Injury and mortality of two Mekong River species exposed to turbulent shear forces
A. H. Colotelo A F , R. P. Mueller A , R. A. Harnish A , J. J. Martinez A , T. Phommavong B , K. Phommachanh C , G. Thorncraft B , L. J. Baumgartner D , J. M. Hubbard A , B. M. Rhode A and Z. D. Deng A E F
A , B. M. Rhode A and Z. D. Deng A E F
A Pacific Northwest National Laboratory, Earth Systems Sciences Division, 902 Battelle Boulevard, Richland, WA 99352, USA.
B Faculty of Agriculture, Forestry and Fisheries, National University of Laos, PO Box 7322, Dongdok, Vientiane, Laos.
C Living Aquatic Resources Research Center, PO Box 9108, Vientiane, Laos.
D Institute of Land, Water and Society, Charles Sturt University, Albury, NSW 2640, Australia.
E Department of Mechanical Engineering, Virginia Tech, 635 Prices Fork Road, MC 0238, Blacksburg, VA 24061, USA.
F Corresponding authors. Email: alisonha.colotelo@pnnl.gov; zhiqun.deng@pnnl.gov
Marine and Freshwater Research 69(12) 1945-1953 https://doi.org/10.1071/MF18126
Submitted: 24 March 2018 Accepted: 19 July 2018 Published: 4 October 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Global hydropower development is one solution proposed to address the increase in energy needs. However, hydropower-related impacts on riverine ecological systems are not well understood. The Mekong River Basin (MRB) is one of the world’s largest waterways and is presently experiencing significant hydropower expansion. It is also one of the most biodiverse rivers; serving as home to many species that are blocked or hindered by the development of dams. One source of injury and mortality for downstream moving fishes is passage through the turbine environment where fishes may be exposed to several physical stressors (e.g. shear forces, rapid decompression, blade strike and turbulence). The current study sought to understand the susceptibility of blue gourami (Trichopodus trichopterus) and iridescent shark (Pangasianodon hypophthalmus) to shear forces. Fishes were exposed to an underwater jet with velocities up to 21.3 m s–1 (equating to strain rates of up to 1185 s–1) and were assessed for behavioural effects, injuries and mortality. Overall, it was determined that both species were susceptible to the shear forces applied in this study and the effects were more pronounced at higher strain rates. Gouramis were more susceptible than sharks. To minimise impacts on these species, shear forces within turbines should not exceed critical limits.
Additional keywords : blue gourami, hydropower, iridescent shark, strain rate.
Introduction
Global hydropower development has helped provide countries with sufficient amounts of clean and inexpensive energy. Efforts to install additional hydro-systems in some of the world’s major waterways, such as the Amazon, Mekong and Congo rivers (Zarfl et al. 2015), promise to produce more energy, but are, in some instances, permanently damaging ecological resources. If not mitigated, such development may lead to large-scale fishery declines and reduced biodiversity (Baumgartner et al. 2017). Fish traveling downstream through hydropower facilities can experience a variety of stressors (e.g. rapid decompression, cavitation, blade strike and collision, turbulence and shear forces, Čada 2001) that may potentially lead to injury or mortality. This downstream passage is of concern, particularly for migratory species (Stephenson et al. 2010) that are predisposed to more frequent encounters with hydropower infrastructure.
The shear forces and associated stresses experienced by fish during downstream movements (including migration and drift) are one of the least understood injury mechanisms, because they are difficult to locate, measure and recreate (Neitzel et al. 2004). Shear occurs when two masses of water, moving at different velocities, intersect (Čada et al. 2007). Alternatively, they can occur when water slows near a solid structure such as turbine blades. Shear forces occur naturally in river systems, and are of little concern. However, elevated levels of shear stress can occur in many locations within a hydropower structure, such as spillways and turbines (Čada et al. 2006), and can exceed critical levels that affect fish welfare.
The prevalence of injury and mortality as a result of exposure to shear forces is difficult to measure in situ because the probability of exposure is dependent on the path that a single fish takes through the turbine unit and because it is nearly impossible to finitely determine the cause of injuries when fish are exposed to multiple stressors (Baumgartner et al. 2017). The probability of exposure to critical shear forces may be estimated using a combination of computational fluid-dynamics models and stream traces (Richmond et al. 2014). The probability of injury and mortality at a range of shear forces can be determined by controlled laboratory experiments (e.g. Neitzel et al. 2004; Deng et al. 2005, 2010). These two probabilities can be combined to determine the relative risk of a given species to shear injuries for a specific project, using tools such as the Biological Performance Assessment tool (Richmond et al. 2014).
Most studies investigating the effects of shear forces have focussed on controlled laboratory studies, because of the uncertainty associated with the magnitude of forces a fish is exposed to in field settings (Baumgartner et al. 2017). The use of this approach has allowed for the ability to control and compare different exposure profiles to better understand the critical factors. Neitzel et al. (2004) found that there were species-specific sensitivities to exposure to shear forces, because mortality and injury were observed in juvenile American shad (Alosa sapidissima) at lower strain rates than in juvenile Chinook salmon, Oncorhynchus tshawytscha, steelhead, O. mykiss, and rainbow trout, O. mykiss. The orientation of fish to the water flow has also been shown to affect the injury rate and severity for a given species. Fish oriented head first into the flow are more susceptible to injury and mortality than those oriented tail first (Neitzel et al. 2004). Finally, the exposure profile that a fish is exposed to is dependent on the hydraulic environment. Deng et al. (2010) compared the response of fish to two hydraulic scenarios, fast-fish-to-slow-water and slow-fish-to-fast-water, and determined that the fish responded differently to the two scenarios. The results of these studies have broadened our understanding of the effects of shear forces on fishes and helped inform future studies to provide conditions representative of those at hydropower projects.
The transboundary Mekong River in South-east Asia is second only to the Amazon River in fish biodiversity (Ziv et al. 2012). Many species inhabiting the Mekong River are migratory and undergo large-scale migrations, combined with downstream drift of eggs and larvae, in both the wet and dry seasons. There are plans to develop several new hydropower plants on the Mekong River mainstem and many of its tributaries, potentially threatening the migratory fish that utilise these portions of the river (Kuenzer et al. 2013; Nielson et al. 2015). The Mekong River Basin (MRB) provides food, transportation and income to ~65 million people, the majority of which rely on the subsistence fisheries as their main source of protein (Mekong River Commission 2010). Sustainability of the fisheries and food security in the basin are already of concern, even without the awaiting impoundment of the basin waterways (Ziv et al. 2012). Although hydropower developers in other river systems around the globe have implemented techniques to try and mitigate the effects of hydropower facilities on fish populations, the success of these projects is largely attributed to the suitability towards local species (Baumgartner et al. 2017). Few Mekong River fish species have been adequately studied to ascertain potential effects of hydropower construction. Designing a turbine that compliments the characteristics of all species would be conceptually difficult, and it has been, alternately, suggested that a design that improves passage for a few key species is the most effective use of time and resources (Nielson et al. 2015).
The objective of the present study was to evaluate the effects of shear stress on two Mekong River native species, the blue gourami, Trichopodus trichopterus (herein referred to as gouramis), and iridescent shark, Pangasianodon hypophthalmus (herein referred to as sharks). Both species are food sources for communities along the Mekong River, making significant movements through the river system and are at risk of population effects because of the construction of hydropower facilities. In fact, sharks have been listed as Endangered under the International Union for Conservation of Nature (ICUN) Red List because of the effects of habitat degradation, fragmentation of the river habitat and over-exploitation (Vidthayanon and Hogan 2011). Fish of both species were exposed to jet velocities ranging from 3.0 to 21.3 m s–1, representing strain rates ranging from 168 to 1185 s–1. This information can be used to determine thresholds of the strain rate that these species can withstand and can be used to inform the design and operation of turbines within the MRB.
Materials and methods
Fish acquisition and holding
Gouramis and sharks were acquired from AquariumFish.net (San Diego, CA, USA) in August 2016. Fish were held in 115-L tanks at Pacific Northwest National Laboratory’s Aquatics Research Laboratory (ARL) supplied with heated and conditioned Columbia River water (mean temperature = 26.1°C) until testing. All fish were subjected to a photoperiod of 12 h light–12 h dark and fed an ad libitum ration of Crave flakes and Nano (gouramis) and FotoTime (sharks).
Test facility
A rectangular fibreglass flume (9 m long, 1.2 m wide and 1.2 m deep) containing a submerged water jet was used to create a quantifiable shear environment consistent with conditions expected within a hydroelectric turbine (see Deng et al. 2010 for description of test apparatus). Flow was generated by using a centrifugal pump with a programmable electronic speed controller that could produce jet velocities in excess of 20 m s–1. Jet velocities were measured with a two-dimensional laser doppler velocimeter and then verified using a pitot tube before testing. A conical stainless-steel nozzle was bolted to a flange inside the flume at one end that had a terminus of 6.35-cm opening. A flow conditioner was incorporated upstream of the nozzle to reduce inlet turbulence. The jet exit was submerged ~0.6 m below the water surface during all tests. We developed a relationship between the pump rotational velocities and the centerline nozzle velocities that were obtained from the pitot-tube measurements at the exit of the nozzle. Exposure strain rate (e) was estimated using the equation
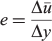
where ū is the mean water velocity (cm s–1) and y is distance (cm) perpendicular to ū. The units of exposure strain rate (cm s–1 cm–1) simplify to per second. The exposure strain rate was calculated according to the method presented by Neitzel et al. (2000). This method assumes that the flow within the deployment tube is zero, making the change in water velocity simply equal to the mean velocity of the jet. The change in distance used in calculating the exposure strain rate was 1.8 cm, which was the value determined by Neitzel et al. (2000) to be a suitable length scale for calculating the strain rate that juvenile salmonids were exposed to. Although the species presented in the study were different, this length scale was utilised to make it possible to compare directly with past studies. More detailed information on the above equation and formulae used to estimate other fluid characteristics of the jet are in Neitzel et al. (2000).
Release mechanisms and test conditions
Slow-fish-to-fast-water
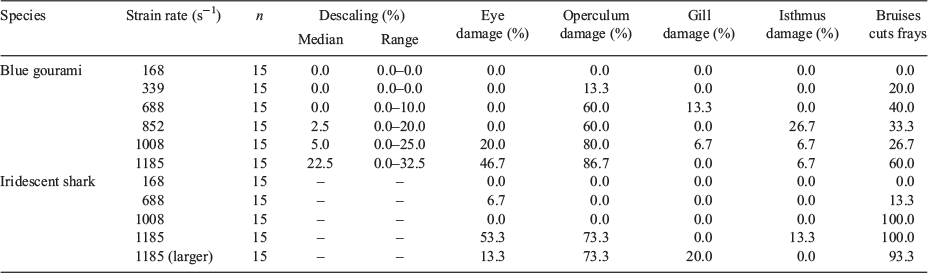
Test fish were introduced into the jet through a 60-cm-long × 3.18-cm-diameter polycarbonate introduction tube, which was fastened above the nozzle at an angle of 30° (Deng et al. 2005). The terminus of the introduction tube was positioned above and in front of the terminus of the nozzle with a 1-cm vertical gap to ensure that test fish contacted the water jet (Fig. 1). Fish were exposed to the following water-jet velocities: 3.0 (considered to be controls on the basis of the results of previous studies; Deng et al. 2005), 6.1 (gouramis only), 12.2, 15.2 (gouramis only), 18.3 and 21.3 m s–1. A group of larger sharks were also exposed to the jet velocity of 21.3 m s–1. The strain rates associated with the jet velocities tested are displayed in Table 1.
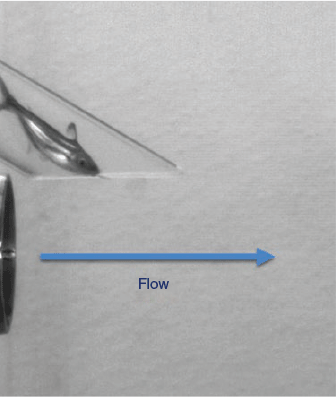
|

|
In total, 165 fish were tested from 4 to 6 October 2016. Test fish were dip-netted (5 at a time) from the holding tanks in the ARL to a 22-L pail supplied with aerated water. The dissolved oxygen (DO) and temperature were recorded in the test tank and in the transport pails before and at the end of testing for each 15-fish test group. For each test group, a fish was randomly captured from the pail using a 15.2-cm-long × 3.18-cm-diameter polycarbonate tubing (cartridge) containing a small volume of water, with removable rubber stoppers at each end (Fig. 2). Prior to subjecting fish to the shear environment, the fish was assessed for any pre-existing external injuries. The pump was started and allowed to achieve the desired water velocity (~3 s), at which point the cartridge containing the fish was placed at the top of the induction tube and one of the stoppers removed, allowing the fish to swim freely out into the introduction tube and, subsequently, into the shear zone. The total duration from fish capture to exposure was usually less than 30 s. As soon as the fish exited the introduction tube and passed through the shear zone, the pump was turned off and the exposed fish was captured using long handled dip nets.

|
Assessment of behaviour, injury and mortality for test fish
Swimming impairments such as loss of equilibrium, lethargy and disorientation were evaluated during recapture. Captured fish were transferred back into the cartridge and re-assessed for external injuries. Injury assessments included eye, gill operculum, isthmus injury, bruising, appendage tearing or hemorrhaging, and descaling. Fish were then placed in individual buckets with aerated water to monitor survival. After the fish were exposed to the shear environment, they were anesthetised using 40 or 120 mg L–1 of buffered MS-222 (for gouramis and sharks respectively), weighed, measured and given a unique fin clip. Fish were also assessed for any injuries while under anaesthesia, and photographs were taken. They were then held in troughs segregated by species and strain rate for 48 h to determine delayed mortality. At the end of the 48-h period, all test fish were euthanased.
Video recording and processing
Two identical high-speed digital cameras (Photron Fastcam Mini UX50, Photron USA, Inc., San Diego, CA, USA) equipped with 50-mm lenses and recording at 1000 frames s–1 were used to simultaneously record the exposure process of each fish. The cameras captured side (X–Y plane) and bottom (X–Z plane) views through polycarbonate viewing windows in the side and bottom of the tank. Halogen and LED lamps were positioned to provide the necessary illumination for high-speed videography. A solid white back panel was placed along the side wall of the tank opposite of the camera, to provide optimal contrast for the tracking of the test fish. The trajectories of two separate points on each fish (head and tail) were tracked manually frame-by-frame, with a motion-tracking software package (Visual Fusion 4.2, Boeing-SVS Inc., Albuquerque, NM, USA). The cameras recorded fish orientation and location from the moment a fish exited the introduction tube and contacted the shear zone until it was swept out of the immediate shear zone (~0.5 m) downstream. An entire exposure sequence lasted only a fraction of a second.
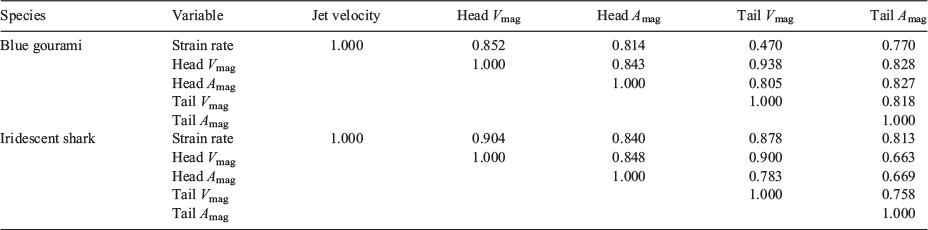
Calibration of the field of view for fish metrics was accomplished by using the outer diameter of the nozzle (63.5 mm) within the Visual Fusion software (Boeing-SVS Inc.) scaling tool. The side- and bottom-view tracks were then combined to form three-dimensional trajectories representing the test fish head and tail velocity and acceleration after exposure. Time series of velocity and acceleration were computed from the three-dimensional trajectories by using a five-point-stencil scheme and smoothed using a zero-phase forward and reverse digital filtering technique based on a running average filter. Finally, the peak values of each variable (velocity and acceleration for head and tail) were computed for each time series and used in the statistical analysis.
Statistical analysis
Multivariate regression was used to evaluate whether or not the velocities and accelerations experienced by the fish were correlated with the strain rate to which they were exposed. Relationships between the predictor variable (strain rate) and response variables (behavioural effects, injury and mortality) were modelled using generalised linear models that incorporated a logit-link function and Bernoulli error structure. Behavioural effects included abnormal or erratic swimming and loss of equilibrium, whereby fish that displayed any of these behaviours were considered ‘affected’ and fish that did not display these behaviours were classified as ‘unaffected’. For the injury evaluation, fish with injured eyes, opercules, gills or isthmus, or those that sustained ≥20% descaling, were considered ‘injured’. All other fish, except mortalities, were considered uninjured. Finally, fish that died within 48 h of shear exposure were considered as a mortality, and fish that did not die within the 48 h were classed as surviving.
Results
Study fish
In total, 165 fish were tested in the present study (90 gouramis and 75 sharks). Overall, gouramis had a median length of 58 mm (range: 45–72 mm) and a median mass of 3.8 g (range: 1.6–6.2 g). The sharks had a median length of 56 mm (43–67 mm) and a median mass of 2.6 g (1.5–4.3 g). The larger sharks had a median length of 76 mm (70–86 mm; Table 1).
Behavioural effects
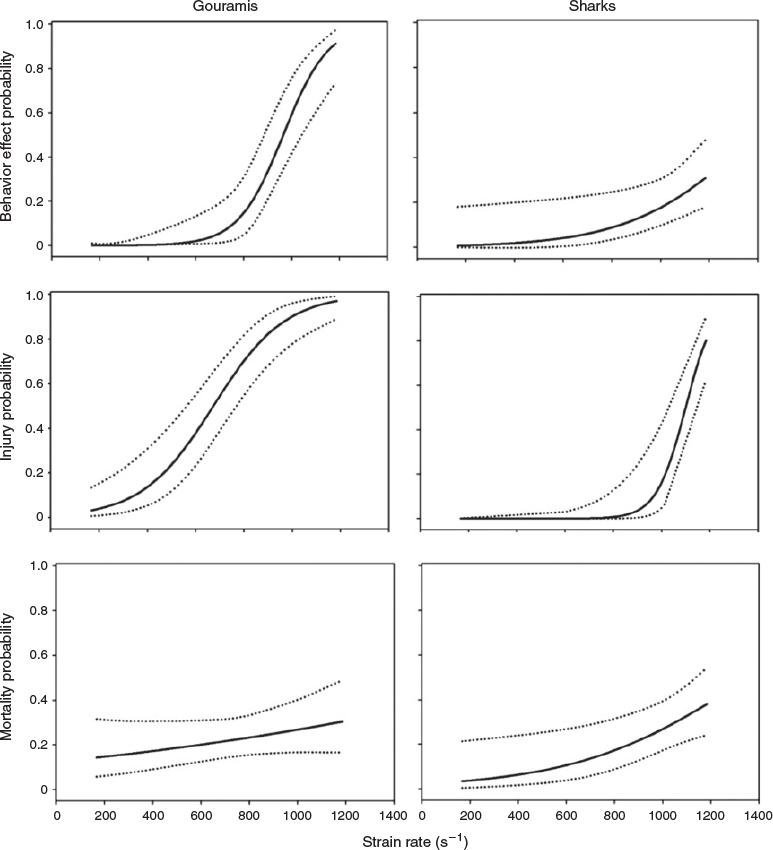
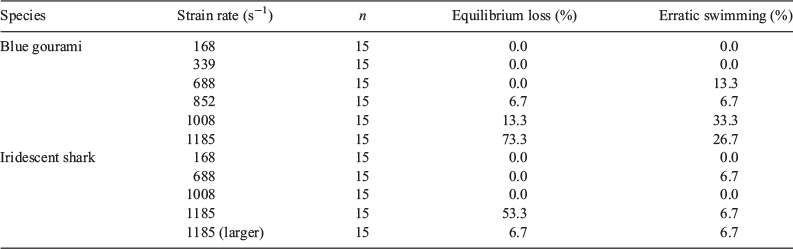
Two different abnormal swimming behaviours were observed immediately following exposure to the shear forces, namely, equilibrium loss and erratic swimming. Erratic swimming was observed following exposure to strain rates of 688 s–1 and greater, whereas equilibrium loss was observed for gouramis exposed to strain rates of 852–1185 s–1 and for sharks exposed to strain rates of 1185 s–1 (Table 2).
|
Strain-rate exposure level had a significant effect on the post-shear behaviour of gouramis and sharks (Table 3). Both species experienced a higher probability of displaying abnormal behaviours at higher strain rates (Fig. 3). Gouramis were more susceptible to behavioural effects at high strain rates, whereas sharks showed a lower probability of suffering such effects (Fig. 3).
Injuries
Individual descaling rates for gouramis exposed to shear forces ranged from 0.0 to 32.5% and increased with strain rate (Table 4). Operculum damage was the most common injury observed for gouramis, and was observed at the highest rate (86.7%) at strain rates of 1185 m s–1 (Table 4). Bruises, cuts and frays were the most common injuries observed for sharks, and were observed at the highest rate (100%) at strain rates of 1008 and 1185 s–1. Gill damage was the least common injury observed for both gouramis (13.3% at 688 s–1 strain rate) and sharks (20.0% at 1185 s–1 for the larger sharks).
Similar to the behaviour effects, the strain rate had a significant effect on the injury rates of gouramis and sharks (Table 3). Both species showed higher probabilities of injuries at higher strain rates (Fig. 3). Gouramis became injured at lower strain rates than did sharks (Fig. 3).
Mortality
Overall, mortality rates ranged from 0 to 67%, with the highest immediate and delayed mortality rates being observed in fish exposed to a strain rate of 1185 s–1 (Table 5). The exception to this trend was for gouramis, which survived at the lowest rate when exposed to a strain rate of 852 s–1. It was observed that both immediate and delayed mortality rates were lower for the larger sharks. Mortality probability was positively correlated with strain rate only for sharks (Table 3), and it reached 40% at the highest strain rate (1185 s–1; Fig. 3).

|
Video analysis
The maximum velocities and accelerations experienced by fish (considering the analysis of the two body points, i.e. head and tail) were highly correlated with the strain rate to which they were exposed (Table 6).
Discussion
Both gouramis and sharks are susceptible to shear forces during hydropower-facility passage. In fact, strain rate was significantly correlated with behavioural effects and injury rates for both species. For sharks, mortality (immediate and delayed) was also correlated with strain rates. When comparing the species tested in this study, gouramis were more susceptible, demonstrating higher rates of behavioural effects and injuries at lower strain rates.
Swimming impairments such as loss of equilibrium and erratic swimming are often indicative of negative whole-body responses to a stressor for fish and have been used to predict mortality (Davis 2010). They can also leave fish susceptible to predation immediately following hydropower facility passage (Walker et al. 2016). In the present study, a loss of equilibrium was observed in more than 50% of the test fish at a strain rate of 1185 s–1, for both species (excluding the larger sharks). Interestingly, the larger sharks did not lose equilibrium at similar rates, indicating that their increased size may aid in their ability to navigate through the turbulent experience (Hockley et al. 2014). Body shape may also have contributed to the incidence of behavioural effects. Gouramis are laterally compressed with deep bodies, providing a larger surface area for high water velocities to influence their movements, whereas sharks are more depressed (similar to other fishes of the order Siluriformes). Regardless of fish size or body form, our results showed that behavioural effects were correlated with strain rates for both species.
Injury was also correlated with the strain rate that fish were exposed to for both species. The injury types included in the present study are consistent with those included in the definition of major injuries from previous studies that investigated the effects of shear stresses on fish (Neitzel et al. 2004). For the purposes of the present analysis, fish that incurred injuries to the eyes, opercules, gills or isthmus, or sustained ≥20% descaling, were considered injured. In the present study, operculum damage was the most frequently occurring injury in both species, especially at the highest strain rates. For gourami, operculum damage was observed for fish exposed to strain rates of 339 s–1 (second-lowest tested) and higher, in as much as 86.7% (strain rate of 1185 s–1) of the test group. Sharks showed signs of operculum damage only at the highest strain rate of 1185 s–1. Operculum provides protection of the gills from physical forces and can also assist with respiration. Therefore, damage to the operculum can leave the fish susceptible to injury, infection, and can hinder the transfer of gases to the blood stream (Hughes 1984).
Cuts, bruises and fin frays were observed for nearly all treatment groups of gouramis and sharks (with the exception of strain rates of 168 s–1). However, the prevalence within each treatment group was highest for the sharks at the higher strain rates. In fact, 100% of the sharks exhibited bruises, cuts and fin fray following exposure to 1008 and 1185 s–1. This was also consistent with the larger sharks, where 93.3% of the test population exhibited these injuries. These injuries may appear to be less harmful than the others described; however, in combination with other injuries and behavioural impairments, they may have effects on the long-term survival of the individuals. Neitzel et al. (2004) identified them as not life-threatening injuries, except when bruises and cuts were large (>0.5 cm in diameter) or with visible bleeding.
Mortality is one of the simplest metrics used to evaluate the effects of passage through hydropower structures for fishes (Colotelo et al. 2017). This also makes it one of the most commonly used metrics in studies examining the effects of hydropower passage on fishes. In the present study, immediate mortality rates (within 15 min of exposure) were as high as 27% for gouramis and 40% for sharks. Delayed mortality rates (within 48 h) were as high as 27% for both species. For gouramis, overall mortality rates (immediate and delayed) were not correlated with strain rates; however, they were highest for the highest strain rate tested (1185 s–1). For sharks, overall mortality was correlated with strain rates, with 67% of the test population exposed to 1185 s–1 succumbing over the 48 h holding period. One-third of the larger sharks exposed to 1185 s–1 also died as a result of the exposure. The lower mortality rate for larger sharks may be due to an increased robustness in larger fish; however, further research is necessary to evaluate this hypothesis. Sharks can grow to be ~130 cm (Van Zalinge et al. 2002) and evaluations of mortality rates for different size classes should be investigated because of the migration patterns of this species.
Mortality of fish in the present study occurred at similar strain rates as those observed for American shad, but occurred at lower strain rates than for juvenile Chinook salmon, rainbow trout and steelhead exposed to similar conditions with the same test apparatus (Neitzel et al. 2004). American shad was the most sensitive species in the Neitzel et al. (2004) study and 10% of the test population was affected (major injury or worse) at a strain rate of 454 s–1, whereas for this study, gouramis had a 27% 48-h delayed mortality and sharks had a 13% 48-h delayed mortality at much lower a strain rate of 168 s–1. The current study lacked true control fish (not exposed to shear forces) and so the delayed mortality rates may be due to a sensitivity of gouramis and sharks to low shear forces, or may be due to a sensitivity to the handling process used.
This study is the second to document the effects of shear forces on species native to the MRB. However, there are many other species that rely on free flowing water within the Mekong to support their migration patterns and are already listed as critically endangered, endangered, vulnerable or near threatened by the International Union for Conservation of Nature and Natural Resources Red List, including the giant salmon Carp (Aaptosyax grypus), Mekong giant catfish (Pangasianodon gigas), and the elephant ear gourami (Osphronemus exodon). Because of the high biodiversity found within the MRB, detailed information on all species is not reasonable, and, so, future studies should focus on economically and ecologically valuable species. Furthermore, prioritisation of species on the basis of key life-history and morphological traits that may make them more susceptible to entrainment and ill effects is warranted (Pracheil et al. 2016).
Conclusions
Overall, it was observed that gouramis and sharks are susceptible to behavioural effects, injury and mortality as a result of exposure to shear forces. The information collected showed that these species responded differently to the same physical environment and, so, further research should focus on evaluating the effects of shear stress on other important species to the MRB. The occurrence of negative effects increased with the severity of shear forces, suggesting that the operation and design of hydroturbines for the MRB should strive to minimise the frequency of high shear-stress events.
Conflicts of interest
Dr Z. Deng is an Associate Editor for Marine and Freshwater Research. Despite this relationship, he did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Acknowledgements
This study is supported by the United States Agency for International Development (USAID). The authors’ views expressed in this publication do not necessarily reflect the views of USAID or the United States government. A. H. Colotelo and Z. D. Deng also thank the US Department of Energy Water Power Technologies Office for their funding support of the Biologically Based Design and Evaluation of Hydro-Turbines (BioDE) project. This research was conducted in compliance with a protocol approved by Pacific Northwest National Laboratory’s Institutional Animal Care and Use Committee. The Pacific Northwest National Laboratory is operated for US Department of Energy by Battelle Memorial institute under contract DE-AC05-76RLO 1830.
References
Baumgartner, L. J., Thorncraft, G., Phonekhampeng, O., Boys, C., Navarro, A., Robinson, W., Brown, R., and Deng, Z. D. (2017). High fluid shear strain causes injury in silver shark: preliminary implications for Mekong hydropower turbine design. Fisheries Management and Ecology 24, 193–198.| High fluid shear strain causes injury in silver shark: preliminary implications for Mekong hydropower turbine design.Crossref | GoogleScholarGoogle Scholar |
Čada, G. F. (2001). The development of advanced hydroelectric turbines to improve fish passage survival. Fisheries 26, 14–23.
| The development of advanced hydroelectric turbines to improve fish passage survival.Crossref | GoogleScholarGoogle Scholar |
Čada, G. F., Loar, J. M., Garrison, L. A., Fisher, R. K., and Neitzel, D. A. (2006). Efforts to reduce mortality to hydroelectric turbine-passed fish: locating and quantifying damaging shear stresses. Environmental Management 37, 898–906.
| Efforts to reduce mortality to hydroelectric turbine-passed fish: locating and quantifying damaging shear stresses.Crossref | GoogleScholarGoogle Scholar |
Čada, G. F., Garrison, L. A., and Fisher, R. K. (2007). Determining the effect of shear stress on fish mortality during turbine passage. Hydro Review 26, 52–57.
Colotelo, A. H., Goldman, A. E., Wagner, K. A., Brown, R. S., Deng, Z. D., and Richmond, M. C. (2017). A comparison of metrics to evaluate the effects of hydro-facility passage stressors on fish. Environmental Reviews 25, 1–11.
| A comparison of metrics to evaluate the effects of hydro-facility passage stressors on fish.Crossref | GoogleScholarGoogle Scholar |
Davis, M. W. (2010). Fish stress and mortality can be predicted using reflex impairment. Fish and Fisheries 11, 1–11.
| Fish stress and mortality can be predicted using reflex impairment.Crossref | GoogleScholarGoogle Scholar |
Deng, Z. D., Guensch, G. R., McKinstry, C. A., Mueller, R. P., Dauble, D. D., and Richmond, M. C. (2005). Evaluation of fish-injury mechanisms during exposure to turbulent shear flow. Canadian Journal of Fisheries and Aquatic Sciences 62, 1513–1522.
| Evaluation of fish-injury mechanisms during exposure to turbulent shear flow.Crossref | GoogleScholarGoogle Scholar |
Deng, Z. D., Mueller, R. P., Richmond, M. C., and Johnson, G. E. (2010). Injury and mortality of juvenile salmon entrained in a submerged jet entering still water. North American Journal of Fisheries Management 30, 623–628.
| Injury and mortality of juvenile salmon entrained in a submerged jet entering still water.Crossref | GoogleScholarGoogle Scholar |
Hockley, F. A., Wilson, C. A. M. E., Brew, A., and Cable, J. (2014). Fish responses to flow velocity and turbulence in relation to size, sex and parasite load. Journal of the Royal Society, Interface 11, 20130814.
| Fish responses to flow velocity and turbulence in relation to size, sex and parasite load.Crossref | GoogleScholarGoogle Scholar |
Hughes, G. M. (1984). General anatomy of the gills. In ‘Fish Physiology’. (Eds W. S. Hoar and D. J. Randall.) Vol. XA, pp. 1–72. (Academic Press: Orlando, FL, USA.)
Kuenzer, C., Campbell, I., Roch, N., Leinenkugel, P., Tuan, C. Q., and Dech, S. (2013). Understanding the impact of hydropower developments in the context of upstream-downstream relations in the Mekong River basin. Sustainability Science 8, 565–584.
| Understanding the impact of hydropower developments in the context of upstream-downstream relations in the Mekong River basin.Crossref | GoogleScholarGoogle Scholar |
Mekong River Commission (2010). State of the basin report 2010. Mekong River Commission, Vientiane, Lao PDR.
Neitzel, D. A., Richmond, M. C., Dauble, D. D., Mueller, R. P., Moursund, R. A., Abernethy, C. S., Guensch, G. R., and Cada, G. F. (2000). Laboratory studies on the effects of shear on fish. Final report 2000. PNNL-13323, Richland, WA, USA.
Neitzel, D. A., Dauble, D. D., Cada, G. F., Richmond, M. C., Guensch, G. R., Mueller, R. P., Abernethy, C. S., and Amidan, B. (2004). Survival estimates for juvenile fish subjected to a laboratory-generated shear environment. Transactions of the American Fisheries Society 133, 447–454.
| Survival estimates for juvenile fish subjected to a laboratory-generated shear environment.Crossref | GoogleScholarGoogle Scholar |
Nielson, N. M., Brown, R. S., and Deng, Z. D. (2015). Review of Existing Knowledge of the Effectiveness and Economics of Fish-Friendly Turbines. Technical Paper number 57, Mekong River Commission, Vientiane, Laos.
Pracheil, B. M., McManamay, R. A., Bevelhimer, M. S., DeRolph, C. R., and Cada, G. F. (2016). A traits-based approach for prioritizing species for monitoring and surrogacy selection. Endangered Species Research 31, 243–258.
| A traits-based approach for prioritizing species for monitoring and surrogacy selection.Crossref | GoogleScholarGoogle Scholar |
Richmond, M. C., Serkowski, J. A., Rakowski, C., Strickler, B., Weisbeck, M., and Dotson, C. (2014). Computational tools to assess turbine biological performance. Hydro Review 33, 88–97.
Stephenson, J. S., Gingerich, A. J., Brown, R. S., Pflugrath, B. D., Deng, Z. D., Carlson, T. J., Langeslay, M. J., Ahmann, M. L., Johnson, R. L., and Seaburg, A. G. (2010). Assessing barotrauma in neutrally and negatively buoyant juvenile salmonids exposed to simulated hydro-turbine passage using a mobile aquatic barotrauma laboratory. Fisheries Research 106, 271–278.
| Assessing barotrauma in neutrally and negatively buoyant juvenile salmonids exposed to simulated hydro-turbine passage using a mobile aquatic barotrauma laboratory.Crossref | GoogleScholarGoogle Scholar |
Van Zalinge, N., Lieng, S., Peng Bun, N., Kong, H., and Valbo Jorgensen, J. (2002). Status of the Mekong Pangasianodon hypophthalmus resources, with species reference to the stock shared between Cambodia and Viet Nam. Technical Paper number 1, Mekong River Commission, Vientiane, Laos.
Vidthayanon, C., and Hogan, Z. (2011). Pangasianodon hypophthalmus (striped catfish). In ‘The IUCN Red List of Threatened Species 2011’, e.T180689A7649971. (International Union for Conservation of Nature and Natural Resources.) Available at http://www.iucnredlist.org/details/180689/0 [Verified 31 March 2017].
Walker, R. W., Ashton, N. K., Brown, R. S., Liss, S. A., Colotelo, A. H., Beirao, B. V., Townsend, R. L., Deng, Z. D., and Eppard, M. B. (2016). Effects of a novel acoustic transmitter on swimming performance and predator avoidance of juvenile Chinook salmon: determination of a size threshold. Fisheries Research 176, 48–54.
| Effects of a novel acoustic transmitter on swimming performance and predator avoidance of juvenile Chinook salmon: determination of a size threshold.Crossref | GoogleScholarGoogle Scholar |
Zarfl, C., Lumsdon, A. E., Berlekamp, J., Tydecks, L., and Tockner, K. (2015). A global boom in hydropower dam construction. Aquatic Sciences 77, 161–170.
| A global boom in hydropower dam construction.Crossref | GoogleScholarGoogle Scholar |
Ziv, G., Baran, E., Nam, S., Rodriguez-Iturbe, I., and Levin, S. A. (2012). Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin. Proceedings of the National Academy of Sciences of the United States of America 109, 5609–5614.
| Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin.Crossref | GoogleScholarGoogle Scholar |