Morphological and molecular identification of indigenous arbuscular mycorrhizal fungi in the rhizosphere of chickpea (Cicer arietinum) and their role in nutrient uptake
Kamran Akbar A , Tabassum Yaseen A # , Banzeer Ahsan Abbasi B , Javed Iqbal A # , Badr Alharthi C , Sajid Fiaz D * , Salma Noureen A , Shumaila Ijaz E , Ejaz Aziz F and Rashid Iqbal G H
D * , Salma Noureen A , Shumaila Ijaz E , Ejaz Aziz F and Rashid Iqbal G H
A
B
C
D
E
F
G
H
# These authors contributed equally to this paper
Handling Editor: Muhammad Zaheer
Abstract
This study aimed to evaluate the effect of arbuscular mycorrhizal fungi (AMF) on the growth, nutrient uptake, and productivity of chickpea (Cicer arietinum). We investigated the diversity of indigenous AMF in their natural habitat and their effect on the plant and elemental characteristics of chickpea by analysing soil physicochemical properties, root colonisation, AMF spore diversity, and elemental composition of chickpea rhizosphere in two locations (Bhakkar and Khushab, Pakistan). Nitrogen levels of 5.47 g/kg and 4.51 g/kg were found in the rhizosphere soils of Bhakkar and Khushab, respectively. Root colonisation was higher (48.5%) in Khushab (Bhakkar, 35.5%), influencing phosphorus absorption in both regions. Molecular analysis identified 21 AMF taxa, with Glomus and Acaulospora being the most dominant genera. Variations in spore sizes were found, with Glomus measuring 10–191 μm, Acaulospora 125–152 μm, Sclerocystis 110–174 μm, and Gigaspora 65–184 μm. Plant analysis revealed that plant materials from Bhakkar had 1.72% ash, 1.16% fat, 3.78% fibre, and 13.05% protein; samples from Khushab had 1.90% ash, 1.25% fat, 3.24% fibre, and 11.5% protein. Elemental concentrations of chickpea plants from Bhakkar were N = 2.68%, P = 32.98 mg/kg, and K = 33.32 mg/kg, whereas those from Khushab were N = 1.94%, P = 1.17 mg/kg, and K = 43.06 mg/kg. Molecular analysis revealed AMF species with a range of 250–1100 bp. Root colonisation was inversely related to soil phosphorus levels but had a positive effect on plant moisture, fats, and carbohydrates. Morphological and molecular identification showed a relatively high AMF taxa in the rhizosphere of chickpea in both regions. Despite their benefits, the potential of AMF as biofertilisers has not been fully utilised due to prevailing agronomic practices.
Keywords: biofertilizer potential, elemental analysis, microbial activity, mycorrhization, root colonization, soil physicochemical properties, spore morphology.
Introduction
Cultivating leguminous species enhances nutrient management by improving phosphorus (P) and potassium (K) availability, reducing the need for synthetic fertilisers (Solangi et al. 2024). It is generally believed that arbuscular mycorrhizal fungi (AMF) form associations in the roots of nearly 75.5% of all plant species, including some important crops (Smith and Read 2010; Brundrett and Tedersoo 2018). In drought-prone soils, AMF can positively affect water uptake and crop yield through rhizosphere interactions (Rehman et al. 2024). When soil is deficient in nutrients, in particular P, AMF can help plants for the uptake of both macro- and micronutrients (Nahar et al. 2021). Chickpea (Cicer arietinum) is the second most consumed legume crop. It is grown in more than 50 countries worldwide (Koul et al. 2022). It is primarily found in semi-arid and temperate regions and is recognised as an affordable, high-protein food source, rich in fibre and carbohydrates (Solangi et al. 2024). Research indicates that utilising AMF is an effective method to promote the growth and enhance the productivity of legumes, specifically C. arietinum. (Hashem et al. 2019). Studies have shown that AMF inoculation can reduce the need for phosphate fertilisers by up to 80% (Solangi et al. 2023). The Government of Punjab (GOP) has reported that Punjab alone contributes 80% of Pakistan’s chickpea production, with 78% of the cultivation occurring in Mianwali, Khushab, Layyah, and Bhakkar districts (Pakistan Ministry of Agriculture Works. Planning Unit 1970; Hassaan et al. 2024).
In Pakistan, the soils in the western and southern parts of the Punjab Province have a pH of 7.0–8.2, making them slightly alkaline. Due to higher humus content and greater microbial activity, the rhizosphere of these soils is more fertile than the non-rhizosphere portions (Makkar et al. 2018). In agricultural environments characterised by a persistent P deficiency, AMF colonisation can significantly enhance P efficiency (Nahar et al. 2021). AMF is widely recognised as an ecosystem engineer that helps plants adapt to adverse conditions (Duan et al. 2021; Abdelaal et al. 2024). Mycorrhizal fungi enhance soil nutrients such as nitrogen (N), phosphorus (P), and organic carbon (OC) (Nahar et al. 2021). For chickpea, the frequency and colonisation rates of mycorrhization were reported to be high (El Hazzat et al. 2018). Glomus and Acaulospora being the two dominant genera (Alimi et al. 2021; Khan 2023). Indeed, Most plants were found to be colonised by species of Glomus including Glomus fasciculatum, Glomus macrocarpum, Glomus etunicatum, Glomus mosseae, and Glomus constrictum; and the species of Acaulospora species such as Acaulospora mellea and Acaulospora reducta. Studies have indicated that soils with high potassium and phosphate levels generally have fewer AMF spore populations, whereas low levels promote higher spore density (Šmilauer et al. 2020).
AMF diversity and strain types have been documented in various environments, including grasslands (Melo et al. 2019; Goldmann et al. 2020), agricultural ecosystems (Wang et al. 2020; Alrajhi et al. 2024), and forests (Rożek et al. 2020; Dos Passos et al. 2021). AMF is ecologically significant as indicators of biodiversity in semi-arid habitats, according to the study. AMF’s great functional diversity and variance in the Medicago truncatula rhizosphere were shown to be tightly related to grazing, soil texture, and nutrient levels (Mahmoudi et al. 2019). The vast mycelium network of AMF enhances phosphorus absorption, supporting community dynamics and plant growth. Furthermore, AMF promotes interspecies relationships, which supports the stability of semi-arid ecosystems (Meng 2023). Molecular biological techniques are increasingly used to investigate AMF diversity across ecosystems, enhancing classification systems and allowing precise measurement of AMF diversity and community distribution. The genus Glomus is particularly prevalent, with a 100% occurrence frequency and relative abundances of 42.268% and 33.048% at the species and operational taxonomic unit (OTU) levels, respectively (Zhang et al. 2021). This study aimed to assess the morphological and molecular diversity of indigenous AMF associated with chickpea; to our knowledge, it is the first investigation of its kind in Punjab, Pakistan. Additionally, we investigated how AMF colonisation influenced the plant and elemental characteristics of C. arietinum in the natural habitats of Punjab’s districts.
Materials and methods
Study sites and sample collection
Several studies were conducted to collect Cicer arietinum L. at the flowering stage, along with rhizospheric soils, from the districts of Bhakkar (31°37′30.90″N, 71°03′56.66″E) and Khushab (32°17′48.01″N, 72°21′9.00″E) in Punjab, Pakistan. These districts are located in lowland semi-arid plains at an elevation of 168 m above sea level, with an average temperature of 35°C (Fig. 1). C. arietinum and rhizospheric soils were collected in sterilised bags from different sites during the spring season (2019–2020). A total of 44 soil and root samples (20 from Bhakkar and 24 from Khushab) were gathered from 20 selected sites in these districts, at a depth of 10–15 cm. The samples were stored for a week at 4°C, while the root samples were kept at −20°C for molecular analysis.
Physiochemical soil analysis
We analysed physiochemical features with the isolation of different AMF spores using the method described by (Trejo-Aguilar and Banuelos 2020).
Soil organic matter: we used the procedure of Schulte and Hoskins (1995):
Soil pH: this was calculated as the negative log of hydrogen ion activity, determined by a pH meter (McLean 1983).
Soil moisture: we used filter paper to clean all of the Petri dishes for the soil moisture analysis. Weight of empty Petri dishes (without lid) (W1) as well as 10 g soil sample (W2) in Petri dishes were noted and measured initial weight before oven (W3 = W1 + W2). This was placed in an oven at 106°C for 4 h and measured the weight (W4 = after drying) (Sparks et al. 2020):
Soil texture: we used hydrometer values used to compute the percentage of sand, silt, and clay, which was used to determine the soil’s textural class according to the USDA soil textural triangle (Gee and Bauder 1986).
Electrical conductivity (EC): we measured the quantity of soluble inorganic salts in each amount of soil is referred to as salinity. An EC bridge is used to test salinity and to determine soil EC (Blake 1965).
Total soil nitrogen: nitrogen determination was made in three steps of digestion, distillation, and titration. In digestion, a mixture of 1 g CuSO4 and 7 g K2SO4 in the ratio of 1:7 was used with a 0.2-g test sample. In distillation, 60 mL of 40% NaOH was dispensed into digested sample along with the 5-mL indicator of boric acid and 200 mL of 0.2% bromocresol for 10 min. Finally, the distillate was analysed for ammonium by titrating against 0.005 N HCl (Bremner and Mulvaney 1982):
AB-DTPA extractable P: we added 10 g of soil and 20 mL of the AB-DTPA solution, filtered the suspension and added 5 mL of the mixed ascorbic reagent. The phosphorus content was then measured at 880 nm using a spectrophotometer (Madurapperuma and Kumaragamage 1999).
AB-DTPA extractable K, Na, Ca, Mg; and micronutrients (Zn, Fe, Mn): 10 g of soil sample was suspended in 20 mL of the AB-DTPA solution. Flame photometer was used to calculate Na and K concentration while atomic absorption spectrophotometer was used to assess the extract’s micronutrient contents with Ca and Mg (McLean 1983):
Root assessment, spores population, isolation, and identification
Chickpea samples were assessed for root colonisation using the procedure described by Phillips and Hayman (1970). The roots were washed to remove soil particles, cut into 1 cm segments, and preserved in 70% formalin acetic acid (FAA). For measuring colonisation, the roots were cleared in 15% (w/v) KOH and placed in a water bath at 90°C for 25–30 min. After cooling, the roots were rinsed with water and stained with 0.5% (w/v) acid fuchsin. Finally, the roots from the collected samples were examined under a compound microscope to observe AMF colonisation. Percentage of root colonisation was calculated by the equation:
Wet sieving and decanting were used to separate coarse soil particles, while organic particles and AMF spores were retained on sieves with various diameters (40 µm, 100 µm, and 150 µm) (Gerdemann and Nicolson 1963). AMF diversity was identified based on spore size, shape, and colour (Perez and Schenck 1989). Single spores were observed and focused under a compound microscope for enumeration, with spore density calculated per 10 g of soil. The INVAM (International Culture Collection of Vesicular Arbuscular Mycorrhizal Fungi) reference species description was used for identifying AMF spore diversity (Gerdemann and Nicolson 1963; Giovannetti and Mosse 1980).
Plant analysis
A total of 1 g of weighed plant powder (WS) was recorded for its initial weight before oven drying (WPS = WP + WS). For ash determination in chickpea., a 1-g sample was placed in a crucible (Wcs) and heated in a furnace at 550°C for 4 h. The crucible with ash was then weighed (WCA), and the ash content was calculated as described by (Bremner and Mulvaney (1982).
Protein percentage in chickpea shoots was determined using the method described by Madurapperuma and Kumaragamage (1999), with protein measured by multiplying the percentage of nitrogen by a factor of 0.25.
For fat determination, 1 g of the sample was wrapped in filter paper and placed in a thimble. The Soxhlet extraction unit was set up with the sample flask containing 70% petroleum ether. The flask was placed in an oven to remove moisture and petroleum ether, with the drying temperature set at 110°C for 30 min (Bremner and Mulvaney 1982). The percentage of fat was determined (Eqn 6).
For fibre analysis, a 2-g sample was treated with 2% HCl solution (4 mL HCl + 196 mL distilled water) and heated in a water bath for 3 h. The mixture was filtered through muslin cloth to separate the residues, which were then treated with 2% NaOH solution for an additional 3 h in a water bath. The residues were transferred to a crucible and placed in an electric oven at 105°C for 3 h, followed by heating in a muffle furnace at 650°C for 3 h, according to the methods described by AOAC (2005).
Elemental analysis
For the analysis of macronutrient (P, K, Na, Ca, Mg) and micronutrient (Zn, Fe, Mn) concentrations, 1 g of the sample was placed in a flask with 10 mL of concentrated HNO3 and allowed to sit for 24 h. After this period, 4 mL of perchloric acid was added, and the mixture was heated until white fumes appeared. The digest was then diluted by adding 100 mL of distilled water and filtered using Whatman 42 filter paper.
A volume of 1 mL of the filtrate was used to measure phosphorus using a photometer, with phosphorus determined by a spectrophotometer at 880 nm. Potassium and sodium concentrations were assessed using a flame photometer, and micronutrient concentrations were measured by atomic absorption spectrophotometer, following the method described in Madurapperuma and Kumaragamage (1999).
Molecular analysis
A total of 1 g of powdered sample (ground with liquid nitrogen) was immediately transferred to a pre-warmed tube containing CTAB extraction buffer (500 μL/sample) and incubated in a water bath at 65°C for 30 min, then allowed to cool. Chloroform and isoamyl alcohol solution (24:1) was added (250 μL/sample) to the mixture for protein denaturation, followed by mild vortexing. The sample was then centrifuged at 11,200g for 15 min at 4°C. After centrifugation, 200 μL of isopropyl alcohol was added to the upper phase of the solution with gentle tapping. The sample was centrifuged again at 11,200g for 15 min at 4°C, and 200 μL of 70% ethanol was added for washing, followed by gentle tapping. The sample was centrifuged a third time at 11,200g for 5 min at 4°C. The resulting DNA pellet was then dissolved in TE buffer (Gerdemann and Nicolson 1963; Stahl and Christensen 1982).
For stock primers, 320 μL of dH2O was added to each set of primers (11 primers). The mixture was then vortexed and centrifuged five times to rehydrate the primers.
To prepare working primers, 10 μL from each AMF specific stock primer was transferred to an Eppendorf tube with 90 μL of dH2O. The PCR mixture was prepared by combining 50 μL of master mix containg separating dye (Ama R One PCR Super Mix, Cat# SM213-0250, Ms: Gene Direx), 28 μL of dH2O, 10 μL of primer (from stock), and 2 μL of Taq DNA polymerase, resulting in a total volume of 90 μL. A volume of 9 μL of the PCR mixture was added to each Eppendorf tube, followed by the addition of 1 μL of AMF extracted DNA to each mixture (Goswami et al. 2018).
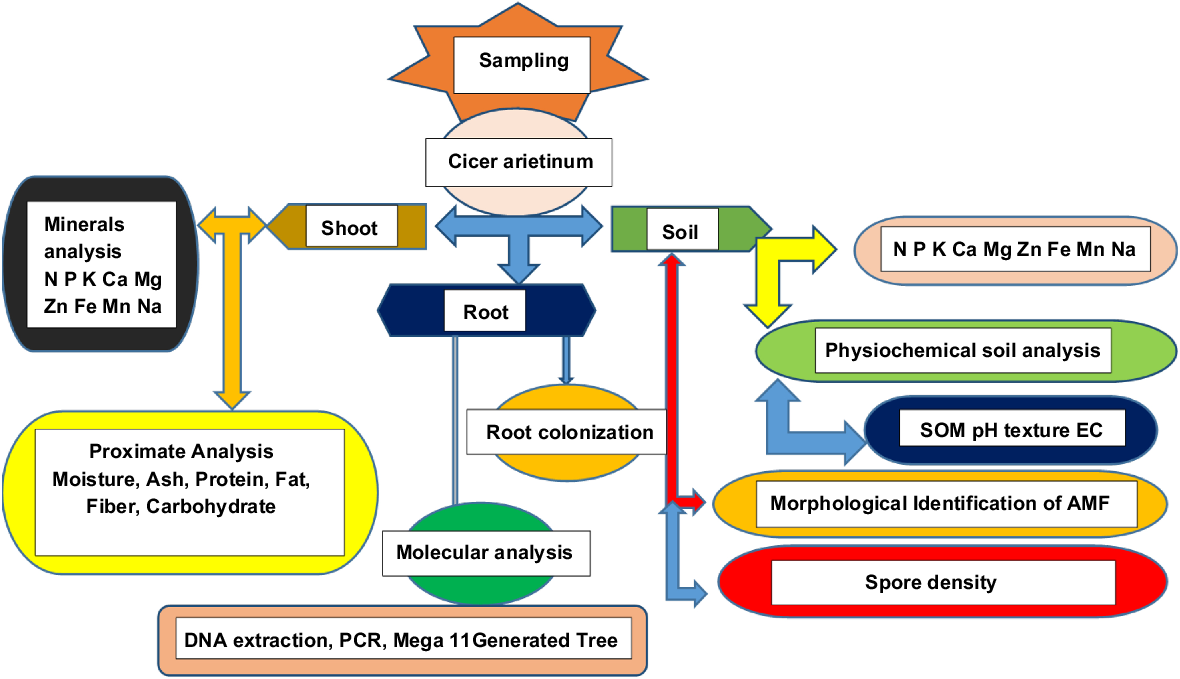
For primers AML1-AML2 and NS1-NS2, the PCR was run for 35 cycles with the following conditions: 95°C for 5 min, 94°C for 30 s, 55°C for 35 s, 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR products were separated using 1.5% agarose gel for AML1-AML2 and 2% agarose gel for NS1-NS2, with 100 mL of 1 × TBE buffer and 3 μL of ethidium bromide. A 100 bp DNA ladder was used as a control. These parameters were applied to all primers except for the annealing temperature. The annealing temperature for Glomus1310-GLOM5.8 and GLOM5.8R-ITS1F was 55°C, while it was 59.5°C for GLOM5.8-ITS1F and 60.7°C for both ARCH1311F-ITS4R and ITS1F-ITS4R (Goswami et al. 2018). Technical replicates use gradient PCR to perform multiple PCR reactions from the same DNA sample, whereas biological replicates use DNA from particular samples that were previously identified visually (Ganguly et al. 2024). Multivariate ANOVA and s.e. at P < 0.05 were used for statistical analysis. Details of the different parameters studied in this research are in Fig. 2.
Results
Physiochemical soil analysis
Chickpea samples were collected from various sites in the districts of Bhakkar and Khushab, Pakistan. Significant physicochemical soil analysis mean values from these sites are in Tables 1 and 2. Mean soil organic matter (SOM) values were 0.65% for Bhakkar and 0.52% for Khushab. Average pH of the 44 samples ranged from 6.12 to 7.88, indicating that the soil was neutral in nature. Observed pH values were statistically significant at P < 0.05, which was a key indicator of AMF spore diversity.
| Year | District | Sites | Soil texture (%) | Texture | EC (dS/m) | SOM (%) | pH | N | P | K | Ca | Mg | Na | Zn | Fe | Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | mg/kg | ||||||||||||||||
| 2020 | Bhakkar | Jandan wala | 37.42 | 29.58 | 33 | CL | 1.5a | 0.075 | 7.31b | 585c | 1.7d | 86.6e | 8.56f | 0.02ab | 330.4bc | 1.36cd | 5.15ef | 8.02fa | |
| 34.1 | 44.2 | 21.7 | L | 1.3a | 0.88 | 7.25b | 1112c | 2.5d | 150e | 664f | 203ab | 94.4 bc | 0.05cd | 6.00ef | 3.7fa | ||||
| Kallurkot | 55.7 | 24.55 | 19.75 | SL | 2.7a | 0.69 | 7.74b | 877c | 4.2d | 144e | 13.5f | 421.1ab | 150 bc | 2.40cd | 5.30ef | 2.5fa | |||
| 46.3 | 10.45 | 43.25 | SC | 2.2a | 0.7 | 7.26b | 594c | 8d | 90e | 17.72f | 88ab | 195 bc | 1.40cd | 4.41ef | 4.2fa | ||||
| Tinda thal | 52.2 | 32.7 | 15.1 | SL | 2.4a | 0.092 | 6.9b | 1342c | 10d | 70e | 42.8f | 385.2ab | 64 bc | 3.30cd | 4.21ef | 4.41fa | |||
| 46.58 | 32 | 21.42 | L | 2.3a | 0.98 | 7.18b | 1134c | 5d | 75.8e | 135.4f | 88.31ab | 55 bc | 112.34cd | 2.25ef | 4.21fa | ||||
| Umar wali | 39.4 | 30.6 | 30 | CL | 1.7a | 1.12 | 7b | 1057c | 4.7d | 95.8e | 88.9f | 163ab | 133 bc | 1.96cd | 6.61ef | 225.66fa | |||
| 36.6 | 40 | 23.4 | L | 1.1a | 0.56 | 7.48b | 1250c | 2.3d | 134.6e | 64.5f | 137ab | 84.6 bc | 2.32cd | 1.05ef | 1.64fa | ||||
| Zamey wala | 40.8 | 38.2 | 21 | L | 1.5a | 0.66 | 6.82b | 3366c | 2.8d | 112e | 342.5f | 102ab | 143bc | 0.00cd | 0.09ef | 2.75fa | |||
| 33.8 | 29.1 | 37.1 | CL | 0.41a | 0.8 | 7.88b* | 5472c | 2.1d | 120e | 8.85f | 91ab | 173bc | 2.44cd | 1.31ef | 7.82fa | ||||
| Khushab | Adhikot | 37.8 | 35.3 | 26.8 | L | 0.56 | 0.44 | 6.44b* | 2763c* | 1.8d* | 105.5e* | 11.2f* | 28.73ab* | 444bc* | 6.00cd* | 1.85ef* | 9.21fa* | ||
| 28.8 | 33.15 | 38.05 | CL | 0.88a* | 0.75 | 6.12b* | 702c* | 0.01d* | 188e* | 172.5f* | 282.6ab* | 413bc* | 5.30cd* | 3.32ef* | 8.88fa* | ||||
| Noorpur | 56.6 | 21.22 | 22.18 | SCL | 1.8a* | 0.95 | 8.1b* | 948c* | 0.04d* | 166e* | 16.85f* | 6.98ab* | 190bc* | 208.70cd* | 2.44ef* | 95.77fa* | |||
| 33.9 | 39.9 | 26.2 | L | 0.5a* | 1.12 | 7.14b* | 3230c* | 1.1d* | 102.4e* | 128.3f* | 53.22ab* | 88bc* | 4.21cd* | 5.21ef* | 5.83fa* | ||||
| Quaiddabad | 30.28 | 38.48 | 31.24 | CL | 0.95a* | 0.49 | 6.14b* | 2175c* | 1.3d* | 107.2e* | 6.9f* | 89.33ab* | 112bc* | 2.25cd* | 4.33ef* | 3.21fa* | |||
| 31.12 | 31.24 | 37.64 | CL | 1.4a* | 0.051 | 7.46b* | 1120v | 2.3d* | 115.8e* | 233f* | 33.11ab* | 342bc* | 1.64cd* | 2.88ef* | 1.9fa* | ||||
| Rakhutra | 18.5 | 56.36 | 25.14 | SL | 0.9a* | 0.12 | 8.14b* | 4510c* | 0.03d* | 122e* | 67.8f* | 43.75ab* | 310bc* | 2.75cd* | 1.95ef* | 142.4fa* | |||
| 31.25 | 32 | 36.75 | CL | 1.2a* | 0.24 | 7.13b* | 955c* | 5.2d* | 136e* | 7.44f* | 11.9ab* | 65bc* | 55.64cd* | 3.76ef* | 7.88fa* | ||||
| Shah Wala | 21.5 | 34.05 | 55.12 | CL | 0.8a* | 0.66 | 13.8b* | 740c* | 4.31d* | 177e* | 6.9f* | 14.44ab* | 13bc* | 82.05cd* | 2.01ef* | 10.41fa* | |||
| 25.42 | 41.24 | 33.34 | CL | 1.4a* | 0.38 | 7.44b* | 2475c* | 0.71d* | 56e* | 13.6f* | 110.8ab* | 122bc* | 2.22cd* | 4.44ef* | 8.21fa* | ||||
CL, clay loam; L, loam; C, clay; SL, silt loam; SCL, silt clay loam. *, significant at P < 0.05.
a = ±0.22, a* = ±0.12, b = ±10, b* = ±0.70, c = ±489, c* = ±406, d = ±0.86, d* = ±0.57, e = ±9.03, e* = ±12.68, f = ±66, f* = ±26, ab = ±42, ab* = ±26, bc = ±25, bc* = ±48, cd = ±11, cd* = ±21, ef = ±0.72, ef* = ±0.38, fa = ±22, fa* = ±15.
| Interaction | d.f. | pH | N % | P | K | Ca | Mg | Na | Zn | Fe | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | ||||||||||||
| Sites | 19 | 3.09 | 7.72 | 3.816 | 85.09 | 5607 | 4564 | 1650 | 2827 | 3.18 | 443.6 | |
| Significance (P < 0.05) | 19 | 0.10 | 0.288 | 0.419 | 0.955 | 0.039 | 0.080 | 0.971 | 0.975 | 0.284 | 0.929 | |
The soil textural class in both districts was loamy. In Bhakkar, the highest EC values recorded were 2.4 dS/m, 1.7 dS/m, and 1.5 dS/m from Tinda Thal, Umar Wali, and Zamey Wala, respectively. In Jandan Wala and Kallurkot, EC values were 1.5 dS/m and 2.7 dS/m, respectively. The concentration of soil nitrogen was high, with values of 5472 mg/kg in Bhakkar and 4510 mg/kg in Khushab, which were not significant (7.72) at P < 0.05.
Table 1 shows the significant relationships among the physicochemical properties of the soil samples from Bhakkar and Khushab. Pphosphorus concentration in Bhakkar soil samples was higher than Khushab, with a notable phosphorus concentration of 4.7 mg/kg measured at Umar Wali in Bhakkar, which had a high organic matter content of 1.12%. Low rhizospheric phosphorus standard is 15 mg/kg; thus both districts indicated phosphorus deficiency (Nair and Sollenberger 2022). Potassium concentrations were reasonable, with 134.6 mg/kg in Bhakkar and 188 mg/kg in Khushab. Other elements such as Ca (0.039), Fe (0.284), and Mg (0.080) showed significantly different results (P < 0.05) (Table 2).
AMF diversity
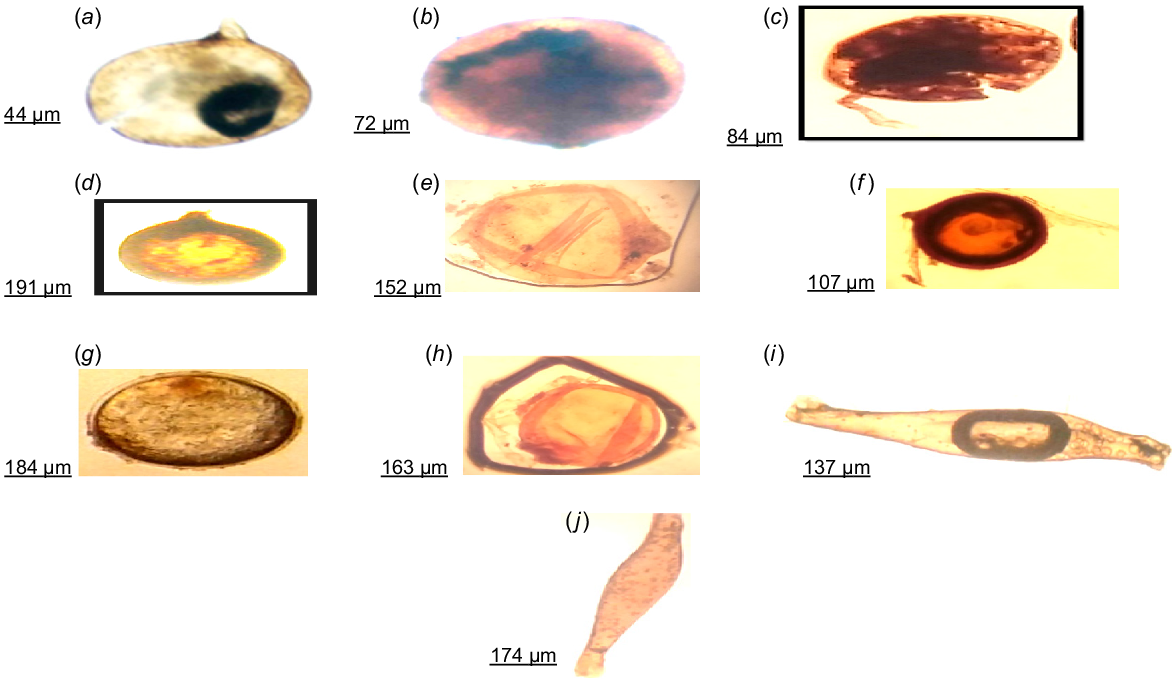
Diversity of AMF depends on spore sizes (AOAC 2005). For example, the size average range across replicates for Glomus spores was 10−40 μm, Acaulospora ranged from 125 μm to 149 μm, Sclerocystis ranged from 110 μm to 155 μm, and Gigaspora ranged from 65 μm to 112 μm. Among the collected samples, Glomus was the smallest in size but the most abundant genus (Fig. 3a–f).
Root colonisation
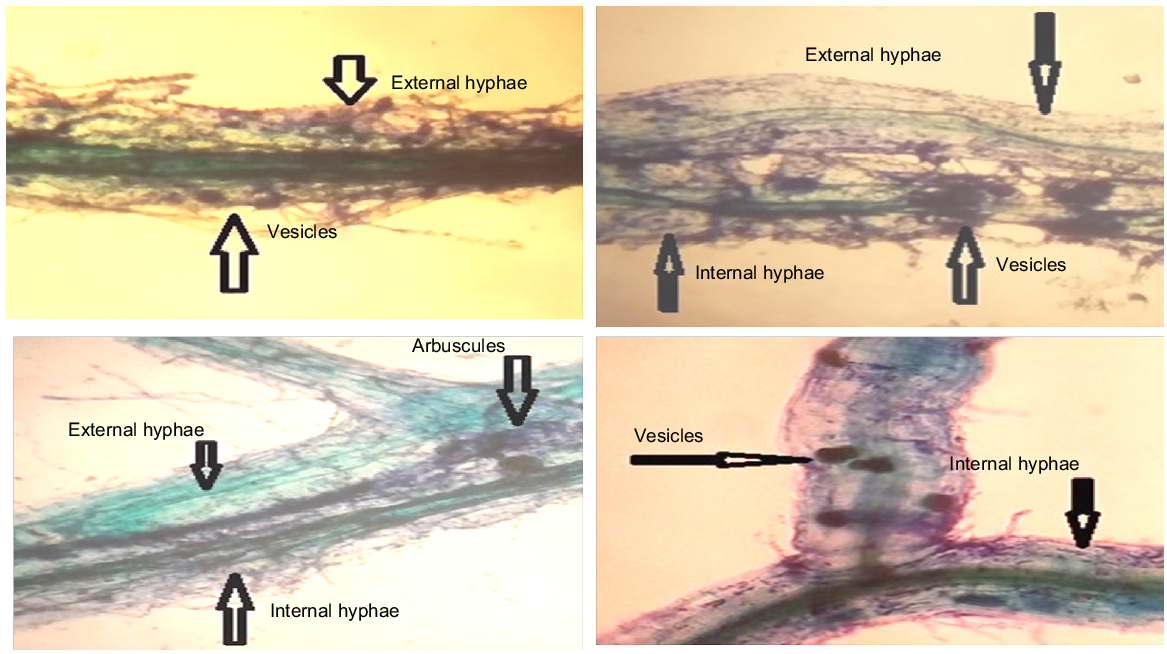
The intensity of root colonisation of chickpea by AMF varied among sampled sites due to differences in the physicochemical properties of the soil (Bhakkar, s.d. 14.31; Khushab, s.d. 17.50) (Perez and Schenck 1989). The Tinda Thal site in Bhakkar had a high root colonisation rate of 75.9%, while the Rakhutra site in Khushab showed a rate of 72.16%, which positively influenced the plant nutrients concentration (Table 3). Kallurkot samples exhibited similar rates of 18.41% and 16.34%, respectively. AMF structures, including arbuscules and vesicles, were identified in the collected samples. Levels of root colonisation varied randomly in both districts of Bhakkar and Khushabin (Figs 4, 5).
| District | Sites | % | mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | Zn | Fe | Mn | |||
| Bhakkar | Jandan wala | 2.38 ± 0.19a | 45.02 ± 5.8b | 38.02 ± 6c | 42.89 ± 5.3d | 0.23 ± 0.01e | 20.40 ± 5.6ab | 14 ± 2.1bc | 11.9 ± 0.84cd | 57.00 ± 5.7da | |
| 2.21 ± 0.19a | 34.30 ± 5.8b | 28.30 ± 6c | 12.21 ± 5.3d | 0.29 ± 01e | 19.50 ± 5.6ab | 11 ± 2.1bc | 3.29 ± 0.84cd | 17.00 ± 5.7da | |||
| Kallurkot | 1.54 ± 0.19a | 15.12 ± 5.8b | 22.38 ± 6c | 34.12 ± 5.3d | 0.21 ± 01e | 2.00 ± 5.6ab | 22 ± 2.1bc | 10.44 ± 0.84cd | 16.00 ± 5.7da | ||
| 1.54 ± 0.19a | 13.08 ± 5.8b | 38.90 ± 6c | 8.00 ± 5.3d | 0.36 ± 01e | 17.20 ± 5.6ab | 15 ± 2.1bc | 8.37 ± 0.84cd | 22.00 ± 5.7da | |||
| Tinda thal | 2.24 ± 0.19a | 68.60 ± 5.8b | 44.65 ± 6c | 52.44 ± 5.3d | 0.26 ± 01e | 66.30 ± 5.6ab | 10 ± 2.1bc | 5.65 ± 0.84cd | 64.00 ± 5.7da | ||
| 3.64 ± 0.19a | 31.23 ± 5.8b | 25.00 ± 6c | 14.10 ± 5.3d | 0.19 ± 01e | 19.40 ± 5.6ab | 12 ± 2.1bc | 6.41 ± 0.84cd | 43.00 ± 5.7da | |||
| Umar wali | 1.8 ± 0.19a | 21.23 ± 5.8b | 14.48 ± 6c | 6.34 ± 5.3d | 0.23 ± 01e | 21.50 ± 5.6ab | 14 ± 2.1bc | 8.41 ± 0.84cd | 32.00 ± 5.7da | ||
| 1.68 ± 0.19a | 50.48 ± 5.8b | 72.96 ± 6c | 25.00 ± 5.3d | 0.27 ± 01e | 44.22 ± 5.6ab | 33 ± 2.1bc | 9.3 ± 0.84cd | 44.00 ± 5.7da | |||
| Zamey wala | 2.1 ± 0.19a | 10.72 ± 5.8b | 4.00 ± 6c | 1.79 ± 5.3d | 0.24 ± 01e | 18.00 ± 5.6ab | 14 ± 2.1bc | 7±±0.84cd | 22.00 ± 5.7da | ||
| 1.68 ± 0.19a | 40.02 ± 5.8b | 44.49 ± 6c | 28.00 ± 5.3d | 0.2 ± 01e | 16.50 ± 5.6ab | 13 ± 2.1bc | 4.48 ± 0.84cd | 13.00 ± 5.7da | |||
| Khushab | Adhikot | 2.8 ± 0.14a* | 0.59 ± 0.2b* | 67.40 ± 7c* | 44.10 ± 5.6d* | 0.16 ± 8.7e* | 17.2 ± 0.3ab* | 08 ± 0.81bc* | 3.96 ± 1.1cd* | 42.00 ± 6.5da* | |
| 1.8 ± 0.14a* | 0.31 ± 0.2b* | 45.40 ± 7c* | 20.00 ± 5.6d* | 72.84 ± 8.7e* | 14.8 ± 0.3ab* | 10 ± 0.81bc* | 12.91 ± 1.1cd* | 28.00 ± 6.5da* | |||
| Noorpur | 1.4 ± 0.14a* | 1.3 ± 0.2b* | 15.12 ± 7 c* | 22.10 ± 5.6d* | 12.00 ± 8.7e* | 16.7 ± 0.3ab* | 15 ± 0.81bc* | 4.44 ± 1.1cd* | 16.08 ± 6.5da* | ||
| 1.5 ± 0.14a* | 0.06 ± 0.2b* | 33.22 ± 7c* | 20.00 ± 5.6d* | 16.06 ± 8.7e* | 17.2 ± 0.3ab* | 14 ± 0.81bc* | 8.06 ± 1.1cd* | 32.40 ± 6.5da* | |||
| Quaiddabad | 1.8 ± 0.14a* | 0.11 ± 0.2b* | 89.30 ± 7c* | 60.22 ± 5.6d* | 78.80 ± 8.7e* | 16.6 ± 0.3ab* | 14 ± 0.81bc* | 8.55 ± 1.1cd* | 64.00 ± 6.5da* | ||
| 2.1 ± 0.14a* | 0.13 ± 0.2b* | 39.50 ± 7c* | 18.94 ± 5.6d* | 30.86 ± 8.7e* | 14.9 ± 0.3ab* | 11 ± 0.81bc* | 5.87 ± 1.1cd* | 58.00 ± 6.5da* | |||
| Rakhutra | 1.5 ± 0.14a* | 0.2 ± 0.2b* | 44.52 ± 7c* | 50.88 ± 5.6d* | 15.66 ± 8.7e* | 15.8 ± 0.3ab* | 12 ± 0.81bc* | 6.18 ± 1.1cd* | 14.00 ± 6.5da* | ||
| 2.1 ± 0.1a* | 2 ± 0.2b* | 49.08 ± 7c* | 45.72 ± 5.6d* | 44.00 ± 8.7e* | 17.2 ± 0.3ab* | 9 ± 0.81bc* | 14.38 ± 1.1cd* | 74.80 ± 6.5da* | |||
| Shah wala | 2.66 ± 0.1a* | 0.06 ± 0.2b* | 17.00 ± 7c* | 5.24 ± 5.6d* | 4.00 ± 8.7e* | 14.3 ± 0.3ab* | 15 ± 0.81bc* | 3.96 ± 1.1cd* | 27.00 ± 6.5da* | ||
| 1.82 ± 0.1a* | 1.1 ± 0.2b* | 30.06v7c* | 45.04 ± 5.6d* | 46.70 ± 8.7e* | 14.8 ± 0.3ab* | 10 ± 0.81bc* | 3.85 ± 1.1cd* | 28.00 ± 6.5da* | |||
*P < 0.05.
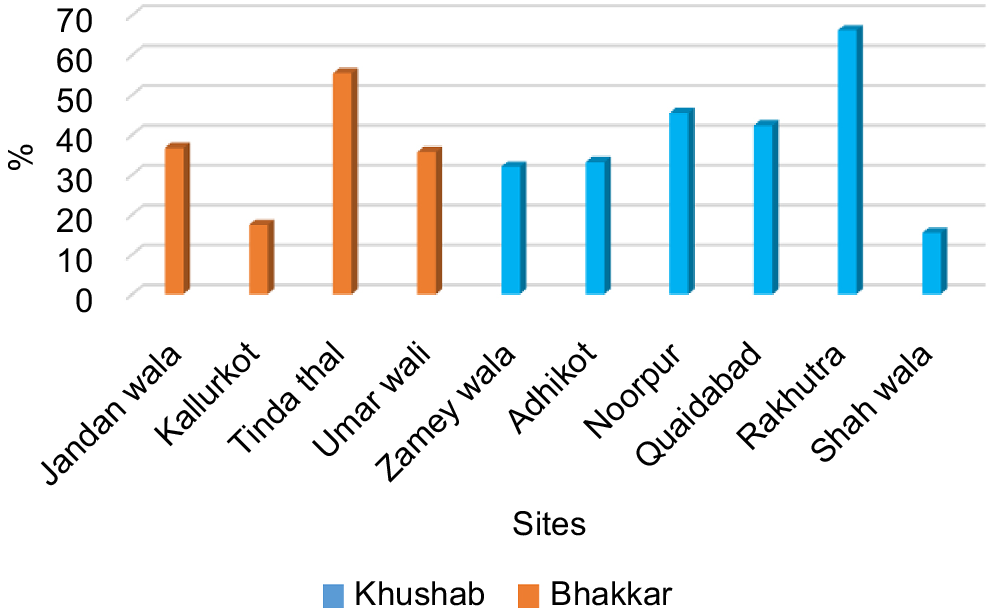
Spore densities
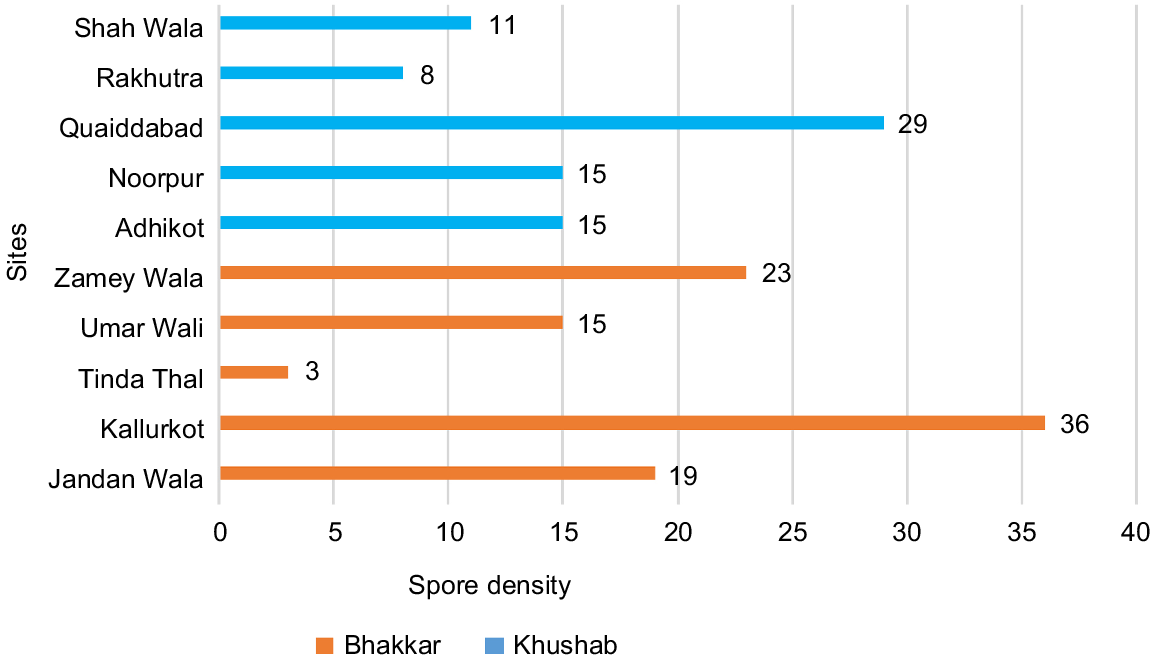
Spore densities of different genera of AMF were enumerated in soil samples collected from the districts of Bhakkar and Khushab (Fig. 6). Although spore abundances varied among sampling sites, the mean spore densities from both districts were nearly the same. The Kallurkot site in Bhakkar had a high spore density of 36 spores/10 g of soil, while a high spore count of 29 at the Quaidabad site in Khushab. Glomus spores were the most abundant in rhizopshere soils of chickpea.
Plant analysis
Moisture content of chickpea samples varied slightly among sites in Bhakkar, with a mean value of 8.62%; the highest being 9.33% from the Jandan Wala site. In Khushab, mean moisture content was 9.20%, ranging from 9.33% (Shah Wala site) to 7.55% (Rakhutra site). Moisture values in Khushab were generally higher than Bhakkar.
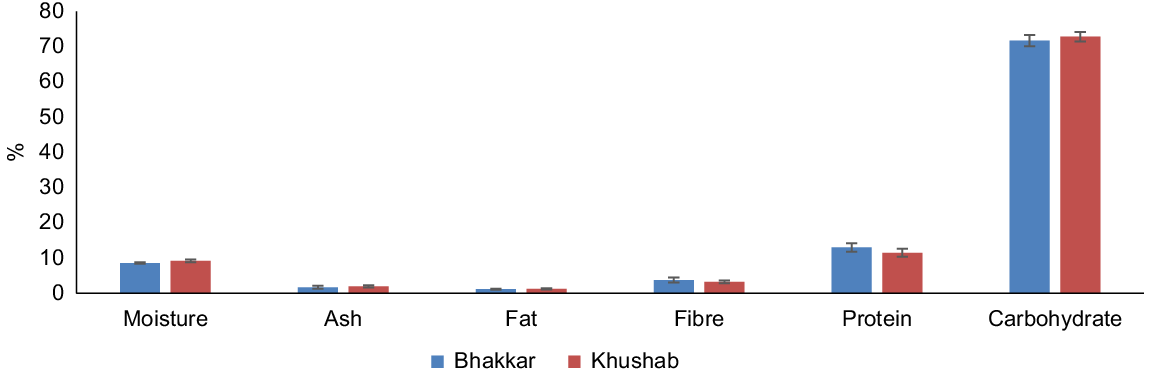
Mean ash content of chickpea samples from Bhakkar was 1.72%; the highest was 4.31% at Zamey Wala and the lowest was 0.57% at Jandan Wala. There were differences in ash content among various sites in Bhakkar. In Khushab, the ash content ranged from 3.15% (Shah Wala) to 0.25% (Quaidabad), showing variations among the selected sites. The mean fat content in chickpea samples from Bhakkar was 1.16%. The Umar Wali site had a high fat value of 1.8%. Fat values varied less among Bhakkar sites, while a high value of 2.1% was obtained from the Rakhutra site in Khushab. In Bhakkar distract, mean fibre content was 3.78%, with highest at Jandan Wala (7.45%) and the lowest at Umar Wali (1.05%). In Khushab district, mean fibre content was 3.24%, with the highest at Rakhutra (5.05%) (Table 4). Similarities in ash, fat, and fibre content are illustrated in the standard error graph. Proteins and carbohydrates were also found to be significantly abundant (Fig. 7).
| District | Sites | Moisture | Ash | Fat | Fiber | Protein | Carbohydrate | |
|---|---|---|---|---|---|---|---|---|
| Bhakkar | Jandan Wala | 9.33 | 1.06 | 0.9 | 7.45 | 14.87 | 66.39 | |
| 9.16 | 0.57 | 0.7 | 2.25 | 13.82 | 73.5 | |||
| Kallurkot | 7.14 | 0.78 | 0.8 | 6.8 | 9.62 | 74.86 | ||
| 8.14 | 2.4 | 1.4 | 4.95 | 9.62 | 73.49 | |||
| Tinda Thal | 8.66 | 0.78 | 1.9 | 3.65 | 14 | 71.01 | ||
| 9.23 | 2.4 | 1.5 | 3 | 22.75 | 61.12 | |||
| Umar Wali | 8.49 | 0.193 | 1.3 | 1.05 | 11.25 | 77.717 | ||
| 9 | 1.77 | 1.8 | 1.15 | 10.5 | 75.78 | |||
| Zamey Wala | 9.09 | 4.31 | 0.7 | 4.8 | 13.12 | 67.98 | ||
| 7.96 | 3 | 0.6 | 2.65 | 10.5 | 75.29 | |||
| Khushab | Adhikot | 7.98 | 2.74 | 0.3 | 4.5 | 17.5 | 66.98 | |
| 9.18 | 2.46 | 0.5 | 3.55 | 11.37 | 72.94 | |||
| Noorpur | 10.26 | 1.41 | 1.4 | 2.85 | 8.75 | 75.33 | ||
| 10.52 | 1.84 | 1.6 | 4.3 | 6.25 | 75.49 | |||
| Quaiddabad | 8.46 | 0.25 | 1.3 | 2.35 | 8.25 | 79.39 | ||
| 7.69 | 0.93 | 1.6 | 1.05 | 13.12 | 75.61 | |||
| Rakhutra | 7.55 | 3.3 | 2.1 | 5.05 | 9.62 | 72.38 | ||
| 9.3 | 2.19 | 1.8 | 3.85 | 13.1 | 69.76 | |||
| Shah Wala | 9.62 | 1.09 | 0.7 | 2.9 | 10.5 | 75.19 | ||
| 11.39 | 3.15 | 1.2 | 2.05 | 16.62 | 65.59 |
Protein content showed less variation among samples from Bhakkar, with the highest protein value of 22.75% obtained from Tinda Thal. In Khushab, protein concentrations were 17.5% at Adhikot and 10.5% at Shah Wala. The highest carbohydrate values were observed in samples from both Bhakkar and Khushab, with mean concentrations of 71.72% and 72.86%, respectively (Table 5 and Fig. 7).
Elemental analysis
Mean nitrogen value was 2.98%, with a high of 3.64% recorded from Tinda Thal in Bhakkar. Kallurkot samples showed a similar nitrogen percentage of 1.54%, which is relatively low for Bhakkar. Mean nitrogen value in Khushab was lower than Bhakkar, with less variation observed among Khushab samples. The Adhikot site in Khushab had a high nitrogen value of 2.8%.
Mean phosphorus values were 32.98 mg/kg for Bhakkar and 0.58 mg/kg for Khushab, indicating that phosphorus concentration is low in Khushab. The Zamey Wala site in Bhakkar had a high phosphorus concentration of 68.20 mg/kg, while the Rakhutra site in Khushab had the highest concentration in samples from that district. Phosphorus content in shoots from Bhakkar was higher than Khushab.
Mean potassium value for Khushab samples was 43.06 mg/kg, higher than Bhakkar (33.32 mg/kg). Zamey Wala site had the lowest potassium content of 4.49 mg/kg in Bhakkar. The highest and lowest shoot potassium values, 89.22 mg/kg and 15.40 mg/kg were observed at the Noorpur and Adhikot sites, respectively, in Khushab.
Mean calcium value was 22.48 mg/kg in Bhakkar, with the highest shoot calcium concentration of 52.00 mg/kg at Zamey Wala. Mean magnesium values were 0.24 mg/kg in Bhakkar and 32.10 mg/kg in Khushab. Magnesium content showed little variation among Bhakkar sites, reflecting similar AMF root colonisation frequencies.
Mean sodium value was 24.50 mg/kg, with a high shoot sodium content of 66.22 mg/kg from Umar Wali and a low value of 2 mg/kg from Kallurkot in Bhakkar. Sodium content showed minimal variation among Khushab sites, with a mean of 15.95 mg/kg and similar values of 17.2 mg/kg observed at Adhikot, Noorpur, and Rakhutra. Rakhutra recorded a high sodium value of 15.8 mg/kg in Khushab.
Mean zinc concentrations were 15.8 mg/kg in Bhakkar and 11.8 mg/kg in Khushab. Umar Wali had the highest zinc value of 33 mg/kg among Bhakkar sites, while Noorpur had the highest zinc concentration of 15 mg/kg in Khushab. Noorpur and Quaidabad both had similar zinc contents of 14 mg/kg in their samples. Zinc content showed little variation among Khushab samples.
Mean iron values were 7.25 mg/kg for Bhakkar and 7.22 mg/kg for Khushab. Jandan Wala samples showed both high and low iron values of 11.9 mg/kg and 3.29 mg/kg, respectively. Iron content varied among sites in both districts.
Mean manganese value was 33.00 mg/kg for Bhakkar, with a high value of 57 mg/kg, while Zamey Wala had a low content of 13 mg/kg. Noorpur had a high manganese content of 64.40 mg/kg. Standard errors for nitrogen and phosphorus showed significant differences, while similar significant values were found for potassium, calcium, and manganese.
Detailed elemental analysis is in Table 3. Elemental significance levels were found to be similar for P, K and Mn (Table 6).
| Interaction | N | P | K | Ca | Mg | Na | Zn | Fe | Mn | |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | ||||||||||
| Sites | 0.294 | 440.724 | 436.507 | 318.517 | 632.295 | 171.399 | 29.642 | 10.230 | 365.609 | |
| Error | 0.000 | 7.035 | 2.931 | 10.091 | 0.096 | 1.85015.760 | 0.000 | 0.000 | 13.084 | |
| d.f. | 19 | |||||||||
| CI (95%) | 2.05 | 16.78 | 38.18 | 27.85 | 16.17 | 20.22 | 13.8 | 7.37 | 35.71 | |
P < 0.0.5.
The relationship between soil spore densities collected from sites in Bhakkar and Khushab districts and plant elemental properties is in Table 7. Spore diversity positively affected the concentrations of plant elements. However, due to minimal differences in spore densities, there were not significant fluctuations in the plant elemental properties.
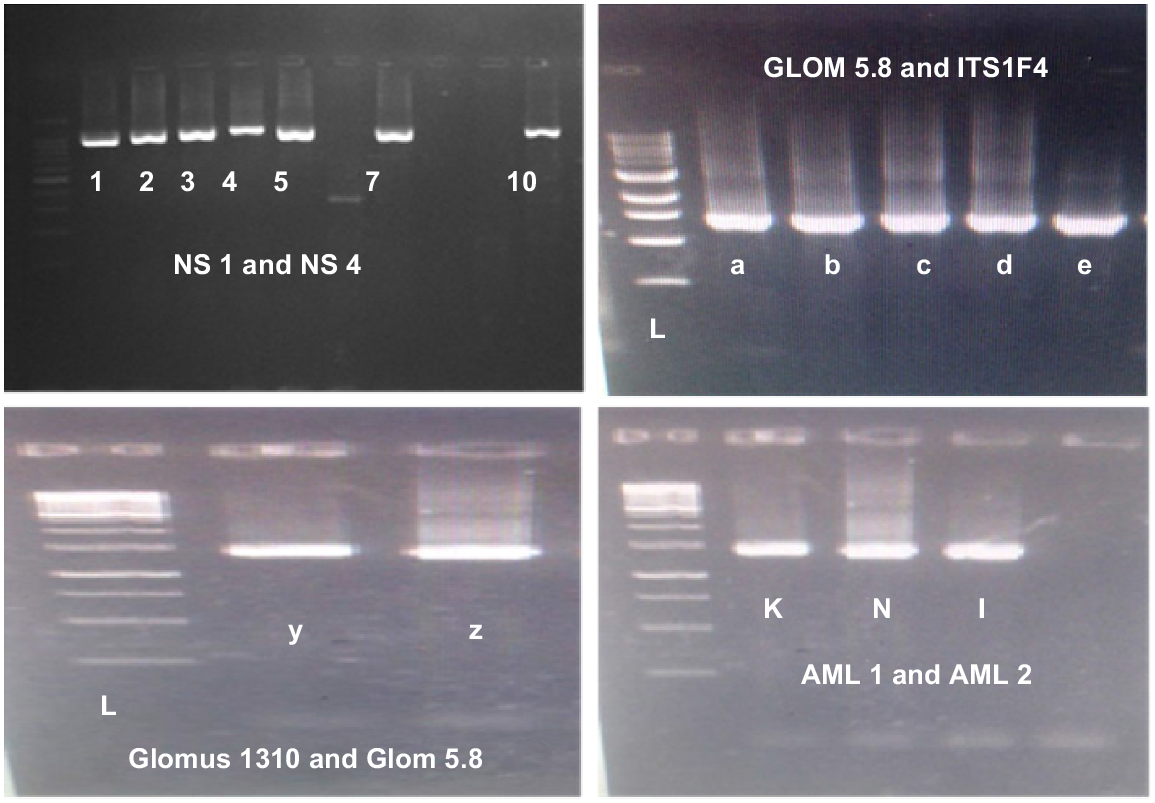
Molecular analysis of arbuscular mycorrhizae fungi in Cicer arietinum L
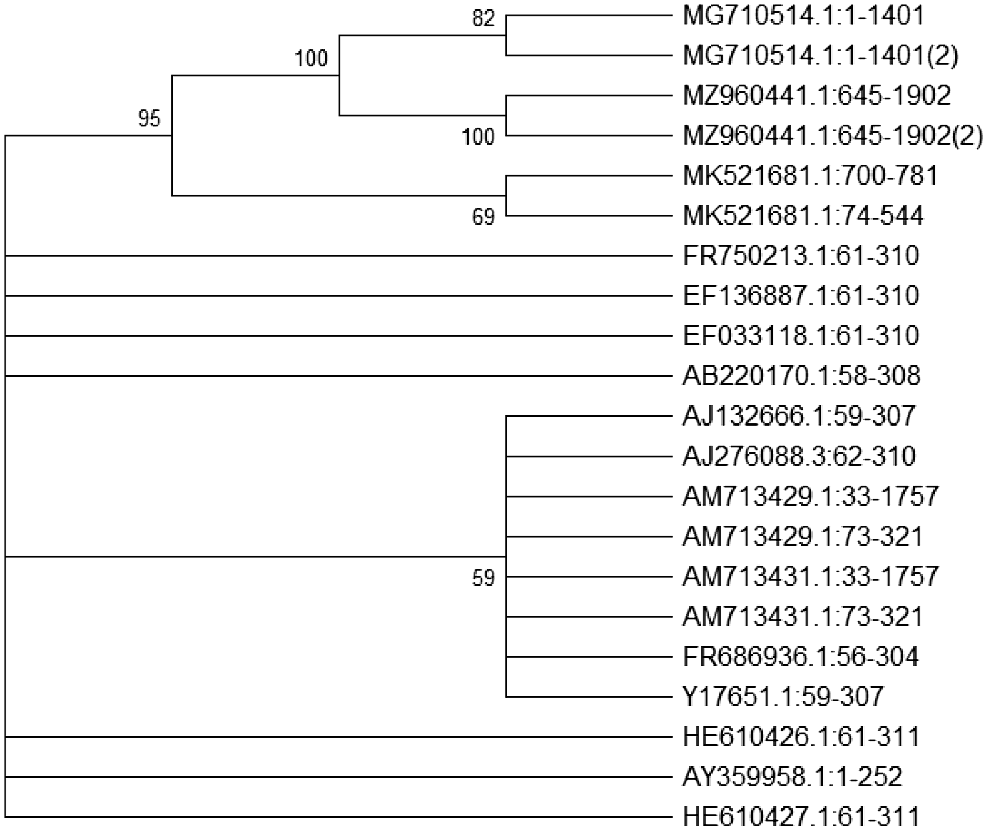
The ladder DNA (L) along primer sets AML1-AML2 generated bands at 700–900 bp, NS1-NS4 at 900–1100 bp, GLOM5.8-ITS1F at 700–1000 bp, Glomus 1310-GLOM 5.8 at 500–700 bp, and ITS1F-ITS4R at 1000–1100 bp (Fig. 8). AML1-AML2 confirmed the presence of AMF diversity in the roots of chickpea collected from both Bhakkar and Khushab districts. The phylogenetic tree illustrating the clades and distances among the AMF genera associated with the primers is in Fig. 9. The evolutionary history was inferred using the Neighbor-Joining method, and evolutionary distances were calculated with the Maximum Composite Likelihood method, expressed in terms of the number of base substitutions per site. The analysis included 24 nucleotide sequences, with ambiguous positions removed for each sequence pair (pairwise deletion option). The final dataset contained 1242 positions. Evolutionary analyses were conducted in MEGA11, producing a cladogram that shows the distances and different traits among the AMF genera Glomus, Archaeospora, Diversispora, Funneliformis, Acaulospora, and Scutellaspora.
Discussion
Physicochemical soil analysis and AMF
In chickpea farming, the application of biofertilisers based on AMF improves crop production, stress tolerance, and nutrient uptake. By lowering reliance on chemical inputs, AMF promotes sustainable farming while simultaneously increasing production and nutritional quality. Including regional AMF strains can increase efficacy even more, providing chickpea production systems with an economical and sustainable option (Pellegrino and Bedini 2014; Saurabh Jha and Songachan 2023). Evidence of increased nutrient absorption induced by AMF symbiosis has been documented (Wang et al. 2023). To explore the potential role of AMF in chickpea in its natural environment, our investigated physicochemical soil properties, AMF spore abundance, diversity, colonisation, and nutrient distribution within the plants. SOM concentrations and EC magnitudes in the districts of Bhakkar and Khushab were similar (Tables 1–2), consistent with previous reports (Krishnamoorthy et al. 2015; Khalid Chaudhry et al. 2016; El Hazzat et al. 2018; Makkar et al. 2018; Saeed et al. 2020). The pH of the investigated sites was neutral, which influenced the abundance of AMF with significance at P < 0.05 (Krishnamoorthy et al. 2015; Saeed et al. 2020). The study results support the view that AMF play a pivotal role in phosphorus acquisition, as the mean phosphorus concentration in Bhakkar soil (4.38 mg/kg) was higher than in Khushab (1.68 mg/kg), attributed to differences in the strength of AMF root colonisation. However, soil nitrogen levels in both districts were not significantly affected by AMF colonisation (Bhatt and Singh 2020; Saeed et al. 2020; Alimi et al. 2021) (Tables 1, 2).
Morphological analysis, root colonisation, and spore density of AMF in rhizospheric soil of chickpeas
Our morphological investigation showed variations in spore sizes among AMF taxa (Glomus, Acaulospora, Gigaspora, Sclerocystis) (Fig. 3), similar to the findings of El Hazzat et al. (2018) andDroh et al. (2023). The study identified Glomus as the dominant and smallest genus, consistent with previous results (Alguacil et al. 2003; Sasvári et al. 2011; Jacquemyn et al. 2011; Hipólito-Piedras et al. 2024) (Fig. 3). Differences in AMF chickpea root colonisation levels at various AMF sampling sites were attributed to the uneven distribution of soil physicochemical properties across the districts (Pepe et al. 2016; Ndeko et al. 2024). Our results highlighted the importance of AMF root colonisation for nutrient acquisition, particularly phosphorus. Variations in AMF root colonisation levels were observed with 40.56% in Khushab compared with 35.45% in Bhakkar (Houngnandan et al. 2009; Marizal and Syariyah 2017) (Figs 4, 5). Average AMF spore densities per 100 g of soil were 19.2 in Bhakkar and 15.6 in Khushab. The density of AMF spores at Bhakkar positively influenced the concentrations of N, P, Na, and Zn in plants of C. arietinum, which was also reported previoulsy (Diagne et al. 2020; Mulyadi and Jiang 2023) (Fig. 6, Table 7).
Plant and elemental analyses of chickpeas
Moisture, fat, and carbohydrate were positively influenced by AMF colonisation in Khushab compared with Bhakkar. Variations in plant ash content observed in this study differed from the findings of Mehrvarz and Chaichi (2008). Mean percentages of moisture, fat, and carbohydrate were 9.10%, 1.25%, and 72.8%, respectively, in Khushab compared with 8.62%, 1.16%, and 71.7%, respectively, in Bhakkar (Table 4). The impact of AMF on these plant components was statistically significant (P < 0.05), aligning with previous studies (Wu et al. 2006; Manoharan et al. 2008; Dobo 2022; Amir and Crossay 2024). Both districts showed similar effects of AMF on protein and fibre concentrations, as reported by (Lester 2009; Egberongbe et al. 2010; Sarah and Burni 2013; Samanhudi et al. 2014) (Tables 4–5). Results from elemental analysis were consistent with previous findings (Xia et al. 2007; Xie et al. 2022), highlighting the role of AMF in nutrient absorption.
Plant potassium levels varied between districts due to differences in AMF colonisation, with Bhakkar showing 33.02 mg/kg and Khushab 43.06 mg/kg (Bai et al. 2008; Subramanian et al. 2008; Naderi et al. 2010; Sajedi and Rejali 2011) (Table 3). N and P concentrations were not significantly affected by AMF in either district (Sajedi and Rejali 2011). P content in Khushab plant samples was notably lower compared to studies on Zea mays (Tian et al. 2004), Gossypium (Sharifi et al. 2007), and Glycine max (Yaseen et al. 2016). Higher calcium concentrations in Khushab compared with Bhakkar further indicated the influence of AMF frequency, as also reported by Ci et al. (2021) and Liu et al. (2019) for Arachis hypogea. Magnesium and sodium concentrations were positively influenced by indigenous AMF taxa, supported by findings from Arias et al. (2015) and Ramakrishnan and Selvakumar (2012), though this contradicted the work of Naderi et al. (2010). Zinc levels were 15.8 mg/kg in Bhakkar and 11.8 mg/kg in Khushab, differing from the study of Maffo et al. (2022) due to difference in AMF colonisation. Iron (Fe) concentrations in plant shoots across both districts under AMF influence have been previously reported (Arias et al. 2015). The standard errors for phosphorus showed significant differences, while potassium, calcium, and manganese also showed significant values. The interactions of AMF with magnesium, zinc, and iron were not significant in either Bhakkar or Khushab (Tables 3, 6). Because of current agronomic techniques, the potential of AMF as biofertilisers has not been completely utilised despite their accepted benefits (Beleri 2023). Increased crop yields and sustainability can result from combining several legume species with AMF, especially in low-input agricultural systems (Zhao et al. 2022).
Molecular analysis of AMF in the roots of chickpeas
Molecular and morphological analyses indicated that Glomus was the predominant AMF genus sporulating with chickpeas in both districts of Punjab. The amplification of the 18S rDNA gene of the small subunit from the chickpea root samples was performed using specific primers (Fig. 8). The goal of the gradient PCR was to isolate different fragments of AMF rDNA based on their size. The primers used enabled the amplification of gene fragments approximately 1100 bp in length. Our results showed that the root samples contained AMF sizes ranging 250–1100 bp (Lee et al. 2008). There was consistency between molecular and morphological investigations in identifying the genera. Primers AML1 and AML2 efficiently amplified AMF sequences from chickpea roots, unlike primers NS1-NS4, GLOM5.8, ITS1F4, and GLOM1310 (Ogoma et al. 2021; Noreen et al. 2023). Phylogenetic analysis indicated that all sequences belonged to the phylum Glomeromycota. The resulting cladogram (Fig. 9) included 10 taxa and 21 species. This included one taxa each from Scutellospora, Gigaspora, Entrophospora, Dentiscutata, Diversispora, and Sclerocystis, along with three from Archaeospora and six each from Acaulospora and Glomus (Noreen et al. (2023).
Conclusions
Plant analysis of chickpea showed significant variations in nutrient composition across sites in Bhakkar and Khushab districts. Moisture content was slightly higher in Khushab, averaging 9.20%, compared with 8.62% in Bhakkar. Ash content varied significantly, ranging from 0.57% to 4.31% in Bhakkar and from 0.25% to 3.15% in Khushab. Fat content was relatively consistent in Bhakkar with a mean of 1.16%, while the highest value of 2.1% was recorded in Khushab. Fibre content showed more variation in Bhakkar with the hightest at 7.45%, whereas Khushab had a slightly lower mean value of 3.24%. Protein and carbohydrates were also found to be significantly abundant, highlighting the nutritional richness of the samples. These findings suggest site-specific differences that may be influenced by environmental conditions and AMF activity. Elemental analysis of chickpeas showed higher nitrogen (up to 3.64%) and phosphorus (32.98 mg/kg) in Bhakkar, while Khushab had more potassium (43.06 mg/kg) and magnesium (32.10 mg/kg). Calcium, sodium, and zinc were higher in Bhakkar; iron levels were similar in both districts (~7.2 mg/kg). Manganese was high in both, reaching 64.40 mg/kg in Khushab. Nutrient levels varied notably across sites. Molecular analysis of the AMF association with soil from Bhakkar and Khushab revealed the presence of 21 AMF taxa, with two genera, Glomus and Acaulospora, identified as the most dominant. This is the first detailed study of AMF and chickpea in these districts of Punjab, Pakistan. Our findings showed the strength of indeginous AMF association with chickpea in natural fields of Punjab, Pakistan with a sign of future work. Our results indicated that mineral concentrations were the primary factor influencing AMF diversity and distribution patterns. The amplification of the 18S gene of AMF rDNA confirmed that chickpeas hosted AMF. The choice of sampling sites was crucial for promoting the establishment of mycorrhizal symbiosis, which is beneficial for chickpea cultivation. AMF colonisation in chickpeas accelaerated the intake of nutrients, particularly P, K and Ca. It is recommended that developing AMF inoculums on a commercial scale could be a transformative step in biofertiliser production, potentially enhancing agricultural productivity. Farmers should also supply commercial-grade AMF biofertiliser inoculum that is cast-free.
Data availability
All the raw data of this research can be obtained from the corresponding authors upon reasonable request.
Declaration of funding
This research was funded by Taif University, Taif, Saudi Arabia, Project No. (TU-DSPP-2024–290).
Author contributions
K.A generated the idea. Experimental facilities and supervision were provided by J.I. The experimental study was performed by K.A. B.A.A, S.N, S.I, T.Y, S.F, and K.A wrote the manuscript. B.A.A, S.N, S.I, S.F, and T.Y helped with software, manuscript editing, and revision. B.A and S.F reviewed, revised, proof-read and edited the manuscript and provided with funds. All authors agreed to the published version of the manuscript.
Acknowledgements
The authors thank Taif University, Saudi Arabia for supporting this work through project number (TU-DSPP-2024-290). We extend our gratitude to the research scholars of the Plant Ecology and Environmental Science Laboratory at the University of Azad Jammu and Kashmir for their invaluable assistance and logistical support throughout this study.
Reference
Abdelaal K, Alaskar A, Hafez Y (2024) Effect of arbuscular mycorrhizal fungi on physiological, bio-chemical and yield characters of wheat plants (Triticum aestivum L.) under drought stress conditions. BMC Plant Biology 24(1), 1119.
| Crossref | Google Scholar | PubMed |
Alguacil MM, Hernández JA, Caravaca F, Portillo B, Roldán A (2003) Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiologia Plantarum 118(4), 562-570.
| Crossref | Google Scholar |
Alimi A, Adeleke R, Moteetee A (2021) Soil environmental factors shape the rhizosphere arbuscular mycorrhizal fungal communities in South African indigenous legumes (Fabaceae). Biodiversitas Journal of Biological Diversity 22(5), 2466-2476.
| Crossref | Google Scholar |
Alrajhi K, Bibi S, Abu-Dieyeh M (2024) Diversity, distribution, and applications of arbuscular mycorrhizal fungi in the Arabian Peninsula. Saudi Journal of Biological Sciences 31(2), 103911.
| Crossref | Google Scholar |
Amir H, Crossay T (2024) Functional and practical importance of AMF-mixed inoculants for plant development. In ‘Arbuscular mycorrhizal fungi in sustainable agriculture: inoculum production and application’. (Eds M Parihar, A Rakshit, A Adholeya, Y Chen) pp. 319–331. (Springer) 10.1007/978-981-97-0296-1_14
Arias MSB, Peña-Cabriales JJ, Alarcón A, Maldonado Vega M (2015) Enhanced Pb absorption by Hordeum vulgare L. and Helianthus annuus L. plants inoculated with an arbuscular mycorrhizal fungi consortium. International Journal of Phytoremediation 17(5), 405-413.
| Crossref | Google Scholar |
Bai J, Lin X, Yin R, Zhang H, Junhua W, Xueming C, Yongming L (2008) The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Applied Soil Ecology 38(2), 137-145.
| Crossref | Google Scholar |
Beleri P (2023) Microbial solutions to soil health: the role of biofertilizers in sustainable agriculture. Environmental Reports 5(2), 6-9.
| Crossref | Google Scholar |
Bhatt R, Singh P (2020) Soil fertility status of Punjab Agricultural University regional research station Kapurthala. Agricultural Research Journal 57(2), 260-265.
| Crossref | Google Scholar |
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist 220(4), 1108-1115.
| Crossref | Google Scholar | PubMed |
Ci D, Tang Z, Ding H, Cui L, Zhang G, Li S, Dai L, Qin F, Zhang Z, Yang J, Xu Y (2021) The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil. Journal of Microbiology 59, 51-63.
| Crossref | Google Scholar | PubMed |
Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S (2020) Roles of arbuscular mycorrhizal fungi on plant growth and performance: importance in biotic and abiotic stressed regulation. Diversity 12(10), 370.
| Crossref | Google Scholar |
Dobo B (2022) Effect of arbuscular mycorrhizal fungi (AMF) and Rhizobium inoculation on growth and yield of Glycine max L. varieties. International Journal of Agronomy 2022(1), 9520091.
| Crossref | Google Scholar |
Dos Passos JH, Maia LC, de Assis DMA, da Silva JA, Oehl F, da Silva IR (2021) Arbuscular mycorrhizal fungal community structure in the rhizosphere of three plant species of crystalline and sedimentary areas in the Brazilian dry forest. Microbial Ecology 82(1), 104-121.
| Crossref | Google Scholar | PubMed |
Droh G, Djezou KM, Tuo S, Touré M, Kouassi A-B (2023) Morphometric characterization of endomycorrhizal fungi (glomeraceae and acaulosporaceae) from the Bouaflé and Niellé Areas in Côte d’Ivoire. American Journal of BioScience 11(1), 1-10.
| Crossref | Google Scholar |
Duan H-X, Luo C-L, Zhu S-Y, Wang W, Naseer M, Xiong Y-C (2021) Density- and moisture-dependent effects of arbuscular mycorrhizal fungus on drought acclimation in wheat. Ecological Applications 31(8), e02444.
| Crossref | Google Scholar |
Egberongbe HO, Akintokun AK, Babalola OO, Bankole MO (2010) The effect of Glomus mosseae and Trichoderma harzianum on proximate analysis of soybean (Glycine max (L.) Merrill.) seed grown in sterilized and unsterilised soil. Journal of Agricultural Extension and Rural Development 2(4), 54-58.
| Google Scholar |
El Hazzat N, Artib M, Touati J, Chliyeh M, Selmaoui K, Ouazzani Touhami A, Benkirane R, Douira A (2018) Diversity of endomycorrhizal fungi in the rhizosphere of chickpea in Morocco. Acta Phytopathologica et Entomologica Hungarica 53(2), 181-193.
| Crossref | Google Scholar |
Ganguly R, Yun M, Lee C-S (2024) Integration of concentration gradient generator with competitive PCR in a droplet for rapid nucleic acid quantification. Process Biochemistry 147, 1-9.
| Crossref | Google Scholar |
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society 46(2), 235-244.
| Crossref | Google Scholar |
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist 84(3), 489-500.
| Crossref | Google Scholar |
Goldmann K, Boeddinghaus RS, Klemmer S, Regan KM, Heintz-Buschart A, Fischer M, Prati D, Piepho H-P, Berner D, Marhan S, Kandeler E, Buscot F, Wubet T (2020) Unraveling spatiotemporal variability of arbuscular mycorrhizal fungi in a temperate grassland plot. Environmental Microbiology 22(3), 873-888.
| Crossref | Google Scholar | PubMed |
Goswami BR, Parakhia MV, Golakiya BA, Kothari CR (2018) Morphological and molecular identification of arbuscular mycorrhizal (AM) fungi. International Journal of Current Microbiology and Applied Sciences 7(1), 2336-2347.
| Crossref | Google Scholar |
Hashem A, Kumar A, Al-Dbass AM, Alqarawi AA, Al-Arjani ABF, Singh G, Farooq M, Abd_Allah EF (2019) Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi Journal of Biological Sciences 26(3), 614-624.
| Crossref | Google Scholar | PubMed |
Hassaan MA, Alishba H, Aslam S, Danyal M, Abbas Z, Ullah A, Babar MM, Haider Z, Iqbal A (2024) Crop rotation as an economic strategy for small-scale farmers: evidence from Punjab, Pakistan. Journal of Oasis Agriculture and Sustainable Development 6(02), 31-39.
| Crossref | Google Scholar |
Hipólito-Piedras RP, Méndez-Cortés H, Ramírez-Tobías HM, Olalde-Portugal V (2024) Glomus nanolumen (Glomeraceae), un hongo micorrízico arbuscular en México. Acta Botanica Mexicana 131, e2226.
| Crossref | Google Scholar |
Houngnandan P, Yemadje RGH, Kane A, Boeckx P, Van Cleemput O (2009) Impact of agricultural practices on the dynamics of soil carbon pools in West Africa: a case study in Benin. Agricultural Systems 100(1), 38-46.
| Google Scholar |
Jacquemyn H, Merckx V, Brys R, Tyteca D, Cammue BPA, Honnay O, Lievens B (2011) Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytologist 192(2), 518-528.
| Crossref | Google Scholar | PubMed |
Khalid Chaudhry U, Shahzad S, Nadir Naqqash M, Saboor A, Yaqoob S, Salim M, Khalid M (2016) Integration of biochar and chemical fertilizer to enhance quality of soil and wheat crop (Triticum aestivum L.). PeerJ PrePrints 4, 1631v1.
| Crossref | Google Scholar |
Khan Z (2023) Root colonization and spore population of AM fungi in cultivated crops. International Journal of Science and Research Archive 10(1), 984-989.
| Crossref | Google Scholar |
Koul B, Sharma K, Sehgal V, Yadav D, Mishra M, Bharadwaj C (2022) Chickpea (Cicer arietinum L.) biology and biotechnology: from domestication to biofortification and biopharming. Plants 11(21), 2926.
| Crossref | Google Scholar | PubMed |
Krishnamoorthy R, Kim C-G, Subramanian P, Kim K-Y, Selvakumar G, Sa T-M (2015) Arbuscular mycorrhizal fungi community structure, abundance and species richness changes in soil by different levels of heavy metal and metalloid concentration. PLoS ONE 10(6), e0128784.
| Crossref | Google Scholar | PubMed |
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiology Ecology 65(2), 339-349.
| Crossref | Google Scholar | PubMed |
Liu W, Zhang Y, Jiang S, Murray PJ, Liao L, Li X, Zhang J (2019) Spatiotemporal differences in the arbuscular mycorrhizal fungi communities in soil and roots in response to long-term organic compost inputs in an intensive agricultural cropping system on the North China Plain. Journal of Soils and Sediments 19, 2520-2533.
| Crossref | Google Scholar |
Madurapperuma WS, Kumaragamage D (1999) Evaluation of AB-DTPA extractant for the estimation of plant available macro and micro nutrients in acidic and neutral soils. Journal of the Soil Science Society of Sri Lanka 11, 29-36.
| Google Scholar |
Maffo AF, Ngonkeu ELE, Chaintreuil C, Temegne CN, Ntsomboh-Ntsefong G, Fall F, Youmbi E (2022) Morphological and molecular diversity of arbuscular mycorrhizal fungi associated to Carica papaya L. rhizosphere in two agro-ecological zones in Cameroon. African Journal of Agricultural Research 18(8), 632-646.
| Crossref | Google Scholar |
Mahmoudi N, Cruz C, Mahdhi M, Mars M, Caeiro MF (2019) Arbuscular mycorrhizal fungi in soil, roots and rhizosphere of Medicago truncatula: diversity and heterogeneity under semi-arid conditions. PeerJ 7(7), e6401.
| Crossref | Google Scholar |
Makkar A, Chatli AS, Sharma A, Kaur P, Kaur N, Goswami E (2018) Analysis of soil samples from various areas of Punjab. International Journal of Research in Engineering, Science and Management 1(11), 496-498.
| Google Scholar |
Manoharan PT, Pandi M, Shanmugaiah V, Gomathinayagam S, Balasubramanian N (2008) Effect of vesicular arbuscular mycorrhizal fungus on the physiological and biochemical changes of five different tree seedlings grown under nursery conditions. African Journal of Biotechnology 7(19), 3431-3436.
| Google Scholar |
Marizal S, Syariyah A (2017) The diversity of arbuscular mycorrhiza fungus (AMF) indigenous in peanuts (Arachis hypogea L.) rhizosphere under different elevation. Journal of Tropical Soils 21(2), 109-114.
| Crossref | Google Scholar |
Mehrvarz S, Chaichi MR (2008) Effect of phosphate solubilizing microorganisms and phosphorus chemical fertilizer on forage and grain quality of barely (Hordeum vulgare L.). American-Eurasian Journal of Agricultural & Environmental Sciences 3(6), 855-860.
| Google Scholar |
Melo CD, Walker C, Krüger C, Borges PAV, Luna S, Mendonça D, Machado AC, Fonseca HMAC, Machado AC (2019) Environmental factors driving arbuscular mycorrhizal fungal communities associated with endemic woody plant Picconiaazorica on native forest of Azores. Annals of Microbiology 69, 1309-1327.
| Crossref | Google Scholar |
Meng EM (2023) Investigating the ecological role of arbuscular mycorrhizal fungi (AMF) in natural ecosystems. International Journal of Science and Research Archive 10(02), 524-534.
| Crossref | Google Scholar |
Mulyadi , Jiang L (2023) The combined application of biochar and arbuscular mycorrhizal fungi (AMF) enhanced the physical and chemical properties of soil and rice productivity in Indonesia. Sustainability 15(12), 9782.
| Crossref | Google Scholar |
Naderi NM, Alizadeh O, Nasr AH (2010) Some macro nutrients uptake optimizing by effect of mycorrhizae fungi in water stress condition in sorghum plant. In ‘Proceedings of the 2010 International Conference on Environmental Engineering and Applications,’ 10–12 September 2010, Singapore. pp. 160–164. (IEEE)
Nahar K, Bovill B, McDonald G (2021) Mycorrhizal colonization in bread wheat varieties differing in their response to phosphorus. Journal of Plant Nutrition 44(1), 29-45.
| Crossref | Google Scholar |
Ndeko AB, Chuma GB, Chokola GM, Kulimushi PZ, Mushagalusa GN (2024) Soil properties shape the arbuscular mycorrhizal status of common bean (Phaseolus Vulgaris) and soil mycorrhizal potential in kabare and walungu territories, Eastern DR Congo. Agricultural Research 13, 287-299.
| Crossref | Google Scholar |
Noreen S, Yaseen T, Iqbal J, Abbasi BA, Farouk Elsadek M, Eldin SM, Ijaz S, Ali I (2023) Morphological and molecular characterizations of arbuscular mycorrhizal fungi and their influence on soil physicochemical properties and plant nutrition. ACS Omega 8(36), 32468-32482.
| Crossref | Google Scholar | PubMed |
Ogoma BO, Omondi SF, Ngaira J, Kimani JW (2021) Molecular diversity of arbuscular mycorrhizal fungi (AMF) associated with Carissa edulis, an endangered plant species along Lake Victoria Basin of Kenya. International Journal of Forestry Research 2021(1), 7792282.
| Google Scholar |
Pellegrino E, Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biology and Biochemistry 68, 429-439.
| Crossref | Google Scholar |
Pepe A, Giovannetti M, Sbrana C (2016) Different levels of hyphal self-incompatibility modulate interconnectedness of mycorrhizal networks in three arbuscular mycorrhizal fungi within the Glomeraceae. Mycorrhiza 26(4), 325-332.
| Crossref | Google Scholar | PubMed |
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55(1), 158-161.
| Crossref | Google Scholar |
Ramakrishnan K, Selvakumar G (2012) Influence of AM fungi on plant growth and nutrient content of tomato (Lycopersicum esculentum Mill). International Journal of Research in Botany 2(4), 24-26.
| Google Scholar |
Rehman MMU, Zhu Y, Abrar M, Khan W, Wang W, Iqbal A, Khan A, Chen Y, Rafiq M, Tufail MA, Ye J-S, Xiong Y-C (2024) Moisture- and period-dependent interactive effects of plant growth-promoting rhizobacteria and AM fungus on water use and yield formation in dryland wheat. Plant and Soil 502(1), 149-165.
| Crossref | Google Scholar |
Rożek K, Rola K, Błaszkowski J, Leski T, Zubek S (2020) How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? Forest Ecology and Management 465, 118091.
| Crossref | Google Scholar |
Saeed M, Khan I, Hameed A, Ullah I, Chaudhry MS, Naveed-Ul-Haq A, Kaleem S (2020) Arbuscular mycorrhizal fungi (AMF) and soil chemical heterogeneity significantly alters nutritional value of tomato fruit. Pakistan Journal of Botany 54(1), 187-193.
| Crossref | Google Scholar |
Sajedi NA, Rejali F (2011) Effects of drought stress, Zinc application and mycorrhiza inoculation on uptake of micro nutrients in maize. Journal of Soil Research 25(2), 83-92.
| Crossref | Google Scholar |
Samanhudi YA, Pujiasmanto B, Rahayu M (2014) Application of organic manure and mycorrhizal for improving plant growth and yield of temulawak (Curcuma xanthorrhiza Roxb). Scientific Research Journal 2(5), 11-16.
| Google Scholar |
Sarah S, Burni T (2013) Symbiotic response of three tropical sorghum varieties to arbuscular mycorrhizal fungal inoculation in marginal soil. International Journal of Agriculture Innovations and Research 1(4), 116-121.
| Google Scholar |
Sasvári Z, Hornok L, Posta K (2011) The community structure of arbuscular mycorrhizal fungi in roots of maize grown in a 50-year monoculture. Biology and Fertility of Soils 47, 167-176.
| Crossref | Google Scholar |
Saurabh Jha S, Songachan LS (2023) The usage of arbuscular mycorrhizal fungi (Amf) as a biofertilizer. Available at https://doi.org/10.21203/rs.3.rs-2559546/v1
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. Journal of Plant Physiology 164(9), 1144-1151.
| Crossref | Google Scholar | PubMed |
Šmilauer P, Košnar J, Kotilínek M, Šmilauerová M (2020) Contrasting effects of host identity, plant community, and local species pool on the composition and colonization levels of arbuscular mycorrhizal fungal community in a temperate grassland. New Phytologist 225(1), 461-473.
| Crossref | Google Scholar | PubMed |
Solangi F, Zhu X, Khan S, Rais N, Majeed A, Sabir MA, Iqbal R, Ali S, Hafeez A, Ali B, Ercisli S, Kayabasi ET (2023) The global dilemma of soil legacy phosphorus and its improvement strategies under recent changes in agro-ecosystem sustainability. ACS Omega 8(26), 23271-23282.
| Crossref | Google Scholar | PubMed |
Solangi F, Zhu X, Solangi KA, Iqbal R, Elshikh MS, Alarjani KM, Elsalahy HH (2024) Responses of soil enzymatic activities and microbial biomass phosphorus to improve nutrient accumulation abilities in leguminous species. Scientific Reports 14(1), 11139.
| Crossref | Google Scholar | PubMed |
Stahl PD, Christensen M (1982) Mycorrhizal fungi associated with Bouteloua and Agropyron in wyoming sagebrush-grasslands. Mycologia 74(6), 877-885.
| Crossref | Google Scholar |
Subramanian KS, Bharathi C, Jegan A (2008) Response of maize to mycorrhizal colonization at varying levels of zinc and phosphorus. Biology and Fertility of Soils 45, 133-144.
| Crossref | Google Scholar |
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Applied Soil Ecology 26(2), 143-148.
| Crossref | Google Scholar |
Trejo-Aguilar D, Banuelos J (2020) Isolation and culture of arbuscular mycorrhizal fungi from field samples. Methods in Molecular Biology 2146, 1-18.
| Crossref | Google Scholar |
Wang Q, Ma M, Jiang X, Guan D, Wei D, Cao F, Kang Y, Chu C, Wu S, Li J (2020) Influence of 37 years of nitrogen and phosphorus fertilization on composition of rhizosphere arbuscular mycorrhizal fungi communities in black soil of northeast China. Frontiers in Microbiology 11, 539669.
| Crossref | Google Scholar | PubMed |
Wang W, Li M-Y, Zhu S-G, Khan A, Tao X-P, Huang G-F, Liu H-Y, Zhang W, Tao H-Y, Gong D-S, Song C, Xiong YC (2023) Plant facilitation improves carbon production efficiency while reducing nitrogen input in semiarid agroecosystem. CATENA 230, 107247.
| Crossref | Google Scholar |
Wu Q-S, Xia R-X, Zou Y-N (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. Journal of Plant Physiology 163(11), 1101-1110.
| Crossref | Google Scholar | PubMed |
Xia Y-S, Chen B-D, Christie P, Andrew SF, Wang Y-S, Li X-L (2007) Arsenic uptake by arbuscular mycorrhizal maize (Zea mays L.) grown in an arsenic-contaminated soil with added phosphorus. Journal of Environmental Sciences 19(10), 1245-1251.
| Crossref | Google Scholar |
Xie K, Ren Y, Chen A, Yang C, Zheng Q, Chen J, Wang D, Li Y, Hu S, Xu G (2022) Plant nitrogen nutrition: the roles of arbuscular mycorrhizal fungi. Journal of Plant Physiology 269, 153591.
| Crossref | Google Scholar | PubMed |
Yaseen T, Khan Y, Rahim F, Wali S, Ahmad I, Begum HA, Ghani SS (2016) Arbuscular mycorrhizal fungi spores diversity and AMF infection in some medicinal plants of District Charsadda KPK. Pure and Applied Biology 5(4), 1176-1182.
| Crossref | Google Scholar |
Zhang M, Shi Z, Yang M, Lu S, Cao L, Wang X (2021) Molecular diversity and distribution of arbuscular mycorrhizal fungi at different elevations in Mt. Taibai of Qinling Mountain. Frontiers in Microbiology 12, 609386.
| Crossref | Google Scholar | PubMed |
Zhao J, Chen J, Beillouin D, Lambers H, Yang Y, Smith P, Zeng Z, Olesen JE, Zang H (2022) Global systematic review with meta-analysis reveals yield advantage of legume-based rotations and its drivers. Nature Communications 13(1), 4926.
| Crossref | Google Scholar |