Green-synthesized ZnO and MgO nanoparticles modulate physiology and antioxidant defense in maize under alkaline stress
Muhammad Iftikhar A * and Anis Ali Shah
A * and Anis Ali Shah  A *
A *
A
Abstract
Alkaline stress severely impairs the growth and yield of Zea mays L. by disrupting physiological and biochemical functions. This study evaluated green-synthesized ZnO and MgO nanoparticles (NPs), prepared using neem and licorice extracts, for mitigating alkaline stress. NPs were nanosized, crystalline, and functionalized by phytochemicals, confirmed by scanning electron microscopy, FT-IR spectroscopy, UV-vis spectroscopy, and energy dispersive X-ray spectroscopy. A pot experiment using NPs (25–200 ppm) under control and alkaline stress assessed morphological, physiological, biochemical, and ionic responses. Alkaline stress reduced root fresh and dry weight to 2.60 and 0.66 g (−59.6%, −31.0%), shoot fresh and dry weight to 2.60 and 0.38 g (−59.6%, −70.0%), and chlorophyll a, b, and carotenoids to 1.31, 0.67, and 2.40 mg g−1 (−62.4%, −54.7%, −62.8%), whereas it increased malondialdehyde (MDA) (244.6%), H₂O₂ (457.7%), and relative membrane permeability (RMP) (55.9%). The combined ZnO (50 ppm) and MgO (50 ppm) treatment improved chlorophyll a, b, and carotenoids to 3.48, 1.48, and 6.45 mg g−1 (165.4%, 120.3%, 168.5%), and total soluble protein (392.8%), total protein (301.0%), proline (105.5%), glutathione (35.6%), and ascorbic acid (44.2%). Antioxidant enzymes increased, with superoxide dismutase at 29.52 U mg−1 (452.8%), peroxidase at 24.44 U mg−1 (862%), and ascorbate peroxidase at 51.62 U mg−1 (560%), whereas MDA, H2O2, and RMP (−78.1%) were reduced. High NP concentrations (ZnO 100 ppm + MgO 100 ppm) were toxic. Moderate ZnO and MgO NP doses enhanced resilience, yield stability, and sustainable agriculture.
Keywords: alkaline stress, antioxidant enzymes, MgONPs, plant physiology, stress markers, Zea mays L., ZnONPs.
Introduction
Soil pH is a critical indicator of nutrient bioavailability, and alkaline soils (pH ≥ 7.0) significantly impair crop productivity due to elevated sodium carbonate and bicarbonate levels (Pérez-Labrada et al. 2024). These soils occur commonly in regions that are mostly arid and semi-arid, where evaporation leads to salt deposition in the soil (Masood et al. 2023; Baloch et al. 2025). Despite their prevalence, the role of nanoparticles, particularly zinc oxide (ZnONPs) and magnesium oxide nanoparticles (MgONPs), in mitigating alkaline stress in plants remains underexplored. This study addresses this gap by examining the physiological, morphological, and antioxidant responses of Z. mays L. under alkaline stress in the presence of these nanoparticles.
High soil pH, especially in Pakistani soils, is a major limiting factor for nutrient availability due to the occurrence of excess CaCO3 and Na2CO3 (Hassan et al. 2022; Khan et al. 2023; Murtaza et al. 2023). In regions such as Punjab and Sindh, soil pH often ranges from 8.1 to 10.4, hindering nutrient uptake and necessitating effective soil amendment strategies (Jamil et al. 2021). Nanoparticles offer promising potential in this regard due to their nanoscale properties and high reactivity (Dutta et al. 2024). About 38% of soils in Tando Allahyar, Sindh, are strongly alkaline, where the soil pH levels range between 6.2 and 8.6 (Jamali et al. 2023). Most soils in the Pakistani Punjab are alkaline, with a median soil pH of 9.2 and it varies between 8.1 and 10.4. In Muzaffargarh district, Punjab, Pakistan, 75% of samples had a soil pH ranging from 8.5 to 9.0 (Akram et al. 2014).
Ion transport is essential for nutrient absorption in plants, but under alkaline stress, imbalances arise. Excess Na+ can inhibit uptake of K+, PO43−, and Ca2+, whereas Cl− suppresses NO3− and H2PO4− uptake, exacerbating nutrient deficiency. Alkaline salts (NaHCO3 and Na2CO3) induce greater damage than neutral salts by disrupting ion homeostasis and photosynthesis (Wang et al. 2022a). Under such stress, the root system is the first responder, transmitting signals to aerial plant parts. This makes the analysis of leaf area, root length, and photosynthetic rate particularly relevant in alkali-stress studies (An et al. 2021).
ZnONPs and MgONPs have shown potential to alleviate alkaline stress by improving photosynthesis, antioxidant enzyme activity, and nutrient uptake. Their nanoscale size facilitates enhanced interaction with plant tissues, supporting stress resilience (Singh et al. 2024a). Despite the growing interest in their agricultural applications, comprehensive evaluations under alkaline stress are lacking. Zinc functions as a crucial antioxidant, involved in reactive oxygen species (ROS) scavenging and metallothionein production (Mierek-Adamska et al. 2025). Studies have demonstrated that ZnONPs reduce effects of salinity on plant height, biomass, and grain yield in Z. mays (Segatto et al. 2023; Ahmed et al. 2025). However, concentrations must be carefully managed; excessive ZnONPs (800 mg kg−1) have shown phytotoxic effects (Tabande et al. 2021).
Similarly, MgONPs enhance protein synthesis, enzyme function, and chlorophyll stability, as Mg2+ is essential for over 300 enzymes and a significant component of chloroplasts (Kaur et al. 2022). Mg also aids in the root uptake of C, N, and S, playing a role in nutrient translocation (de Sousa Ferreira et al. 2023). However, high-pH soils may hinder magnesium availability due to MgCO3 precipitation and ion interactions (Broadley and White 2010). Field studies have shown that Mg2+ application significantly improves plant height, grain yield, and biomass in calcareous soils (Ahmed et al. 2023), yet MgONPs remain underexplored for their ability to help plants cope with alkaline stress, especially in maize.
Z. mays (maize) is a food, feed, and industrial crop of global relevance that supports food, feed, and industrial sectors, such as pharmaceutical and nutraceutical use (Deepak and Jayadeep 2022). Maize grown in the arid or semi-arid areas of Punjab, where alkaline soils are also abundant in Pakistan, is a highly-planted crop (Nadeem et al. 2022; Taj et al. 2023). These soils have reduced access to nutrients and decreased water retention, which affects important physiological functions and biochemical processes, ultimately limiting the yield of crops (Rathod and Verma 2023). In addition, alkaline stress is an unexplored abiotic limitation of economic and nutritional importance, making it necessary to develop mitigation measures that are efficient and sustainable.
Conventional strategies such as organic amendment and chemical fertilizer have proved not effective enough in terms of poor nutrient mobility, cost, and environmental-related issues (Zhang et al. 2022; Proshad et al. 2024). Thus, current breakthroughs in nanotechnology provide possible opportunities to manage crops sustainably. Specifically, green-synthesized ZnONPs and MgONPs can have a high surface area, and are biocompatible and less phytotoxic than traditional formulations or nanomaterials such as CuO and TiO2 (Du et al. 2024; Syed et al. 2024). This enables them to penetrate through the foliage, increase nutrient uptake in alkaline soils, and improve the release of nutrients in a controlled fashion, minimizing leaching and environmental hazard (Ahmad et al. 2024; Alves et al. 2024).
Moreover, zinc and magnesium are the most necessary micronutrients involved in the role of membrane stabilization, chlorophyll biosynthesis, and enzyme activation activity that is significant to tolerate stress conditions (Ahmed et al. 2023). The application of ZnONPs and MgONPs has been reported to promote growth, physiology, and the antioxidant defenses of plants against salinity and drought stress (Segatto et al. 2023; Seleiman et al. 2023a; Inam et al. 2024), but their combined application in maize grown in alkaline soil conditions has not been investigated. This study addresses that gap by hypothesizing that alone and combined, ZnONPs and MgONPs can improve maize tolerance by enhancing nutrient uptake, maintaining physiological stability, and upregulating antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)) to mitigate oxidative stress. This work aims to establish an eco-friendly, nanotechnology-based approach to mitigate alkaline stress in maize, contributing to improved yield stability. Collectively, this eco-friendly nano-intervention represents a novel and sustainable strategy to strengthen maize resilience, stabilize yields, and support food security in alkaline soil agroecosystems.
Materials and methods
Green synthesis of ZnONPs
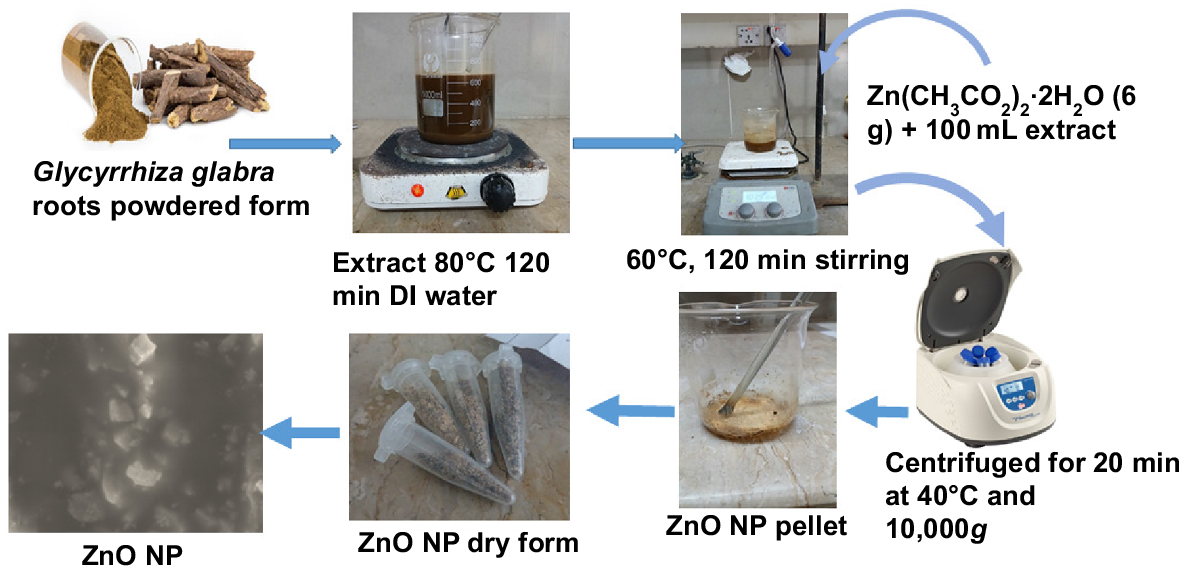
Plant extract was prepared by followed the protocol of Wittschier et al. (2009). After cleaning the roots of Glycyrrhiza glabra with purified water, they were dried in a shady area for a few days. Once the roots had dried, they were turned into a powder using a mortar and pestle. Ten grams of powdered roots was accurately weighed on a digital balance. A conical flask was used to combine 100 mL of deionized (DI) water with the powder which was then heated to 80°C and stirred continuously for 2 h. Control of the temperature was a major concern as the object was being heated. The cooled solution was poured through Whatman filter paper three times to eliminate any small particles. The pH of the solution after filtration was tested and noted to make sure it was suitable for the chemical reaction. The filtered extract was kept at 4°C for later use (Fig. 1).
The ZnONPs were synthesized following the protocol of Ogunyemi et al. (2020) (Fig. 1). The process of synthesizing ZnONPs first involved dissolving 6 g of zinc acetate dihydrate (from Sigma-Aldrich) in 100 mL of G. glabra root extract. The mixture was stirred for 2 h at 60°C to promote the reaction. NaOH (1 mol L−1) was added dropwise to the solution to bring the pH to 7.5, the perfect pH for making nanoparticles. A pale white color in the solution indicated that ZnONPs had been produced. The reaction mixture was centrifuged for 20 min at 10,000g and 40°C to collect the nanoparticles. The supernatant was discarded and the pellet washed twice using double-distilled water by centrifuging at 10,000g for 15 min to remove impurities. The nanoparticles were placed in a furnace and heated from 100 to 500°C for 2 h at a rate of 3°C min−1 to remove all remaining organic materials. The dried nanoparticles were then ground in a mortar and pestle to obtain a fine powder, ready for further exploration and uses. G. glabra (licorice) root extract contains many different phytochemicals. Natural reducing agents include phytochemicals that are flavonoids, phenolics, and glycyrrhizin. They donate electrons to zinc ions (Zn2+), to turn them into ZnONPs. This procedure not only avoids the use of harsh chemical reductions but also renders the synthesis environmentally friendly (Upadhyay et al. 2020). Phenolic compounds are adsorbed on the surface of the newly prepared ZnONPs as capping reagents. This inhibits aggregation, regulates the distribution of particle size, and increases the stability and dispersibility of the nanoparticle (Hasan et al. 2021). Since G. glabra contains strong antioxidants, phytochemicals help reduce the free radicals formed when infusing a nanoparticle, additionally stabilizing them and extending their biocompatibility (El-Saber Batiha et al. 2020).
Green synthesis of MgONPs
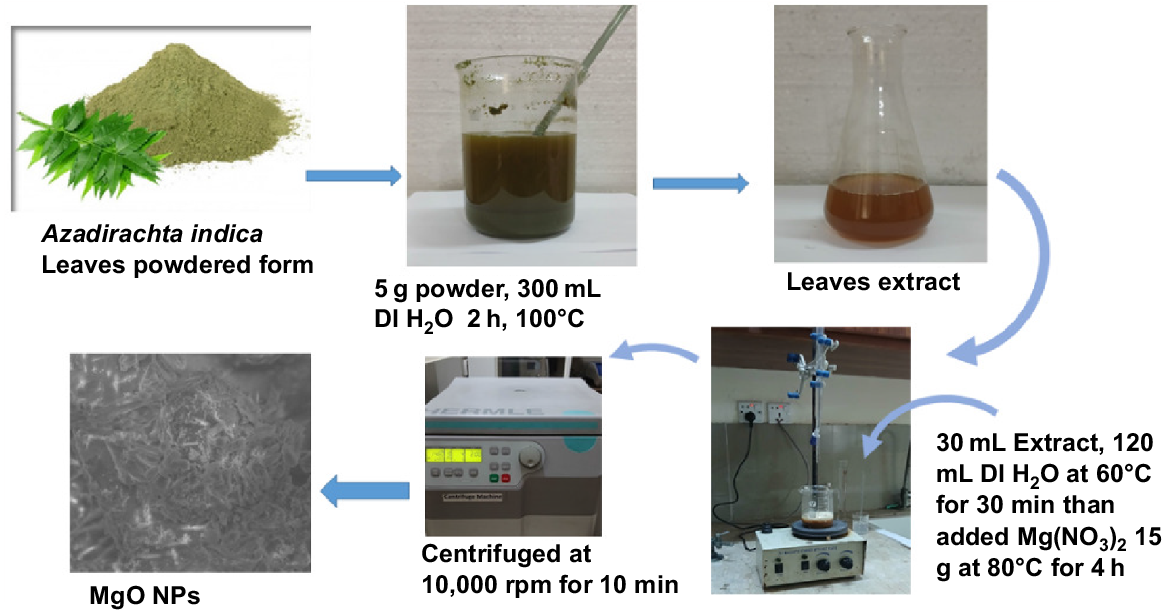
The plant leaf extract was prepared following the procedure of Akhtar et al. (2024). The leaves of Azadirachta indica (neem) were collected, fully cleaned with a solution of 1% sodium hypochlorite, and allowed to dry. Distilled water was used to rinse the leaves and they were left to dry in shade for 8 days. When the leaves were dry, they were finely ground with a mortar and pestle. Five grams of powdered neem leaves were added to 300 mL of DI water in a conical flask and boiled for 2 h at a temperature of 100°C. While heating the solution, its pH was constantly observed and set to pH 7.4. After boiling, the mixture was passed through Whatman filter paper three times to eliminate any noticeable debris. After the extraction, a yellowish-brown mixture was obtained and stored for the formation of MgONPs (Fig. 2).
The MgONPs were synthesized using the process that Al-Harbi et al. (2024) described with minor modifications (Fig. 2). A 500 mL conical flask was filled with 30 mL of fresh A. indica leaf extract and 120 mL of DI water. The flask was then heated to 60°C for 30 min. After adding 30 g of Mg(NO3)2 (Fischer Scientific, purity 99.5%), the mixture was heated to 80°C for 4 h while being constantly stirred. NaOH (1 mol L−1) was then gradually added until the pH of the solution was 7.4. The solution’s yellow color changed to yellowish-brown, indicating the synthesis of MgONPs. The extract of Azadirachta indica (neem) leaves has a high concentration of phytochemicals, which are essential for the green synthesis of magnesium MgONPs. Polyphenols and other phytochemicals in green A. indica leaves act as natural reducing agents. They also donate electrons to magnesium ions (Mg2+) and turn them into MgONPs without using harsh reagents (Silva et al. 2022). The phytochemicals, flavonoids, tannins, and other antioxidants, are present on the surface of the MgONPs (Silva et al. 2022). This capping action avoids aggregation, regulates the size of the particles, and increases the stability and homogeneity of the nanoparticles (Chatepa et al. 2024). The MgONPs are small, uniform, and highly crystalline, and their shape, size, and dispersity depends on the particular composition and concentration of the phytochemicals in the leaf extract (Ikhuoria et al. 2024).
Various advanced techniques were used to investigate the structure, appearance, and composition of the synthesized ZnONPs and MgONPs. The nanoparticles were analyzed by UV-vis absorption spectroscopy within the range of 200–800 nm. Unique absorption peaks in the UV-Vis spectrum confirmed that the nanoparticles were formed (Kumar et al. 2022). Scanning electron microscopy (SEM) was used to examine the surface appearance and size of the nanoparticles. The high-quality images revealed the shape, size, and grouping of the nanoparticles (Faisal et al. 2022). The elemental composition of the nanoparticles was determined by energy-dispersive X-ray (EDX) analysis. The use of this technique revealed that the nanoparticles held zinc and magnesium (Kangathara et al. 2022). FTIR spectroscopy (500–4000 cm−1) was used to study both the functional groups and the chemical bonds in the synthesized nanoparticles. It allowed determination of any functional groups on the nanoparticles that could connect with plant cells (Seghir et al. 2023).
Soil collection and analysis
A detailed soil analysis was done before starting the experiment to determine the soil’s physical and chemical properties. The soil was tested with a multiparameter meter (HI 2211 from China) to make sure the pH was within the alkaline range. The ETL-01 EC meter was used to measure the soil’s electrical conductivity (EC). The method outlined by Abbasi Surki et al. (2020) was used to determine the soil organic content. A method set by Ni et al. (2021) and Johnson-Beebout et al. (2009) was applied to test whether essential nutrients such as calcium, magnesium, and zinc were readily available and measured their available amount. The molybdenum–antimony anti-colorimetric method was used to assess phosphorus, and potassium was measured by a flame photometer using the ammonium acetate method.
Nitrogen in the soil was released by alkali hydrolysis and measured. The CEC (cation exchange capacity) was evaluated in this study by using neutral ammonium acetate for extraction (Wang et al. 2021). The total alkalinity, carbonates, and bicarbonates were all tested by using the titration method mentioned by Alexakis et al. (2012) (Table 1).
| Parameters | Control soil (pH 7.0) | Stress soil (pH 8.9) | |
|---|---|---|---|
| pH | 7.0 | 8.9 | |
| EC (dS m−1) | 0.32 | 2.33 | |
| Organic matter (%) | 3.4 | 0.56 | |
| CEC (cmol kg−1) | 6 | 1.81 | |
| Available Phosphorus (mg kg−1) | 5.78 | 4.5 | |
| Available Potassium (mg kg−1) | 98 | 56 | |
| Available Na+ (mg kg−1) | 23 | 164 | |
| Available nitrogen (mg kg−1) | 18 | 9 | |
| Available Ca2+ (mg kg−1) | 8.2 | 12 | |
| Available Mg2+ (mg kg−1) | 11.7 | 6 | |
| Available Zn (mg kg−1) | 4.3 | 1.92 | |
| Total alkalinity | <1.6 | >3.5 |
Parameters: pH, electrical conductivity (EC), organic matter, cation exchange capacity (CEC), and availability of macro- and micronutrients reflect the growing conditions of Z. mays L. in soil.
First, ZnONPs and MgONPs were weighed using a weight balance to achieve various final concentrations of 25, 50, 75, 100, 125, 150, and 200 ppm with great accuracy. The nanoparticles were applied to the soil without being mixed in water, to prevent clumping and coagulation in the soil. The soil was divided into three parts to ensure it could be mixed well. For each layer, a third of the weighed nanoparticles to give the required concentration was sprinkled over the top of the soil which was then stirred with sterilized tools and hands wearing gloves. After that, another layer of soil was added and more nanoparticles were mixed with it until everything was well-mixed again. Last, the third layer of soil was added and the remaining nanoparticles were evenly mixed into it. The soil was mixed for a further 15 min to spread the nanoparticles evenly throughout it.
Two experimental groups were established, the alkaline stress group and the control group. Each group consisted of five replicates, with the alkaline stress group treated with soil from a field with an alkaline pH of 8.9 and nanoparticles (ZnONPs and MgONPs, both individually and in combination) as per the treatment plan (Table S2). The control group was planted in healthy soil with a neutral pH of 7.0 and treated with nanoparticles in the same manner to provide a baseline for comparison. The mixing process was performed in a controlled environment at room temperature to minimize any external environmental influences. The soil was added to the pots in small portions to avoid over-compaction, which could reduce aeration and negatively affect root growth. Each pot was filled with a standardized amount of soil to ensure uniform depth across all experimental units. The soil samples were then stored for subsequent plant physiology and antioxidant analyses, which were conducted to assess the broader physiological and biochemical impacts of the nanoparticle treatments.
The experiment was done using Z. mays L. (DK-9108 variety) seeds purchased from Bayer distributing center in Lahore, Pakistan. The experiment was conducted in the botanical garden of the University of Education, Lahore, Pakistan. One hundred and sixty pots were included in a completely randomized design (CRD) and each group received five replicates. The soil in the experiment came from Tehsil Chunian in District Kasur, Punjab, Pakistan, where the soil pH is 8.9. Before planting, important properties of the soil such as pH, EC, organic content, and nutrient availability were tested (Table 1).
The maize seeds were cleaned by placing them for 5 min in a solution consisting of 0.1% mercuric chloride to kill any harmful organisms on the surface. After sterilizing the seeds, they were thoroughly cleaned with distilled water and those that looked healthy were picked out. When the soil was prepared, the seeds were sown in the pots. It took about 1 week for the seeds to germinate and 2 weeks later, the plants were thinned so that three survived with uniform distance in each pot for even growth. The areas in which the experiment was performed were under normal environmental conditions, 18°C average temperature, 65% humidity, and 23 mm of rain throughout the experiment. ZnONPs and MgONPs were applied at certain doses, as reported in the results and mentioned in Table S2. The experiment was conducted to test if using ZnONPs or MgONPs could help maize plants to withstand the damage from high pH stress.
After 25 days of plant growth (Fig. 3), one plant from each replicate was selected for morphological analysis. Root, shoot, and leaf lengths were measured using a calibrated scale to evaluate the overall growth of the plants. The root and shoot were then carefully separated using a sharp cutter to minimize any damage, and their fresh weights were recorded using an electronic balance. To determine the dry weight, the plant samples were placed in an oven set at 75°C for 24 h or until they reached a constant weight. After drying, the dry weight of the root and shoot were measured again using an electronic balance.
Growth variation of Zea mays L. plants grown under alkaline stress with different treatments of ZnONPs and MgONPs both alone and combined.
These morphological attributes were measured for both the alkaline stress group and the control group. This comparison allowed for an evaluation of how ZnONPs and MgONPs influenced plant growth under alkaline stress (pH 8.9) compared to normal soil conditions (pH 7.0). The plant samples were collected and stored at −80°C to preserve their biochemical integrity for subsequent physiological assessments and antioxidant analyses. These plants were used to assess the further biochemical and stress tolerance responses to the nanoparticles under these conditions.
An LCpro-SD Infrared Gas Analyzer (IRGA) was used to assess the Z. mays L. leaves photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (tr), and intercellular CO2 concentration (Ci). Each replicate’s enlarged young leaf was put inside the open leaf chamber. The jaws of the chamber were then closed. After the following modifications, readings were obtained: the leaf surface area was 6.25 cm2, the ambient CO2 concentration (Cref) was 430 μmol mol−1, the leaf chamber’s molar gas flow rate (U) was 200 μmol−1, the leaf chamber’s temperature (Tch) ranged between 23 and 28°C, the ambient pressure (P) was 998 kPa, and the photosynthetically active radiation (PAR/Qleaf) at the leaf surface peaked at 926 μmol m−2 s−1 (Fernandes et al. 2021; Roque et al. 2022).
An OS-30p+ chlorophyll fluorimeter was used to detect the variable fluorescence (Fv), maximal fluorescence (Fm), maximum quantum yield of photosystem II (PSII) (Fv/Fm), and minimal fluorescence (Fo).
Before readings were taken, each fully grown leaf was positioned between the clips of the apparatus to allow for 20 min of dark adaptation. After 25 days of growth, this process was also carried out in full sunshine during the day (Arrobas et al. 2017).
A renowned procedure was used to measure the amount of chlorophyll at the tillering stage (Arnon 1949). Fresh leaves were taken from each pot using an analytical scale and weighed equally up to 0.5 g. Using a mortar and pestle, leaves were crushed in 10 mL of 80% acetone solution. Whatman Grade 42 paper was used to filter off the solid material. After that, the filtrate was stored in the refrigerator for 24 h at 4°C. The absorbance was measured in a quartz cuvette, using a double-beam UV-vis spectrophotometer (Metash-Model UV-9000), measurements were made at 480, 645, and 663 nm.
For chlorophyll a, b, total chlorophyll, and carotenoid determination, the below formulas were used:
where V is the volume, W is the weight, and OD is optical density at the indicated wavelength.
The Jones and Turner (1978) method was used to assess the RWC of the leaves. Each replicate’s leaf size was the same, and an electronic balance was used to record the fresh weight (FW) of each leaf. The leaves were placed directly in distilled water in Petri plates, allowing them to soak thoroughly in water. The leaf turgid weight (TW) was calculated after 3 h of room temperature storage in a dark area. After that, the leaves were incubated for 24 h at 80°C to determine their dry weight (DW).
The following formula was used to determine the RWC:
The method of Yang et al. (1996) was used to calculate the RMP. The size of the young and fully-grown leaves removed from each replicate was uniform. After being cut into small pieces, the leaves were put in test tubes with 20 mL of distilled DI water.
After vortexing the test tubes for 10 s, an electrical conductivity meter (Hanna HI-9811-5 EC portable meter) was used to measure the initial electrical conductivity (EC0). After being wrapped in aluminum foil, the test tubes were stored in a refrigerator at 4°C for 24 h before being examined for EC1. To find the EC2, these samples were covered and autoclaved for 20 min at 121°C.
The calculation of RMP was as follows:
Biochemical attributes
The Bradford (1976) methodology was used to examine the amount of soluble proteins. After 500 mg of leaves were weighed, they were finely powdered in 10 mL of 50 mM potassium phosphate buffer, with a pH of 7.8 at 4°C. The ground material was centrifuged for 20 min in a cool environment at 6000g using a Hermle Z 326 K. Bradford reagent (100 mg of Coomassie brilliant blue, 100 mL of 85% phosphoric acid, and 50 mL of 95% ethanol) was then synthesized as a dissolving reagent with distilled water added to obtain a volume of 1000 mL. A double-beam UV-vis spectrophotometer (Metash-Model UV-9000) was used to measure the OD at 595 nm after mixing 2 mL of Bradford reagent and 100 μL of plant extract.
The Kamath et al. (2015) method was used to evaluate phenolic proportions. Using a mortar and pestle, 50 mg of fresh leaves from each replicate were crushed using 5 mL of 80% acetone solution. The homogenized mixture was centrifuged for 10 min at 10,000g at 4°C. A 0.1 mL of clear supernatant was taken, diluted with 1 mL of Folin-Ciocalteau’s phenol reagent and 2 mL of distilled purified water, and shaken vigorously. Finally, 5 mL of 20% Na2CO3 was added, and 10 mL of distilled pure water made up the entire volume. After the mixture had been stirred, the OD was measured at 750 nm using a double-beam UV-vis spectrophotometer (Matesh-Model UV-9000). The results were expressed in milligrams per kilogram of fresh leaves.
The Bates et al. (1973) method was used to calculate proline. The process involved homogenizing 0.5 g of leaves in 10 mL of sulfuric acid at a concentration of 3%. The homogenate was filtered using Whatman filter paper. The acid ninhydrin reagent was formed by combining 1.25 g of ninhydrin with 30 mL of glacial acetic acid and 20 mL of orthophosphoric acid (6 M, 20 mL). After dissolving 2 mL of homogenate filtrate in 2 mL of glacial acetic acid and ninhydrin acid in a glass tube, the sample solution was incubated at 100°C for 60 min. Subsequently, the liquid was placed in an ice bath. After centrifuging the sample mixture at 10,000g for 10 min at 4°C, 2 mL of the supernatant was extracted. A steady stream of air was passed across the reaction mixture while 4 mL of toluene was added for 1–2 min. The chromophore was extracted from the upper aqueous phase. Using toluene as a blank, the absorbance was measured at 520 nm using a UV-vis spectrophotometer.
The method of Irigoyen et al. (1992) was slightly modified to determine the amount of soluble sugar in leaves. Solutions that contained 0, 20, 40, 60, 80, and 100 g mL−1 of sucrose were used to prepare standards. After adding 0.5 mL of anthrone ethyl acetate solution and 5 mL of sulfuric acid to a total of 0.5 mL of each standard solution, the mixture was heated for 1 min in a water bath. After the sample had cooled to room temperature the absorbance was measured at 630 nm (25–28°C) by a UV-visible spectrophotometer, and a standard curve was produced. Leaf extracts were prepared using 0.3 g of fresh leaf segments, which were placed in a centrifuge tube with 10 mL of distilled water and heated for 30 min. The extracts were then filtered through two layers of cheesecloth A 0.5 mL aliquot of the filtrate was treated as above for the standards and used to measure the absorbance at 630 nm. The standard curve was used to estimate the amounts of soluble sugar. After deducting the water content from the leaf weight (FW), the sugar content was reported as milligrams of sucrose equivalents per gram of DW.
One milliliter of lactose-containing MCW (methanol/chloroform/water) extraction buffer was combined with 100 mg of frozen leaf tissue that had been finely powdered. After being moved to a microcentrifuge tube, this mixture was incubated for 30 min in a water bath at 50°C. Following incubation, the material was centrifuged at room temperature for 5 min at 14,000g. The extracted soluble sugars were present in the resultant supernatant, which was pipetted into a 15 mL polypropylene conical tube with a label and kept on ice. Three extractions were performed by repeating this procedure twice more using fresh MCW buffer that was lactose-free. The pellet left over after the extractions was employed to determine insoluble (starch) substances rather than to analyze soluble sugars.
The starch was also measured using the pellet that was produced during the extraction of soluble sugar. The tissue pellet was re-suspended in 330 mL of 100% dimethyl sulfoxide (DMSO). The reconstituted sample was then submerged in a boiling water bath for 5 min. A 50 μL aliquot of the starch slurry was combined with 950 μL of 100 mM sodium acetate buffer (pH 5.0) in a fresh microcentrifuge tube to create a 1:20 dilution of the solubilized starch solution. To the diluted starch solution, 100 μL of an α-amylase working solution was added. After quickly vortexing the liquid to ensure complete mixing, it was incubated for 15 min in a boiling water bath. Based on the glucose generated during the enzymatic reaction, the absorbance at 340 nm was applied to calculate the starch concentration (Leach and Braun 2016).
Following the methodology of Miller (1959), the α-amylase activity was measured by combining 0.25 mL of the suitably diluted enzyme with 0.5 mL of 0.2 M acetate buffer (pH 5.0) and 1.25 mL of soluble starch (1%). The concentration of glucose released from starch by α-amylase was measured spectrophotometrically at 575 nm after 10 min of incubation at 50°C. The amount of enzyme that releases 1 μmol of reducing sugar (glucose) per minute under assay conditions was known as one international unit (IU) of α-amylase activity. The results were represented as IU per gram of dry substrate (IU g−1 ds).
Ten leaves from each replication plant were ground with a pestle and mortar after being oven-dried for 3 days at 500°C. After three extractions using 10 mL 80% aqueous ethanol, the tissues were extracted 5 mL distilled water and centrifuged at 5000g. Samples were centrifuged at 3500g following active charcoal treatment, and the supernatant was kept at −80°C for storage. A glucose HK test kit and a MultiScan Ascent Microplate Reader were used to measure sucrose and hexose. After being incubated at 50°C for 40 min, a 10 μL extract aliquot was combined with 10 μL water and 100 μL glucose assay reagent, and it was then incubated at 30°C for 15 min. The absorbance was measured at 340 nm. After adding 0.25 phosphoglucose isomerase enzyme units, the absorbance was measured once more. This was followed by the addition of 83 invertase enzyme units, 60 min of incubation at 30°C, and a final absorbance measurement at 340 nm (Opsahl and Benner 1999).
The Cakmak and Horst (1991) description of the thiobarbituric acid (TBA) reaction was used to evaluate the MDA concentration. A 0.5 g sample of fresh leaves was homogenized with 5% trichloroacetic acid (TCA) and centrifuged for 10 min at 4000g. After mixing 2 mL of extract with 2 mL of 0.6% TBA, the mixture was put in a boiling water bath for 10 min. The absorbance at 532, 600, and 450 nm was then measured.
The following formula was used to determine the MDA content:
The hydrogen peroxide content was determined using the Velikova et al. (2000) technique. Fresh, fully grown leaves were removed from each sample, and 0.5 g was weighed. After the leaves were ground in a prechilled mortar and pestle, the sample was put into a conical tube and combined with 5 mL (0.1%) of TCA. A similar process was performed for each sample. After that, the extracts were centrifuged for 15 min at 4°C and 12,000g. Using a pipette, a 0.5 mL aliquot of the supernatant was removed and 0.5 mL of phosphate buffer at pH 7 and 1 mL of potassium iodide were added. A UV-vis spectrophotometer was used to take readings at 390 nm after each mixture was vortexed.
EL was calculated following Dionisio-Sese and Tobita (1998). First, 20 leaf discs were placed in deionized water and examined for electrical conductivity (ECa). The electrical conductivity (ECb) of the samples was then measured after the test tubes containing the leaf discs were incubated in a water bath at 50–60°C for 25 min. The electrical conductivity (ECc) of these test tubes was then measured after they were boiled for 10 min at 100°C.
The following formula was used to determine the EL:
According to Law et al. (1983), leaf samples (0.5 g) were homogenized with 10% (w/v) TCA (5 mL) and centrifuged at 15,000g for 15 min to measure reduced glutathione (GSH). A 150 μL aliquot of supernatant was combined with 100 μL of 6 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), 50 μL of glutathione reductase (10 units mL−1), and 700 μL of 0.3 mM NADPH. The concentration of total glutathione was calculated using a standard curve with UV-vis 412 nm. At pH 7.5, all of the reagents were made in 125 mM NaH2PO4 buffer with 6.3 mM EDTA.
After crushing 1 g of leaf in 5% TCA, it was centrifuged at 15,000g for 15 min. Plant homogenate (0.2 mL), phosphate buffer pH 7.4 (0.6 mL, 0.2 M), TCA (1 mL, 10%), H3PO4 (0.8 mL, 42%), α,α′-dipyridyl (0.8 mL, 4%), and FeCl3 (0.4, of 3%) made up the reaction mixture. Instead of using plant material, 0.2 mL of TCA (5%) was utilized as a blank. The OD was measured at 525 nm. A calibration curve created using standard solutions was used to determine AsA (Łukasik et al. 2021).
Leaves were collected from each replicate, and 500 mg of fresh leaf tissue was finely ground in 10 mL of 50 mM potassium phosphate buffer (pH 7.8) at 4°C. The homogenate was centrifuged at 10,000g for 10 min at 4°C, and the resulting supernatant was used for the SOD assay. The reaction mixture comprised 50 mM phosphate buffer (pH 7.8), 20 μM riboflavin, 75 mM nitro blue tetrazolium (NBT), 13 mM methionine, and 0.1 mM EDTA. For 10 min, the mixture was exposed to two fluorescent light tubes at a rate of 40 μmol m−1 s−1. The absorbance was measured at 560 nm using a UV-vis spectrophotometer. Additionally, blank and controls were performed in the same way, but without enzyme and illumination, respectively. One unit of SOD activity was defined as the amount of SOD activity that produced half-maximal inhibition of NBT decrease (Giannopolitis and Ries 1977).
To measure APX activity, a different buffer was made. In summary, 50 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM sodium ascorbate, 0.1 mM H2O2, and 50 μL of enzyme extract and 500 mg of fresh leaf tissue extract were all present in the reaction mixture (1 mL). Using the method of Nakano and Asada (1981), APX activity was measured by observing a decrease in absorbance at 290 nm caused by the oxidation of ascorbic acid. The absorbance was measured every 30 s after the addition of H2O2 for 2 min.
Similarly, 0.1 mL of leaf extract of 500 mg fresh leaves extract, 0.6 mL of 20 mM guaiacol, 0.7 mL of 50 mM phosphate buffer (pH 5.0), and 0.6 mL of 40 mM H2O2 were combined to create a solution for the POD determination. The absorbance of POD was measured using a spectrophotometer at a wavelength of 470 nm every 30 s for 150 s (Chance and Maehly 1955).
A 0.1 mL sample of leaf extract, 1.9 mL of 5.9 mM H2O2, and 1 mL of 50 mM phosphate buffer (pH 7.0) were mixed to create a solution in order to measure the CAT activity. Over a period of 120 s, the catalase absorbance was measured at 240 nm every 30 s (Chance and Maehly 1955).
Following the collection of dried shoot and root samples, they were thoroughly ground in a mortar and pestle. Root and shoot samples, each weighing 0.1 g, were digested using H2SO4. Samples were put into test tubes, and then 2 mL of H2SO4 was added. After a day, samples were heated on a hot plate and then progressively combined with H2O2 until the liquid lost its color. This was followed by the addition of distilled water to a final volume of 50 mL. After this combination was filtered, Na+, K+, and Ca2+ levels were measured using a flame-photometer (Ward and Carpenter 2010).
Statistical analysis
Data were compiled and organized using Microsoft Excel (Microsoft 365). Statistical analyses were performed in R software (RStudio, Ver. 2024.09.0+375). Data normality was verified using the Shapiro–Wilk test (shapiro.test()), and the homogeneity of variances was assessed using Levene’s test (leveneTest() from the car package). Three-way analysis of variance (ANOVA) was conducted using the aov() function, and mean comparisons were performed with Duncan’s multiple range test (duncan.test() from the agricolae package) at a significance level of P < 0.05. Pearson’s correlation analysis was carried out, and principal component analysis (PCA) was conducted using the prcomp() function. Results are presented as mean values of five replicates ± standard error.
Results
FTIR of MgONPs
The FTIR spectrum of MgONPs prepared using neem leaf extract exhibits a mixture of signals coming from both the inorganic MgO core and the organic biomolecules that are part of the extract. The clear peak at 560 cm−1 confirms the unequivocal existence of MgO (Fig. 4a). These results were reinforced by similar findings in the literature Shanmugam et al. (2023) present confirmation of MgONPs by FTIR peaks from 601 to 890 cm−1. It is noteworthy that MgONPs display a characteristic peak at ~407 cm−1. Kiran et al. (2023) observed a stretching frequency peak at 550 cm−1, which confirms the Mg–O bond.
Characterization of MgONPs synthesized using Azadirachta indica leaf extract: (a) FTIR, (b1, b2) EDX, (c) SEM, (d) SEM histogram, and (e) UV contribution of the peak at 1064 cm−1.
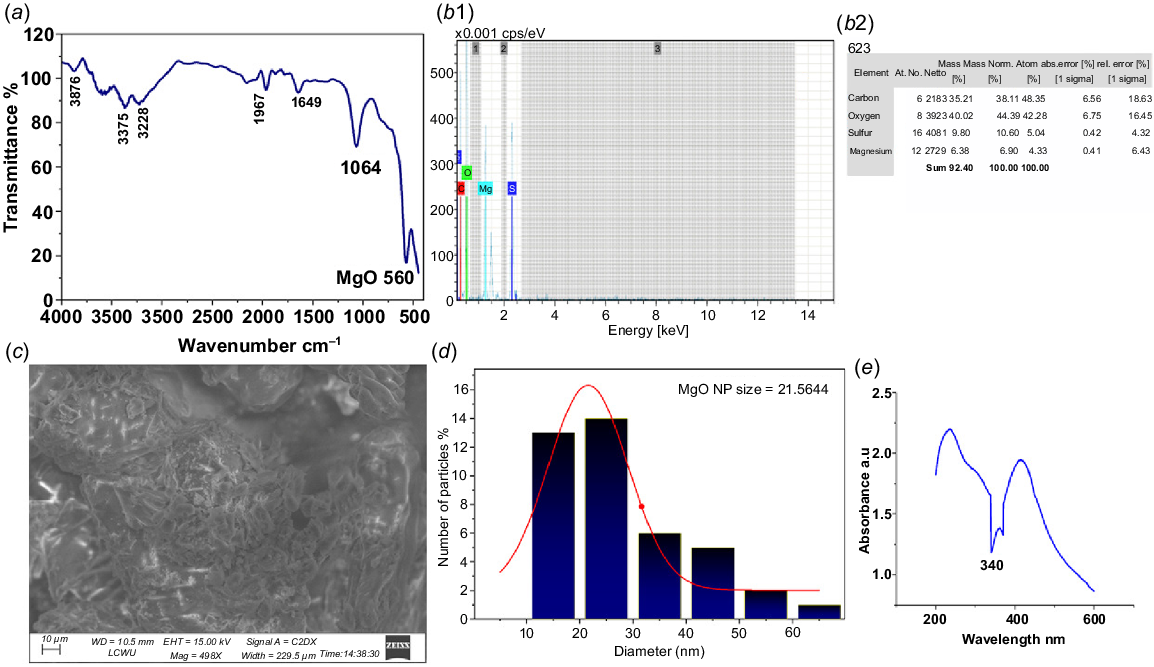
The absorption at 3375 cm−1 was a characteristic peak of O–H groups from phenolic compounds, alcohols, carboxylic acids, and surface hydroxy groups on the MgONPs. A peak at 3639 cm−1 corresponding to alcohols or phenols was reported by Mazher et al. (2023) on CaO nanoparticles. This peak also lies in the same range as the peak at 3375 cm−1 (Mazher et al. 2023). At the upper range, peaks at 2967–2850 cm−1 were observed, corresponding to the C–H stretches of terpenoids and fatty acids. A peak at 2917.81 cm−1 for Combretum molle leaves corresponds to the stretching of C–H of alkanes which hints that the leaves may have terpenoids and fatty acids (Ntshanka et al. 2020; Parusnath et al. 2023).
The peak at 1649 cm−1 points to water or carbonates on MgO and to C=O bonds in carboxylic acids, esters, or amides in residual proteins. Wetting a single crystal of MgO causes it to form hydrated magnesium carbonates (Weber et al. 2023). The main peak seen here (1064 cm−1) indicates C–O stretching in alcohols, ethers, and esters from neem leaf extract. The peaks in the 1000–1200 cm−1 range for the MgONPs were consistent with the C–O stretching vibrations of alcohols, ethers, and esters in the biomolecular substances, as reported by Kanwal et al. (2018). The peaks at 3876 and 1967 cm−1 are likely noise or artefacts, since 1967 cm−1 is much higher than expected for a C=O stretch (Fig. 4a).
FTIR of ZnONPs
The stretching vibrations of the ZnO core and biomolecules of the extract of Glycyrrhiza glabra (licorice) root at 580 and 839 cm−1 validate the presence of ZnONPs (Fig. 5a). The FTIR spectrum of the synthesized ZnONPs (531 cm−1, 550 cm−1) matched that in the literature (Al-Kordy et al. 2021; Pare et al. 2022). The absorption at 3344 cm−1 is due to O–H stretching from the phenolic compounds, alcohols, and carboxylic acids in the licorice root, which again suggests that the extract acts as a capping or stabilizing agent for the ZnONPs.
Characterization of ZnONPs synthesized using the extract of Glycyrrhiza glabra L. (licorice) root: (a) FTIR, (b1, b2) EDX, (c) SEM, (d) SEM histogram, and (e) UV spectrum.
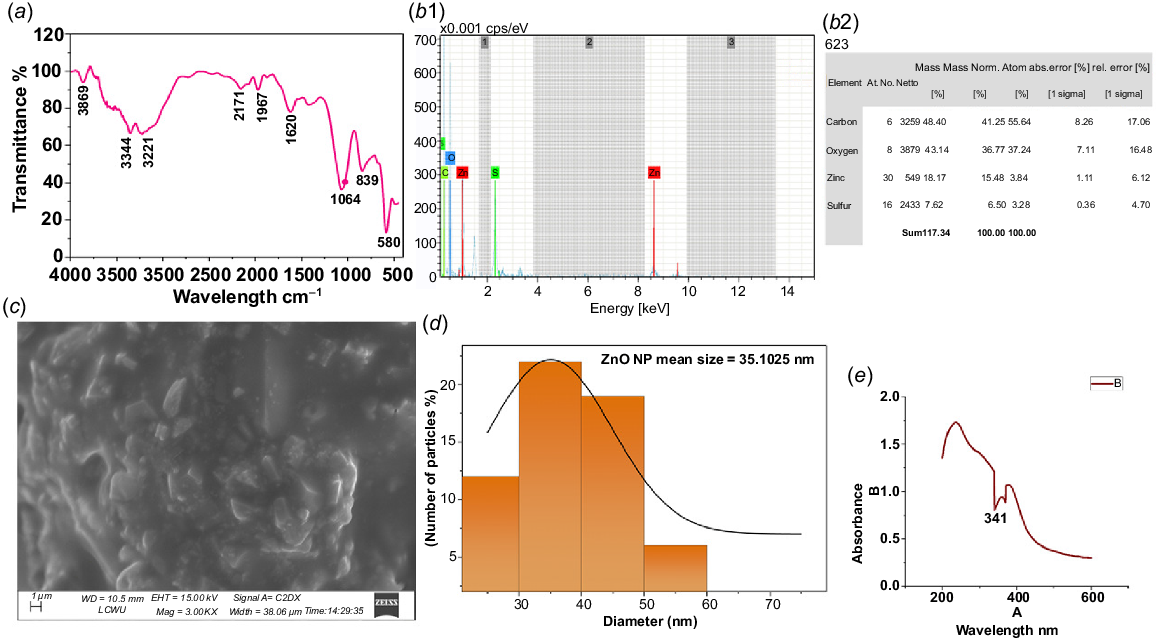
Similarly to Amin et al. (2023), during the biochemical synthesis of ZnONPs, the FTIR spectrum exhibited appearance of O–H stretching in the range of 3550–3200 cm−1. A weak absorbance at 2171 cm−1 is probably from adsorbed CO2 that can potentially form carbonate species at the ZnO surface. A small peak at 1967 cm−1 is probably an artefact, whereas the significant peak at 1620 cm−1 indicates a mix of O–H bending from surface-bound water and C=O stretches (carboxylic acids, esters), probably derived from some triterpenoid components of G. glabra root.
Our analysis is strongly associated with the results of Dangana et al. (2023), which found O–H bending vibrations at 1620 cm−1 in ZnO nanoparticles prepared with spinach plant extract. The peak at 1064 cm−1 is attributed to the bending of Zn–O–Zn and C–O stretches of ethers, alcohols, and esters present in the various biomolecules of the extract, such as carbohydrates and triterpenoids. C–O stretching vibrations for ethers, alcohols, and esters are typically located at 1000–1300 cm−1, which could explain the contribution of the peak (Amin et al. 2023; Dai et al. 2023) at 1064 cm−1 (Fig. 5a).
EDX of MgONPs
The presence of magnesium (Mg) and oxygen (O) in the EDX results proves that the reaction formed MgONPs. Because we found carbon (C) at 35.21 wt% and sulfur (S) at 9.80 wt%, it can be noted that organic matter is associated with the procedure due to the neem leaf extract. The existence of Mg (6.38 wt%) and O (41.02 wt%) demonstrates that MgO nanoparticles have been formed. These residues mean that nanoparticles prepared by this process consist of MgO as the main component along with some neem-based compounds which could stabilize them (as seen in Fig. 4b1, b2).
EDX of ZnONPs
Two elements, zinc (Zn) and oxygen (O), were detected in the EDX analysis, confirming the formation of ZnONPs. The content of Zn and O is 18.17 wt% and 43.14 wt%, respectively. The non-stoichiometric presence of Zn and O is further supported by significant carbon (C) and sulfur (S) signals (48.40 wt% and 7.62 wt%, respectively), which can be attributed to organic residues from the G. glabra root extract used in the synthesis. This is common in green synthesis, where biomolecules such as triterpenoids, flavonoids, and polysaccharides from the G. glabra root serve as both reducing and stabilizing agents, resulting in the coating of ZnONPs. These organic components contribute to the elevated carbon content, whereas sulfur likely originates from other biomolecules in the licorice root. Thus, the formation of ZnO is suggested by the presence of Zn and O, although the EDX data indicate that the final product is a composite of ZnO and organic components from G. glabra (Fig. 5b1, b2).
SEM of MgONPs
Fig. 4c, d were obtained using SEM to determine the morphology and size of the green-synthesized MgONPs. SEM revealed a fused, irregular crystalline structure of the MgONPs synthesized from neem leaf extract. The size of the nanoparticles, determined by SEM images using Image J and Origin software (Fig. 4d), varied between 17–40 nm, with an average size of 21.56 nm. This suggests a narrow distribution; however, multiple SEM images were examined, and particle size distribution analysis was performed using the software for accurate measurement.
SEM of ZnONPs
The morphology and size of the G. glabra root extract ZNONPs were obtained by SEM (Fig. 5c). Scanning was conducted at approximately 40,000× magnification, enabling an inspection of the surface features of individual nanoparticles. Fig. 5c, d show a heterogeneous environment of particles with some level of agglomeration. Based on the SEM results, the ZnONPs were mostly spherical, rough in texture, evenly sized, well-dispersed, and crystalline in nature. According to SEM examinations, the particle size is in the order of 35.10 nm. ImageJ and Origin software were used to estimate the size and generate the histogram shown in Fig. 5d. This suggests a narrow distribution; however, multiple SEM images were examined, and particle size distribution analysis was performed using the software for accurate measurement.
UV of MgONPs
The optical absorption spectrum of MgONPs dispersed in deionized water is shown in Fig. 4e. A distinct absorption peak at 341 nm was detected within the UV region of 200–800 nm, which is characteristic of MgONPs. Similarly, MgONPs synthesized by Caccinia macranthera extract exhibited absorption peaks close to the bio-assisted MgONPs peak at 357 nm (Barzegar et al. 2024). This similarity also suggests the purity of the synthesized MgONPs, as no other peaks were detected in the UV-vis spectrum.
UV of ZnONPs
The synthesis of ZnONPs using G. glabra root extract was confirmed by UV-vis spectroscopic analysis. For this standard analysis, the sample was dissolved in DI water. The UV-vis spectrum was recorded over a wavelength range of 200–800 nm. A significant peak observed at around 341 nm indicated the successful formation of ZnONPs in the mixture (Fig. 5e). This broad absorption band, which shifts towards longer wavelengths, may be attributed to the migration of the electronic cloud within the ZnONPs framework. Additionally, the plant extract contains starch, proteins, and antioxidant molecules, which are responsible for reducing the Zn metal salt to ZnONPs.
Growth parameters (root FW, root DW, shoot FW, and shoot DW, root length, shoot length, leaf length)
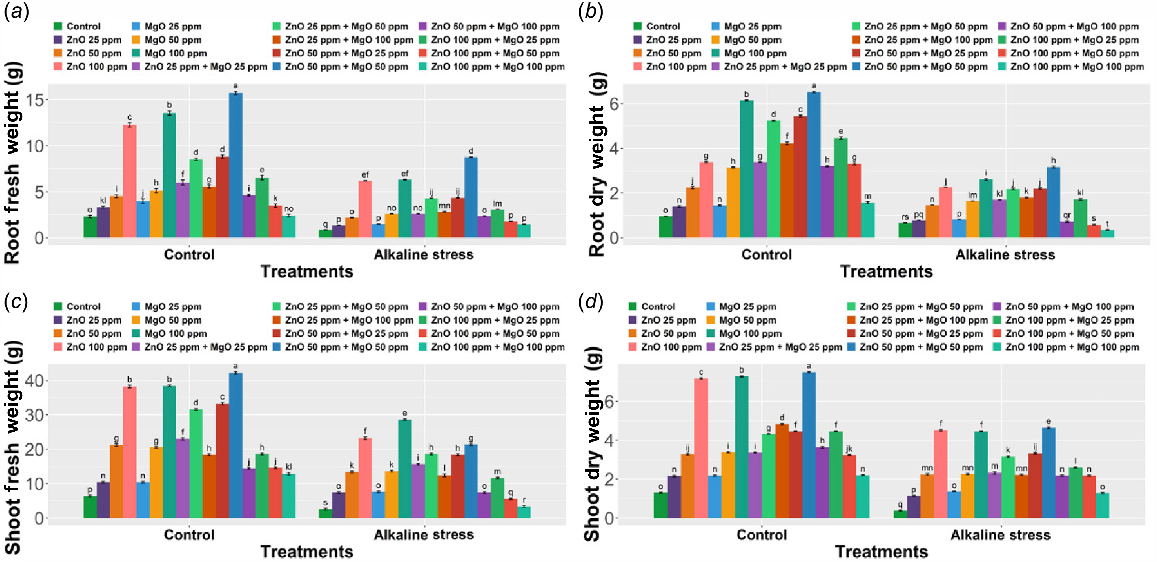
When green-synthesized ZnONPs and MgONPs were applied, root FW, root DW, shoot FW, and shoot DW of Z. mays varied depending on whether plants were grown under control or alkaline stress. Without ZnONPs and MgONPs, the control treatment had standard and good growth. Root FW was best improved in the combined ZnONPs (50 ppm) and MgONPs (50 ppm) treatment at 15.74 ± 0.18 g, almost five times the untreated control (P < 0.0001). Similarly, 100 ppm ZnONPs increased root FW four-fold (12.26 ± 0.37 g) and root DW two-fold (3.38 ± 0.19 g), whereas 100 ppm MgONPs increased the shoot by 125% and 238.46% compared with control and stress, respectively (P < 0.0001). When both ZnONPs (100 ppm) and MgONPs (100 ppm) were applied together, the results were less impressive: the root FW was 2.396 ± 0.11 g (+4.17%), root DW 3.20 ± 0.17 g (+231.95%), and shoot FW 12.80 ± 0.36 g. Furthermore, root length, shoot length, and leaf length also increased with a moderate dose 50 ppm of each nanoparticle compared with the control and stress only group (Supplementary Fig. S1). Under alkaline stress, the biomass levels in the stress control group declined significantly. Root DW dropped to 0.66 ± 0.16 g (−31%), shoot DW to 0.38 ± 0.03 g (−70.01%), and shoot FW to 2.60 ± 0.21 g (−59.63%) compared with the control. Treatment with 100 ppm ZnONPs under stress boosted shoot FW by 262.5% and shoot DW by 255.77% (P < 0.0001), whereas 100 ppm MgONPs gave only a small improvement (Fig. 6a–d). Plants treated with high doses of combined ZnONPs (100 ppm) and MgONPs (100 ppm) had suboptimal recovery under stress, with root FW 1.78 ± 0.09 g (−22.61%) and root DW 0.83 ± 0.07 g (−13.55%) less compared with the stress control (P < 0.0001) (Fig. 6a–d). In both control and stress conditions, the combination of both ZnONPs (50 ppm) and MgONPs (50 ppm) resulted in the greatest positive changes, likely due to complementary roles of Zn (enzyme activation) and Mg (chlorophyll biosynthesis), enhancing photosynthesis and biomass under stress. However, higher combined doses (ZnONPs 100 ppm + MgONPs 100 ppm) caused negative effects, emphasizing the need for optimal nanoparticle concentrations. Results were consistent across replicates, showing similar trends in all parameters. Overall, the moderate combination of ZnONPs (and MgO (50 ppm + 50 ppm) significantly improved maize biomass under alkaline stress, whereas excessive doses reduced growth performance.
Morphological attributes of Zea mays L. grown under alkaline stress with the effect of ZnONP and MgONP soil amendment. The data represent the mean of five replicates ± s.e., with different letters indicating significant differences between treatments (P < 0.05) based on Duncan’s test. (a) Root fresh weight. (b) Root dry weight. (c) Shoot fresh weight. (d) Shoot dry weight.
Physiological traits (photosynthetic pigments: chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids)
Both green-synthesized ZnONPs and MgONPs significantly affected photosynthetic pigments of Z. mays under both control and alkaline stress. In control conditions, the combination of ZnONPs (50 ppm) and MgONPs (50 ppm) increased chlorophyll a by 40.91%, chlorophyll b by 55.39%, and carotenoids by 785.3%, with total chlorophyll reaching its maximum of 6.50 ± 0.11 mg g−1, a 239.23% increase from control. This enhancement may reflect complementary roles of Zn (enzyme activation) and Mg (chlorophyll biosynthesis), resulting in more efficient photosynthesis. ZnONPs (100 ppm) alone also improved pigments, raising chlorophyll a (5.62 ± 0.10 mg g−1), chlorophyll b (2.04 ± 0.03 mg g−1), carotenoids (6.71 ± 0.14 mg g−1), and total chlorophyll (6.96 ± 0.08 mg g−1), whereas MgONPs (100 ppm) produced slightly lower values. In contrast, a high combined dose (ZnONPs 100 ppm and MgONPs 100 ppm) showed toxicity, reducing chlorophyll a to 1.25 ± 0.06 mg g−1, chlorophyll b to 0.66 ± 0.02 mg g−1, and carotenoids by ~15% (P < 0.0001) (Fig. S2a–d). Under alkaline stress, the most effective treatment was the combined ZnONPs (50 ppm) and MgONPs (50 ppm), which increased chlorophyll a to 3.48 ± 0.07 mg g−1, chlorophyll b to 1.48 ± 0.05 mg g−1, total chlorophyll to 5.15 ± 0.09 mg g−1, and carotenoids to 6.45 ± 0.12 mg g−1. However, MgONPs (100 ppm) produced the highest values under stress, with chlorophyll a 5.44 ± 0.08 mg g−1, total chlorophyll 6.62 ± 0.10 mg g−1, and carotenoids 6.52 ± 0.11 mg g−1. Excessive nanoparticle concentrations (ZnONPs 100 ppm + MgONPs 100 ppm) reduced pigment contents by half, indicating nanoparticle toxicity at high doses (Fig. S2a–d, Table S1). ANOVA revealed highly significant differences (P < 0.0001), and DMRT confirmed that ZnONPs (50 ppm) and MgONPs (50 ppm) combined and ZnONPs (100 ppm) alone were grouped among the best-performing treatments (‘a’, ‘ab’), and high combined doses were least effective (‘e’, ‘f’). Replicate measurements showed uniform trends across treatments, confirming stability and reliability of these findings. Overall, moderate nanoparticle doses improved pigment contents under both control and alkaline stress, whereas high concentrations negatively affected photosynthetic efficiency.
Chlorophyll fluorescence attributes (Fo, Fm, Fv) in Z. mays L. exposed to alkaline stress and the effect of ZnONPs and MgONPs
Alkaline stress altered chlorophyll fluorescence parameters in Z. mays. The combination of ZnONPs (50 ppm) and MgONPs (50 ppm) produced the highest values under control conditions, increasing Fo by 55.85%, Fm by 42.52%, and Fv by 4.90% compared with the control (P < 0.0001). ZnONPs (100 ppm) alone raised Fo by 63.96%, Fm by 34.88%, and Fv by 24.24%, whereas MgONPs (100 ppm) increased Fo (157.20 ± 3.92), Fm (524.20 ± 6.81), and Fv (313.00 ± 5.47) by 46.49%, 30.26%, and 4.10%, respectively (P < 0.0001). High combined doses (ZnONPs 100 ppm + MgONPs 100 ppm) reduced fluorescence values, indicating toxicity at elevated concentrations (Fig. S3a–d). Under alkaline stress, the most effective treatment was again ZnONPs (50 ppm) and MgONPs (50 ppm) combined, which increased Fo (209.80 ± 4.68), Fm (487.80 ± 6.22), and Fv (282.20 ± 5.86) by 1124.35%, 187.65%, and 124.37%, respectively, compared with stressed control (P < 0.0001). ZnONPs (100 ppm) and MgONPs (100 ppm) alone also improved fluorescence but to a lesser extent. In contrast, high combined doses (ZnONPs 100 ppm + MgONPs 100 ppm) reduced Fo, Fm, and Fv below stressed controls, highlighting the risk of overdosing. The improvements in fluorescence likely reflect the synergistic effects of Zn in electron transport chain efficiency and Mg in chlorophyll structure stability, resulting in improved photosystem performance. All parameters exhibited consistent patterns across replicates, supporting the robustness of these observations. Overall, moderate nanoparticle doses enhanced fluorescence under both control and stress, whereas high concentrations impaired photosystem efficiency.
Gas exchange parameters: photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (gs), and intercellular CO2 (Ci) in Z. mays L. exposed to alkaline stress and the effect of ZnONPs and MgONPs
Application of green-synthesized ZnONPs and MgONPs improved photosynthetic gas exchange under both control and alkaline stress conditions. The combined dose of ZnONPs (50 ppm) and MgONPs (50 ppm) produced the highest values, increasing photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (gs), and intercellular CO2 (Ci) by 98.90%, 478.81%, 309.56%, and 92.77% compared with control (P < 0.0001). ZnONPs (100 ppm) alone also enhanced gas exchange (Pn 30.43 ± 0.52; Tr 6.96 ± 0.14; gs 0.51 ± 0.02; Ci 321.68 ± 8.45), whereas high combined doses (ZnONPs 100 ppm + MgONPs 100 ppm) reduced Pn by 37.38%, gs by 53%, and Ci by 35.32% under control conditions (Fig. S4a–d). Under alkaline stress, combined ZnONPs (50 ppm) and MgONPs (50 ppm) also produced the best response, with Pn 22.82 ± 0.55, Tr 5.95 ± 0.13, gs 0.58 ± 0.01, and Ci 345.26 ± 7.83, showing increases of at least 50% over stressed controls (P < 0.0001). ZnONPs (100 ppm) improved all traits but less strongly, whereas high combined doses further suppressed photosynthetic activity, indicating a phytotoxic effect at excessive nanoparticle concentrations. These enhancements likely result from synergistic effects where Zn supports enzyme activity in photosynthetic pathways and Mg facilitates chlorophyll stability and CO2 assimilation. The observed responses were reproducible across replicate measurements, ensuring reliability of the presented data. Collectively, results highlight that optimum nanoparticle combinations enhance gas exchange efficiency under stress, whereas overdosing reverses these benefits.
RWC and RMP in Z. mays L. plants exposed to alkaline stress and the effect of ZnONPs and MgONPs
The application of green-synthesized ZnONPs and MgONPs significantly affected water content and membrane stability in Z. mays under both control and alkaline stress. In control plants, untreated samples had an RWC of 71.2 ± 0.37 and an RMP of 23.8 ± 0.42. The combination ZnONPs (50 ppm) and MgONPs (50 ppm) maximally enhanced RWC to 109.8 ± 0.37 (54.21% increase) and minimized RMP to 5.2 ± 0.32 (78.15% reduction) (P < 0.0001). ZnONPs (100 ppm) alone also increased RWC (106.2 ± 0.37; +49.16%) and lowered RMP (12.2 ± 0.37; −19.33%), whereas MgONPs (100 ppm) alone had moderate effects (RWC 90.8 ± 0.37; +27.53%; RMP 20.4 ± 0.34; −14.29%). High combined doses (ZnONPs 100 ppm + MgONPs 100 ppm) decreased RWC to 22.2 ± 0.37 and slightly lowered RMP to 17.2 ± 0.28, showing phytotoxicity at excessive concentrations (Fig. S5a–d). Under alkaline stress, untreated plants exhibited impaired water balance (RWC 27.0 ± 3.60) and severe membrane damage (RMP 37.0 ± 0.44). The ZnONPs (50 ppm) and MgONPs (50 ppm) treatment improved RWC to 109.6 ± 1.72 (+328%) and reduced RMP to 12.2 ± 0.31 (−67.03%), outperforming all other treatments (P < 0.0001). ZnONPs (100 ppm) alone also protected membranes (RWC 90.8 ± 0.37, RMP 14.2 ± 0.35), whereas MgONPs (100 ppm) had moderate effects (RWC 82.2 ± 1.20, RMP 15.2 ± 0.33). The highest combined dose under stress conditions performed poorly, with RWC falling to 45.2 ± 1.09 and RMP remaining high at 34.6 ± 0.36, indicating toxicity (Fig. S5a, b). These changes likely reflect the synergistic effect of Zn improving osmotic regulation and Mg stabilizing cell membranes, jointly enhancing water balance under stress. All parameters showed consistent patterns across replicates, confirming the reliability of these responses. Overall, moderate nanoparticle applications improved water status and reduced membrane damage, whereas higher doses had toxic effects.
Secondary metabolites (proteins, proline, amylase, phenolics) in Z. mays L. exposed to alkaline stress and the effect of ZnONPs and MgONPs
Exposure of Z. mays to green-synthesized ZnONPs and MgONPs significantly affected total protein, total soluble protein, amylase activity, and proline levels under both control and alkaline stress conditions. The combined dose of ZnONPs (50 ppm) and MgONPs (50 ppm) maximally increased total soluble protein (162.0 ± 2.61 mg g−1; +735.05%), total phenolics (6.04 ± 0.04 μg g−1; +85.28%), and proline (263.6 ± 2.38 mg g−1; +171.19%) compared with control (P < 0.0001). Under alkaline stress, the same combined treatment improved total soluble protein (120.6 ± 2.20 mg g−1; +392.78%), total phenolics (6.2 ± 0.03 μg g−1; +301.03%), and proline (199.75 ± 4.66 mg g−1; +105.50%) relative to stressed plants. High nanoparticle doses (ZnONPs 100 ppm + MgONPs 100 ppm) caused reductions, with total soluble protein falling to 25.0 ± 0.52 mg g−1 (−31.69%) and total phenolics to 0.8 ± 0.02 μg g−1 (−77.97%), whereas proline slightly increased (62.0 ± 2.65 mg g−1; +33.54%) (Fig. S6a–d). The enhanced protein metabolism and osmoprotectant (proline) buildup likely result from synergistic effects. Zn stimulates enzyme activity while Mg supports protein stability and amylase function, improving stress resilience. Patterns were consistent across replicate measurements, confirming data reliability. Overall, moderate nanoparticle doses enhanced protein synthesis and stress-related metabolites, whereas higher doses had inhibitory effects.
Soluble sugars, insoluble sugars, α-amylase activity, and total carbohydrates in Z. mays L. exposed to alkaline stress and the effect of ZnONPs and MgONPs
Under normal (non-stressed) conditions, applying ZnONPs and MgONPs at 50 ppm each produced a marked improvement in maize biochemical traits compared with untreated plants. α-Amylase activity increased from 0.07 ± 0.01 U g−1 FW in the control to 0.53 ± 0.02 U g−1 FW with treatment, reflecting a 657.14% rise. Soluble sugars increased from 16.2 ± 1.2 mg g−1 FW to 23.8 ± 1.3 mg g−1 FW (46.91% increase) and insoluble sugars rose from 10.5 ± 1.0 mg g−1 FW to 23.5 ± 1.2 mg g−1 FW (123.81% increase). Total carbohydrate content also improved significantly, increasing from 21.8 ± 1.3 mg g−1 FW in the control to 30.0 ± 1.5 mg g−1 FW (37.61% increase). Under alkaline stress, the same combined treatment showed even stronger effects compared to stress-only plants. α-Amylase activity increased from 0.05 ± 0.01 U g−1 FW to 0.26 ± 0.01 U g−1 FW (420.00% increase). Soluble sugars rose from 8.2 ± 1.0 mg g−1 FW to 19.0 ± 1.1 mg g−1 FW (131.71% increase) and insoluble sugars increased from 5.5 ± 0.9 mg g−1 FW to 18.2 ± 1.1 mg g−1 FW (230.91% increase). Similarly, total carbohydrate levels improved from 12.3 ± 1.2 mg g−1 FW to 24.2 ± 1.4 mg g−1 FW, indicating a 96.75% enhancement (Fig. S7a–d). These improvements are primarily attributed to the functional roles of zinc and magnesium provided by ZnONPs and MgONPs, respectively. Zinc acts as a structural and catalytic cofactor for several enzymes, including α-amylase, thus improving carbohydrate breakdown and energy supply. Magnesium is essential for ATP stabilization and acts as a central atom in chlorophyll, supporting energy metabolism and enhancing carbon fixation. Together, these nanoparticles improved enzyme activity, carbohydrate accumulation, and stress resilience, demonstrating their synergistic role in mitigating the adverse effects of alkaline stress and enhancing plant physiological performance.
Stress markers (MDA, H2O2, and EL) in Z. mays L. exposed to alkaline stress and the effect of ZnONPs and MgONPs
Exposure of Z. mays to alkaline stress significantly increased oxidative damage markers. MDA levels rose to 154.4 ± 2.14 nmol g−1 FW (+244.6%) compared with the control (44.8 ± 2.06 nmol g−1 FW, P < 0.001), indicating enhanced lipid peroxidation. H2O2 levels also increased from 31.4 ± 1.33 μmol g−1 in control plants to 174.8 ± 1.85 μmol g−1 under stress (+457%, P < 0.001), and EL doubled from 38.8% to 81.2% (+109%, P < 0.001), highlighting severe membrane injury. Application of ZnONPs (100 ppm) significantly reduced oxidative stress. In control plants, MDA declined to 13.6 ± 0.93 nmol g−1 (−69.6%) and in stressed plants to 44.0 ± 1.41 nmol g−1 (−71.5%). H2O2 dropped by 72.6% in control (8.6 ± 0.51 μmol g−1) and by 65.1% in stressed plants (61.0 ± 1.79 μmol g−1). Similarly, MgONPs (100 ppm) slightly lowered MDA under control (12.4 ± 1.94 nmol g−1 FW) and reduced stress-induced MDA by 92.1%, whereas H2O2 levels declined by 73.2% in controls without stress (54.4 ± 1.36 μmol g−1) relative to with stress only. EL also improved with nanoparticle treatments. In non-stressed plants, EL decreased by 34.0% with 50 ppm ZnONPs and by 52.6% with 50 ppm MgONPs. The combined treatment (ZnONPs 50 ppm + MgONPs 50 ppm) further reduced EL to 11.8 ± 0.37% (−69.6%). Under stress, the same treatment lowered EL by 81.2% (15.25 ± 0.48%). However, the highest dose (ZnONPs 100 ppm + MgONPs 100 ppm) caused membrane injury in non-stressed plants, elevating EL to 61.6 ± 0.51% (+58.8%) and maintaining high values (70.33 ± 11.27%) under stress (Fig. S8a–d). These outcomes suggest that moderate nanoparticle doses reduce oxidative damage by limiting lipid peroxidation, scavenging ROS, and stabilizing membranes. The synergy likely stems from Zn-mediated antioxidant enzyme activation and Mg-supported membrane stabilization. All the results were consistent across replicates, confirming reliability. Overall, moderate nanoparticle concentrations alleviated oxidative stress effectively, whereas excessive doses exerted phytotoxic effects.
Non-enzymatic antioxidants (GSH and AsA) in Z. mays L. plants exposed to alkaline stress and the effect of ZnONPs and MgONPs
ZnONPs and MgONPs treatments significantly altered the antioxidant metabolites under normal and alkaline stress (P < 0.001). The total combination of ZnONPs (50 ppm) and MgONPs (50 ppm) showed a maximum increase (402.4 μmol g−1 FW glutathione, +35.6%) in control plants (296.8 μmol g−1 FW glutathione), which implies higher redox capabilities. In a similar manner, MgONPs (100 ppm) alone showed an increase in glutathione of 25.5%, whereas the high dose combination (ZnONPs 100 ppm + MgONPs 100 ppm) decreased glutathione by 56.8%, indicating dose dependent phytotoxicity. In alkaline stress, glutathione levels were highly reduced in the untreated plants, yet, nanoparticle treatment partially restored them and the highest recovery and stabilization of antioxidant defense occurred with combined ZnONPs (50 ppm) and MgONPs (50 ppm) treatment. With regard to ascorbic acid, compared to the control plants (143.4 μmol g−1 FW), values were improved significantly using ZnONPs (100 ppm) (+43.5%), as well as ZnONPs (50 ppm) + MgONPs (50 ppm) (+44.2%) combined, showing the effectiveness of both individual stressors and combinations. On the other hand, the simple administration of MgONPs (100 ppm) alone decreased ascorbic acid by 18.1%, and the high dose of these two systems (ZnONPs 100 ppm + MgONPs 100 ppm) contributed to the additional reduction of ascorbic acid of 90.6 μmol g−1 FW (−36 Ascorbic acid declined drastically (57.0 ± 0.71 μmol g−1 FW) but was considerably enhanced to 132.4 ± 0.51 μmol g−1 FW (+132.3%) with ZnONPs (100 ppm) and 127.2 ± 0.86 μmol g−1 FW (+123.2%) with ZnONPs (50 ppm) and MgONPs (50 ppm) (Fig. S9a–d). These enhancements suggest that Zn and Mg have complementary roles such that Zn affects enzyme activation and Mg acts in chlorophyll biosynthesis and thus boosts redox homeostasis and antioxidant defense during stress. On the whole, moderate doses of nanoparticles (50 ppm each) enhanced the antioxidant response, and high doses (100 ppm each) did result in phytotoxicity. All values are the means of five replicates and the variations are minimal supporting the identical responses.
Enzymatic antioxidants in Z. mays L. plants exposed to alkaline stress and the effect of ZnONPs and MgONPs
Application of ZnONPs and MgONPs in comparison to the control group and alkaline stress group greatly influences the activities of SOD, POD, CAT, and APX (P < 0.001). The three antioxidants enzyme activities of SOD, POD, and APX, were recorded at 15.18 ± 0.22 units, 5.32 ± 0.30 units, and 12.3 ± 0.30 U mg−1 protein in the untreated (control group) plants. Stress caused by high pH decreased these activities (P < 0.001), reducing SOD to 5.34 ± 0.29 units, POD to 2.54 ± 0.10 units, and APX to 7.84 ± 0.49 U mg−1 with percentage decreases of 64.8%, 52.2%, and 36.3%, respectively, compared with the control plants (Fig. 7a–d).
Enzymatic antioxidant of Zea mays L. grown under alkaline stress with the effect of ZnONP and MgONP soil amendment. The data represent the mean of five replicates ± s.e., with different letters indicating significant differences between treatments (P < 0.05) based on Duncan’s test. (a) SOD. (b) POD. (c) APX. (d) CAT.
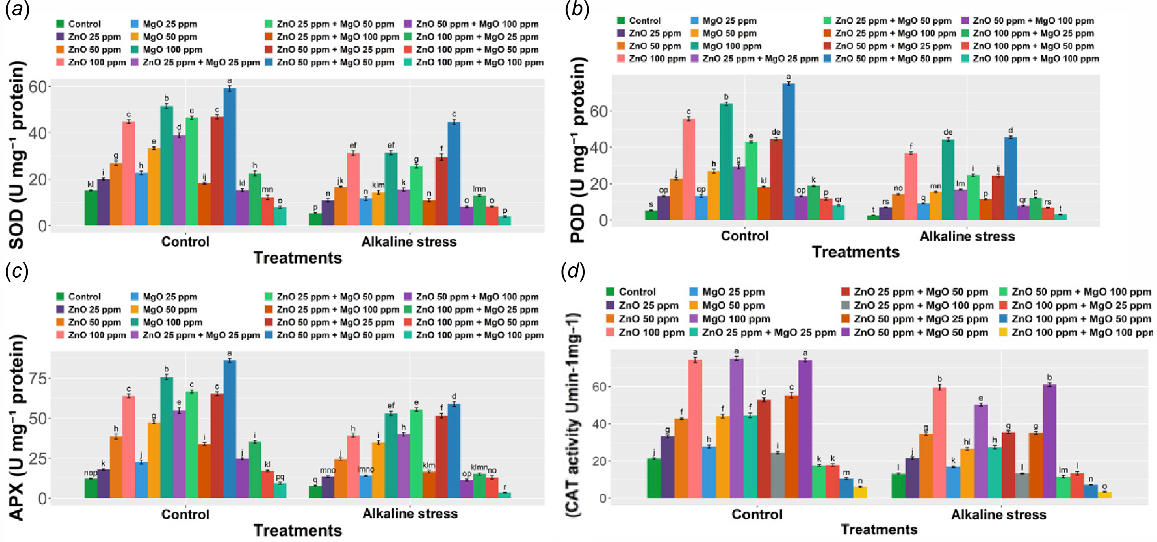
Nanoparticles applied under alkaline stress helped reduce the effects that alkalinity had on the enzyme activities (P < 0.001). The highest boost to activity of the enzymes was always achieved with the moderate combined dose of ZnONPs (50 ppm) and MgONPs (50 ppm). Compared to the control, the combined treatment caused a dramatic rise in SOD to 46.84 ± 0.85 units (208.6%), POD to 44.68 ± 0.79 units (740%), and APX to 65.22 ± 1.17 U mg−1 protein (430.2%). Compared to the plants under alkaline stress, the treatment caused a substantial increase in SOD to 29.52 ± 1.32 units (P < 0.001) (+452.8%), POD to 24.44 ± 0.89 units (P < 0.001) (+862%), and APX to 51.62 ± 1.33 U mg (+185%).
The ZnONPs (100 ppm) treatment brought noticeable increases in enzyme functions in both the control and alkaline stressed plants. With this treatment, the APX level under stress conditions increased to 63.86 ± 1.05 U mg−1 (+419.2%) (P < 0.001) and under control to 39.3 ± 0.95 U mg−1 (+401.3%) (P < 0.001). All enzyme activity showed an increase following MgONPs (100 ppm) treatment that was significant, but less effective than those shown by ZnONPs (100 ppm) or the combination of ZnONPs (50 ppm) and MgONPs (50 ppm) with values of P < 0.05 to P < 0.01 (Fig. 7a–d).
Compared to other treatments, the combined ZnONPs (100 ppm) and MgONPs (100 ppm) treatment generally did not cause significant changes. Under the control group, SOD and APX activities were greatly reduced (P < 0.05) to 7.92 ± 0.43 units (–47.8%) and 9.5 ± 0.66 U mg−1 (–22.8%), respectively. There was a minor but reliable increase in POD activity to 8.08 ± 0.41 units (+52%). Under alkaline stress conditions, this treatment brought about changes that were random and only sometimes significant. Even though an increase was seen for POD to 10.57 ± 7.47 U mg−1 (+99%) and for APX to 13.32 ± 9.80 U mg−1 (+69.9%), the results were not statistically significant (P > 0.05), suggesting that the dose could lead to inconsistency and might not be safe. CAT also improved under stress conditions, 63% increased with 100 ppm treatment under stress plants compared with only stress. Combine effect 50 ppm each of both nanoparticles improved slightly higher compared with all treatments with stress plants only. The results validate the explanations that low or moderate doses of nanoparticles exhibit synergistic enhancement of antioxidant defense but higher doses lead to marked instability and potential phytotoxicity. The resilience was probably promoted by the complementary nature of the roles of Zn (enzyme cofactor) and Mg (chlorophyll stabilization, metabolic support). All presented numbers are an average of five replicates that indicates stable experimental responses.
Mineral nutrients Ca2+, K+, and Na+ in Z. mays L. plants exposed to alkaline stress and the effect of ZnONPs and MgONPs
Z. mays L. showed a decrease in Ca2+, K+, and Na+ when exposed to alkaline stress. Following stress treatment, adding ZnONPs and MgONPs allowed the mineral content in plants to rise from 25 ppm to 100 ppm, whereas it was not the case for plants that had only stress treatment, as seen in Fig S10 (a–c). The group treated with 100 ppm of ZnONPs recorded the top results for Ca2+ of 76% and K+ of 83% under stress conditions versus the control stressed group. MgONPs (100 ppm) led to 16% higher K+ and 5% higher Na+ in stress plants than in those in the stress control. Applying a combined treatment of ZnONPs (50 ppm) and MgONPs (50 ppm) resulted in higher Na+ and Ca2+ content in stressed plants than in those in the stress control. Direct application of ZnONPs and MgONPs with 100 ppm alone increased Ca2+, Mg2+, and K+ concentration, which was less than the level observed in the 200 ppm treatment control group. However, the combination of 100 ppm ZnONPs and 100 ppm MgONPs, all together, gave the least amount of mineral nutrients in both control and stress treated plants. It means that at concentrations higher than 100 ppm, plants do not benefit from enhanced mineral ion levels under alkaline stress (Fig. S10a–c).
Pearson correlation and principal component analysis
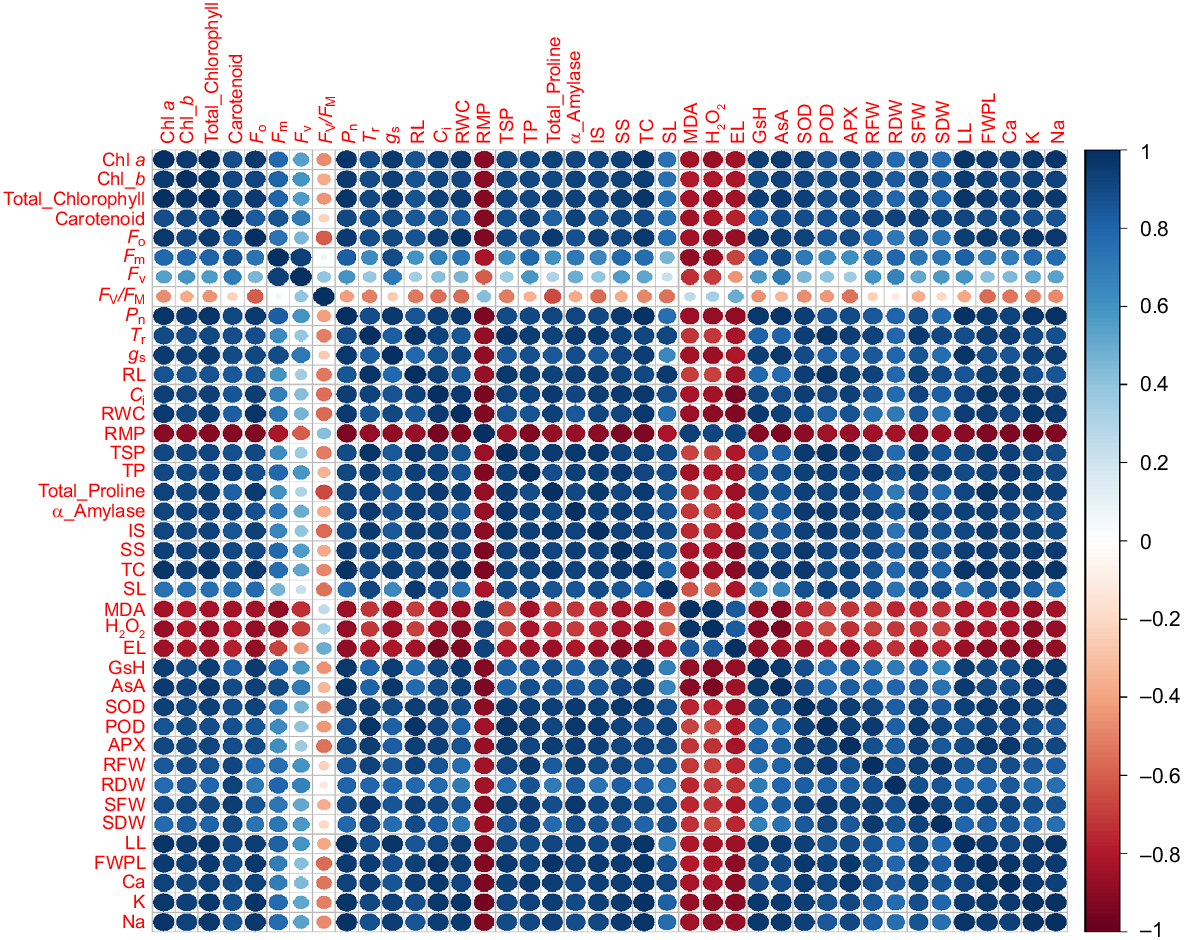
The PCA biplot (Figs 8 and 9) supports the Pearson’s correlation results. PCA was performed to identify the major contributing traits associated with stress tolerance and nanoparticle treatments. The first two principal components (PC1 and PC2) accounted for a cumulative 91.5% of the total variability, with PC1 explaining 85.1% and PC2 accounting for 6.4%. This high explained variance indicates a strong multivariate structure in the dataset. Parameters such as photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 (Ci), antioxidant enzymes (SOD, POD, CAT, APX), non-enzymatic antioxidants (AsA, GSH), osmolytes (proline, total soluble proteins), chlorophyll pigments, and ions (K+, Ca2+) were all positively loaded along PC1. These traits clustered together, indicating strong mutual association and shared contribution to stress mitigation and improved growth. This pattern is especially prominent in plants treated with combined moderate levels of ZnONPs and MgONPs (50 ppm each), suggesting this dose combination promoted coordinated enhancement of physiological functions under alkaline stress. On the opposite side of PC1, variables such as MDA, H2O2, EL, and RMP loaded negatively, showing inverse relationships with growth-promoting and protective traits. These stress indicators were closely aligned, reflecting enhanced oxidative damage in the untreated stress group or under high nanoparticle concentrations.
Pearson’s correlation for all studied parameters of Zea mays L. treated with ZnONPs and MgONPs in control and alkaline stress conditions. Various abbreviations used are as follows; SL, shoot length; RL, root length; SFW, shoot fresh weight; SDW, shoot dry weight; LL, leaf length; FWPL, fresh weight per leaf; EL, electrolyte leakage; TC, total carbohydrates; SS, soluble protein; IS, insoluble protein; TP, total protein; Chl, chlorophyll; Cart, carotenoids; Pn, net photosynthesis rate; Tr, transpiration rate; gs, stomatal conductance; Ci, intercellular CO2; Fv/Fm, maximum quantum efficiency of PSII; F0, minimal florescence; Fm, maximal florescence; MDA, malondialdehyde; H2O2, hydrogen peroxide; RWC, relative water content; RMP, relative membrane permeability; GsH, glutathione; AsA, ascorbate; TSP, total soluble proteins; CAT, catalase; POD, peroxidase; SOD, superoxide dismutase; APX, ascorbate peroxidase; Na, sodium; K, potassium; Ca, calcium.
PCA and Scree plot showed the PCA studied parameters distribution: Principal component analysis for all studied parameters of Zea mays L. under the alkaline stress and soil amendments with ZnONPs and MgONPs. (Various abbreviations used are same as given in Fig. 8).
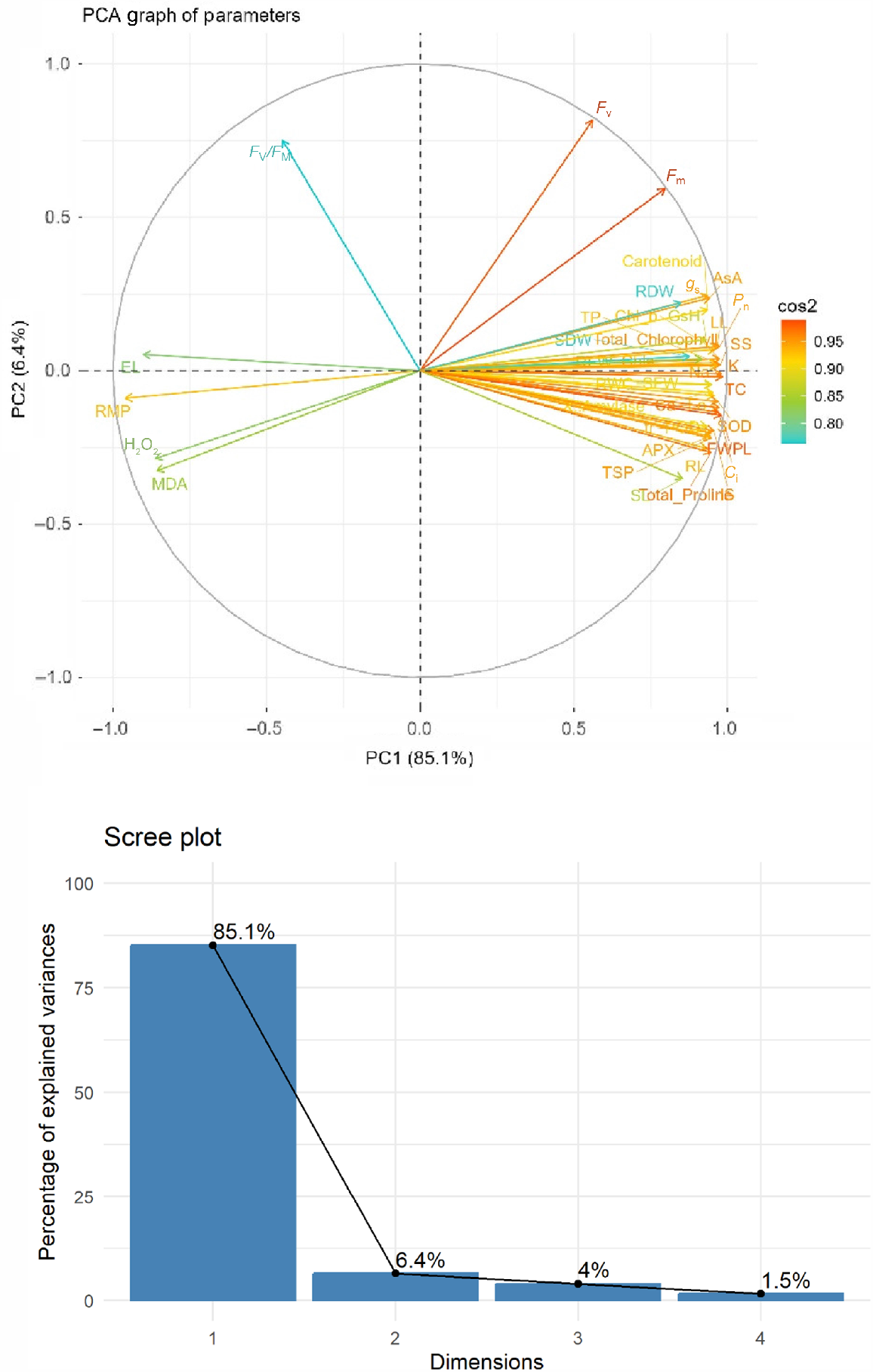
PC2 helped further distinguish traits such as Fv/Fm, Fo, and Fm, which were plotted more vertically and had distinct vectors. These chlorophyll fluorescence attributes maintained moderate separation from other traits, indicating their specific contribution to photosynthetic efficiency independent of antioxidant or ionic regulation. In summary, the PCA clearly revealed how moderate nanoparticle dosages improved stress resilience by enhancing antioxidant activity, osmotic balance, and ionic uptake, while reducing oxidative damage. In contrast, untreated stress and higher nanoparticle concentrations grouped with damage-related parameters, indicating physiological impairment. The clustering of multiple beneficial traits under moderate treatments underscores the integrated plant response facilitated by optimal nanoparticle application.
Discussion
Alkaline stress is extremely detrimental to high-yield grain crops, especially because alkalinity causes ion poisoning, nutrient imbalances, and oxidative damage that disrupt physiological processes and decrease yield (Hasanuzzaman 2020; Kumar et al. 2024; Yang et al. 2024). Arid and semi-arid areas are particularly vulnerable to this problem. Extensive research has been conducted on ZnONPs and MgONPs for stress mitigation against salinity, drought, and heavy metal stresses (Ali et al. 2024; Taj et al. 2024; Singh et al. 2024b). However, their role in alkaline soil stress mitigation is unexplored. Earlier reports have shown that these nanoparticles can increase the absorption of nutrients, stabilize cell membranes, and increase antioxidant defenses, but how they work together or alone in alkaline soils is unknown.
Our results support the alkaline-stress-inhibitory effects of ZnONPs and MgONPs in potentially enhancing nutrient absorption, antioxidant (SOD, CAT, POD, and APX) enzyme expression, and reducing oxidative stress, H2O2, EL, and membrane permeability. The same pattern was observed in wheat plants under salinity stress and coriander under drought stress treated with ZnONPs and MgONPs, respectively, indicating their ability to mitigate a wide range of stresses (Gupta et al. 2024; Olasan et al. 2024). These findings are specifically applicable to maize production in the alkaline soils, where conventional amendments could be expensive and less effective. As an alternative strategy, nanoparticle application can offer a sustainable solution that farmers may apply to enhance crop stability, yield, and farm profitability as a whole.
The biomass of grain crops is significantly reduced under alkaline conditions. In this respect, we have shown that ZnONPS and MgONPs increased the Z. mays biomass substantially under normal and alkaline stresses. Importantly, ZnONPs promoted cell division, nutrition intake, and photosynthesis under stress (Rizwan et al. 2019; Ali et al. 2024), and MgONPs promoted growth of root and oxidative stress tolerance (Liu et al. 2023). Mechanistically, such an increase can be clarified by the fact that ZnONPs release Zn2+ to act as a cofactor in the biosynthesis of chlorophyll and antioxidant defense systems (Gupta et al. 2024). Mg2+ ions stabilize the structure of the ribosome and enable the synthesis of ATP, they also promote the synthesis of proteins, energy transfer, and general cellular homeostasis during stressful conditions (Liu et al. 2023). Moreover, in this study, ZnONPs enhanced the length of maize roots and shoots and critically, the combination of ZnONPs and MgONPs has improved growth and biomass, in line with Wu et al. (2020) and Bayat et al. (2022). The synergistic effects of these nanoparticles likely increased enzyme activity, protein synthesis, and chlorophyll associated photosynthesis leading to biomass increase during alkaline stress (Gautam et al. 2023).
Nevertheless, consistent with other studies, alkaline stress alone decreased fresh and dry biomass, as well as leaf length (Sriramachandrasekharan et al. 2022). This is mainly due to the fact that excess alkalinity alters the structure of enzymes, reduces cell division, lowers photosynthesis, and also leads to nutrient deficiencies, all of which collectively inhibit growth (Ma et al. 2023; Pérez-Labrada et al. 2024). Likewise, Kumar et al. (2024) observed decreased shoot, root length, biomass, and root–shoot ratios in chickpea subjected to alkaline stress. Furthermore, it is interesting that in this experiment, the high concentration of nanoparticles (ZnONPs 100 ppm + MgONPs 100 ppm) also diminished root, shoot, and leaf generation, which is consistent with the findings of Elbasiouny et al. (2022) and Gowtham et al. (2024), who have also establishing that higher doses of nanoparticles provoke ROS-related cell death. Based on these observations, it is deemed that optimal nanoparticle concentrations lead to better plant health, whereas higher concentrations are phytotoxic.
Coupled with this finding, the most effective ZnONPs concentration (100 ppm) significantly enhanced root and shoot growth in stressed maize and wheat whereas high concentrations (150–200 ppm) resulted in toxicity (Srivastav et al. 2021; Raza et al. 2025). Similarly, MgONPs stimulated growth at low concentrations in maize (Abbas et al. 2024) and carrots (Mirrani et al. 2024) under stress, but on the contrary, phytotoxicity at higher doses in spinach was observed (Gautam et al. 2023). In contrast to earlier works on the application of ZnO or MgO alone, our findings indicated that synergistic effects were higher than individual nanoparticle treatments, thus suggesting a complementary role in nutrient uptake and antioxidant effects under the combined application of 50 ppm each.
Previously reported, when nanoparticles are applied in combination, they had synergistic effects, making plants grow better under stress (Lian et al. 2022). Characteristically, ZnONP applications enhanced the root and shoot mass of common beans (Gupta et al. 2024) and okra (Alabdallah and Alzahrani 2020), and MgONPs at 100 ppm maximized the root and shoot mass of Sesamum indicum (Olasan et al. 2024). Consolidated, these findings indicate that combined ZnO and MgO treatments often yielded synergistic effects in biomass under stress (Alves et al. 2024), and this finding directly affirms our results of a combined effect of ZNONPs and MgONPs with alkaline stress. Given their practical implications, these results indicate the possibility for alkaline soil agriculture in which moderate doses of nanoparticles may be included in fertilization strategies or seed priming to increase biomass and lower crop losses to provide farmers with a sustainably priced alternative.
In our study, photosynthetic pigments such as chlorophyll a, chlorophyll b, and carotenoids were significantly decreased by alkaline stress, and this finding is in line with previous reports (Abdel Latef and Tran 2016). Interestingly, this reduction was probably due to limited Mg2+ availability, oxidative stress, and impaired chloroplast membranes, which are essential to photosynthetic activity (Wang et al. 2022b; Shi et al. 2024). In this respect, it is worth noting that the application of ZnONPs and MgONPs alleviated these adverse effects, which contributed to pigment stability and photosynthetic efficacy. This was further confirmed by the fact that the combined application of ZnONPs (50 ppm) and MgONPs (50 ppm) improved the chlorophyll a by 26.09% and carotenoids by 1124.35% as compared to the stressed control, a powerful ability to improve pigment degradation. This may be attributed to ZnONPs liberating Zn2+ which serves as a cofactor of δ-aminolevulinic acid dehydratase required to manufacture chlorophyll (Šulinskienė et al. 2019). Whereas, the Mg2+ from MgONPs is a part of the chlorophyll center and sustains the stability of PSII and pigment integrity with stress conditions (Taj et al. 2024). These findings are also in line with prior studies of Tanweer et al. (2022) and Zhai et al. (2025) who also found an increase in the stability of chlorophyll pigments and a better photosynthesis capability under stress when nanoparticles were applied. As an example, under salinity stress, ZnONPs enhanced root and shoot biomass and the stability of the pigments in okra (Alabdallah and Alzahrani 2020), whereas in Brassica napus, the pigment values and chlorophyll were elevated by 14–27% with the use of 10 mg L−1 MgONPs and enhanced net photosynthesis, while 200 mg L−1 MgONPs decreased photosynthesis (Ali et al. 2024).
Likewise, wheat exposed to salinity stress of 10 dS m−1 and treated with ZnONPs showed a 24.6% and 10% increase in chlorophyll a and b, respectively (Adil et al. 2022), and common bean showed a 27–100% increase in chlorophyll (Gupta et al. 2024). Similar improvements were also observed with rice in terms of pigmentation, with synergistic effects of combined ZnONPs and MgONPs also being observed in terms of chlorophyll content and gas exchange in maize (Jampilek and Kralova 2022). In contrast, very high concentrations (ZnONPs 100 ppm + MgONPs 100 ppm) in our study led to reduced pigments: total chlorophyll a and b declined by 51.61% and 13.46%, respectively, emphasizing the dose-dependent effect of nanoparticle exposure.
This enhancement is probably due to an increase in the expression of genes associated with chlorophyll biosynthesis (CHLH, CHLD, CHLM) by the combined ZnONPs and MgONPs and an increase in the availability of Mg2+ which is vital in chlorophyll production. Moreover, ZnONPs have been shown to activate thiols and phytochelatins which preserve pigment stability against oxidative damage caused by excessive alkalinity (Mardi et al. 2022; Mai et al. 2024). This demonstrates that retaining the chlorophyll pigments during alkaline stress helps in photosynthesis which provides improved biomass and yield potential to farmers farming under alkaline environment.
Enhanced gas exchange performance Pn, tr, gs and Ci may be attributed to Zn-driven enzyme activation and Mg-facilitated ATP synthesis, which improved stomatal regulation, CO2 assimilation, and energy transfer, thereby supporting greater photosynthetic carbon fixation under stress (Faizan et al. 2023). Notably, the results of photosynthetic gas exchange showed clearly that both ZnONPs and MgONPs assist the plants in taking up more CO2 and efficiently utilizing water in photosynthesis (Rizwan et al. 2019). This aligns with prior evidence, where it was demonstrated that ZnONPs can boost photosynthesis by bringing greater efficiency to RubisCo and carbonic anhydrase, which subsequently results in increased fixation of CO2 and sugar synthesis (Faizan et al. 2018). Interestingly, in our investigation, the combined ZnONPs (50 ppm) and MgONPs (50 ppm) treatment showed the most improvements, the photosynthetic rate was 304.84% greater and stomatal conductance improved by 344.83% compared to stressed control plants. These findings are consistent with previous studies that show that nanoparticle treatments increase the opening of stomata and promote the uptake of CO2 (Liang et al. 2023). Likewise, ZnONP application to stressed soybean enhanced the stomatal conductance considerably compared with non-treated plants (Ahmad et al. 2020), and MgONPs stimulated the promotion of both transpiration and intercellular CO2 (Faizan et al. 2023) under arsenic stress. In combination, enhanced stomatal conductance and photosynthesis rate signify effective CO2 assimilation and water-use efficiency under alkaline stress. Practically, this improved gas exchange will lead to an improved vegetative growth and grain filling that will give farmers a cheap solution to increase yields and profits. Altogether, these findings indicate that ZnONPs and MgONPs combined have synergistic effects on the gas exchange, which provides the maximum photosynthetic activity under stress conditions.
As we observed in our study, the affect exerted by ZnONPs and MgONPs enhanced the Fv/Fm and chlorophyll fluorescence and it assisted PSII in retaining its efficiency (Tryfon et al. 2023). Likewise, in earlier maize studies, it was revealed that ZnONPs can enhance photosynthesis in salt stress conditions confirming their significance in enhancing photosynthetic rates (Seleiman et al. 2023b). In line with these findings, the treatment of ZnONPs during salinity stress increased chlorophyll fluorescence in plants, which is a sign of better photosynthesis (Rasouli et al. 2022). Moreover, the increase in chlorophyll fluorescence and Fv/Fm ratios indicate that ZnONPs enhance PSII functions, photoprotection, and the presence of photoinhibition induced by stress in plants (Moustakas et al. 2024). In agreement, Fv/Fm increased at the mechanistic level, which is attributed to improved PSII activity due to possible regulation of photosystem structural genes (PSAD2, PSAE2) by ZnONPs as well as stabilization of the light-harvesting complexes by MgONPs, which are essential to guarantee efficient electron transfer and energy dissipation (Qu et al. 2024). According to these mechanistic implications, ZnONPs in common beans sustained quantum yield (Phi PSII) at near-control levels (Gupta et al. 2024). MgONPs coupled with arbuscular mycorrhizal fungi (AMF) in Dracocephalum moldavica L. and other crops enhanced chlorophyll fluorescence, stomatal conductance, and photosynthesis rate (Ghaffari Yaichi et al. 2025). On the same note, MgONPs alone increased chlorophyll fluorescence in Coriandrum sativum when exposed to drought (Kaleem et al. 2025). All of these trends fit our results, which showed that a combined application of ZnONPs and MgONPs boosted Fv/Fm and beneficial gas exchange characteristics, thus enhancing photosynthetic utilization under alkaline stress.
When alkaline stress happens, RWC falls, RMP goes up, and ROS increases, leading to both oxidative stress and damage to the cell membrane. Our research shows that ZnONPs treatment under alkaline stress resulted in an increased RWC, from 57.1 ± 0.37% to 106.2 ± 0.37% (49.16% increase) and a decreased RMP from 29.4 ± 0.34% to 19.2 ± 0.37% (19.33% decrease). Notably, the combined ZnONP (50 ppm) and MgONP (50 ppm) treatment not only enhanced the cell hydration and dynamically stabilized root membrane potential under alkaline stress but also increased the RWC, which positively affected membrane integrity. ZnONPs can release ions and stabilize soil pH, supply nutrients, and enhance crop membrane bonding (Verma et al. 2022), and MgONPs normalize the pH and assist in maintaining membrane activity and other the metabolism processes (Ahmed et al. 2023). Mechanistically, these effects are probably compensated by the increased expression of aquaporins and ROS-scavenging enzymes (SOD, CAT) which in combination are able to ensure osmotic stability and limit membrane damage in case of stress (Abbas et al. 2024).
It has been previously reported that the RWC increased significantly up to 25% in wheat in the presence of ZnONPs (Raza et al. 2025). ZnONPs in potatoes (Al-Selwey et al. 2023) and MgONPs in maize (Abbas et al. 2024) caused an increase in cellular water maintenance during drought due to membrane stabilization, aquaporin activation, and mitigation of oxidative stress. Agriculturally, all these synergistic impacts amount to improved crop anchoring and survival in alkaline soils and a stable yield as well as national food security. These effects positively impact production of fewer ROS, the proper functioning of mitochondria and chloroplasts, and hydration (Song et al. 2023). However, nanoparticle aggregation in the soil can also lead to toxicity and result in an increase in ROS, membrane injury, and disruption of nutrient uptake, which once again highlights that dosing of nanoparticles is critically important in terms of stress tolerance.
Growth of the plant is highly limited under alkaline stress because it interferes with water relations, photosynthetic efficiency, and cellular redox (Yang et al. 2024). Excessive production of ROS such as superoxide anions (O2−) and H2O2 are among the main outcomes of high ROS generation, which oxidize lipids, proteins, and nucleic acids resulting in unstable membranes and poor metabolic function (Sachdev et al. 2021). In our study, alkaline stress significantly increased oxidative stress markers; MDA levels rose by 244.6% and H2O2 levels by 457.7%. EL also increased, indicating severe membrane damage. Alkaline stress significantly decreased the activity of antioxidant enzymes; the SOD activity decreased by 64.8% and POD activity by 52.2%. A reduction of SOD and POD activities was seen in a previous study of salinity stress in rice (Hu et al. 2022). Such a decrease can be taken as an indicator of impaired ROS-scavenging ability, which leads to the intensification of lipid peroxidation, an increase in the level of MDA, loss of membrane integrity, and a decrease in the efficiency of photosynthesis (Girotti and Korytowski 2023). Moreover, in this study, under alkaline stress in maize, the non-enzymatic antioxidants GSH and AsA that are essential to redox homeostasis were decreased and thus the cells became particularly susceptible to oxidative damage. This is in accordance with a previous study in which salinity stress reduced the non-enzymatic antioxidants in Pisum sativum and this effect was reduced with the application of ZnONPs (Mustafa et al. 2024). The damage that occurred impaired the functions of the chloroplast and mitochondria, lowered the level of water-use efficiency, and slowed the growth of plants (Rajput et al. 2021; Mansoor et al. 2022).
Despite this, green-synthesized ZnONPs and MgONPs have been shown to reverse such trends by increasing both the enzymatic and the non-enzymatic antioxidant defenses. Our research work reported, that individually, the application of ZnO (100 ppm) reduced MDA content by 69.6% under control and 71.5% under stress, while H2O2 decreased by 72.6% under control and 65.1% under stress. Similarly, the combination of ZnO 50 ppm and MgO 50 ppm (total 100 ppm) decreased EL by 69.6% under control and 81.2% under stress, indicating improved membrane stability and reduced oxidative stress. These treatments enhanced SOD to 29.52 U mg−1 (452.8%), POD to 24.44 U mg−1 (862%), and APX to 51.62 U mg−1 (560%) and replenished ROS detoxification systems, which preserved cell membranes and photosynthetic pigments. Our findings indicate a decrease in MDA, H2O2, and leakage of electrolytes on moderate exposure to ZnONPs and MgONPs in agreement with previous findings. The application of ZnONPs on Phaseolus vulgaris under salt stress decreased MDA by 17–35% and H2O2 by 16–20% and increased SOD, POD, and APX (Gupta et al. 2024). Similarly, MgONPs enhanced antioxidant protection and decreased oxidative stress indicators in Coriandrum sativum and Spinacia oleracea under drought and cadmium stress (Taj et al. 2024; Kaleem et al. 2025). All these similarities show that ZnONPs and MgONPs enhance the antioxidant-protective system and prevent membrane damage, validating our results about alkaline stress tolerance improvement in maize.
In contrast, the application of high nanoparticle doses (ZnO 100 ppm + MgO 100 ppm) increased EL and ROS accumulation, indicating nanoparticle toxicity at elevated levels due to excess ion release and aggregation. These findings correlate with earlier reports that the optimum dose of ZnONPs enhanced salt tolerance in rice and common beans by increasing the activity of antioxidant enzymes, preserving chlorophyll level, and reducing MDA and H2O2 (Singh et al. 2025). MgONPs (50 ppm) also increased the antioxidant capacity of other crops exposed to drought stress (Kaleem et al. 2025), showing a potential to mitigate various stresses. Mechanistically, Zn2+ released from ZnONPs activates stress-responsive genes and serves as a co-factor to SOD, whereas Mg2+ provides structural stabilization to antioxidant enzymes and metabolic enzymes, which ensure optimal detoxification of ROS (Azarin et al. 2022; Liu et al. 2023). In contrast our study showed that ZnONPs and MgONPs have a strong ability to mitigate the alkaline stress, to retain stability under severe conditions. This was confirmed in a previous report; nanotechnology strengthens the optical abilities of materials which could help make plants more resistant to stress (Javed et al. 2025). Our study is consistent with previous research; under salt stress in maize 100 mg L−1 MgONPs reduced MDA and H2O2 and enhanced the SOD, POD, and APX (Seleiman et al. 2023a).
ZnONP and MgONP treatments also exhibited a positive effect on non-enzymatic osmolytes and metabolic markers. Moderate doses of treatments (ZnONPs 50 ppm + MgONPs 50 ppm) showed a great jump in the content of total soluble protein (95.60 2.20 μg g−1, +392.78%), total protein (4.90 0.03 μg g−1, +301.03%), proline (199.75 4.66 μg g−1, +105.50%), and amylase as an osmoprotectant. Proline stabilizes membranes and proteins, scavenges ROS, and controls the redox balance in a cell, whereas soluble proteins and amylase enhance metabolic stability and the release of energy in response to a stress (Mansour and Salama 2020; Kaczorowski et al. 2024). This may be attributed to the fact that Zn2+ is a cofactor to RNA polymerases and transcription factors that upregulate protein production whereas Mg2+ is required in ATP production, assembly of ribosomes, as well as activation of RubisCO all of which enhance enzymatic and metabolic efficiency (Liu et al. 2023; Ahmad et al. 2024). The combined effects enhanced the carbohydrate mobilization and osmotic response to alkaline stress (Zhai et al. 2025).
In our study, a high concentration of nanoparticles (ZnONPs 100 ppm + MgONPs 100 ppm) was toxic, which results in a significant decrease of the total soluble protein (25.0 ± 0.52 μg g−1, –31.69%) and total protein (0.8 ± 0.02 μg g−1, –77.97%) compared with stress only. Proline (62.0 ± 2.65) (–48%) also decreased. This toxicity must have been occasioned by the overproduction of ROS, agglomeration of nanoparticles, and electrolytic disorders that interrupt metabolic usages and protein homeostasis. These results support the value of dose optimization to maximize the advantages of nanoparticles in reducing potential adverse outcomes and minimizing possible adverse effects.
Nanoparticle treatments also had extremely positive effects on ion homeostasis. In alkaline stress, plants often over-accumulate Na+ which in turn alters cellular ionic balance, damages membranes, and inhibits enzyme activity (Almeida et al. 2017). ZnONP and MgONP treatments reduced Na+ levels and enriched the levels of Ca2+ and K+, which suggests better selective passage and absorption of ions. These findings supported our work that a combination moderate dose (ZnONPs 50 ppm + MgONPs 50 ppm) had the highest level of Ca2+ (44.75 ± 1.40 mg g−1 DW) and K+ (52.3 ± 1.85 mg g−1 DW) which established the synergistic effect of Zn and Mg on cellular ionic homeostasis (Lalarukh et al. 2022). Mechanically, zinc governs Na+/H+ antiporters and K+ channels, enhancing Na+ efflux and K+ uptake, whereas magnesium supports phospholipid stabilization of membranes and activating Ca2+ ATPase to guarantee best Ca2+ signaling and maintenance. These nanoparticles can prevent ion toxicity, stabilize cell integrity, and promote metabolism functions under alkaline stress due to their ability to enhance the ionic balance (Eissa et al. 2022).
There are significant agricultural implications of these findings in the real world. Alkaline soils, prevalent in the arid and semi-arid lands, inhibit the productivity of crops because of the poor nutritive status, alkalinity, and ionic imbalance. Our findings show that low concentrations of ZnONPs and MgONPs synthesized via the green process induce a significant increase in stress tolerance due to activation of the antioxidative defenses, elevation of osmolyte and protein metabolisms, and stabilizing the ionic balance. To growers it translates to better plant establishment, greater photosynthetic capacity, and better grain fill in tough soil environments, which ultimately equates to greater harvest and revenue. The lower amount of nanoparticles, using soil amendments or via foliar spray, also promotes the applicability of nanoparticles as a scalable, low cost benefit to alkaline soil management. Furthermore, the application of green synthesis technologies leads to an environmentally friendly farming practice, which reduces environmental risks of chemically mediated nanomaterials.
Such results are consistent with prior work establishing dose-related responses of nanoparticles on plants (Mustafa and Komatsu 2022) and expand their applicability to field-scale alkaline soil treatment. The most effective doses seem to be moderate (ZnONPs 50 ppm + MgONPs 50 ppm), as they balance the enhanced metabolic stability with a lack of nanoparticle-induced toxicity. Overall, the combination of ZnONPs and MgONPs with alkaline soil management is a potentially effective method to increase crop sustainability, maintaining the stability of yields and contributing to food security in areas where alkaline soil endangers food production.
Conclusion
Alkaline stress is one of the major constraints affecting maize production in arid and semi-arid regions, including Punjab, Pakistan, where it reduces plant growth and yield. This study demonstrated that green-synthesized ZnO and MgO nanoparticles, applied individually and in combination within the 25–200 ppm range, improved morphological, physiological, biochemical, and antioxidant enzyme responses in maize under alkaline stress by reducing oxidative damage and supporting stress tolerance. The combined ZnONPs (50 ppm) and MgONPs (50 ppm) treatment exhibited a clear synergistic effect, providing the greatest overall improvement, whereas the highest dose (ZnONPs 100 ppm + MgONPs 100 ppm) resulted in phytotoxic effects under both control and stress conditions. These findings indicate that green-synthesized nanoparticles could serve as environmentally friendly alternatives to chemical fertilizers for mitigating alkaline stress, enhancing crop growth, and improving yield stability. In addition, their role in regulating antioxidant enzyme responses under stress conditions provides a baseline for advancing sustainable nanotechnology-based solutions in agriculture. However, further studies are needed focusing on field-scale validation, dose optimization, nanoparticle–plant molecular interactions, and environmental safety to ensure safe and effective application in real-world agricultural systems.
Data availability
All data is presented in this manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
MI: idea formulation, methodology and design the experiment, performed data analysis and designed the manuscript. Validation and supervision by AAS. MI and AAS: involved in drafting article, revision, arranged the figures, tables and manuscript description. All the authors read and approved the finalized manuscript for publication.
Acknowledgements
This research work was conducted as part of the Ph.D. degree of MI at Department of Botany, University of Education, Lahore, Pakistan.
References
Abbas Z, Hassan MA, Huang W, Yu H, Xu M, Chang X, Fang X, Liu L (2024) Influence of Magnesium Oxide (MgO) nanoparticles on maize (Zea mays L.). Agronomy 14(3), 617.
| Crossref | Google Scholar |
Abbasi Surki A, Nazari M, Fallah S, Iranipour R, Mousavi A (2020) The competitive effect of almond trees on light and nutrients absorption, crop growth rate, and the yield in almond–cereal agroforestry systems in semi-arid regions. Agroforestry Systems 94(3), 1111-1122.
| Crossref | Google Scholar |
Abdel Latef AA, Tran L-SP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Frontiers in Plant Science 7, 243.
| Crossref | Google Scholar |
Adil M, Bashir S, Bashir S, Aslam Z, Ahmad N, Younas T, Asghar RMA, Alkahtani J, Dwiningsih Y, Elshikh MS (2022) Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Frontiers in Plant Science 13, 932861.
| Crossref | Google Scholar |
Ahmad I, Ahmad B, Boote K, Hoogenboom G (2020) Adaptation strategies for maize production under climate change for semi-arid environments. European Journal of Agronomy 115, 126040.
| Crossref | Google Scholar |
Ahmad I, Ahmad W, Nepal J, Junaid MB, Bukhari NA, Usman M, Ahmad N, Khan RN (2024) Synergistic enhancement of maize crop yield and nutrient assimilation via zinc oxide nanoparticles and phosphorus fertilization. Journal of the Science of Food and Agriculture 104(11), 6733-6745.
| Crossref | Google Scholar | PubMed |
Ahmed N, Zhang B, Bozdar B, Chachar S, Rai M, Li J, Li Y, Hayat F, Chachar Z, Tu P (2023) The power of magnesium: unlocking the potential for increased yield, quality, and stress tolerance of horticultural crops. Frontiers in Plant Science 14, 1285512.
| Crossref | Google Scholar |
Ahmed AF, Fawzy M, Al-zahrani M, Abdelkader M (2025) Enhancing metabolic processes and water deficit stress tolerance of maize plants through biochar addition and foliar application of zinc nanoparticles. Russian Journal of Plant Physiology 72(1), 10.
| Crossref | Google Scholar |
Akhtar MS, Fiaz S, Aslam S, Chung S, Ditta A, Irshad MA, et al. (2024) Green synthesis of magnetite iron oxide nanoparticles using Azadirachta indica leaf extract loaded on reduced graphene oxide and degradation of methylene blue. Scientific Reports 14(1), 18172.
| Crossref | Google Scholar |
Akram Z, Hussain S, Mansoor M, Afzal M, Waqar A, Shabbir I (2014) Soil fertility and salinity status of Muzaffargarh District, Punjab Pakistan. Universal Journal of Agricultural Research 2, 242-249.
| Crossref | Google Scholar |
Al-Harbi LM, Ezzeldien M, Elhenawy AA, Said AH (2024) Assessment of the bioactivity of bioinspired magnesium oxide nanoparticles from the Azadirachta indica extract. Frontiers in Bioengineering and Biotechnology 12, 1480694.
| Crossref | Google Scholar |
Al-Kordy HMH, Sabry SA, Mabrouk MEM (2021) Statistical optimization of experimental parameters for extracellular synthesis of zinc oxide nanoparticles by a novel haloalaliphilic Alkalibacillus sp.W7. Scientific Reports 11, 10924.
| Crossref | Google Scholar |
Al-Selwey WA, Alsadon AA, Ibrahim AA, Labis JP, Seleiman MF (2023) Effects of zinc oxide and silicon dioxide nanoparticles on physiological, yield, and water use efficiency traits of potato grown under water deficit. Plants 12(1), 218.
| Crossref | Google Scholar |
Alabdallah NM, Alzahrani HS (2020) The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi Journal of Biological Sciences 27(11), 3132-3137.
| Crossref | Google Scholar | PubMed |
Alexakis D, Gotsis D, Giakoumakis S (2012) Assessment of drainage water quality in pre- and post-irrigation seasons for supplemental irrigation use. Environmental Monitoring and Assessment 184, 5051-5063.
| Crossref | Google Scholar | PubMed |
Ali S, Ulhassan Z, Ali S, Kaleem Z, Yousaf MA, Sheteiwy MS, Ali S, Waseem M, Jalil S, Wang J, Zhou W (2024) Differential responses of Brassica napus cultivars to dual effects of magnesium oxide nanoparticles. Environmental Science and Pollution Research 31(8), 12446-12466.
| Crossref | Google Scholar | PubMed |
Almeida DM, Oliveira MM, Saibo NJM (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genetics and Molecular Biology 40(1 suppl 1), 326-345.
| Crossref | Google Scholar | PubMed |
Alves TEP, Diniz AGA, Safadi GMVV, Silva-Neto CM (2024) Absorption of commercial and nanoparticulate ZnO and MgO synthesized by combustion reaction applied to maize soil. Environmental Nanotechnology, Monitoring & Management 22, 101005.
| Crossref | Google Scholar |
Amin ZS, Afzal M, Ahmed N, Zeshan B, Hashim NHHN, Yean CY (2023) Synthesis, characterization and biological activities of zinc oxide nanoparticles derived from secondary metabolites of Lentinula edodes. Molecules 28(8), 3532.
| Crossref | Google Scholar |
An Y, Gao Y, Tong S, Liu B (2021) Morphological and physiological traits related to the response and adaption of Bolboschoenus planiculmis seedlings grown under salt-alkaline stress conditions. Frontiers in Plant Science 12, 567782.
| Crossref | Google Scholar |
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology 24, 1-15.
| Crossref | Google Scholar | PubMed |
Arrobas M, Afonso S, Ferreira IQ, Moutinho-Pereira J, Correia CM, Rodrigues MÂ (2017) Liming and application of nitrogen, phosphorus, potassium, and boron on a young plantation of chestnut. Turkish Journal of Agriculture and Forestry 41(6), 441-451.
| Crossref | Google Scholar |
Azarin K, Usatov A, Minkina T, Plotnikov A, Kasyanova A, Fedorenko A, Duplii N, Vechkanov E, Rajput VD, Mandzhieva S, Alamri S (2022) Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 287, 132167.
| Crossref | Google Scholar |
Baloch Q, Kubar KA, Korai PK, Kalhoro SA, Junaid MB, Baloch MA, Zia-ul-hassan , Alkahtani J, Abrar M (2025) Evaluating vertical distribution of soil salinity patterns across multiple soil depths in a semi-arid dry region. Journal of Soil Science and Plant Nutrition 25, 5173-5185.
| Crossref | Google Scholar |
Barzegar M, Ahmadvand D, Sabouri Z, Darroudi M (2024) Phytoextract-mediated synthesis of magnesium oxide nanoparticles using Caccinia macranthera extract and examination of their photocatalytic and anticancer effects. Materials Research Bulletin 169, 112514.
| Crossref | Google Scholar |
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205-207.
| Crossref | Google Scholar |
Bayat M, Zargar M, Murtazova KM-S, Nakhaev MR, Shkurkin SI (2022) Ameliorating seed germination and seedling growth of nano-primed wheat and flax seeds using seven biogenic metal-based nanoparticles. Agronomy 12(4), 811.
| Crossref | Google Scholar |
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248-254.
| Crossref | Google Scholar | PubMed |
Broadley MR, White PJ (2010) Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proceedings of the Nutrition Society 69(4), 601-612.
| Crossref | Google Scholar | PubMed |
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum 83(3), 463-468.
| Crossref | Google Scholar |
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods in Enzymology 2, 764-775.
| Crossref | Google Scholar |
Chatepa LEC, Mwamatope B, Chikowe I, Masamba KG (2024) Effects of solvent extraction on the phytoconstituents and in vitro antioxidant activity properties of leaf extracts of the two selected medicinal plants from Malawi. BMC Complementary Medicine and Therapies 24(1), 317.
| Crossref | Google Scholar |
Dai F, Zhuang Q, Huang G, Deng H, Zhang X (2023) Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega 8(19), 17064-17076.
| Crossref | Google Scholar | PubMed |
Dangana RS, George RC, Shittu UO, Agboola FK (2023) Facile biosynthesis, characterisation and biotechnological application of ZnO nanoparticles mediated by leaves of Cnidoscolus aconitifolius. Artificial Cells, Nanomedicine, and Biotechnology 51(1), 309-317.
| Crossref | Google Scholar | PubMed |
de Sousa Ferreira L, de Souza Oliveira V, de Paula Marchiori JJ, Ferreira TC, Bernabé ACB, Boone GTF, dos Santos Pereira LL, Carriço E (2023) The nutrient magnesium in soil and plant: a review. International Journal of Plant & Soil Science 35(8), 136-144.
| Crossref | Google Scholar |
Deepak TS, Jayadeep PA (2022) Prospects of maize (corn) wet milling by-products as a source of functional food ingredients and nutraceuticals. Food Technology and Biotechnology 60(1), 109-120.
| Crossref | Google Scholar |
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Science 135(1), 1-9.
| Crossref | Google Scholar |
Du J, AL-Huqail A, Cao Y, Yao H, Sun Y, Garaleh M, El Sayed Massoud E, Ali E, Assilzadeh H, Escorcia-Gutierrez J (2024) Green synthesis of zinc oxide nanoparticles from Sida acuta leaf extract for antibacterial and antioxidant applications, and catalytic degradation of dye through the use of convolutional neural network. Environmental Research 258, 119204.
| Crossref | Google Scholar |
Dutta S, Sinelshchikova A, Andreo J, Wuttke S (2024) Nanoscience and nanotechnology for water remediation: an earnest hope toward sustainability. Nanoscale Horizons 9(6), 885-899.
| Crossref | Google Scholar | PubMed |
Eissa D, Hegab RH, Abou-Shady A, Kotp YH (2022) Green synthesis of ZnO, MgO and SiO2 nanoparticles and its effect on irrigation water, soil properties, and Origanum majorana productivity. Scientific Reports 12(1), 5780.
| Crossref | Google Scholar |
El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, M. Abdel-Daim M, Prasad Devkota H (2020) Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L.(Fabaceae). Biomolecules 10(3), 352.
| Crossref | Google Scholar |
Faisal S, Naqvi S, Ali M, Lin L (2022) Comparative study of multifunctional properties of synthesised ZnO and MgO NPs for textiles applications. Pigment & Resin Technology 51(3), 301-308.
| Crossref | Google Scholar |
Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56, 678-686.
| Crossref | Google Scholar |
Faizan M, Alam P, Rajput VD, Faraz A, Afzal S, Ahmed SM, Yu F-Y, Minkina T, Hayat S (2023) Nanoparticle mediated plant tolerance to heavy metal stress: what we know? Sustainability 15(2), 1446.
| Crossref | Google Scholar |
Fernandes EA, dos Anjos Soares LA, de Lima GS, de Sousa Silva Neta AM, Roque IA, da Silva FA, et al. (2021) Cell damage, gas exchange, and growth of Annona squamosa L. under saline water irrigation and potassium fertilization. Semina: Ciências Agrárias, Londrina 42(3), 999-1018.
| Crossref | Google Scholar |
Gautam A, Sharma P, Ashokhan S, Yaacob JS, Kumar V, Guleria P (2023) Inhibitory impact of MgO nanoparticles on oxidative stress and other physiological attributes of spinach plant grown under field condition. Physiology and Molecular Biology of Plants 29(12), 1897-1913.
| Crossref | Google Scholar | PubMed |
Ghaffari Yaichi Z, Hassanpouraghdam MB, Rasouli F, Aazami MA, Vojodi Mehrabani L, Jabbari SF, Asadi M, Esfandiari E, Jimenez-Becker S (2025) Zinc oxide nanoparticles foliar use and arbuscular mycorrhiza inoculation retrieved salinity tolerance in Dracocephalum moldavica L. by modulating growth responses and essential oil constituents. Scientific Reports 15(1), 492.
| Crossref | Google Scholar |
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology 59(2), 309-314.
| Crossref | Google Scholar | PubMed |
Girotti AW, Korytowski W (2023) Trafficking of oxidative stress-generated lipid hydroperoxides: pathophysiological implications. Free Radical Research 57(2), 130-139.
| Crossref | Google Scholar | PubMed |
Gowtham HG, Shilpa N, Singh SB, Aiyaz M, Abhilash MR, Nataraj K, Amruthesh KN, Ansari MA, Alomary MN, Murali M (2024) Toxicological effects of nanoparticles in plants: mechanisms involved at morphological, physiological, biochemical and molecular levels. Plant Physiology and Biochemistry 210, 108604.
| Crossref | Google Scholar |
Gupta A, Bharati R, Kubes J, Popelkova D, Praus L, Yang X, Severova L, Skalicky M, Brestic M (2024) Zinc oxide nanoparticles application alleviates salinity stress by modulating plant growth, biochemical attributes and nutrient homeostasis in Phaseolus vulgaris L. Frontiers in Plant Science 15, 1432258.
| Crossref | Google Scholar |
Hasan MK, Ara I, Mondal MSA, Kabir Y (2021) Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 7(6), e07240.
| Crossref | Google Scholar |
Hassan ZA, Sarwar G, Aftab M, Manzoor MZ, Zafar A, Shehzad I, Riaz A, Hussain S, Sattar A, Niaz A (2022) Noxious impact of sodium bicarbonate (NaHCO3) on soil properties and ionic composition of rice plants. Journal of Agricultural Research 60(3), 193-202.
| Google Scholar |
Hu D, Li R, Dong S, Zhang J, Zhao B, Ren B, Ren H, Yao H, Wang Z, Liu P (2022) Maize (Zea mays L.) responses to salt stress in terms of root anatomy, respiration and antioxidative enzyme activity. BMC Plant Biology 22(1), 602.
| Crossref | Google Scholar |
Ikhuoria EU, Uwidia IE, Otabor GO, Ifijen IH (2024) Comparative analysis of magnesium oxide nanoparticles biosynthesized from rubber seed shell and rubber leaf extracts. Biomedical Materials & Devices 2(2), 1078-1088.
| Crossref | Google Scholar |
Inam A, Javad S, Naseer I, Alam P, Almutairi ZM, Faizan M, Zauq S, Shah AA (2024) Efficacy of chitosan loaded zinc oxide nanoparticles in alleviating the drastic effects of drought from corn crop. Plant Stress 14, 100617.
| Crossref | Google Scholar |
Irigoyen JJ, Einerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiologia Plantarum 84(1), 55-60.
| Crossref | Google Scholar |
Jamali K, Sahito F, Shah Z-u-H, Talpur NA, Rajpar I, Babar SK, Talpur KH, Afzal J, Amur A, Leghari Z (2023) Spatial variability mapping of selected soil properties of District Tando Allahyar, Sindh, Pakistan. Journal of Applied Research in Plant Sciences 4, 625-636.
| Crossref | Google Scholar |
Jamil M, Akhtar N, Iqbal MM, Khan MUH, Muslim N, Qazi MA (2021) Indexing of physico-chemical variables and fertility status of district Sahiwal soils, Punjab, Pakistan. Soil & Environment 40(1), 95-101.
| Crossref | Google Scholar |
Jampilek J, Kralova K (2022) Advances in biologically applicable graphene-based 2D nanomaterials. International Journal of Molecular Sciences 23(11), 6253.
| Crossref | Google Scholar |
Johnson-Beebout SE, Lauren JG, Duxbury JM (2009) Immobilization of zinc fertilizer in flooded soils monitored by adapted DTPA soil test. Communications in Soil Science and Plant Analysis 40(11–12), 1842-1861.
| Crossref | Google Scholar |
Jones MM, Turner NC (1978) Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiology 61(1), 122-126.
| Crossref | Google Scholar | PubMed |
Kaczorowski R, Forenc T, Hunia J, Górny J, Janiszewski M, Jurek J, Komorowski M, Kapciak A, Pelczarska A (2024) Magnesium and zinc as vital micronutrients enhancing athletic performance and recovery–a review. Quality in Sport 33, 56021.
| Crossref | Google Scholar |
Kaleem M, Shah AA, Usman S, Xu W, Alsahli AA (2025) Magnesium oxide nanoparticles improved drought resilience in Coriander sativum L. through lifting antioxidant level, redox balancing, and improving photosynthetic efficiency. ACS Omega 10, 32813-32828.
| Crossref | Google Scholar | PubMed |
Kamath SD, Arunkumar D, Avinash NG, Samshuddin S (2015) Determination of total phenolic content and total antioxidant activity in locally consumed food stuffs in Moodbidri, Karnataka, India. Advances in Applied Science Research 6(6), 99-102.
| Google Scholar |
Kangathara N, Sabari V, Saravanan L, Elangovan S (2022) Synthesis, characterization, and comparison of pure zinc oxide and magnesium-doped zinc oxide nanoparticles and their application on ethanol sensing activities. Journal of Nanomaterials 2022(1), 1769278.
| Crossref | Google Scholar |
Kanwal S, Ilyas N, Shabir S, Saeed M, Gul R, Zahoor M, Batool N, Mazhar R (2018) Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). Journal of Plant Nutrition 41(4), 526-538.
| Crossref | Google Scholar |
Kaur K, Jayarambabu N, Rao KV (2022) Comparative study of chemo-bio synthesized mgo nanoparticle on maize seed germination. IOP Conference Series: Materials Science and Engineering 1225, 012045.
| Crossref | Google Scholar |
Khan KS, Naveed M, Qadir MF, Yaseen M, Siddiqui MH (2023) Bio-organically acidified product-mediated improvements in phosphorus fertilizer utilization, uptake and yielding of Zea mays in calcareous soil. Plants 12(17), 3072.
| Crossref | Google Scholar |
Kiran S, Albargi HB, Afzal G, Aimun U, Anjum MN, Qadir MB, Khaliq Z, Jalalah M, Irfan M, Abdullah MM (2023) Azadirachta indica-assisted green synthesis of magnesium oxide nanoparticles for degradation of Reactive Red 195 dye: a sustainable environmental remedial approach. Applied Water Science 13(10), 193.
| Crossref | Google Scholar |
Kumar N, Nigam A, Pathak AK (2022) Assessment of genetic variability and path analysis in carrot (Daucus carota L.) genotypes. International Journal of Horticulture and Food Science 4, 1-9.
| Crossref | Google Scholar |
Kumar K, Jaiswal A, Koppolu UMK, Kumar KRR (2024) Alkaline stress disrupts growth, biochemistry, and ion homeostasis of chickpea (Cicer arietinum L.) roots. Frontiers in Agronomy 6, 1497054.
| Crossref | Google Scholar |
Lalarukh I, Zahra N, Al Huqail AA, Amjad SF, Al-Dhumri SA, Ghoneim AM, Alshahri AH, Almutari MM, Alhusayni FS, Al-Shammari WB, Poczai P, Mansoora N, Ayman M, Abbas MHH, Abdelhafez AA (2022) Exogenously applied ZnO nanoparticles induced salt tolerance in potentially high yielding modern wheat (Triticum aestivum L.) cultivars. Environmental Technology & Innovation 27, 102799.
| Crossref | Google Scholar |
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochemical Journal 210(3), 899-903.
| Crossref | Google Scholar | PubMed |
Leach KA, Braun DM (2016) Soluble sugar and starch extraction and quantification from maize (Zea mays) leaves. Current Protocols in Plant Biology 1(1), 139-161.
| Crossref | Google Scholar | PubMed |
Lian J, Cheng L, Zhai X, Wu R, Liu W, Pan J, Shohag MJI, Xin X, He Z, Yang X (2022) Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of Cd in wheat (Triticum aestivum L.). Journal of Hazardous Materials 440, 129857.
| Crossref | Google Scholar |
Liang Y, Liu H, Fu Y, Li P, Li S, Gao Y (2023) Regulatory effects of silicon nanoparticles on the growth and photosynthesis of cotton seedlings under salt and low-temperature dual stress. BMC Plant Biology 23(1), 504.
| Crossref | Google Scholar |
Liu Z, Yuan D, Qin X, He P, Fu Y (2023) Effect of Mg-modified waste straw biochar on the chemical and biological properties of acidic soils. Molecules 28(13), 5225.
| Crossref | Google Scholar |
Ma Q, Wang H, Wu E, Yuan Y, Feng Y, Zhao L, Feng B (2023) Comprehensive physiological, transcriptomic, and metabolomic analysis of the response of Panicum miliaceum L. roots to alkaline stress. Land Degradation & Development 34(10), 2912-2930.
| Crossref | Google Scholar |
Mai Y, Ren Y, Deng S, Ashraf U, Tang X, Duan M, Mo Z (2024) Influence of ZnO nanoparticles on early growth stage of fragrant rice at low temperature (LT) stress. Journal of Soil Science and Plant Nutrition 24(1), 1301-1317.
| Crossref | Google Scholar |
Mansoor S, Ali Wani O, Lone JK, Manhas S, Kour N, Alam P, Ahmad A, Ahmad P (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11(2), 225.
| Crossref | Google Scholar |
Mardi A, Mohajjel Shoja H, Mohajel Kazemi E (2022) Comparative study of growth responses, photosynthetic pigment content, and gene expression pattern in tobacco plants treated with ZnO nano and ZnO bulk particles. Journal of Nanoparticle Research 24(10), 208.
| Crossref | Google Scholar |
Masood N, Hudson-Edwards KA, Zafar T, Farooqi A (2023) Natural carbon mineralization and its control on the geochemical evolution of coal-based aquifers in the Salt Range, Punjab, Pakistan. Environmental Geochemistry and Health 45(10), 7033-7050.
| Crossref | Google Scholar | PubMed |
Mazher M, Ishtiaq M, Hamid B, Haq SM, Mazhar A, Bashir F, Mazhar M, Mahmoud EA, Casini R, Alataway A, Dewidar AZ, Elansary HO (2023) Biosynthesis and characterization of calcium oxide nanoparticles from Citrullus colocynthis fruit extracts; their biocompatibility and bioactivities. Materials 16(7), 2768.
| Crossref | Google Scholar |
Mierek-Adamska A, Kulasek M, Dąbrowska GB, Blindauer CA (2025) Type 4 plant metallothioneins–players in zinc biofortification? Biological Reviews 100, 1229-1249.
| Crossref | Google Scholar | PubMed |
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3), 426-428.
| Crossref | Google Scholar |
Mirrani HM, Noreen Z, Usman S, Shah AA, Mahmoud EA, Elansary HO, Aslam M, Waqas A, Javed T (2024) Magnesium nanoparticles extirpate salt stress in carrots (Daucus carota L.) through metabolomics regulations. Plant Physiology and Biochemistry 207, 108383.
| Crossref | Google Scholar |
Moustakas M, Dobrikova A, Sperdouli I, Hanć A, Moustaka J, Adamakis I-DS, Apostolova E (2024) Photosystem II tolerance to excess zinc exposure and high light stress in Salvia sclarea L. Agronomy 14(3), 589.
| Crossref | Google Scholar |
Mustafa G, Chaudhari SK, Manzoor M, Batool S, Hatami M, Hasan M (2024) Zinc oxide nanoparticles mediated salinity stress mitigation in Pisum sativum: a physio-biochemical perspective. BMC Plant Biology 24(1), 835.
| Crossref | Google Scholar |
Nadeem M, Nazer Khan M, Abbas G, Fatima Z, Iqbal P, Ahmed M, Ali Raza M, Rehman A, Ul Haq E, Hayat A, Ali M, Ahmad S (2022) Application of CSM-CANEGRO model for climate change impact assessment and adaptation for sugarcane in semi-arid environment of Southern Punjab, Pakistan. International Journal of Plant Production 16(3), 443-466.
| Crossref | Google Scholar |
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22(5), 867-880.
| Crossref | Google Scholar |
Ni Y, Yang T, Ma Y, Zhang K, Soltis PS, Soltis DE, et al. (2021) Soil pH determines bacterial distribution and assembly processes in natural mountain forests of eastern China. Global Ecology and Biogeography 30(11), 2164-2177.
| Crossref | Google Scholar |
Ntshanka NM, Ejidike IP, Mthunzi FM, Moloto MJ, Mubiayi KP (2020) Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomedical and Pharmacology Journal 13(4), 1683-1694.
| Crossref | Google Scholar |
Ogunyemi SO, Zhang M, Abdallah Y, Ahmed T, Qiu W, Ali MA, et al. (2020) The bio-synthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and their antibacterial activity against the bacterial leaf blight pathogen. Frontiers in Microbiology 11, 588326.
| Crossref | Google Scholar |
Olasan JO, Uzoma AC, Thomas AT, Ndidiamaka A, Mary DD (2024) Effect of magnesium oxide (MgO) nanoparticles on the performance of black-seeded beniseed (Sesamum indicum L.). Acta Chemica Malaysia (ACMY) 8(2), 107-113.
| Crossref | Google Scholar |
Opsahl S, Benner R (1999) Characterization of carbohydrates during early diagenesis of five vascular plant tissues. Organic Geochemistry 30(1), 83-94.
| Crossref | Google Scholar |
Pare B, Barde VS, Solanki VS, Agarwal N, Yadav VK, Alam MM, Gacem A, Alsufyani T, Khedher NB, Park J-W, Park S, Jeon B-H (2022) Green synthesis and characterization of LED-Irradiation-responsive nano ZnO catalyst and photocatalytic mineralization of malachite green dye. Water 14, 3221.
| Crossref | Google Scholar |
Parusnath M, Naidoo Y, Singh M, Rihan H, Dewir YH (2023) Phytochemical composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels leaf and stem extracts. Plants 12(8), 1702.
| Crossref | Google Scholar |
Pérez-Labrada F, Espinoza-Acosta JL, Bárcenas-Santana D, García-León E, López-Pérez MC (2024) Underlying mechanisms of action to improve plant growth and fruit quality in crops under alkaline stress. In ‘Abiotic Stress in crop plants-ecophysiological responses and molecular approaches’. (Eds M Hasanuzzaman, K Nahar) (IntechOpen)
Proshad R, Li J, Sun G, Zheng X, Yue H, Chen G, Zhang S, Li Z, Zhao Z (2024) Field application of hydroxyapatite and humic acid for remediation of metal-contaminated alkaline soil. Environmental Science and Pollution Research 31(9), 13155-13174.
| Crossref | Google Scholar | PubMed |
Qu R, Liu N, Wen Q, Guo J, Ge F (2024) Molecular mechanism of dissolvable metal nanoparticles-enhanced CO2 fixation by algae: metal-chlorophyll synthesis. Environmental Pollution 349, 123987.
| Crossref | Google Scholar |
Rajput VD, Harish , Singh RK, Verma KK, Sharma L, Quiroz-Figueroa FR, et al. (2021) Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 10(4), 267.
| Crossref | Google Scholar |
Rasouli F, Asadi M, Hassanpouraghdam MB, Aazami MA, Ebrahimzadeh A, Kakaei K, Dokoupil L, Mlcek J (2022) Foliar application of ZnO-NPs influences chlorophyll fluorescence and antioxidants pool in Capsicum annum L. under salinity. Horticulturae 8(10), 908.
| Crossref | Google Scholar |
Rathod A, Verma NS (2023) Impact of abiotic stress on agronomical crops. Frontiers of Agronomy 27,.
| Google Scholar |
Raza MAS, Muhammad F, Farooq M, Aslam MU, Akhter N, Toleikienė M, Binobead MA, Ali MA, Rizwan M, Iqbal R (2025) ZnO-nanoparticles and stage-based drought tolerance in wheat (Triticum aestivum L.): effect on morpho-physiology, nutrients uptake, grain yield and quality. Scientific Reports 15(1), 5309.
| Crossref | Google Scholar |
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, et al. (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269-277.
| Crossref | Google Scholar | PubMed |
Roque IA, Soares LADA, Lima GSD, Lopes IAP, Silva LDA, Fernandes PD (2022) Biomass, gas exchange and production of cherry tomato cultivated under saline water and nitrogen fertilization. Revista Caatinga 35(3), 686-696.
| Crossref | Google Scholar |
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10, 277.
| Crossref | Google Scholar |
Segatto C, Souza CA, Fiori MA, Lajús CR, Silva LL, Riella HG (2023) Seed treatment with magnesium nanoparticles alters phenology and increases grain yield and mineral content in maize. Australian Journal of Crop Science 17(2), 165-178.
| Crossref | Google Scholar |
Seghir BB, Hima M, Moulatti F, Sahraoui I, Ben Amor I, Zeghoud S, et al. (2023) Exploring the antibacterial potential of green-synthesized MgO and ZnO nanoparticles from two plant root extracts. Nanomaterials 13(17), 2425.
| Crossref | Google Scholar |
Seleiman MF, Ahmad A, Alhammad BA, Tola E (2023a) Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy 13(10), 2645.
| Crossref | Google Scholar |
Seleiman MF, Ahmad A, Alshahrani TS (2023b) Integrative effects of zinc nanoparticle and PGRs to mitigate salt stress in maize. Agronomy 13(6), 1655.
| Crossref | Google Scholar |
Shanmugam J, Sharmili Sundararaj A, Shanmugasundaram R, Ravichandran B, Mani M, Mohammed Riyaz SU, Dhayalan M, Cid-Samamed A, Simal-Gandara J (2023) Green preparation of bract extract (Musa acuminate) doped magnesium oxide nanoparticles and their bioefficacy. Applied Organometallic Chemistry 37(5), e7063.
| Crossref | Google Scholar |
Shi Y, Jin X, Ackah M, Amoako FK, Li J, Tsigbey VE, Li H, Cui Z, Sun L, Zhao C, Zhao C (2024) Comparative physio-biochemical and transcriptome analyses reveal contrasting responses to magnesium imbalances in leaves of mulberry (Morus alba L.) plants. Antioxidants 13(5), 516.
| Crossref | Google Scholar |
Silva AA, Sousa AMF, Furtado CRG, Carvalho NMF (2022) Green magnesium oxide prepared by plant extracts: synthesis, properties and applications. Materials Today Sustainability 20, 100203.
| Crossref | Google Scholar |
Singh A, Rajput VD, Lalotra S, Agrawal S, Ghazaryan K, Singh J, Minkina T, Rajput P, Mandzhieva S, Alexiou A (2024a) Zinc oxide nanoparticles influence on plant tolerance to salinity stress: insights into physiological, biochemical, and molecular responses. Environmental Geochemistry and Health 46(5), 148.
| Crossref | Google Scholar |
Singh A, Sengar RS, Rajput VD, Al-Ghzawi AL, Shahi UP, Ghazaryan K, Minkina T, Tawaha ARMA, Al Zoubi OM, Habeeb T (2024b) Impact of salinity stress and zinc oxide nanoparticles on macro and micronutrient assimilation: unraveling the link between environmental factors and nutrient uptake. Journal of Ecological Engineering 25(2), 1-9.
| Crossref | Google Scholar |
Singh KM, Jha AB, Dubey RS, Sharma P (2025) Nanoparticle-mediated mitigation of salt stress-induced oxidative damage in plants: insights into signaling, gene expression, and antioxidant mechanisms. Environmental Science: Nano 12, 2983-3017.
| Crossref | Google Scholar |
Song J, Yu S, Yang R, Xiao J, Liu J (2023) Opportunities for the use of selenium nanoparticles in agriculture. NanoImpact 31, 100478.
| Crossref | Google Scholar |
Sriramachandrasekharan MV, Priya NG, Manivannan R, Prakash M (2022) Ameliorative role of Silicon on osmoprotectants, antioxidant enzymes and growth of maize grown under alkaline stress. Silicon 14, 6577-6585.
| Crossref | Google Scholar |
Srivastav A, Ganjewala D, Singhal RK, Rajput VD, Minkina T, Voloshina M, Srivastava S, Shrivastava M (2021) Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants 10(12), 2556.
| Crossref | Google Scholar |
Šulinskienė J, Bernotienė R, Baranauskienė D, Naginienė R, Stanevičienė I, Kašauskas A, Ivanov L (2019) Effect of zinc on the oxidative stress biomarkers in the brain of nickel-treated mice. Oxidative Medicine and Cellular Longevity 2019(1), 8549727.
| Crossref | Google Scholar |
Syed S, Islam A, Shabeer M, Nadhman A, Ahmad F, Irfan N, Mehwish S, Khan A (2024) Biomedical applications of green synthesized zinc oxide and magnesium-doped zinc oxide nanoparticles using aqueous extract of ziziphus oxyphylla leaves. IEEE Transactions on NanoBioscience 23(3), 418-427.
| Crossref | Google Scholar | PubMed |
Tabande L, Sepehri M, Yasrebi J, Zarei M, Ghasemi-Fasaei R, Khatabi B (2021) The root endophytic fungus Serendipita indica decreased the phytotoxicity of zinc oxide nanoparticles to alfalfa (Medicago sativa L.). 10.21203/rs.3.rs-219701/v1
Taj A, Bibi H, Akbar WA, Rahim HU, Iqbal M, Ullah S (2023) Effect of poultry manure and NPK compound fertilizer on soil physicochemical parameters, NPK availability, and uptake by spring maize (Zea mays L.) in alkaline-calcareous soil. Gesunde Pflanzen 75(2), 393-403.
| Crossref | Google Scholar |
Taj H, Noreen Z, Aslam M, Usman S, Shah AA, Rafique M, Raja V, El-Sheikh MA (2024) Effects of SNP, MgSO4, and MgO-NPs foliar application on Spinacia oleracea L. growth and physio-biochemical responses under cadmium stress. Scientific Reports 14(1), 26687.
| Crossref | Google Scholar |
Tanweer T, Rana NF, Saleem I, Shafique I, Alshahrani SM, Almukhlifi HA, Alotaibi AS, Alshareef SA, Menaa F (2022) Dental composites with magnesium doped zinc oxide nanoparticles prevent secondary caries in the alloxan-induced diabetic model. International Journal of Molecular Sciences 23(24), 15926.
| Crossref | Google Scholar |
Tryfon P, Sperdouli I, Adamakis I-DS, Mourdikoudis S, Moustakas M, Dendrinou-Samara C (2023) Impact of coated zinc oxide nanoparticles on photosystem II of tomato plants. Materials 16(17), 5846.
| Crossref | Google Scholar |
Upadhyay S, Mantha AK, Dhiman M (2020) Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. Journal of Ethnopharmacology 258, 112690.
| Crossref | Google Scholar |
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science 151(1), 59-66.
| Crossref | Google Scholar |
Verma Y, Singh SK, Jatav HS, Rajput VD, Minkina T (2022) Interaction of zinc oxide nanoparticles with soil: insights into the chemical and biological properties. Environmental Geochemistry and Health 44, 221-234.
| Crossref | Google Scholar | PubMed |
Wang C, Wang H, Li Y, Li Q, Yan W, Zhang Y, Wu Z, Zhou Q (2021) Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microbial Biotechnology 14, 726-736.
| Crossref | Google Scholar | PubMed |
Wang W, Zhang F, Sun L, Yang L, Yang Y, Wang Y, Siddique KHM, Pang J (2022a) Alkaline salt inhibits seed germination and seedling growth of canola more than neutral salt. Frontiers in Plant Science 13, 814755.
| Crossref | Google Scholar |
Wang J, Li Q, Zhang M, Wang Y (2022b) The high pH value of alkaline salt destroys the root membrane permeability of Reaumuria trigyna and leads to its serious physiological decline. Journal of Plant Research 135(6), 785-798.
| Crossref | Google Scholar |
Weber J, Starchenko V, Yuan K, Anovitz LM, Ievlev AV, Unocic RR, Borisevich AY, Boebinger MG, Stack AG (2023) Armoring of MgO by a passivation layer impedes direct air capture of CO2. Environmental Science & Technology 57(40), 14929-14937.
| Crossref | Google Scholar | PubMed |
Wittschier N, Faller G, Hensel A (2009) Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. Journal of Ethnopharmacology 125(2), 218-223.
| Crossref | Google Scholar | PubMed |
Wu F, Fang Q, Yan S, Pan L, Tang X, Ye W (2020) Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environmental Science and Pollution Research 27, 26974-26981.
| Crossref | Google Scholar | PubMed |
Yang G, Rhodes D, Joly RJ (1996) Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Functional Plant Biology 23(4), 437-443.
| Crossref | Google Scholar |
Yang S, Xu Y, Tang Z, Jin S, Yang S (2024) The impact of alkaline stress on plant growth and its alkaline resistance mechanisms. International Journal of Molecular Sciences 25(24), 13719.
| Crossref | Google Scholar |
Zhai J, Xian X, Zhang Z, Wang Y (2025) Nano-zinc oxide can enhance the tolerance of apple rootstock M9-T337 seedlings to saline alkali stress by initiating a variety of physiological and biochemical pathways. Plants 14(2), 233.
| Crossref | Google Scholar |
Zhang D, Tang Y, Zhang C, Huhe FNU, Wu B, Gong X, Chuang SSC, Zheng J (2022) Formulating zwitterionic, responsive polymers for designing smart soils. Small 18(38), 2203899.
| Crossref | Google Scholar |
Łukasik I, Sytykiewicz H, Goławska S (2021) Effect of the cereal aphid infestation on the oxidative damages of protein in the maize (Zea mays L.). Acta Scientiarum Polonorum Hortorum Cultus 20(1), 81-89.
| Crossref | Google Scholar |