Sea catfishes (Ariidae) feeding on freshwater floodplains of northern Australia
Bradley J. Pusey A B C E , Timothy D. Jardine D , Stuart E. Bunn A and Michael M. Douglas C
A B C E , Timothy D. Jardine D , Stuart E. Bunn A and Michael M. Douglas C
A Australian Rivers Institute, Griffith University, Kessels Road, Nathan, Qld 4111, Australia.
B Charles Darwin University, Ellengowan Drive, Casuarina, NT 0810, Australia.
C School of Biological Sciences, The University of Western Australia, Stirling Highway, Crawley, WA 6009, Australia.
D School of Environment and Sustainability, Global Institute for Water Security, University of Saskatchewan, Preston Road, Saskatoon, SK, S7H 4J6, Canada.
E Corresponding author. Email: bpusey@westnet.com.au
Marine and Freshwater Research 71(12) 1628-1639 https://doi.org/10.1071/MF20012
Submitted: 9 January 2020 Accepted: 8 April 2020 Published: 11 June 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Flooding of the terminal floodplains of northern Australian rivers provides a greatly expanded, productive habitat accessed by both freshwater and estuarine fishes. This study aimed to determine the extent to which sea catfishes (Ariidae) make use of floodplains and the reasons for doing so (i.e. spawning, feeding). Nine species were collected from floodplains and adjacent distributaries of the Mitchell and Flinders rivers; floodplain use was largely restricted to freshwater species. Evidence of prior wet season spawning was recorded for some species, and mesenteric lipid deposits indicated that fish were in good condition. However, little evidence of spawning on floodplains was found. Stomach content analysis and stable isotope analysis indicated dietary partitioning, particularly between freshwater and estuarine species, but also within freshwater species, and indicated that some species were responsive to variations in food availability. Isotope analyses suggest extensive movement between freshwater, estuarine and marine habitats at different life history stages for the catfish assemblage studied. Terminal floodplains of northern Australian rivers provide important temporary habitat for adult sea catfishes to feed upon, but do not appear to be used as spawning grounds.
Additional keywords: dietary partitioning, stable isotopes, stomach contents, wet–dry tropics.
Introduction
Floodplains are among the most biologically productive and diverse ecosystems on Earth and are of enormous economic importance (Tockner and Stanford 2002). Periodic rhythmic flooding links aquatic and terrestrial ecosystems and enhances productivity during both the flooded and dry phases (Pettit et al. 2011; Jardine et al. 2015). This period of increased aquatic production, driven largely by algal production, and the provision of an expanded and structurally complex habitat ensure that floodplains are used by riverine fishes for adult growth and reproduction, and as nursery grounds for juvenile fish (Bayley 1995; Winemiller 2004; Jardine et al. 2012a, 2012b; Villamarín et al. 2016). When river floodplains are located close to the river terminus, there is increased potential for inundation to expand the links between terrestrial, riverine and marine ecosystems. For example, when inundated, the vast terminal floodplains of northern Australia are contiguous with, and grade into, the receiving marine environment (Ward et al. 2011), creating the opportunity for marine, estuarine and freshwater fish to access elevated primary and secondary floodplain production (Pettit et al. 2011; Adame et al. 2017). However, floodplain inundation across much of northern Australia is often short and does not necessarily occur every year (Jardine et al. 2012a, 2012b); these characteristics may therefore constrain the extent to which fish do indeed make use of floodplain habitats, particularly as spawning areas.
Ariidae (sea catfishes), a cosmopolitan family of ~30 genera and more than 150 species (Nelson et al. 2016), is among the most abundant of fishes in tropical estuarine habitats (Barletta et al. 2005), often dominating commercial and artisanal fishery catches (Coates 1991; Dantas et al. 2010). Although mainly marine and estuarine in habit, many species occur in fresh waters (Betancur-R 2010) and make use of floodplains (Pusey et al. 2017). Ariid catfishes are, in general, flexible opportunistic feeders (Yáñez-Arancibia and Lara-Domínguez 1988; Bachok et al. 2004; Pusey et al. 2004; Krumme et al. 2008). Ontogenetic (Yáñez-Arancibia and Lara-Domínguez 1988; Hajisamae et al. 2004; Mendoza-Carranza and Vieira 2009), spatial (Mendoza-Carranza and Vieira 2009) and phenotypic (Krumme et al. 2008) variation in prey choice within species contribute collectively to a perception of flexible feeding within the family. Such flexibility may allow ariid catfishes to make use of a variety of food types when and where they become available (such as occurs during flood inundation) and, as a consequence, lead to high overlap in resource use. Conversely, this flexibility may allow closely related species to effectively partition resources; dietary partitioning within sympatric ariid assemblages has been reported (Blaber et al. 1994; Maitra et al. 2020). However, Blaber et al. (1994) demonstrated that interspecific variation in prey consumption was largely explained by interspecific differences in head and mouth morphology (dentition, mouth size and shape). Thus, in this case, partitioning may be phylogenetically based rather than driven by the dynamics of predator and prey abundance. Similarly, partitioning within an ariid catfish assemblage (four species) of the Cochin Estuary was limited to one species only, and this species, for which molluscs were an important prey item, used the estuary on a seasonal basis only (Maitra et al. 2020).
Ariid catfishes are known to disperse widely and make use of floodplain habitats (Bishop et al. 2001; Crook et al. 2020), and to transport accumulated nutrients and energy elsewhere throughout the riverine landscape (Jardine et al. 2012a). The present study was undertaken as part of a larger program that examined food web variation in rivers of tropical northern Australia (Warfe et al. 2011; Jardine et al. 2012a, 2012b, 2015). Importantly, components of this research were conducted during the wet season when rivers were in short, but intense, periods of flood. Ecological studies during this phase of the hydrological cycle are logistically difficult due to the remoteness of the area (accessibility is limited and expensive to overcome) and infrequently undertaken. Consequently, our understanding of ecological dynamics during this phase of the hydrological cycle remains an important knowledge gap (Davis et al. 2018), highlighting the value of information gathered incidentally during the conduct of any research activities. We opportunistically gathered information on the diet and condition of catfishes collected from the lower-most reaches of main river channels and the adjacent inundated floodplain habitats during short fieldwork campaigns in the Mitchell and Flinders rivers, northern Australia, described elsewhere (e.g. Jardine et al. 2012a, 2012b). The aims of the study were to determine: (1) the diversity, diet and condition of sea catfishes present within flooded lower river and adjacent floodplain habitats; and (2) the manner and extent to which catfishes make use of floodplain production. We hypothesised that: (1) both freshwater and estuarine catfishes would access floodplain habitats; (2) both freshwater and estuarine catfish would spawn in floodplain habitats; and (3) the generalist nature of catfish feeding would result in little interspecific differences in diet.
Materials and methods
Study area
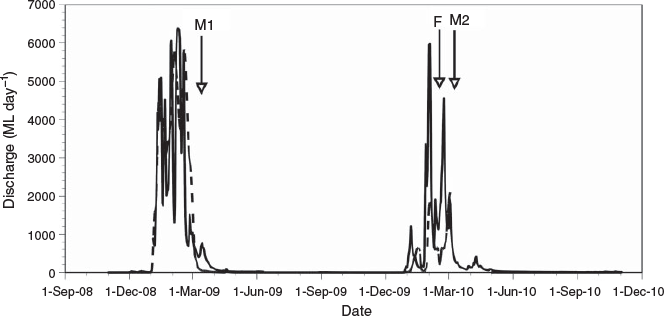
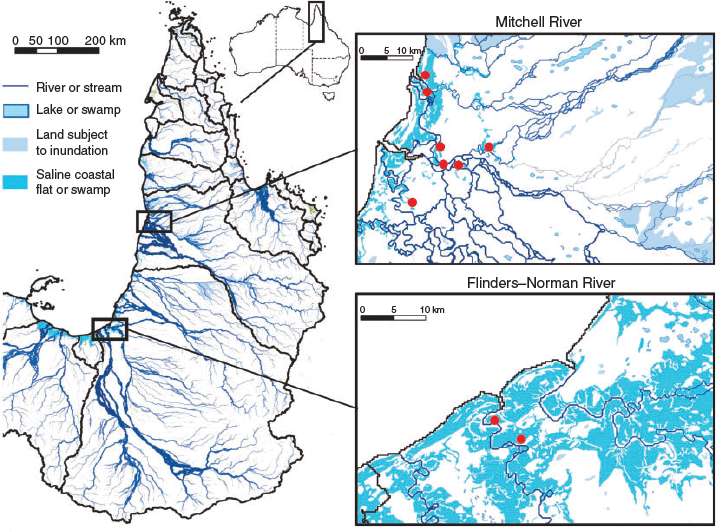
The Mitchell and Flinders rivers of northern Australia are large systems (72 000 and 109 000 km2 respectively) emptying into the Gulf of Carpentaria (Fig. 1) that experience strong wet–dry seasonality in discharge (Kennard et al. 2010; Warfe et al. 2011). The catchments of both rivers are largely covered by monsoonal dry sclerophyllous vegetation, and land use is dominated by free-range grazing. Both rivers are distinguished by large terminal floodplains that are inundated for less than 2 months a year by a combination of direct rainfall capture and local flooding during the wet season (Ward et al. 2011; Jardine et al. 2012a). The present study occurred in the late wet seasons of 2009 and 2010 after peak flood flows (Fig. 2) but while floodplains remained connected to the main river channel (Fig. 3). In the first year of the study (2009), discharge in the Mitchell River (at the most downstream gauge, Koolatah) was above average (peak discharge equal to the 10-year recurrence interval flood). In the second year, similarly high flows occurred in the Mitchell River, whereas flood flows in the Flinders River were close to average. In each year and river, floodplain and adjacent estuarine habitats were accessed by boat (from a mother ship moored near the river mouth) for a short period (Mitchell River: 5–7 March 2009 and 5–7 March 2010; Flinders River: 14–16 February 2010). Sample locations were located within 40 km of the river mouth in both rivers. Conductivity was low in the Mitchell River (35–110 µS cm–1) and higher in the Flinders River (typically 195–688 µS cm–1), although pockets of brackish water (9660 µS cm–1) occurred in poorly flushed mangrove zones of this river. Near-neutral pH (6.75–7.66) was recorded in both rivers and turbidity was much greater in the Flinders River (672–821 nephelometric turbidity units, NTU) than in the Mitchell River (11–107 NTU). Mean minimum and maximum air temperatures were 23.8 and 32.8°C respectively.
|
|
Sample collection
The care and use of experimental animals used in this study complied with guidelines and policies approved by Griffith University and ethics permit ENV/08/11/AEC.
Samples for stomach content (SCA) and stable isotope analyses (SIA) were collected by gill and seine netting during daylight hours in inundated floodplain and flooded estuarine habitats. It is important to note that the sampling of fishes was primarily intended to collect a diverse array of material for SIA and that a sample design focused solely on catfish and intended to quantify abundance across different habitats was precluded by time constraints. Note also that the Mitchell River was sampled on two occasions, whereas the Flinders River was sampled only once. Upon capture, fish were killed (by pithing) and placed on ice. Each individual was measured (standard length (SL) in millimetres) and weighed (g), and a 1-cm3 tissue sample was taken from the lateral portion of the body and frozen immediately. The abdominal cavity was dissected open, the stomach removed and the reproductive status and body condition were noted. Reproductive status was scored categorically according to the eight-point scheme of Kesteven (1960): I, virgin; II, maturing virgin; III, developing; IV, developing, occupying >50% of the cavity; V, gravid; VI, spawning; VII, spawning but partially spent; VIII, spent; and 0, recovering but with evidence of prior spawning. Condition was scored by reference to the extent of mesenteric lipid deposits as follows: L1, no evidence of mesenteric lipid stores; L2, mesenteric lipid deposits visible but filling <10% of the body cavity; L3, mesenteric lipid deposits visible but filling <20% of the body cavity; and L4, mesenteric lipid deposits present and filling <50% of the body cavity. This scheme does not account for lipids stored within muscle; however, mean (±s.d.) C : N ratios determined in muscle for the fish examined here were low (3.7 ± 1.5 in the Flinders River (n = 52), 3.9 ± 1.7 in the Mitchell River in 2009 (n = 23) and 3.9 ± 1.7 in the Mitchell River in 2010 (n = 24)), indicative of low within-muscle lipid content (Logan et al. 2008). Fullness of the excised stomach was scored on a scale of 0–100%; contents were examined macroscopically and allocated to 1 or more of 14 categories, including an unidentified fraction following Hyslop (1980). Dietary categories, although broad, were intended to maximise distinction between allochthonous and autochthonous sources, plants and animals, and to reflect major differences in prey size. The category ‘Fish’ also included fish scales. Dietary composition was expressed in terms of proportion of volume (%).
Potential source material (e.g. primary producers such as benthic algae, epiphytic algae, epipelon and terrestrial plants), primary consumers (e.g. aquatic invertebrates such as chironomid and trichopteran larvae, crabs, prawns and molluscs) and secondary consumers (e.g. fish) were collected and frozen (either whole or as an excised tissue or fin clip sample). Samples were thawed in the laboratory, oven-dried at 50°C for 48 h and ground to a powder for isotope analysis of carbon and nitrogen. Samples were weighed to ~0.6 mg and combusted in an EA 3000 elemental analyser (Eurovector, Milan, Italy), and sample gases were delivered to an Isoprime mass spectrometer (GV Instruments, Manchester, UK). Working standards were liquids calibrated against International Atomic Energy Agency (IAEA) CH6, CH7, N1, N2 and NBS-127, and data are presented herein as parts per thousand deviations from international standards (Pee Dee Belemnite carbonate and atmospheric nitrogen). A full description, including estimates of precision, may be found in Jardine et al. (2012b). δ13C data presented here are not corrected for lipid content given the low lipid content (C : N < 4) reported above. Six individuals of Neoarius graeffei (Kner & Steindachner, 1867) less than 70 mm SL (<8 g) from the Flinders River were included in SIA.
Data analysis
Stomach content data were averaged (and standard errors calculated) for each species and normalised (i.e. summed to 100%) after exclusion of the unidentified fraction. Permutational tests (i.e. permutational multivariate analysis of variance (PERMANOVA); Anderson et al. 2008) based on a Bray–Curtis sample by sample resemblance matrix were used to test for specific and spatial differences in diet in Primer 7 (ver. 7.0.13, see www.primer-e.com, accessed 28 April 2020). Because the design was unbalanced (i.e. not all species occurred in each river), two separate analyses were undertaken, the first in which species (random) was nested within river (fixed) and the second in which river was nested within species. Data for Hexanematichthys mastersi (Ogilby, 1898), Netuma proxima (Ogilby, 1898), Hemiarius dioctes (Kailola, 2000) and Cinetodus froggatti (Ramsay & Ogilby, 1887) were not included because sample sizes were low (<5) and preliminary ordination analysis indicated that the diet of these species was very different to that of other species (i.e. they were extreme outliers in ordination space). Moreover, these species were never collected from flooded floodplain habitats, but were collected from the flooded riverine section only. PERMANOVA was similarly used to test for significant differences in isotope (δ13C and δ15N) values, but was performed using a resemblance matrix based on Euclidean distance. All species were included, but the six very small individuals of N. graeffei were not.
Results
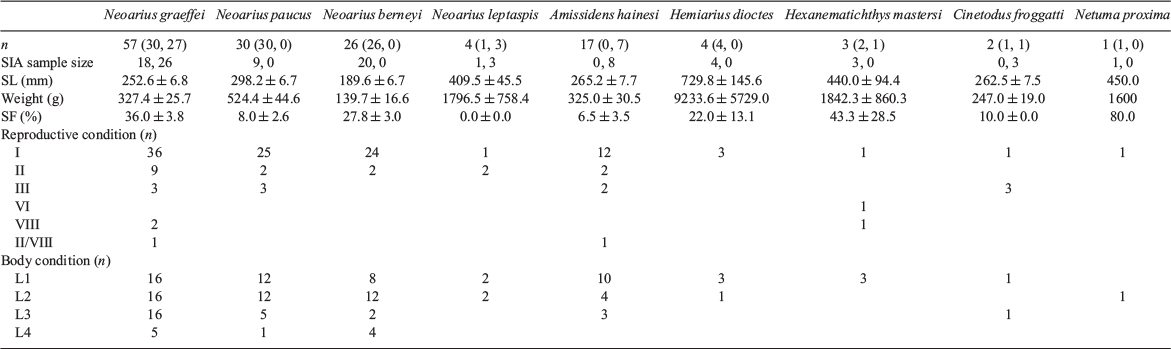
In all, 143 individuals from nine catfish species within 6 genera were collected. More species were collected from the Mitchell River than the Flinders River (nine v. six), but note that sampling effort was not equivalent for each river. The most abundant species were from within the predominantly freshwater genus Neoarius. Most fish examined ranged from 150 to 350 mm SL and were less than 750 g in weight; however, H. dioctes, He. mastersi and Ne. proxima were larger (often >500 mm SL and >2000 g; Table 1). These three species, and C. froggatti, were collected only from flooded estuarine sections and were absent from the inundated floodplain itself. All individuals examined were adults (i.e. in at least the 1+ year-class) except for the six N. graeffei <70 mm SL (i.e. age-0+).
|
Little evidence of reproductive activity was observed at the time of sampling (Table 1). However, two individuals of N. graeffei were classed as spent (VIII) or recovering or spent (II/VIII), one individual of Amissidens hainesi (Kailola, 2000) was classed as recovering or spent and two individuals of H. mastersi were classed as either spent or in Stage VI (spawning). The presence of spent individuals suggests recent spawning, as does the inclusion in our samples of small juveniles (<70 mm SL). Most individuals collected had visible mesenteric fat deposits indicative of good body condition. Moreover, some individuals of N. graeffei, Neoarius paucus (Kailola, 2000) and Neoarius berneyi (Whitley, 1941) contained extensive lipid deposits (Stages L3 and L4; Table 1).
Stomach content analysis
Stomach fullness was typically low (<40% for most species; Table 1). Nonetheless, the presence of material in the stomachs of fish collected from floodplains confirmed that they were feeding in this habitat. N. graeffei and N. berneyi consumed a wide variety of food items, differing in that N. graeffei consumed more large items, such as crabs, prawns, gastropods and terrestrial plant matter. Five N. graeffei contained the seeds (minus the hard outer cover) of the mangrove Avicennia marina. N. berneyi consumed more aquatic insect larvae (predominantly chironomid larvae) and, as a result, more detritus and sediment. Both species consumed terrestrial invertebrates in equivalent small amounts. Compared with its congeners, N. paucus consumed a less diverse array of items, consisting of larger mobile prey such as crabs and other macrocrustacea. Only 3 of the 17 A. hainesi collected contained identifiable food items and, of those that did, chironomid larvae occurred in two and small crabs (carapace width <10 mm) in the other. All Neoarius leptaspis (Bleeker, 1862) that were collected had empty stomachs. C. froggatti consumed bivalves, He. mastersi consumed bivalves and prawns, Ne. proxima consumed crabs and H. dioctes consumed fish. Bivalve molluscs and prawns were absent from the diet of other catfish species collected from the floodplain of the Mitchell River. Neither prey type was present in fish from floodplain habitats, but both were found in fish collected in the flooded estuarine sections of the Mitchell River. Although fish (as a category) was present in the stomachs of a small number of N. graeffei, N. berneyi and N. paucus (5, 3 and 1 respectively), whole fish rather than fish scales were present in only one individual of each species. By contrast, H. dioctes consumed whole fish (ariid catfish and a waspfish (Apistidae: Cheroscorpaena sp.)).
Significant dietary partitioning was detected by PERMANOVA when the factor species was nested with the factor river (Table 3); all three species of Neoarius within the Mitchell River differed significantly from one another. Similarly, the diet of N. graeffei and A. hainesi differed significantly in the Flinders River. Although not included in multivariate analyses, a distinction between the diet of estuarine species and those occurring on the floodplain was noted. The diet of N. graeffei, the only species commonly caught in both rivers, differed significantly between rivers (Table 3). Individuals from the Flinders River consumed more prawns (28.9 v. 0%), fewer crabs (8.4 v. 19.8%) and less terrestrial insect material (14.8 v. 29.6%) than did those from the Mitchell River. Equivalent amounts of terrestrial vegetation were consumed in each river (15.4 v. 16.9%), although the nature of that vegetation differed, being dominated by leaves, twigs and flowers in fish from the Flinders River and by mangrove seeds in the Mitchell River.
|
Stable isotope analysis
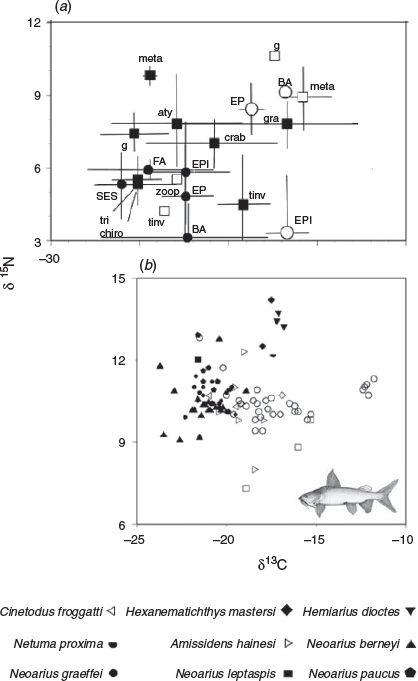
Isotope values of primary producers (i.e. algae) differed consistently between rivers (Fig. 4a). Seston, filamentous algae, epiphytic algae, benthic algae and epipelon from the Mitchell River ranged from –26 to –22‰ δ13C and from 3 to 6‰ δ15N. By contrast, equivalent samples from the Flinders River ranged from –19 to –12‰ δ13C and from 3 to 9‰ δ15N. With the exception of terrestrial insects, the δ13C values of most consumer organisms were similar to algal source values from their respective rivers of origin (approximately –22‰ for the Mitchell River and –18‰ for the Flinders River). Terrestrial invertebrates, particularly grasshoppers, from the Mitchell River were not as depleted in 13C as potential aquatic source material. A distinction between Metapenaeus prawns from each river was evident, as was (albeit to a lesser extent) a distinction between crabs from the Mitchell River and prawns from either river. Prawns were more 15N enriched than potential source materials and other consumer organisms.
|
A clear distinction between rivers was evident in the isotope biplot for catfish samples (Fig. 4b). In general, catfish from the Mitchell River, like primary producer and consumer organisms from this river, were characterised by δ13C values less than –19‰. H. dioctes, He. mastersi and N. proxima from the Mitchell River were an exception, having δ13C values of between –18 and –16‰ and unlike any measured potential source material from this river. These species were also distinguished by δ15N values greater than those recorded for other species. A significant effect of species (nested within river) on isotope values was detected by PERMANOVA with many significant pairwise comparisons (Table 3). All comparisons between freshwater species (i.e. N. graeffei, N. berneyi or N. paucus) and estuarine species (H. dioctes or He. mastersi) in the Mitchell River were significant. No between-species comparison within the estuarine species set was significant. Within the freshwater species, only the comparison between N. berneyi and N. paucus was significant.
Catfish from the Flinders River were characterised by δ13C values typically greater than –20‰, similar to those of available primary producers. The six small individuals of N. graeffei, all less than 70 mm SL, had δ13C values of approximately –12‰ and unlike any measured potential aquatic source material. Between-species distinction in isotope values was recorded for comparisons between N. graeffei and A. hainesi, between N. graeffei and C. froggatti, and between N. leptaspis and N. hainesi (Table 3). As demonstrated for SCA, the isotope values of N. graeffei from the Mitchell River differed significantly from those of N. graeffei from the Flinders River (Table 3).
Significant relationships between isotope values and size (bodyweight in grams) were detected. In the Mitchell River and when all species were considered, δ13C became more positive with increasing size (δ13C = 0.7768 × ln(weight) –25.165; r2 = 0.5042, P < 0.001, n = 49), as did δ15N (δ15N = 0.5744 × ln(weight) + 7.6901; r2 = 0.5438, P < 0.001, n = 49). The significant relationship for δ15N was driven largely by inclusion of the large predatory estuarine species H. dioctes, He. mastersi and Ne. proxima because no significant relationship between weight and δ15N was observed when the analysis was restricted to N. graeffei. However, δ13C values in this species remained positively related to size (δ13C = 0.8329 × ln(weight) –25.313; r2 = 0.3625, P < 0.001, n = 17). No significant relationship between size and either isotope was detected for N. berneyi or N. paucus. In the Flinders River (all species considered but without the subset of very small juveniles identified above), δ13C similarly increased with size (δ13C = 0.0032 × ln(weight) –19.621; r2 = 0.2784, P < 0.001, n = 38), but no relationship between size and δ15N was detected. However, when the analysis was restricted to N. graeffei only, significant relationships between size and both isotopes were detected (δ13C = 0.8019 × ln(weight) –22.818 (r2 = 0.4616, P < 0.001, n = 19) and δ15N = 0.493 × ln(weight) + 13.045 (r2 = 0.4212, P < 0.001, n = 19)). That is, as N. graeffei grew, they became enriched in both 13C and 15N. The slope of the δ13C–size relationship was very similar to that seen in the Mitchell River, suggesting a similar ontogenetic process.
Discussion
The present study examined floodplain use by freshwater and estuarine sea catfishes (Ariidae) during a short period of inundation and addressed three hypotheses concerning use, namely that: 1) both estuarine and freshwater species of ariid would feed upon the inundated floodplain; (2) the floodplain would be used as a spawning area; and (3) the abundance of food on the floodplain would result in little interspecific partitioning of diet. Of the 11 ariid catfishes known to occur in northern Australian freshwaters (Pusey et al. 2017), 9 were collected from the Mitchell and Flinders rivers in the present study. Of these, species within Neoarius (which numerically dominated the total catch) are predominantly riverine (Pusey et al. 2017), although occasionally recorded from estuaries and the near-shore marine (i.e. N. graeffei and N. leptaspis; Blaber et al. 1994). A. hainesi occurs in the near-shore marine environment, very infrequently in freshwaters (Kailola 2000), and the records here are, to the best of our knowledge, the first account of its presence on freshwater floodplains. C. froggatti, Ne. proxima, H. dioctes and He. mastersi are primarily estuarine and, when present in freshwaters, are typically restricted to the very lower reaches of northern rivers (Pusey et al. 2017). Our hypothesis that both freshwater and estuarine catfishes would make use of inundated floodplain habitats can be largely rejected. With the exception of A. hainesi, estuarine species do not appear to use the inundated floodplain itself, but remain in the highly dilute flooded estuarine sections of the river adjacent to the floodplains.
Most freshwater ariids of northern Australia spawn during, or just before, the wet season Pusey et al. 2004). Peak abundance of juvenile estuarine ariids, including H. dioctes and C. froggatti, occurs during the wet season in the South Alligator River, another northern Australian river with a terminal floodplain (Pusey et al. 2016). The absence of gravid females, the presence of spent individuals and the collection of small juveniles within some species (i.e. N. graeffei) in the present study suggests that spawning occurred before our sampling (i.e. at the beginning of the wet season). Small juveniles were not collected from floodplain habitats, further suggesting that spawning did not occur on the floodplain. Moreover, the distinct isotope values recorded for juvenile N. graeffei from the Flinders River suggest an estuarine or near-shore early juvenile phase (see below). Thus, our second hypothesis that ariid catfishes spawn on the floodplain has little support. Although it does not appear that floodplains of the Mitchell and Flinders rivers were used for spawning, high rates of primary and secondary floodplain production (Pettit et al. 2011; Adame et al. 2017) may enhance juvenile development and support postpartum adult growth. Many fish had visible mesenteric fat deposits indicative of energy storage and good condition (Brown and Murphy 2004). Villamarín et al. (2016) similarly found higher body condition and the development of mesenteric fat bodies in diamond mullet (Planiliza (=Liza) alata) on northern Australian floodplains during the wet season. Moreover, the close match between isotope values of primary production on the floodplain and of the catfishes foraging there suggests rapid assimilation of floodplain resources.
Limited dietary specialisation has been reported elsewhere for both freshwater and marine ariid catfishes and, when present, is most strongly related to interspecific differences in mouth size and shape of the palatine teeth (Allen 1991; Coates 1991; Blaber et al. 1994) or phenological differences in habitat use (Maitra et al. 2020). In the present study, and notwithstanding that sample sizes were low for some species and that within-species variability was high, different species consumed a broadly separate array of food types. This was particularly so for the larger estuarine species (H. dioctes, He. mastersi and Ne. proxima), which foraged on fish, molluscs and large crustaceans. These species were also distinguished by isotope values unlike any measured potential floodplain carbon source, likely reflecting prior foraging in coastal or estuarine habitats (i.e. slow elemental turnover; Mont’Alverne et al. 2016). Strictly marine predators from these systems (e.g. threadfin and queenfish) have similar δ13C values ranging from –20 to –16‰ (Jardine et al. 2012b). Thus, these estuarine species retained isotope values from prior feeding over long time scales (i.e. months) and, given their large size, it is unlikely that SIA of muscle tissue would detect more recent short-term feeding on a distinctive set of new food resources. That is not to say that floodplain production plays no role in their growth; the presence of these species in freshwater riverine habitats adjacent to inundated floodplains may enable them to prey upon species moving off the floodplain, particularly as the extent of inundation of the floodplains decreases.
Differences in diet also occurred for species collected from the floodplain, with all possible comparisons (three in the Mitchell River and one in the Flinders River) being significantly different. As reported for marine and estuarine ariids (Blaber et al. 1994), these differences in prey use probably relate to differences in overall body size and absolute differences in mouth size. N. paucus is both larger and has a larger mouth (in both relative and absolute terms) than its congeners or A. hainesi (Pusey et al. 2004; Kailola 2000). In the present study, N. paucus consumed large crustaceans, whereas the smaller and small-mouthed N. berneyi and A. hainesi consumed small aquatic invertebrates. N. graeffei, intermediate in size, consumed a wide array of prey items, but most food items were only recovered from few individuals. N. graeffei is a widespread and abundant species with a flexible foraging habitat (Pusey et al. 2004). The importance of items of a terrestrial origin (insects and plant material) to the diet of N. graeffei and N. berneyi (32.5 and 27.1% respectively) suggests this flexibility extends to feeding at different levels in the water column. Particularly notable in this regard is the consumption of mangrove seeds by N. graeffei. Although the consumption of seeds by fishes is not uncommon (see Correa et al. 2007; Pollux 2011) and occurs in catfishes (e.g. the channel catfish Ictalurus punctatus; Adams et al. 2007), it has not been reported extensively for estuarine fishes. We could find no prior reports of seed consumption in tropical estuarine fishes, let alone consumption of mangrove seeds.
Carbon stable isotope values for aquatic primary producers differed consistently between the two rivers, being enriched in 13C in the Flinders River (δ13C ranging from –18 to –16‰ for epipelon, epiphytes and benthic algae) compared with the Mitchell River (range –23 to –22‰). Invertebrate secondary consumers similarly differed between rivers, as did catfish. These findings strongly suggest that local floodplain production, primarily algal, is important in supporting invertebrate biomass and is rapidly translated up the food chain to consumer species such as catfish. The distinction between the two local food webs with regard to δ13C values suggests river-specific environmental features play a role. Algal δ13C variation is driven by a complicated mix of factors, including productivity, salinity, flow and the δ13C of dissolved inorganic carbon (Fry 2002; Finlay 2004). The size of the wet season flood in the Flinders River was much less than that in the Mitchell River in either year of the study, and the floodplain and saline flats of the former may have not been extensively flushed by fresh waters. For example, conductivity was very low on the Mitchell River floodplain (typically <100 µS cm–1), whereas higher values (195–688 µS cm–1), and occasionally much higher values (9660 µS cm–1), were recorded on the Flinders River floodplain. Variation in salinity has been linked to variation in element turnover and isotope discrimination in fish (Mont’Alverne et al. 2016) and food web structure in estuarine ecosystems (Matson and Brinson 1990; Deegan and Garritt 1997), as has the magnitude of wet season discharge from rivers into their estuaries (Nelson et al. 2015). The likelihood that the Flinders River floodplain functioned as a transitional area between a freshwater floodplain and an estuarine or saltflat system also explains the increased importance of Metapenaeus prawns in the diet of catfish from the Flinders River, where they were highly abundant, relative to the Mitchell River. Isotope values for Metapenaeus in this study were similar to the δ13C values of the epipelon, epiphyton and benthic algae of their respective rivers, suggesting aquatic floodplain production sustained prawn biomass, which, in turn, provided an abundant food source for some catfish species.
SCA and SIA provided different indications of the extent of dietary separation within catfish species. Although PERMANOVA of stable isotope variation detected many significant between-species differences, most were restricted to comparisons between estuarine and freshwater species (as for comparisons based on stomach contents). A significant difference between freshwater species was limited to the comparison between N. berneyi, which consumed small aquatic insects, detritus and terrestrial insects, and N. paucus, which consumed large crustaceans. No significant difference in isotope values was detected for N. berneyi and N. graeffei in the Mitchell River, despite a significant difference in diet being detected. This is perhaps not surprising if the prey consumed, although differing between species, is similarly reliant on the same carbon source (i.e. algal derived). However, the isotope values for N. graeffei and A. hainesi in the Flinders River were significantly different, supporting the significant difference observed in stomach contents. However, the δ13C values of A. hainesi were more negative than those of N. graeffei and dissimilar to any measured primary consumer other than a single zooplankton sample. There is no published information on any aspect of the ecology of the former species, and thus no means to assess whether this difference reflects prior specialised feeding (i.e. in an estuarine habitat) on a distinctive food source. SCA and SIA information indicates that, contrary to our expectations, species of Ariidae do, indeed, partition food resources in the terminal floodplain section of these northern Australian rivers. Large estuarine species remained within the flooded riverine section of the river, whereas freshwater species, and A. hainesi, consume different food items derived from the floodplain.
The distinction between adult and young-of-the-year N. graeffei in the Flinders River (4–8‰ 13C) suggests that these small individuals had not foraged extensively on the floodplain itself before capture, but had been foraging further downstream in fully estuarine or marine habitats. The δ13C values for these small catfish (ranging from –13 to –12‰) are similar to those of seagrass carbon reported by Loneragan et al. (1997). Seagrass beds within the river channel may constitute an important food-producing habitat for small juvenile ariids, as well as providing refuge from predation once they have left the relative safety of the paternal buccal cavity. Significant relationships between δ13C and size for N. graeffei from both rivers indicate either growth-related metabolic differences in carbon fractionation (Martino et al. 2019) or a change in diet or habitat with increasing size. The effects of the former are typically much smaller (~1‰) than those we observed for N. graeffei (3–6‰), implying that habitat or diet shifts were more likely responsible for the observed patterns. That the rate of change in carbon isotope values with size was virtually identical for fish from both rivers despite significant differences in diet suggests that habitat shifts play an important role in generating size-related differences. The observed changes in isotope composition with size (across the gradient from small juveniles to large adults) suggest a complex pattern of movement and habitat use over the wet season period. Moreover, given that N. graeffei returns to upstream refugial habitats at the end of the wet season after a period of floodplain-supported growth (Jardine et al. 2012b), movement over the entire life history is complex and extensive in this species.
A diverse array of fishes, both estuarine and freshwater, has been recorded from these terminal floodplain habitats (Pusey et al. 2017). For example, 51 species of teleost fishes, 22 of which were predominantly estuarine, were collected during the present study (B. J. Pusey, unpubl. data). The presence of estuarine species on freshwater floodplains and adjacent flooded riverine sections of the Mitchell and Flinders rivers indicates not only a well-developed tolerance to low salinity, but also that the highly productive terminal floodplains of northern Australia provide an important habitat for both estuarine and freshwater fishes. Energy and nutrients derived from these floodplains support reproduction and juvenile development of a range of freshwater and estuarine forage species such as the diamond mullet (Villamarín et al. 2016), clupeid gizzard shads (Nematalosa spp.) and large predatory species of economic significance such as barramundi (Lates calcarifer; Jardine et al. 2012b). At the regional scale, the extent and duration of floodplain inundation is an important determinant of spatial variation in biodiversity of freshwater organisms (Jardine et al. 2015). Moreover, energy and nutrients derived from floodplains are transported elsewhere throughout the riverine network and help support freshwater fish biomass during the dry season when habitat extent is greatly diminished (Jardine et al. 2012a, 2012b). Elsewhere in northern Australia, the richness and abundance of juvenile forms of estuarine species is greatest during the wet season, coincident with inundation of terminal floodplains (Blaber et al. 1994; Pusey et al. 2016); floodplain production may also play an important role in sustaining estuarine biodiversity of northern Australian rivers.
Ariid catfishes comprise a significant component of the biodiversity of northern Australian rivers; they occur widely within individual rivers, attain large size and often dominate fish biomass (Jardine et al. 2012a, 2012b; Pusey et al. 2017). The present study found that although a diverse array of ariids was present in the two rivers when in flood, typically estuarine species, with the exception of A. hainesi, foraged in the highly dilute flooded river channel but not on the floodplain. By contrast, typically freshwater species did forage on the inundated floodplain, but did not make use of this habitat as a spawning area. Dietary partitioning between freshwater and estuarine species, and within freshwater species, was observed. SIA confirmed the dietary differences between estuarine and freshwater species. SIA provided less support for partitioning between freshwater species, likely because although the identity of prey species may have differed, most potential prey items were feeding on a similar array of items (e.g. periphyton). The present study indicates that ariid catfishes may occupy a variety of trophic roles, moving between such roles as they grow and as they make use of different habitats. Ariid catfishes likely play an important, but largely unrecognised, role in the ecology of northern Australian aquatic systems, particularly within riverine food webs. We acknowledge that the findings of the present study are provisional given that sample sizes were small for some species and that the study took place over a limited period. Our understanding of the trophic ecology of the family in northern Australia is limited, as is our understanding of what factors drive the dietary partitioning observed here and, indeed, whether it extends to other rivers and to other times of the year. A greater research focus on this group of fishes would provide substantial insights into the dynamics of northern Australian rivers in general and of their floodplains in particular.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was conducted under the Tropical Rivers and Coastal Knowledge (TRaCK) Research Hub. TRaCK received major funding for its research through the Australian Government’s Commonwealth Environment Research Facilities initiative, the Australian Government’s Raising National Water Standards Program, Land and Water Australia, the Fisheries Research and Development Corporation and the Queensland Government’s Smart State Innovation Fund.
Acknowledgements
The authors are grateful for the assistance in the field provided by S. Hamilton, N. Pettit, M. Burford, M. Pusey, C. Perna, R. Greenswool, A. Frank, R. Hunt and M. Gater. The authors thank D. Valdez, R. Diocares, V. Fry and L. Jardine for help with sample processing and isotope analysis, and Mark Kennard for help with figure production. The authors are also grateful for the contributions of two anonymous referees.
References
Adame, M. F., Pettit, N. E., Valdez, D., Ward, D., Burford, M. A., and Bunn, S. E. (2017). The contribution of epiphyton to the primary production of tropical floodplain wetlands. Biotropica 49, 461–471.| The contribution of epiphyton to the primary production of tropical floodplain wetlands.Crossref | GoogleScholarGoogle Scholar |
Adams, S. B., Hamel, P. B., Connor, K., Burke, B., Gardiner, E. S., and Wise, D. (2007). Potential roles of fish, birds, and water in swamp privet (Forestiera acuminata) seed dispersal. Southeastern Naturalist (Steuben, ME) 6, 669–682.
| Potential roles of fish, birds, and water in swamp privet (Forestiera acuminata) seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Allen, G. R. (1991). ‘Field guide to the freshwater fishes of New Guinea.’ (Christensen Research Institute: Madang, Papua New Guinea.)
Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). ‘PERMANOVA for PRIMER: Guide to Software and Statistical Methods.’ (PRIMER-E: Plymouth, UK.)
Bachok, Z., Mansor, M. I., and Noordin, R. M. (2004). Diet composition and food habits of demersal and pelagic marine fishes from Terengganu waters, east coast of Peninsular Malaysia. NAGA. WorldFish Center Quarterly 27, 41–47.
Barletta, M., Barletta-Bergan, A., Saint-Paul, U., and Hubold, G. (2005). The role of salinity in structuring the fish assemblages in a tropical estuary. Journal of Fish Biology 66, 45–72.
| The role of salinity in structuring the fish assemblages in a tropical estuary.Crossref | GoogleScholarGoogle Scholar |
Bayley, P. B. (1995). Understanding large river: floodplain ecosystems. Bioscience 45, 153–158.
| Understanding large river: floodplain ecosystems.Crossref | GoogleScholarGoogle Scholar |
Betancur-R, R. (2010). Molecular phylogenetics supports multiple evolutionary transitions from marine to freshwater habitats in ariid catfishes. Molecular Phylogenetics and Evolution 55, 249–258.
| Molecular phylogenetics supports multiple evolutionary transitions from marine to freshwater habitats in ariid catfishes.Crossref | GoogleScholarGoogle Scholar | 20045737PubMed |
Bishop, K. A., Allen, S. A., Pollard, D. A., and Cook, M. G. (2001). Ecological studies on the freshwater fishes of the Alligator Rivers region, Northern Territory: autecology. Report 145. (Supervising Scientist: Darwin, NT, Australia.) Available at https://www.environment.gov.au/system/files/resources/dd133e7c-2565-4683-991f-db30856a50ca/files/ssr145.pdf [Verified 28 April 2020].
Blaber, S. J., Brewer, D. T., and Salini, J. P. (1994). Diet and dentition in tropical ariid catfishes from Australia. Environmental Biology of Fishes 40, 159–174.
| Diet and dentition in tropical ariid catfishes from Australia.Crossref | GoogleScholarGoogle Scholar |
Brown, M. L., and Murphy, B. R. (2004). Seasonal dynamics of direct and indirect condition indices in relation to energy allocation in largemouth bass Micropterus salmoides (Lacepede). Ecology Freshwater Fish 13, 23–36.
| Seasonal dynamics of direct and indirect condition indices in relation to energy allocation in largemouth bass Micropterus salmoides (Lacepede).Crossref | GoogleScholarGoogle Scholar |
Coates, D. (1991). Biology of fork-tailed catfishes from the Sepik River, Papua New Guinea. Environmental Biology of Fishes 31, 55–74.
| Biology of fork-tailed catfishes from the Sepik River, Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Correa, S. B., Winemiller, K. O., Lopez-Fernandez, H., and Galetti, M. (2007). Evolutionary perspectives on seed consumption and dispersal by fishes. A.I.B.S. Bulletin 57, 748–756.
Crook, D. A., Buckle, D. J., Morrongiello, J. R., Allsop, Q. A., Baldwin, W., Saunders, T. M., and Douglas, M. M. (2020). Tracking the resource pulse: movement responses of fish to dynamic floodplain habitat in a tropical river. Journal of Animal Ecology 89, 795–807.
| Tracking the resource pulse: movement responses of fish to dynamic floodplain habitat in a tropical river.Crossref | GoogleScholarGoogle Scholar | 31750933PubMed |
Dantas, D. V., Barletta, M., Costa, M. F., Barbosa-Cintra, S. C. T., Possatto, F. E., Ramos, J. A., Lima, A. R. A., and Saint-Paul, U. (2010). Movement patterns of catfishes (Ariidae) in a tropical semi-arid estuary. Journal of Fish Biology 76, 2540–2557.
| Movement patterns of catfishes (Ariidae) in a tropical semi-arid estuary.Crossref | GoogleScholarGoogle Scholar | 20557607PubMed |
Davis, A. M., Pusey, B. J., and Pearson, R. G. (2018). Big floods, big knowledge gap: food web dynamics in a variable river system. Ecology Freshwater Fish 27, 898–909.
| Big floods, big knowledge gap: food web dynamics in a variable river system.Crossref | GoogleScholarGoogle Scholar |
Deegan, L. A., and Garritt, R. H. (1997). Evidence for spatial variability in estuarine food webs. Marine Ecology Progress Series 147, 31–47.
| Evidence for spatial variability in estuarine food webs.Crossref | GoogleScholarGoogle Scholar |
Finlay, J. C. (2004). Patterns and controls of lotic algal stable carbon isotope ratios. Limnology and Oceanography 49, 850–861.
| Patterns and controls of lotic algal stable carbon isotope ratios.Crossref | GoogleScholarGoogle Scholar |
Fry, B. (2002). Conservative mixing of stable isotopes across estuarine salinity gradients: a conceptual framework for monitoring watershed influences on downstream fisheries production. Estuaries 25, 264–271.
| Conservative mixing of stable isotopes across estuarine salinity gradients: a conceptual framework for monitoring watershed influences on downstream fisheries production.Crossref | GoogleScholarGoogle Scholar |
Hajisamae, S., Chou, L. M., and Ibrahim, S. (2004). Feeding habits and trophic relationships of fishes utilizing an impacted coastal habitat, Singapore. Hydrobiologia 520, 61–71.
| Feeding habits and trophic relationships of fishes utilizing an impacted coastal habitat, Singapore.Crossref | GoogleScholarGoogle Scholar |
Hyslop, E. J. (1980). Stomach contents analysis – a review of methods and their application. Journal of Fish Biology 17, 411–429.
| Stomach contents analysis – a review of methods and their application.Crossref | GoogleScholarGoogle Scholar |
Jardine, T. D., Pusey, B. J., Hamilton, S. K., Pettit, N. E., Davies, P. M., Douglas, M. M., Sinnamon, V., Halliday, I. A., and Bunn, S. E. (2012a). Fish mediate high foodweb connectivity in the lower reaches of a tropical floodplain river. Oecologia 168, 829–838.
| Fish mediate high foodweb connectivity in the lower reaches of a tropical floodplain river.Crossref | GoogleScholarGoogle Scholar | 21983712PubMed |
Jardine, T. D., Pettit, N. E., Warfe, D. M., Pusey, B. J., Ward, D. P., Douglas, M. M., Davies, P. M., and Bunn, S. E. (2012b). Consumer–resource coupling in wet–dry tropical rivers. Journal of Animal Ecology 81, 310–322.
| Consumer–resource coupling in wet–dry tropical rivers.Crossref | GoogleScholarGoogle Scholar | 22103689PubMed |
Jardine, T. D., Bond, N. R., Burford, M. A., Kennard, M. J., Ward, D. P., Bayliss, P., Davies, P. M., Douglas, M. M., Hamilton, S. K., Melack, J. M., Naiman, R. J., Pettit, N. E., Pusey, B. J., Warfe, D. M., and Bunn, S. E. (2015). Does flood rhythm drive ecosystem responses in tropical riverscapes? Ecology 96, 684–692.
| Does flood rhythm drive ecosystem responses in tropical riverscapes?Crossref | GoogleScholarGoogle Scholar | 26236865PubMed |
Kailola, P. J. (2000). Six new species of fork-tailed catfishes (Pisces, Teleostei, Ariidae) from Australia and New Guinea. The Beagle: Records of the Museums and Art Galleries of the Northern Territory 16, 1–27.
Kennard, M. J., Pusey, B. J., Olden, J. D., Mackay, S. J., Stein, J. L., and Marsh, N. (2010). Classification of natural flow regimes in Australia to support environmental flow management. Freshwater Biology 55, 171–193.
| Classification of natural flow regimes in Australia to support environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Kesteven, G. L. (1960). ‘Manual of Field Methods in Fisheries Biology.’ (Food and Agriculture Organization of the United Nations: Rome, Italy.)
Krumme, U., Brenner, M., and Saint-Paul, U. (2008). Spring–neap cycle as a major driver of temporal variations in feeding of intertidal fishes: evidence from the sea catfish Sciades herzbergii (Ariidae) of equatorial west Atlantic mangrove creeks. Journal of Experimental Marine Biology and Ecology 367, 91–99.
| Spring–neap cycle as a major driver of temporal variations in feeding of intertidal fishes: evidence from the sea catfish Sciades herzbergii (Ariidae) of equatorial west Atlantic mangrove creeks.Crossref | GoogleScholarGoogle Scholar |
Logan, J. M., Jardine, T. D., Miller, T. J., Bunn, S. E., Cunjak, R. A., and Lutcavage, M. E. (2008). Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. Journal of Animal Ecology 77, 838–846.
| Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods.Crossref | GoogleScholarGoogle Scholar | 18489570PubMed |
Loneragan, N. R., Bunn, S. E., and Kellaway, D. M. (1997). Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Marine Biology 130, 289–300.
| Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study.Crossref | GoogleScholarGoogle Scholar |
Maitra, S., Harikrishnan, M., and Nidhin, B. (2020). Feeding strategy, dietary overlap and resource partitioning among four mesopredatory catfishes of a tropical estuary. Journal of Fish Biology 96, 130–139.
| Feeding strategy, dietary overlap and resource partitioning among four mesopredatory catfishes of a tropical estuary.Crossref | GoogleScholarGoogle Scholar | 31697401PubMed |
Martino, J. C., Doubleday, Z. A., and Gillanders, B. M. (2019). Metabolic effects on carbon isotope biomarkers in fish. Ecological Indicators 97, 10–16.
| Metabolic effects on carbon isotope biomarkers in fish.Crossref | GoogleScholarGoogle Scholar |
Matson, E. A., and Brinson, M. M. (1990). Stable carbon isotopes and the C : N ratio in the estuaries of the Pamlico and Neuse rivers, North Carolina. Limnology and Oceanography 35, 1290–1300.
| Stable carbon isotopes and the C : N ratio in the estuaries of the Pamlico and Neuse rivers, North Carolina.Crossref | GoogleScholarGoogle Scholar |
Mendoza-Carranza, M., and Vieira, J. P. (2009). Ontogenetic niche feeding partitioning in juvenile of white sea catfish Genidens barbus in estuarine environments, southern Brazil. Journal of the Marine Biological Association of the United Kingdom 89, 839–848.
| Ontogenetic niche feeding partitioning in juvenile of white sea catfish Genidens barbus in estuarine environments, southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Mont’Alverne, R., Jardine, T. D., Pereyra, P. E., Oliveira, M. C., Medeiros, R. S., Sampaio, L. A., Tesser, M. B., and Garcia, A. M. (2016). Elemental turnover rates and isotopic discrimination in a euryhaline fish reared under different salinities: implications for movement studies. Journal of Experimental Marine Biology and Ecology 480, 36–44.
| Elemental turnover rates and isotopic discrimination in a euryhaline fish reared under different salinities: implications for movement studies.Crossref | GoogleScholarGoogle Scholar |
Nelson, J. A., Deegan, L., and Garritt, R. (2015). Drivers of spatial and temporal variability in estuarine food webs. Marine Ecology Progress Series 533, 67–77.
| Drivers of spatial and temporal variability in estuarine food webs.Crossref | GoogleScholarGoogle Scholar |
Nelson, J. S., Grande, T. C., and Wilson, M. V. (2016). ‘Fishes of the World.’ (Wiley: Hoboken, NJ, USA.)
Pettit, N. E., Bayliss, P., Davies, P. M., Hamilton, S. K., Warfe, D. M., Bunn, S. E., and Douglas, M. M. (2011). Seasonal contrasts in carbon resources and ecological processes on a tropical floodplain. Freshwater Biology 56, 1047–1064.
| Seasonal contrasts in carbon resources and ecological processes on a tropical floodplain.Crossref | GoogleScholarGoogle Scholar |
Pollux, B. J. A. (2011). The experimental study of seed dispersal by fish (ichthyochory). Freshwater Biology 56, 197–212.
| The experimental study of seed dispersal by fish (ichthyochory).Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Kennard, M. J., and Arthington, A. H. (2004). ‘Freshwater Fishes of North-eastern Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Pusey, B. J., Kennard, M. J., Larson, H. K., Alsop, Q., Hammer, M., and Buckle, D. J. (2016). Estuarine fishes of the south Alligator River, Kakadu National Park, northern Australia. Marine and Freshwater Research 67, 1797–1812.
| Estuarine fishes of the south Alligator River, Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Pusey, B. J., Burrows, D. W., Kennard, M. J., Perna, C. N., Unmack, P. J., Allsop, Q., and Hammer, M. P. (2017). Freshwater fishes of northern Australia. Zootaxa 4253, 1–104.
| Freshwater fishes of northern Australia.Crossref | GoogleScholarGoogle Scholar | 28609989PubMed |
Tockner, K., and Stanford, J. A. (2002). Riverine floodplains: present state and future trends. Environmental Conservation 29, 308–330.
| Riverine floodplains: present state and future trends.Crossref | GoogleScholarGoogle Scholar |
Villamarín, F., Magnusson, W. E., Jardine, T. D., Valdez, D., Woods, R., and Bunn, S. E. (2016). Temporal uncoupling between energy acquisition and allocation to reproduction in a herbivorous–detritivorous fish. PLoS One 11, e0150082.
| Temporal uncoupling between energy acquisition and allocation to reproduction in a herbivorous–detritivorous fish.Crossref | GoogleScholarGoogle Scholar | 26938216PubMed |
Ward, D., Pusey, B., Brooks, A., Olley, J., Shellberg, J., Spencer, J., and Tews, K. (2011). River landscapes and aquatic systems diversity. In ‘Aquatic Biodiversity of the Wet–Dry Topics of Northern Australia: Patterns, Threats and Future’. (Ed. B. J. Pusey.) pp. 5–22. (CDU Press: Darwin, NT, Australia.)
Warfe, D. M., Pettit, N. E., Davies, P. M., Pusey, B. J., Hamilton, S. K., Kennard, M. J., Townsend, S. A., Bayliss, P., Ward, D. P., Douglas, M., Burford, M. A., Finn, M., Bunn, S. E., and Halliday, I. (2011). The ‘wet-dry’ in the wet–dry tropics drives river ecosystem structure and processes in northern Australia. Freshwater Biology 56, 2169–2195.
| The ‘wet-dry’ in the wet–dry tropics drives river ecosystem structure and processes in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (2004). Floodplain river food webs: generalizations and implications for fisheries management. In ‘Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries Volume 2’, 11–14 February 2003, Phnom Penh, Cambodia. (Eds R. L. Welcomme and T. Petr.) pp. 285–309. (Food and Agriculture Organization and Mekong River Commission, FAO Regional Office for Asia and the Pacific: Bangkok, Thailand.)
Yáñez-Arancibia, A., and Lara-Domínguez, A. L. (1988). Ecology of three sea catfishes (Ariidae) in a tropical coastal ecosystem – southern Gulf of Mexico. Marine Ecology – Progress Series 49, 215–230.
| Ecology of three sea catfishes (Ariidae) in a tropical coastal ecosystem – southern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |