Evaluation of the diffusive gradients in thin-films (DGT) technique for measuring nitrate and ammonium in soil
Krishantha Kodithuwakku A , Jianyin Huang A * , Casey L. Doolette
A * , Casey L. Doolette  B , Sean Mason C , John Boland
B , Sean Mason C , John Boland  D , Enzo Lombi
D , Enzo Lombi  A B , Niklas J. Lehto
A B , Niklas J. Lehto  E and Peter R. Teasdale A A
E and Peter R. Teasdale A A
A STEM, University of South Australia, Mawson Lakes Campus, Mawson Lakes, SA 5095, Australia.
B Future Industries Institute, University of South Australia, Mawson Lakes Campus, Mawson Lakes, SA 5095, Australia.
C Agronomy Solutions Pty. Ltd., 3/11, Ridley Street, Hindmarsh, SA 5007, Australia.
D Industrial Al research centre, STEM, University of South Australia, Mawson Lakes Campus, Mawson Lakes, SA 5095, Australia.
E Department of Soil and Physical Science, Faculty of Agriculture and Life Sciences, Lincoln University, New Zealand.
Environmental Chemistry 19(8) 483-494 https://doi.org/10.1071/EN22107
Submitted: 21 October 2022 Accepted: 19 January 2023 Published: 20 February 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Nitrate (NO3−) and ammonium (NH4+) are the most important soil nitrogen forms for plant growth. However, conventional extraction techniques may introduce artefacts affecting the measurement of plant-available N concentrations following sampling and sample preparation processes. This is the first study of the DGT technique being used to measure NO3-N and NH4-N in a wide range of soils, compared with conventional KCl extraction, and examined different factors that contribute to the plant-availability of these ions in soils. The knowledge would help to optimise soil nitrogen management practices, increase economic benefits and reduce environmental impacts.
Rationale. The availability of soil nitrogen for plant uptake can be affected by numerous soil factors such as soil texture, moisture and organic matter content, temperature and microbial activity. Conventional extraction techniques may affect the measurement of plant-available N concentrations following sampling and sample preparation processes, including drying, sieving, homogenising, freezing and thawing. The diffusive gradients in thin-films (DGT) technique can overcome some limitations of the conventional extraction techniques and has been used to successfully estimate the plant-available fractions of nutrients, such as P, K, Zn, Cu and Mn in soils. Therefore, it is important to evaluate the use of DGT for measuring NO3− and NH4+ in a wide variety of soils and examine the factors that contribute to the plant-availability of these ions in soils.
Methodology. The experiment evaluated the ability of the DGT technique to measure NO3-N and NH4-N in soils using binding layers containing A520E anion exchange resin or Microlite® PrCH cation exchange resin, respectively. The DGT results were compared to those from conventional KCl extraction.
Results. The A520E- and PrCH-DGTs showed good detection limits for NO3-N (6.90 µg L−1) and NH4-N (6.23 µg L−1) and were able to measure potentially available NO3-N and NH4-N in unfertilised soils. The mass of NO3-N and NH4-N that accumulated on the DGT device increased linearly across soil concentrations ranging from 5 to 300 mg kg−1 NO3-N (depending on soil type) and 5–300 mg kg−1 NH4-N; which is equivalent to fertiliser rates of 75–450 kg ha−1 N. DGTs were used to measure potentially available NO3-N and NH4-N in ten soils with various physical and chemical properties. The DGT results were compared with conventional KCl extraction used to determine soil mineral N. DGT and KCl extraction measured values were significantly correlated with each other for NO3-N (R2 = 0.53; P-value < 0.001), but the relationship between the two measurements was weaker for NH4-N (R2 = 0.20, P-value = 0.045).
Discussion. The results suggest that the two methods sample different N pools in the soils, with DGT targeting the NO3-N and NH4-N that are available in soil pore water and attached to labile solid phases.
Keywords: accumulation, binding capacity, conventional extraction, correlation, DGT technique, fertiliser efficiency, limit of detection, soil nitrogen availability.
Introduction
Nitrogen (N) is critical for plant growth and, of all the plant macronutrients, has the most complex biogeochemical cycle in soils (Montemurro and Diacono 2016). Among the variety of nitrogen species in soil, nitrate (NO3−) and ammonium (NH4+) are the most important nitrogen forms for plant growth (Sharma and Bali 2018) and ecosystem production (Canfield et al. 2010). Between 50 and 70% of soil nitrogen is prone to loss (Belete et al. 2018) mainly by volatilisation, denitrification, runoff and leaching (Montemurro and Diacono 2016). Increasing global populations require a significant increase in food production (Godfray et al. 2010), which in turn requires maintaining plant-available N stocks in agricultural lands (Gruber and Galloway 2008). These can be sustained through fertiliser inputs but minimising losses must also play a key role. Multiple studies have shown that the application of synthetic N fertilisers has increased continuously since the 1960s (Yuan and Peng 2017), with around a 1.5% annual increase from 2015 to 2020 (FAO 2017) forecasted in 2017. Simultaneously, N fertiliser prices have also increased over previous decades; for instance, the global average urea price was USD 187 per metric tonne in 1990 compared to USD 602 in 2013 (Adjesiwor and Islam 2016). However, the over-application of fertiliser is not economically viable (Goulding et al. 2008) and leads to negative environmental consequences (Gruber and Galloway 2008). Since the 1960s, nitrogen concentrations in surface waters have increased significantly due to the widespread use of fertiliser, leading to eutrophication (Moss 2008). Additionally, soil acidification (Guo et al. 2010), nitrous oxide (N2O) emission (a type of greenhouse gas) (De Laporte et al. 2021) and ozone depletion (Gruber and Galloway 2008) are other issues related to over-fertilisation with N. Accurate measurement of plant-available soil nitrogen concentrations across various soil types would help to optimise soil nitrogen management practices, increase economic benefits and reduce environmental impacts (Giles et al. 1975; Du et al. 2020).
The availability of soil nitrogen for plant uptake can be affected by numerous soil factors such as soil texture (Tremblay et al. 2012), moisture and organic matter content (Qian and Schoenau 1994), temperature and microbial activity (Maynard et al. 2007; Canali et al. 2011). Conventional extraction techniques may introduce artefacts affecting plant-available N concentrations following sampling and sample preparation processes (e.g. drying, sieving, homogenising, freezing and thawing) (Stark and Hart 1997; Cai et al. 2016). Furthermore, the methods to perform these measurements encompass a wide range of different sieving procedures, extractant types and concentrations, soil/extractant ratios and extraction times. This diversity provides a wide range of estimates of N availability and confounds comparisons between studies (McTaggart and Smith 1993; Li et al. 2012; Carrillo-Gonzalez et al. 2013; Inselsbacher 2014; Homyak et al. 2015). One common method to sample mineral N (NH4+ and NO3−) in soils is to extract these analytes with a 2 mol L−1 KCl solution, using a dry soil/extractant ratio of 1:5, 1:10 or 1:20 (w/v) (Schroeder et al. 1985; Maynard et al. 2007; Rayment and Lyons 2011). This extraction has been used historically to estimate plant-available N (Saha et al. 2018) despite suggestions that this method overestimates NH4+ availability because it can extract strongly bound ions that may not be available in the soil porewater for plant uptake (Mengel 1982; Maynard et al. 2007). The extensive sample processing used to extract the NH4+ from the soil has also been suggested to be an important contributing factor to this limitation (Stark and Hart 1997; Cai et al. 2016).
Diffusive gradients in thin-films (DGT) technique has been used to successfully estimate the plant-available fractions of nutrients, such as P, K, Zn, Cu and Mn in soils (Zhang et al. 1995; Mundus et al. 2012; Six et al. 2012; Zhang et al. 2013, 2014b; Guan et al. 2022). The amount of analyte sampled by DGT depends on the analyte concentration in the soil porewater and its resupply rate from solid into porewater (Harper et al. 1998; Sochaczewski et al. 2007). These factors can also affect the rate of nutrient uptake by plant roots in soils (Marschner and Rengel 2012). Moreover, DGT deployments in soils are often carried out under similar moisture conditions to those experienced in the field, without the addition of a chelant or solute to promote the release of analytes of interest from the solid phases that may go on to affect soil chemical characteristics (Lehto 2016). It follows that DGT has been found to be effective at predicting plant-available nutrient concentrations, especially when the supply of nutrients to plants is diffusion-limited (e.g. P, Zn and Cu) (Mason et al. 2010; Tandy et al. 2011; Sun et al. 2014; Vogel et al. 2020; Guan et al. 2022).
The DGT techniques for measuring NO3− and NH4+ in freshwaters were developed by Huang et al. (2016a, 2016b) using A520E and PrCH ion exchange resins, respectively. The authors noted the high capacity of the two resins to bind NO3− and NH4+ ions, which in turn highlighted the potential of these resins for undertaking DGT measurements of mineral N species in soils. Cai et al. (2016) tested the use of SIR-100-HP resin in DGT for measuring NO3− in the porewaters of three soils and found good agreement (<10% error) between DGT devices and porewater NO3− values from soil solutions that were collected by centrifuging the soils at 5000g for 15 min. Vogel et al. (2020) showed that soil NO3− and NH4+ concentrations measured in one soil using DGT devices equipped with A520E and PrCH resins were comparable with KCl-extracted N under different soil-N application rates and in the presence of nitrification inhibitors. Furthermore, they showed that KCl and DGT extractions performed before and after plant harvesting gave similar patterns of soil NO3− and NH4+ measurements. However, the experiment only used one soil type and to date, the ability of DGT with A520E and PrCH binding resins to measure soil NO3− and NH4+ has not been tested across a wide range of soils. Furthermore, a detailed comparison of DGT-available and KCl-extractable N-species and an analysis of the important factors that cause differences between them is lacking. Given the different mechanisms by which the two techniques sample mineral N in soils, a comparison across a range of soil types will increase the understanding of factors that govern NO3− and NH4+ solubility. This study aimed to validate the use of DGT for measuring NO3− and NH4+ in soils with a variety of physical and chemical properties and examine the factors that contribute to the plant-availability of these ions in soils.
Experimental
The DGT technique was evaluated by (1) testing the capability of DGT to measure NO3− and NH4+ in three unamended soils, (2) measuring the accumulation of these ions in DGTs deployed in the same three soils spiked at 12 N concentrations, and (3) comparing DGT to conventional KCl extraction in ten contrasting soils spiked with two N concentrations.
Soil preparation
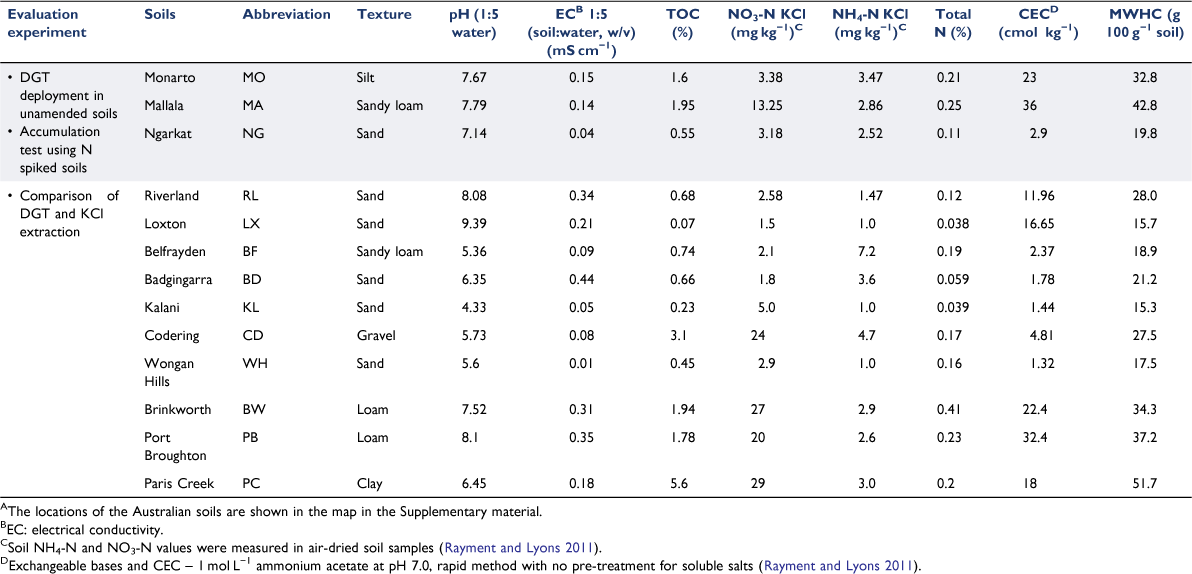
Thirteen topsoils (0–10 cm depth) listed in Table 1, were selected from Australian agricultural sites to provide a range of chemical and physical soil properties for evaluating DGT performance. Soils were ground, sieved (<2 mm) and dried in an oven at 40°C for 2 days until constant weight (Rayment and Lyons 2011). The soil maximum water holding capacity (MWHC) was determined by the gravitational method (Margesin and Schinner 2005). Soil chemical and physical characteristics (pH, electrical conductivity (EC), cation exchange capacity (CEC), texture, total organic carbon (TOC) and total nitrogen) were determined for each soil (Table 1). Extractable mineral nitrogen species (NO3− and NH4+) were measured in the air-dried soils using a 2 mol L−1 KCl extraction, as described by Rayment and Lyons (2011). Briefly, a 1:5 soil/extractant ratio and 1 h of extraction were employed and the supernatant was analysed after filtering (polyethersulfone, 0.45 µm pore size; Sterlitech Corp., USA). To assist comparison between NO3− and NH4+ measurements, forthwith their concentrations will be reported as NO3-N and NH4-N.
|
DGT preparation
Reagents
The A520E anion exchange resin and the PrCH cation exchange resin (Purolite Co. Ltd, Asia Pacific, Zhejiang, China) were used as the binding agents in the DGT binding layers. Ultrapure agarose (Life Technologies Corp., USA) was used to prepare the diffusive layer and PrCH binding layer (see below) and bisacrylamide (acrylamide/bis-acrylamide – 40%, Sigma-Aldrich, USA) was used for the A520E binding layer. All reagents were of analytical grade and were purchased from Sigma-Aldrich, Merck and Chem-Supply. All samples and standard solutions were prepared in ultrapure water (Milli-Q® Advantage A10, Merck; 18.2 MΩ resistivity). All plastic containers used for the experiments, DGT devices and glass plates were cleaned in 10% (v/v) HCl (AR-grade, Merck) for a minimum of 24 h, and rinsed thoroughly with ultrapure water prior to use.
Pre-treatment of A520E resin
Preliminary work identified high concentrations of NO3-N in the A520E resin, and this needed to be removed to minimise the possibility of it interfering with the DGT analyses. To optimise the washing procedure, three concentrations of NaCl (1, 2 and 3 mol L−1) were tested over three wash times (24, 48 and 72 h) at two temperatures (20 and 60°C). First, the A520E resin beads were finely ground using an electric grinder and 2.8 (± 0.05) g was measured in triplicate into 50 mL centrifuge tubes and 50 mL of NaCl was added. Tubes were then shaken (end-over-end for 20°C samples, manually every 2–4 h for 60°C samples) for the designated time, and centrifuged for 20 min at 8116g, after which the supernatant was removed. The NaCl solution was refreshed after each 24 h period for the two longer wash times. After washing, the resin was rinsed with ultrapure water to remove any excess NaCl by shaking manually for 2 min and the supernatant was removed after centrifugation (8116g for 20 min). This was repeated until the EC of the supernatant was less than 100 µS cm−1. The washed A520E resin in the centrifuge tubes was frozen (−18°C), freeze-dried for 24 h and then homogenised by manually shaking in a clean polyethylene container prior to use in the binding gels (see below).
Gel layer and membrane preparation
Agarose diffusive gels (0.075 cm thick) were prepared as described previously (Davison and Zhang 1994; Huang et al. 2016b). PrCH binding layers were prepared as described by Huang et al. (2016b) to a final thickness of 0.075 cm. The A520E binding layer was prepared using the washed A520E resin (washed with 3 mol L−1 NaCl for 72 h, at 20°C), as described by Huang et al. (2016a) and cast to a thickness of 0.050 cm. Agarose diffusive gels were stored in 0.001 mol L−1 NaCl, while the A520E and PrCH binding layers were stored in ultrapure water at 4°C before the DGT devices were assembled. Polyethersulfone filter membranes were soaked in ultrapure water for 24 h before use (0.45 µm pore size; Sterlitech Corp., USA).
DGT device preparation
Properly washed DGT devices with an exposure area of 2.54 cm2 (DGT Research Ltd., Lancaster, UK) were assembled by layering (1) a binding layer (either A520E or PrCH) at the base, (2) an agarose diffusive layer in the middle and (3) a polyethersulfone filter membrane at the top. The cap of the DGT device was used to seal the assembly and ensure that any solute flux into the resin was through the filter only. For the PrCH-DGT, a 0.025 cm thick spacer ring was used between the base of the device housing and the cap to accommodate the thicker binding layer. Assembled devices were stored in double-sealed plastic bags with 1–2 mL of ultrapure water at 4°C until deployment. Forthwith, the DGT devices used to measure NH4-N and NO3-N will be referred to as PrCH-DGT and A520E-DGT, respectively. DGT blank concentrations were measured (see below) from randomly sampled, undeployed devices of both types. The limit of detection (LoD) for both devices was calculated as three times the standard deviation of these blanks (Luo et al. 2010).
DGT deployment, elution and analysis
Between 50 and 60 g of each soil was weighed into a Petri dish to a soil bulk density of 1.5 g cm−3. The water content was raised to 80% MWHC using ultrapure water and left to equilibrate for 48 h. The DGT devices were then deployed, at 24°C, onto the soil surface, ensuring complete contact with the DGT device window. The deployment time was 20 h (±5 min), unless stated otherwise. At the end of the deployment, the devices were removed and washed thoroughly with ultrapure water, after which the devices were stored in sealed plastic bags with 1–2 mL of ultrapure water at 4°C until disassembly.
The DGT devices were opened using a clean screwdriver and the binding layers were removed with clean plastic forceps. Both types of binding layers were eluted in 2 mL of 2 mol L−1 NaCl for 24 h. The eluents were diluted at least 10 times with ultrapure water and the concentrations of NH4-N and NO3-N were measured using a segmented flow analyser, with a high-resolution digital photometer using a colorimetric method (Seal AA3, Seal Analytical, USA). Instrument detection limits were 2.31 µg L−1 for NH4-N and 1.96 µg L−1 for NO3-N (n = 10). The eluent concentrations were used to determine the total masses of NH4-N and NO3-N bound to the resin gel, assuming that 87.2% of the NH4+ and 82.7% of the NO3− bound to the two types of binding layers were eluted (Huang et al. 2016a, 2016b). The DGT-measured concentrations of NH4-N and NO3-N in the soil porewater were calculated from the accumulated analyte mass using Eqns 1, 2, respectively (Davison and Zhang 1994); forthwith, these will be expressed as CNH4-N and CNO3-N.
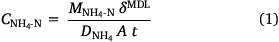
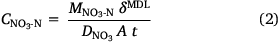
Here, MNH4-N and MNO3-N are the masses of NH4-N or NO3-N (ng) bound to the PrCH and A520E binding layers, respectively, δMDL is the total thickness of the material diffusion layer (i.e. filter and diffusion layer: 0.089 cm), DNH4 and DNO3 are the diffusion coefficients of NH4+ (Huang et al. 2016b) and NO3− (Huang et al. 2016a) through the material diffusion layer (cm2 s−1, corrected for temperature and EC), t is the deployment time (s) and A is the exposure area of the device to the soil (2.54 cm2).
DGT validation for ammonium and nitrate measurement in soils
DGT deployment in unamended soils
The ability of PrCH- and A520E-DGT to measure low concentrations of NH4-N and NO3-N was assessed by deploying them in three contrasting unamended topsoils (Monarto, Mallala and Ngarkat) (Table 1). They were selected based on different soil textures, EC, CEC, inorganic nitrogen and organic matter concentrations. Soils were incubated as described above in DGT deployment, elution and analysis, after which subsamples were collected for DGT deployment and KCl extraction. The DGT devices were deployed in triplicate for 24 h to measure CNH4-N and CNO3-N, as described earlier. A 2 mol L−1 KCl extraction (see below in Mineral nitrogen species measurement using 2 mol L−1 KCl extraction of moist soils) was carried out on a separate moist subsample, 12 h after the start of the DGT deployment.
Accumulation of NH4-N and NO3-N in PrCH- and A520E-DGT
The performance of the PrCH- and A520E-DGT to measure NH4-N and NO3-N across a wide range of soil-N concentrations was tested using the same three soils as for the DGT deployment in unamended soils (see above). Two stock solutions containing either 2000 or 5000 mg L−1 of NO3-N were prepared using calcium nitrate, and a further two stock solutions with the same NH4-N concentrations were prepared using ammonium sulfate. The stock solutions were used to prepare a range of N spike solutions that contained NO3-N and NH4-N in concentrations of 5, 10, 20, 30, 40, 50, 75, 100, 150, 200, 250 and 300 mg kg−1. The pHs of the spike (NO3-N and NH4-N) solutions were adjusted to pH 7 using 0.01 mol L−1 NaOH, after which they were used to increase the N content of 150 g subsamples of the three different soils. The soil moisture levels were then raised to 80% of MWHC and the soils were left to equilibrate for 48 h. PrCH- and A520E-DGT were deployed in triplicate in N treated soils for 20 h in an incubator (at 24 °C) to measure CNH4-N and CNO3-N, as described previously. These deployments were shorter than the typical 24 h to minimise the loss of mineral N from soils through microbial assimilation, denitrification or volatilisation and reduce the possibility of saturating the binding layers. Subsamples of each treatment underwent 2 mol L−1 KCl extraction (see below for details) and pH and EC measurements, in triplicate, 10 h after the start of the DGT deployment.
DGT deployment in a wide range of soils
To further explore the effect of soil characteristics on DGT and 2 mol L−1 KCl-extracted N concentrations, and the differences between them, an experiment was undertaken in ten contrasting soils (Table 1) varying in texture, pH (4.33–9.39), EC (0.01–0.44 mS cm−1), organic matter content (0.07–3.1%) and CEC (1.32–32.4 cmol kg−1). The soils were incubated at 24°C for 2 weeks at 60% of MWHC, after which the N stock solutions (described above) were used to increase NH4-N and NO3-N concentrations to either 50 or 100 mg kg−1 of NH4-N and NO3-N (i.e. 100 and 200 mg kg−1 increase in total N, respectively). The soil moisture level was then raised to 80% of MWHC and samples were incubated for another 24 h. CNH4-N and CNO3-N were measured in triplicate following 20 h DGT deployment at 24 °C, as described previously. Subsamples of each treatment underwent 2 mol L−1 KCl extraction (see next) and pH and EC measurements in triplicate at the beginning and end of the DGT deployments (Supplementary Fig. S3).
Mineral nitrogen species measurement using 2 mol L−1 KCl-extraction of moist soils
Moist soil (80% of MWHC) samples were extracted with 2 mol L−1 KCl using a 1:5 (dry soil/KCl, w/v) ratio. The volume of the extractant was adjusted (<8%) to compensate for the soil moisture content and to ensure the soil/solution ratio was 1:5 on a dry soil basis. The samples were shaken on an end-over-end shaker for 1 h, after which the supernatant was settled for 1 h and filtered (0.45 µm pore size) (Rayment and Lyons 2011). The filtered extracts were diluted (10–100 times) with ultrapure water prior to analysis with the Seal AA3 segmented flow analyser, as described above. The soil concentrations of NH4-N and NO3-N extracted using 2 mol L−1 KCl are reported forthwith as ENH4-N and ENO3-N, respectively.
Statistical analysis
The Kolmogorov–Smirnov test was used to confirm that the data were normally distributed and homogeneity of variance between factors was confirmed using Levene’s test. The different resin purification methods were compared by the T-test based on resin NO3-N per device. Regression analysis was used to identify the linear response of DGT to soil nitrogen concentrations and compare two soil-N measuring methods (DGT and KCl). The coefficient of determination (R2) was used to determine the strength of relationships. Multiple regression analysis was used to examine the effect of various soil factors on NH4-N measured using different tests. For all analyses, the significance level was 0.05. Analyses were performed using Excel (Microsoft Office 365) and SPSS (IBM, version: 26).
Results and discussion
Blanks and LoD of DGTs and KCl
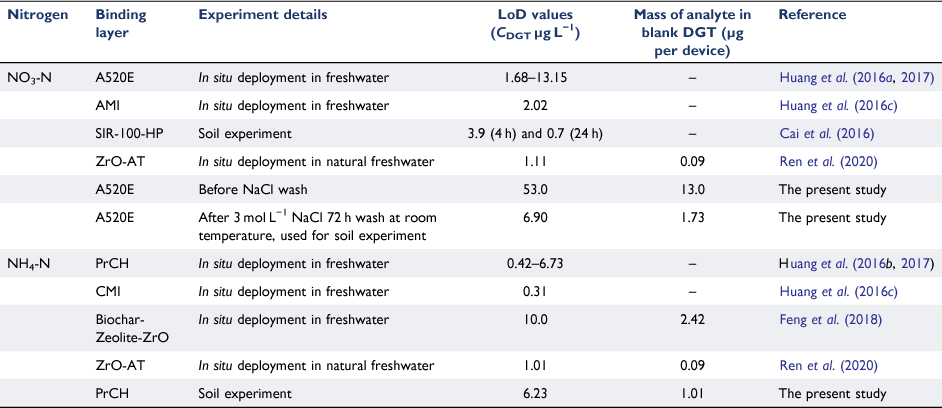
Initially, the mass of NO3-N in the blank DGT device with unwashed A520E resin was 13.0 ± 0.79 µg per device (n = 10), corresponding to a LoD of 53.0 µg L−1. These values are substantially higher than those from previous reports (e.g. blank = 1.68 µg L−1 and LoD of 13.15 µg L−1 Huang et al. 2016a, 2017), and may have been caused by contamination during manufacturing, subsequent packaging, transport or any combination of these. Therefore, the resin was washed before it was used in the DGT. Washing with 3 mol L−1 NaCl for 72 h at 60°C resulted in the lowest average mass of NO3-N per device (1.31 µg, Supplementary Fig. S2). Performing this washing at room temperature resulted in slightly less NO3-N being removed from the resin and thus, a slightly higher mass of NO3-N per device (1.73 µg). However, the difference was not significant (P-value = 0.56), so the resins were washed at room temperature for all subsequent experiments as it is more practical and economical. The LoD for the A520E-DGT (using washed resin) was 6.90 µg L−1 (limit of quantitation (LoQ) of 23 µg L−1) which is within the range of previous studies (1.11–13.15 µg L−1; Table 2) (Huang et al. 2016a, 2017; Ren et al. 2020).
|
The average mass of NH4-N in the blank PrCH resin binding layers was 1.01 ± 0.03 µg, giving the CNH4-N a LoD of 6.23 µg L−1 (LoQ 20.7 µg L−1). These values are within the range of values determined in previous studies (Huang et al. 2016b, 2017; Ren et al. 2020) (Table 2).
For KCl extraction, the LoD (1.97 and 2.22 mg kg−1 for ENH4-N and ENO3-N, respectively) and LoQ (6.57 and 7.42 mg kg−1 for ENH4-N and ENO3-N, respectively) were calculated as three times (LoD) and ten times (LoQ) the standard deviation of three blank 2 mol L−1 KCl extracts.
Measuring low concentrations of N in unamended agricultural soils
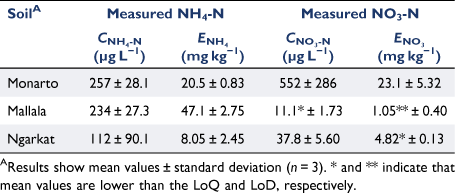
Measurements with PrCH- and A520E-DGT confirmed that these binding layers can be used to measure low concentrations of NH4+ and NO3− in soils. The lowest NH4-N concentration identified using PrCH-DGT and the 2 mol L−1 KCl extraction tests was in the Ngarkat soil (112 µg L−1 and 8.05 mg kg−1 for CNH4-N and ENH4-N, respectively) (Table 3). The mean CNH4-N and ENH4-N concentrations in this soil were 5.4 and 1.2 times higher than their respective LoQs. The two tests disagreed on the soil with the highest available NH4-N concentration: the highest CNH4-N was measured in the Monarto soil (257 µg L−1), while the Mallala soil had the highest ENH4 (47.1 mg kg−1) (Table 3). This indicates that the two tests measure different pools of NH4-N in the soils. This is further discussed below when comparing DGT to conventional KCl extraction. The NO3-N concentrations measured in the three soils by the A520E-DGT and 2 mol L−1 KCl extraction followed the same trend: the highest concentrations were found in the Monarto soil (CNO3-N 552 µg L−1; ENO3-N 23.1 mg kg−1), while the lowest concentrations were measured in the Mallala soil (CNO3-N 11.1 µg L−1; ENO3-N was below the LoD) (Table 3). In the Mallala soil, the mean CNO3-N was 50% of the LoQ.
|
Because DGT accumulates analyte over its full deployment time, the LoD and LoQ can be improved for many analytes by using longer deployments to allow for greater accumulation on the binding layer (Österlund et al. 2016). This ostensibly also applies to NH4+ and NO3−; however, the extent to which long deployment times can be employed to provide accurate concentrations of these analytes in soils at a specific point in time can be limited by the variety of natural processes that can change their concentrations during the deployment. For example, Li et al. (2020) reported a mean global nitrification rate of 3.82 mg kg−1 day−1, with higher rates in warm, moist and basic soils with high microbial biomass and abundant nitrogen. It follows that, when nitrification is the dominant process and occurring at a fast rate, measurements of CNH4-N and CNO3-N are likely to result in progressively lower and higher values, respectively, for the two analytes when increasing deployment times. However, this does provide the opportunity to use different-length DGT deployments to provide unique information on the dynamics of these N species, where possible artefacts caused by extensive soil manipulation are minimised.
Operational NH4-N and NO3-N soil concentration range for PrCH- and A520E-DGT
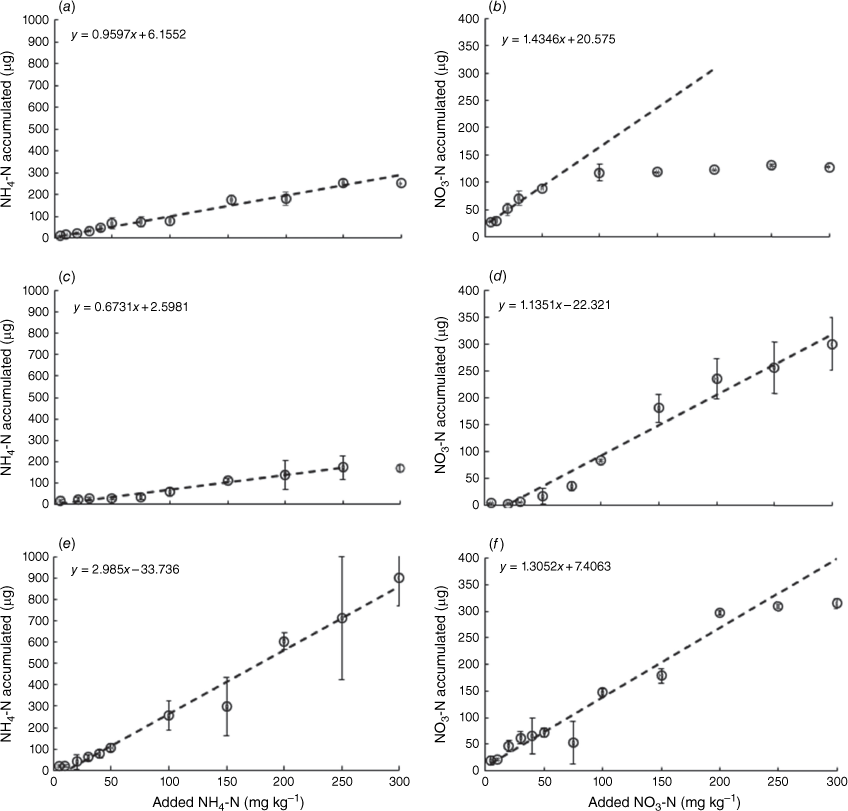
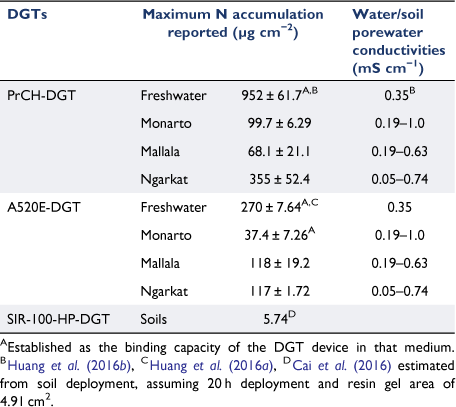
Accumulation of NH4-N on the PrCH-DGT increased linearly in all three soils when the soil concentration increased to 300 mg kg−1 (P-value = 0.001; Fig. 1a, c, e). However, the rate of NH4-N mass accumulation to the device varied between the soils in the order Mallala < Monarto < Ngarkat (0.67, 0.95 and 2.98 µg NH4-N accumulated per mg kg−1 increase in soil NH4-N concentration, respectively: Fig. 1). These differences in accumulation rate are most likely due to differences in the soils’ capacity to supply NH4+ to the DGT (see next section). At the highest NH4-N application rate, the DGT device had accumulated between 170 and 900 µg NH4-N per device in the three soils without evidence to suggest that the mass accumulated in the binding layer of the DGT device was approaching maximum capacity. The maximum amounts of NH4-N bound onto the PrCH-DGT here are over three times lower than what was observed in fresh waters by Huang et al. (2016b) (2990 µg device−1 equivalent to 952 µg cm−2 Table 4). The 300 mg kg−1 NH4-N application rate here corresponds to an area application rate of 450 kg N ha−1 (assuming a topsoil depth of 10 cm and a bulk density of 1.5 g cm−3), which is at the upper end of N application rates to most agricultural soils (Di and Cameron 2002; GRDC 2017). This suggests that the PrCH-DGT can be used to measure soil NH4-N concentrations in most agricultural soils.
|
The accumulation was linear between 0 and 300 mg kg−1 added NO3-N in the Mallala and Ngarkat soils (P-value < 0.05) (Fig. 1d, f), and in both soils up to 300 µg NO3-N was bound in the DGT without evidence that the binding capacity of the DGT device had been exceeded. However, the binding capacity was reached in the Monarto soil once the mass accumulated NO3-N exceeded 95 µg (Fig. 1b). The NO3-N accumulation in the binding layer was linear only until that point (between 0 and 50 mg kg−1 added NO3-N; P-value = 0.001). This value is over three times higher than the highest reported NO3-N accumulation in a soil deployment by Cai et al. (2016) using the SIR-100-HP-DGT (Table 4). Within the linear ranges of the three soils, the accumulation rate of NO3-N on the A520E-DGT was similar in all three soils: 1.43, 1.13 and 1.30 µg NO3-N accumulated per mg kg−1 increase in soil NO3-N concentration for Monarto, Mallala and Ngarkat soils, respectively (Fig. 1).
Accumulation of NO3-N into the A520E-DGT can be limited by competition for the resin binding sites with other anions (Huang et al. 2016a). While the A520E-DGT has a greater affinity for NO3− than other commonly found anions in soils, such as HCO3−, SO42− and Cl− (Gu et al. 2004; Huang et al. 2016a), these ions limit the binding of NO3− when present together in high concentrations. Soil EC can be used as a proxy for the total dissolved ionic solids’ concentration in soil (Friedman 2005). For all NO3-N concentrations added to the Monarto soil, EC values (0.19–1.0 mS cm−1) were higher than those measured in the Ngarkat (EC: 0.19–0.74 mS cm−1) and Mallala (EC: 0.05–0.63 mS cm−1) soils. The EC values in the Monarto soil align with the previously identified upper conductivity value of 1 mS cm−1 where the resin’s capacity for binding NO3− is reduced due to ion competition (Huang et al. 2016a). A conservative upper limit of A520E-DGT for measuring NO3-N in soils is therefore 50 mg NO3-N kg−1 (equivalent to 75 kg ha−1 of NO3-N, in the 10 cm of topsoil with a bulk density of 1.5 g cm−3), which is generally above the range of soil NO3-N concentrations recommended for agricultural production (Department of Employment Economic Development and Innovation 2010) and among the highest application rates of USA and China (Zhang et al. 2015). In most cases, a soil-N test is performed prior to sowing to estimate potential soil-N supply before soil-N application; therefore, the maximum binding capacity is sufficient for soil-N tests. Concentrations exceeding this limit are rare, except under specific circumstances such as animal urine patches (Di et al. 2009; Orwin et al. 2009). In such cases, EC measurements should be used to confirm that ion competition is unlikely to affect the CNO3-N measurement.
Because the capacity of the PrCH-DGTs was not exceeded, and the capacity of A520-DGTs was only exceeded in one instance (NO3-N in Monarto soil), it is unsurprising that the highest amounts of solute bound to the resin layers are lower than the capacities reported in solution measurements by Huang et al. (2016a, 2016b) (Table 4). However, the results reported here can inform the upper operational limit for using these binding layers in DGT soil-N measurements. Any results where the mass of N accumulated exceeds these values should be interpreted with caution.
Comparison of DGT to conventional KCl-extraction for measuring NH4-N and NO3-N in contrasting soil types
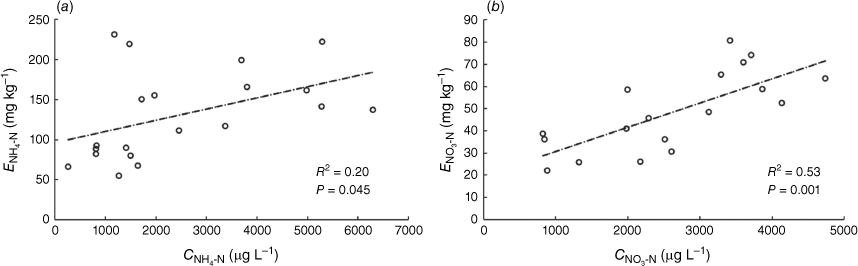
Positive correlations were found between CNH4-N and ENH4-N measurements (R2 = 0.20; P-value = 0.045) and between CNO3-N and ENO3-N measurements (R2 = 0.52; P-value <0.001) in the ten soils amended with two rates of N (50 and 100 mg kg−1 of NH4-N and NO3-N) (Fig. 2). The stronger correlation between CNO3-N and ENO3-N is likely to be due to NO3− anions generally adsorbing weakly to soil solid phases: mainly as diffuse-ion swarm species or as outer-sphere surface complexes (Sposito 2008). Consequently, differences in the mechanisms by which the ion is sampled by the two techniques may be expected to have a less pronounced effect on the amount measured.
Analysis of ENH4-N and CNH4-N among the different soils revealed that soil CEC can play a significant role in the relative amounts of NH4+ sampled by the two techniques. In the soils with low CEC (1.32–4.81 cmol kg−1), the correlation between CNH4-N and ENH4-N measurements increased (R2 = 0.53, P-value < 0.05, 10 soil samples: five soils at two concentrations, shown in Supplementary Fig. S4), while the relationship was not significant in the soils where CEC was greater than 11.9 cmol kg−1 (R2 = 0.12, P-value > 0.05; 10 soil samples: five soils at two concentrations, shown in Supplementary Fig. S4). A comparison of the amount of NH4-N measured by the two techniques in soils with different CECs found that the soils’ ability to supply NH4+ to the PrCH-DGT decreases with higher CEC when compared to the amount sampled by the 2 mol L−1 KCl extract (Fig. 3). Soil CEC also co-varied negatively with the rate of NH4-N mass accumulation by DGT when different amounts of NH4-N were applied to three soils (see the previous section for details). In that experiment, the CEC of the three soils Mallala (36 cmol kg−1), Monarto (23 cmol kg−1) and Ngarkat (2.9 cmol kg−1) was inversely related to the rate at which NH4-N supply to the DGT increased with NH4-N application to the soil (Table 2, Fig. 1 a, c, e).
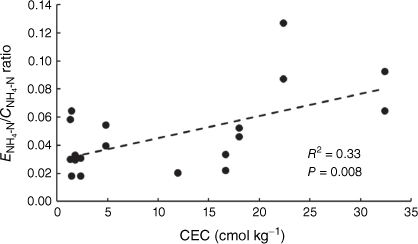
|
The role of CEC may be rationalised by the dynamics of soil chemical processes. The supply of solute to DGT is thought to be determined partly by the resupply from sorbed solute when porewater concentrations are depleted (Zhang et al. 2014a; Lehto 2016; Marrugo-Madrid et al. 2021), but the extent of this resupply can be limited by the rate of desorption during the measurement time (Harper et al. 1998; Lehto et al. 2008). On the other hand, the 2 mol L−1 KCl extraction assumes an equilibrium between solid and solution phases and relies on ion-exchange between K+ and the similar size NH4+ ions sorbed to soil colloids (Mulvaney 1996). The difference in the amount of NH4+ measured by the two techniques may be partly due to the ion exchange reaction in the extraction overcoming the kinetic limitation to solid phase NH4-N supply to the DGT. A further factor contributing to the difference may be the role of soil pH that had an exponential relationship with CEC (CEC = 0.086 × e0.6413pH, P-value < 0.05, Supplementary Fig. S5). Four of the ten soils tested had pH > 7 and may have lost some NH4+ through volatilisation as ammonia gas during measurements. Losses through volatilisation may have been exacerbated by the warm temperature, high soil moisture content, the use of ammonium sulfate to add NH4-N and that pH has a stronger effect than CEC in affecting volatilisation (Powlson and Dawson 2021). In addition, there would have been more time for NH4+ to be lost during the 20 h DGT deployment than the shorter KCl extraction (usually < 2 h). This suggests that DGT measurements of NH4-N should be ideally carried out quickly, under cooler conditions and in acidic soils to minimise losses of the analyte during the deployment.
Conclusions
This is the first study in which PrCH- and A520E-DGT were evaluated to measure NH4-N and NO3-N in a variety of soils with a wide range of soil-N concentrations and soil properties. The PrCH- and A520E-DGT were able to detect and quantify low concentrations of the two mineral N species in low fertility soils. The two types of DGT, PrCH- and A520E-DGT, can also be used to measure soil NH4-N and NO3-N concentrations at most agriculturally relevant soil concentrations; however, for NO3-N measurements, complementary measurements of EC are recommended to help ensure that resin binding saturation does not affect the results. When compared against the more commonly used 2 mol L−1 KCl extraction, DGT appears to sample a different pool of NH4-N in soils and the strengths of the technique may lie in investigations into the sorption dynamics of the solute. However, such measurements should seek to minimise losses of the solute during the measurement through volatilisation to ensure accurate results.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in [repository name e.g. FigShare] at [doi].
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors thank UniSA STEM for providing a PhD scholarship to conduct the research on this topic. They also honour Professor Peter Teasdale’s contribution to conceptualising the research.
References
Adjesiwor AT, Islam MA (2016). Rising nitrogen fertilizer prices and projected increase in maize ethanol production: the future of forage production and the potential of legumes in forage production systems. Grassland Science 62, 203–212.| Rising nitrogen fertilizer prices and projected increase in maize ethanol production: the future of forage production and the potential of legumes in forage production systems.Crossref | GoogleScholarGoogle Scholar |
Belete F, Dechassa N, Molla A, Tana T (2018). Effect of split application of different N rates on productivity and nitrogen use efficiency of bread wheat (Triticum aestivum L.). Agriculture & Food Security 7, 92
| Effect of split application of different N rates on productivity and nitrogen use efficiency of bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Cai C, Williams PN, Li H, Davison W, Wei T-J, Luo J, Zhu Y-G, Zhang H (2016). Development and application of the diffusive gradients in thin films technique for the measurement of nitrate in soils. Analytical Chemistry 89, 1178–1184.
| Development and application of the diffusive gradients in thin films technique for the measurement of nitrate in soils.Crossref | GoogleScholarGoogle Scholar |
Canali S, Di Bartolomeo E, Tittarelli F, Montemurro F, Verrastro V, Ferri D (2011). Comparison of different laboratory incubation procedures to evaluate nitrogen mineralization in soils amended with aerobic and anaerobic stabilized organic materials. Journal of Food, Agriculture & Environment 9, 540–546.
Canfield DE, Glazer AN, Falkowski PG (2010). The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196.
| The evolution and future of Earth’s nitrogen cycle.Crossref | GoogleScholarGoogle Scholar |
Carrillo-Gonzalez R, Gonzalez-Chavez MCA, Aitkenhead-Peterson JA, Hons FM, Loeppert RH (2013). Extractable DOC and DON from a dry-land long-term rotation and cropping system in Texas, USA. Geoderma 197–198, 79–86.
| Extractable DOC and DON from a dry-land long-term rotation and cropping system in Texas, USA.Crossref | GoogleScholarGoogle Scholar |
Davison W, Zhang H (1994). In situ speciation measurements of trace components in natural waters using thin-film gels. nature 367, 546–548.
| In situ speciation measurements of trace components in natural waters using thin-film gels.Crossref | GoogleScholarGoogle Scholar |
De Laporte A, Banger K, Weersink A, Wagner-Riddle C, Grant B, Smith W (2021). Economic and environmental consequences of nitrogen application rates, timing and methods on corn in Ontario. Agricultural Systems 188, 103018
| Economic and environmental consequences of nitrogen application rates, timing and methods on corn in Ontario.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC (2002). Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutrient Cycling in Agroecosystems 64, 237–256.
| Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC, Shen J-P, Winefield CS, O’Callaghan M, Bowatte S, He J-Z (2009). Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience 2, 621–624.
| Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils.Crossref | GoogleScholarGoogle Scholar |
Du S, Pan Q, Xu Y, Cao S (2020). Comparison of three measurement models of soil nitrate-nitrogen based on ion-selective electrodes. International Journal of Agricultural and Biological Engineering 13, 211–216.
| Comparison of three measurement models of soil nitrate-nitrogen based on ion-selective electrodes.Crossref | GoogleScholarGoogle Scholar |
FAO (2017) ‘World fertilizer trends and outlook to 2020.’ (FAO)
Feng Z, Wang N, He M, Yang L, Wang Y, Sun T (2018). Simultaneous sampling of dissolved orthophosphate and ammonium in freshwaters using diffusive gradients in thin films with a mixed binding phase. Talanta 186, 176–182.
| Simultaneous sampling of dissolved orthophosphate and ammonium in freshwaters using diffusive gradients in thin films with a mixed binding phase.Crossref | GoogleScholarGoogle Scholar |
Friedman SP (2005). Soil properties influencing apparent electrical conductivity: a review. Computers and Electronics in Agriculture 46, 45–70.
| Soil properties influencing apparent electrical conductivity: a review.Crossref | GoogleScholarGoogle Scholar |
Giles JF, Reuss JO, Ludwick AE (1975). Prediction of Nitrogen Status of Sugarbeets by Soil Analysis 1. Agronomy Journal 67, 454–459.
| Prediction of Nitrogen Status of Sugarbeets by Soil Analysis 1.Crossref | GoogleScholarGoogle Scholar |
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010). Food security: the challenge of feeding 9 billion people. Science 327, 812–818.
| Food security: the challenge of feeding 9 billion people.Crossref | GoogleScholarGoogle Scholar |
Goulding K, Jarvis S, Whitmore A (2008). Optimizing nutrient management for farm systems. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 667–680.
| Optimizing nutrient management for farm systems.Crossref | GoogleScholarGoogle Scholar |
GRDC (2017) ‘Growthnotes.’ (Grain Research & Development Corporation) Available at https://grdc.com.au/resources-and-publications/grownotes/crop-agronomy/wheatgrownoteswestern
Gruber N, Galloway JN (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296.
| An Earth-system perspective of the global nitrogen cycle.Crossref | GoogleScholarGoogle Scholar |
Gu B, Ku Y-K, Jardine PM (2004). Sorption and binary exchange of nitrate, sulfate, and uranium on an anion-exchange resin. Environmental Science & Technology 38, 3184–3188.
| Sorption and binary exchange of nitrate, sulfate, and uranium on an anion-exchange resin.Crossref | GoogleScholarGoogle Scholar |
Guan D-X, He S-X, Li G, Teng HH, Ma LQ (2022). Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils. Critical Reviews in Environmental Science and Technology 52, 3035–3079.
| Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils.Crossref | GoogleScholarGoogle Scholar |
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010). Significant acidification in major Chinese croplands. Science 327, 1008–1010.
| Significant acidification in major Chinese croplands.Crossref | GoogleScholarGoogle Scholar |
Harper MP, Davison W, Zhang H, Tych W (1998). Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochimica et Cosmochimica Acta 62, 2757–2770.
| Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes.Crossref | GoogleScholarGoogle Scholar |
Homyak PM, Vasquez KT, Sickman JO, Parker DR, Schimel JP (2015). Improving nitrite analysis in soils: Drawbacks of the conventional 2 M KCl extraction. Soil Science Society of America Journal 79, 1237–1242.
| Improving nitrite analysis in soils: Drawbacks of the conventional 2 M KCl extraction.Crossref | GoogleScholarGoogle Scholar |
Huang J, Bennett WW, Teasdale PR, Gardiner S, Welsh DT (2016a). Development and evaluation of the diffusive gradients in thin films technique for measuring nitrate in freshwaters. Analytica Chimica Acta 923, 74–81.
| Development and evaluation of the diffusive gradients in thin films technique for measuring nitrate in freshwaters.Crossref | GoogleScholarGoogle Scholar |
Huang J, Bennett WW, Welsh DT, Li T, Teasdale PR (2016b). Development and evaluation of a diffusive gradients in a thin film technique for measuring ammonium in freshwaters. Analytica chimica acta 904, 83–91.
| Development and evaluation of a diffusive gradients in a thin film technique for measuring ammonium in freshwaters.Crossref | GoogleScholarGoogle Scholar |
Huang J, Bennett WW, Welsh DT, Teasdale PR (2016c). Determining time-weighted average concentrations of nitrate and ammonium in freshwaters using DGT with ion exchange membrane-based binding layers. Environmental Science: Processes & Impacts 18, 1530–1539.
| Determining time-weighted average concentrations of nitrate and ammonium in freshwaters using DGT with ion exchange membrane-based binding layers.Crossref | GoogleScholarGoogle Scholar |
Huang J, Bennett WW, Teasdale PR, Kankanamge NR, Welsh DT (2017). A modified DGT technique for the simultaneous measurement of dissolved inorganic nitrogen and phosphorus in freshwaters. Analytica Chimica Acta 988, 17–26.
| A modified DGT technique for the simultaneous measurement of dissolved inorganic nitrogen and phosphorus in freshwaters.Crossref | GoogleScholarGoogle Scholar |
Inselsbacher E (2014). Recovery of individual soil nitrogen forms after sieving and extraction. Soil Biology and Biochemistry 71, 76–86.
| Recovery of individual soil nitrogen forms after sieving and extraction.Crossref | GoogleScholarGoogle Scholar |
Lehto NJ (2016) Principles and Application in Soils and Sediments. In ‘Diffusive Gradients in Thin-Films for Environmental Measurements’. (Ed. W Davison) (Cambridge University Press: Cambridge, UK)
Lehto NJ, Sochaczewski Ł, Davison W, Tych W, Zhang H (2008). Quantitative assessment of soil parameter (KD and TC) estimation using DGT measurements and the 2D DIFS model. Chemosphere 71, 795–801.
| Quantitative assessment of soil parameter (KD and TC) estimation using DGT measurements and the 2D DIFS model.Crossref | GoogleScholarGoogle Scholar |
Li K-y, Zhao Y-y, Yuan X-l, Zhao H-b, Wang Z-h, Li S-x, Malhi SS (2012). Comparison of factors affecting soil nitrate nitrogen and ammonium nitrogen extraction. Communications in Soil Science and Plant Analysis 43, 571–588.
| Comparison of factors affecting soil nitrate nitrogen and ammonium nitrogen extraction.Crossref | GoogleScholarGoogle Scholar |
Li Z, Zeng Z, Tian D, Wang J, Fu Z, Zhang F, Zhang R, Chen W, Luo Y, Niu S (2020). Global patterns and controlling factors of soil nitrification rate. Global Change Biology 26, 4147–4157.
| Global patterns and controlling factors of soil nitrification rate.Crossref | GoogleScholarGoogle Scholar |
Luo J, Zhang H, Santner J, Davison W (2010). Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic (V), selenium (VI), vanadium (V), and antimony (V). Analytical Chemistry 82, 8903–8909.
| Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic (V), selenium (VI), vanadium (V), and antimony (V).Crossref | GoogleScholarGoogle Scholar |
Margesin R, Schinner F (2005) ‘Manual for soil analysis-monitoring and assessing soil bioremediation.’ (Springer Science & Business Media)
Marrugo-Madrid S, Turull M, Zhang H, Díez S (2021). Diffusive gradients in thin films for the measurement of labile metal species in water and soils: a review. Environmental Chemistry Letters 19, 3761–3788.
| Diffusive gradients in thin films for the measurement of labile metal species in water and soils: a review.Crossref | GoogleScholarGoogle Scholar |
Marschner P, Rengel Z (2012) Nutrient availability in soils. In ‘Marschner’s mineral nutrition of higher plants’. (Ed. P Marschner) pp. 315–330. (Elsevier)
Mason S, McNeill A, McLaughlin MJ, Zhang H (2010). Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant and Soil 337, 243–258.
| Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods.Crossref | GoogleScholarGoogle Scholar |
Maynard DG, Kalra YP, Crumbaugh JA (2007) Nitrate and Exchangeable Ammonium Nitrogen. In ‘Soil sampling and methods of analysis’. (Ed. MR Carter, EG Gregorich) pp. 71–80. (CRC Press: Boca Raton)
McTaggart IP, Smith KA (1993). Estimation of potentially mineralisable nitrogen in soil by KCl extraction. Plant and Soil 157, 175–184.
| Estimation of potentially mineralisable nitrogen in soil by KCl extraction.Crossref | GoogleScholarGoogle Scholar |
Mengel K (1982) Factors of plant nutrient availability relevant to soil testing. In ‘Application of Electro-Ultrafiltration (EUF) in Agricultural Production’. (Ed. K Németh) pp. 129–138. (Springer)
| Crossref |
Montemurro F, Diacono M (2016). Towards a better understanding of agronomic efficiency of nitrogen: Assessment and improvement strategies. Agronomy 6, 31
| Towards a better understanding of agronomic efficiency of nitrogen: Assessment and improvement strategies.Crossref | GoogleScholarGoogle Scholar |
Moss B (2008). Water pollution by agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 659–666.
| Water pollution by agriculture.Crossref | GoogleScholarGoogle Scholar |
Mulvaney RL (1996). Nitrogen—inorganic forms. Methods of Soil Analysis: Part 3 Chemical Methods 5, 1123–1184.
| Nitrogen—inorganic forms.Crossref | GoogleScholarGoogle Scholar |
Mundus S, Lombi E, Holm PE, Zhang H, Husted S (2012). Assessing the plant availability of manganese in soils using Diffusive Gradients in Thin films (DGT). Geoderma 183–184, 92–99.
| Assessing the plant availability of manganese in soils using Diffusive Gradients in Thin films (DGT).Crossref | GoogleScholarGoogle Scholar |
Orwin KH, Bertram JE, Clough TJ, Condron LM, Sherlock RR, O’Callaghan M (2009). Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes. Applied Soil Ecology 42, 271–278.
| Short-term consequences of spatial heterogeneity in soil nitrogen concentrations caused by urine patches of different sizes.Crossref | GoogleScholarGoogle Scholar |
Österlund H, Widerlund A, Ingri J, Davison W, Zhang H (2016) ‘Applications in Natural Waters ‘Diffusive Gradients in Thin-Films for Environmental Measurements’. (Ed. W Davison) pp.123–145. (Cambridge University Press: Cambridge, UK)
Powlson DS, Dawson CJ (2021). Use of ammonium sulphate as a sulphur fertilizer: Implications for ammonia volatilization. Soil Use and Management 38, 622–634.
| Use of ammonium sulphate as a sulphur fertilizer: Implications for ammonia volatilization.Crossref | GoogleScholarGoogle Scholar |
Qian P, Schoenau JJ (1994). Assessing nitrogen mineralization from soil organic matter using anion exchange membranes. Fertilizer Research 40, 143–148.
| Assessing nitrogen mineralization from soil organic matter using anion exchange membranes.Crossref | GoogleScholarGoogle Scholar |
Department of Employment, Economic Development and Innovation (2010) ‘Soil health for vegetable production in Australia.’ (Department of Employment, Economic Development and Innovation)
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods: Australasia.’ (CSIRO publishing)
Ren M, Ding S, Shi D, Zhong Z, Cao J, Yang L, Tsang DCW, Wang D, Zhao D, Wang Y (2020). A new DGT technique comprised in a hybrid sensor for the simultaneous measurement of ammonium, nitrate, phosphorus and dissolved oxygen. Science of The Total Environment 725, 138447
| A new DGT technique comprised in a hybrid sensor for the simultaneous measurement of ammonium, nitrate, phosphorus and dissolved oxygen.Crossref | GoogleScholarGoogle Scholar |
Saha UK, Sonon L, Biswas BK (2018). A comparison of diffusion-conductimetric and distillation-titration methods in analyzing ammonium-and nitrate-nitrogen in the KCl-extracts of Georgia soils. Communications in Soil Science and Plant Analysis 49, 63–75.
| A comparison of diffusion-conductimetric and distillation-titration methods in analyzing ammonium-and nitrate-nitrogen in the KCl-extracts of Georgia soils.Crossref | GoogleScholarGoogle Scholar |
Schroeder BL, Farina MPW, Fey MV (1985). A comparison of several nitrogen- availability indices on four natal maize-producing soils. South African Journal of Plant and Soil 2, 164–166.
| A comparison of several nitrogen- availability indices on four natal maize-producing soils.Crossref | GoogleScholarGoogle Scholar |
Sharma LK, Bali SK (2018). A review of Methods to Improve Nitrogen Use Efficiency in Agriculture. Sustainability 10, 51
| A review of Methods to Improve Nitrogen Use Efficiency in Agriculture.Crossref | GoogleScholarGoogle Scholar |
Six L, Pypers P, Degryse F, Smolders E, Merckx R (2012). The performance of DGT versus conventional soil phosphorus tests in tropical soils-an isotope dilution study. Plant and Soil 359, 267–279.
| The performance of DGT versus conventional soil phosphorus tests in tropical soils-an isotope dilution study.Crossref | GoogleScholarGoogle Scholar |
Sochaczewski Ł, Tych W, Davison B, Zhang H (2007). 2D DGT induced fluxes in sediments and soils (2D DIFS). Environmental Modelling & Software 22, 14–23.
| 2D DGT induced fluxes in sediments and soils (2D DIFS).Crossref | GoogleScholarGoogle Scholar |
Sposito G (2008) ‘The chemistry of soils’, 2nd edn. (Oxford university press)
Stark JM, Hart SC (1997). High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 385, 61–64.
| High rates of nitrification and nitrate turnover in undisturbed coniferous forests.Crossref | GoogleScholarGoogle Scholar |
Sun Q, Chen J, Ding S, Yao Y, Chen Y (2014). Comparison of diffusive gradients in thin film technique with traditional methods for evaluation of zinc bioavailability in soils. Environmental Monitoring and Assessment 186, 6553–6564.
| Comparison of diffusive gradients in thin film technique with traditional methods for evaluation of zinc bioavailability in soils.Crossref | GoogleScholarGoogle Scholar |
Tandy S, Mundus S, Yngvesson J, de Bang TC, Lombi E, Schjoerring JK, Husted S (2011). The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant and Soil 346, 167–180.
| The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Tremblay N, Bouroubi YM, Bélec C, Mullen RW, Kitchen NR, Thomason WE, Ebelhar S, Mengel DB, Raun WR, Francis DD, Vories ED, Ortiz‐Monasterio I (2012). Corn response to nitrogen is influenced by soil texture and weather. Agronomy Journal 104, 1658–1671.
| Corn response to nitrogen is influenced by soil texture and weather.Crossref | GoogleScholarGoogle Scholar |
Vogel C, Sekine R, Huang J, Steckenmesser D, Steffens D, Huthwelker T, Borca CN, Pradas del Real AE, Castillo-Michel H, Adam C (2020). Effects of a nitrification inhibitor on nitrogen species in the soil and the yield and phosphorus uptake of maize. Science of the Total Environment 715, 136895
| Effects of a nitrification inhibitor on nitrogen species in the soil and the yield and phosphorus uptake of maize.Crossref | GoogleScholarGoogle Scholar |
Yuan S, Peng S (2017). Exploring the trends in nitrogen input and nitrogen use efficiency for agricultural sustainability. Sustainability 9, 1905
| Exploring the trends in nitrogen input and nitrogen use efficiency for agricultural sustainability.Crossref | GoogleScholarGoogle Scholar |
Zhang H, Davison W, Miller S, Tych W (1995). In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochimica et Cosmochimica Acta 59, 4181–4192.
| In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Mason S, McNeill A, McLaughlin MJ (2013). Optimization of the diffusive gradients in thin films (DGT) method for simultaneous assay of potassium and plant-available phosphorus in soils. Talanta 113, 123–129.
| Optimization of the diffusive gradients in thin films (DGT) method for simultaneous assay of potassium and plant-available phosphorus in soils.Crossref | GoogleScholarGoogle Scholar |
Zhang C, Ding S, Xu D, Tang Y, Wong MH (2014a). Bioavailability assessment of phosphorus and metals in soils and sediments: a review of diffusive gradients in thin films (DGT). Environmental Monitoring and Assessment 186, 7367–7378.
| Bioavailability assessment of phosphorus and metals in soils and sediments: a review of diffusive gradients in thin films (DGT).Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Mason S, McNeill A, McLaughlin MJ (2014b). Application of the diffusive gradients in thin films technique for available potassium measurement in agricultural soils: Effects of competing cations on potassium uptake by the resin gel. Analytica Chimica Acta 842, 27–34.
| Application of the diffusive gradients in thin films technique for available potassium measurement in agricultural soils: Effects of competing cations on potassium uptake by the resin gel.Crossref | GoogleScholarGoogle Scholar |
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015). Managing nitrogen for sustainable development. Nature 528, 51–59.
| Managing nitrogen for sustainable development.Crossref | GoogleScholarGoogle Scholar |