Otolith chemistry delineates the influence of natal origin, dispersal and flow on the population dynamics of golden perch (Macquaria ambigua) in a regulated river
Brenton P. Zampatti A B I , Sandra J. Leigh
A B I , Sandra J. Leigh  A B , Phillipa J. Wilson
A B , Phillipa J. Wilson  A C , David A. Crook
A C , David A. Crook  D E , Bronwyn M. Gillanders
D E , Bronwyn M. Gillanders  B , Roland Maas
B , Roland Maas  F , Jed I. Macdonald
F , Jed I. Macdonald  G H and Jon Woodhead
G H and Jon Woodhead  F
F
A Inland Waters and Catchment Ecology Program, South Australian Research and Development Institute (SARDI) – Aquatic Sciences, PO Box 120, Henley Beach, SA 5022, Australia.
B School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
C Australian Institute of Marine Science, Indian Ocean Marine Research Centre, The University of Western Australia (M096), Perth, WA 6009, Australia.
D Research Institute for the Environment and Livelihoods, Engineering Health Science & Environment, Charles Darwin University, Darwin, NT 0909, Australia.
E Centre for Freshwater Ecosystems, La Trobe University, Wodonga, Vic. 3689, Australia.
F School of Geography, Earth and Atmospheric Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
G Arthur Rylah Institute for Environmental Research, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
H Oceanic Fisheries Programme, Pacific Community (SPC), BP D5 98848, Nouméa, New Caledonia.
I Corresponding author. Present address: CSIRO Land and Water, Locked Bag 2, Glen Osmond, SA 5064, Australia. Email: brenton.zampatti@csiro.au
Marine and Freshwater Research - https://doi.org/10.1071/MF20280
Submitted: 20 September 2020 Accepted: 27 April 2021 Published online: 29 June 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
For riverine fishes threatened by fragmentation and flow modification, effective management requires an understanding of when and where key life history processes (spawning, recruitment and movement) take place. The structural and chemical properties of otoliths provide a unique means to recount a fish’s life in time and space. We investigated the age structure of the migratory, pelagic-spawning golden perch (Macquaria ambigua) in the Murray River, Australia, and used water and otolith 87Sr/86Sr ratios to delineate the natal origin and movement of fish from discrete cohorts. Water 87Sr/86Sr was distinct among the Darling River (a major tributary) and lower and mid-Murray River. Otolith chemistry revealed golden perch collected in the lower Murray River were progeny of spawning in either the Murray or Darling rivers, during years characterised by within-channel rises in flow, or in both rivers in a year of overbank flooding. Movement of juvenile fish from the Darling River substantially influenced population structure in the lower Murray River, whereby post-flood population growth was largely due to the immigration of age-1+ fish. This study demonstrates the potential importance of tributary recruitment sources, dispersal and connectivity on main-stem population dynamics and the utility of otolith chemistry for spatially reconciling population structure and the life histories of freshwater fishes.
Keywords: freshwater fish, Murray–Darling Basin, migration, river regulation, strontium.
Introduction
Globally, modification of riverine habitats and flow regimes alters ecosystem function, with related effects on population resilience and biodiversity (Dudgeon et al. 2006; Poff et al. 2007). Freshwater fishes are particularly vulnerable to river regulation (Nilsson et al. 2005), and effective management requires a spatiotemporal context for the key life history processes (spawning, recruitment and movement) that determine population dynamics. Rivers are dendritic in nature and riverine fish may disperse among the branches of these networks (Fagan 2002; Koster et al. 2014). Ontogenetic variation in habitat use is also commonplace, with alternative locations potentially used for spawning, rearing and refuge (Amoros and Bornette 2002; King 2004). As such, dispersal and migration between disparate locations can be an important determinant of metapopulation structure and function (Jager et al. 2001).
In lotic ecosystems, migratory, pelagic-broadcast spawning fishes (pelagophils) are particularly affected by fragmentation and flow modification (Hoagstrom and Turner 2015). Hydrological cues and hydraulic habitats for spawning are altered by flow regulation, and spawning migrations and the obligate downstream drift of eggs and larvae, essential for the development of early life stages, are interrupted by physical barriers and the hydraulic effects of dams and weirs (Welcomme et al. 2006; Perkin et al. 2015). Consequently, in regulated rivers, these fishes may demonstrate episodic recruitment and low demographic resilience (Zampatti and Leigh 2013a). Conservation and rehabilitation of pelagophils requires an understanding of habitat and hydrological requirements across a fish’s lifetime, including characterising natal, juvenile and adult habitats, and movement among these (Dudley and Platania 2007).
In Australia’s Murray–Darling Basin (MDB), flow regulation has negatively affected native fish populations (Barrett 2004). To redress this, fish form a primary objective for environmental water delivery under contemporary river rehabilitation programs (Koehn et al. 2014). Golden perch (Macquaria ambigua) is one of a few species in the MDB that are migratory, pelagic-broadcast spawners, and where spawning, recruitment and movement have been explicitly associated with flow variability (Mallen-Cooper and Stuart 2003; Mallen-Cooper and Brand 2007; King et al. 2009; Zampatti and Leigh 2013a; Koster et al. 2014; 2017). Accordingly, these aspects of golden perch life history form a focus for environmental flow management in the MDB.
To inform flow restoration, knowledge regarding the spatial structure of populations and the effects of flow on population processes is vital. Nevertheless, few investigations relating fish recruitment to flow have considered precisely when and where fish originated (although, see Limburg et al. 2013; Macdonald and Crook 2014). In the lower Murray River, golden perch spawning and recruitment are associated with in-channel and overbank rises in flow, nominally >15 000 ML day–1 (Zampatti and Leigh 2013a). Following extensive flooding in the lower Murray River in 2010–11, golden perch abundance increased significantly, compared with six previous years of generally low, in-channel flows (Zampatti and Leigh 2013b). Age structure analysis revealed that the increased abundance was due to high numbers of young-of-the-year (hereafter referred to as age-0+) and age-1+ fish born in the flood year and in the year prior respectively. Indeed, ~50% of the juvenile golden perch collected after flooding were age-1+ fish born during a period of low flow in the lower Murray River (<10 000 ML day–1, 44th percentile exceedance flow; MDBA, unpubl. data). Zampatti and Leigh (2013b) speculated that the age-1+ cohort did not originate in the Murray River and, instead, originated in the Darling River, the major tributary of the Murray River, potentially in association with a substantial rise in discharge (0–11 000 ML day–1) in the lower Darling River 1 year before the 2010–11 flood. Confirming the provenance of golden perch in the lower Murray River and integrating this with information on migration history will improve understanding of the spatial ecology of golden perch in the MDB and relationships between flow, key life history processes and population dynamics.
The chemical composition of fish otoliths (ear stones) can be used to study the origin and movement of fish (Elsdon et al. 2008). A fish’s historical movement, including its place of birth and death, can potentially be determined by comparing geochemical signatures in otoliths with ambient signatures in water, if there is geographic variability in water chemistry. Stable isotopes of strontium (Sr) have been used successfully to discern the natal habitats and movements of numerous diadromous and freshwater fishes (Kennedy et al. 2002; Crook et al. 2013; Brennan and Schindler 2017). Unlike metal : Ca ratios (e.g. Sr/Ca, Ba/Ca), Sr isotope ratios are not biologically fractionated; therefore, 87Sr/86Sr ratios measured in otoliths directly reflect the ambient water 87Sr/86Sr ratio (Kennedy et al. 2000). As a result, spatiotemporal ‘isoscapes’ of dissolved 87Sr/86Sr in water can provide a template for determining the spatial origin and movement history of fish (Barnett-Johnson et al. 2008; Muhlfeld et al. 2012).
The aims of this study were to characterise the age structure of golden perch populations in the lower Murray River and to use water and otolith strontium isotope ratios to elucidate the natal origin and migration history of fish from discrete cohorts. Our specific objectives were to: (1) demonstrate the persistence of specific age classes in the lower Murray River population associated with distinct flow events; (2) characterise spatiotemporal variability in water 87Sr/86Sr ratios at sites in the Murray and Darling rivers; (3) determine otolith core 87Sr/86Sr ratios in distinct cohorts of golden perch; (4) compare 87Sr/86Sr ratios in otolith cores with water 87Sr/86Sr ratios in the study region to elucidate fish provenance; and (5) measure 87Sr/86Sr ratios along transects from otolith core to edge to investigate the migration history of golden perch from the specific cohorts.
Study region
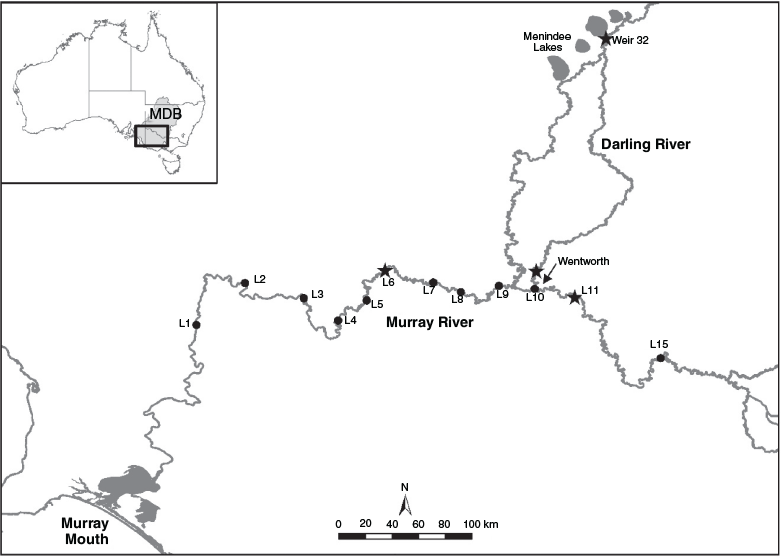
The MDB drains an area of 1 073 000 km2. The combined length of the two major rivers, the Murray and the Darling, is ~5500 km and both rivers flow through predominantly semi-arid and arid landscapes. River regulation, comprising headwater storages, weirs, floodplain levees and tidal barrages, and consumptive use for irrigation and domestic supply, have markedly reduced the magnitude and variability of discharge in the Murray River (Maheshwari et al. 1995) and most of its tributaries (Kingsford 2003).
This study was conducted in the lower and mid-reaches of the Murray River, and the lower Darling River. The lower Murray River extends upstream from the river mouth to the Darling River junction (Fig. 1). This 830-km reach of river is fragmented by 10 low-level (~3 m) weirs that, under low flows, transform a historically dynamic lotic system into a homogeneous series of lentic environments (Walker 2006; Mallen-Cooper and Zampatti 2018). The mid-Murray extends upstream from the Darling River junction for 1155 km to Yarrawonga (Fig. 1). This region is less fragmented by weirs and retains long reaches (hundreds of kilometres) of lotic habitat (Mallen-Cooper and Zampatti 2018). However, it is subject to regulated discharge and, in some reaches, seasonal inversion of flow (Maheshwari et al. 1995). The lower Darling River extends for 510 km upstream from the junction with the Murray River to the Menindee Lakes, an extensive series of off-channel lakes (457 km2; 1731-GL capacity) used to regulate and store Darling River flows for consumptive use.
Materials and methods
Fish collection and ageing
Sampling of golden perch occurred annually from 2010 to 2014, in main-channel and anabranch habitats between Lock 3 and Lock 6 in the lower Murray River. In each year, golden perch were sampled in March–May by boat electrofishing using either a 5- or 7.5-kW Smith Root (Model GPP 5 or 7.5) electrofishing unit. At each site, electrofishing was conducted during daylight hours and all available littoral habitats were fished. All fish were measured to the nearest millimetre (total length, TL) and a subsample of fish (n = 50–70) proportionally representative of the length structure was retained for ageing. Fish were killed in the field by AQUI-S (AQUI-S, Lower Hutt, New Zealand) overdose before removal of the sagittal otoliths.
Whole sagittae were embedded in clear resin and sectioned transversely through the primordium (400–600 μm). Sections were mounted on microscope slides and examined under a dissecting microscope (×25) under transmitted light. Annual increment formation in golden perch otoliths has been validated (Anderson et al. 1992; Mallen-Cooper and Stuart 2003; Zampatti and Leigh 2013a). Independent estimates of age were made by three readers by counting the number of opaque zones (annuli) from the primordium to the otolith edge. Age-0+ fish were defined as individuals lacking clearly discernible annuli.
All sampling and study procedures were conducted under an exemption (Number 9902132) of Section 115 of the Fisheries Management Act 2007 and in accordance with the South Australian Animal Welfare Act 1985.
Water collection and Sr isotope analysis
To investigate spatiotemporal variability in 87Sr/86Sr ratios in water, surface water samples were collected fortnightly to monthly over a 4-year period from December 2011 to December 2014 in the three study reaches: the mid-Murray River (Mildura, Lock 11), the lower Murray River (Lock 6) and the lower Darling River (Weir 32). One water sample was also collected in the Darling River at Pomona (immediately upstream of Wentworth) in December 2011 (Fig. 1). Water samples were collected in new polypropylene 114- × 44-mm sample containers (SARSTEDT, Nümbrecht, Germany) and refrigerated.
An aliquot (20 mL) of each water sample was filtered through a 0.2-µm Acrodisc syringe-mounted filter into a clean polystyrene beaker and dried overnight in a HEPA-filtered fume cupboard. Filtering in the laboratory, rather than after sample collection in the field, does not affect measurement of 87Sr/86Sr (Palmer and Edmond 1989). Strontium was extracted using a single pass over 0.15-mL (4 × 12 mm) beds of EICHROM Sr resin (50–100 µm). Following Pin et al. (1994), 2- and 7-M nitric acids were used to wash matrix elements off the resin, followed by elution of clean Sr in 0.05-M nitric acid. The total blank, including syringe filtering, was ≤0.1 ng, indicating sample to blank ratios of ≥4000; therefore, blank corrections were deemed unnecessary. Strontium isotope analyses were performed on a Nu Plasma multi-collector–inductively coupled plasma–mass spectrometer (MC-ICP-MS; Nu Instruments, Wrexham, UK) interfaced with an ARIDUS desolvating system and low-uptake (0.05 mL min–1) nebuliser (Teledyne CETAC, Omaha, NE, USA). Instrumental mass bias was corrected by normalizing to 88Sr/86Sr = 8.37521 and results are reported relative to a value of 0.710230 for the SRM987 Sr isotope standard. Based on at least thirty 10-s integrations, internal precisions (2 s.e.) averaged ±0.00002 and mean reproducibility (2 s.d.) was ±0.00004.
Otolith preparation and Sr isotope analysis
Otoliths for Sr isotope analysis were obtained from golden perch collected for age determination, and prior collections (2005–10) of golden perch juveniles and adults sampled by electrofishing, as per samples collected for age structure analysis, in main-channel and anabranch habitats in the lower Murray River between Lock 3 and Lock 6 (Fig. 1; Table 1). Post-larval fish (age-0+) were collected in riverine and anabranch habitats in 2005 using the methods outlined in Zampatti and Leigh (2013a). Briefly, drift nets (mesh 500 µm, opening 0.5 m in diameter, length 1.5 m) and modified quatrefoil light traps (Floyd et al. 1984) were set concurrently, overnight.
|
Otolith preparation and analysis for Sr isotope ratios (87Sr/86Sr) generally followed the methods outlined in Woodhead et al. (2005) and Zampatti et al. (2015). Specifically, sagittal otoliths were dissected and retained whole (age-0+ fish) or embedded in clear casting resin (≥age 1+ fish) and sectioned transversely (400–600 μm). Whole otoliths and sections were then mounted on acid-washed glass slides using Crystalbond. Whole otoliths were mounted proximal surface downwards and polished to the primordium using a series of wetted lapping films (9, 5 and 3 μm), whereas transverse sections were polished using wetted 9-μm lapping film. To remove potential for systematic bias during analysis, slides were reheated and the polished otolith or section removed and arranged randomly on a ‘master’ slide, which was subsequently rinsed in ultrapure water (Millipore) and dried overnight in a Class 100 laminar flow cabinet.
Laser ablation (LA)–ICPMS was used to measure 87Sr/86Sr in otoliths. The analytical system consisted of a Nu Plasma MC-ICP-MS, coupled to an Applied Spectra RESOlution excimer laser ablation system (Applied Spectra, West Sacramento, CA, USA) operating at 193 nm. Master slides were placed in the sample cell and the primordium of each otolith was located visually with a 400× objective and a video imaging system. For each otolith, the intended ablation path was digitised using GeoStar software (ver. 6.14, Applied Spectra). For age-0+ fish collected in 2005 and 2010, and age-1+ fish collected in 2007, a 55-µm ablation spot was used to measure 87Sr/86Sr in the region of the otolith core (area incorporating early life history information; i.e. the first few days of a fish’s life) and edge (area incorporating information from the capture location). The laser was operated to provide a fluence of ~2–3 J cm–2 at the sample surface and pulsed at 10 Hz. Ablation was performed under pure He to minimise the re-deposition of ablated material, and the sample was then rapidly entrained into the Ar carrier gas flow. Each analysis consisted of 60 s of data acquisition, following 30 s of baseline (gas blank) measurement.
For all other samples, otoliths were ablated along a transect from the primordium to the dorsal margin using a 6 × 100-μm rectangular laser slit. The laser was operated to provide a fluence of 2–3 J cm–2, pulsed at 10 Hz and, depending on the size of the otolith, scanned across the sample at 5 or 10 μm s–1. To remove any surface contaminants, each otolith transect was pre-ablated and a 30-s background was measured prior to acquiring data. Corrections for instrumental mass bias and Kr, Rb and Ca argide/dimer interferences were made using the Iolite software for deconvolution of time resolved mass spectrometry data (Paton et al. 2011).
To calculate external precision, a modern marine carbonate standard composed of mollusc shells was analysed after every 10 otolith samples. According to long-term laboratory measurements, this standard has an 87Sr/86Sr value of 0.70916, identical to the accepted modern seawater value of 0.709160 (McArthur and Howarth 2004). Mean (±s.d.) values of 87Sr/86Sr values in the modern marine carbonate standard (n = 24) run throughout the analyses were 0.70918 ± 0.00017, with external precision (expressed as ±2 s.e.) calculated as ±0.00006. Mean within-run precision, measured as ±2 s.e., was ±0.00005.
Results
Golden perch age demographics 2010–14
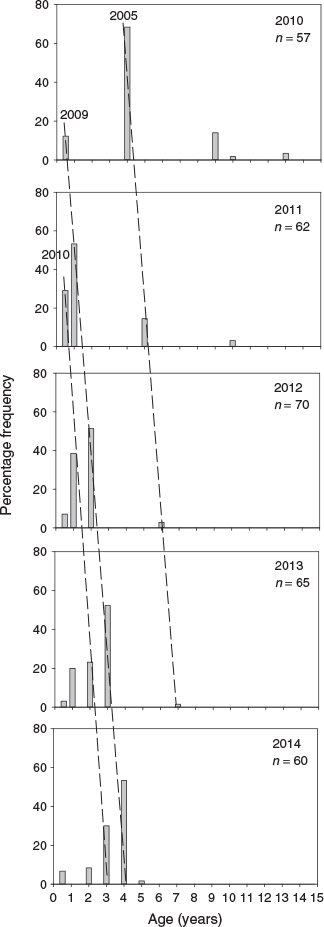
From 2010 to 2014, three distinct cohorts of golden perch were present in the lower Murray River, corresponding with birth years of 2005–06, 2009–10 and 2010–11 (Fig. 2). In 2010, age-4+ fish, from the 2005–06 cohort, comprised ~70% of population, but this cohort diminished in 2011 to represent <20% of the population as age-5+ fish (Fig. 2) and, by 2014, was absent (Fig. 2). In 2011, the age-0+ and age-1+ fish from the respective 2010–11 and 2009–10 cohorts comprised >70% of the population, and continued to dominate through 2012–14 (Fig. 2). The most prominent cohort in the lower Murray River from 2010 to 2014, was that of the 2009–10 recruits, which comprised at least 50% of the population from 2011 to 2014, as age-1+ to age-4+ fish respectively (Fig. 2).
|
Spatiotemporal variation in water 87Sr/86Sr
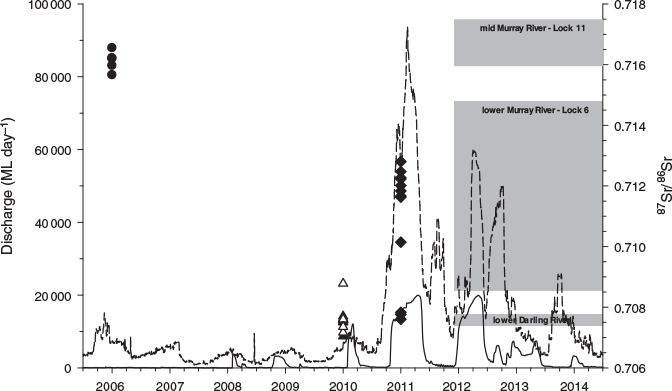
Water 87Sr/86Sr showed substantial variation among both years and regions. In the lower Darling River, 87Sr/86Sr values ranged from 0.707434 to 0.707592 and exhibited considerable intra- and interannual stability (Fig. 3; Table S1 of the Supplementary material). Water 87Sr/86Sr values in the lower Darling River were considerably lower and clearly distinct from the mid-Murray and lower Murray, which were in the range 0.715954–0.717482 and 0.708619–0.714719 respectively (Fig. 3; Table S1). Water 87Sr/86Sr values in the lower Murray River reflected mixed water sources and variability in the contribution of discharge from the Murray and Darling river catchments (Fig. 3; Table S1). For example, in February 2012, flow from the Darling River comprised >95% of flow to the lower Murray River, and water 87Sr/86Sr in this region (0.708619) approached that of the Darling River (~0.7075; Fig. 3; Table S1). By contrast, in November 2014, flow from the Darling River was negligible and water 87Sr/86Sr in the lower Murray River (Lock 6; 0.714130) more closely resembled that in the mid Murray River (Lock 11; 0.716920; Fig. 3; Table S1).
Provenance of golden perch
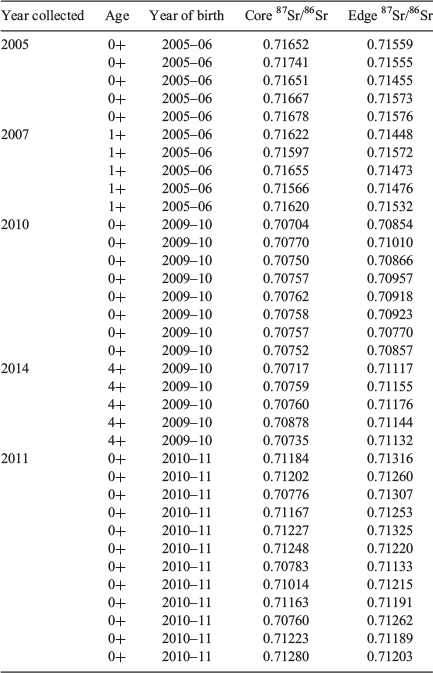
Golden perch from the 2005–06 cohort exhibited otolith core 87Sr/86Sr ranging from 0.71651 to 0.71741 for age-0+ fish and from 0.71566 to 0.71655 for age-1+ fish (Fig. 3; Table 1). These otolith 87Sr/86Sr corresponded with water 87Sr/86Sr in the mid Murray River at Lock 11 (Fig. 3; Table 1), indicating a Murray River natal origin for the 2005–06 cohort of golden perch. By contrast, golden perch from the 2009–10 cohort exhibited otolith core 87Sr/86Sr ranging from 0.70704 to 0.70878 for age-0+ and age-4+ fish (Fig. 3; Table 1), suggesting that the majority of fish had originated in the Darling River (Fig. 3; Table S1). Of the fish from the 2010–11 cohort, 75% (n = 9) had otolith core 87Sr/86Sr values ranging from 0.71163 to 0.71280 and the remaining 25% (n = 3) had otolith core 87Sr/86Sr values ranging from 0.70760 to 0.70783 (Fig. 3; Table S1), corresponding to water 87Sr/86Sr in the lower Murray and Darling River respectively (Fig. 3; Table S1). As such, the 2010–11 cohort of golden perch differed from the 2005–06 (Murray River origin) and 2009–10 (Darling River origin) cohorts in that fish originated from both the lower Murray River and Darling River. Golden perch from the 2005–06 and 2009–10 cohorts originated in association with spatially distinct flow pulses in the Murray and Darling rivers respectively (Fig. 3). By contrast, fish comprising the 2010–11 cohort originated in both the Murray and Darling rivers in association with broad-scale flooding across the southern MDB (Fig. 3).
Migration history of fish
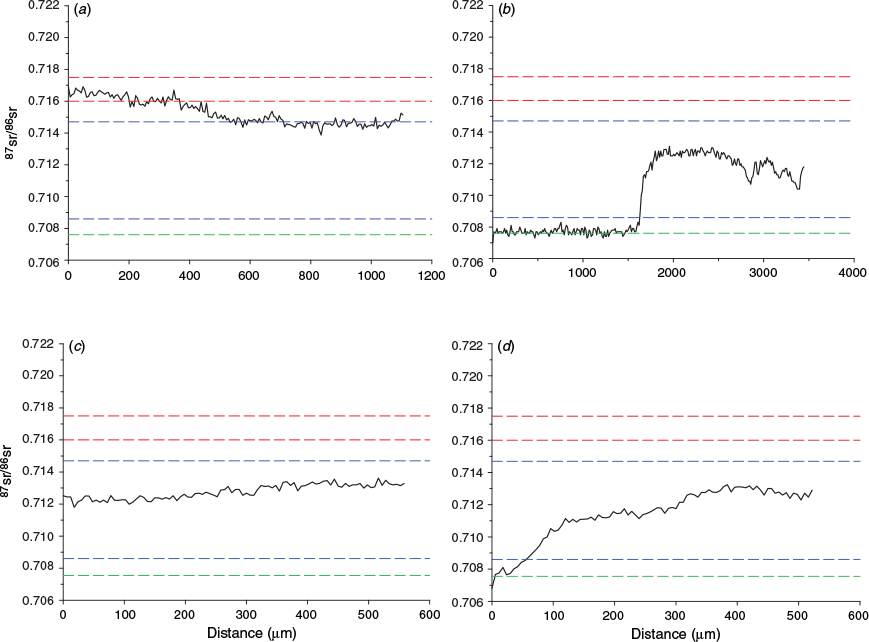
To investigate the migration history of golden perch we analysed 87Sr/86Sr profiles from otolith core to edge, thus elucidating lifetime variability in 87Sr/86Sr. Age-1+ golden perch from the 2005–06 cohort exhibited otolith 87Sr/86Sr transects indicative of spawning and residence in the Murray River. At a finer spatial scale, early in their lives, these fish had otolith 87Sr/86Sr comparable to water 87Sr/86Sr in the mid-Murray River and then transition to a lower Murray River 87Sr/86Sr (Fig. 4a; Fig. S1 of the Supplementary material). Age-4+ golden perch from the 2009–10 cohort predominantly exhibited otolith 87Sr/86Sr transects indicative of a Darling River origin and subsequent transition into the Murray River as age-1+ fish (Fig. 4b; Fig. S1). Of the 12 age-0+ golden perch from the 2010–11 cohort, 75% exhibited otolith 87Sr/86Sr indicative of a lower Murray River spawning origin and continued residence in the lower Murray River until capture (Fig. 4c; Fig. S2 of the Supplementary material). The remaining 25% exhibited otolith core 87Sr/86Sr values comparable to that of water in the Darling River, but rapid transition to values representative of water 87Sr/86Sr in the lower Murray River early in their lives (Fig. 4d; Fig. S2), indicating a lower Darling River natal origin and subsequent dispersal into the lower Murray River as larvae.
Discussion
Population demographics
Data on population age structure are fundamental for fisheries management, including understanding how interventions, such as flow augmentation, influence populations (Berkeley et al. 2004; Cowx and Van Zyll de Jong 2004). In 2010, the golden perch population in the lower Murray River was dominated (~70%) by a single age class (age-4+ fish) that originated in association with a spring flow pulse in the Murray River 4 years previously (late 2005). Although fish ranged in age from 0+ to 13+, the population was characterised by a depauperate age structure, with an absence of recruitment during an extended period (2001–09) of drought (hereafter the ‘Millennium Drought’; van Dijk et al. 2013; Zampatti and Leigh 2013a). Long-lived freshwater fishes with periodic life histories, like golden perch, characteristically demonstrate large interannual variation in recruitment, and distinct cohorts may dominate populations for many years (Winemiller 2005). In regulated rivers, hydrological alteration and fragmentation can compromise the demographic resilience of species with periodic life history traits (Olden and Kennard 2010), and climate variability, including drought, may further exacerbate this effect (Bond et al. 2015). As such, the appearance of a cohort of age-0+ golden perch in early 2010, during the later stages of the Millennium Drought and in association with unprecedented low flows in the Murray River, was unexpected and, at the time, the provenance of these fish was unclear (Zampatti and Leigh 2013a).
In subsequent years, several cohorts that emanated from years characterised by drought and overbank flooding dominated the population (Zampatti and Leigh 2013b). Concurrently, older age classes declined and were absent by 2014. The mechanisms for this decline may include mortality and emigration. Golden perch can live for >20 years (Stuart 2006), so age-related mortality is unlikely, but anoxic blackwater during flooding in 2011 may have affected survival (Leigh and Zampatti 2013; Thiem et al. 2017). Recreational fishing mortality also occurs, but has not been quantified in the Murray River. Reproductively mature golden perch also migrate upstream in the Murray and Darling rivers (Reynolds 1983; Mallen-Cooper 1999; Zampatti et al. 2018a), and movement rates of freshwater fish may increase in association with higher flows (Albanese et al. 2004). Indeed, investigations of the abundance and size structure of golden perch populations in the mid-upper reaches of the Murray River have shown an influx of larger, adult fish after flooding (Lyon et al. 2019). In association with a decrease in reproductively mature age classes in the lower Murray River, this suggests that upstream movement during floods may have substantial effects on population structure in donor and receiving populations. Given the fundamental roles of survival and movement in determining population structure, rates of mortality and movement for golden perch in the Murray River represent essential knowledge.
Integrating water and otolith chemistry
Spatial heterogeneity in water chemistry among rivers allowed the spatial origin and migration histories of fish to be determined. Catchment lithology and geological variation were sufficient to distinguish river water 87Sr/86Sr (Douglas et al. 1995; Gingele and De Deckker 2005), although the lower Murray River showed substantial variation in water 87Sr/86Sr over 4 years. Temporal variation in water 87Sr/86Sr is expected in rivers that receive inputs from heterogeneous subcatchments or groundwater (Crook et al. 2013, 2017); nevertheless, 87Sr/86Sr ratios in the lower Murray River in 2011–14 (~0.7086–0.7147) were distinctly lower than the mid-Murray River, primarily due to the effect of low-87Sr/86Sr water from the Darling River. Longer-term (2011–20) investigations of water 87Sr/86Sr in the Murray and Darling rivers (Ye et al. 2020) and a Bayesian mixing isotope model that uses discharge, 87Sr/86Sr and Sr concentrations (Zampatti et al. 2019) also indicate that the mixed sources of water that comprise flow in the lower Murray River generate a consistently unique 87Sr/86Sr signature. Consequently, the lower and mid-Murray River, and Darling River, exhibited distinctive isotope signatures, and fish originating in, or moving between, these regions are discernible based on otolith 87Sr/86Sr.
Otolith chemistry revealed that golden perch from the lower Murray River were the progeny of spawning in either the Murray River or Darling River. Recruitment occurred during years characterised by within-channel rises in flow, in either river, or by extensive overbank flooding, across both catchments. Fish from the 2005–06 year-class, when there was an in-channel rise in discharge in the Murray River, originated in the mid-Murray River before transitioning to the lower Murray River. Relatively low otolith 87Sr/86Sr observed early in the lives of the fish suggests a natal origin in the lower reaches of the mid-Murray River (i.e. the Lock 11 region) followed by drift as early stage juveniles into the lower Murray River. During the same period, flows in the lower Darling River were negligible and unlikely to promote spawning and recruitment of golden perch.
From 2010 to 2014, golden perch from the 2009–10 year-class formed the dominant cohort in the lower Murray River, and these fish exhibited otolith core 87Sr/86Sr commensurate with a Darling River natal origin. In 2009–10, the Murray River was in the latter stages of the Millennium Drought (van Dijk et al. 2013), and low flows in the river were unlikely to promote golden perch spawning and recruitment (Zampatti and Leigh 2013a). In the Darling River, however, golden perch spawning was associated with a substantial increase in discharge (from 0 to 11 000 ML day–1). In early 2010, low abundances of young-of-year golden perch (2009–10 cohort) were collected in the lower Murray River, but it was not until 2011 that these fish, as 1 year olds, contributed to a significant increase in golden perch abundance in this region (Zampatti and Leigh 2013b). Otolith 87Sr/86Sr transects from age-4+ (2009–10 cohort) golden perch collected in the lower Murray River in 2014 predominantly demonstrated a distinct transition from the Darling River to the Murray River at age-1+, indicating movement between these rivers in association with widespread flooding in the Murray and Darling rivers in 2010–11.
Flooding in 2010–11 was also associated with an additional cohort of golden perch that, like the 2009–10 cohort, contributed substantially to the lower Murray River age structure over subsequent years (Zampatti and Leigh 2013b). Otolith 87Sr/86Sr analysis of this cohort indicated two potential natal origins: the Darling River and the lower Murray River. For fish with a Darling River origin, transects of otolith 87Sr/86Sr showed a transition in 87Sr/86Sr early in the fish’s life indicative of movement from the Darling River to the Murray River. This suggests a lower Darling River natal origin and subsequent larval drift into the lower Murray River. The presence and progression of this cohort, in association with overbank flooding, accords with: (1) contemporary models of golden perch spawning and recruitment, whereby both flooding and in-channel flows may promote strong cohorts (Mallen-Cooper and Stuart 2003; Zampatti and Leigh 2013b); and (2) concepts of increased productivity and ecosystem response in floodplain rivers, corresponding with flooding (Puckridge et al. 1998).
Dispersal and movement
Understanding the spatial organisation of recruitment sources and subsequent dispersal of fish is essential to fish population management (Cooke et al. 2016; Abell et al. 2018). The present study demonstrates that the Darling River is an important source of golden perch to the lower Murray River, with fish dispersing from natal habitats either in the year of birth, as eggs and early stage juveniles, or at age-1+, in association with high flows (flooding) in the Darling River and Murray River.
Spawning and recruitment of golden perch have been documented throughout the Darling River system (e.g. Ebner et al. 2009; Sharpe 2011; Rolls et al. 2013), and rivers in the northern catchment have been proposed as recruitment sources that influence population structure in the southern catchment, including the 2009–10 cohort in the Murray River (Stuart and Sharpe 2020). Our results confirm the Darling River as a recruitment source for the lower Murray River but, based on otolith 87Sr/86Sr, fish comprising the 2009–10 cohort likely originated from the lower to mid-Darling River, which exhibits water 87Sr/86Sr distinct from the northern tributaries (Zampatti et al. 2019). A lower Darling River origin is also supported by the movement of early stage juveniles from the Darling River to the lower Murray River, and the reflection of this transition in otolith 87Sr/86Sr (Zampatti et al. 2018b). Ultimately, recruitment sources in the Darling River system may be spatially diverse and, as such, the protection of longitudinally intact flow pulses and contiguous lotic habitats over hundreds to thousands of kilometres will aid in maintaining the integrity of golden perch populations in the MDB (Mallen-Cooper and Zampatti 2020; Stuart and Sharpe 2020).
The dispersal of pelagic eggs and early stage juveniles is mediated by the hydraulic characteristics of flowing water. Worldwide, pelagophilic fishes are disadvantaged by fragmentation and flow regulation, with obligate downstream drift of early life stages interrupted by the physical and hydraulic effects of dams and weirs (Dudley and Platania 2007; Perkin et al. 2015). The lower Darling River is unconstrained by major weirs and characterised by lotic habitats for hundreds of kilometres, even under low discharges (e.g. 200 ML day–1; Mallen-Cooper and Zampatti 2020). These conditions may facilitate the development of golden perch eggs and larvae to a juvenile stage that can then tolerate the lentic, weir pool environments of the lower Murray River (Mallen-Cooper and Zampatti 2018). Comparative hydraulic conditions are re-established in the weir-pool-constrained lower Murray River at discharges exceeding ~20 000 ML day–1, a discharge that corresponds with golden perch spawning and recruitment in this region (Zampatti and Leigh 2013a).
Interactions between main-stem rivers and tributary streams are increasingly recognised as important determinants of riverine ecological function, particularly in regulated rivers where tributaries may retain native habitats and hydrological characteristics (Kiffney et al. 2006; Rice et al. 2008; Pracheil et al. 2013). For native fish populations, tributary spawning and rearing habitats, and main stem–tributary movements, may confer benefits to main-stem fish populations and, in some cases, be integral to population persistence (Pollux et al. 2006; Pracheil et al. 2009). Certainly, in the case of golden perch in the southern MDB, population demographics in the lower Murray River are substantially influenced by interaction with the Darling River. The hydrological and hydraulic characteristics of the Darling River, and connectivity between the Darling River and lower Murray River, promote greater demographic resilience to golden perch populations than would occur if populations were dependent wholly on the Murray River. Rehabilitation of flow-affected rivers will benefit from considering main stem–tributary interactions and their effect on the spatial structuring and dynamics of fish populations (Galat and Zweimüller 2001; Koster et al. 2014).
Although the importance of drift for early life stages (eggs and larvae) of riverine fish is increasingly considered (Lechner et al. 2016), the downstream movement of juvenile fish (e.g. age-1+ and 2+) has received less attention; although the downstream migration of juvenile diadromous salmonids (smolts) has been studied extensively (e.g. McDonald 1960; McCormick et al. 1998). In the present study, the flood-mediated downstream movement of age-1+ golden perch from the Darling River substantially affected population structure in the lower Murray River. Flooding has been shown to displace juvenile and small-bodied freshwater fish and increase active movement, with subsequent effects on assemblage structure (Albanese et al. 2004; Walton et al. 2017). Nevertheless, for iteroparous, potamodromous, non-salmonid fishes, there is a paucity of studies considering the flood-associated downstream dispersal of juvenile fish and the consequences of these movements on receiving population dynamics (Kraabøl et al. 2009). This process, which may be a fundamental driver of riverine fish population dynamics in large floodplain rivers, warrants further investigation.
Management implications and conclusions
Knowledge of the demographics of populations, including variability through space and time, is essential to understanding the stability and resilience of populations (Winemiller 2005; Kerr et al. 2010). However, basic age structure data are deficient for many of the world’s riverine fish, thus impeding conservation (Reynolds et al. 2005). In Australia’s MDB, contemporary demographic data for most riverine fishes has been lacking and, despite a motivation to improve native fish populations (Barrett 2004), demographic targets have not, until recently (e.g. Commonwealth Environmental Water Office 2016), formed part of population monitoring or management.
Critical to managing fish populations is an understanding of the processes that determine population structure. In this study we used the chemistry of otoliths as a natural tag to investigate natal origin and trace the movements of larval, juvenile and adult fish, an ontological approach not possible with traditional mark–recapture or telemetric techniques (Gillanders 2005). The findings of this study support Zampatti and Leigh (2013b), who suggested that a dominant cohort of golden perch in the lower Murray River, spawned during a drought, originated in the Darling River. The findings also support the premise that spawning and recruitment of golden perch in the lower Murray River do not generally occur in years with low spring–summer flow (e.g. <15 000 ML day–1; Zampatti and Leigh 2013a). Yet, conspicuous cohorts of golden perch in the lower Murray River may align with low-flow years due to immigration from disparate regions that have experienced appropriate hydrological conditions to promote spawning and recruitment. This complex structuring of populations that incorporates spatiotemporal variability in population processes and associated environmental drivers highlights the importance of considering the range of factors that may affect population structure (e.g. spawning, recruitment and movement) to effectively manage riverine fish populations.
In the MDB, the movement of reproductively mature golden perch between river catchments has long been recognised (Reynolds 1983), and contemporary genetic studies indicate high rates of dispersal and genetic diversity (Faulks et al. 2010). Nevertheless, golden perch are traditionally managed as individual jurisdictional stocks. In concert with previous studies, the results of this study suggest that golden perch, at least in the southern MDB, need to be managed holistically as one metapopulation (stock) over a large spatial scale (thousands of kilometres). Furthermore, although both within-channel flow pulses and overbank floods may promote golden perch recruitment (Mallen-Cooper and Stuart 2003; Sharpe 2011; Zampatti and Leigh 2013a), it appears that large-scale flooding is an important driver of population growth, facilitated by: (1) localised spawning and recruitment; and (2) dispersal of early life stages and juvenile fish (e.g. age-1+) from disparate regions.
Concepts of provenance and migration form essential questions for understanding population dynamics and the management of freshwater fishes (Kennedy et al. 2002; Brennan and Schindler 2017). Ultimately, to promote population persistence, research and management need to be undertaken at scales commensurate with population processes. This study has shown that integrating water and otolith 87Sr/86Sr enables determination of spawning regions and the age-related movement of a migratory, pelagic-spawning fish. This approach has broad utility for understanding the ecology and population dynamics of riverine fishes, as well as providing insights into the processes that structure populations, the scales over which these operate and associated environmental conditions.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This project was funded by the South Australian Department of Environment and Water and the Commonwealth Environmental Water Office.
Acknowledgements
The authors thank the many staff from SARDI Aquatic Sciences who provided field and laboratory support throughout the project, including Ian Magraith, Paul Jennings, Arron Strawbridge and Neil Wellman. Thanks also to the following individuals for the collection of water samples: Warren Beer and Tony Waye (SA Water, Lock 6), Jeff Galasso (Goulburn Murray Water, Lock 11) and James Philp (State Water Corporation, NSW, Weir 32). The authors also appreciate the valuable comments provided by Martin Mallen-Cooper, Chris Bice, Leah Beesley and an anonymous reviewer on earlier versions of this manuscript.
References
Abell, N. J., Oliver, D. C., and Whitledge, G. W. (2018). Recruitment sources and spatial patterns of population demographics of spotted bass in a large river–tributary network. Fisheries Management and Ecology 25, 339–349.| Recruitment sources and spatial patterns of population demographics of spotted bass in a large river–tributary network.Crossref | GoogleScholarGoogle Scholar |
Albanese, B., Angermeier, P. L., and Dorai-Raj, S. (2004). Ecological correlates of fish movement in a network of Virginia streams. Canadian Journal of Fisheries and Aquatic Sciences 61, 857–869.
| Ecological correlates of fish movement in a network of Virginia streams.Crossref | GoogleScholarGoogle Scholar |
Amoros, C., and Bornette, G. (2002). Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biology 47, 761–776.
| Connectivity and biocomplexity in waterbodies of riverine floodplains.Crossref | GoogleScholarGoogle Scholar |
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Validation of the use of thin-sectioned otoliths for determining age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia. Australian Journal of Marine and Freshwater Research 43, 1103–1128.
| Validation of the use of thin-sectioned otoliths for determining age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Barnett-Johnson, R., Pearson, T. E., Ramos, F. C., Grimes, C. B., and MacFarlane, R. B. (2008). Tracking natal origins of salmon using isotopes, otoliths, and landscape geology. Limnology and Oceanography 53, 1633–1642.
| Tracking natal origins of salmon using isotopes, otoliths, and landscape geology.Crossref | GoogleScholarGoogle Scholar |
Barrett, J. (2004). Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches. Ecological Management & Restoration 5, 15–23.
| Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches.Crossref | GoogleScholarGoogle Scholar |
Berkeley, S. A., Hixon, M. A., Larson, R. J., and Love, M. S. (2004). Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries (Bethesda, Md.) 29, 23–32.
| Fisheries sustainability via protection of age structure and spatial distribution of fish populations.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Balcombe, S. R., Crook, D. A., Marshall, J. C., Menke, N., and Lobegeiger, J. S. (2015). Fish population persistence in hydrologically variable landscapes. Ecological Applications 25, 901–913.
| Fish population persistence in hydrologically variable landscapes.Crossref | GoogleScholarGoogle Scholar | 26465032PubMed |
Brennan, S. R., and Schindler, D. E. (2017). Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins. Ecological Applications 27, 363–377.
| Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins.Crossref | GoogleScholarGoogle Scholar | 27875020PubMed |
Commonwealth Environmental Water Office (2016). Commonwealth Environmental Water Portfolio Management Plan: Lower Murray–Darling 2016–17. Commonwealth of Australia, Canberra, ACT, Australia.
Cooke, S. J., Martins, E. G., Struthers, D. P., Gutowsky, L. F., Power, M., Doka, S. E., Dettmers, J. M., Crook, D. A., Lucas, M. C., Holbrook, C. M., and Krueger, C. C. (2016). A moving target—incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environmental Monitoring and Assessment 188, 239.
| A moving target—incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations.Crossref | GoogleScholarGoogle Scholar | 27004432PubMed |
Cowx, I. G., and Van Zyll de Jong, M. (2004). Rehabilitation of freshwater fisheries: tales of the unexpected? Fisheries Management and Ecology 11, 243–249.
| Rehabilitation of freshwater fisheries: tales of the unexpected?Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Macdonald, J. I., McNeil, D. G., Gilligan, D. M., Asmus, M., Mass, R., and Woodhead, J. (2013). Recruitment sources and dispersal of an invasive fish in a large river system as revealed by otolith chemistry analysis. Canadian Journal of Fisheries and Aquatic Sciences 70, 953–963.
| Recruitment sources and dispersal of an invasive fish in a large river system as revealed by otolith chemistry analysis.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Lacksen, K., King, A. J., Buckle, D. J., Tickell, S. J., Woodhead, J. D., Maas, R., Townsend, S. A., and Douglas, M. M. (2017). Temporal and spatial variation in strontium in a tropical river: implications for otolith chemistry analyses of fish migration. Canadian Journal of Fisheries and Aquatic Sciences 74, 533–545.
| Temporal and spatial variation in strontium in a tropical river: implications for otolith chemistry analyses of fish migration.Crossref | GoogleScholarGoogle Scholar |
Douglas, G. B., Gray, C. M., Hart, B. T., and Beckett, R. (1995). A strontium isotopic investigation of the origin of suspended particulate matter (SPM) in the Murray–Darling River system, Australia. Geochimica et Cosmochimica Acta 59, 3799–3815.
| A strontium isotopic investigation of the origin of suspended particulate matter (SPM) in the Murray–Darling River system, Australia.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z., Knowler, D. J., Leveque, C., Naiman, R. J., Prieur-Richard, A., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar | 16336747PubMed |
Dudley, R. K., and Platania, S. P. (2007). Flow regulation and fragmentation imperil pelagic spawning fishes. Ecological Applications 17, 2074–2086.
| Flow regulation and fragmentation imperil pelagic spawning fishes.Crossref | GoogleScholarGoogle Scholar | 17974342PubMed |
Ebner, B. C., Scholz, O., and Gawne, B. (2009). Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia. New Zealand Journal of Marine and Freshwater Research 43, 571–578.
| Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillanders, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences.Crossref | GoogleScholarGoogle Scholar |
Fagan, W. F. (2002). Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83, 3243–3249.
| Connectivity, fragmentation, and extinction risk in dendritic metapopulations.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010). Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua). Molecular Ecology 19, 4723–4737.
| Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 20887362PubMed |
Floyd, K. B., Courtenay, W. H., and Hoyt, R. D. (1984). A new larval fish light trap: the quatrefoil trap. Progressive Fish-Culturist 46, 216–219.
| A new larval fish light trap: the quatrefoil trap.Crossref | GoogleScholarGoogle Scholar |
Galat, D. L., and Zweimüller, I. (2001). Conserving large-river fishes: is the highway analogy an appropriate paradigm? Journal of the North American Benthological Society 20, 266–279.
| Conserving large-river fishes: is the highway analogy an appropriate paradigm?Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (2005). Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources 18, 291–300.
| Otolith chemistry to determine movements of diadromous and freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Gingele, F. X., and De Deckker, P. (2005). Clay mineral, geochemical and Sr–Nd isotopic fingerprinting of sediments in the Murray–Darling fluvial system, southeast Australia. Australian Journal of Earth Sciences 52, 965–974.
| Clay mineral, geochemical and Sr–Nd isotopic fingerprinting of sediments in the Murray–Darling fluvial system, southeast Australia.Crossref | GoogleScholarGoogle Scholar |
Hoagstrom, C. W., and Turner, T. F. (2015). Recruitment ecology of pelagic-broadcast spawning minnows: paradigms from the ocean advance science and conservation of an imperilled freshwater fauna. Fish and Fisheries 16, 282–299.
| Recruitment ecology of pelagic-broadcast spawning minnows: paradigms from the ocean advance science and conservation of an imperilled freshwater fauna.Crossref | GoogleScholarGoogle Scholar |
Jager, H. I., Chandler, J. A., Lepla, K. B., and Van Winkle, W. (2001). A theoretical study of river fragmentation by dams and its effects on white sturgeon populations. Environmental Biology of Fishes 60, 347–361.
| A theoretical study of river fragmentation by dams and its effects on white sturgeon populations.Crossref | GoogleScholarGoogle Scholar |
Kennedy, B. P., Blum, J. D., Folt, C. L., and Nislow, K. H. (2000). Using natural strontium isotopic signatures as fish markers: methodology and application. Canadian Journal of Fisheries and Aquatic Sciences 57, 2280–2292.
| Using natural strontium isotopic signatures as fish markers: methodology and application.Crossref | GoogleScholarGoogle Scholar |
Kennedy, B. P., Klaue, A., Blum, J. D., Folt, C. L., and Nislow, K. H. (2002). Reconstructing the lives of fish using Sr isotopes in otoliths. Canadian Journal of Fisheries and Aquatic Sciences 59, 925–929.
| Reconstructing the lives of fish using Sr isotopes in otoliths.Crossref | GoogleScholarGoogle Scholar |
Kerr, L. A., Cadrin, S. X., and Secor, D. H. (2010). The role of spatial dynamics in the stability, resilience, and productivity of an estuarine fish population. Ecological Applications 20, 497–507.
| The role of spatial dynamics in the stability, resilience, and productivity of an estuarine fish population.Crossref | GoogleScholarGoogle Scholar | 20405802PubMed |
Kiffney, P. M., Greene, C. M., Hall, J. E., and Davies, J. R. (2006). Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers. Canadian Journal of Fisheries and Aquatic Sciences 63, 2518–2530.
| Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers.Crossref | GoogleScholarGoogle Scholar |
King, A. J. (2004). Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. Journal of Fish Biology 65, 1582–1603.
| Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Mahoney, J. (2009). Environmental flow enhances native fish spawning and recruitment in the Murray River, Australia. River Research and Applications 25, 1205–1218.
| Environmental flow enhances native fish spawning and recruitment in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2003). Ecological impacts and institutional and economic drivers for water resource development – a case study of the Murrumbidgee River, Australia. Aquatic Ecosystem Health & Management 6, 69–79.
| Ecological impacts and institutional and economic drivers for water resource development – a case study of the Murrumbidgee River, Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., King, A. J., Beesley, L., Copeland, C., Zampatti, B. P., and Mallen-Cooper, M. (2014). Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management. Ecological Management & Restoration 15, 40–50.
| Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Dawson, D. R., O’Mahony, D. J., Moloney, P. D., and Crook, D. A. (2014). Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua). PLoS One 9, e96044.
| Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 24788137PubMed |
Koster, W. M., Dawson, D. R., Liu, C., Moloney, P. D., Crook, D. A., and Thomson, J. R. (2017). Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia. Journal of Fish Biology 90, 93–108.
| Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 27734494PubMed |
Kraabøl, M., Johnsen, S. I., Museth, J., and Sandlund, O. T. (2009). Conserving iteroparous fish stocks in regulated rivers: the need for a broader perspective! Fisheries Management and Ecology 16, 337–340.
| Conserving iteroparous fish stocks in regulated rivers: the need for a broader perspective!Crossref | GoogleScholarGoogle Scholar |
Lechner, A., Keckeis, H., and Humphries, P. (2016). Patterns and processes in the drift of early developmental stages of fish in rivers: a review. Reviews in Fish Biology and Fisheries 26, 471–489.
| Patterns and processes in the drift of early developmental stages of fish in rivers: a review.Crossref | GoogleScholarGoogle Scholar |
Leigh, S. J., and Zampatti, B. P. (2013). Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia. Australian Journal of Zoology 61, 160–169.
| Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., Hayden, T. A., Pine, W. E., Yard, M. D., Kozdon, R., and Valley, J. W. (2013). Of travertine and time: otolith chemistry and microstructure detect provenance and demography of endangered humpback chub in Grand Canyon, USA. PLoS One 8, e84235.
| Of travertine and time: otolith chemistry and microstructure detect provenance and demography of endangered humpback chub in Grand Canyon, USA.Crossref | GoogleScholarGoogle Scholar | 24358346PubMed |
Lyon, J. P., Bird, T. J., Kearns, J., Nicol, S., Tonkin, Z., Todd, C. R., O’Mahony, J., Hackett, G., Raymond, S., Lieschke, J., Kitchingman, A., and Bradshaw, C. J. A. (2019). Increased population size of fish in a lowland river following restoration of structural habitat. Ecological Applications 29, e01882.
| Increased population size of fish in a lowland river following restoration of structural habitat.Crossref | GoogleScholarGoogle Scholar | 30946514PubMed |
Macdonald, J. I., and Crook, D. A. (2014). Nursery sources and cohort strength of young-of-the-year common carp (Cyprinus carpio) under differing flow regimes in a regulated floodplain river. Ecology Freshwater Fish 23, 269–282.
| Nursery sources and cohort strength of young-of-the-year common carp (Cyprinus carpio) under differing flow regimes in a regulated floodplain river.Crossref | GoogleScholarGoogle Scholar |
Maheshwari, B. L., Walker, K. F., and Mcmahon, T. A. (1995). Effects of regulation on the flow regime of the River Murray, Australia. Regulated Rivers 10, 15–38.
| Effects of regulation on the flow regime of the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M. (1999). Developing fishways for nonsalmonid fishes: a case study from the Murray River in Australia. In ‘Innovations in Fish Passage Technology’. (Ed. M. Odeh.) pp. 173–196. (American Fisheries Society: Bethesda, MD, USA.)
Mallen-Cooper, M., and Brand, D. A. (2007). Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage? Fisheries Management and Ecology 14, 319–332.
| Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage?Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Stuart, I. G. (2003). Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Research and Applications 19, 697–719.
| Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2020). Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow. Ecological Management & Restoration 21, 218–228.
| Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow.Crossref | GoogleScholarGoogle Scholar |
McArthur, J. M., and Howarth, R. J. (2004). Sr-isotope stratigraphy: the Phanerozoic 87Sr/86Sr-curve and explanatory notes. In ‘A Geologic Timescale 2004’. (Eds F. M. Gradstein, J. G. Ogg, and A. G. Smith.) pp. 96–105. (Cambridge University Press: Cambridge, UK.)
McCormick, S. D., Hansen, L. P., Quinn, T. P., and Saunders, R. L. (1998). Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55, 77–92.
| Movement, migration, and smolting of Atlantic salmon (Salmo salar).Crossref | GoogleScholarGoogle Scholar |
McDonald, J. (1960). The behaviour of Pacific salmon fry during their downstream migration to freshwater and saltwater nursery areas. Journal of the Fisheries Board of Canada 17, 655–676.
| The behaviour of Pacific salmon fry during their downstream migration to freshwater and saltwater nursery areas.Crossref | GoogleScholarGoogle Scholar |
Muhlfeld, C. C., Thorrold, S. R., McMahon, T. E., and Marotz, B. (2012). Estimating westslope cutthroat trout (Oncorhynchus clarkia lewisi) movements in a river network using strontium isoscapes. Canadian Journal of Fisheries and Aquatic Sciences 69, 906–915.
| Estimating westslope cutthroat trout (Oncorhynchus clarkia lewisi) movements in a river network using strontium isoscapes.Crossref | GoogleScholarGoogle Scholar |
Nilsson, C., Reidy, C. A., Dynesius, M., and Revenga, C. (2005). Fragmentation and flow regulation of the world’s large river systems. Science 308, 405–408.
| Fragmentation and flow regulation of the world’s large river systems.Crossref | GoogleScholarGoogle Scholar | 15831757PubMed |
Olden, J. D., and Kennard, M. J. (2010). Intercontinental comparison of fish life history strategies along a gradient of hydrologic variability. American Fisheries Society Symposium 73, 83–107.
Palmer, M. R., and Edmond, J. M. (1989). The strontium isotope budget of the modern ocean. Earth and Planetary Science Letters 92, 11–26.
| The strontium isotope budget of the modern ocean.Crossref | GoogleScholarGoogle Scholar |
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J. (2011). Iolite: freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry 26, 2508–2518.
| Iolite: freeware for the visualisation and processing of mass spectrometric data.Crossref | GoogleScholarGoogle Scholar |
Perkin, J. S., Gido, K. B., Cooper, A. R., Turner, T. F., Osborne, M. J., Johnson, E. R., and Mayes, K. B. (2015). Fragmentation and dewatering transform Great Plains stream fish communities. Ecological Monographs 85, 73–92.
| Fragmentation and dewatering transform Great Plains stream fish communities.Crossref | GoogleScholarGoogle Scholar |
Pin, C., Briot, D., Bassin, C., and Poitrasson, F. (1994). Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography. Analytica Chimica Acta 298, 209–217.
| Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., Olden, J. D., Merritt, D. M., and Pepin, D. M. (2007). Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences of the United States of America 104, 5732–5737.
| Homogenization of regional river dynamics by dams and global biodiversity implications.Crossref | GoogleScholarGoogle Scholar | 17360379PubMed |
Pollux, B. J. A., Pollux, P. M. J., Korosi, A., Verberk, W. C. E. P., and Van der Velde, G. (2006). Reproduction, growth, and migration of fishes in a regulated lowland tributary: potential recruitment to the river Meuse. Hydrobiologia 565, 105–120.
| Reproduction, growth, and migration of fishes in a regulated lowland tributary: potential recruitment to the river Meuse.Crossref | GoogleScholarGoogle Scholar |
Pracheil, B. M., Pegg, M. A., and Mestl, G. E. (2009). Tributaries influence recruitment of fish in large rivers. Ecology Freshwater Fish 18, 603–609.
| Tributaries influence recruitment of fish in large rivers.Crossref | GoogleScholarGoogle Scholar |
Pracheil, B. M., McIntyre, P. B., and Lyons, J. D. (2013). Enhancing conservation of large-river biodiversity by accounting for tributaries. Frontiers in Ecology and the Environment 11, 124–128.
| Enhancing conservation of large-river biodiversity by accounting for tributaries.Crossref | GoogleScholarGoogle Scholar |
Puckridge, J. T., Sheldon, F., Walker, K. F., and Boulton, A. J. (1998). Flow variability and the ecology of large rivers. Marine and Freshwater Research 49, 55–72.
| Flow variability and the ecology of large rivers.Crossref | GoogleScholarGoogle Scholar |
Reynolds, L. F. (1983). Migration patterns of five fish species in the Murray–Darling River system. Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Reynolds, J. D., Webb, T. J., and Hawkins, L. A. (2005). Life history and ecological correlates of extinction risk in European freshwater fishes. Canadian Journal of Fisheries and Aquatic Sciences 62, 854–862.
| Life history and ecological correlates of extinction risk in European freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Rice, S. P., Kiffney, P., Greene, C., and Pess, G. R. (2008). The ecological importance of tributaries and confluences. In ‘River Confluences, Tributaries and the Fluvial Networks’. (Eds S. P. Rice, A. G. Roy, and B. L. Rhoads.) pp. 209–242. (Wiley: Chichester, UK.)
Rolls, R. J., Growns, I. O., Khan, T. A., Wilson, G. G., Ellison, T. L., Prior, A., and Waring, C. C. (2013). Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes. Freshwater Biology 58, 1804–1819.
| Fish recruitment in rivers with modified discharge depends on the interacting effects of flow and thermal regimes.Crossref | GoogleScholarGoogle Scholar |
Sharpe, C. P. (2011) Spawning and recruitment ecology of golden perch (Macquaria ambigua Richardson 1845) in the Murray and Darling rivers. Ph.D. Thesis, Griffith University.
Stuart, I. G. (2006). Validation of otoliths for determining age of golden perch, a long-lived freshwater fish of Australia. North American Journal of Fisheries Management 26, 52–55.
| Validation of otoliths for determining age of golden perch, a long-lived freshwater fish of Australia.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Sharpe, C. P. (2020). Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia. Aquatic Conservation 30, 675–690.
| Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
van Dijk, A. I., Beck, H. E., Crosbie, R. S., de Jeu, R. A., Liu, Y. Y., Podger, G. M., Timbal, B., and Viney, N. R. (2013). The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resources Research 49, 1040–1057.
| The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society.Crossref | GoogleScholarGoogle Scholar |
Walker, K. F. (2006). Serial weirs, cumulative effects: the lower River Murray, Australia. In ‘Ecology of Desert Rivers’ (Ed. R. Kingsford) pp. 248–279. (Cambridge University Press: Cambridge, UK).
Walton, S. E., Nunn, A. D., Probst, W. N., Bolland, J. D., Acreman, M., and Cowx, I. G. (2017). Do fish go with the flow? The effects of periodic and episodic flow pulses on 0+ fish biomass in a constrained lowland river. Ecohydrology 10, e1777.
| Do fish go with the flow? The effects of periodic and episodic flow pulses on 0+ fish biomass in a constrained lowland river.Crossref | GoogleScholarGoogle Scholar |
Welcomme, R. L., Winemiller, K. O., and Cowx, I. G. (2006). Fish environmental guilds as a tool for assessment of ecological condition of rivers. River Research and Applications 22, 377–396.
| Fish environmental guilds as a tool for assessment of ecological condition of rivers.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O. (2005). Life history strategies, population regulation, and implications for fisheries management. Canadian Journal of Fisheries and Aquatic Sciences 62, 872–885.
| Life history strategies, population regulation, and implications for fisheries management.Crossref | GoogleScholarGoogle Scholar |
Woodhead, J., Swearer, S., Hergt, J., and Maas, R. (2005). In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies. Journal of Analytical Atomic Spectrometry 20, 22–27.
| In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies.Crossref | GoogleScholarGoogle Scholar |
Ye, Q., Giatas, G., Brookes, J., Furst, D., Gibbs, M., Oliver, R., Shiel, R., Zampatti, B. P., Aldridge, K., Bucater, L., Busch, B., Hipsey, M., Lorenz, Z., Maas, R., and Woodhead, J. (2020) Commonwealth Environmental Water Office Long-Term Intervention Monitoring Project 2014–2019: Lower Murray River Technical Report. South Australian Research and Development Institute, Aquatic Sciences, Adelaide, SA, Australia.
Zampatti, B. P., and Leigh, S. J. (2013a). Effects of flooding on recruitment and abundance of Golden Perch (Macquaria ambigua ambigua) in the lower River Murray. Ecological Management & Restoration 14, 135–143.
| Effects of flooding on recruitment and abundance of Golden Perch (Macquaria ambigua ambigua) in the lower River Murray.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., and Leigh, S. J. (2013b). Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua, implications for environmental flow management in the River Murray, Australia. Marine and Freshwater Research 64, 618–630.
| Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua, implications for environmental flow management in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., Wilson, P. J., Baumgartner, L., Koster, W., Livore, J. P., McCasker, N., Thiem, J. D., Tonkin, Z., and Ye, Q. (2015). Reproduction and recruitment of golden perch (Macquaria ambigua ambigua) in the southern Murray–Darling Basin in 2013–2014: an exploration of river-scale response, connectivity and population dynamics. SARDI Publication number F2014/000756–1. South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B. P., Leigh, S. J., Bice, C. M., and Rogers, P. J. (2018a). Multiscale movements of golden perch (Percichthyidae: Macquaria ambigua) in the River Murray, Australia. Austral Ecology 43, 763–774.
| Multiscale movements of golden perch (Percichthyidae: Macquaria ambigua) in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., Strawbridge, A., Thiem, J., Tonkin, Z., Maas, R., Woodhead, J., and Fredberg, J. (2018b). Golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus) age demographics, natal origin and migration history in the River Murray, Australia. SARDI Publication number F2018/000116–1, SARDI Research Report Series number 993. South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B., Fanson, B., Strawbridge, A., Tonkin, Z., Thiem, J., Butler, G., Balcombe, S., Koster, W., King, A., Crook, D., Woods, R., Brooks, S., Lyon, J., Baumgartner, L., and Doyle, K. (2019). Basin-scale population dynamics of Golden Perch and Murray Cod: relating flow to provenance, movement and recruitment in the Murray–Darling Basin. In ‘Murray–Darling Basin Environmental Water Knowledge and Research Project – Fish Theme Research Report’. (Eds A. Price, S. Balcombe, P. Humphries, A. King, and B. Zampatti.) pp. 26–30. (Centre for Freshwater Ecology, La Trobe University: Wodonga, Vic., Australia.)