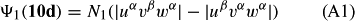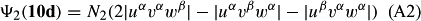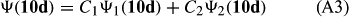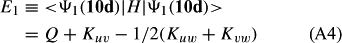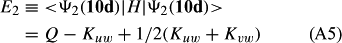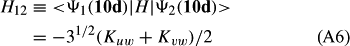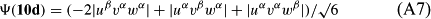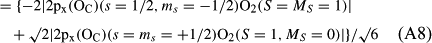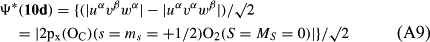Valence Bond Formulations of Mechanisms for the Formation and Decomposition of N2O5
Richard D. Harcourt A C and Thomas M. Klapötke BA School of Chemistry, University of Melbourne, Parkville, Vic. 3010, Australia.
B Department of Chemistry and Biochemistry, Ludwig-Maximilian University Munich (LMU), Munich D-81377, Germany.
C Corresponding author. Email: r.harcourt@unimelb.edu.au
Environmental Chemistry 3(5) 355-363 https://doi.org/10.1071/EN06058
Submitted: 4 September 2006 Accepted: 9 October 2006 Published: 26 October
Environmental Context. N2O5 is an important nitrogen reservoir in polar stratospheric clouds found in Antarctica and involved with the ozone hole. Here we provide valence bond representations for the gas-phase formation and decomposition of this molecule.
Abstract. Qualitative valence bond considerations are used to suggest how electronic reorganization could proceed for (a) the formation of N2O5 via the reactions NO2 + O3 → NO3 + O2, and NO2 + NO3 → N2O5, and (b) the thermal decomposition of N2O5 via the following sets of reactions: (i) N2O5 → NO2 + NO3, 2NO3 → O2NOONO2 →2NO2 + O2; (ii) NO2 + NO3 → ONOONO2 → NO + O2 + NO2, NO + NO3 → 2NO2. Increased-valence structures, which possess one-electron bonds and fractional electron-pair bonds as well as ‘normal’ electron-pair bonds, are used to represent the electronic structures of the molecules.
Keywords. : atmospheric chemistry — nitrogen oxides — ozone — valence bond structures
Acknowledgements
T.M.K. gratefully acknowledges financial support from the University of Munich (LMU), the Fonds der Chemischen Industrie and the European Research Office (ERO) of the USA Army Research Laboratory (ARL) under contract no. N 62558–05-C-0027. We thank Mrs. Carmen Nowak, at the LMU, for drawing the VB structures, and Professor Brian Duke for reading the paper and for his helpful comments. R.D.H. also thanks Dr Jonathan White for the provision of a workstation.
In ref. [10a], it has been deduced that X + O3 → XO + O2 generates O2(3Σ–g). We shall re-describe the theory here, using real rather than the complex MOs of ref. [10a] for the O2. To describe the O2–NO3 dissociation of the OAOB–OCNO2 product-like structure 10d of Fig. 10, the primary active space involves three electrons, which singly occupy the 2px(OC) AO, the π*x(OAOB) MO and the π*y(OAOB) MO. We shall designate these (assumed normalized) orbitals as u, v and w, respectively. Well before the conclusion of the reaction, the u and v orbitals overlap, to form the fractional OAOB–OC bond in 10d when their electrons are singlet spin-paired. The resulting S = MS = 1/2 spin wave function for the u, v and w electrons is given by Eqn (A1). A second, orthogonal S = MS = 1/2 spin wave function for the three electrons, is that of Eqn (A2). At any stage along the reaction coordinate, the wave function of Eqn (A3) can be constructed. Near the conclusion of the reaction, the u–v overlap is negligible, and therefore the orbital overlap integral < u|v > can be ignored. The resulting values for the normalization constants of Eqns (A1) and (A2) are then N1 = 1/√2 and N2 = 1/√6. We can then construct the energy matrix elements of Eqns (A4)–(A6). in which Q is the energy of three Slater determinants without exchange (i.e. with no antisymmetry), and the Kij = < i(1)j(2)|1/r12|j(1)i(2) > are exchange integrals for pairs of the u, v and w orbitals. When dissociation of the OAOB–OCNO2 bond occurs, the exchange integrals Kuv and Kuw are equal to zero, to give E1 = Q – 1/2Kvw, E2 = Q + 1/2Kvw and H12 = –31/2Kvw/2. Using these matrix elements, the lower-energy solution of the associated secular equation gives E = Q - Kvw, C1 = 31/2/2 and C2 = 1/2. The resulting expression for the Ψ(10d) of Eqn (A3) is that of Eqn (A7), which is equivalent to Eqn (A8). The O2 (as OAOB) component of Eqn (A8) involves the O2(3Σ–g) ground-state with MS = +1 and 0 spin components. The higher-energy solution of the secular equation gives E* = Q + Kvw, C1 = 1/2, C2 = –31/2/2 and Eqn (A9) The O2 component of Eqn (A9) involves the O2(1Δg) excited-state.
[1]
[2]
[3]
[4]
[5]
A. R. Ravishankara,
Faraday Discuss. 2005, 130, 9.
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
[and refs [13,47] therein].
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |

Appendix: Electronic States for the O2 of X + O3 → XO + O2