Changes in proportions of arsenic species within an Ecklonia radiata food chain
Simon Foster A B , William Maher A and Frank Krikowa AA Ecochemistry Laboratory, Institute of Applied Ecology, University of Canberra, Belconnen, ACT 2601, Australia.
B Corresponding author. Email: simon.foster@canberra.edu.au
Environmental Chemistry 5(3) 176-183 https://doi.org/10.1071/EN07063
Submitted: 6 September 2007 Accepted: 20 May 2008 Published: 19 June 2008
Environmental context. The present study examines arsenic species in kelp and associated grazing animals of an Ecklonia radiata food chain. The study focusses on the changes in proportions of arsenoribosides obtained from E. radiata and mechanisms are proposed to explain the transformations of arsenoribosides observed in the organisms that graze on it.
Abstract. Total arsenic and arsenic species in the tissues of three growth stages of the macroalgae Ecklonia radiata and within organisms that feed on it are reported. Arsenic concentrations in E. radiata tissues varied from 40 to 153 μg g–1. Growth stage did not influence arsenic concentrations or arsenic species. E. radiata contained glycerol arsenoriboside (1–8.5%), phosphate arsenoriboside (10–22%) and sulfonate arsenoriboside (73–91%). Arsenic concentrations varied significantly among animal species and between tissues (5–123 μg g–1). Animals contained variable quantities of arsenobetaine (14–83%). Haliotis rubra tissues contained high concentrations of glycerol trimethylarsonioriboside (0.7–22%) and the fish Odax cyanomelas contained large quantities of phosphate arsenoriboside (25–64%) with little arsenobetaine (1.5–15%).
Arsenoribosides consumed from macroalgae are substantially converted or differentially accumulated as glycerol and phosphate arsenoribosides in animal tissues. In all animals, phosphate arsenoriboside would appear to be conserved or synthesised de novo. In gastropods, glycerol trimethylarsonioriboside and thio arsenic species are formed in the digestive system. Thus, the intermediate arsenic species that form a plausible pathway for the formation of arsenobetaine from dimethylarsenoribosides are present.
Additional keywords: Ecklonia radiata ecosystem, herbivores, macroalgae, total arsenic.
Introduction
Most arsenic in marine macroalgae is present as dimethylarsenoribosides (arsenoribosides),[1] whereas in marine animals, arsenic is in the form of arsenobetaine (Me3As–CH2–COO–, AB) (see Fig. 1 for relevant structures).[1]
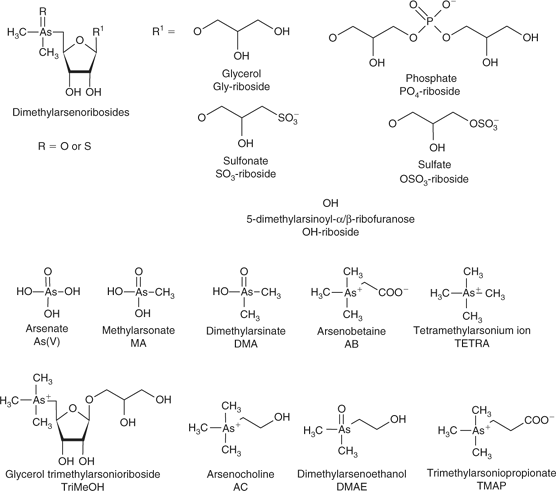
|
Little work has reported on the transfer and conversion of arsenoribosides in organisms. In non-digestive tissues, 3′-[(2″,3″-dihydroxypropyl)hydroxyphosphinyloxy]-2′hydroxypropyl 5-deoxy-5-dimethylarsinoyl-β-d-riboside (PO4-riboside) is commonly found, either alone or together with 2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinoyl-β-d-riboside (Gly-riboside).[2] Trimethylarsonioriboside (2′,3′-dihydroxypropyl 5-deoxy-5-trimethyl-arsonioriboside; TriMeOH) has been shown to form arsenocholine under anaerobic conditions.[3] It is also likely that TriMeOH is also formed in the digestive system of marine animals, especially gastropods.[4,5] Thioarsenoribosides have also been found in molluscs.[6–8] At low pH (~1), arsenoribosides degrade to 5-dimethylarsinoyl-α/β-ribofuranose (OH-riboside)[9] whereas at high pH (~11), they are stable.[10] Anaerobic decomposition of arsenoribosides produces 2-dimethylarsinoyl ethanol (Me2As(O)–CH2–CH2OH; DMAE),[11] whereas aerobic decomposition produces OH-riboside, dimethylarsinate (DMA) and inorganic arsenic.[12] These degradation studies were conducted in sediments, however, are microbially mediated, and would be similar to what occurs in the digestive systems of herbivorous animals. Shrimp (Crangon crangon) have been fed Gly-riboside and TriMeOH but accumulated little of these arsenoribosides (0.9% and 4.2%, respectively), with ~50% of the TriMeOH being converted to AB.[13] The conversion of TriMeOH to AB indicates that when TriMeOH is produced in situ in marine animals, some conversion to AB would be expected.
Ecklonia radiata ecosystems are common on the south-east coast of New South Wales, Australia. E. radiata forms dense monocultures that contain few other algae species, owing to competition for light and space.[14] E. radiata is a laminarian alga with a single erect stipe, one primary blade and numerous secondary blades.[15] The meristem is located at the tip of the stipe; hence, elongation of the primary blade occurs from the base, whereas erosion occurs at the tips.[16] E. radiata is present in various stages of growth (the growth stages have been described as juvenile (stage 1), intermediate (stage 2), and mature plants (stage 3)).[14] E. radiata can remain in the juvenile state for long periods, waiting for a break in the canopy allowing further growth.[14] In E. radiata forests, animals have a diet of live E. radiata or detritus derived from E. radiata.[17]
The dominant animal species inhabiting E. radiata ecosystems on the south-east coast of New South Wales, Australia, are the gastropods Haliotis rubra and Turbo torquatus, the sea urchins Heliocidaris erythrogramma and Centrostephanus rodgersii, and the fish Odax cyanomelas.[18,19] H. rubra are predominantly detritivores, mainly consuming macroalgae detritus.[20] The gastropod T. torquatus tends to consume early stage macoralgal gametophytes, filamentous algae, and microalgae from rock surfaces.[21] The sea urchins H. erythrogramma and C. rodgersii are the most significant consumers of brown macroalgae in rocky reefs.[22] O. cyanomelas tend to consume the blades of E. radiata.[18]
Materials and methods
Sampling and sample preparation
Samples were collected from three locations (Broulee, Long Beach and Rosedale) on the south-east coast of New South Wales, Australia. Samples were collected by hand in 3–7 m of water. E. radiata, H. rubra, T. torquatus, H. erythrogramma, and C. rodgersii were removed from the rock with a flat-bladed knife, O. cyanomela were caught with the use of a single prong hand-spear. O. cyanomela were placed into separate plastic bags and kept on ice till dissected. The algae and remaining animals were maintained alive in aerated seawater until dissection of the organisms into their various tissues. E. radiata was cleaned of any epiphytic growth by scrapping with razor blades and rinsing in deionised water before dissection. Animal tissues were dissected with a stainless steel surgical scalpel, rinsed in deionised water and placed into acid-cleaned polyethylene vials. All samples were processed and frozen in dry ice on the day of collection.
On arrival at the laboratory, all samples were lyophilised (Labconco, Sydney, Australia). Tissues were homogenised using either a Retsch ZM100 mill (0.2-mm stainless steel mesh, Retsch, Sydney, Australia), or homogenised using liquid nitrogen in an agate mortar and pestle, and stored in clean polyethylene vials in a desiccator until analysed.
Total arsenic and arsenic species analysis
Tissues were digested with nitric acid using a microwave digestion procedure described previously by Baldwin et al.[23] After cooling, digests were diluted to 10 mL in polyethylene vials with deionised water. Total arsenic concentrations were determined by inductively coupled plasma mass spectrometry.[24] Certified reference materials analysed for arsenic were NIST SRM 1566a Oyster tissue, NRCC DORM-2 Dogfish muscle and BCR 279 Ulva lactuca. Measured arsenic concentrations (mean ± s.d.) for Oyster tissue were (n = 12): measured, 13.5 ± 0.8 μg As g–1, certified 14.0 ± 1.2 μg As g–1; DORM-2 (n = 14): measured, 18.4 ± 1.2 μg As g–1, certified 18.0 ± 1.1 μg As g–1 and Ulva lactuca (n = 12): measured 3.3 ± 1.5 μg As g–1, certified 3.09 ± 0.20 μg As g–1.
Water-soluble arsenic species were extracted from biological materials by a microwave extraction procedure developed by Kirby et al.[25] Separation and measurement of arsenic species were made by high pressure liquid chromatography–inductively coupled plasma mass spectrometry. The chromatographic conditions and arsenic standards used have been reported previously.[6]
The accuracy of the arsenic speciation procedure was determined by the analysis of the certified reference material, DORM-2. The concentrations (mean ± s.d.) of AB (17.1 ± 0.9 μg g–1) and tetramethylarsonium ion (TETRA) (0.236 ± 0.011 μg g–1) measured in DORM-2 tissue (n = 3) were similar to certified values (AB, 16.4 ± 1.1 μg g–1; TETRA, 0.248 ± 0.054 μg g–1). The reproducibility of the peak times is presented in electronic data Tables A4, A6–A8 (N.B. all tables are contained in an Accessory publication).
Data analysis
Significant differences in arsenic concentrations between locations, tissues and growth stages were determined by univariate analysis of variance with a significance level of P = 0.05 applied to log-transformed data (SPSS 14, Sydney, Australia). Cluster analysis and principal component analysis were used to classify groups with similar proportions of arsenic species (Primer 5; PRIMER-E PTY LTD, Plymouth, UK).[26]
Results and discussion
Total arsenic concentrations and species in Ecklonia radiata
Significant differences in arsenic concentrations were found between macroalgal tissue types (Table A1) (d.f. = 3, mean squared (MS) = 6.253, F distribution = 19.522, P < 0.001) with arsenic concentrations of blades = meristem = stipe < holdfast. Arsenic concentrations in blades measured in the present study were similar to those reported for Stage 3 plants (25–53 μg g–1 dry mass) with holdfasts excluded (Table A2).[27] The higher arsenic concentrations in holdfasts may be due to the presence of the large abundance of macro and microorganisms living and decaying within the holdfast area contributing to the arsenic content.[28]
Arsenic concentrations of similar tissues in the three growth stages (Table A1) did not significantly differ (d.f. = 2, MS = 0.153, F = 1.434, P = 0.283). However, considerable variability in arsenic concentrations of Stage 1 blades may have masked any significant differences between these tissues. Differential accumulation of arsenic with growth is not evident.
Little arsenic (0.1–1.2%) was extracted into acetone whereas 73–107% of arsenic was extracted with methanol/water (Table A3). Although differences in arsenic concentrations are evident between the tissues and growth stages (Table A3), proportionately only minor differences in arsenic species were found between any tissue and growth stage (Table A4). SO3-riboside ((S)-2′-hydroxy-3′-sulfooxypropyl 5-deoxy-5-dimethylarsinoyl-β-d-riboside) was the major arsenic species in tissues of all growth stages (Table A4). OSO3-riboside ((R,S)-2′-hydroxy-3′-sulfonylpropyl 5-deoxy-5-dimethylarsinoyl-β-d-riboside) was only found in the holdfasts of stage 1 and stage 2 plants (Table A4). Overall in all the tissues and stages, the ratio of Gly-riboside, PO4-riboside, SO3-riboside, and OSO3-riboside was 4 : 16 : 79 : 0.4, similar to those found by Tukai et al.[27] with a Gly-riboside, PO4-riboside, SO3-riboside ratio of 2 : 22 : 71; OSO3-riboside was not detected. This is also the same general pattern of arsenic species distribution in E. radiata reported by Francesconi and Edmonds.[29] The large amount of inorganic arsenic measured in the stipe of the Stage 1 plant (13%) is noted (Table A4) but does not appear to be a general phenomenon and may be associated with microscopic epiphytes that could not be removed. Thioarsenic species were found in small quantities (0.1–1.8%, Table A4) with thiophosphate arsenoriboside (thio-PO4-riboside) accounting for ~60% of the thioarsenic species. Thioarsenoriboside concentrations measured in the present study are similar to those found by Meier et al.[30] in fresh Fucus vesiculosus. In our experience, thioarsenic species comprise little of the arsenic present in fresh healthy macroalgae; however, substantial concentrations of thioarsenic species are found in decaying and damaged macroalgae (15–17%),[31] thus are mainly formed during decomposition. No TriMeOH was found in any macroalgae samples. Only one study has ever reported the presence of TriMeOH in macroalgae.[32] An unidentified cationic species eluting at 5.7 min was also found, corresponding to less than 1% of arsenic, in nearly all samples (Table A4).
These results show that animals that eat E. radiata blades, meristems and stipes (holdfasts are rarely eaten) will ingest similar quantities of arsenic and arsenic species irrespective of the tissue or growth stage consumed.
Total arsenic concentrations and species in animals
Arsenic concentrations varied significantly between animal species and tissues (Tables A1 and A2) with arsenic body burdens varying in the order (lowest to high As concentration): O. cyanomelas < H. erythrogramma = T. torquatus < C. rodgersii < H. rubra. Mean arsenic concentrations in H. rubra muscle were within the same range as that found by Kirby et al.[4] (38–96 μg g–1) for H. rubra. However, in the present study, H. rubra intestinal arsenic concentrations were much higher (112 ± 1 μg g–1; Table A2) than previously reported (60 ± 23 μg g–1).[4] Arsenic concentrations in the gastropods H. rubra and T. torquatus were within the range usually found in marine gastropods.[1,4,5] Arsenic concentrations in the tissues of the fish O. cyanomelas are similar to those previously found in this species.[4] High arsenic concentrations in the intestinal tissues of the animal species analysed in the present study are consistent with a macroalgal diet high in arsenic.
Little arsenic (0.4–5.7%) was extracted into acetone, with the exception of the fatty tissues of H. erythrogramma and C. rodgersii gonads and O. cyanomelas liver where 8.9, 16 and 12%, respectively, were extracted (Table A3). Methanol/water extraction of arsenic was highly variable for the different animal tissues (Table A3). Low extraction recoveries (25–36%) were found for food pellets from the gut of both H. erythrogramma and C. rodgersii. It is likely the food pellets are partly insoluble geological material dislodged from rocky surfaces during grazing. Based on our experience in extracting non-methanol/water soluble arsenic species, the remaining arsenic species in these samples are likely to be inorganic and simple methylated arsenic species.[33] Total column recoveries were generally high, illustrating that little of the extracted arsenic is uncharacterised (Table A3).
Principle component analysis did not discriminate the data well as most of the animal tissues were similar in composition (Table A5 and Fig. A1).
Arsenoribosides
Gastropods H. rubra and T. torquatus
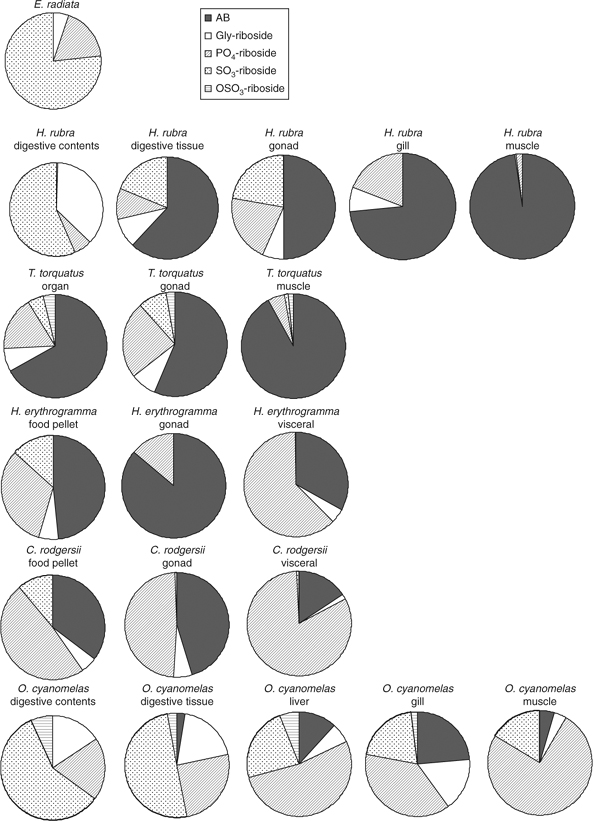
The proportion of arsenoribosides in the digestive contents and digestive tissue of H. rubra reflect the relative proportion of arsenoribosides in E. radiata (Fig. 2; Tables A6 and A8), assuming some degradation of the arsenoribosides (Tables A6 and A8). In non-digestive tissues, however, Gly-riboside and PO4-riboside increase proportionally relative to SO3-riboside, which may reflect conservation of the former or de novo synthesis of PO4-riboside by the animals (Fig. 2; Tables A6 and A8).
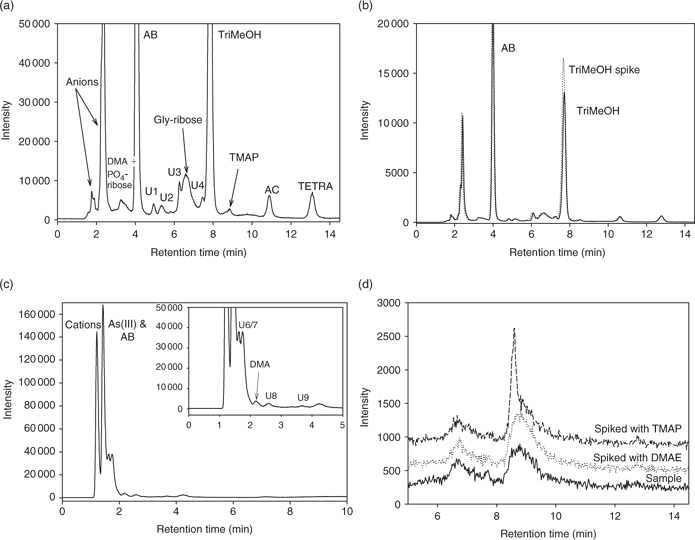
|
TriMeOH in the digestive tissues indicates that further methylation of the dimethylarsenoribosides is occurring during digestion or shortly after absorption through the gut wall (Fig. 3a, b; Table A6). As TriMeOH is also present in the gut contents, the former is more likely. Two unidentified arsenic species (U6/7), which could not be separated, were also found in the digestive tissues (Fig. 3c). Thioarsenoribosides are present in all tissues (Table A7) with the major species being thio-PO4-riboside.
Sea urchins H. erythrogramma and C. rodgersii
PO4-riboside was the major arsenoriboside in sea urchin tissues (Fig. 2; Table A8), which for H. erythogramma and C. rodgersii amounts to 23 ± 3 and 42 ± 5 μg g–1, respectively. In contrast, Kirby et al.[4] reported only minor concentrations of PO4-riboside in all tissues.
Fish O. cyanomelas
Digestive tissues and gut contents contained similar proportions of arsenoribosides as found in E. radiata (Fig. 2). However, OSO3-riboside is also found in fish tissues and not in the E. radiata blades and meristem tissues that comprise the diet of this species. In liver, gill and muscle tissues, PO4-riboside (25–64%) was again the major arsenoriboside (Fig. 2; Table A8). In contrast to Kirby et al.,[4] we found no evidence of TriMeOH formation (Table A6), and it may be that this is an intermediate formed during the digestive process and rapidly metabolised or excreted.
Other arsenic species
AB and trimethylarsoniopropionate (TMAP)
The gastropods and sea urchins contained large quantities of AB in muscle, gonad and gill tissues (46–92%), with lower amounts in digestive and visceral tissues (14–25%), whereas O. cyanomelas had low amounts of AB in all tissues (1.5–15%). AB was also found in large amounts in sea urchin food pellets (Table A6), providing a direct source of AB. Sea urchins eat E. radiata but also graze on rock surfaces and ingest microalgae and bacteria that are formed into a pellet in the oesophagus.[34] We have found that algae growing on the surface of rocks contains AB,[6] thus providing a direct source. TMAP was found in higher concentrations in H. rubra digestive tissues, and in low concentrations in other animals and tissues (Table A6).
Arsenocholine (AC)
AC was found in moderate amounts in the gastropod tissues, particularly H. rubra (Table A6).
DMA
DMA was present in non-digestive tissues in small amounts (<11%, Table A8). The presence of high concentrations of DMA in gut contents, food pellets and some tissues (19–27%, Table A8) indicated that consumed arsenoribosides are being degraded and possibly excreted.
Tetramethylarsonium ion (TETRA)
In T. torquatus organs, TETRA accounts for 17% of the arsenic (Table A6). TETRA has been found in high concentrations in gastropods and is probably formed by the methylation of DMA within the gut or digestive tissues in gastropods.[1]
DMAE
DMAE (tentatively identified by cochromatography) was found in sea urchin food pellets (12–14%) and some tissues (<2.5%) (Fig. 3d; Table A6). DMAE has been found in a range of marine fish tissues;[35] however, never in large concentrations.
Inorganic arsenic (AsV)
Substantial amounts of AsV were only found in the gut contents of H. rubra (Table A8) and AsV probably is a degradation product of arsenoribosides.
Possible mechanisms for the metabolism of arsenoribosides and formation of AB
Before arsenic metabolism can be discussed, an appreciation of the processes that occur in animal digestive systems is needed. The gastropods and O. cyanomelas have mildly acidic foreguts and alkaline hindguts (pH 5.6–6.5, 8.4–9.2 respectively);[36,37] however, the pH in the foregut may drop to pH 2–3 during digestion.[38] Foreguts are aerobic and hindguts are anaerobic. The physiochemical conditions in sea urchin digestive systems have not been described, but as aerobic and facultative anaerobic bacteria have been isolated from their digestion system,[39] they must also experience aerobic and anaerobic conditions. The sea urchins’ digestive tract also contain alginase that requires pH > 4 to function.[40] All these animals are fermentors with gut microbial communities that convert carbohydrates to alcohols or acids under anaerobic conditions.[36,41]
Arsenoribosides are likely to be degraded during fermentation; however, unlike glucose-based sugars, pentose fermentation is incomplete.[42] DMAE, DMA, and AsV are common degradation products of macroalgae (Fig. 4a).[11] DMA was present in all tissues (Table A8). Maher et al.[43] found DMA in the blood of the fish Mugil cephalus; thus, absorbed DMA can potentially be circulated via the blood to other tissues. AsV is generally not found in animal tissues (Table A8). Given that AsV interferes with phosphorylation,[44] the uptake and circulation of AsV would not be favoured and mechanisms would exist to exclude the uptake of AsV. Edmonds et al.[11] have shown that anaerobic decomposition of arsenoribosides produces DMAE. This arsenic species was only found in sea urchin tissues (Table A6). We have found that in anaerobic environments, decaying macroalgae produce DMAE, which is subsequently rapidly degraded to DMA and AsV.[31]
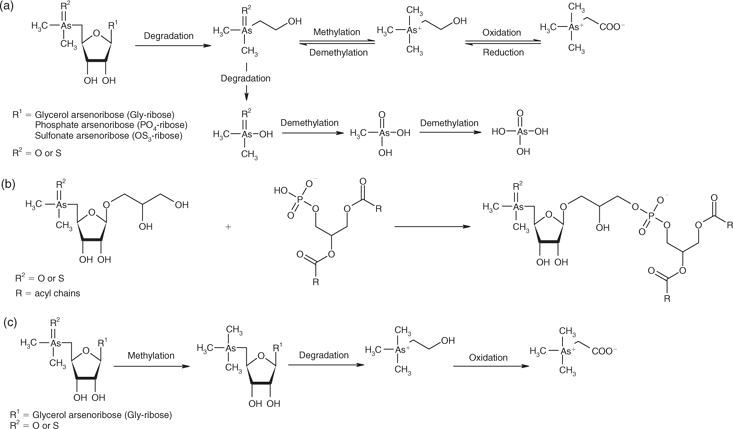
|
In the present study, Gly-riboside and PO4-riboside were the major arsenoribosides found in animal tissues (Tables A6 and A8). As the animals’ digestive systems are mainly alkaline to neutral and not likely to experience low pH for long periods, degradation of arsenoribosides to OH-riboside, shown to occur slowly at low pH,[9,45] would probably not occur in these organisms.
The prevalence of SO3-riboside in E. radiata and of Gly- and PO4-ribosides in the animals that feed on it suggests that the transformation of the former into the latter might occur in the food chain. Literature reports of the microbial conversion of sulfonates into alcohols offer some support for this suggestion.[46] Anaerobic desulfonation has been demonstrated during fermentation, resulting in the loss of SO3 as sulfide or thiosulfate.[47]
Although differential accumulation of PO4-riboside cannot be discounted, it is unlikely that high PO4-riboside concentrations in animal tissues are the result of direct ingestion, as their diet is composed of ~79% SO3-riboside from E. radiata, (Table A4). PO4-riboside has been found previously as the sole arsenoriboside in the digestive gland of the western rock lobster Panulirus cygnus, and in mussels.[1]
PO4-riboside is likely to be synthesised in vivo as it is found in almost all marine organisms. Suwalsky and coworkers[48] have shown that inorganic and methylated arsenic compounds interact with dimyristoylphosphatidylethanolamine under model conditions. Therefore, it could be possible that Gly-riboside could bond with phosphatidic acid in cell membranes (Fig. 4b). The likelihood of Gly-riboside participating in this type of reaction is supported by the arsenic species formed when fish were fed AC, and small quantities of glycerophosphosphorylarsenocholine (R1P(OO)OCH2CH(OR)CH2(OR) where R1 = AC) were produced.[49] By analogy, if R1 is Gly-riboside, PO4-riboside is formed following hydrolysis. Animals that have a low AB content have a high PO4-riboside content, indicating that storage of this sugar may be occurring rather than conversion to AB.
Thioarsenoribosides and TriMeOH (Table A6) could be formed in the digestive tissues of animals (Fig. 4c) because they are anaerobic and contain thiols and hydrogen sulfide. E. radiata had small quantities of thioarsenoribosides (Table A7) but TriMeOH was not present (Table A6) and is rarely reported in other seaweeds.[32] It has been shown that conversion of thio-Gly-riboside to TriMeOH occurs.[50] As thioarsenoribosides are entering through the diet and probably being produced in digestive tissues, formation of TriMeOH maybe occurring via this route (Fig. 4c). The majority of arsenic in H. rubra muscle tissue is AB (Table A6), yet there is no evidence of sufficient concentrations of AB in the abalones’ diet to yield these high concentrations. The presence of high concentrations of TriMeOH and AC in digestive tissues supports the direct synthesis of AB from dimethylarsenoribosides, via cleavage of the riboside ring and subsequent decarboxylation to give AC with further oxidation to form AB (Fig. 4c), such as outlined by McSheey et al.[51] Similarly, the presence of DMAE and AC in sea urchins (Table A6) also provides a possible pathway for the formation of AB from dimethylarsenoribosides (Fig. 4a) via the mechanisms proposed by Edmonds et al.[11] The metabolism of DMAE is, however, ambiguous as degradation to DMA and AsV readily occurs.[31,52]
Gastropods, sea urchins and O. cyanomelas accumulate and/or metabolise arsenoribosides differently as indicated by the different proportions of AB, arsenoribosides and other minor arsenic species. We are at present synthesising labelled arsenoribosides (and other species) found in animals to confirm the pathways of arsenoriboside metabolism and the biosynthesis of AB in marine organisms.
Acknowledgements
We would like to thank the Ecochemistry class for assistance with field sampling and sample preparation. The University of Canberra’s Vice Chancellor’s scholarship for S. Foster is gratefully acknowledged.
All tables and Fig. A1 are contained in an Accessory publication, which is available from the Environmental Chemistry website.
[1]
[2]
J. S. Edmonds ,
Y. Shibata ,
K. A. Francesconi ,
R. J. Rippingale ,
M. Morita ,
Arsenic transformations in short marine food chains studied by HPLC–ICP-MS.
Appl. Organomet. Chem. 1997
, 11, 281.
| Crossref | GoogleScholarGoogle Scholar |

[3]
K. Francesconi ,
J. S. Edmonds ,
R. V. Stick ,
Arsenocholine from anaerobic decomposition of a trimethylarsonioriboside.
Appl. Organomet. Chem. 1992
, 6, 247.
| Crossref | GoogleScholarGoogle Scholar |

[4]
J. Kirby ,
W. Maher ,
D. Spooner ,
Arsenic occurrence and species in near-shore macroalgae-feeding marine animals.
Environ. Sci. Technol. 2005
, 39, 5999.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[5]
K. A. Francesconi ,
W. Goessler ,
S. Panutrakul ,
K. J. Irgolic ,
A novel arsenic-containing riboside (arsenosugar) in three species of gastropod.
Sci. Total Environ. 1998
, 221, 139.
| Crossref | GoogleScholarGoogle Scholar |

[6]
S. Foster ,
W. Maher ,
E. Schmeisser ,
A. Taylor ,
F. Krikowa ,
S. C. Apte ,
Arsenic species in a rocky intertidal marine food chain in NSW, Australia, revisited.
Environ. Chem. 2006
, 3, 304.
| Crossref | GoogleScholarGoogle Scholar |

[7]
E. Schmeisser ,
R. Raml ,
K. Francesconi ,
D. Kuehnelt ,
A.-L. Lindberg ,
C. Sörös ,
W. Goessler ,
Thio arsenosugars identified as natural constituents of mussels by liquid chromatography–mass spectrometry.
Chem. Commun. 2004
, 16, 1824.
| Crossref | GoogleScholarGoogle Scholar |

[8]
V. Nischwitz ,
K. Kanaki ,
S. A. Pergantis ,
Mass spectrometric identification of novel arsinothioylsugars in marine bivalves and algae.
J. Anal. At. Spectrom. 2006
, 21, 33.
| Crossref | GoogleScholarGoogle Scholar |

[9]
B. M. Gamble ,
P. A. Gallagher ,
J. A. Shoemaker ,
X. Wei ,
C. A. Schwegel ,
J. T. Creed ,
An investigation of the chemical stability of arsenosugars in simulated gastric juice and acidic environments using IC–ICP-MS and IC-ESI-MS/MS.
Analyst 2002
, 127, 781.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[10]
B. M. Gamble ,
P. A. Gallagher ,
J. A. Shoemaker ,
A. N. Parks ,
D. J. Freeman ,
C. A. Schwegel ,
J. T. Creed ,
An investigation of the chemical stability of arsenosugars in basic environments using IC–ICP-MS and IC-ESI-MS/MS.
Analyst 2003
, 128, 1458.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[11]
J. S. Edmonds ,
K. A. Francesconi ,
J. A. Hansen ,
Dimethyloxarsylethanol from anaerobic decomposition of brown kelp (Ecklonia radiata): a likely precursor of arsenobetaine in marine fauna.
Experientia 1982
, 38, 643.
| Crossref | GoogleScholarGoogle Scholar |

[12]
H. Castlehouse ,
C. Smith ,
A. Raab ,
C. Deacon ,
A. A. Meharg ,
J. Feldmann ,
Biotransformation and accumulation of arsenic in soil amended with seaweed.
Environ. Sci. Technol. 2003
, 37, 951.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[13]
K. A. Francesconi ,
D. A. Hunter ,
B. Bachmann ,
G. Raber ,
W. Goessler ,
Uptake and transformation of arsenosugars in the shrimp Crangon crangon.
Appl. Organomet. Chem. 1999
, 13, 669.
| Crossref | GoogleScholarGoogle Scholar |

[14]
H. Kirkman ,
The first year in the life history and the survival of the juvenile marine macrophyte, Ecklonia radiata (Turn.) J. Agardh.
J. Exp. Mar. Biol. Ecol. 1981
, 55, 243.
| Crossref | GoogleScholarGoogle Scholar |

[15]
W. J. Fletcher ,
R. W. Day ,
The distribution of epifauna on Ecklonia radiata (C. Agardh) J. Agardh and the effect of disturbance.
J. Exp. Mar. Biol. Ecol. 1983
, 71, 205.
| Crossref | GoogleScholarGoogle Scholar |

[16]
T. Wernberg ,
M. A. Coleman ,
A. Fairhead ,
S. Miller ,
M. Thomsen ,
Morphology of Ecklonia radiata (Phaeophyta: Laminarales) along its geographic distribution in south-western Australia and Australasia.
Mar. Biol. 2003
, 143, 47–55.
| Crossref | GoogleScholarGoogle Scholar |

[17]
H. Kirkman ,
Standing stock and production of Ecklonia radiata (C.Ag.): J. Agardh.
J. Exp. Mar. Biol. Ecol. 1984
, 76, 119.
| Crossref | GoogleScholarGoogle Scholar |

[18]
G. P. Jones ,
Interactions between herbivorous fishes and macro-algae on a temperate rocky reef.
J. Exp. Mar. Biol. Ecol. 1992
, 159, 217.
| Crossref | GoogleScholarGoogle Scholar |

[19]
N. L. Andrew ,
G. P. Jones ,
Patch formation by herbivorous fish in a temperate Australian kelp forest.
Oecologia 1990
, 85, 57.
| Crossref | GoogleScholarGoogle Scholar |

[20]
M. Guest ,
P. Nichols ,
S. Frusher ,
A. Hirst ,
Evidence of abalone (Haliotis rubra) diet from combined fatty acid and stable isotope analyses.
Mar. Biol. 2008
, 153, 579.
| Crossref | GoogleScholarGoogle Scholar |

[21]
N. L. Andrew ,
Changes in subtidal habitat following mass mortality of sea urchins in Botany Bay, New South Wales.
Aust. J. Ecol. 1991
, 16, 353.
| Crossref | GoogleScholarGoogle Scholar |

[22]
G. P. Jones ,
N. L. Andrew ,
Herbivory and patch dynamics on rocky reefs in temperate Australasia: the roles of fish and sea urchins.
Aust. J. Ecol. 1990
, 15, 505.
| Crossref | GoogleScholarGoogle Scholar |

[23]
S. Baldwin ,
M. Deaker ,
W. Maher ,
Low-volume microwave digestion of marine biological tissues for the measurement of trace elements.
Analyst 1994
, 119, 1701.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[24]
W. Maher ,
F. Krikowa ,
J. Kirby ,
A. T. Townsend ,
P. Snitch ,
Measurement of trace elements in marine environmental samples using solution ICP-MS. Current and future applications.
Aust. J. Chem. 2003
, 56, 103.
| Crossref | GoogleScholarGoogle Scholar |

[25]
J. Kirby ,
W. Maher ,
Measurement of water-soluble arsenic species in freeze-dried marine animal tissues by microwave-assisted extraction and HPLC–ICP-MS
J. Anal. At. Spectrom. 2002
, 17, 838.
| Crossref | GoogleScholarGoogle Scholar |

[26]
[27]
R. Tukai ,
W. A. Maher ,
I. J. McNaught ,
M. J. Ellwood ,
M. Coleman ,
Occurrence and chemical form of arsenic in marine macroalgae from the east coast of Australia.
Mar. Freshwater Res. 2002
, 53, 971.
| Crossref | GoogleScholarGoogle Scholar |

[28]
S. D. A. Smith ,
The macrofaunal community of Ecklonia radiata holdfasts: variation associated with sediment regime, sponge cover and depth.
Aust. J. Ecol. 1996
, 21, 144.
| Crossref | GoogleScholarGoogle Scholar |

[29]
K. A. Francesconi ,
J. S. Edmonds ,
Arsenic species in marine samples.
Croat. Chem. Acta 1998
, 71, 343.

[30]
J. Meier ,
N. Kienzl ,
W. Goessler ,
K. A. Francesconi ,
The occurrence of thio-arsenosugars in some samples of marine algae.
Environ. Chem. 2005
, 2, 304.
| Crossref | GoogleScholarGoogle Scholar |

[31]
[32]
Y. Shibata ,
M. Morita ,
A novel trimethylated arsenic-sugar isolated from the brown algae Sargassum thunbergia.
Agric. Biol. Chem. 1988
, 52, 1087.

[33]
S. Foster ,
W. Maher ,
F. Krikowa ,
J. Kirby ,
S. C. Apte ,
A microwave-assisted technique for the sequential extraction of water and non-water soluble arsenic compounds from estuarine plant and marine animal tissue using dilute nitric acid.
Talanta 2007
, 71, 537.
| Crossref | GoogleScholarGoogle Scholar |

[34]
J. C. Ogden ,
P. S. Label ,
The role of herbivorous fish and urchins in coral reef communities.
Environ. Biol. Fishes 1978
, 3, 49.
| Crossref | GoogleScholarGoogle Scholar |

[35]
J. J. Sloth ,
E. H. Larsen ,
K. Julshamn ,
Report on three aliphatic dimethylarsinoyl compounds as common minor constituents in marine samples. An investigation using high-performance liquid chromatography/inductively coupled plasma mass spectrometry and electrospray ionisation tandem mass spectrometry.
Rapid Commun. Mass Spectrom. 2005
, 19, 227.
| Crossref | GoogleScholarGoogle Scholar |

[36]
K. D. Clements ,
Endosymbiotic communities of two herbivorous labioid fishes, Odax cyanomelas and O. pullus.
Mar. Biol. 1991
, 109, 223.
| Crossref | GoogleScholarGoogle Scholar |

[37]
J. H. Erasmus ,
P. A. Cook ,
V. E. Coyne ,
The role of bacteria in the digestion of seaweed by the abalone Haliotis midae.
Aquaculture 1997
, 155, 377.
| Crossref | GoogleScholarGoogle Scholar |

[38]
T. A. Anderson ,
Mechanisms of digestion in the marine herbivore, the luderick, Girella tricuspidata (Quoy and Gaimard).
J. Fish Biol. 1991
, 39, 535.
| Crossref | GoogleScholarGoogle Scholar |

[39]
J. M. Harris ,
The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis.
Microb. Ecol. 1993
, 25, 195.
| Crossref | GoogleScholarGoogle Scholar |

[40]
R. W. Eppley ,
R. Lasker ,
Alginase in the sea urchin Strongylocentrotus purpuratus.
Science 1959
, 129, 214.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[41]
T. Sawabe ,
Y. Oda ,
Y. Shiomi ,
Y. Ezura ,
Alginate degradation by bacteria isolated from the gut of sea urchins and abalones.
Microb. Ecol. 1995
, 30, 193.
| Crossref | GoogleScholarGoogle Scholar |

[42]
F. Dickens ,
Yeast fermentation of pentose phosphoric acids.
Biochem. J. 1938
, 32, 1645.
| PubMed |

[43]
W. Maher ,
W. Goessler ,
J. Kirby ,
G. Raber ,
Arsenic concentrations and speciation in the tissues and blood of sea mullet (Mugil cephalus) from Lake Macquarie NSW, Australia.
Mar. Chem. 1999
, 68, 169.
| Crossref | GoogleScholarGoogle Scholar |

[44]
H. F. ter Welle ,
E. C. Slater ,
Uncoupling of respiratory-chain phosphorylation by arsenate.
Biochim. Biophys. Acta 1967
, 143, 1.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[45]
H. R. Hansen ,
M. Jaspars ,
J. Feldmann ,
Arsinothioyl-sugars produced by in vitro incubation of seaweed extract with liver cytosol analysed by HPLC coupled simultaneously to ES-MS and ICP-MS.
Analyst 2004
, 129, 1058.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[46]
W. Reichenbecher ,
D. P. Kelly ,
J. C. Murrell ,
Desulfonation of propanesulfonic acid by Comamonas acidovorans strain P53: evidence for an alkanesulfonate sulfonatase and an atypical sulfite dehydrogenase.
Arch. Microbiol. 1999
, 172, 387.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[47]
A. M. Cook ,
H. Laue ,
F. Junker ,
Microbial desulfonation.
FEMS Microbiol. Ecol. 1998
, 22, 399.

[48]
M. Suwalsky ,
C. Rivera ,
C. P. Sotomayor ,
M. Jemiola-Rzeminska ,
K. Strzalka ,
Monomethylarsonate (MMAV) exerts stronger effects than arsenate on the structure and thermotropic properties of phospholipids bilayers.
Biophys. Chem. 2008
, 132, 1.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[49]
K. A. Francesconi ,
R. V. Stick ,
J. S. Edmonds ,
Glycerolphosphorylarsenocholine and phosphatidylarsenocholine in yelloweye mullet (Aldrichetta forsteri) following oral administration of arsenocholine.
Experientia 1990
, 46, 464.
| Crossref | GoogleScholarGoogle Scholar |

[50]
K. Francesconi ,
J. S. Edmonds ,
R. V. Stick ,
Synthesis, NMR spectra and chromatographic properties of five trimethylarsonioriboses
Appl. Organomet. Chem. 1994
, 8, 517.
| Crossref | GoogleScholarGoogle Scholar |

[51]
S. McSheehy ,
J. Szpunar ,
R. Lobinski ,
V. Haldys ,
J. Tortajada ,
J. S. Edmonds ,
Characterization of arsenic species in kidney of the clam Tridacna derasa by multidimensional liquid chromatography-ICPMS and electrospray time-of-flight tandem mass spectrometry.
Anal. Chem. 2002
, 74, 2370.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[52]
Accessory publication