A look at the CLAW hypothesis from an atmospheric chemistry point of view
Roland von GlasowSchool of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK. Email: R.Von-Glasow@uea.ac.uk
Environmental Chemistry 4(6) 379-381 https://doi.org/10.1071/EN07064
Submitted: 7 September 2007 Accepted: 25 October 2007 Published: 6 December 2007
Environmental context. Feedbacks in the climate system as suggested by, for example, the CLAW hypothesis have often been accepted as fact whereas many open questions remain. In this manuscript some of these uncertainties and their implications are discussed and additional processes that might drastically change the importance of this suggested feedback loop are highlighted.
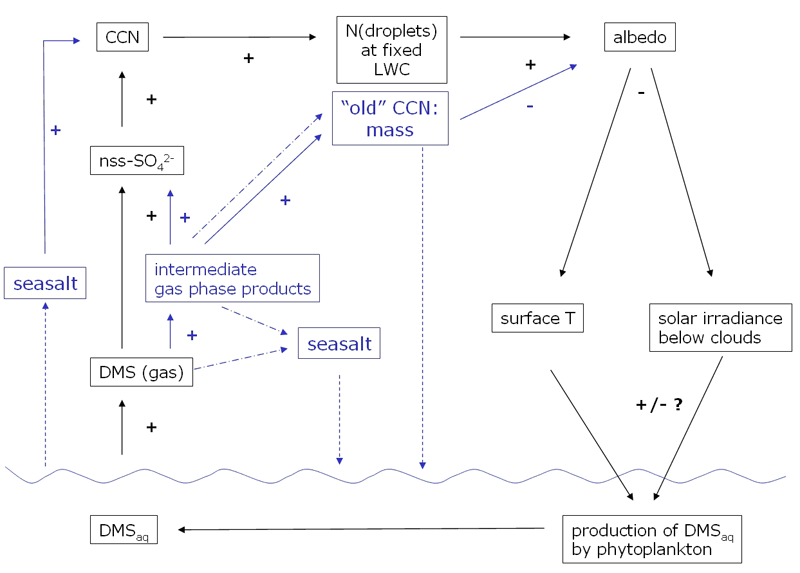
As detailed in Ayers[1] the CLAW hypothesis[2] suggests a negative (climate stabilising) feedback between the production of dimethyl sulfide (DMS) in the ocean, its oxidation in the atmosphere and cloud properties. From an atmospheric point of view some of the simplifications implied in the CLAW hypothesis attract attention. Important intermediate steps and feedbacks in the breakdown of DMS and the cycling of sulfur have been neglected in the CLAW hypothesis or have become apparent only in recent years. Fig. 1 illustrates the CLAW hypothesis and includes key additional atmospheric processes that I will briefly explain in the following paragraphs.
DMS chemistry – oxidation pathways and final products
The atmospheric chemistry of DMS is a lot more complicated than initially thought or implied in the CLAW hypothesis. Depending on the oxidant, the first oxidation step leads to hydrogen abstraction or oxygen addition. The abstraction pathway leads by several intermediates to SO2, which can be taken up to particles or further oxidised to H2SO4 or methanesulfonic acid (MSA). The addition pathway leads to intermediate products that are water soluble (dimethyl sulfoxide (DMSO), dimethyl sulfone (DMSO2), methylsulfinic acid (MSIA) and MSA) and, therefore, contribute to the growth of existing particles. MSIA can react in the gas phase to SO2.[3] It is important to note that only H2SO4 can lead to new particle formation: other products like MSA cannot produce new aerosol particles.[4] The yield of SO2 and MSA from DMS depends on many chemical and meteorological conditions, also many of the assumptions that are used for the derivation of reduced reaction mechanisms might not be valid under some or even most conditions.[5–8]
OH is usually assumed to be the main oxidant for DMS. However, NO3 can be a relevant oxidant as well, especially in winter when OH concentrations are reduced by a factor of 5–10 compared with summer or in more polluted regions.[5,9,10] The oxidation of DMS by O3 in cloud droplets can also be fairly efficient, again especially in winter.[5,11]
A potentially very important oxidant is bromine oxide, BrO, which leads to O addition.[12] Toumi,[13] based on kinetic data from Barnes et al.,[12] was the first to suggest that BrO might play a role in the oxidation of DMS. The presence of BrO increases the rate of DMS oxidation and also the rate of SO2 formation, however, the conversion efficiency of DMS to SO2, the key precursor for H2SO4 and, therefore, new particle formation, is always reduced when BrO is relevant as oxidant.[5] Furthermore, as many of the intermediate products in the addition pathway are water soluble, the yield of SO2 and, therefore, of new aerosol particles is strongly reduced if the addition pathway is of importance especially under cloudy conditions. BrO might play an important role on the global scale with a contribution to DMS oxidation of up to 30% even if present at mixing ratios of only a few tenths of a pmol mol–1.[11,14] To highlight the importance of NO3 and BrO as DMS oxidants in addition to OH, a look at the DMS lifetime (at T = 280 K) with regard to oxidation by the main gas-phase oxidants is useful: τ(OH = 106 molecules cm–3) = 29 h, τ(NO3 = 5 pmol mol–1) = 18 h, τ(BrO = 1 pmol mol–1) = 21 h, τ(Cl = 104 molecules cm–3) = 84 h.
Most of the global aerosol models do not include a detailed treatment of the atmospheric chemistry of DMS (they usually only include OH and sometimes NO3 as oxidant) and, therefore, cannot yet investigate chemistry–cloud climate feedback in detail as they rely on rather simplistic assumptions about the oxidation of DMS and the oxidation products.
Fate of SO2
As already mentioned, H2SO4 is the only sulfur species that can lead to the formation of new particles under atmospheric conditions. H2SO4, however, is only formed by reaction of SO2 with OH. A competing process for SO2 oxidation in the gas phase is uptake by aerosol particles and cloud droplets and subsequent aqueous phase oxidation by H2O2, HOBr, HOCl and, at high pH values, by O3.[15,16] This has several important implications as it leads not only to the growth of existing particles but also to the increased uptake of SO2 from the gas phase and, therefore, a further reduction in the precursors for new particle formation.
The oxidation of SO2 in sea salt aerosol is especially noteworthy as the lifetime of large sea salt aerosol particles is short, which effectively leads to a ‘shortcut’ in the sulfur cycle in the marine boundary layer (MBL) by rapidly depositing the DMS-derived sulfur back to the ocean. In this regard the reaction of SO2,aq with O3,aq in fresh, alkaline sea salt is key[15] as it has a strong pH dependence. Furthermore, there are indications that, at least under some conditions, the alkalinity in sea spray aerosol particles might be greater than in sea water,[17] which would increase the role of O3 as oxidant in sea salt aerosol and the ‘short cut’ in the sulfur cycle.
Relevance for the ‘big picture’
With regard to the overall effects as suggested in the CLAW hypothesis, it seems to be clear that atmospheric DMS concentrations are highest in summer, which is also when its gas phase oxidants are highest as well as the number of CCN in the remote MBL.[18] So an important question – from a global perspective – that remains is: Do we need to know all the above-mentioned details when there is such a clear correlation between these factors that eventually have a strong influence on cloud properties? I think we do.
When focussing on microphysical properties of MBL clouds, it is not relevant where in the atmosphere the nucleation of new aerosol particles occurs. Only a change in cloud condensation nuclei (CCN) number in the MBL is of importance, which is (at a given supersaturation) a function of the particle size spectrum and their solubility. The overall effect of particle growth by, for example, aqueous phase formation of sulfate on the number of CCN depends on the size of the particles that grow. If the aerosol particles are already in the size range where they activate to CCN under the given supersaturation, further growth results in fewer but bigger cloud droplets, which reduces the albedo of the cloud and potentially increases the rate of drizzle and, therefore, decreases cloud lifetime.[19] The growth of these small particles (typically on the order of several 10 nm) would only increase the number of CCN, and therefore possibly that of cloud droplets, if there is a significant number of aerosol particles that do not act as CCN under the given supersaturation. In many cases in the remote MBL this process might not be too relevant, however, as a lot of the particle growth by uptake of sulfur compounds seems to happen in large, very hygroscopic sea salt particles.[5] In order to investigate the change in CCN number, a reliable quantification of aerosol growth as a function of particle size (and type) is needed.
Many studies focussed on observed seasonalities to investigate elements of the CLAW hypothesis, which is a very relevant thing to do as many of the involved steps are expected to show distinct seasonal variations. Not quantitatively included in these studies so far have been the possibility of primary[20] and secondary[21] sources of organic marine aerosol particles and their relevance as CCN that would have a seasonal dependence peaking with biological activity as well. While the focus on seasonalities of processes that influence the chemistry is important, one should not forget about the driving influence of meteorology on clouds. The study by Klein and Hartmann,[22] for example, suggested a strong correlation of stratiform cloud cover and stability, both peaking in summer.
In general, however, it is important to understand cause and effect relationships and not to rely on correlations to assess possible feedbacks and to derive parameterisations for (global) models. Furthermore, we need to achieve a detailed understanding of the processes behind the single steps of the CLAW hypothesis in order to disentangle potentially compensating effects that might not be compensating anymore under different conditions, e.g. past or future climate. When using global models it has to be ensured that the choice of the parameterisations and simplifications used does not implicitly prescribe the outcome of a feedback in a model by, for example, using too simplistic chemical reaction schemes or parameterisations for the aerosol indirect effects. Even complex global models (e.g., Earth System Models) that treat DMS production in the ocean explicitly, as well as the atmospheric oxidation of DMS and aerosol physics and feedbacks with the clouds, have to make a fair amount of assumptions which, in the end, might implicitly (and unintentionally) prescribe the resulting feedbacks.
In summary, there are strong indications that processes that do not lead to the formation of new aerosol particles but to the growth of pre-existing particles play an important role in the atmospheric cycling of DMS. The resulting effect on cloud albedo is possibly the opposite of what was originally suggested in the CLAW hypothesis.

|
Acknowledgements
I would like to thank Ken Carslaw and Peter Liss for helpful comments.
[1]
G. P. Ayers ,
J. M. Cainey ,
The CLAW hypothesis: a review of the major developments.
Environ. Chem. 2007
, 4, 366.
| Crossref | GoogleScholarGoogle Scholar |

[2]
R. J. Charlson ,
J. E. Lovelock ,
M. O. Andreae ,
S. G. Warren ,
Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate.
Nature 1987
, 326, 655.
| Crossref | GoogleScholarGoogle Scholar |

[3]
A. Kukui ,
D. Borissenko ,
G. Laverdet ,
G. L. Bras ,
Gas phase reactions of OH radicals with dimethyl sulfoxide and methane sulfonic acid using turbulent flow reactor and chemical ionization mass spectrometry.
J. Phys. Chem. A 2003
, 107, 5732.
| Crossref | GoogleScholarGoogle Scholar |

[4]
S. M. Kreidenweis ,
J. H. Seinfeld ,
Nucleation of sulfuric acid-water solution particles: Implications for the atmospheric chemistry of organosulfur species.
Atmos. Environ. 1988
, 22, 283.
| Crossref | GoogleScholarGoogle Scholar |

[5]
R. von Glasow ,
P. J. Crutzen ,
Model study of multiphase DMS oxidation with a focus on halogens.
Atmos. Chem. Phys. 2004
, 4, 589.

[6]
D. D. Lucas ,
R. G. Prinn ,
Mechanistic studies of dimethylsulfide oxidation products using an observationally constrained model.
J. Geophys. Res. 2002
, 107, 4201.
| Crossref | GoogleScholarGoogle Scholar |

[7]
D. D. Lucas ,
R. G. Prinn ,
Parametric sensitivity and uncertainty analysis of dimethylsulfide oxidation in the remote marine boundary layer.
Atmos. Chem. Phys. 2005
, 5, 1505.

[8]
I. Barnes ,
J. Hjorth ,
N. Mihalopoulos ,
Dimethyl sulfide and dimethyl sulfoxide and their oxidation in the atmosphere.
Chem. Rev. 2006
, 106, 940.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[9]
S. Koga ,
H. Tanaka ,
Numerical study of the oxidation process of dimethylsulfide in the marine atmosphere.
J. Atmos. Chem. 1993
, 17, 201.
| Crossref | GoogleScholarGoogle Scholar |

[10]
S. Koga ,
H. Tanaka ,
Simulation of seasonal variations of sulfur compounds in the remote marine atmosphere.
J. Atmos. Chem. 1996
, 23, 163.
| Crossref | GoogleScholarGoogle Scholar |

[11]
O. Boucher ,
C. Moulin ,
S. Belviso ,
O. Aumont ,
L. Bopp ,
E. Cosme ,
R. von Kuhlmann ,
M. G. Lawrence ,
et al. Sensitivity study of dimethylsulphide (DMS) atmospheric concentrations and sulphate aerosol indirect radiative forcing to the DMS source representation and oxidation.
Atmos. Chem. Phys. 2003
, 3, 49.

[12]
I. Barnes ,
V. Bastian ,
K. H. Becker ,
R. D. Overath ,
Kinetic studies of the reactions of IO, BrO and ClO with DMS.
Int. J. Chem. Kinet. 1991
, 23, 579.
| Crossref | GoogleScholarGoogle Scholar |

[13]
R. Toumi ,
BrO as a sink for dimethylsulphide in the marine atmosphere.
Geophys. Res. Lett. 1994
, 21, 117.
| Crossref | GoogleScholarGoogle Scholar |

[14]
R. von Glasow ,
R. von Kuhlmann ,
M. G. Lawrence ,
U. Platt ,
P. J. Crutzen ,
Impact of reactive bromine chemistry in the troposphere.
Atmos. Chem. Phys. 2004
, 4, 2481.

[15]
H. Sievering ,
J. Boatman ,
E. Gorman ,
Y. Kim ,
L. Anderson ,
G. Ennis ,
M. Luria ,
S. Pandis ,
Removal of sulphur from the marine boundary layer by ozone oxidation in sea-salt aerosols.
Nature 1992
, 360, 571.
| Crossref | GoogleScholarGoogle Scholar |

[16]
R. Vogt ,
P. J. Crutzen ,
R. Sander ,
A mechanism for halogen release from sea-salt aerosol in the remote marine boundary layer.
Nature 1996
, 383, 327.
| Crossref | GoogleScholarGoogle Scholar |

[17]
H. Sievering ,
J. Cainey ,
M. Harvey ,
J. McGregor ,
S. Nichol ,
P. Quinn ,
Aerosol non-sea-salt sulfate in the remote marine boundary layer under clear-sky and normal cloudiness conditions: Ocean-derived biogenic alkalinity enhances sea-salt sulfate production by ozone oxidation.
J. Geophys. Res. 2004
, 109, D19317.
| Crossref | GoogleScholarGoogle Scholar |

[18]
G. P. Ayers ,
R. W. Gillett ,
DMS and its oxidation products in the remote marine atmosphere; implications for climate and atmospheric chemistry.
J. Sea Res. 2000
, 43, 275.
| Crossref | GoogleScholarGoogle Scholar |

[19]
B. A. Albrecht ,
Aerosols, cloud microphysics, and fractional cloudiness.
Science 1989
, 245, 1227.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[20]
C. D. O’Dowd ,
M. C. Facchini ,
F. Cavalli ,
D. Ceburnis ,
M. Mircea ,
S. Decesari ,
S. Fuzzi ,
Y. J. Yoon ,
et al. Biogenically driven organic contribution to marine aerosol.
Nature 2004
, 431, 676.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[21]
N. Meskhidze ,
A. Nenes ,
Phytoplankton and cloudiness in the southern ocean.
Science 2006
, 314, 1419.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[22]
S. A. Klein ,
D. L. Hartmann ,
The seasonal cycle of low stratiform clouds.
J. Clim. 1993
, 6, 1587.
| Crossref | GoogleScholarGoogle Scholar |