Past atmospheric composition and chemistry from ice cores – progress and prospects
Eric W. Wolff A B , Manuel A. Hutterli A and Anna E. Jones AA British Antarctic Survey, High Cross, Madingley Road, Cambridge CB3 0ET, UK.
B Corresponding author. Email: ewwo@bas.ac.uk
Environmental Chemistry 4(4) 211-216 https://doi.org/10.1071/EN07031
Submitted: 5 April 2007 Accepted: 4 June 2007 Published: 16 August 2007
Environmental context. Investigating the past is often the only way we have of determining whether we have included all processes correctly into models, and then of verifying their behaviour. Ice cores provide an excellent way of finding out about the past. Air bubbles trapped in the ice allow us to directly access the concentration of stable trace gases, including important greenhouse gases. However, there are also tantalising possibilities to learn about aerosols and shorter-lived gases. This article describes some of the information we have already learnt from ice cores, but also describes the challenges that require understanding of atmospheric chemistry in the polar regions today in order to extract the full value of the records of the past trapped in the ice sheet.
Abstract. Ice cores provide the most direct evidence available about the past atmosphere. For long-lived trace gases, ice cores have provided clear evidence that in the last two centuries, concentrations of several greenhouse gases have risen well outside the natural range observed in the previous 650 000 years. Major natural changes are also observed between cold and warm periods. Aerosol components have to be interpreted in terms of changing sources, transport and deposition. When this is done, they can also supply evidence about crucial aspects of the past environment, including sea ice extent, trace element deposition to the ocean, and about the aerosols available for cloud nucleation, for example. It is much more difficult to extract information about shorter-lived chemical species. Information may be available in components such as nitrate and formaldehyde, but to extract that information, detailed modern atmospheric studies about air to snow transfer, preservation in the ice, and the link between the polar region boundary layer and other parts of the atmosphere are urgently required.
Additional keywords: Antarctic, Arctic, ice cores, paleoclimate.
The chemical composition of the present-day atmosphere is increasingly well recorded and understood. It has remained a challenge to obtain data at all heights in the atmosphere, and only recently have some areas, such as the polar regions, been studied in detail.[e.g. 1,2] However, we have a good knowledge of the spatial distributions of long-lived trace gases, of aerosol concentrations, and an increased understanding of radicals and short-lived gases. Increasingly, atmospheric chemistry is being considered as a component of Earth System models, because of the feedbacks between biogeochemical cycles, atmospheric chemistry and climate. However, our knowledge of atmospheric chemical compositions in the past is severely limited. Direct measurements are almost entirely absent beyond the last few decades, so that there is little direct evidence of what even a pre-industrial atmosphere contained, and even less knowledge of the more distant past, when different climates certainly altered the rates of emissions and of processes in the atmosphere. One result is that there is little constraint on the atmospheric chemistry or climate–chemistry feedbacks in model simulations of the past, and little certainty that processes occurring in climates other than those of today are correctly represented.
Among palaeoenvironmental archives, only ice cores directly record some aspects of past atmospheric composition. They are particularly powerful for long-lived trace gases, where a time-series at a single location is representative for the state of the global atmosphere. For aerosols and shorter lived gases, extensive studies are needed to understand the relationship between what has been retained in the ice and the atmospheric composition above the site, and between the composition at the site and a more regionally or globally meaningful value. Most ice core studies come from the polar regions, so that chemical processes, transport and deposition in polar regions has become a particularly important area of study. Ice cores can also be collected from high altitude mid and low latitude sites: these tend to cover a shorter time period, and require even more careful study to determine the region of the atmosphere that they represent. Some very interesting insights into changing emissions have been gained from recent studies in, for example, the European Alps;[3,4] however, this paper will concentrate on polar region ice cores.
Here, we will describe the kind of information that can be recovered from ice cores, and will give examples of the atmospheric chemical insights that have been gained from such work. However, we will also discuss the difficulties in interpreting many of the measurements and the need for further research to recover the invaluable archives that certainly exist in ice cores.
Signals in ice cores
Ice cores record information in three distinct forms.[5] The isotopic composition of the water molecules themselves acts as a proxy for past temperature. Numerous chemicals that are wet deposited with snowfall or dry deposited to the snow surface, as well as gases that adsorb onto ice surfaces, are recorded in the composition of the snow itself. In this category, for example, are the large spikes of sulfuric acid that are deposited in the years following a major volcanic eruption, and sea salt that originates from sea water and sea ice surfaces. Finally, at depth (typically 60–100 m) snow is compressed and sintered under the weight of overlying layers into solid ice with trapped air bubbles. These bubbles contain a sample of an ancient atmosphere. By cracking open the bubbles (for example by crushing the ice into tiny pieces) we can recover air for analysis of stable gases as if it was a modern sample held in a flask (although only limited volumes can be obtained). To date, ice core records are available that extend back some 800 000 years for Antarctica[6] and 123 000 years for Greenland.[7]
Ice core records of components deposited at the snow surface (e.g. aerosol components) can, at suitable sites, give seasonal temporal resolution. At such sites precise dating is also possible. However, at sites with low snow accumulation (which includes those with the longest records), such resolution (and such certain dating) is not possible. For the trace gases, resolution is determined mainly by the fact that the depth at which air bubbles become closed off from the atmosphere is not fixed: air of a particular age may become closed off over a depth range of a few metres. The corollary of this is that ice from a given depth contains air bubbles with a spread of ages. By choosing a site with a very high snow accumulation rate, we can reduce this effective resolution for the trace gases to a decade or two, but at sites with low snow accumulation rate, the age spread of each sample may be centuries.
Stable trace gases
Continuous measurements in the atmosphere began only in the 1950s for CO2, and even later for other trace gases, so that most of our knowledge of the rise in concentrations during the industrial period comes from ice cores. Sites with a high snow accumulation rate provide well resolved records of recent decades, while sites with a low snow accumulation rate offer low-resolution reconstructions of concentrations over much longer timescales. Some artefacts do occur for CO2 for Greenland ice that has high concentrations of impurities such as organic matter and carbonate-rich dust, so that Antarctic cores are preferred for this gas. For CH4, ice cores from both Greenland and Antarctica show identical patterns of change in concentration over both short and long timescales, but with a gradient in concentrations between the hemispheres that is expected from the modern distribution of concentrations (and that derives from the predominance of northern sources).
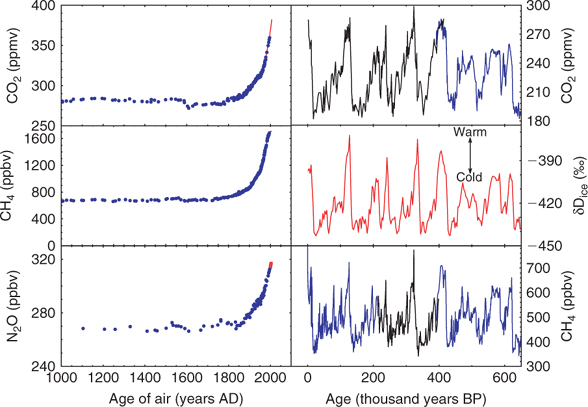
Ice core data for recent centuries[8] are shown in Fig. 1 (left). This clearly shows that both CO2 and CH4 began their recent increase in the early 19th century; CO2 has risen 35% above its pre-industrial concentration, while CH4 has more than doubled. N2O has risen 18% in the same period.
|
These changes need to be placed in the context of the much longer record of natural changes accessible from ice cores (Fig. 1, right).[9,10] Over 650 000 years the Earth has shown a repeated pattern of long cold glacial periods interspersed with short warm interglacials such as the last 11 000 years. These glacial/interglacial cycles recur roughly every 100 000 years. It turns out that during the cold periods both CO2 and CH4 were at considerably lower concentrations than in the pre-industrial period. For CO2 the reasons for change probably lie mainly in the physics and biology of the oceans; for CH4, both changes in terrestrial sources and in atmospheric sinks may be involved.[11] This raises interesting issues in atmospheric chemistry involving OH (the primary sink for CH4), its precursors and reactants. Unfortunately there are still few data to constrain the relative importance of source and sink changes, an issue that both models and ice cores might in the future address.
Chemicals with shorter atmospheric lifetimes
Even CH4, with an atmospheric lifetime of approx. 10 years, shows a significant interhemispheric difference in concentration (that can be useful as a diagnostic of source and sink locations[12]). Gases with a shorter lifetime, and aerosols with typical lifetimes against deposition of days to weeks, will show very strong regional differences that may vary with climate and other factors, and this requires a more complicated interpretation of ice cores.
In ice cores, we measure what is preserved as the snow layer is buried and compressed. Working backwards, we have to be concerned first with losses and diffusional processes in the snowpack. Re-volatilisation of impurities, photochemistry in the snowpack,[1] and slow chemical reactions can all lead to changes from the initially deposited material. Deposition processes are also important: the deposited concentration as well as losses of a gas-phase species may depend on temperature and snow accumulation rate, while aerosols are deposited in both wet and dry processes that can also change with time.[13] The processes described in this paragraph all complicate the relationship between the ice core concentration of a chemical and its local atmospheric concentration.
However, the local atmospheric concentration is also usually not the quantity of environmental interest. First, we measure an ice concentration that may depend on the concentration in the atmosphere at a range of altitudes (e.g. cloud level and below for wet deposition, ground level for dry deposition). If there are strong vertical gradients in the atmosphere, the significance of the ice measurement must be carefully considered. Vertical profiling of the chemical composition of the polar regions troposphere is still in its infancy, and most studies to date have considered only the relationship between snow concentrations and the near surface atmospheric concentration. Second, transport and deposition on route from the source region will have a strong influence on the concentrations of chemicals that reach remote polar sites, particularly for aerosol species. We are often most interested in source strengths and how they have changed with time. If the transport time or the residence time of aerosol has changed this may affect the concentrations we measure, and often a combination of modelling and prior knowledge is required to separate these effects from those of the changing source. Finally, for short-lived gases such as H2O2 or NOx we have to consider the relationship between the concentrations we deduce or measure in the polar atmosphere and more regionally relevant concentrations that may be diagnostic for significant changes in the atmosphere.
In the following sections, we discuss some time series of shorter-lived chemicals and the factors that influence them, before considering what new research is needed to allow us to decipher these records.
Irreversible aerosols
For time periods where it is reasonable to assume that climatic changes have not significantly altered the strength of transport or deposition, changes in aerosol concentrations can be assumed to be dominated by changes in source strength. Thus concentrations of Pb in Greenland snow were shown to have increased by a factor >100 from prehistoric times to the late 1960s and then decreased again by a factor >10 by the 1990s.[14,15] Such large changes can only have been a result of increased emissions followed by the effect of abatement measures (principally the introduction of unleaded gasoline). Similarly, a factor of 2 increase in sulfate, and a factor of 3 increase in nitrate concentrations in Greenland snow[16] must reflect increases in the precursor gases being transported from industrial areas of Europe and North America.
The situation is more complicated when we investigate glacial–interglacial timescales where the entire climate has altered, so that both transporting circulation and the hydrological cycle have altered significantly. For example, the huge increase in concentrations of calcium (representing terrestrial dust) recorded in Greenland cores in the last glacial period has been ascribed to a combination of changes in source, transport and lifetime.[17]
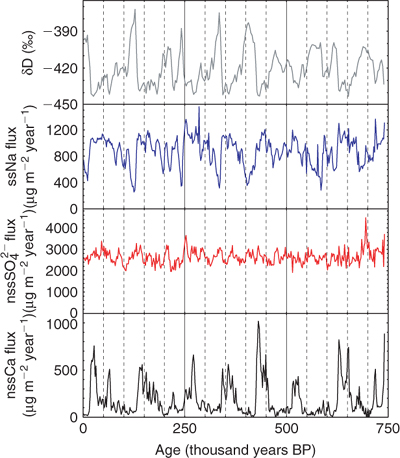
Recently, records of aerosol concentrations over the last 740 000 years were presented from the Dome C ice core produced by the European Project for Ice Coring in Antarctica (EPICA).[18] Because the snow accumulation rate is so low at this site, dry deposition is expected to dominate, and in this case it is expected that fluxes rather than ice concentrations represent atmospheric composition.[19] Models of atmospheric transport have suggested that neither transport nor residence time should have altered appreciably between the glacial and interglacial periods,[e.g. 20] although this assumption certainly requires further study. If we accept this conclusion, then we conclude that the atmospheric concentrations of terrestrial dust (represented by Ca and Fe) were increased by a factor of 20 at the last glacial maximum and other cold periods compared to the present, and that this must reflect changed conditions at the source in Patagonia (Fig. 2). Such increases in terrestrial aerosol load (matched by the increased northern high latitude aerosol load inferred from Greenland ice cores) have important implications for climate (through aerosol radiative forcing), for biogeochemistry (through deposition of Fe to the ocean) and for atmospheric chemistry (through changing the surfaces available for heterogeneous reactions). The factor of 2 increase in sea salt loading implied by the ice core record for glacial maxima has been interpreted as an indicator of increased sea ice extent,[21] although this interpretation remains somewhat controversial.
|
Both non-sea-salt (nss) sulfate and methanesulfonate (MSA) in Antarctica derive mainly from oxidation of dimethylsulfide (DMS). In the past, MSA has been used as an indicator of DMS production because of its apparent simplicity: sulfate requires a sea salt correction and also has a volcanic source, while MSA is produced only from DMS. The finding that MSA in ice from central Antarctica (Vostok) had much higher concentrations in the last glacial period than in the present[22] was taken to imply that there was much greater biological production of DMS in the Southern Ocean at that time. This would have been an interesting case study for the so-called CLAW hypothesis,[23] in which it was hypothesised that a climate-related increase in DMS production might lead to increased concentrations of sulfate aerosol capable of acting as cloud condensation nuclei (CCN), which would in turn have been expected, through cloud, to cause a climate feedback. However, in the last decade, it has been shown convincingly that MSA is partly re-emitted from the snowpack at sites with very low snow accumulation rate,[24,25] so that under present-day conditions, the concentration recorded in the ice is much reduced compared to that initially deposited. In contrast, MSA is retained by dust-laden glacial age snow, so that the glacial–interglacial contrast in MSA concentrations almost certainly results from a post-depositional artefact.
Because nss sulfate undergoes no such re-emission, it can be used reliably as an indicator of glacial-interglacial changes, despite the other complications involved. It was found that the nss sulfate flux has been almost constant (within ±20%) over the last 740 000 years (Fig. 2).[18] The conclusion is that, while the hypothesised feedback[23] may be possible, the Antarctic ice cores provide no evidence that it actually occurred, at least in the sector of the Southern Ocean sampled by the Dome C measurements.[26]
More complex and reversible chemicals
There would be huge interest in learning about the past strength of some of the processes that control atmospheric chemistry, such as the oxidative capacity of the atmosphere, or its NOx status. However, the short-lived species of most interest are obviously not directly accessible, and only indirect indicators can be measured. Here we discuss two case studies of the progress in this area, as an indication of where further research may yield success.
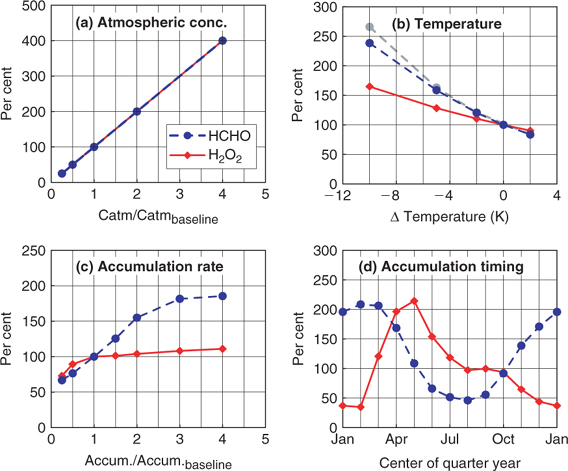
Earlier, it was proposed that HCHO in ice could be used (in combination with known concentration changes in CH4) as an indicator of the oxidative status of the atmosphere.[27] Unfortunately, it turns out that HCHO is strongly affected by post-depositional processes, both revolatilisation[28,29] and photochemical production.[1,30] Only intense study over the last decade has identified the environmental factors (temperature, snow accumulation rate, seasonal distribution of snow accumulation) that control the preservation of HCHO in the snowpack (Fig. 3).[29] In Greenland snow, the concentration of HCHO has increased by around 90% in the last century. Only by accounting through modelling for the environmental factors discussed above can it be determined whether a part of the change in concentration in ice reflects a changing atmospheric concentration of HCHO. Taking into account the known increase in CH4 concentration (factor of >2) over this time period, it may be possible through atmospheric chemical modelling to place limits on the change in OH concentration in some particular regions of the atmosphere that are consistent with this result. This would be an interesting result, but not on the grand scale originally hoped for. In addition, it is currently not possible to make the much more intense correction that would be required over longer timescales. There may be possibilities to gain knowledge of larger scale information about oxidants in the atmosphere through recently developed techniques such as measurement of the mass-independent 17O anomaly in oxidation products such as nitrate, but the detailed transfer function from the ice measurement to global product is again likely to be complicated. Detailed studies of the present-day atmosphere will be required to establish the significance of such data.
|
Nitrate has been extensively measured in polar ice cores, and there have been hopes that it might be possible to link it to atmospheric NOx concentrations. However, nitrate undergoes loss from the snowpack through both revolatilisation[31] and photolysis;[e.g. 32] the effect is particularly strong at inland Antarctic sites with low snow accumulation rates, for example Dome C, where very high concentrations in surface snow (up to 1000 μg kg–1) can be reduced to as little as 10 μg kg–1 by a depth of 1 m.[33] However at sites with much more modest losses, it is again reasonable to assume that, under conditions where climate has not changed significantly, major changes in ice core nitrate concentration probably do represent changes in atmospheric nitrate concentrations. On this basis, increasing nitrate concentrations in Greenland snow deposited during the period from ~1930 to 1980[34,35] probably do result from increases in NOx emissions in Europe and North America. However, where much larger climate changes occur, such as from interglacial to glacial climate with increased dust concentrations, it is likely that a changed strength of deposition processes and losses must be accounted for quantitatively before any broad significance can be drawn from changing concentrations.
In fact, it appears as if, through photolysis of snow nitrate, there is a relationship between snow nitrate concentrations and local boundary layer NOx concentrations. Effectively the photolysis of nitrate in snow is (at least in spring and summer) the main source of NOx to the boundary layer.[36] The relationship is, therefore, in the opposite sense to that assumed: snow nitrate controls boundary layer NOx, but this simple statement has only local significance. Whether it will be possible, through better understanding, modelling and maybe isotopic measurements[37] to relate the deposition to snow to some large-scale input from above the boundary layer, remains to be seen.
Conclusion
Ice cores have already provided a significant window into past atmospheric composition, both in the pre-industrial period and over glacial–interglacial timescales. They have been particularly powerful in respect of long-lived trace gases, where measurements made in ice have global significance, and for aerosol components that can be related to particular and important environmental sources. For shorter-lived species, it has proven difficult to relate the concentrations measured in ice to a relevant atmospheric concentration. However, ice cores still represent our best chance of placing further constraints on past atmospheric composition. If this potential is to be realised, extensive study of air–snow transfer, of polar atmospheric chemistry, and of transport of chemistry to the polar regions is needed. In addition, models that connect global atmospheric chemistry to the polar boundary layer and ice will be required so that different scenarios of the past atmosphere can be confronted with the measurements available from polar ice. Some of this work will be carried out in several projects during the International Polar Year of 2007–2008.
[1]
A. M. Grannas,
A. E. Jones,
J. Dibb,
M. Ammann,
C. Anastasio,
H. J. Beine,
M. Bergin,
J. Bottenheim,
Atmos. Chem. Phys. Discuss. 2007, 7, 4165.
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |
| Crossref | GoogleScholarGoogle Scholar |



