Insights into interactions of chlorine-based cleaning products with indoor relevant surfaces
Michael R. Alves A , Cholaphan Deeleepojananan A , Victor W. Or A , Izaac Sit B and Vicki H. Grassian
A , Cholaphan Deeleepojananan A , Victor W. Or A , Izaac Sit B and Vicki H. Grassian  A *
A *
A Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093, USA.
B Department of Nanoengineering, University of California San Diego, La Jolla, CA 92093, USA.
Environmental Chemistry 19(6) 343-349 https://doi.org/10.1071/EN22031
Submitted: 23 April 2022 Accepted: 3 October 2022 Published: 1 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. The chemistry that occurs in indoor environments and the role that indoor surfaces play have recently received increased attention in the scientific community. Here we have investigated the chemistry of chlorine-based cleaning products and their interactions with indoor relevant surfaces and find that these surfaces react with these cleaning products to yield surface adsorbed chlorine oxides and other surface-bound species.
Rationale. Indoor chemistry has recently received increased attention in the scientific community due to the fact that there is relatively little known given its unique environment including point combustion sources (candles, gas stoves, etc.) resulting in high aerosol concentrations, high surface to volume ratios and the impact of humans on indoor air quality. Recently, surface-initiated reactions during chlorine cleaning events have been proposed.
Methodology. In this study, we probe the interaction of bleach headspace gas with high surface area silica as a proxy for window glass – an ‘inert’ and impervious surface – using attenuated total reflectance Fourier Transform infrared (ATR-FTIR) spectroscopy, atomic force microscopy photothermal infrared (AFM-PTIR) spectroscopy and transmission electron microscopy (TEM) to observe surface chemical and physical changes.
Results. The results suggest chemical transformations occur at the silica surface forming surface adsorbed chlorine oxides (ClOx). Conductivity and ion chromatography methods support the presence of adsorbed chloride after surfaces have been exposed to bleach and HOCl.
Discussion. Interactions between HOCl and indoor surfaces have not been previously studied with molecular based techniques. The possibility of surface-mediated reactions has been relatively unexplored on indoor surfaces and this study shows the chemistry of chlorine-containing cleaning products on indoor relevant surfaces.
Keywords: bleach cleaning, chlorine oxides, hypochlorous acid, indoor chemistry, indoor air quality, indoor surfaces, surface chemistry.
Introduction
While people spend the large majority of their lives in indoor spaces containing a chemical ‘cocktail’ comprising a myriad of different surfaces, gases and combustion and light sources, our understanding of how these unique components interact with each other is greatly lacking (Weschler and Shields 1999; Nazaroff and Weschler 2004; Gligorovski and Abbatt 2018). The possible implications for human health concerns necessitate the study of indoor environments to constrain the many variables that control indoor chemistry. The use of strong household cleaners indoors is a significant topic of interest due to their powerful oxidising or reducing capabilities – likely producing a variety of byproducts with unknown properties (Wong et al. 2017). Overall, strong oxidisers such as quaternary ammonia compounds, hypochlorite (OCl−) and other oxychlorides, peroxygens (H2O2 or peracetic acid), phenolic compounds and alcohols make up the vast majority of household disinfection products in the United States (Fu et al. 2007). These disinfection products can be incredibly effective in their purpose, are widely used and yet they are one of the least understood aspects of indoor air chemistry and health (Weschler et al. 2006). A few studies have significantly correlated usage of caustic cleaners in the home to adverse health effects such as asthma and wheezing in children (Quirce and Barranco 2010; Parks et al. 2020). Conversely, some studies showed no significant connection between cleaners and respiratory issues with the possible differences in conclusions being due to the age of subjects, location or other unknown factors (Bukalasa et al. 2019). Whether these negative health effects are because of the primary ejection of the cleaner sprays as aerosols, the active disinfection chemical products themselves, the gas-phase form of the cleaners and the secondary organic aerosol they can form, or the oxidised/reacted products of the initial active component, is widely unknown. Studying the mechanism by which these strong and caustic chemical components interact with the indoor environment could facilitate a more accurate and holistic understanding of how these chemicals impact human health.
Hypochlorous acid (HOCl) is produced in the gas phase during and after cleaning events with bleach products. This oxidative compound is formed from the natural acidification of the main active ingredient, OCl−, present in the bleach solution. As the aqueous solution takes up CO2, the pH of the solution decreases leading to HOCl emission as shown in Eqn 1.
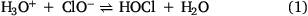
How HOCl interacts in indoor spaces, via gas-phase reactions, condensed- and surface-phase interactions, is poorly understood. Other important gas phase compounds can also be produced from bleach products, from the reaction between HOCl/OCl− and ammonia (NH3) or dinitrogen pentoxide (N2O5), including chloramines (NHxCly) and nitryl chloride (ClNO2) (Jafvert and Valentine 1992; Wong et al. 2017; Wang et al. 2019). In addition, secondary organic aerosol formation increases in the gas-phase when volatile organic compounds and bleach are present at the same time (Mattila et al. 2020a; Patel et al. 2020). Most interesting for this current study is the work of Mattila et al. (2020b) which shows the presence of a large unidentified reservoir for hypochlorous acid indoors during the 2018 HOMEChem campaign (Farmer et al. 2019) – larger than what could be attributed to gases and aerosols. Tentatively, this reservoir was hypothesised to be due to surfaces. Whether or not these surface interactions are reversible, reactive or produce other unique products is currently not known.
Silica, or SiO2, has been studied extensively over the past century and continues to be an area of interest for material and surface scientists. Although window glass (amorphous SiO2) can be commonly considered as a relatively inert substrate which is an insulator and not a semiconductor such as titanium dioxide (TiO2), surface hydroxy groups formed in the presence of water (relative humidity > 5%) gives the surface adsorptive properties (Suh et al. 2000; Fang et al. 2019). Despite SiO2 being a ubiquitous surface indoors, its interaction with HOCl, a common oxidative gas molecule, under room temperature conditions is not well studied. In this study, we look at the interaction between SiO2 and HOCl.
Experimental methods
To probe the chemistry of HOCl on an indoor relevant substrate, high surface area silica (SiO2, OX50 Aerosil) was used as a proxy for window glass. The sample was baked at 500°C for 12 h to remove trace organics prior to exposure. Thin films of the silica were created by suspending the silica in water and were allowed to dry on an AMTIR crystal (Amorphous Material Transmitting IR radiation, Pike Technologies) overnight. The appearance of the 1140 and 1235 cm−1 peaks and lack of contamination peaks confirmed the presence of clean deposited silica nanoparticles on the crystal surface. Hypochlorous acid was generated by either buffering a 10–15% sodium hypochlorite solution (Sigma Aldrich) or commercial bleach, with monosodium phosphate to achieve a slightly acidic pH of 6.4. The method was adapted from Schwartz-Narbonne and coworkers (Schwartz-Narbonne et al. 2019).
A flow of 10 sccm of HOCl gas was transferred into a dilution line containing 0.1 slpm of zero air, achieving a concentration of 61 +/− 1 ppm. The concentration of HOCl and Cl2 was measured using a 53.5 cm gas-phase UV-vis cell, using wavelengths of 242 and 330 nm respectively to calculate concentration. The absorption cross-sections used to calculate the concentration of HOCl and Cl2 were 2.03 × 10−19 and 2.55 × 10−19 cm2 molecule−1 respectively (Burkholder et al. 2015). A pulsed Xe lamp (Excelitas Technologies Corp.) was used as a source and a diode array detector (Ocean Optics USB 3000) was used to measure the change in absorbance respective to the background. ATR-FTIR spectra were obtained using a Nicolet iS10 FTIR spectrometer (Thermo Fisher) equipped with an MCT/A detector and the AMTIR crystal base at 4 cm−1 resolution using an average of 256 scans over a spectroscopic range of 600–4000 cm−1. Measurements of chlorine oxyanions were carried out using ion chromatography (IC) (ICS-2000 ThermoFisher) and TraceCERT™ IC standards including chlorite, chlorate and perchlorate (Sigma Aldrich).
To measure nanoparticle size, 15 μL of a sonicated 0.01 g L–1 silica suspension was dropped on a formvar/carbon-coated 100 mesh copper grid and dried. The copper grid was analysed using a JEM-1400 Plus transmission electron microscope at 80 kV. The silica was exposed to HOCl using conditions as described above for 1 h.
Atomic force microscopy photothermal infrared (AFM-PTIR) spectroscopic measurements were performed using a commercial AFM-IR microscope (nanoIR2, Bruker) with a tunable mid-IR quantum cascade laser (QCL MIRcat-QT, Daylight solutions). Images and spectra were collected at ~5–10% relative humidity (RH) and ambient temperature (20–25°C). Analysis was conducted using silicon nitride probes with a chromium-gold coating (HQ: NSC19/CR-AU, MikroMasch) with a typical tip radius of 30 nm and a nominal spring constant range of 0.05–2.3 N m–1. AFM imaging was conducted in tapping mode at a scan rate of 0.5 Hz. AFM-PTIR spectra were collected with a nominal spatial resolution below 35 nm and a spectroscopic resolution of 5 cm−1, co-averaging over 128 laser pulses per wavenumber. The silica solution was dried onto the quartz substrates under similar conditions to the sample preparation for ATR-FTIR.
Results and discussion
Due to the complex nature of the indoor environment, studying surface-level and mechanistic chemistry can be challenging. To find which surface (or surfaces) can act as a sink for gas-phase HOCl, silica, a model for window glass, was used as a simple starting point. Similar to adsorbed H2O, (Goodman et al. 2001; Rubasinghege and Grassian 2013) it was initially hypothesised that HOCl would interact with surface hydroxy groups via hydrogen-bonding and then upon removal of HOCl from the gas phase the relatively weakly adsorbed HOCl would desorb upon evacuation. Instead, ATR-FTIR spectroscopy shows that products are formed at the surface, with infrared absorption peaks appearing mostly in the low frequency region below 1000 cm−1, as shown in Fig. 1. In this region, it is seen that peaks in the spectra grow in intensity as a function of time as the silica surface is exposed to HOCl.
|
In particular, these infrared data show that multiple peaks are present at 963, 859 and 730 cm−1 upon adsorption. These peaks suggest there are multiple species being produced and that HOCl, a volatile compound, reacts with the surface to yield chlorine oxyanions or functionalised silica (e.g. Si–Cl, Si–ClOx, etc.). For reference, the spectrum of gas-phase HOCl shows the νO–Cl stretch is measured to be around 725 cm−1, suggesting that ClOx species are being formed. Thus, this was an unexpected result due to the volatility of HOCl and provides evidence that a different chemical process is occurring, namely the formation of new surface bound chlorine oxides.
The appearance of these chlorine oxide products is well-correlated with the decrease of Si–O–Si bands around 1200 cm−1, a site for surface reaction. It should be noted that this experiment occurred at a concentration of HOCl around 64 ppm during exposure, higher than typical HOCl concentrations in indoor spaces reported at levels between 0.1 and 5 ppm during cleaning events (Wong et al. 2017; Wang et al. 2019; Mattila et al. 2020a, 2020b). In addition, the gas stream also contains Cl2 thus deriving kinetic parameters from these data is not possible.
Following these adsorption measurements, the HOCl was removed from the gas flow and clean dry air was introduced into the ATR-FTIR cell for 60 min. It is seen in the spectra shown in Fig. 1 that upon this desorption step, the peaks remain, showing that these products remain on the surface.
The presence of surface-bound products was further proven through the use of conductivity measurements and ion chromatography. Silica was exposed to HOCl under similar conditions for the ATR-FTIR measurements for 2 h. After a 1 h period of desorption using dry zero air, the exposed silica nanoparticles were immersed in Milli-Q water and sonicated for 2 h. Blanks containing just Milli-Q water as well as clean silica nanoparticles were also sonicated as a solvent blank and method blank. Conductivity of the HOCl exposed samples increased above twice the background and the ion concentration of chloride was enhanced by a factor of two in comparison to the blank concentration of non-exposed silica.
Though AMTIR crystals are normally used for somewhat caustic and/or acidic systems, it was evident throughout this experiment that the crystal itself was slowly reacting with the HOCl/Cl2 gas. Not only does a singular peak increase over time around 850 cm−1, eventually after many uses and long term exposures, an oxidised layer will form on the crystal. Diamond powder (Pike Technologies) at various grit sizes was used to clean this oxide layer and regenerate the flat AMTIR crystal surface. The crystal is made up of a brittle alloy of selenium, arsenic and germanium. The similarity between germanium and silicon possibly led to a similar interaction with the oxidant. A ZnSe crystal (Pike Technologies) exposed to the same conditions did not show any unwanted byproducts, however due to its limitations in pH range and weak signal below 1000 cm−1, it was not further used for this study.
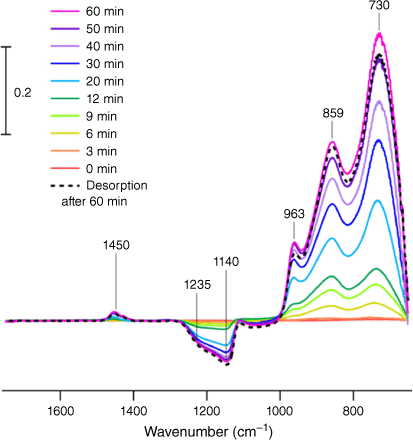
To show that the product peaks could be formed independent of the AMTIR crystal surface, a silica substrate with a thin film of silica nanoparticles was exposed to similar conditions of HOCl and Cl2 gas for about 2 h. After the period of desorption, the infrared spectra of the surface were measured using AFM-PTIR spectroscopy. In the absence of the AMTIR crystal, the interaction between the gas and silica surface shows similar results as shown in Fig. 2 – namely the 963 and 853 cm−1 peaks. The third peak at 730 cm−1 could not be seen due to the spectroscopic limitations of the AFM-PTIR below 800 cm−1. The height comparisons between the two peaks shown in Fig. 2 are clearly different due to differences in operating principles between these two different infrared methods. Or and coworkers highlight how the sample thickness and non-linearity of the PTIR technique can lead to deviations in spectroscopic intensities (Or et al. 2018). The broadness of these peaks indicate the presence of multiple binding modes and conformations at the surface, which is explored a bit further in more detail below.
|
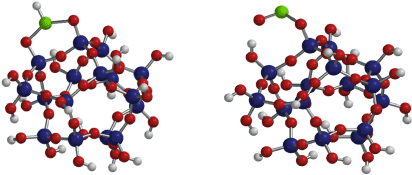
To further understand these data, an amorphous SiO2 pseudo structure was formed using a total of 62 atoms using Spartan Software (Wavefunction Inc.). The geometries of the energy minimised cluster were calculated at the B3LYP/6-311+G** level of theory. Molecular vibrational frequency calculations were performed at the EDF2 DFT level of theory (Lin et al. 2004). Scaling factors to account for anharmonicity in the calculated frequencies were not used in this study. Similar to the B3LYP model, the EDF2 functional provides slightly more accurate results for vibrational spectra while also decreasing computation time as discussed in Lin et al. (2004).
|
Of the different possible binding modes, the monodentate form and the bridging bidentate forms (in this case of bridging ClO2 with protonation) as shown in Fig. 3, were found to have the most similar vibrational modes to the experimental data (see Table 1). The monodentate form had intense major vibrational modes calculated at 988 and 827 cm−1. The bridging bidentate configuration shows major vibrational modes at 726, 743 and 844 cm−1, where 726 and 743 cm−1 were defined as a Cl–H wagging mode with contributions from the mode at 844 cm−1. As scaling factors which account for anharmonicity were not used, it should be noted that the agreement between the theoretical and experimental frequencies for the v(SiO–H) mode is a result of these lower frequency broad peaks. While it would be expected that the difference in these frequencies at the higher energies should be larger, on the order of 100–300 cm−1, the variance in frequencies below 1000 cm−1 should be quite close to accurate (around 20–80 cm−1) (Baltrusaitis et al. 2006). Thus, for the purposes of this study, product vibrational frequencies between 700 and 1000 cm−1 for the calculated model systems are consistent with the experimental data (Table 1).
|
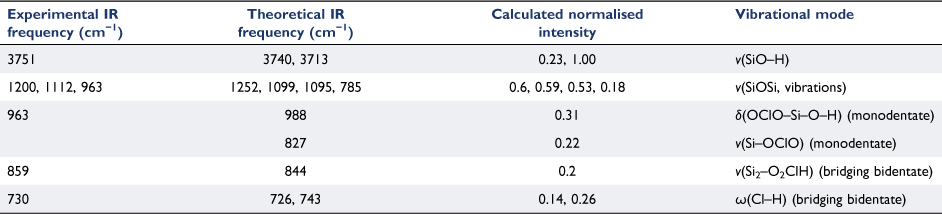
An AFM image of the exposed silica thin film is seen in Fig. 2c. Transmission electron microscopy (TEM) images of individual particles are shown in Fig. 4. The unexposed silica particles in Fig. 4a show smooth edges whereas the images of the particles taken after exposure to the HOCl gas show an increase in surface roughness. These data suggest the possibility of surface roughening due to surface segregation of the chlorine oxides that form or even etching due to the presence of the strong oxidant.
|
Most interesting is that analysis of these exposed silica surfaces several weeks after exposure to HOCl show X-ray photoelectron spectra (XPS) with no Cl signal in the Cl 2p region indicating that these products desorb from the surface over time (see Supplementary Fig. S1). A comparison of the XPS data for HOCl exposed SiO2 particles compared to TiO2 particles shows that weeks after HOCl exposure there is chloride present on the TiO2 particle surfaces but not the SiO2 particle surfaces, showing there is different bleach chemistry on these two different particle surfaces with surface products more strongly adsorbing to the TiO2 surface (see Supplementary Fig. S1).
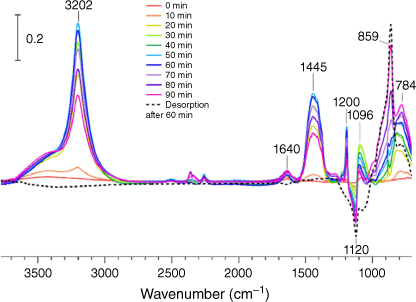
Commercial bleach products contain a multitude of other ingredients besides hypochlorite, including (but not limited to): anionic and non-ionic organic surfactants, carbonates, dye transfer inhibitors using polyamine N-oxide polymers or various other imidazole/pyrrolidone based polymers, organic acids and more. To measure the impact this realistic model of chlorine cleaning would have on the silica, a commercial bleach solution was acidified and its headspace (measured RH < 20%) was allowed to interact with a silica thin film. As shown in Fig. 5, similar partially and irreversibly bound chlorine oxide peaks appear, albeit in different relative intensities compared to the model HOCl plus silica experiment. However, unlike the model experiment, many other additional peaks are observed to reversibly adsorb onto the silica thin film and possibly compete for active sites with the produced HOCl gas. The largest peak at 3202 cm−1 clearly indicates the presence of a secondary, and likely aliphatic, amine perhaps derived from one of the nitrogen-containing polymers and compounds commonly found in bleach patents. Additional new peaks between 1700 and 1200 cm−1 show some absorbed water at 1640 cm−1, methylene groups (surfactants/polymers) at 1445 cm−1 and other modes at 1200 and 1096 cm−1 or other various organic species such as the N–H bending mode, that all eventually desorb upon the use of dry zero air an hour after the experiment. The change in relative intensities of the chlorine oxyanion peaks between 1000 and 700 cm−1 in the bleach experiment compared to the silica samples exposed to HOCl in the silica experiment could be due to many reasons, including the presence of additional adsorbed water and the presence of a large amount of heteroatom-containing organic molecules. Nevertheless, it seems that even with water and a significant number of organic species nearby on the surface, HOCl interacts with the silica surface to form chlorine oxides as seen by the low frequency (below 1000 cm−1) region.
|
Thus, these bleach cleaning experiments show that several different chemical species partition onto the surface. Several of these species are similar to when HOCl absorbs onto SiO2 to form the suspected ClOx surface bound products that remain on the surface after the bleach headspace gas is no longer in the flow system. In contrast, all other chemical species, including secondary amines, desorb from the surface after a flow of bleach-free dry air is directed over the sample and thus are more weakly adsorbed to the surface.
Conclusions
SiO2 surfaces exposed to gas-phase HOCl form surface bound chlorine oxides as identified by vibrational spectroscopy. In addition, the enrichment of chloride signal in IC analysis along with the decrease in the Si–O–Si bonds as indicated from infrared spectroscopy, highlight that there are unique surface sites for reaction and possibly multiple mechanisms involved in these interactions. Calculations using a model of a SiO2 cluster shows that ClOx species associated with the silica surface have absorptions in the low frequency region between 725 and 1000 cm−1 due to several vibrations consistent with the ATR-FTIR and AFM-PTIR spectra. TEM images of the silica particles show evidence that the surface is being roughened as the silica is exposed to HOCl. The studies of bleach with SiO2 show similar interactions with HOCl and other components including buffers, secondary amines, polymers, aromatics and more – showing the complexity of indoor surface chemistry. While the data shown here was done purely between gas and solid phases, liquid bleach is commonly applied directly onto surfaces. Other common surfaces are expected to also have unique interactions with HOCl and the different organics and nitrogen-containing species present. Overall, these results show that the use of chlorine cleaning products leads to complex surface chemistry. Future work should aim to qualitatively determine the compositions of such systems so that a quantitative understanding of the sources and sinks of indoor relevant oxidants may be further developed along with information on rates of reactions as a funciton of relative humidity and temperature.
Supplementary material
Supplemental material contains one figure with XPS data. Supplementary material is available online.
Data availability
The data that support this study are available in Dryad at https://doi.org/10.6078/D1PQ7C.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This material is based upon work supported by the Alfred P. Sloan Foundation under grant G-2020-12675 as well as support from the National Science Foundation Graduate Research Fellowship Program (DGE-1650112).
Acknowledgements
The authors gratefully acknowledge the support of the Alfred P. Sloan Foundation under grant number G‐2020‐12675 and the National Science Foundation Graduate Research Fellowship Program (DGE1650112). The low-resolution TEM images were acquired at the UCSD Cellular and Molecular Medicine Electron Microscopy Core Facility (UCSD-CMM-EM Core, RRID:SCR_022039) which is supported in part by the NIH award S10 OD023527. The authors also thank Professor Jonas Baltrusaitis at Lehigh University for the collection of XPS data.
References
Baltrusaitis J, Jensen JH, Grassian VH (2006). FTIR spectroscopy combined with isotope labeling and quantum chemical calculations to investigate adsorbed bicarbonate formation following reaction of carbon dioxide with surface hydroxyl groups on Fe2O3 and Al2O3. Journal of Physical Chemistry B 110, 12005–12016.| FTIR spectroscopy combined with isotope labeling and quantum chemical calculations to investigate adsorbed bicarbonate formation following reaction of carbon dioxide with surface hydroxyl groups on Fe2O3 and Al2O3.Crossref | GoogleScholarGoogle Scholar |
Bukalasa JS, Brunekreef B, Koppelman GH, Vonk JM, Gehring U (2019). Use of cleaning agents at home and respiratory and allergic symptoms in adolescents: The PIAMA birth cohort study. Environment International 128, 63–69.
| Use of cleaning agents at home and respiratory and allergic symptoms in adolescents: The PIAMA birth cohort study.Crossref | GoogleScholarGoogle Scholar |
Burkholder JB, Sander SP, Abbatt JPD, Barker JR, Huie RE, Kolb CE, Kurylo MJ, Orkin VL, Wilmouth DM, Wine PH (2015) Chemical kinetics and photochemical data for use in atmospheric studies: evaluation number 18. JPL Publication, California, pp. 4–215. https://hdl.handle.net/2014/45510
Fang Y, Lakey PSJ, Riahi S, McDonald AT, Shrestha M, Tobias DJ, Shiraiwa M, Grassian VH (2019). A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: limonene adsorption on SiO2. Chemical Science 10, 2906–2914.
| A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: limonene adsorption on SiO2.Crossref | GoogleScholarGoogle Scholar |
Farmer DK, Vance ME, Abbatt JPD, Abeleira A, Alves MR, Arata C, Boedicker E, Bourne S, Cardoso-Saldaña F, Corsi R, Decarlo PF, Goldstein AH, Grassian VH, Hildebrandt Ruiz L, Jimenez JL, Kahan TF, Katz EF, Mattila JM, Nazaroff WW, Novoselac A, O’Brien RE, Or VW, Patel S, Sankhyan S, Stevens PS, Tian Y, Wade M, Wang C, Zhou S, Zhou Y (2019). Overview of HOMEChem: House observations of microbial and environmental chemistry. Environmental Science: Processes & Impacts 21, 1280–1300.
| Overview of HOMEChem: House observations of microbial and environmental chemistry.Crossref | GoogleScholarGoogle Scholar |
Fu E, McCue K, Boesenberg D (2007) Chemical disinfection of hard surfaces – household, industrial and institutional settings. In ‘Handbook for Cleaning/Decontamination of Surfaces’. (Eds I Johansson, P Somasundaran) pp. 573–592. (Elsevier)
| Crossref |
Gligorovski S, Abbatt JPD (2018). An indoor chemical cocktail. Science 359, 632–633.
| An indoor chemical cocktail.Crossref | GoogleScholarGoogle Scholar |
Goodman AL, Bernard ET, Grassian VH (2001). Spectroscopic study of nitric acid and water adsorption on oxide particles: Enhanced nitric acid uptake kinetics in the presence of adsorbed water. Journal of Physical Chemistry A 105, 6443–6457.
| Spectroscopic study of nitric acid and water adsorption on oxide particles: Enhanced nitric acid uptake kinetics in the presence of adsorbed water.Crossref | GoogleScholarGoogle Scholar |
Jafvert CT, Valentine RL (1992). Reaction scheme for the chlorination of ammoniacal water. Environmental Science and Technology 26, 577–586.
| Reaction scheme for the chlorination of ammoniacal water.Crossref | GoogleScholarGoogle Scholar |
Lin CY, George MW, Gill PMW (2004). EDF2: A density functional for predicting molecular vibrational frequencies. Australian Journal of Chemistry 57, 365–370.
| EDF2: A density functional for predicting molecular vibrational frequencies.Crossref | GoogleScholarGoogle Scholar |
Mattila JM, Arata C, Wang C, Katz EF, Abeleira A, Zhou Y, Zhou S, Goldstein AH, Abbatt JPD, DeCarlo PF, Farmer DK (2020a). Dark chemistry during bleach cleaning enhances oxidation of organics and secondary organic aerosol production indoors. Environmental Science and Technology Letters 7, 795–801.
| Dark chemistry during bleach cleaning enhances oxidation of organics and secondary organic aerosol production indoors.Crossref | GoogleScholarGoogle Scholar |
Mattila JM, Lakey PSJ, Shiraiwa M, Wang C, Abbatt JPD, Arata C, Goldstein AH, Ampollini L, Katz EF, DeCarlo PF, Zhou S, Kahan TF, Cardoso-Saldaña FJ, Ruiz LH, Abeleira A, Boedicker EK, Vance ME, Farmer DK (2020b). Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning. Environmental Science and Technology 54, 1730–1739.
| Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning.Crossref | GoogleScholarGoogle Scholar |
Nazaroff WW, Weschler CJ (2004). Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmospheric Environment 38, 2841–2865.
| Cleaning products and air fresheners: Exposure to primary and secondary air pollutants.Crossref | GoogleScholarGoogle Scholar |
Or VW, Estillore AD, Tivanski AV, Grassian VH (2018). Lab on a tip: Atomic force microscopy-photothermal infrared spectroscopy of atmospherically relevant organic/inorganic aerosol particles in the nanometer to micrometer size range. Analyst 143, 2765–2774.
| Lab on a tip: Atomic force microscopy-photothermal infrared spectroscopy of atmospherically relevant organic/inorganic aerosol particles in the nanometer to micrometer size range.Crossref | GoogleScholarGoogle Scholar |
Parks J, McCandless L, Dharma C, Brook J, Turvey SE, Mandhane P, Becker AB, Kozyrskyj AL, Azad MB, Moraes TJ, Lefebvre DL, Sears MR, Subbarao P, Scott J, Takaro TK (2020). Association of use of cleaning products with respiratory health in a Canadian birth cohort. Canadian Medical Association Journal 192, E154–E161.
| Association of use of cleaning products with respiratory health in a Canadian birth cohort.Crossref | GoogleScholarGoogle Scholar |
Patel S, Sankhyan S, Boedicker EK, DeCarlo PF, Farmer DK, Goldstein AH, Katz EF, Nazaroff WW, Tian Y, Vanhanen J, Vance ME (2020). Indoor particulate matter during HOMEChem: Concentrations, size distributions, and exposures. Environmental Science and Technology 54, 7107–7116.
| Indoor particulate matter during HOMEChem: Concentrations, size distributions, and exposures.Crossref | GoogleScholarGoogle Scholar |
Quirce S, Barranco P (2010). Cleaning agents and Asthma. Journal of Investigational Allergology and Clinical Immunology 20, 542–550.
Rubasinghege G, Grassian VH (2013). Role(s) of Adsorbed Water in the Surface Chemistry of Environmental Interfaces. Chemical Communications 49, 3071–3094.
| Role(s) of Adsorbed Water in the Surface Chemistry of Environmental Interfaces.Crossref | GoogleScholarGoogle Scholar |
Schwartz-Narbonne H, Wang C, Zhou S, Abbatt JPD, Faust J (2019). Heterogeneous chlorination of squalene and oleic acid. Environmental Science and Technology 53, 1217–1224.
| Heterogeneous chlorination of squalene and oleic acid.Crossref | GoogleScholarGoogle Scholar |
Suh M, Bagus PS, Pak S, Rosynek MP, Lunsford JH (2000). Reactions of hydroxyl radicals on titania, silica, alumina, and gold surfaces. Journal of Physical Chemistry B 104, 2736–2742.
| Reactions of hydroxyl radicals on titania, silica, alumina, and gold surfaces.Crossref | GoogleScholarGoogle Scholar |
Wang C, Collins DB, Abbatt JPD (2019). Indoor illumination of terpenes and bleach emissions leads to particle formation and growth. Environmental Science and Technology 53, 11792–11800.
| Indoor illumination of terpenes and bleach emissions leads to particle formation and growth.Crossref | GoogleScholarGoogle Scholar |
Weschler CJ, Shields HC (1999). Indoor ozone/terpene reactions as a source of indoor particles. Atmospheric Environment 33, 2301–2312.
| Indoor ozone/terpene reactions as a source of indoor particles.Crossref | GoogleScholarGoogle Scholar |
Weschler CJ, Shields HC (2000). The influence of ventilation on reactions among indoor pollutants: Modeling and experimental observations. Indoor Air 10, 92–100.
| The influence of ventilation on reactions among indoor pollutants: Modeling and experimental observations.Crossref | GoogleScholarGoogle Scholar |
Weschler CJ, Wells JR, Poppendieck D, Hubbard H, Pearce TA (2006). Workgroup report: Indoor chemistry and health. Environmental Health Perspectives 114, 442–446.
| Workgroup report: Indoor chemistry and health.Crossref | GoogleScholarGoogle Scholar |
Wong JPS, Carslaw N, Zhao R, Zhou S, Abbatt JPD (2017). Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air 27, 1082–1090.
| Observations and impacts of bleach washing on indoor chlorine chemistry.Crossref | GoogleScholarGoogle Scholar |


