Origin of arsenolipids in sediments from Great Salt Lake
Ronald A. Glabonjat A D , Georg Raber A , Kenneth B. Jensen A , Florence Schubotz B , Eric S. Boyd C and Kevin A. Francesconi A
A D , Georg Raber A , Kenneth B. Jensen A , Florence Schubotz B , Eric S. Boyd C and Kevin A. Francesconi A
A Institute of Chemistry, NAWI Graz, University of Graz, 8010 Graz, Austria.
B MARUM – Center for Marine Environmental Sciences and Department of Geosciences, University of Bremen, 28359 Bremen, Germany.
C Department of Microbiology and Immunology, Montana State University, Bozeman, MT 59717, USA.
D Corresponding author. Email: ronald.glabonjat@uni-graz.at
Environmental Chemistry 16(5) 303-311 https://doi.org/10.1071/EN19135
Submitted: 10 May 2019 Accepted: 17 June 2019 Published: 19 July 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Environmental context. Arsenic is a globally distributed element, occurring in various chemical forms with toxicities ranging from harmless to highly toxic. We examined sediment samples from Great Salt Lake, an extreme salt environment, and found a variety of organoarsenic species not previously recorded in nature. These new compounds are valuable pieces in the puzzle of how organisms detoxify arsenic, and in our understanding of the global arsenic cycle.
Abstract. Arsenic-containing lipids are natural products found predominantly in marine organisms. Here, we report the detection of known and new arsenolipids in sediment samples from Great Salt Lake, a hypersaline lake in Utah, USA, using high-performance liquid chromatography in combination with both elemental and molecular mass spectrometry. Sediments from four investigated sites contained appreciable quantities of arsenolipids (22–312 ng As g−1 sediment) comprising several arsenic-containing hydrocarbons and 20 new compounds shown to be analogues of phytyl 2-O-methyl dimethylarsinoyl riboside. We discuss potential sources of the detected arsenolipids and find a phytoplanktonic origin most plausible in these algal detritus-rich salt lake sediments.
Lipid-soluble arsenic compounds (arsenolipids) are natural products found in many marine organisms. They contain arsenic bound into common lipids such as fatty acids (Rumpler et al. 2008; Sele et al. 2014), hydrocarbons (Taleshi et al. 2008; Sele et al. 2014), fatty alcohols (Amayo et al. 2013) and phospholipids (García-Salgado et al. 2012; Viczek et al. 2016; Yu et al. 2018). An intriguing addition to natural arsenolipids was recently found in the marine microalga Dunaliella tertiolecta (Glabonjat et al. 2017). This organism contained an arsenosugar-phytol in which the arsenic is bound to a 2-O-methyl ribose moiety. A methoxy group in the 2-O-position of the ribose ring is a configuration found in major classes of ribonucleic acid (RNA) (Poole et al. 2000; Birkedal et al. 2015), but has never before been reported for simple carbohydrates, an observation relevant to the hypothesised evolutionary link between RNA and DNA in early life’s ancient metabolism (Poole et al. 2000; Rana and Ankri 2016; Glabonjat et al. 2017). A second interesting aspect of the new arsenolipid is the ether-bound phytyl group. Such ether linkages to isoprenoid hydrocarbons are characteristic for membrane lipids of Archaea; however, similar phytyl moieties are also found attached to chlorophylls anchoring them to the chloroplast membrane in photoautotrophs, including phytoplankton.
Guided by these two observations, we hypothesised that the new arsenosugar-phytol could be produced by planktonic algae or Archaea and might therefore be abundant in extreme environments rich in these two life forms. Here, we report an investigation using high-performance liquid chromatography (HPLC)–mass spectrometry to examine the arsenolipid content in sediments from Great Salt Lake (GSL), Utah, USA. Archaea are abundant components of GSL sediments (Tenchov et al. 2006; Boyd et al. 2017) and abundant halophilic algae, including Dunaliella species, have also been reported (Stephens 1973; Brock 1975; Meuser et al. 2013).
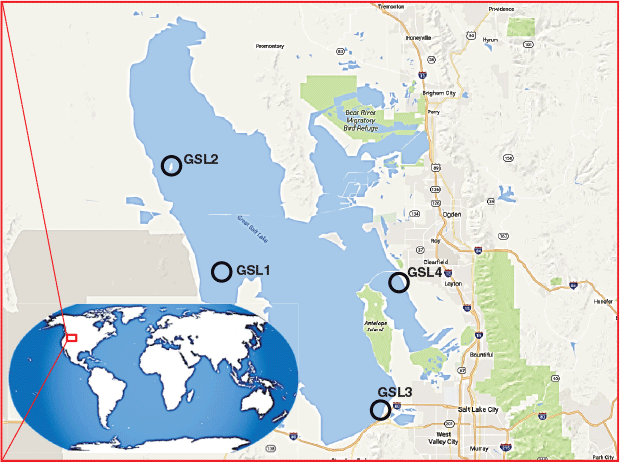
Surface sediments (0–2 cm) were sampled from the GSL on 7–14 November 2010 from four locations (Fig. 1): GSL1, North Basin; GSL2, Gunnison Island; GSL3, South Shore; and GSL4, Farmington Bay. Details of the collection, storage and preparation of the samples are reported in Boyd et al. (2017) and further details relevant to the analyses in the current study are presented in the Supplementary Material. In brief, the lipids were extracted from lyophilised sediments by a modified Bligh and Dyer method using a mixture of dichloromethane/methanol/aqueous buffer (2 × phosphate buffer at pH 7.2; and 2 × trichloroacetic acid at pH 2.0). The dry extracts were stored at −20 °C, and later redissolved in absolute ethanol (300 µL) before analysis by using HPLC-elemental mass spectrometry (inductively coupled plasma mass spectrometry, ICPMS), and HPLC-electrospray ionisation high-resolution mass spectrometry (HR-ESMS), following the method of Glabonjat et al. (2018). An aliquot of the total lipid extract was analysed on a Bruker maXis Plus ultra-high-resolution quadrupole time-of-flight mass spectrometer coupled to a Dionex Ultimate 3000RS ultra-high-performance liquid chromatography system with an electrospray ionisation source after Woermer et al. (2013).
|
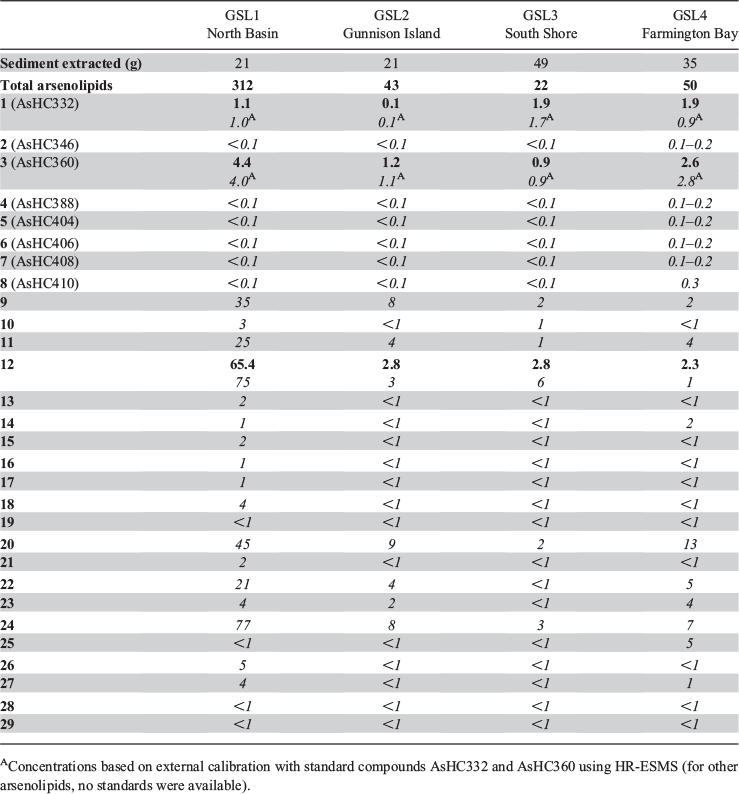
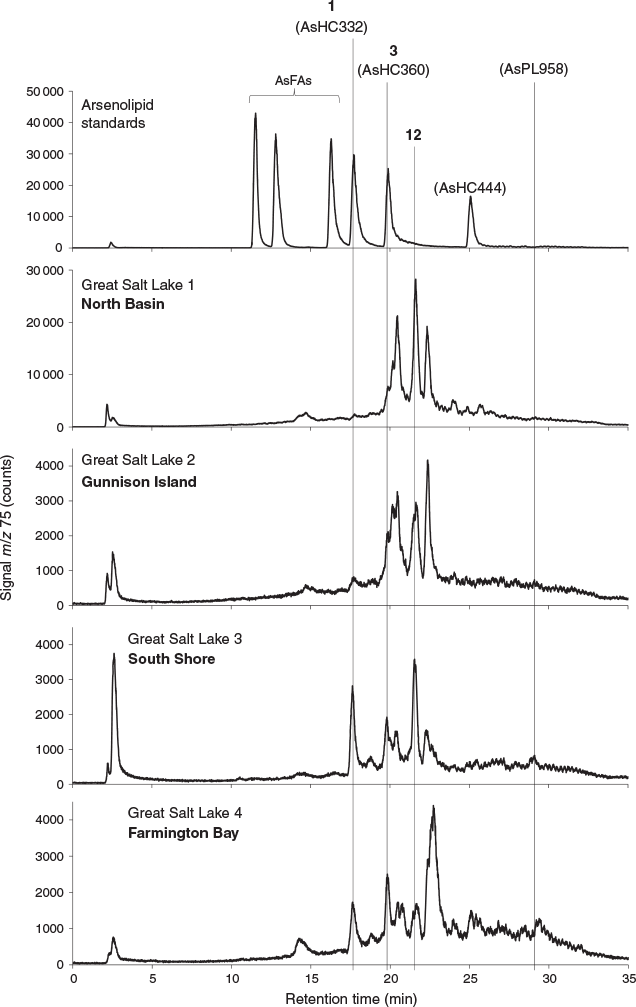
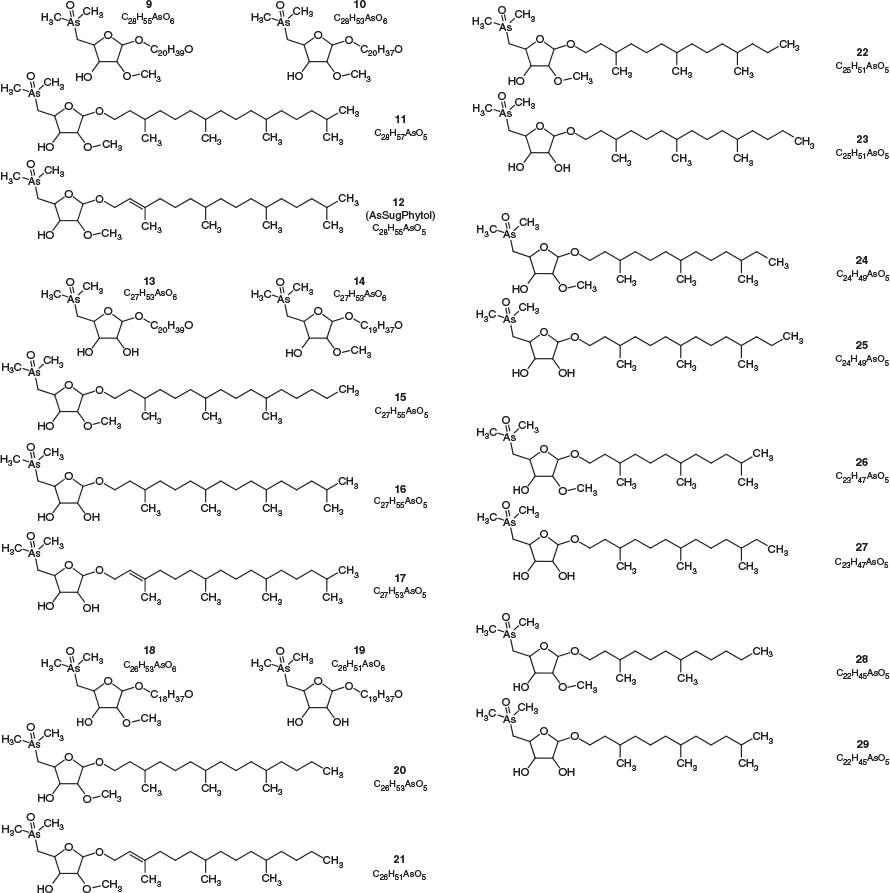
All four sediment samples from GSL showed the presence of lipid-soluble arsenic (22–312 ng As g−1 extracted sediment) comprising many arsenolipids (Table 1 and Fig. 2). Five known (1–5) and three newly discovered (6–8) arsenic hydrocarbons (AsHCs), the main ones being the frequently reported AsHC332 (1) and AsHC360 (3), were present in all samples (see Fig. S1 in the Supplementary Material for structures of the eight AsHCs found). The major arsenolipids, however, comprised a group of 21 ether terpenoids containing an arsenosugar moiety, of which only one (phytyl 2-O-methylriboside 12) has previously been reported (Glabonjat et al. 2017; Glabonjat et al. 2018). Structures for these compounds were assigned based on their mass spectral data as described below, and with analogy to naturally occurring non-arsenic terpenoids.
|
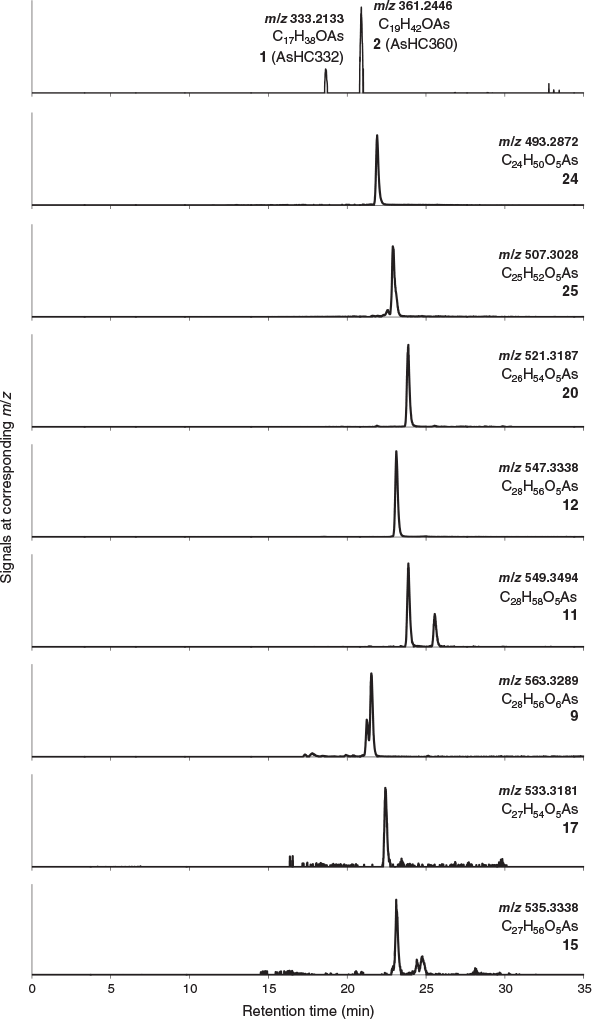
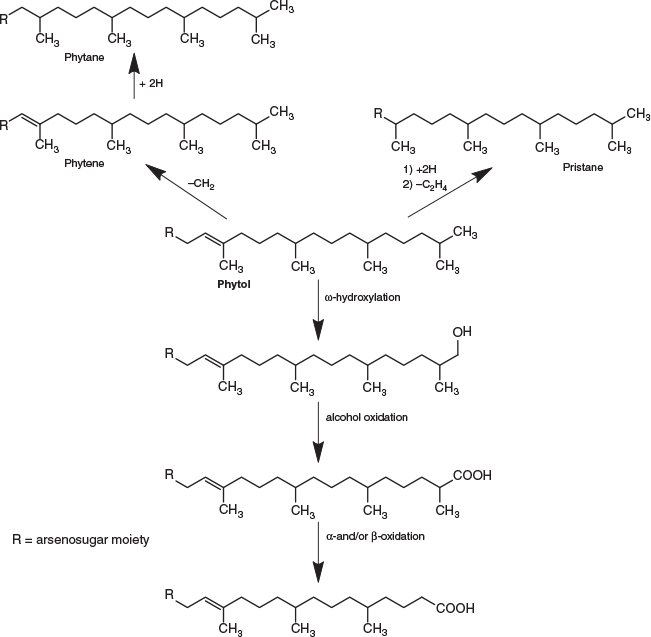
The various compounds separated well during chromatography (Fig. 3 and Fig. S2, Supplementary Material). They produced fragmentation patterns homologous to the pattern obtained for 12 (Fig. 4, Fig. S3 and Table S1, Supplementary Material). In conjunction with the measured masses of the protonated molecular ions, [M + H]+, the various compounds could be assigned distinct molecular formulae. These data strongly indicated the presence of similar isoprenoyl 2-O-methylriboside moieties in 12 of the newly discovered arsenolipids; for another eight isoprenoyl compounds, the data corresponded to a non-methylated ‘normal’ riboside moiety. Although the identities of the lipophilic portions of the molecules could not be established by mass spectrometry, likely structures can be proposed (Fig. 5) based on naturally occurring non-arsenic lipids and what is known of phytol metabolism (Fig. 6) in aquatic systems (Rontani and Volkman 2003; Sturt et al. 2004; Rontani and Bonin 2011). The mass spectral data for all compounds identified in the present study are shown in Table S2 (Supplementary Material); in all cases, the obtained molecular mass agreed with the calculated value to within 2 ppm or less.
|
|
|
The longest side chain we found in GSL sediments was equivalent to a phytyl moiety. A likely biosynthetic pathway towards the corresponding arsenolipid compound 12 was proposed by Glabonjat et al. (2018), and comprises the substitution of methionine by dimethylarsinous acid in S-adenolsylmethionine, followed by 2-O-methylation of the riboside by methionine, hydrolytic adenine removal, and finally, condensation of the 2-O-methylriboside with phytyl diphosphate. Shorter side chains could be explained by ω-hydroxylation with subsequent alcohol oxidation to the corresponding aldehyde (both intermediate products were detected in GSL samples; Figs 3–5 and Fig. S4, Supplementary Material) and finally to carboxylic acid as previously described for phytanic acid (Xu et al. 2006). This ω-terminal carboxylated isoprenoid can then undergo α- or β-oxidation (Jansen and Wanders 2006; Watkins et al. 2010), as typically found in phytol degradation pathways (Fig. 6), resulting in shorter-C-chain products (e.g. pristanal, pristanic acid, phytone), which were also present in the sediment samples from GSL (Figs 3–5).
Quantification of arsenolipids can be performed when ICPMS is used as the detector for HPLC, but only when the compounds have been adequately separated. When many arsenolipids are present, as in these sediment samples, it is not possible to confidently quantify the individual compounds. Nevertheless, we were able to estimate that in the four sediment samples, compound 12, the phytyl 2-O-methyl arsenoriboside previously found in D. tertiolecta, accounted for 5–20 % of the total arsenolipids present. Despite traces of arsenic eluting in the region of arsenosugar phospholipids, neither these compounds nor their possible O-methyl-analogues could be detected by means of accurate mass measurements. The absence of these compounds in GSL sediment extracts can be explained by rapid degradation of arsenosugar phospholipids in microbial-rich sediment environments (Glabonjat et al. 2019). In the four GSL sediment samples, we determined concentrations of three major arsenolipids – two hydrocarbons, 1 (0.1–1.9 ng g−1) and 3 (0.9–4.4 ng g−1), and the phytyl 2-O-methylriboside, 12 (2.3–65.4 ng g−1) – based on reversed phase (RP) HPLC-ICPMS data. The two arsenic hydrocarbons, 1 and 3, were also quantified by means of RP-HPLC-HR-ESMS, after external calibration with standard compounds (Table 1). Furthermore, we estimated the concentrations of all 21 arsenosugar terpenoids (9–29) and eight arsenic hydrocarbons (1–8) detected in the present study using the obtained HR-ESMS data based on the assumption that related compounds give a similar ESMS response (Table 1).
We hypothesise the origin of the arsenolipids in these GSL sediments to be either halophilic Archaea or algae in the form of detritus derived from unicellular algae inhabiting the overlaying water column (Meuser et al. 2013). The arsenolipid abundance in the sediments with ether linkages and C20 isoprenoidal hydrocarbon units intuitively suggest an archaeal origin because archaeal membrane lipids are composed of glycerol ethers and isoprenoids (Kates 1993; Angelini et al. 2012). The ether linkages of Archaea impart greater stability to the lipids compared with glycerol esters and fatty acids, which are the major components of membrane lipids in Bacteria and Eukarya. In hypersaline environments, archaeal lipid contribution increases with increasing salinity (Tenchov et al. 2006), and GSL sediments have been shown to host plentiful and diverse Archaea (Boyd et al. 2017; Baxter 2018). However, one of the major arsenolipids in the sediments, the phytol derivative 12, has recently been identified in the unicellular alga D. tertiolecta (Glabonjat et al. 2017), and Dunaliella species have been reported as abundant planktonic organisms in the overlaying water body of the GSL (Stephens 1973; Stephens and Gillespie 1976; Meuser et al. 2013). Planktonic detritus, including detritus from Dunaliella species therefore represents an important source of organic material continuously depositing on the lake bottom.
To provide further clues about the primary source of arsenolipids identified in the sediments, we determined the profile of normal lipids in the same sediment extracts. Although archaeal lipids were present in GSL sediments, lipids characteristic of unicellular algae (e.g. chloroplast lipids and betaine lipids) (Dembitsky 1996) were one to four orders of magnitude more abundant (Table S3, Supplementary Material). Furthermore, the archaeal lipids in the sediments contained a significant proportion (10–18 %) of extended phytyl chains, i.e. isoprenoid hydrocarbons with 25 carbons, which are characteristic for halophilic Archaea (Fig. S5, Supplementary Material) (Angelini et al. 2012). Notably, the arsenolipids in GSL sediments contained only isoprenoidal hydrocarbons with 20 carbons or less, and arsenic analogues of archaeal lipids with isoprenoid hydrocarbons of 25 carbons were not found among the arsenolipids. We therefore find it more likely that the isoprenoidal arsenolipids, and other arsenolipids, in GSL sediments are primarily derived from halophilic algae such as Dunaliella spp. (Glabonjat et al. 2017). This interpretation is consistent with the suggestion by Rosenberg (1967) that galactolipids, necessary for full photosynthetic activity, form hydrophobic complexes through interactions of their double bonds with isoprenoid methyl groups in chlorophylls, carotenes and quinones, and thereby ensure appropriate spacing and molecular orientation necessary for photosynthetic reactions.
Whether the presence of the isoprenoid arsenolipids in these unicellular halotolerant algae reflects a detoxification response to elevated environmental arsenic concentrations or if the arsenolipids serve other purposes in these organisms remains to be determined. However, As is elevated in GSL, with recent studies reporting a mean annual concentration of 112 µg L−1 in filtered water from 24 sites sampled over a 14-year period (Adams et al. 2015). Little variation in As concentrations was noted across sites, although a slight seasonal variation of As concentration was noted. This suggests that the organisms sampled in the present study were likely exposed to similar amounts of As. Further work is required to evaluate potential links between the prevalence of arsenolipids in GSL sediments or waters and the availability of As.
In summary, we identified 29 arsenolipids including 23 new compounds in sediments from GSL, a hypersaline lake, thereby demonstrating the widespread abundance of arsenolipids, which were hitherto thought to be limited to marine ecosystems. Most of the new arsenic compounds contain isoprenoid chains and an ether bond, which are characteristics of membrane lipids from Archaea. Nevertheless, here we suggest that the primary source of the various arsenolipids in GSL sediments is most likely sedimented detritus of halotolerant phytoplankton, with only a minor contribution from the microbial community inhabiting the benthic sediments themselves.
Supplementary material
The supplementary material contains further details about sample collection, preparation and determination. Table S1 shows proposed MS/MS fragmentation products of arsenosugar isoprenoids present in GSL sediment extracts; Table S2 provides details on high resolution mass spectral data of all the 29 detected arsenolipids; Table S3 gives an overview on ‘normal’ (arsenic-free) membrane lipids present in the GSL sediment extracts; Fig. S1 shows structures of the eight detected AsHCs; Fig. S2 shows RP-HPLC-HR-ESMS chromatograms of all 29 arsenolipids detected in GSL sediment extracts; Fig. S3 shows high resolution fragmentation spectra of all 29 detected arsenolipids; Fig. S4 gives an overview on possible oxidised arsenosugar lipids; and Fig. S5 shows examples of detected ‘normal’ archaeal lipids determined in the GSL sediment extracts in this study.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Austrian Science Fund (FWF) project number I2412-B21. We also thank NAWI Graz for supporting the Graz Central Laboratory – Environmental Metabolomics. Florence Schubotz acknowledges funding from the Central Research Development Fund of the University of Bremen. Eric Boyd acknowledges funding from the NASA Astrobiology Institute (project no. NNA15BB02A). The authors are grateful to David Naftz and Mark Marvin-DiPasquale for sample collection.
References
Adams WJ, DeForest DK, Tear LM, Payne K, Brix KV (2015). Long-term monitoring of arsenic, copper, selenium, and other elements in Great Salt Lake (Utah, USA) surface water, brine shrimp, and brine flies. Environmental Monitoring and Assessment 187, 118| Long-term monitoring of arsenic, copper, selenium, and other elements in Great Salt Lake (Utah, USA) surface water, brine shrimp, and brine fliesCrossref | GoogleScholarGoogle Scholar | 25690606PubMed |
Amayo KO, Raab A, Krupp EM, Gunnlaugsdottir H, Feldmann J (2013). Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMS. Analytical Chemistry 85, 9321–9327.
| Novel identification of arsenolipids using chemical derivatizations in conjunction with RP-HPLC-ICPMS/ESMSCrossref | GoogleScholarGoogle Scholar | 23984920PubMed |
Angelini R, Corral P, Lopalco P, Ventosa A, Corcelli A (2012). Novel ether lipid cardiolipins in archaeal membranes of extreme haloalkaliphiles. Biochimica et Biophysica Acta 1818, 1365–1373.
| Novel ether lipid cardiolipins in archaeal membranes of extreme haloalkaliphilesCrossref | GoogleScholarGoogle Scholar | 22366205PubMed |
Baxter BK (2018). Great Salt Lake microbiology: a historical perspective. International Microbiology 21, 79–95.
| Great Salt Lake microbiology: a historical perspectiveCrossref | GoogleScholarGoogle Scholar | 30810951PubMed |
Birkedal U, Christensen-Dalsgaard M, Krogh N, Sabarinathan R, Gorodkin J, Nielsen H (2015). Profiling of ribose methylations in RNA by high-throughput sequencing. Angewandte Chemie International Edition 54, 451–455.
| Profiling of ribose methylations in RNA by high-throughput sequencingCrossref | GoogleScholarGoogle Scholar | 25417815PubMed |
Boyd ES, Yu R-Q, Barkay T, Hamilton TL, Baxter BK, Naftz DL, Marvin-DiPasquale M (2017). Effect of salinity on mercury methylating benthic microbes and their activities in Great Salt Lake, Utah. The Science of the Total Environment 581–582, 495–506.
| Effect of salinity on mercury methylating benthic microbes and their activities in Great Salt Lake, UtahCrossref | GoogleScholarGoogle Scholar | 28057343PubMed |
Brock TD (1975). Salinity and the ecology of Dunaliella from Great Salt Lake. Journal of General Microbiology 89, 285–292.
| Salinity and the ecology of Dunaliella from Great Salt LakeCrossref | GoogleScholarGoogle Scholar |
Dembitsky VM (1996). Betaine ether-linked glycerolipids: chemistry and biology. Progress in Lipid Research 35, 1–51.
| Betaine ether-linked glycerolipids: chemistry and biologyCrossref | GoogleScholarGoogle Scholar | 9039425PubMed |
García-Salgado S, Raber G, Raml R, Magnes C, Francesconi KA (2012). Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae. Environmental Chemistry 9, 63–66.
| Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgaeCrossref | GoogleScholarGoogle Scholar |
Glabonjat RA, Raber G, Jensen KB, Guttenberger N, Zangger K, Francesconi KA (2017). A 2-O-methylriboside unknown outside the RNA world contains arsenic. Angewandte Chemie International Edition 56, 11963–11965.
| A 2-O-methylriboside unknown outside the RNA world contains arsenicCrossref | GoogleScholarGoogle Scholar | 28763144PubMed |
Glabonjat RA, Ehgartner J, Duncan EG, Raber G, Jensen KB, Krikowa F, Maher WA, Francesconi KA (2018). Arsenolipid biosynthesis by the unicellular alga Dunaliella tertiolecta is influenced by As/P ratio in culture experiments. Metallomics 10, 145–153.
| Arsenolipid biosynthesis by the unicellular alga Dunaliella tertiolecta is influenced by As/P ratio in culture experimentsCrossref | GoogleScholarGoogle Scholar | 29251312PubMed |
Glabonjat RA, Duncan EG, Francesconi KA, Maher WA (2019). Transformation of arsenic lipids in decomposing Ecklonia radiata. Journal of Applied Phycology
| Transformation of arsenic lipids in decomposing Ecklonia radiataCrossref | GoogleScholarGoogle Scholar |
Jansen GA, Wanders RJA (2006). Alpha-oxidation. Biochimica et Biophysica Acta 1763, 1403–1412.
| Alpha-oxidationCrossref | GoogleScholarGoogle Scholar | 16934890PubMed |
Kates M (1993). Biology of halophilic bacteria, Part II. Membrane lipids of extreme halophiles. Experientia 49, 1027–1036.
| Biology of halophilic bacteria, Part II. Membrane lipids of extreme halophilesCrossref | GoogleScholarGoogle Scholar | 8270029PubMed |
Meuser JE, Baxter BK, Spear JR, Peters JW, Posewitz MC, Boyd ES (2013). Contrasting patterns of community assembly in the stratified water column of Great Salt Lake, Utah. Microbial Ecology 66, 268–280.
| Contrasting patterns of community assembly in the stratified water column of Great Salt Lake, UtahCrossref | GoogleScholarGoogle Scholar | 23354179PubMed |
Poole A, Penny D, Sjöberg B-M (2000). Methyl-RNA: an evolutionary bridge between RNA and DNA?. Chemistry & Biology 7, R207–R216.
| Methyl-RNA: an evolutionary bridge between RNA and DNA?Crossref | GoogleScholarGoogle Scholar |
Rana AK, Ankri S (2016). Reviving the RNA world: an insight into the appearance of RNA methyltransferases. Frontiers in Genetics 7, 99
| Reviving the RNA world: an insight into the appearance of RNA methyltransferasesCrossref | GoogleScholarGoogle Scholar | 27375676PubMed |
Rontani J-F, Bonin P (2011). Production of pristane and phytane in the marine environment: role of prokaryotes. Research in Microbiology 162, 923–933.
| Production of pristane and phytane in the marine environment: role of prokaryotesCrossref | GoogleScholarGoogle Scholar | 21288485PubMed |
Rontani J-F, Volkman JK (2003). Phytol degradation products as biogeochemical tracers in aquatic environments. Organic Geochemistry 34, 1–35.
| Phytol degradation products as biogeochemical tracers in aquatic environmentsCrossref | GoogleScholarGoogle Scholar |
Rosenberg A (1967). Galactosyl diglycerides: their possible function in Euglena chloroplasts. Science 157, 1191–1196.
| Galactosyl diglycerides: their possible function in Euglena chloroplastsCrossref | GoogleScholarGoogle Scholar | 6038692PubMed |
Rumpler A, Edmonds JS, Katsu M, Jensen KB, Goessler W, Raber G, Gunnlaugsdottir H, Francesconi KA (2008). Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?. Angewandte Chemie International Edition 47, 2665–2667.
| Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?Crossref | GoogleScholarGoogle Scholar | 18306198PubMed |
Sele V, Sloth JJ, Holmelid B, Valdersnes S, Skov K, Amlund H (2014). Arsenic-containing fatty acids and hydrocarbons in marine oils – determination using reversed-phase HPLC–ICP-MS and HPLC–qTOF-MS. Talanta 121, 89–96.
| Arsenic-containing fatty acids and hydrocarbons in marine oils – determination using reversed-phase HPLC–ICP-MS and HPLC–qTOF-MSCrossref | GoogleScholarGoogle Scholar | 24607114PubMed |
Stephens DW (1973). A summary of biological investigations concerning the Great Salt Lake, Utah (1861–1973). The Great Basin Naturalist 34, 221–229.
Stephens DW, Gillespie DM (1976). Phytoplankton production in the Great Salt Lake, Utah, and a laboratory study of algal response to enrichment. Limnology and Oceanography 21, 74–87.
| Phytoplankton production in the Great Salt Lake, Utah, and a laboratory study of algal response to enrichmentCrossref | GoogleScholarGoogle Scholar |
Sturt HF, Summons RE, Smith K, Elvert M, Hinrichs K-U (2004). Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry – new biomarkers for biogeochemistry and microbial ecology. Rapid Communications in Mass Spectrometry 18, 617–628.
| Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry – new biomarkers for biogeochemistry and microbial ecologyCrossref | GoogleScholarGoogle Scholar | 15052572PubMed |
Taleshi MS, Jensen KB, Raber G, Edmonds JS, Gunnlaugsdottir H, Francesconi KA (2008). Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus. Chemical Communications 39, 4706–4707.
| Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosusCrossref | GoogleScholarGoogle Scholar |
Tenchov B, Vescio EM, Sprott GD, Zeidel ML, Mathai JC (2006). Salt tolerance of archaeal extremely halophilic lipid membranes. The Journal of Biological Chemistry 281, 10016–10023.
| Salt tolerance of archaeal extremely halophilic lipid membranesCrossref | GoogleScholarGoogle Scholar | 16484230PubMed |
Viczek SA, Jensen KB, Francesconi KA (2016). Arsenic-containing phosphatidylcholines: a new group of arsenolipids discovered in herring caviar. Angewandte Chemie International Edition 55, 5259–5262.
| Arsenic-containing phosphatidylcholines: a new group of arsenolipids discovered in herring caviarCrossref | GoogleScholarGoogle Scholar | 26996517PubMed |
Watkins PA, Moser AB, Toomer CB, Steinberg SJ, Moser HW, Karaman MW, Ramaswamy K, Siegmund KD, Lee DR, Ely JJ, Ryder OA, Hacia JG (2010). Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions. BMC Physiology 10, 19
| Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functionsCrossref | GoogleScholarGoogle Scholar | 20932325PubMed |
Woermer L, Lipp JS, Schroeder JM, Hinrichs K-U (2013). Application of two new LC-ESI-MS methods for improved detection of intact polar lipids (IPLs) in environmental samples. Organic Geochemistry 59, 10–21.
| Application of two new LC-ESI-MS methods for improved detection of intact polar lipids (IPLs) in environmental samplesCrossref | GoogleScholarGoogle Scholar |
Xu F, Ng VY, Kroetz DL, Montellano PRO (2006). CYP4 isoform specificity in the omega-hydroxylation of phytanic acid, a potential route to elimination of the causative agent of Refsum’s disease. The Journal of Pharmacology and Experimental Therapeutics 318, 835–839.
| CYP4 isoform specificity in the omega-hydroxylation of phytanic acid, a potential route to elimination of the causative agent of Refsum’s diseaseCrossref | GoogleScholarGoogle Scholar | 16707724PubMed |
Yu X, Xiong C, Jensen KB, Glabonjat RA, Stiboller M, Raber G, Francesconi KA (2018). Mono-acyl arsenosugar phospholipids in the edible brown alga kombu (Saccharina japonica). Food Chemistry 240, 817–821.
| Mono-acyl arsenosugar phospholipids in the edible brown alga kombu (Saccharina japonica)Crossref | GoogleScholarGoogle Scholar | 28946346PubMed |