thiram toxicity.
Yield cannot normally be used as an indication of plant nodulation and N fixation success, because of the myriad of factors that are involved in determining seed production and yield. In chickpea, root disease, time of sowing and environmental factors (e.g. soil type, rainfall, disease) have huge implications for flowering and seed production (GRDC 2017). Each field site had unique environmental constraints such as dry soil (Angas Valley and Mallala), low growing-season temperatures (Gymbowen) and sandy soils (Angas Valley and Ouyen), which would have affected seed yield (Table 1). At Gymbowen (2019), there was an increase in yield in the plants with seeds coated with P-Pickel T, which was not reflected by an increase in shoot N fixation. A possible explanation for this discrepancy might be in the presence of plant disease, such as ascochyta blight, which can be controlled by P-Pickel T (as well as Botrytis, Pythium and Fusarium). It has been shown that fungicide control of disease in chickpea can result in yield benefits of 96% or higher (Fanning et al. 2022). Therefore, plant disease can have impacts on plant growth and yield sometimes beyond the benefits of nodulation and N fixation.
Improvement of nodulation through use of fungicides non-toxic to rhizobia
The use of fungicides containing metalaxyl and other non-toxic active ingredients could improve nodulation and N fixation of pulses in soils and environments where there is a high risk of seedling root disease. Increasing severity of root disease symptoms and incidence of the legume pathogen Aphanomyces euteiches has been found to be negatively correlated with nodulation in field pea (Wu et al. 2019). Often fungal root pathogens impair root length and development, in particular a reduction in root hairs, which are essential for nodule formation (Lawrence and Harvey 2006), and the control of these pathogens with a fungicide non-toxic to rhizobia could improve root growth and development and enhance nodulation.
The experiments conducted at Blyth, Hart and Ouyen (in 2020 and 2021) were designed to investigate whether nodulation could be increased in chickpea and lentil by using fungicides Apron, Uniform and Vibrance to reduce disease and improve root health. Metalaxyl is an active ingredient in all of these fungicides and has systemic properties that allow control of many root pathogens, including Pythium and Phytophthora that are significant pathogens of pulses in southern Australia (Gerard et al. 2017). It is generally accepted that fungicides containing metalaxyl have low to no toxicity at recommended rates across rhizobia species both in vitro (Kyei-Boahen et al. 2001; Ahemad and Khan 2012) and in nodulation and N fixation (Edmisten et al. 1988; Sartori et al. 2023), which was also found in the present study both in vitro and in the field. In addition, the application of Uniform and Vibrance as liquid in-furrow led to a reduced risk of direct contact with the rhizobia inoculated onto the seed. The combination of these factors favour root disease control and improved nodulation of pulses.
Fungicide efficacy is mostly related to the type of disease present, so the extent to which nodulation can be improved by fungicides such as Apron, Uniform and Vibrance may be observable only in the presence of the relevant pathogen inoculum. For example, an investigation in faba bean found that Apron Maxx was more effective against Rhizoctonia solani than against Fusarium avenaceum (Chang et al. 2014). We found that nodulation of chickpea was improved using Apron and Uniform, compared with the control at the Blyth site in 2020, but not at the other sites in the same year. In the present study, very little pathogen inoculum was found both in the soil and in plant roots through Predicta B disease testing. This may explain variability among sites in the response of plants treated with the different fungicides. Plant growth measurements were taken at only one point during the growing season (peak biomass), and disease incidence and severity may have changed quickly during this time and not have been accurately captured (e.g. emergence data could indicate seedling disease). This is supported by the increase in shoot weight with Apron (Blyth site, 2020) and Vibrance (Hart site, 2021) which was related only to the nodulation response to Apron application at Blyth. Further research using added pathogen inoculum or sites with confirmed disease infestation is required to investigate this hypothesis further.
Conclusions
Compatibility of fungicides with rhizobia has been investigated in the laboratory, greenhouse and field for many decades with varying conclusions. Our study showed that thiram-containing fungicides such as P-Pickel T are toxic to mesorhizobia under different environments and inoculation methods and with a low background resident soil rhizobial population. It is recommended that farmers do not expose rhizobial inoculant to direct contact with thiram-containing fungicides, and that separation of the fungicide from rhizobia through use of a granular inoculant is the best option to reduce toxic effects and therefore to avoid reduction in nodulation and N fixation. Although there was a clear reduction in the nodulation of chickpea following P-Pickel T fungicide treatment, this effect did not always translate into lowered plant shoot growth or yield. Shoot growth and yield can be greatly influenced by specific disease pressures, and chickpea is particularly susceptible to ascochyta blight caused by Didymella rabiei (and controlled by P-Pickel T), and environmental conditions.
The use of fungicides that are non-toxic to rhizobia, such as those based on metalaxyl, may provide an opportunity to increase nodulation and N fixation through improving root growth in soils and environments with root disease inoculum. These fungicides were shown both in vitro and in the field to have low or no toxicity to both Mesorhizobium and Rhizobium inoculants. The fungicides Uniform and Vibrance (applied in-furrow) are not registered in Australia for use on pulses, but in our study showed potential to increase nodulation in some environments. Apron applied on the seed with the inoculant also showed improved nodulation at one site sown to chickpea. The use of fungicides to improve nodulation in pulses is therefore worth investigating further with more pulse types, environments and fungicide products.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Matthew Denton is an Associate Editor of Crop & Pasture Science, but was not involved in the peer review or any decision-making process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
The authors wish to acknowledge the financial support provided by the Grains Research and Development Corporation project UOA1805-017RTX (9176500).
References
Aamil M, Zaidi A, Khan MS (2004) Fungicidal impact on chickpea-Mesorhizobium symbiosis. Journal of Environmental Science and Health. Part B, Pesticides, Food Contaminants, and Agricultural Wastes 39(5/6), 779-790.
| Crossref | Google Scholar | PubMed |
Ahemad M, Khan MS (2012) Effects of pesticides on plant growth promoting traits of Mesorhizobium strain MRC4. Journal of the Saudi Society of Agricultural Sciences 11(1), 63-71.
| Crossref | Google Scholar |
Anglade J, Billen G, Garnier J (2015) Relationships for estimating N2 fixation in legumes: incidence for N balance of legume-based cropping systems in Europe. Ecosphere 6(3), 1-24.
| Crossref | Google Scholar |
Atieno M, Wilson N, Casteriano A, Crossett B, Lesueur D, Deaker R (2018) Aqueous peat extract exposes rhizobia to sub-lethal stress which may prime cells for improved desiccation tolerance. Applied Microbiology and Biotechnology 102, 7521-7539.
| Crossref | Google Scholar | PubMed |
Cardillo BEdS, Oliveira DP, Soares BL, Martins FAD, Rufini M, Da Silva JS, Neto GGF, De Andrade MJB, Moreira FMdS (2019) Nodulation and yields of common bean are not affected either by fungicides or by the method of inoculation. Crop Economics, Production and Management 111(2), 694-701.
| Crossref | Google Scholar |
Casteriano A, Wilkes MA, Deaker R (2013) Physiological changes in rhizobia after growth in peat extract may be related to improved desiccation tolerance. Applied and Environmental Microbiology 79(13), 3998-4007.
| Crossref | Google Scholar |
Chang KF, Conner RL, Hwang SF, Ahmed HU, McLaren DL, Gossen BD, Turnbull GD (2014) Effects of seed treatments and inoculum density of Fusarium avenaceum and Rhizoctonia solani on seedling blight and root rot of faba bean. Canadian Journal of Plant Science 94(4), 693-700.
| Crossref | Google Scholar |
Corbin EJ, Brockwell J, Gault RR (1977) Nodulation studies on chickpea (Cicer arietinum). Australian Journal of Experimental Agriculture and Animal Husbandry 17(84), 126-134.
| Crossref | Google Scholar |
De Mendiburu F (2023) Agricolae: statistical procedures for agricultural research. The Comprehensive R Archive Network. Available at https://cran.r-project.org/web/packages/agricolae/index.html
Deaker R, Roughley RJ, Kennedy IR (2004) Legume seed inoculation technology—a review. Soil Biology and Biochemistry 36(8), 1275-1288.
| Crossref | Google Scholar |
Denton MD, Phillips LA, Peoples MB, Pearce DJ, Swan AD, Mele PM, Brockwell J (2017) Legume inoculant application methods: effects on nodulation patterns, nitrogen fixation, crop growth and yield in narrow-leaf lupin and faba bean. Plant and Soil 419, 25-39.
| Crossref | Google Scholar |
Edmisten KL, Wolf DD, Stromberg EL (1988) Compatibility of metalaxyl with Rhizobium meliloti on alfalfa seed to control pythium damping off. Crop Science 28(3), 568-570.
| Crossref | Google Scholar |
Evans J, McNeill AM, Unkovich MJ, Fettel NA, Heenan DP (2001) Net nitrogen balances for cool-season grain legume crops and contributions to wheat nitrogen uptake: a review. Australian Journal of Experimental Agriculture 41(3), 347-359.
| Crossref | Google Scholar |
Fanning J, Brand J, Munoz Santa I, McDonald L, Taylor J, Hollaway G (2022) Management of chickpea Ascochyta blight using fungicides and cultivar resistance improves grain yield, quality, and grower profitability. Frontiers in Plant Science 13, 942220.
| Crossref | Google Scholar | PubMed |
Gaind S, Rathi MS, Kaushik BD, Nain L, Verma OP (2007) Survival of bio-inoculants on fungicides-treated seeds of wheat, pea and chickpea and subsequent effect on chickpea yield. Journal of Environmental Science and Health, Part B 42(6), 663-668.
| Crossref | Google Scholar |
Gerard T, Hill K, Linsell K, McKay A (2017) Emerging pulse root diseases in the South East Region of South Australia. Grains Research and Development Corporation. Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2019/02/emerging-pulse-root-diseases-in-the-south-east-region-of-south-australia [accessed 2 November 2023]
Graham PH, Ocampo G, Ruiz LD, Duque A (1980) Survival of Rhizobium phaseoli in contact with chemical seed protectants. Agronomy Journal 72(4), 625-627.
| Crossref | Google Scholar |
GRDC (2017) Chickpea southern region – GrowNotes™. Grains Research and Development Corporation. Available at https://grdc.com.au/resources-and-publications/grownotes/crop-agronomy/chickpea-southern-region-grownotes
Isoi T, Yoshida S (1988) Effect of thiram (tetramethyl-thiuram-disulphide) application on nodulation in soybean and kidney bean plants: observation using the root-box-culture technique. Soil Science and Plant Nutrition 34(4), 633-637.
| Crossref | Google Scholar |
Kutcher HR, Lafond G, Johnston AM, Miller PR, Gill KS, May WE, Hogg T, Johnson E, Biederbeck VO, Nybo B (2002) Rhizobium inoculant and seed-applied fungicide effects on field pea production. Canadian Journal of Plant Science 82(4), 645-661.
| Crossref | Google Scholar |
Kyei-Boahen S, Slinkard AE, Walley FL (2001) Rhizobial survival and nodulation of chickpea as influenced by fungicide seed treatment. Canadian Journal of Microbiology 47(6), 585-589.
| Crossref | Google Scholar | PubMed |
Lawrence L, Harvey P (2006) Rooting out Pythium and its destructive allies. Outlooks on Pest Management 17(5), 195-196.
| Crossref | Google Scholar |
Matus A, Sadleir J, Cronkwright J, McLeod R (2003) Effects of Crown (carbathiin and thiabendazole), Allegiance FL (metalaxyl), Vitaflo 280 (carbathiin and thiram), and Apron Maxx (fluodioxonil and metalaxyl) on N2 fixation of chickpea, dry bean, lentil, and pea. In ‘Soils and Crops Workshop’, University of Saskatchewan. Available at https://harvest.usask.ca/handle/10388/9542
Odeyemi O, Alexander M (1977) Use of fungicide-resistant rhizobia for legume inoculation. Soil Biology and Biochemistry 9(4), 247-251.
| Crossref | Google Scholar |
Osman A, Sherif A, Elhussein A, Mohamed A (2012) Sensitivity of some nitrogen fixers and the target pest Fusarium oxysporum to fungicide thiram. Interdisciplinary Toxicology 5(1), 25-29.
| Crossref | Google Scholar | PubMed |
Pande S, Siddique KHM, Kishore GK, Bayaa B, Gaur PM, Gowda CLL, Bretag TW, Crouch JH (2005) Ascochyta blight of chickpea (Cicer arietinum L.): a review of biology, pathogenicity, and disease management. Australian Journal of Agricultural Research 56(4), 317-322.
| Crossref | Google Scholar |
Panwar A, Sharma YK, Meena RS, Aishwath OP, Choudhary S, Jethra G (2015) In-vitro interaction between fungicides and rhizobacteria isolated from fenugreek root nodules. Annals of Plant and Soil Research 17(4), 336-341.
| Google Scholar |
R Core Team (2024) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org
Rathjen JR, Ryder MH, Riley IT, Lai TV, Denton MD (2020) Impact of seed-applied pesticides on rhizobial survival and legume nodulation. Journal of Applied Microbiology 129(2), 389-399.
| Crossref | Google Scholar | PubMed |
Rennie RJ, Dubetz S (1984) Effect of fungicides and herbicides on nodulation and N2 fixation in soybean fields lacking indigenous Rhizobium japonicum. Agronomy Journal 76(3), 451-454.
| Crossref | Google Scholar |
Rennie RJ, Howard RJ, Swanson TA, Flores GHA (1985) The effect of seed-applied pesticides on growth and N2 fixation in pea, lentil, and fababean. Canadian Journal of Plant Science 65(1), 23-28.
| Crossref | Google Scholar |
Revellin C, Leterme P, Catroux G (1993) Effect of some fungicide seed treatments on the survival of Bradyrhizobium japonicum and on the nodulation and yield of soybean [Gycine max. (L) Merr.]. Biology and Fertility of Soils 16, 211-214.
| Crossref | Google Scholar |
Sartori FF, Engroff TD, Godoy Sanches TH, Soave JM, Victório Pessotto M, Felisberto G, Hilgemberg Jr VE, De Borja Reis AF, Hungria M, Nogueira MA, Jaccoud-Fillho DdS, Andreote FD, Neto DD (2023) Potentially harmful effects of seed treatment and pre-inoculation on soybean biological nitrogen fixation and yield. European Journal of Agronomy 142, 126660.
| Crossref | Google Scholar |
Wu L, Chang K-F, Hwang S-F, Conner R, Fredua-Agyeman R, Feindel D, Strelkov SE (2019) Evaluation of host resistance and fungicide application as tools for the management of root rot of field pea caused by Aphanomyces euteiches. The Crop Journal 7(1), 38-48.
| Crossref | Google Scholar |


