Experimental determination of the dissolution kinetics of zero-valent iron in the presence of organic complexants
Eric M. Pierce A B , Dawn M. Wellman A , Alexander M. Lodge A and Elsa A. Rodriguez AA Environmental Technology Directorate, Pacific Northwest National Laboratory, P.O. Box 999, MS: K6-81, Richland, WA 99352, USA.
B Corresponding author. Email: Eric.Pierce@pnl.gov
Environmental Chemistry 4(4) 260-270 https://doi.org/10.1071/EN07004
Submitted: 16 January 2007 Accepted: 30 June 2007 Published: 16 August 2007
Environmental context. Iron metal is being considered as a material to be used for the treatment of groundwater contaminated with toxic metals and organics. Although time-dependant information is available, predicting the long-term behaviour of this material has been complicated by the build-up of rust or other alteration phases on the surface of Fe metal. In addition to the build-up of rust, changes to important environmental factors also complicate these types of predictions. The research discussed in this paper uses a non-traditional experimental technique to isolate the impact of specific environmental factors (i.e. pH, temperature) and organic complexants on the dissolution of Fe metal.
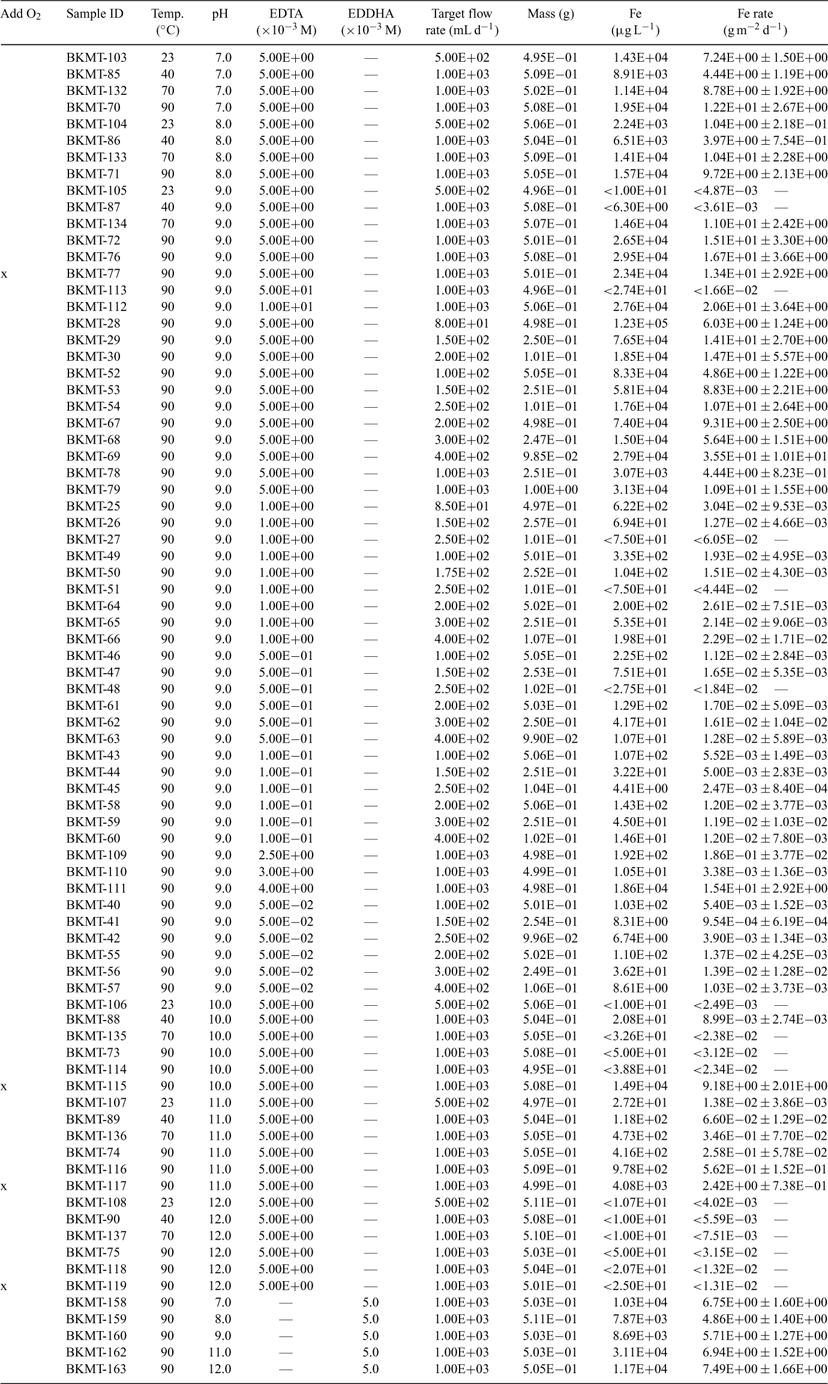
Abstract. The geochemical cycling of iron, the reactivity of iron minerals and, more recently, the reactivity of zero valent iron (α-Fe), have been the subject of numerous investigations for over more than three decades. These investigations provide a wealth of knowledge regarding the effect of pH, temperature, chelating agents etc. on the reactivity and mechanism(s) of dissolution for α-Fe and iron oxide/oxyhydroxide minerals. However, most investigations have been conducted under static conditions that promote the formation of a partially oxidised surface film (e.g. passivating layer). In the presence of a passivating layer, the proposed dissolution mechanisms are vastly different and are based on the composition of the partially oxidised surface film. The objective of this study was to quantify the dissolution of α-Fe under conditions that maintain the pO2 at a relatively constant level and minimise the formation of a passivating layer on the metal surface. Single-pass flow-through tests were conducted under conditions of relatively constant dissolved O2 [O2(aq)] over the pH(23°C) range from 7 to 12 and temperature range from 23 to 90°C in the presence of ethylenediamine tetraacetic acid (EDTA) and ethylenediamine di-O-hydroxyphenylacetic acid (EDDHA) to maintain dilute conditions and minimise the formation of a partially oxidised surface film and Fe-bearing secondary phase(s) during testing. Although more information is needed, these results suggest the adsorption of EDTA and EDDHA, or the diffusion of the oxidised Fe–organic complex from the surface of α-Fe, is the rate-limiting step in the dissolution reaction. Results also suggest that the rate of dissolution is independent of pH, has a non-linear dependence on the concentration of organic complexant, and the forward dissolution rate for α-Fe is as much as three orders of magnitude greater than when a passive film and corrosion products are present.
Acknowledgements
The authors would like to express gratitude to K. N. Geiszler, S.R. Baum, and E. Clayton, of Pacific North-west National Laboratory (PNNL), for providing high quality analytical data from sample solutions. PNNL is operated by Battelle for the DOE under Contract DE-AC05–76RL01830.
[1]
[2]
W. Stumm ,
B. Sulzberger ,
The cycling of iron in natural environments: Considerations based on laboratory studies of heterogeneous redox processes.
Geochim. Cosmochim. Acta 1992
, 56, 3233.
| Crossref | GoogleScholarGoogle Scholar |

[3]
J. Bruno ,
W. Stumm ,
P. Wersin ,
F. Brandberg ,
On the influence of carbonate in mineral dissolution. 1. The thermodynamics and kinetics of hematite dissolution in bicarbonate solutions at T = 25°C.
Geochim. Cosmochim. Acta 1992
, 56, 1139.
| Crossref | GoogleScholarGoogle Scholar |

[4]
V. Zutic ,
W. Stumm ,
Effect of organic acids and fluoride on the dissolution kinetics of hydrous α-FeOOH. A model study using the rotating disc electrode.
Geochim. Cosmochim. Acta 1984
, 48, 1493.
| Crossref | GoogleScholarGoogle Scholar |

[5]
S. B. Yabusaki ,
K. J. Cantrell ,
B. Sass ,
C. I. Steefel ,
Multicomponent reactive transport in an in-situ zero-valent iron cell.
Environ. Sci. Technol. 2001
, 35, 1493.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[6]
J. Klausen ,
P. J. Vikesland ,
T. Kohn ,
W. P. Ball ,
A. L. Roberts ,
Longevity of granular iron in groundwater treatment processes: solution composition effects on reduction of organohalides and nitroaromatic compounds
Environ. Sci. Technol. 2003
, 37, 1208.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[7]
J. S. Fruchter ,
In situ treatment of chromium-contaminated groundwater
Environ. Sci. Technol. 2002
, 36, 465.

[8]
B. Gu ,
T. J. Phelps ,
L. Liang ,
M. J. Dickey ,
Y. Roh ,
B. L. Kinsall ,
A. V. Palumbo ,
G. K. Jacobs ,
Biogeochemical dynamics in zero-valent iron columns: implications for permeable reactive barriers.
Environ. Sci. Technol. 1999
, 33, 2170.
| Crossref | GoogleScholarGoogle Scholar |

[9]
B. Gu ,
L. Liang ,
M. J. Dickey ,
X. Yin ,
S. Dai ,
Reductive precipitation of uranium (VI) by zero-valent iron.
Environ. Sci. Technol. 1998
, 32, 3366.
| Crossref | GoogleScholarGoogle Scholar |

[10]
A. Abdelouas ,
W. Lutze ,
H. E. Nuttall ,
W. L. Gong ,
Remediation of UVI-contaminated water using zero-valent iron.
Cr. Acad. Sci. II A 1999
,
, 315.

[11]
K. J. Cantrell ,
D. I. Kaplan ,
T. W. Wietsma ,
Zero-valent iron for the in situ remediation of selected metals in groundwater.
J. Hazard. Mater. 1995
, 42, 201.
| Crossref | GoogleScholarGoogle Scholar |

[12]
[13]
B. Zinder ,
G. Furrer ,
W. Stumm ,
Coordination chemistry of weathering II: dissolution of Fe(III) oxides
Geochim. Cosmochim. Acta 1986
, 50, 1861.
| Crossref | GoogleScholarGoogle Scholar |

[14]
B. Sulzberger ,
D. Suter ,
C. Siffert ,
S. Banwart ,
W. Stumm ,
Dissolution of Fe(III) (hydr)oxides in natural waters; laboratory assessment on the kinetics controlled by surface coordination.
Mar. Chem. 1989
, 28, 127.
| Crossref | GoogleScholarGoogle Scholar |

[15]
[16]
C. E. Noradoun ,
I. F. Cheng ,
EDTA degradation induced by oxygen activation in a zerovalent iron/air/water system.
Environ. Sci. Technol. 2005
, 39, 7158.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[17]
[18]
A. J. Davenport ,
L. J. Oblonsky ,
M. P. Ryan ,
M. F. Toney ,
The structure of the passive film that forms on iron in aqueous environments.
J. Electrochem. Soc. 2000
, 147, 2162.
| Crossref | GoogleScholarGoogle Scholar |

[19]
M. Büchler ,
P. Schmuki ,
H. Böhni ,
Formation and dissolution of the passive film on iron studied by a light reflectance technique.
J. Electrochem. Soc. 1997
, 144, 2307.
| Crossref | GoogleScholarGoogle Scholar |

[20]
J. Liu ,
D. Macdonald ,
The passivity of iron in the presence of ethylenediaminetetraacetic acid.
J. Electrochem. Soc. 2001
, 148, B425.
| Crossref | GoogleScholarGoogle Scholar |

[21]
B. Wehrli ,
B. Sulzberger ,
W. Stumm ,
Redox processes catalyzed by hydrous oxide surfaces.
Chem. Geol. 1989
, 78, 167.
| Crossref | GoogleScholarGoogle Scholar |

[22]
J. M. Santana-Casiano ,
M. Gonzalez-Davila ,
M. J. Rodriguez ,
F. J. Millero ,
The effect of organic compounds in the oxidation kinetics of Fe(II).
Mar. Chem. 2000
, 70, 211.
| Crossref | GoogleScholarGoogle Scholar |

[23]
B. Nowack ,
L. Sigg ,
Adsorption of EDTA and metal-EDTA complexes onto goethite.
J. Colloid Interface Sci. 1996
, 177, 106.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[24]
G. Bondietti ,
J. Sinniger ,
W. Stumm ,
The reactivity of Fe(III) hydroxides: effect of ligands in inhibiting the dissolution.
Colloid Surf., A 1993
, 79, 157.
| Crossref | GoogleScholarGoogle Scholar |

[25]
J. Rubio ,
E. Matijevic ,
Interaction of metal hydrous oxides with chelating agents I. β-FeOOH-EDTA.
J. Colloid Interface Sci. 1979
, 68, 408.
| Crossref | GoogleScholarGoogle Scholar |

[26]
H.-C. Chang ,
E. Matijevic ,
Interactions of metal hydrous oxides with chelating agents IV. Dissolution of hematite.
J. Colloid Interface Sci. 1983
, 92, 479.
| Crossref | GoogleScholarGoogle Scholar |

[27]
M. C. Ballesteros ,
E. H. Rueda ,
M. A. Blesa ,
The influence of iron (II) and (III) on the kinetics of geothite dissolution by EDTA.
J. Colloid Interface Sci. 1998
, 201, 13.
| Crossref | GoogleScholarGoogle Scholar |

[28]
M. A. Blesa ,
E. B. Borghi ,
A. J. G. Maroto ,
A. E. Regazzoni ,
Adsorption of EDTA and iron EDTA complexes on magnetite and the mechanism of dissolution of magnetite by EDTA.
J. Colloid Interface Sci. 1984
, 98, 295.
| Crossref | GoogleScholarGoogle Scholar |

[29]
W. Sunda ,
S. Huntsmann ,
Effect of pH, light, and temperature on Fe-EDTA chelation and Fe hydrolysis in seawater.
Mar. Chem. 2003
, 84, 35.
| Crossref | GoogleScholarGoogle Scholar |

[30]
F. Gambardella ,
I. J. Ganzevald ,
J. G. M. Winkelman ,
E. J. Heeres ,
Kinetics of the reaction of Fe(II)-EDTA with oxygen in aqueous solutions.
Ind. Eng. Chem. Res. 2005
, 44, 8190.
| Crossref | GoogleScholarGoogle Scholar |

[31]
W. Stumm ,
Reactivity at the mineral-water interface: dissolution and inhibition.
Colloid Surf., A 1997
, 120, 143.
| Crossref | GoogleScholarGoogle Scholar |

[32]
B. Nowack ,
L. Sigg ,
Dissolution of Fe(III) hydroxides by metal-EDTA complexes.
Geochim. Cosmochim. Acta 1997
, 61, 951.
| Crossref | GoogleScholarGoogle Scholar |

[33]
E. H. Rueda ,
R. L. Grassi ,
M. A. Blesa ,
Adsorption and dissolution in the system goethite/aqueous EDTA.
J. Colloid Interface Sci. 1985
, 106, 243.
| Crossref | GoogleScholarGoogle Scholar |

[34]
[35]
H. M. Nabhan ,
J. Vanderdeelen ,
A. Cottenie ,
Chelate behavior in saline-alkaline soil conditions.
Plant Soil 1977
, 46, 603.
| Crossref | GoogleScholarGoogle Scholar |

[36]
H. Siebner-Freidbach ,
Y. Hadar ,
Y. Chen ,
Interaction of iron chelating agents with clay minerals.
Soil Sci. Soc. Am. J. 2004
, 68, 470.

[37]
A. L. Underwood ,
Spectrophotometric determination of iron with ethylenediamine di(o-hydroxyphenylacetic acid).
Anal. Chem. 1958
, 30, 44.
| Crossref | GoogleScholarGoogle Scholar |

[38]
[39]
[40]
[41]
B. P. McGrail ,
W. L. Ebert ,
A. J. Bakel ,
D. K. Peeler ,
Measurement of kinetic rate law parameters on a Na-Ca-Al borosilicate glass for low-activity waste.
J. Nuc. Mater. 1997
, 249, 175.
| Crossref | GoogleScholarGoogle Scholar |

[42]
[43]
C. P. Huang ,
H. W. Wang ,
P. C. Chiu ,
Nitrate reduction by metallic iron.
Water Res. 1998
, 32, 2257.
| Crossref | GoogleScholarGoogle Scholar |

[44]
L. L. Zawaideh ,
T. C. Zhang ,
The effects of pH and addition of an organic buffer (HEPES) on nitrate transformation in Fe0 water systems.
Water Sci. Technol. 1998
, 38, 107.
| Crossref | GoogleScholarGoogle Scholar |

[45]
[46]
E. Sikora ,
D. Macdonald ,
The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior.
J. Electrochem. Soc. 2000
, 147, 4087.
| Crossref | GoogleScholarGoogle Scholar |

[47]
B. Nowack ,
Environmental chemistry of aminopolycarboxylate chelating agents.
Environ. Sci. Technol. 2002
, 36, 4009.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[48]
E. M. Pierce ,
J. P. Icenhower ,
R. J. Serne ,
J. Catalano ,
Experimental determination of UO2(cr) dissolution kinetics: effects of solution saturation state and pH.
J. Nuc. Mater. 2005
, 345, 206.
| Crossref | GoogleScholarGoogle Scholar |

[49]
J. P. Icenhower ,
D. M. Strachan ,
B. P. McGrail ,
R. D. Scheele ,
E. A. Rodriguez ,
J. L. Steele ,
V. L. Legore ,
Dissolution kinetics of pyrochlore ceramics for the disposition of plutonium.
Am. Mineral. 2006
, 91, 39.
| Crossref | GoogleScholarGoogle Scholar |

[50]
D. M. Wellman ,
J. P. Icenhower ,
A. P. Gamerdinger ,
S. W. Forrester ,
Effects of pH, temperature, and aqueous organic material on the dissolution kinetics of meta-autunite minerals, (Na,Ca)2–1[(UO2)(PO4)]2•3H2O.
Am. Mineral. 2006
, 91, 143.
| Crossref | GoogleScholarGoogle Scholar |

Results from SPFT Tests with α-Fe [Conditioned Amasteel (low carbon steel)]