Multiproxy approach to track changes in the ecological condition of wetlands in the Gunbower Forest, a Ramsar site
Neeraj Mall A G , Peter Gell A B , Giri R. Kattel
A G , Peter Gell A B , Giri R. Kattel  C D E , Patricia Gadd F and Atun Zawadzki F
C D E , Patricia Gadd F and Atun Zawadzki F
A School of Sciences, Psychology and Sport, Federation University Australia, Mount Helen, Vic., Australia.
B Diponegoro University, Semarang, Indonesia.
C School of Geographical Sciences, Nanjing University of Information Science and Technology, Nanjing, P.R. China.
D Department of Infrastructure Engineering, The University of Melbourne, Melbourne, Vic., Australia.
E Department of Hydraulic Engineering, Tsinghua University, Beijing, P.R. China.
F Australian Nuclear Science and Technology Organisation, Lucas Heights, Sydney, NSW, Australia.
G Corresponding author. Email: neeraj.mll@gmail.com
Marine and Freshwater Research 73(10) 1196-1211 https://doi.org/10.1071/MF21249
Submitted: 28 August 2021 Accepted: 30 January 2022 Published: 12 April 2022
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Gunbower Forest is bordered by the Murray River and Gunbower Creek and hosts several floodplain wetlands listed under the Ramsar Convention. Sediment cores were retrieved from three wetlands to trace changes to their ecological state over time. The basal sediments of the wetlands date back to the beginning of river regulation in the 1930s, suggesting that only after then were they inundated sufficiently often to allow for net sediment accumulation. The diatoms preserved in the lower levels of all cores suggest clear, freshwater conditions prevailed during that period. Increased sediment and nutrient loads are inferred by increased epiphytic forms and nutrient indicators. Over recent decades the wetlands have transitioned to plankton dominance, reflecting greater connectivity to the river and distributary, and a reduced light environment. This pattern resembles to that recorded both upstream and downstream, suggesting a regional-scale change in the wetlands of the southern Murray–Darling Basin.
Keywords: diatoms, ecological condition, Gunbower Forest, X-ray fluorescence, XRF, Murray River, palaeolimnology, Ramsar wetland, stable isotopes.
Introduction
The functioning and ecological condition of freshwater ecosystems have been affected by anthropogenic and environmental changes that pose formidable challenges for global water resource management practices (Bhaduri et al. 2016; Large et al. 2017). In particular, inland water systems have been identified among the most threatened ecosystems worldwide (Finlayson et al. 2005; Dudgeon et al. 2006; Huziy and Sushama 2017) because they are closed systems and everything that happens within a catchment can affect ecosystem function. Approximately 6% of the global land area is occupied by wetlands (Junk et al. 2013). Wetlands play an indispensable role in hydrological and biogeochemical cycles, thus harbouring a large part of the world’s biodiversity, providing multiple services to humankind (Junk et al. 2013). However, many of world’s wetlands have been altered substantially, or have disappeared (Davidson 2014; Darrah et al. 2019), in response to anthropogenic pressures and climate change (Chen et al. 2018; Dubois et al. 2018), particularly with the diversion of water for human and industrial use (Tockner et al. 2010; Pittock and Finlayson 2011; Finlayson 2013). Globally, palaeolimnological evidence from shallow lakes indicates such changes, including lakes in China (Lake Erhai; Wang et al. 2012), North America (Gregory-Eaves and Beisner 2011; Velghe et al. 2012), the Arctic (Smol et al. 2005), as well as in the artificial wetlands of Norfolk in the UK (Boyle et al. 2016).
The Murray–Darling Basin (MDB) is Australia’s largest river system, and it has come under the influence of anthropogenic stressors for more than 150 years (Davies et al. 2018). By the early 21st century, it was recognised as being widely degraded (Norris et al. 2001). The MDB wetlands are threatened by altered hydrology, soil disturbance, degraded water quality and changes to marsh and swamp habitats that provide for wildlife (Papas and Moloney 2012). In addition, natural cycles, such as floods and droughts, have periodic impacts on both humans and wildlife. The threats to the Basin are of such great magnitude that the waterways of the MDB are today recognised as among 10 Australian ecosystems most at risk from tipping points (Laurance et al. 2011). This widely recognised degraded state of the waterways of the Basin has driven the formulation of the MDB Plan, which seeks to restore waterway health through the recovery of environmental water (Murray–Darling Basin Authority 2012). The first water quality monitoring was conducted in 1951, and there are little or no preregulation water quality data available that could provide a reasonable understanding of the earliest phase of water resource development (Gell and Reid 2016; Gillson et al. 2021).
The historical ecological evidence of human impacts on the aquatic systems of the MDB and the relatively short duration of field monitoring reveal that there is likely much change that has occurred that is obscured by time (Gell and Reid 2014). Palaeoecological approaches can reveal the changes before the first monitoring programs. Further, the integration of modern and palaeoecological approaches can place modern data in the context of this evidence more powerfully (Kattel et al. 2020). The benefit of this longer-term perspective includes the capacity to understand the influence of low-frequency cycles of change, to identify past critical transitions in the state that may preclude restoration, to understand natural baseline conditions (Finlayson et al. 2016) and to better establish the current trajectory of change. Together, this evidence can inform the restoration challenges of the MDB (Gell et al. 2018b).
Numerous studies have investigated rapid palaeo-environmental change over centennial to millennial time scales in inland freshwater wetlands of south-eastern Australia (Thoms et al. 1999; Gell et al. 2005). In the MDB, many studies have been conducted on wetlands in the Murray–Murrumbidgee region as far back as 200 years since European settlement began (Gell and Reid 2014). Most of the palaeoecological research has used diatoms as indicators of salinity, pH and water level changes (Gell et al. 2002, 2005, 2009; Tibby 2003, 2004; Reid and Ogden 2009), or diatoms and invertebrates to infer vegetation (e.g. macrophyte) and land use changes (Ogden 2000; Reid et al. 2007; Kattel et al. 2017). In addition, the palaeolimnological records from the southern MDB have revealed substantial human impact following European settlement during the late 19th century, with 80% of wetlands affected by sediment, 48% affected by nutrients and 34% affected by salinisation (Gell and Reid 2014; Gell et al. 2019). These syntheses of studies have documented that, in many sites, elevated pollution loads began soon after regulation was installed on the Murray River from the 1930s (Gell and Reid 2016).
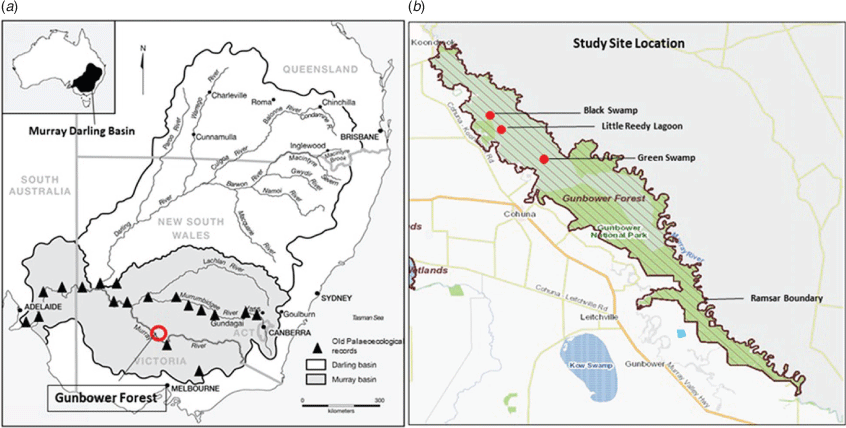
Several MDB palaeoecological records lack dating models and rarely are such studies supported by the additional evidence of stable isotope analysis and X-ray fluorescence (XRF) stratigraphy data (Gell and Reid 2014; Gell et al. 2019). Furthermore, palaeolimnological analyses from the Murray River system are focused on the upstream reaches (Reid et al. 2007) or those wetlands below the junction of the Murrumbidgee River (Fig. 1). So far, only a few studies have focused on the middle reaches of the Murray River. This study will help to fill a geographical gap in previous palaeoecological records in the MDB. The Gunbower Forest wetlands of northern Victoria are situated midway between the tributary and upland sites and those in the lower reaches. Hence, the wetlands of Gunbower Forest were selected to reconstruct palaeolimnological history to investigate changes specific to the mid-reaches of the Murray River. The wetlands in this study were listed as wetlands of international importance under the Ramsar Convention in 1982 and are listed as iconic sites under The Living Murray (TLM) Program (Hale and Butcher 2011). However, the site has been influenced by natural and anthropogenic activities, such as climate change, river regulation, nutrient enrichment and sedimentation for over a century. Palaeoecological evidence in numerous Ramsar sites elsewhere has shown alteration of ecological character even before they were listed as international Ramsar sites (Gell et al. 2016), suggesting the significance of a study of this kind. This study explored the degree to which the ecological condition of the wetlands at the time of its Ramsar listing was representative of its long-term state and assessed the causes of wetland changes and any deviation from its original ecological character that may have occurred in the wetlands of the Gunbower Forest.
Study area
In this study, three wetlands were selected to investigate changes in the aquatic ecology of the Gunbower wetland system over time. The selected research sites (Little Reedy Lagoon and the Black (BS) and Green (GS) swamps) are located in the Gunbower Forest area, which is among the largest natural red gum forests in the MDB (North Central Catchment Management Authority 2015). These sites are recognised under the Ramsar Convention listing and are located at the lower forest near Gunbower Creek (Fig. 1). These wetlands are seasonal distributaries that carry floodwaters through the forest. The highly regulated Gunbower Creek carries water for local irrigation and its water level is now high through the dry season, limiting the capacity of these wetlands to drain naturally.
Methods
In October 2017, sediment cores were collected from each of BS (84 cm), GS (86 cm) and Little Reedy Lagoon (45 cm) using a Russian (D-section) corer (Jowsey 1966). After each core was extracted, it was carefully packed and transported to the sediment core repository at Federation University and stored at ~4°C. Few samples from each site were stored at –20°C for stable isotope analysis. Cores were analysed in two stages. First, preliminary analysis was conducted in which sediment samples were taken at coarse intervals (5 cm) and prepared for diatom and pollen analysis (Gell et al. 2018a). These preliminary analyses confirmed the preservation of diatoms and pollen grains throughout the sedimentary record and that there were substantial changes in the fossil diatom and vegetation records. Second, high-resolution analysis was performed on the longer cores (BS and GS), which were subsampled at contiguous 1-cm intervals. These subsamples were treated for detailed diatom analysis. Further, frozen samples were freeze-dried and homogenised for stable isotope analysis. Then, BS and GS cores were repacked and taken to the Australian Nuclear Science and Technology Organisation (ANSTO) laboratories (Lucas Heights, NSW, Australia) for XRF, stable isotope and 210Pb analysis. The shortest core from Little Reedy Lagoon was only used for preliminary analysis and the results have been reported elsewhere (Gell et al. 2018a).
To reconstruct the ecological history of BS and GS, four major palaeolimnological approaches were used: (1) 210Pb dating was performed to understand sedimentation rates and the age of the cores over the recent past (mostly a century scale) lacustrine records; (2) elemental composition was analysed using XRF scanning; (3) biological and chemical indicators, such as diatoms and stable isotopes of bulk sediments, were analysed; and (4) once the biological and XRF data were collated, ordination techniques were used to explore the patterns of change in the chemical and biological indicators.
Chronology of the cores with 210Pb dating
The chronology of the cores was established using the 210Pb dating approaches described by Appleby (2002). Both the constant rate of supply (CRS) and constant flux constant sedimentation (CFCS) models were used to calculate estimated ages and mass accumulation rates for each wetland (Appleby and Oldfield 1983). In order to establish a 210Pb age model, 18 subsamples (depth levels between 0 and 86 cm) of the BS and GS cores were analysed for 210Pb activity. Sediment samples were processed at ANSTO using α spectrometry following the methods outlined in Harrison et al. (2003).
XRF scanning
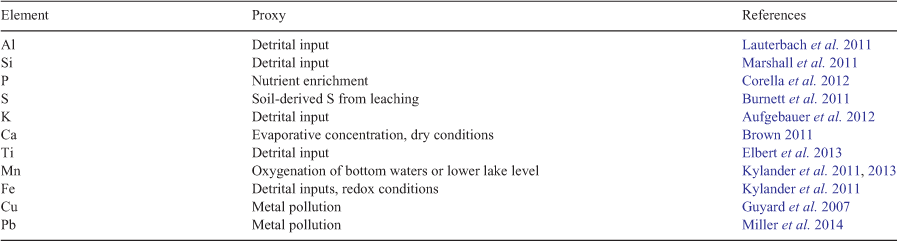
The surface of the cores for XRF analysis was cleaned, levelled and covered with an ultrathin conductive plastic film for scanning, allowing for radiographs and elemental composition to be obtained. The relative abundance of 34 elements was determined for each site, with a few elements (Table 1) selected for further analysis (Guyard et al. 2007; Brown 2011; Burnett et al. 2011; Lauterbach et al. 2011; Marshall et al. 2011; Corella et al. 2012; Elbert et al. 2013; Kylander et al. 2011, 2013). The elemental composition of the sediment cores was explored using in-built software (Itrax) to assess changes in geochemistry.
|
Stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) analyses of bulk sediment
Four samples from BS and five from GS cores were subjected to stable isotope analysis. These samples were pretreated separately for carbon and nitrogen analysis at the ANSTO laboratory. Each sample was divided into two fractions (Fernandes and Krull 2008) and analysed using a Thermo Fisher Delta V isotope ratio mass spectrometer (IRMS). The δ13C ratios were obtained from the comparison of CO2 standard gas reference (99.996%, δ13CVPDB = –6.317‰), and all values stated are relative to this reference (Vienna Pee Dee Belemnite (VPDB) standard). Ratios of the δ15N isotope were derived by comparing a N2 standard gas reference (99.99% δ15NAIR = –1.706‰), with all values stated being relative to this reference. Two quality control references were also included in each run and samples were repeatedly analysed to obtain similar results from two replicate samples.
Analyses of diatoms
Following the method of Battarbee et al. (2002), the samples for diatom analysis were digested in 10% HCl and 10% H2O2. Aliquots (800 μL) were dried on coverslips and mounted on microscope slides using Naphrax. A minimum of 200 valves per slide, following Bate and Newall (1998), were identified. Where diatoms were sparse, at least three transects were traversed and all entire valves counted. Counting was undertaken using a Nikon Eclipse 80i microscope at a magnification of 1000× under differential interference contrast. Diatom identification was based on photomicrographs from the following references: Krammer and Lange-Bertalot (1986, 1991, 2004), Krammer (1988), Gell et al. (1999) and Sonneman et al. (1999).
Statistical analysis
Statistical analyses such as indirect ordination (e.g. detrended correspondence analysis, DCA, principal component analysis, PCA) were undertaken for sediment core samples using CANOCO software for Windows (ver. 4.5, Microcomputer Power, Ithaca, NY, USA, see www.canoco.com), RStudio (ver. 1.2.5042, RStudio PBC, Boston, MA, USA, see www.rstudio.com), C2 software (ver. 1.7.7, Newcastle University, Newcastle upon Tyne, UK; Juggins 2007) and Microsoft Excel (ver. 12.0, Microsoft Corporation). The PCA ordination method was used to investigate the gradients of change and the constrained incremental sum of squares (CONISS) method was used to assess the clustering of adjacent samples (Grimm 1987). The percentage of diatom abundance relative to the depth of the core and periods of change was visually explored using Tilia software (ver. 2.6.1, E. C. Grimm, Illinois State Museum Research and Collections Center, Springfield, IL, USA). Mass accumulation rates and ages of cores are presented as the mean ± s.d. All statistical analyses for XRF data were undertaken using the statistical coding interface R (ver. 1.2.5042, RStudio PBC), with the exception of the stable isotope plots produced using C2 (ver. 1.7.7; Juggins 2007). The relationship between elemental compositions was explored using RStudio software.
Results
Chronology
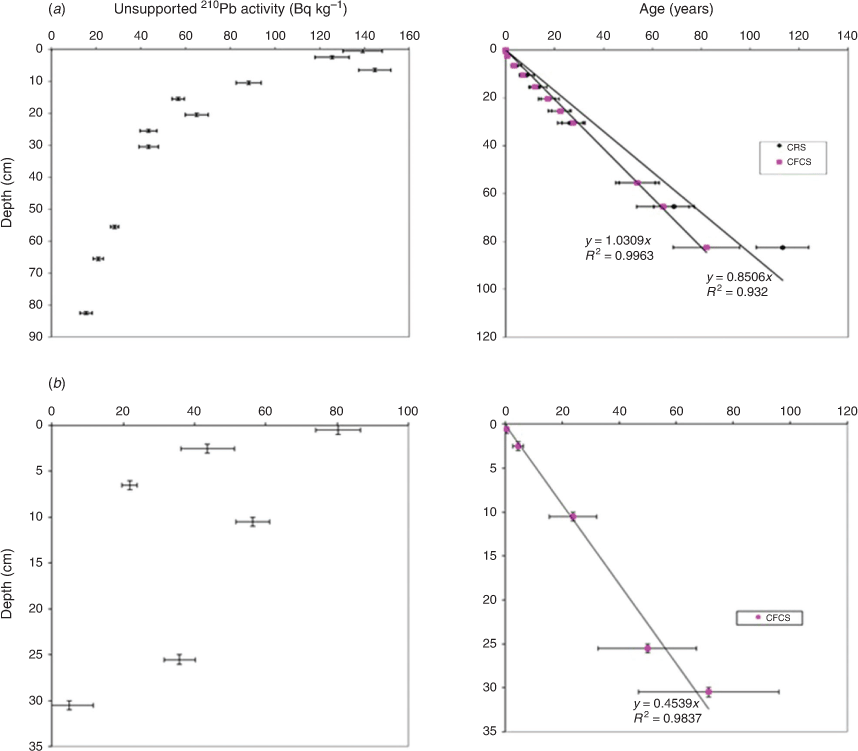
Eleven and eight 210Pb dates were obtained from the BS and GS cores respectively (Fig. 2). For BS, the CFCS and CRS core chronology models were applied to the 210Pb data to determine the mass accumulation rates and ages of the sediment cores (Fig. 2a). The CFCS and CRS age depth models were found to be largely consistent in age estimation, except for the deepest sample. The CFCS model estimated a constant sediment mass accumulation rate of 0.287 ± 0.047 g cm–2 year–1 (r2 = 0.8393). The CRS-based sediment mass accumulation rate ranged from 0.2–0.38 g cm–2 year–1 in the 0- to 30-cm depth layers to 0.21–0.06 g cm–2 year–1 in the 55- to 83-cm depth layers. However, the CFCS model estimated the age of the deepest sample (82 cm) to be 82 ± 13 years old, dated to c. 1936 ± 13 CE, whereas the CRS-based age for this deepest layer was 113 ± 11 years and dated to c. 1904 ± 11 CE (Fig. 2a).
|
The CFCS 210Pb dating model was used to calculate the sediment core chronology for the GS core. The unsupported 210Pb activity had not reached the background level at the bottom of the core (at a depth of 75 cm; Fig. 2b); hence, the CRS model cannot be used to determine the core chronology. Unsupported 210Pb activities showed declining values towards the bottom of the core, but only between depths of 0 and 31 cm. The estimated sediment mass accumulation rate between 0 and 31 cm was 0.141 ± 0.054 g cm–2 year–1 (r2 = 0.7383). An age of 71 ± 25 years was estimated for the depth of 30–31 cm, which means the core, at this depth, was dated back to 1943 ± 25 CE (Fig. 2b). Below 31 cm, the ages of the core samples were calculated by extrapolating the CFCS sediment ages between 0 and 30 cm against depth; thus, this needs to be regarded with some uncertainty.
The point of first arrival of exotic pollen provides evidence for the post-European phase. Pinus pollen first appeared at 20 cm, equating to 2000, in the BS core and at 80 cm, equating to 1860, in the GS core (Gell et al. 2018a).
Geochemical analysis
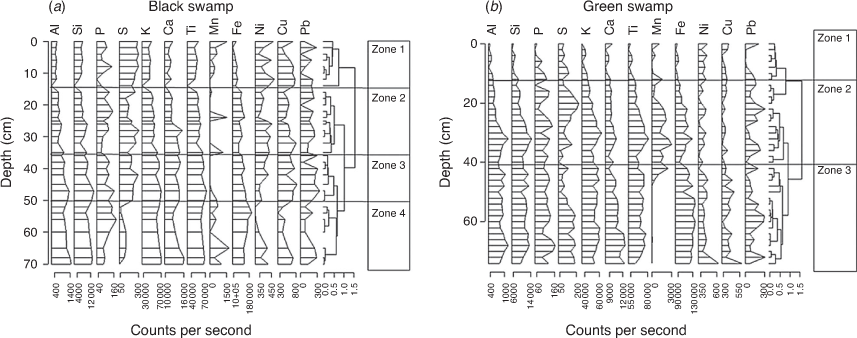
The Itrax XRF core scanning results for the BS and GS cores are presented in Fig. 3. There were noticeable differences in elemental composition of metals such as Pb, Ca, Al, K, Fe, Mn, Cu, S and P, and biogenic Si) Indicators of erosional (detrital) inputs, which include the lithogenic elements Al, Si, K, Ti and Fe, were commonly recorded. These elements are geochemically stable, usually hosted by resistant minerals and are conservative to most geochemical environments (Boës et al. 2011). For instance, the values of Ti, an erosion indicator on account of its association with fine particles (Kylander et al. 2011), showed enrichment in Zones 2, 3 and 4 (70–12 cm) of the BS core (Fig. 3a) and in Zone 3 (70–50 cm) of the GS core (Fig. 3b). Detrital enrichment in these cores declined towards the top. S, a proxy for organic matter (Croudace et al. 2006), remained stable in all the BS zones (Fig. 3a). Increased values of Fe between BS Zone 4 and BS Zone 3 (70–35 cm) inform on reducing conditions (Kylander et al. 2013), whereas low values of Fe were noticed in BS Zones 1 and 2. Values of Mn were observed only at a few depths at BS, whereas Mn declined in GS Zone 2a through to GS Zone1 (Fig. 3b). There was considerable variation in P values through the BS and GS cores, with values declining at the top of the cores. In comparison, slightly increased values of metals Cu, Pb and Ni were noticed at 15 cm and above 5 cm in the BS core and between 12.5 cm and the top of the GS core (see Fig. S1 in the Supplementary material).
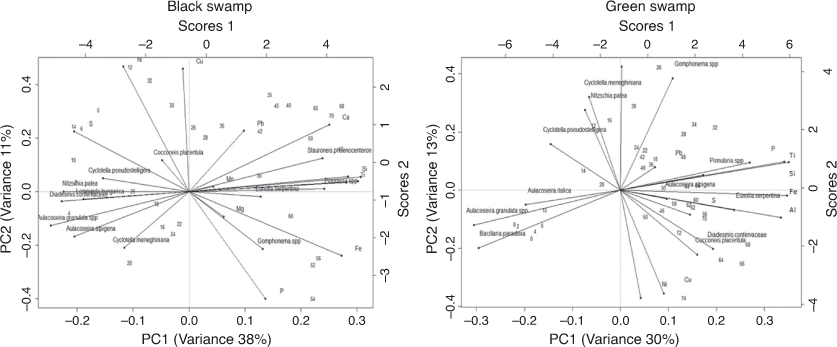
PCA of elements
The PCA results showed that the variation in the elemental components is mostly described by the first two independent axes, namely Axes 1 and 2. In the BS core, the first axis (PC1 was closely aligned with the elements Fe, Si, Al, Ti, Ni, K, Mn and S which together accounted for 46% of the total variance (Fig. 4a). The second axis (PC2) was closely aligned with the elements Ni, Cu, P and Pb, which together accounted for a further 17% of the total variation (Fig. 4a). PC1 revealed a clustering of the detrital (Ti, Fe, Si, Al, K) and pedogenic (Ca) elements, whereas the organic matter indicator S was negatively correlated with these detrital elements, suggesting the input of S from dead remains (Ivanov 1981). In the GS core, the first two axes accounted for 66% of the total variance. The first axis represented 48% of the total variance and was correlated with detrital and pedogenic elements, including Fe, K, Ti, Si and Ca, and some redox elements, such as Cu, Ni and Fe. The second axis accounted for ~18.51% of the total variance and was shown by the presence of Ni and Cu at the positive end and by only Mn at its negative end (Fig. 4b).
|
Stable isotope analysis of C and N of bulk sediments
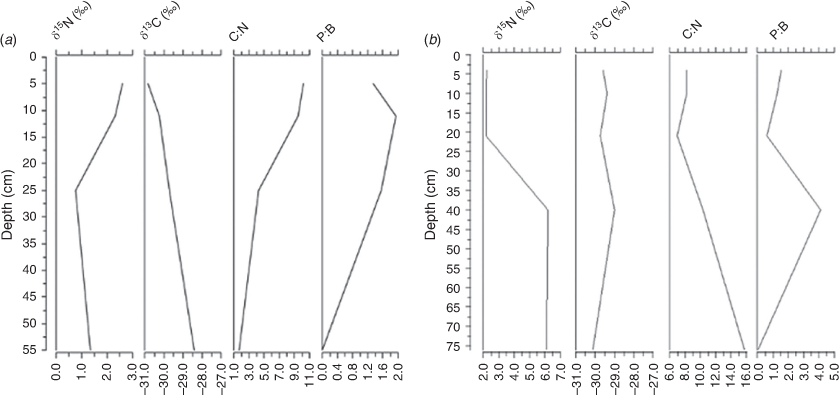
The dynamics of carbon and nitrogen in BS is revealed by the response of δ13C and δ15N values (i.e. when the δ13C values declined consistently, the δ15N values fluctuated over time; Fig. 5a). The δ13C values for the BS showed as discrete samples, which were between –28.4 and –30.8‰ from 55 to 5 cm in the core. A decrease of 2.4‰ in δ13C was noted from the bottom to the top samples of the BS core between 55 and 5 cm. By contrast, the δ13C values in the GS core varied little, between –29 and –30.1‰, with slightly lower values recorded at the base of the core (Fig. 5b). The C/N ratios were lower at the base of the BS core (below 55 cm) and greater at the core top (5 cm), reflecting a recent increase in nitrogen input to the wetland, whereas the C/N ratios for GS core were between 6.9 and 15 at depths between 4 and 76 cm (Fig. 5b). The results obtained from the stable isotope analysis did not reveal any specific changes, so were plotted with planktonic to benthic (P:B) ratios to relate nutrient inputs with planktonic-rich conditions.
|
Diatom analysis
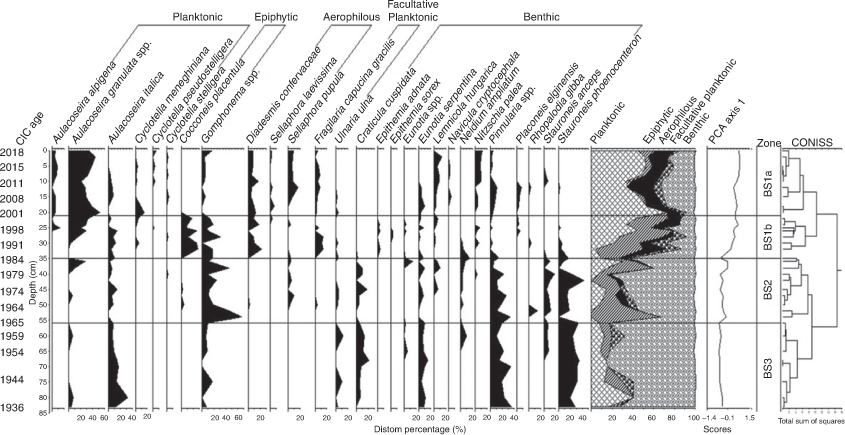
The diatoms from 36 core samples were counted for both the cores and are shown in Fig. 6 and 7. The diatom diagrams were separated into three zones for BS cores (BS1, BS2 and BS3; Fig. 6) and four zones for GS cores (GS1, GS2, GS3, and GS4; Fig. 7) based on the quantitative statistical analysis in the diatom assemblages using the CONISS function in the Tilia software (Grimm 1987). Zones BS1 and GS2 were further subdivided into BS1a and BS1b and GS2a and GS2b respectively. Diatom species were classified in accordance with their known habitat preferences (i.e. being either planktonic, aerophilous, benthic, epiphytic or facultative planktonic). Planktonic diatoms largely represent open water, whereas aerophilous diatoms tend to colonise very shallow waters or wet sediment and may be transported in erosion events. Benthic and epiphytic forms are associated with littoral zones, the former more with sediment, whereas the latter is associated with macrophytes. Facultative planktonic diatoms can exploit multiple habitats and so are common in turbid waters. Zones with different diatoms species representation are discussed below in chronological order.
BS diatom analysis
Zone BS3 (85–56 cm)
This basal zone was dominated by benthic forms (74%) such as Craticula cuspidata, Eunotia serpentina, Pinnularia spp. and Stauroneis phoenicenteron, with limited occurrences of plankton (mostly Aulacoseira italica; Fig. 6). At 80 cm, the planktonic A. italica reached a peak of 32%, but it declined to 7% at 56 cm. Epiphytes and facultative planktonic taxa were uncommon, contributing an average of 6.3 and 3.3% respectively to the total valve count. Epiphytes were increasingly observed through the zone before increasing in BS2. Aerophilous species were absent.
Zone BS2 (56–35 cm)
Throughout this zone benthic taxa (Pinnularia spp. and S. phoenicenteron) and the epiphyte Gomphonema spp. were predominant. S. phoenicenteron showed a peak of 43% at 42 cm, whereas Stauroneis anceps had low values (Fig. 6). Pinnularia spp. declined gradually upwards from 56 to 35 cm in the zone. C. cuspidata, E. serpentina, Lemnicola hungarica, Neidium ampliatum and Rhopalodia gibba were recorded in low numbers. The nutrient indicator Nitzschia palea first appeared at 40 cm. The planktonic form A. italica declined through this zone, whereas the abundance of Aulacoseira granulata ssp. increased to a peak of 28% at 36 cm. The epiphytes Gomphonema spp. accounted for an average of 26% of all taxa (between 56 and 35 cm), reaching a peak of 60% at 55 cm and 40% at 38 cm. The aerophilous form Sellaphora pupula appeared in this zone but was in low abundance.
Zone BS1b (35–21 cm)
In this zone epiphytes were common (Fig. 6). A notable peak of epiphytes at 27 cm in Zone BS1b and a high abundance of Cocconeis placentula were observed, but they declined upwards to ~10% at 20 cm and decreased further in Zone BS1a. The river plankton A. granulata ssp. showed a gradual increase from 36 cm (3%) to 20 cm, where it reached 52% of the count, whereas A. italica and Aulacoseira alpigena showed lower percentage values. Generally, above 30 cm, benthic diatoms were less abundant, being replaced mostly by epiphytic and planktonic forms. L. hungarica, an epiphyte on floating plants (e.g. Lemna, Azolla) was persistent and increased in abundance to the top of Zone BS1a. The nutrient indicators Cyclotella meneghiniana and N. palea were evident in several samples, whereas the acidophilous Pinnularia spp. and Eunotia spp. were rare.
Zone BS1a (21–0 cm)
This zone was characterised by the dominance of planktonic species (47%), mainly A. granulata ssp. (Fig. 6). C. meneghiniana showed peaks between 15 and 20 cm, and N. palea increased from 21 cm to the top of the core. L. hungarica increased gradually, reaching a peak of 14% at 2 cm. Benthic diatoms represented, on average, ~26% of all taxa. Aerophilous forms were common in this zone, with Diadesmis confervaceae and S. pupula occurring with average abundance of 8 and 9% respectively. Epiphytic forms were uncommon.
GS diatom analysis
Zone GS4 (75–65 cm)
This zone was characterised by the dominance of benthic (Pinnularia spp., Gyrosigma spp., E. serpentina), planktonic (A. italica, A. granulata) and aerophilous (D. confervaceae) species (Fig. 7). The aerophilous species (D. confervaceae and S. pupula) showed higher percentage values (24%) than planktonic species.
Zone GS3 (65–50 cm)
This part of the core was dominated by the planktonic species A. alpigena and Aulacoseira ambigua, constituting as much as 80% of the diatom sum (Fig. 7). A. alpigena reached its maximum abundance of 68% at 60 cm, whereas A. ambigua peaked at 48% at 55 cm. C. meneghiniana was observed above 55 cm. A rapid decrease in the abundance of most aerophilous and benthic data was seen in Zone GS4 and continued in Zone GS3. Eunotia spp. were not recorded above 60 cm. This zone is considered a transition zone, marking a shift from benthic to planktonic diatoms.
Zone GS2b (50–25 cm)
In this zone the species composition changed rapidly. This zone was characterised by a shift in the dominance of the planktonic diatoms to A. granulata, which reached a maximum abundance of 78% at a depth of 35 cm (Fig. 7). Lower percentages were recorded in the remaining planktonic diatoms, such as A. alpigena, A. ambigua and A. italica. However, aerophilous species declined to 4% and benthic species were at a low of 19%. C. meneghiniana and Cyclotella pseudostelligera were regularly observed in this zone.
Zone GS2a (25–11 cm)
This part of the zone continued to show a dominance of planktonic diatoms, and these accounted for up to 66% of valves, whereas some benthic and epiphytic forms persisted with average values of 20 and 8.4% respectively (Fig. 7). N. palea became common in this zone, rising to 10% of valves.
Zone GS1 (11–0 cm)
Although benthic values increased, planktonic forms remained dominant, with over 70% of valves (Fig. 7). Typical planktonic taxa here included A. ambigua, A. granulata angustissima, A. italica and C. meneghiniana. A. granulata angustissima reached its maximum abundance (43%) at 12 cm. Of the benthic forms, Bacillaria paradoxa was at 22% of benthic species, which may be reflective of salt input to the wetland. Other benthic diatoms were persistent but in low abundances. Epiphytic taxa were largely absent.
PCA of diatoms
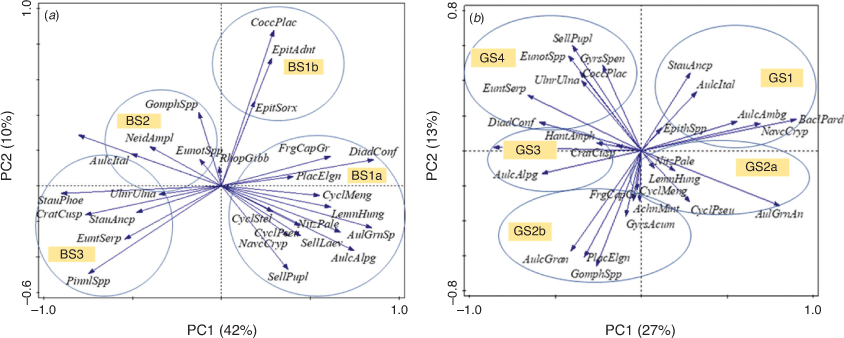
In BS, the PCA of diatom species showed that PC1 and PC2 explained 42% and 10% of the total variation respectively (Fig. 8a). Diatom results were plotted with PC1 and PC2 scores, and demonstrated a transition from benthic and epiphytic diatoms with negative scores to planktonic, facultative planktonic and aerophilous diatoms with positive PC1 scores (Fig. 6). The main shifts in PC1 scores corresponded to the changes in distinct zones identified by the cluster analysis (Fig. 6). Zones BS2 and BS3 showed the lowest PC 1 sample scores (between –1.3 and –0.1). By contrast, BS1a and BS1b zones showed high and positive score values of the PC 1 ranging between 0.3 and 1.51. This showed a shift in diatom assemblages between zones.
In GS, PC1 represented 27% of variance and PC2 a total variance of 13% (Fig. 8b). PC1 scores were used to interpret shifts in diatom assemblages in distinct zones identified by cluster analysis (Fig. 7). In GS4 and GS3, PC 1 scores ranged between –0.4 and –1.4, whereas a peak of 1.3 score was obtained in zone GS3 at 58 cm corresponding to a shift to planktonic diatoms. The scores remained consistent between Zones GS2a and GS2b, whereas higher values were observed in Zone GS1 that ranged between 1.7 and 2.2. The PCA diagram showed overlap between the clustering of zones, suggesting mixing conditions in the wetland.
Discussion
Chronology and sediment accumulation rates
In addition to the chronology based on 210Pb analyses, dating inferences can be made by interpretation of diatom taxa changes with known environmental changes, and by analysis of Pinus pollen. For example, A. alpigena (syn. Aulacoseira subborealis; Aulacoseira pusilla) appears to arrive in several Murray River wetland records soon after river regulation towards the mid-20th century (Fluin et al. 2010). The planktonic species reflect relatively permanent or deeper water, as well as enriched or turbid conditions. The record from Junction Park Billabong (Tibby et al. 2020) downstream suggests that A. alpigena peaked much earlier, but this is based on low levels of exotic pollen and a record that lacks radiometric dating across the relevant period. The record from near the Perricoota Forest, across the river from Gunbower (Gell et al. 2018a), records A. alpigena from the mid-19th century, confirming it as a reliable indicator of post-regulation times in this vicinity. So, early increases in this taxon provide a temporal tie-point c. 1940 CE (e.g. Gell et al. 2018b). Its presence likely confirms post-regulation time.
The arrival of Pinus pollen in western Victoria sediments has generally been inferred to represent a date of 1850 CE (Mooney et al. 2001; Tibby et al. 2007). Along the Murray River Tibby et al. (2020) used it to ascribe an age of 1880 CE. However, due to the spatial variability in the development of landscapes around south-east Australia, it is likely that, in some cores, there are many decades of post-European sediment accumulation that are without Pinus (Bickford et al. 2008). Therefore, across much of south-east Australia, although the presence of pine pollen definitively recognises post-European sediment, the first arrival in a sediment core does not necessarily equate to 1850 CE (Gell et al. 1993; Mooney et al. 2001). The town of Cohuna, the nearest to the Gunbower Forest, was not settled until 1880, and so it is likely that the first detection of Pinus pollen in these wetlands represents a date in the 20th, rather than the 19th, century.
For the BS core, the CFCS and CRS models suggested an age for the deepest sample of 82 ± 13 and 113 ± 11 years respectively. Following Mooney et al. (2001), the absence of exotic pollen in the lower sections of the BS core may suggest the record extends as far back as the onset of river regulation and possibly into times before (Gell et al. 2018a). However, given the timing of local European settlement, and that BS is surrounded by native forest and has no extensive pine plantations in its vicinity, a later date is likely. So, this suggests that the sediments above this point are deposited after 1880, but are likely to be much younger (Bickford et al. 2008). Further, although Reid et al. (2007) observed increases in fine sediments causing muddy water conditions that triggered aerophilous diatoms soon after European settlement, followed by a shift to plankton dominance, elsewhere the shift to plankton follows the onset of river regulation (Kattel et al. 2017). In this record, an aerophilous taxon increased at 30 cm and the plankton rise began at 35 cm. This may suggest these sediments were deposited c. 1930; however, for these depths, 210Pb dating suggests the age of the core to be between 1980 and 1990. The 210Pb records would be more reliable because diffuse catchment-based drivers are harder to reconcile within the palaeoecological record. So, these shifts in diatom groups are more likely attributable to more recent changes (e.g. 1970s) consistent with Kings Billabong downstream (Kattel et al. 2015). The appearance of the nutrient indicator C. meneghiniana at 35 cm supports the notion that the upper sediment layers represent the recent high-impact period in the Gunbower Forest Island. Although it cannot be ruled out that sediments accumulated in the wetlands at a time before regulation led to an increase in the permanence of inundation of Gunbower wetlands, it is likely that the basal age relates to some time after regulation (allowing for the ~10 cm of sediment penetrated by the corer nose but not sampled), following the release of water for irrigation from Torrumbarry Weir via Gunbower Creek from 1923.
For the GS core, the CFCS model suggested an age of 71 ± 25 years for 31 cm. The age below this depth was calculated by extrapolation of the CFCS model, and this yielded an age of 165 years ago at the deepest sample at 75 cm equating to 1853 ± 25 CE. Exotic Pinus was first observed at 80 cm in the GS core (Gell et al. 2018a), which confirms its post-European age, but is likely younger than the often-used age of 1850 CE. At the base, the diatom assemblage had low numbers of plankton and was mostly dominated by large benthic species (E. serpentina, Gyrosigma spp., Pinnularia spp.) that were soon replaced by aerophilous and planktonic diatoms, as observed in Hogan’s Billabong (Reid et al. 2007). However, the nutrient-indicating diatom (C. meneghiniana) was observed at 55 cm of the core, suggesting an age much after European settlement. Although the extrapolated basal age suggests net sediment deposition may have occurred before regulation in this case, the high 210Pb activity at the bottom of the core and the appearance of A. alpigena at 70 cm contradicts the evidence of the extrapolated age and the arrival of Pinus in GS core, suggesting an old age. So, like BS, this record may extend only as far as the early 20th century regardless of the extrapolated age.
There is much uncertainty in establishing sound chronologies in these wetlands given the fluvial setting, the low concentrations of exotic pollen and the uncertainty of the stratigraphic age of their first arrival. Although not conclusive, several factors (river regulation, sedimentation rates and diatom assemblages) suggest a basal age in the 20th, rather than the 19th, century. River regulation (1923 CE) provides a clear mechanism for altered hydrology, with increased volume of water and reduced drainage due to elevated water levels above Torrumbarry Weir and in Gunbower Creek. Typically, post-European sedimentation rates are ~1 cm year–1 (Gell and Reid 2014), including at Perricoota (Gell et al. 2018a) and in wetlands in the dairy zone of Gunbower (N. Mall, unpubl. data). The application of rates such as these yield maximum ages for GS and BS of 1930 CE. Finally, the emerging diatom biostratigraphy (e.g. the rise in A. alpigena) from floodplain wetlands suggests a common timing of change across several hundred river kilometres.
Thus, it is likely that before regulation, these wetlands were not permanently wet in the case of BS, and perhaps not until regulation in the case of GS. This suggests that the preregulation condition included frequent phases where the wetlands were dry and the accumulated sediment was lost (Kattel et al. 2015). The diatom valves preserved in the lower part of each core were mostly broken, indicating, at most, dry sediments and very shallow water. In summary, the average sedimentation rates at these sites were 0.9 cm year–1 for BS and 0.45 cm year–1 for GS (between 0 and 30 cm), and the basal sediments are likely no older than 100 years.
Change in the Gunbower wetlands
Based on knowledge of historical river flows (Mallen-Cooper and Zampatti 2018), rainfall data (Bureau of Meteorology 2019) and the MDB Commission Monthly Simulation Model (Cooling et al. 2002), before regulation, under natural conditions, the Murray system experienced drying and flooding at regular intervals. However, after regulation the system has faced many episodes of drought seasons, except the flood events of 1956–58, 1973–75 and 2010. The frequency of drought has increased recently, resulting in dry phases shifting from one to three in 10 years to three to seven in 10 years. So, the shift to more regular inundation in the wetlands is likely a consequence of the artificial elevation of Gunbower Creek, maintained for water supply purposes, which limits drainage, rather than an increase in overbank inputs from the main river.
The cores extracted from BS (84 cm) and GS (86 cm), as well as those from Little Reedy Lagoon (45 cm) reported in Gell et al. (2018a), are short relative to several extracted upstream (e.g. Hogan’s Billabong, 320 cm) and downstream (e.g. Tareena Billabong, 460 cm; Murroondi, 1200 cm; Gell and Reid 2014, 2016). Typically, based on over 60 sediment records across the southern MDB, post-European sedimentation rates are 1–5 cm year–1. So, it is unlikely that these short records extend into the 19th century. Similar to Kings Billabong (Kattel et al. 2017, 2020), it is possible that net sediment accumulation commenced only after the wetlands became relatively permanent after river regulation from the late 1920s, and so their natural state was intermittent or seasonal. This is consistent with the majority of sites in the southern MDB, with long-term permanent wetlands confined to the upper reaches, near large river confluences, in large meander cut-offs downstream of the Darling River and towards the coast (Gell 2020).
The diatom records from both BS and GS cores show a consistent transition mainly from the large, benthic diatoms to epiphytic forms to plankton dominance at the surface. This suggests that the early phase of permanency was of waters that were sufficiently transparent to allow for light to penetrate to the substrate and for submerged aquatic plants to grow. The gradual switch to plankton dominance reflects the contraction of the photic zone to the surface waters providing an advantage to floating forms. The rise and fall in epiphytes likely mark the onset of nutrient enrichment, but ultimately the decline in the aquatic macrophyte communities that have been lost through a critical transition or on account of an ongoing supply of fine sediments (Kattel et al. 2017). This pattern of change is consistent with those records in MDB floodplain records from the lower reaches (e.g. Sinclair flat; Grundell et al. 2012) to the upper reaches (e.g. Hogan’s Billabong; Reid et al. 2007).
According to the Itrax XRF data, detrital inputs at the base of the cores indicated significant erosion. These detrital elements would likely have brought P into the system and stimulated organic matter production because P is a limiting nutrient (Arai and Livi 2013). Higher enrichment of detrital elements would indicate periods of higher surface water input that coincided with the high values of Ca at the basal sediments and declined towards the top of the core. The concentrations of Ca closely follow the total inorganic carbon of the sediment. Generally, during dry periods, high inorganic carbonate precipitation is observed due to the concentration of dissolved ions under higher evaporative conditions as well as higher calcium carbonate preservation (Brown 2011). So, the low values of Ca in the upper sections of cores (20–0 cm) can be due to high-flow conditions.
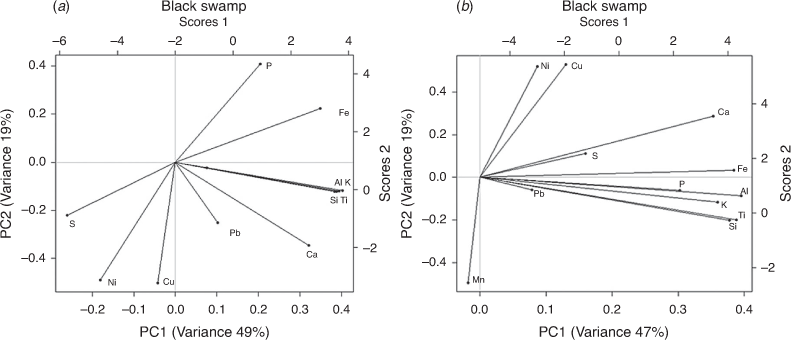
In addition, a PCA synthesis diagram of diatom species and elements (Fig. 9) for both cores showed a significant correlation between metals (e.g. Ni, Cu) and diatoms (mainly planktonic, aerophilous and eutrophic). On the basis of habitat preferences of the individual diatom species, inferences can be made regarding the environmental factors that the dominant principal components most likely represent. These inferences were combined with elements to analyse conditions of the wetlands. For the BS core, Ni and Cu showed a positive correlation with planktonic (A. granulata ssp., A. alpigena, C. pseudostelligera, C. meneghiniana), disturbance-indicating (D. confervaceae) and nutrient-indicating (N. palea) diatoms. In the GS core, PC scores of Ni and Cu were similar to epiphytic (C. placentula) and aerophilous (D. confervaceae) diatoms. This correlation represented catchment disturbance and turbid conditions of the wetland at different depths, as indicated by depth numbers in the synthesis diagram. These wetland conditions could be reflecting their connection to river or Gunbower Creek. By contrast, the negative relationship between planktonic diatoms and erosional indicators (such as Ti, K, Fe) may be reflecting a shift to turbid conditions favoured by planktonic diatoms (mainly Aulacoseira granulata ssp.). These inferences were further supported by high δ15N values reflecting high nutrient levels in the top 15 cm of the BS core and at the 40-cm point of the GS core that could be associated with animal waste or irrigation activities. For example, 15N enrichment was found in bulk sediments of Lake Alexandrina, South Australia (Herczeg et al. 2001) and Kings Billabong (Kattel et al. 2017) due to land use activities. The high (>10) C/N ratio for the surface sediments of the BS core and for the basal sediments (75–65 cm) of the GS core could be related to input from terrestrial sources, which is supported by the presence of the aerophilous species D. confervaceae, an indicator of catchment disturbance.
A relatively high contribution of organic matter from aquatic sources was consistent with macrophyte loss and correlated with the increasing numbers of the diatom L. hungarica (c. 1975 CE), which is an epiphyte on floating plants (Fig. 6). Such a rise in floating plants could be due to nutrients or turbidity. So, these changes reflect a shift to turbid waters and a plankton-dominated system with variable pH conditions. Further, the δ13C-enriched macrophyte in the lower sections of the BS core may be replaced by depleted δ13C phytoplankton in the upper sections of cores under hydrological variation (e.g. Krull et al. 2009), consistent with other studies (e.g. Kattel et al. 2020), indicating algae as the likely main source of organic matter (Lamb et al. 2006). This is also shown by the high P/B ratio in the upper sections of the BS core.
The research sites are distant from the typical sources of nutrients, such as human settlements or zones of intensive irrigation. The diatom record reveals a rise in species that are clear indicators of nutrient enrichment (e.g. Cyclotella spp. and Nitzschia spp.). Further, the transition from benthic to planktonic forms is consistent with a state transition that may arise from sudden or consistent increases in nutrient or sediment loads. As such, it appears that, although it may be expected that the condition of these wetlands may be afforded protection from impacts on account of the neighbouring forests, the records show that nutrients, presumably from outside the forest reserve, are entering the waterways. The Gunbower system is flooded by Murray River water, which would carry nutrients from highly developed agricultural areas upstream. So, it is expected that benthic diatoms at the base of the cores suggest preregulation clear water conditions. However, a shift to planktonic-rich diatoms in the upper sections of the cores suggests nutrient increases likely well after the more frequent connection following the Torrumbarry weir regulation. These changes that appear typical of those across the broader basin suggest there is a regional-scale driver of change influencing these wetlands.
Wetland Change in the MDB
As noted above, these records of change for the Gunbower wetlands are consistent with other floodplain records, and the post-regulation phases of naturally permanent wetlands, across the southern MDB (Gell and Reid 2016). This suggests that rather than a proximal source, there is a regional-scale increase in the supply of sediments and nutrients to basin-wide wetlands, and that the main river is the dominant agent for this supply. The sediment loads may be attributable to any of the phases of erosion following European arrival (Rutherfurd et al. 2020), including the high volumes of sediment shed during the gold rush (Davies et al. 2018). High nutrient loads are known to have affected the rivers for more than a century (Ogden 2000; Reid et al. 2007; Gell and Reid 2016; Tibby et al. 2020), but a range of records (e.g. Kings Billabong; Kattel et al. 2017) suggest this has been exacerbated recently. It is possible that earlier enrichment elsewhere is due to local inputs, but these forested sites (BS and GS) may have been less influenced by early development.
The Gunbower record corroborates the interpretation of the loss of submerged aquatic macrophyte communities both upstream (Reid et al. 2007; Tibby et al. 2020) and downstream (Grundell et al. 2012; Kattel et al. 2017), and reveals that this phenomenon has occurred throughout the main river system. Changes to wetlands, as recorded in sediment records, are influenced by climate and hydrological management. Variations in the timing of the change may reflect local changes in these factors, as well as the relative sensitivity of the respective wetlands to regional-scale fluxes. In the Gunbower system, and likely elsewhere, the increase in permanency precludes the benefits of drying, which are likely to allow for ‘resetting’ and the re-establishment of plant communities (Gell and Reid 2016). The documented shift from submerged plant forms to floating or emergent (Kattel et al. 2020), appears to be reflected in the Gunbower records and suggests the influence of turbidity on changing plant communities. This has implications for the crustacean fauna (Kattel et al. 2017), and likely these conditions would also influence consumers in the higher trophic levels of the food web.
Implications for the Gunbower Forest Ramsar site
The Gunbower Forest wetlands were listed by Australia in 1982 as a Wetland of International Importance (Ramsar Site) under the Ramsar Convention on Wetlands. The ecological character description at the time of listing was of a periodically inundated, forested floodplain. Its four listing criteria (Hale and Butcher 2011) highlight its quality as a freshwater tree-dominated wetland (Criterion 1), its role in supporting five threatened wetland species (Criterion 2), its role as habitat for waterbirds and as a breeding site for birds and fish (Criterion 4) and its role in providing for the ecological requirements of populations of native fish (Criterion 8). Water resource development and climate change are identified as threats to the site, but nutrient and sediments inputs are not (Hale and Butcher 2011).
Although the indicators analysed in these sediment records do not directly assess change in the biota that underpin this listing, they do reveal ecological change in the wetlands over time. The state of some of the wetlands at the site then appear to have already shifted, on account of declining water quality, by the time they were listed under Ramsar. So, this site provides an example where palaeoecology has revealed that the long-term changes to a wetland have been underestimated (Pritchard 2021).
Nations have an obligation to report to the Ramsar secretariat if a wetland ‘has changed, is changing or is likely to change’ (Davidson 2016). Australia, being one of the prominent Ramsar Parties, is obliged to adopt the Ramsar Convention Guidelines for maintaining degraded ecological characters of the wetlands listed under the convention. Kumar et al. (2021) have explored some of the important issues of ecological character of wetlands undergoing change (whether human induced or not). The ecological character is a description of the contemporary ecosystem components of the site and their interactions. Although the Gunbower Forest description identifies its present state and contemporary or future threats, it does not record past changes to the waterbodies and the influence of changing water quality. So, the longer-term record attests to considerable change, albeit in indicators not noted in the ecological character description (Davidson et al. 2020).
This evidence reveals that a deleterious, anthropogenic change has occurred to the ecological character of at least some of the site’s wetlands. That the changes occurred before listing entertains Pritchard’s (2021) question of what is the right baseline. Should managers adhere to the time-of-listing baseline, then no reportable change has occurred (Gell 2017). However, as Finlayson et al. (2016) note, if this was applied universally it would excuse all the degradation that occurred before the signing of the Convention. In seeking to restore ‘the site’s true value as seen from a longer-term perspective’ (Pritchard 2021), it may be appropriate for the Australian government to identify water quality decline as a threat and to seek means by which the present phytoplankton state, in these and similar wetlands, is returned to one dominated by aquatic macrophytes.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
The preliminary study of Gunbower wetlands was funded by the North Central Catchment Management Authority and was reported in Gell et al. (2018a). Geochemical (Itrax XRF), 210Pb and stable isotope analysis were funded by an ANSTO research grant.
Acknowledgements
The North Central Catchment Management Authority and the Barapa Barapa supported the initial phase of core collection. Michael Shawn-Fletcher and Kristen Beck undertook the initial pollen analyses. Cameron Gell assisted with field work.
References
Appleby, P. (2002) Chronostratigraphic techniques in recent sediments. In ‘Tracking environmental change using lake sediments’. pp. 171–203. (Springer: Dordrecht, Netherlands.)Appleby, P., and Oldfield, F. (1983). The assessment of 210 Pb data from sites with varying sediment accumulation rates. Hydrobiologia 103, 29–35.
| The assessment of 210 Pb data from sites with varying sediment accumulation rates.Crossref | GoogleScholarGoogle Scholar |
Arai, Y., and Livi, K. (2013). Underassessed phosphorus fixation mechanisms in soil sand fraction. Geoderma 192, 422–429.
| Underassessed phosphorus fixation mechanisms in soil sand fraction.Crossref | GoogleScholarGoogle Scholar |
Aufgebauer, A., Panagiotopoulos, K., Wagner, B., Schaebitz, F., Viehberg, F. A., Vogel, H., Zanchetta, G., Sulpizio, R., Leng, M. J., and Damaschke, M. (2012). Climate and environmental change in the Balkans over the last 17 ka recorded in sediments from Lake Prespa (Albania/F.Y.R. of Macedonia/Greece). Quaternary International 274, 122–135.
| Climate and environmental change in the Balkans over the last 17 ka recorded in sediments from Lake Prespa (Albania/F.Y.R. of Macedonia/Greece).Crossref | GoogleScholarGoogle Scholar |
Bate, N., and Newall, P. (1998) Techniques for the use of diatoms in water quality assessment: how many valves. In ‘Proceedings of the 15th international diatom symposium’, 28 September–2 October 1998, Perth, WA, Australia. (Ed. J. John.) pp. 153–160. (Curtis University: Perth, WA, Australia.)
Battarbee, R. W., Jones, V. J., Flower, R. J., Cameron, N. G., Bennion, H., Carvalho, L., and Juggins, S. (2002) Diatoms. In ‘Tracking environmental change using lake sediments’. pp. 155–202. (Springer: Dordrecht, Netherlands.)
Bhaduri, A., Bogardi, J., Siddiqi, A., Voigt, H., Vörösmarty, C., Pahl-Wostl, C., Bunn, S. E., Shrivastava, P., Lawford, R., Foster, S., Kremer, H., Renaud, F. G., Bruns, A., and Osuna, V. R. (2016). Achieving sustainable development goals from a water perspective. Frontiers in Environmental Science 4, 64.
| Achieving sustainable development goals from a water perspective.Crossref | GoogleScholarGoogle Scholar |
Bickford, S., Gell, P., and Hancock, G. J. (2008). Wetland and terrestrial vegetation change since European settlement on the Fleurieu Peninsula, South Australia. The Holocene 18, 425–436.
| Wetland and terrestrial vegetation change since European settlement on the Fleurieu Peninsula, South Australia.Crossref | GoogleScholarGoogle Scholar |
Boës, X., Rydberg, J., Martinez-Cortizas, A., Bindler, R., and Renberg, I. (2011). Evaluation of conservative lithogenic elements (Ti, Zr, Al, and Rb) to study anthropogenic element enrichments in lake sediments. Journal of Paleolimnology 46, 75–87.
| Evaluation of conservative lithogenic elements (Ti, Zr, Al, and Rb) to study anthropogenic element enrichments in lake sediments.Crossref | GoogleScholarGoogle Scholar |
Boyle, J. F., Sayer, C. D., Hoare, D., Bennion, H., Heppel, K., Lambert, S. J., Appleby, P. G., Rose, N. L., and Davy, A. J. (2016). Toxic metal enrichment and boating intensity: sediment records of antifoulant copper in shallow lakes of eastern England. Journal of Paleolimnology 55, 195–208.
| Toxic metal enrichment and boating intensity: sediment records of antifoulant copper in shallow lakes of eastern England.Crossref | GoogleScholarGoogle Scholar |
Brown, E. T. (2011). Lake Malawi’s response to “megadrought” terminations: sedimentary records of flooding, weathering and erosion. Palaeogeography, Palaeoclimatology, Palaeoecology 303, 120–125.
| Lake Malawi’s response to “megadrought” terminations: sedimentary records of flooding, weathering and erosion.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2019). Climate of the 2018–19 financial year. (Commonwealth of Australia) http://www.bom.gov.au/climate/updates/articles/a034.shtml [Verified 10 July 2019].
Burnett, A. P., Soreghan, M. J., Scholz, C. A., and Brown, E. T. (2011). Tropical East African climate change and its relation to global climate: a record from Lake Tanganyika, Tropical East Africa, over the past 90+ kyr. Palaeogeography, Palaeoclimatology, Palaeoecology 303, 155–167.
| Tropical East African climate change and its relation to global climate: a record from Lake Tanganyika, Tropical East Africa, over the past 90+ kyr.Crossref | GoogleScholarGoogle Scholar |
Chen, H., Zhang, W., Gao, H., and Nie, N. (2018). Climate change and anthropogenic impacts on wetland and agriculture in the Songnen and Sanjiang Plain, Northeast China. Remote Sensing 10, 356.
| Climate change and anthropogenic impacts on wetland and agriculture in the Songnen and Sanjiang Plain, Northeast China.Crossref | GoogleScholarGoogle Scholar |
Cooling, M. P., Lloyd, L. N., Rudd, D. J., and Hogan, R. P. (2002). Environmental water requirements and management options in Gunbower Forest, Victoria. Australasian Journal of Water Resources 5, 75–88.
| Environmental water requirements and management options in Gunbower Forest, Victoria.Crossref | GoogleScholarGoogle Scholar |
Corella, J. P., Brauer, A., Mangili, C., Rull, V., Vegas‐Vilarrúbia, T., Morellón, M., and Valero‐Garcés, B. L. (2012). The 1.5-ka varved record of Lake Montcortès (southern Pyrenees, NE Spain). Quaternary Research 78, 323–332.
| The 1.5-ka varved record of Lake Montcortès (southern Pyrenees, NE Spain).Crossref | GoogleScholarGoogle Scholar |
Croudace, I., Rindby, A., and Guy Rothwell, R. (2006). ITRAX: description and evaluation of a new multi-function X-ray core scanner. Geological Society of London, Special Publications 267, 51–63.
| ITRAX: description and evaluation of a new multi-function X-ray core scanner.Crossref | GoogleScholarGoogle Scholar |
Darrah, S. E., Shennan-Farpón, Y., Loh, J., Davidson, N. C., Finlayson, C. M., Gardner, R. C., and Walpole, M. J. (2019). Improvements to the Wetland Extent Trends (WET) index as a tool for monitoring natural and human-made wetlands. Ecological Indicators 99, 294–298.
| Improvements to the Wetland Extent Trends (WET) index as a tool for monitoring natural and human-made wetlands.Crossref | GoogleScholarGoogle Scholar |
Davidson, N. C. (2014). How much wetland has the world lost? Long-term and recent trends in global wetland area. Marine and Freshwater Research 65, 934–941.
| How much wetland has the world lost? Long-term and recent trends in global wetland area.Crossref | GoogleScholarGoogle Scholar |
Davidson, N. C. (2016) Wetland losses and the status of wetland-dependent species. In ‘The Wetland Book: II: Distribution, Description and Conservation’. (Eds C. M. Finlayson, G. R. Milton, R. C. Prentice, and N. C. Davidson.) pp. 1–14. (Springer: Dordrecht, Netherlands.)
Davidson, N. C., Dinesen, L., Fennessy, S., Finlayson, C. M., Grillas, P., Grobicki, A., McInnes, R. J., and Stroud, D. A. (2020). A review of the adequacy of reporting to the Ramsar Convention on change in the ecological character of wetlands. Marine and Freshwater Research 71, 117–126.
| A review of the adequacy of reporting to the Ramsar Convention on change in the ecological character of wetlands.Crossref | GoogleScholarGoogle Scholar |
Davies, P., Lawrence, S., Turnbull, J., Rutherfurd, I., Grove, J., Silvester, E., Baldwin, D., and Macklin, M. (2018). Reconstruction of historical riverine sediment production on the goldfields of Victoria, Australia. Anthropocene 21, 1–15.
| Reconstruction of historical riverine sediment production on the goldfields of Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Dubois, N., Saulnier-Talbot, É., Mills, K., Gell, P., Battarbee, R., Bennion, H., Chawchai, S., Dong, X., Francus, P., Flower, R., Gomes, D. F., Gregory-Eaves, I., Humane, S., Kattel, G., Jenny, J., Langdon, P., Massaferro, J., McGowan, S., Mikomägi, A., Ngoc, N. T. M., Ratnayake, A. S., Reid, M., Rose, N., Saros, J., Schillereff, D., Tolotti, M., and Valero-Garcés, B. (2018). First human impacts and responses of aquatic systems: a review of palaeolimnological records from around the world. The Anthropocene Review 5, 28–68.
| First human impacts and responses of aquatic systems: a review of palaeolimnological records from around the world.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar | 16336747PubMed |
Elbert, J., Wartenburger, R., von Gunten, L., Urrutia, R., Fischer, D., Fujak, M., Hamann, Y., Greber, N. D., and Grosjean, M. (2013). Late Holocene air temperature variability reconstructed from the sediments of Laguna Escondida, Patagonia, Chile (45°30′S). Palaeogeography, Palaeoclimatology, Palaeoecology 369, 482–492.
| Late Holocene air temperature variability reconstructed from the sediments of Laguna Escondida, Patagonia, Chile (45°30′S).Crossref | GoogleScholarGoogle Scholar |
Fernandes, M., and Krull, E. (2008). How does acid treatment to remove carbonates affect the isotopic and elemental composition of soils and sediments? Environmental Chemistry 5, 33–39.
| How does acid treatment to remove carbonates affect the isotopic and elemental composition of soils and sediments?Crossref | GoogleScholarGoogle Scholar |
Finlayson, C. M. (2013). Climate change and the wise use of wetlands: information from Australian wetlands. Hydrobiologia 708, 145–152.
| Climate change and the wise use of wetlands: information from Australian wetlands.Crossref | GoogleScholarGoogle Scholar |
Finlayson, M., Cruz, R., Davidson, N., Alder, J., Cork, S., de Groot, R., Lévêque, C., Milton, G., Peterson, G., and Pritchard, D. (2005) ‘Millennium Ecosystem Assessment: Ecosystems and Human Well-being: Wetlands and Water Synthesis.’ (Island Press.)
Finlayson, C. M., Clarke, S. J., Davidson, N. C., and Gell, P. (2016). Role of palaeoecology in describing the ecological character of wetlands. Marine and Freshwater Research 67, 687–694.
| Role of palaeoecology in describing the ecological character of wetlands.Crossref | GoogleScholarGoogle Scholar |
Fluin, J., Tibby, J., and Gell, P. (2010). Testing the efficacy of electrical conductivity (EC) reconstructions from the lower Murray River (SE Australia): a comparison between measured and inferred EC. Journal of Paleolimnology 43, 309–322.
| Testing the efficacy of electrical conductivity (EC) reconstructions from the lower Murray River (SE Australia): a comparison between measured and inferred EC.Crossref | GoogleScholarGoogle Scholar |
Gell, P. A. (2017). Using paleoecology to understand natural ecological character in Ramsar wetlands Past Global Changes Magazine 25, 86–87.
| Using paleoecology to understand natural ecological character in Ramsar wetlandsCrossref | GoogleScholarGoogle Scholar |
Gell, P. A. (2020). Restoring Murray River floodplain wetlands: does the sediment record inform on watering regime? River Research and Applications 36, 620–629.
| Restoring Murray River floodplain wetlands: does the sediment record inform on watering regime?Crossref | GoogleScholarGoogle Scholar |
Gell, P., and Reid, M. (2014). Assessing change in floodplain wetland condition in the Murray–Darling Basin, Australia. Anthropocene 8, 39–45.
| Assessing change in floodplain wetland condition in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P. A., and Reid, M. A. (2016). Muddied waters: the case for mitigating sediment and nutrient flux to optimize restoration response in the Murray–Darling Basin, Australia. Frontiers in Ecology and Evolution 4, 16.
| Muddied waters: the case for mitigating sediment and nutrient flux to optimize restoration response in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P. A., Stuart, I.-M., and Smith, J. D. (1993). The response of vegetation to changing fire regimes and human activity in East Gippsland, Victoria, Australia. The Holocene 3, 150–160.
| The response of vegetation to changing fire regimes and human activity in East Gippsland, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P., Sonneman, J., Reid, M., Illman, M., and Sincock, A. (1999) An illustrated key to the common diatom genera from southern Australia – identification guide number 26. Murray–Darling Freshwater Research Centre, Albury, NSW, Australia.
Gell, P. R., Sluiter, I., and Fluin, J. (2002). Seasonal and interannual variations in diatom assemblages in Murray River connected wetlands in north-west Victoria, Australia. Marine and Freshwater Research 53, 981–992.
| Seasonal and interannual variations in diatom assemblages in Murray River connected wetlands in north-west Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P., Tibby, J., Fluin, J., Leahy, P., Reid, M., Adamson, K., Bulpin, S., MacGregor, A., Wallbrink, P., and Hancock, G. (2005). Accessing limnological change and variability using fossil diatom assemblages, south‐east Australia. River Research and Applications 21, 257–269.
| Accessing limnological change and variability using fossil diatom assemblages, south‐east Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P., Fluin, J., Tibby, J., Hancock, G., Harrison, J., Zawadzki, A., Haynes, D., Khanum, S., Little, F., and Walsh, B. (2009). Anthropogenic acceleration of sediment accretion in lowland floodplain wetlands, Murray–Darling Basin, Australia. Geomorphology 108, 122–126.
| Anthropogenic acceleration of sediment accretion in lowland floodplain wetlands, Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Gell, P. A., Finlayson, C. M., and Davidson, N. C. (2016). Understanding change in the ecological character of Ramsar wetlands: perspectives from a deeper time – synthesis. Marine and Freshwater Research 67, 869–879.
| Understanding change in the ecological character of Ramsar wetlands: perspectives from a deeper time – synthesis.Crossref | GoogleScholarGoogle Scholar |
Gell, P., Beck, K., Fletcher, M., Kattel, G., and Doan, P. (2018a) Gunbower Forest Wetlands–Paleoecological History. North Central Catchment Management Authority. Federation University and University of Melbourne, Australia.
Gell, P., Perga, M.-E., and Finlayson, C. M. (2018b) Changes over time. In ‘Freshwater Ecology and Conservation: Approaches and Techniques’. (Ed. J. M. R. Hughes.) pp. 283–305. (Oxford University Press: Oxford, UK.)
Gell, P. A., Reid, M. A., and Wilby, R. L. (2019). Management pathways for the floodplain wetlands of the southern Murray–Darling Basin: lessons from history. River Research and Applications 35, 1291–1301.
| Management pathways for the floodplain wetlands of the southern Murray–Darling Basin: lessons from history.Crossref | GoogleScholarGoogle Scholar |
Gillson, L., Dirk, C., and Gell, P. (2021). Using long-term data to inform a decision pathway for restoration of ecosystem resilience. Anthropocene 36, 100315.
| Using long-term data to inform a decision pathway for restoration of ecosystem resilience.Crossref | GoogleScholarGoogle Scholar |
Gregory-Eaves, I., and Beisner, B. E. (2011). Palaeolimnological insights for biodiversity science: an emerging field. Freshwater Biology 56, 2653–2661.
| Palaeolimnological insights for biodiversity science: an emerging field.Crossref | GoogleScholarGoogle Scholar |
Grimm, E. C. (1987). CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers & Geosciences 13, 13–35.
| CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares.Crossref | GoogleScholarGoogle Scholar |
Grundell, R., Gell, P., Mills, K., and Zawadzki, A. (2012). Interaction between a river and its wetland: evidence from the Murray River for spatial variability in diatom and radioisotope records. Journal of Paleolimnology 47, 205–219.
| Interaction between a river and its wetland: evidence from the Murray River for spatial variability in diatom and radioisotope records.Crossref | GoogleScholarGoogle Scholar |
Guyard, H., Chapron, E., St-Onge, G., Anselmetti, F. S., Arnaud, F., Magand, O., Francus, P., and Mélières, M.-A. (2007). High-altitude varve records of abrupt environmental changes and mining activity over the last 4000 years in the Western French Alps (Lake Bramant, Grandes Rousses Massif). Quaternary Science Reviews 26, 2644–2660.
| High-altitude varve records of abrupt environmental changes and mining activity over the last 4000 years in the Western French Alps (Lake Bramant, Grandes Rousses Massif).Crossref | GoogleScholarGoogle Scholar |
Hale, J., and Butcher, R. (2011) Ecological character description for the Gunbower Forest Ramsar Site. Report to the Department of Sustainability, Environment, Water. Population and Communities, Canberra, ACT, Australia.
Harrison, J., Heijnis, H., and Caprarelli, G. (2003). Historical pollution variability from abandoned mine sites, greater blue mountains world heritage area, New South Wales, Australia. Environmental Geology 43, 680–687.
| Historical pollution variability from abandoned mine sites, greater blue mountains world heritage area, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Herczeg, A., Smith, A., and Dighton, J. (2001). A 120 year record of changes in nitrogen and carbon cycling in Lake Alexandrina, South Australia: C: N, δ15N and δ13C in sediments. Applied Geochemistry 16, 73–84.
| A 120 year record of changes in nitrogen and carbon cycling in Lake Alexandrina, South Australia: C: N, δ15N and δ13C in sediments.Crossref | GoogleScholarGoogle Scholar |
Huziy, O., and Sushama, L. (2017). Lake–river and lake–atmosphere interactions in a changing climate over Northeast Canada. Climate Dynamics 48, 3227–3246.
| Lake–river and lake–atmosphere interactions in a changing climate over Northeast Canada.Crossref | GoogleScholarGoogle Scholar |
Ivanov, M. V. (1981). The global biogeochemical sulphur cycle. In ‘SCOPE 17: Some Perspectives of the Major Biogeochemical Cycles’. (Ed. G. E. Likens.) pp. 61–78. (Wiley: New York, NY, USA.)
Jowsey, P. C. (1966). An improved peat sampler. New Phytologist 65, 245–248.
| An improved peat sampler.Crossref | GoogleScholarGoogle Scholar |
Juggins, S. (2007) ‘C2 Version 1.5 User guide. Software for ecological and palaeoecological data analysis and visualisation.’ (Newcastle University: Newcastle upon Tyne, UK.)
Junk, W. J., An, S., Finlayson, C. M., Gopal, B., Květ, J., Mitchell, S. A., Mitsch, W. J., and Robarts, R. D. (2013). Current state of knowledge regarding the world’s wetlands and their future under global climate change: a synthesis. Aquatic Sciences 75, 151–167.
| Current state of knowledge regarding the world’s wetlands and their future under global climate change: a synthesis.Crossref | GoogleScholarGoogle Scholar |
Kattel, G., Gell, P., Perga, M. E., Jeppesen, E., Grundell, R., Weller, S., Zawadzki, A., and Barry, L. (2015). Tracking a century of change in trophic structure and dynamics in a floodplain wetland: integrating palaeoecological and palaeoisotopic evidence. Freshwater Biology 60, 711–723.
| Tracking a century of change in trophic structure and dynamics in a floodplain wetland: integrating palaeoecological and palaeoisotopic evidence.Crossref | GoogleScholarGoogle Scholar |
Kattel, G., Gell, P., Zawadzki, A., and Barry, L. (2017). Palaeoecological evidence for sustained change in a shallow Murray River (Australia) floodplain lake: regime shift or press response? Hydrobiologia 787, 269–290.
| Palaeoecological evidence for sustained change in a shallow Murray River (Australia) floodplain lake: regime shift or press response?Crossref | GoogleScholarGoogle Scholar |
Kattel, G. R., Eyre, B. D., and Gell, P. A. (2020). Integration of palaeo-and-modern food webs reveal slow changes in a river floodplain wetland ecosystem. Scientific Reports 10, 12955.
| Integration of palaeo-and-modern food webs reveal slow changes in a river floodplain wetland ecosystem.Crossref | GoogleScholarGoogle Scholar | 32737428PubMed |
Krammer, K. (1988). ‘Susswasserflora von Mitteleuropa. Bd 2. Bacillariophyceae. Teil 2. Bacillariaceae, Epithemiaceae, Surirellaceae.’ (Gustav Fischer.)
Krammer, K., and Lange-Bertalot, H. (1986) ‘Susswasserflora von Mitteleuropa. Bd 2, Bacillariophyceae, Teil 1, Naviculaceae.’ (Eds H. Ettl, H. Gerloff, H. Heynig, D. Mollenhauer.) (Gustav Fischer.)
Krammer, K., and Lange-Bertalot, H. (1991) ‘Süsswasserflora von Mitteleuropa. Bd 2(3). Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae.’ (Gustav Fischer.)
Krammer, K., and Lange-Bertalot, H. (2004) ‘Süsswasserflora von Mitteleuropa 2: Bacillariophyceae. Teil 4: Achnanthaceae. Kritische Ergänzungen zu Achnanthes sl, Navicula s. str., Gomphonema.’ (Spektrum Akademischer Verlag/Gustav Fischer: Heidelberg, Germany.)
Krull, E., Haynes, D., Lamontagne, S., Gell, P., McKirdy, D., Hancock, G., McGowan, J., and Smernik, R. (2009). Changes in the chemistry of sedimentary organic matter within the Coorong over space and time. Biogeochemistry 92, 9–25.
| Changes in the chemistry of sedimentary organic matter within the Coorong over space and time.Crossref | GoogleScholarGoogle Scholar |
Kumar, R., McInnes, R., Finlayson, C. M., Davidson, N., Rissik, D., Paul, S., Cui, L., Lei, Y., Capon, S., and Fennessy, S. (2021). Wetland ecological character and wise use: towards a new framing. Marine and Freshwater Research 72, 633–637.
| Wetland ecological character and wise use: towards a new framing.Crossref | GoogleScholarGoogle Scholar |
Kylander, M. E., Ampel, L., Wohlfarth, B., and Veres, D. (2011). High‐resolution X‐ray fluorescence core scanning analysis of Les Echets (France) sedimentary sequence: new insights from chemical proxies. Journal of Quaternary Science 26, 109–117.
| High‐resolution X‐ray fluorescence core scanning analysis of Les Echets (France) sedimentary sequence: new insights from chemical proxies.Crossref | GoogleScholarGoogle Scholar |
Kylander, M., Klaminder, J., Wohlfarth, B., and Löwemark, L. (2013). Geochemical responses to paleoclimatic changes in southern Sweden since the late glacial: the Hässeldala Port lake sediment record. Journal of Paleolimnology 50, 57–70.
| Geochemical responses to paleoclimatic changes in southern Sweden since the late glacial: the Hässeldala Port lake sediment record.Crossref | GoogleScholarGoogle Scholar |
Lamb, A., Wilson, G., and Leng, M. (2006). A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Science Reviews 75, 29–57.
| A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material.Crossref | GoogleScholarGoogle Scholar |
Large, A., Gilvear, D., and Starkey, E. (2017). Ecosystem service-based approaches for status assessment of Anthropocene riverscapes. In ‘Rivers of the Anthropocene’. (Eds J. M. Kelly, P. Scarpino, H. Berry, J. Syvitski, and M. Meybeck.) pp. 23–42. (University of California Press: Oakland, CA, USA.)
Laurance, W. F., Dell, B., Turton, S. M., Lawes, M. J., Hutley, L. B., McCallum, H., Dale, P., Bird, M., Hardy, G., and Prideaux, G. (2011). The 10 Australian ecosystems most vulnerable to tipping points. Biological Conservation 144, 1472–1480.
| The 10 Australian ecosystems most vulnerable to tipping points.Crossref | GoogleScholarGoogle Scholar |
Lauterbach, S., Brauer, A., Andersen, N., Danielopol, D., Dulski, P., Huels, M., Milecka, K., Namiotko, T., Obremska, M., Von Grafenstein, U., and Participants, D. (2011). Environmental responses to Lateglacial climatic fluctuations recorded in the sediments of pre‐Alpine Lake Mondsee (northeastern Alps). Journal of Quaternary Science 26, 253–267.
| Environmental responses to Lateglacial climatic fluctuations recorded in the sediments of pre‐Alpine Lake Mondsee (northeastern Alps).Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Marshall, M. H., Lamb, H. F., Huws, D., Davies, S. J., Bates, R., Bloemendal, J., Boyle, J., Leng, M. J., Umer, M., and Bryant, C. (2011). Late Pleistocene and Holocene drought events at Lake Tana, the source of the Blue Nile. Global and Planetary Change 78, 147–161.
| Late Pleistocene and Holocene drought events at Lake Tana, the source of the Blue Nile.Crossref | GoogleScholarGoogle Scholar |
Miller, H., Croudace, I. W., Bull, J. M., Cotterill, C. J., Dix, J. K., and Taylor, R. N. (2014). Sediment lake record of anthropogenic and natural inputs to Windermere (English Lake District) using double-spike lead isotopes, radiochronology, and sediment microanalysis. Environmental Science & Technology 48, 7254–7263.
| Sediment lake record of anthropogenic and natural inputs to Windermere (English Lake District) using double-spike lead isotopes, radiochronology, and sediment microanalysis.Crossref | GoogleScholarGoogle Scholar |
Mooney, S. D., Radford, K. L., and Hancock, G. (2001). Clues to the ‘burning question’: pre‐European fire in the Sydney coastal region from sedimentary charcoal and palynology. Ecological Management & Restoration 2, 203–212.
| Clues to the ‘burning question’: pre‐European fire in the Sydney coastal region from sedimentary charcoal and palynology.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Authority (2012) ‘Murray–Darling Basin Authority annual report 2011–12.’ (MDBA: Canberra, ACT, Australia.)
Norris, R. H., Liston, P., Davies, N., Coysh, J., Dyer, F., Linke, S., Prosser, I., and Young, B. (2001) ‘Snapshot of the Murray–Darling Basin river condition.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
North Central Catchment Management Authority (2015). Gunbower Creek System Environmental Water Management Plan. Document Version 7, NCCMA, Huntly, Vic., Australia.
Ogden, R. W. (2000). Modern and historical variation in aquatic macrophyte cover of billabongs associated with catchment development. Regulated Rivers 16, 497–512.
| Modern and historical variation in aquatic macrophyte cover of billabongs associated with catchment development.Crossref | GoogleScholarGoogle Scholar |
Papas, P., and Moloney, P. (2012) Victoria’s wetlands 2009–2011: statewide assessments and condition modelling. Technical Report Series 229, Arthur Rylah Institute for Environmental Research.
Pittock, J., and Finlayson, C. M. (2011). Australia’s Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change. Marine and Freshwater Research 62, 232–243.
| Australia’s Murray–Darling Basin: freshwater ecosystem conservation options in an era of climate change.Crossref | GoogleScholarGoogle Scholar |
Pritchard, D. (2021). The ‘ecological character’ of wetlands: a foundational concept in the Ramsar Convention, yet still cause for debate 50 years later. Marine and Freshwater Research , .
| The ‘ecological character’ of wetlands: a foundational concept in the Ramsar Convention, yet still cause for debate 50 years later.Crossref | GoogleScholarGoogle Scholar |
Reid, M. A., and Ogden, R. W. (2009). Factors affecting diatom distribution in floodplain lakes of the southeast Murray Basin, Australia and implications for palaeolimnological studies. Journal of Paleolimnology 41, 453–470.
| Factors affecting diatom distribution in floodplain lakes of the southeast Murray Basin, Australia and implications for palaeolimnological studies.Crossref | GoogleScholarGoogle Scholar |
Reid, M. A., Sayer, C. D., Kershaw, A. P., and Heijnis, H. (2007). Palaeolimnological evidence for submerged plant loss in a floodplain lake associated with accelerated catchment soil erosion (Murray River, Australia). Journal of Paleolimnology 38, 191–208.
| Palaeolimnological evidence for submerged plant loss in a floodplain lake associated with accelerated catchment soil erosion (Murray River, Australia).Crossref | GoogleScholarGoogle Scholar |
Rutherfurd, I. D., Kenyon, C., Thoms, M., Grove, J., Turnbull, J., Davies, P., and Lawrence, S. (2020). Human impacts on suspended sediment and turbidity in the River Murray, south eastern Australia: multiple lines of evidence. River Research and Applications 36, 522–541.
| Human impacts on suspended sediment and turbidity in the River Murray, south eastern Australia: multiple lines of evidence.Crossref | GoogleScholarGoogle Scholar |
Smol, J. P., Wolfe, A. P., Birks, H. J., Douglas, M. S., Jones, V. J., Korhola, A., Pienitz, R., Rühland, K., Sorvari, S., Antoniades, D., Brooks, S. J., Fallu, M. A., Hughes, M., Keatley, B. E., Laing, T. E., Michelutti, N., Nazarova, L., Nyman, M., Paterson, A. M., Perren, B., Quinlan, R., Rautio, M., Saulnier-Talbot, E., Siitonen, S., Solovieva, N., and Weckström, J. (2005). Climate-driven regime shifts in the biological communities of arctic lakes. Proceedings of the National Academy of Sciences of the United States of America 102, 4397–4402.
| Climate-driven regime shifts in the biological communities of arctic lakes.Crossref | GoogleScholarGoogle Scholar | 15738395PubMed |
Sonneman, J., Sincock, A., Fluin, J., Reid, M., Newall, P., Tibby, J., and Gell, P. (1999) An illustrated guide to common stream diatom species from temperate Australia. Identification Guide number 33, Cooperative Research Centre for Freshwater Ecology, Sydney, NSW, Australia.
Thoms, M., Ogden, R., and Reid, M. (1999). Establishing the condition of lowland floodplain rivers: a palaeo-ecological approach. Freshwater Biology 41, 407–423.
| Establishing the condition of lowland floodplain rivers: a palaeo-ecological approach.Crossref | GoogleScholarGoogle Scholar |
Tibby, J. (2003). Explaining lake and catchment change using sediment derived and written histories: an Australian perspective. The Science of the Total Environment 310, 61–71.
| Explaining lake and catchment change using sediment derived and written histories: an Australian perspective.Crossref | GoogleScholarGoogle Scholar | 12812731PubMed |
Tibby, J. (2004). Development of a diatom-based model for inferring total phosphorus in southeastern Australian water storages. Journal of Paleolimnology 31, 23–36.
| Development of a diatom-based model for inferring total phosphorus in southeastern Australian water storages.Crossref | GoogleScholarGoogle Scholar |
Tibby, J., Gell, P. A., Fluin, J., and Sluiter, I. R. K. (2007). Diatom–salinity relationships in wetlands: assessing the influence of salinity variability on the development of inference models. Hydrobiologia 591, 207–218.
| Diatom–salinity relationships in wetlands: assessing the influence of salinity variability on the development of inference models.Crossref | GoogleScholarGoogle Scholar |
Tibby, J., Adamson, K., and Kershaw, P. (2020). An 1800-year water-quality and vegetation record from Junction Park Billabong, Murray River, Australia: an assessment of European impacts and sensitivity to climate. Journal of Paleolimnology 63, 159–175.
| An 1800-year water-quality and vegetation record from Junction Park Billabong, Murray River, Australia: an assessment of European impacts and sensitivity to climate.Crossref | GoogleScholarGoogle Scholar |
Tockner, K., Pusch, M., Borchardt, D., and Lorang, M. S. (2010). Multiple stressors in coupled river–floodplain ecosystems. Freshwater Biology 55, 135–151.
| Multiple stressors in coupled river–floodplain ecosystems.Crossref | GoogleScholarGoogle Scholar |
Velghe, K., Vermaire, J. C., and Gregory‐Eaves, I. (2012). Declines in littoral species richness across both spatial and temporal nutrient gradients: a palaeolimnological study of two taxonomic groups. Freshwater Biology 57, 2378–2389.
| Declines in littoral species richness across both spatial and temporal nutrient gradients: a palaeolimnological study of two taxonomic groups.Crossref | GoogleScholarGoogle Scholar |
Wang, R., Dearing, J. A., Langdon, P. G., Zhang, E., Yang, X., Dakos, V., and Scheffer, M. (2012). Flickering gives early warning signals of a critical transition to a eutrophic lake state. Nature 492, 419.
| Flickering gives early warning signals of a critical transition to a eutrophic lake state.Crossref | GoogleScholarGoogle Scholar | 23160492PubMed |