Examining annual catch trends and gear selectivity of bull sharks (Carcharhinus leucas): implications for Queensland’s coastal management
Riley W. Banaghan A * , Alexis L. Levengood A and Bonnie J. Holmes A
A * , Alexis L. Levengood A and Bonnie J. Holmes A
A
Abstract
In coastal waters of eastern Australia, the bull shark (Carcharhinus leucas) is one of the most frequently caught shark species within the Queensland Shark Control Program (QSCP).
An analysis of 27 years of QSCP catch data (1996–2022) involving 2352 bull sharks from nine locations spanning 1700 km captured in gill-nets or on drumlines identified changes in catch composition and gear selectivity.
Linear regression tested trends in length and catchability, and selectivity and bias were evaluated through mean and distribution comparison tests.
Drumlines caught more sharks (80.1%), indicating stronger selectivity for C. leucas of all size classes. A decline in standardised catch per unit effort (CPUE) was noted in tropical regions for each gear type, yet there were increases of bull sharks caught within subtropical gill-nets (P < 0.001). The sex ratio was female biased on drumlines (1.85:1) and in gill-nets (1.53:1).
Our findings corroborated previous research, highlighting the significance of considering regional variations in CPUE, sex ratios and size compositions of sharks caught in shark control programs.
Catch rates provide insights into shark population trends, particularly where gear configurations have remained relatively consistent. Downward trends may indicate unsustainable harvest, or external influences.
Keywords: bather protection, bull shark, Carcharhinus leucas, conservation, drumlines, elasmobranchs, fisheries, gill-net.
Introduction
Elasmobranchs, including sharks, rays and skates, are globally distributed and play multifaceted roles in marine ecosystems (Myers et al. 2007; Pimiento et al. 2023). However, their life-history traits, such as slow growth, delayed reproduction, and low reproductive rates, make many species highly susceptible to anthropogenic exploitation (Hoenig 1990; Dulvy et al. 2017, 2021). Overfishing, habitat degradation and climate change have contributed to widespread declines, with recent global assessments estimating that nearly a third of all elasmobranch species face extinction risk (Dulvy et al. 2008, 2021; Pacoureau et al. 2021), making them one of the most vulnerable vertebrate classes (Ward-Paige et al. 2012). These declines have triggered significant ecological consequences, including trophic cascades and shifts in prey populations (Ferretti et al. 2010; Estes et al. 2011; Ripple et al. 2014; Hammerschlag et al. 2025). As apex predators, sharks exert a top-down influence on food webs, ecosystems and habitats (Heupel et al. 2014; Dulvy et al. 2017), underscoring the urgency of addressing ongoing population declines and the lack of recovery (Ferretti et al. 2010; Roff et al. 2018). Monitoring shark populations is often hindered by sharks’ elusive and wide-ranging nature (Heithaus et al. 2002; Werry et al. 2014) and the expansive geographic scale of marine ecosystems (Baum et al. 2003).
The bull shark (Carcharhinus leucas) belongs to the family Carcharhinidae, commonly known as whaler sharks in Australia (Last 2009), and requiem sharks globally. Among the species within this family, the genus Carcharhinus is the most diverse, comprising 35 of 57 species (Collareta et al. 2022). Being one of the most adaptable large-bodied shark species, bull sharks occupy marine, estuarine and freshwater environments across warm temperate, subtropical and tropical regions (Thorson 1972; Thomerson et al. 1977; Pillans et al. 2005; Smoothey et al. 2023). Currently listed as ‘vulnerable’ by the International Union for Conservation of Nature (IUCN) (Rigby et al. 2021), bull sharks exhibit natal philopatry, with females returning to specific estuarine and freshwater nurseries to give birth (Tillett et al. 2012; Heupel et al. 2015; Smoothey et al. 2019). In Australia, bull sharks are widely distributed along the northern, eastern and western coastlines, with genetic studies indicating high connectivity driven by large-scale male movements and female site fidelity (Devloo-Delva et al. 2023). Ontogenetic shifts in habitat use are well-documented, with juveniles primarily occupying low-salinity nursery areas, whereas subadults and adults undertake extensive seasonal migrations along the coast (Simpfendorfer et al. 2005; Heupel et al. 2010; Heupel and Simpfendorfer 2011).
Bull sharks frequently overlap with human activities, particularly in estuarine and nearshore environments. This overlap has led to increasing human–wildlife conflict, particularly in areas with high recreational and commercial fishing activity (Werry et al. 2012; Smoothey et al. 2023). For example, recent studies on shark depredation in Australia have indicated that bull sharks are contributing to the increasing depredation of fish catches (Mitchell et al. 2018; Vardon et al. 2021; Mitchell et al. 2023). Additionally, their presence in urban waterways, such as the Sydney Harbour and the Brisbane River, has led to concerns over public safety, with a small number of recorded shark attacks in these areas (Smoothey et al. 2019).
Across their range, bull sharks are susceptible to anthropogenic pressures relating to subsistence, recreational and commercial fishing activities, including those related to bather protection programs (Harry et al. 2011; Werry et al. 2012; Dulvy et al. 2021). Illegal targeting of large sharks by foreign fisheries throughout Australian waters (Field et al. 2009; Marinac 2022), along with unregulated, unmonitored exploitation, has played major roles in the over-exploitation of shark fisheries resources (Sumaila et al. 2006). Globally, it is estimated that ~1 million bull sharks are caught annually, equating to ~30,000 tonnes (Mg) (Clarke et al. 2006), and declines in bull shark lengths and population sizes have been reported in many locations (Cliff and Dudley 1991; O’Connell et al. 2007; Haig et al. 2018). Concomitantly, declines in several ‘whaler’ shark populations, which included bull sharks, have been reported in Queensland (Qld) (Dudley 1997; Roff et al. 2018; Henderson et al. 2024). For example, Haig et al. (2018) conducted a species-specific analysis on bull shark populations by using shark control data in Qld (1996–2012), reporting widespread and localised declines in size and abundance. However, despite these historical declines, recent reports suggest a potential increase in juvenile bull shark populations in some regions, possibly linked to warming temperatures and urbanisation (Mullins et al. 2024). Similar trends have been noted in Qld, where both recreational and commercial fishers report an increase in bull shark encounters in local waterways (Vardon et al. 2021). The recent rise in depredation rates in Australian fisheries further highlights the need to reassess bull shark populations and their interactions with human activities (Mitchell et al. 2018, 2023).
Given the scarcity of fisheries-independent monitoring for large sharks, shark control programs provide a valuable long-term dataset for assessing population trends (Sumpton et al. 2011; Haig et al. 2018; Lee et al. 2018, 2019; Niella et al. 2020, 2021; Lopes et al. 2024). Here, we analysed long-term temporal and spatial trends associated within bull shark catch across nine locations spanning 1700 km within the QSCP over a 27-year period (1996–2022). The objectives of this study were to (1) determine whether widespread declines in catch per unit effort (CPUE) within the QSCP are currently evident for bull sharks, (2) identify whether shifts in the average length of bull sharks are occurring within Qld waters, and (3) assess changes in relative catchability and relationships among gear type, location, sex and size of bull sharks. The outcomes of this study will enable a greater understanding of the stock status of bull sharks within Qld, and identify if shark numbers and size-shifts are occurring in coastal waters of Qld.
Methods
Queensland Shark Control Program (QSCP)
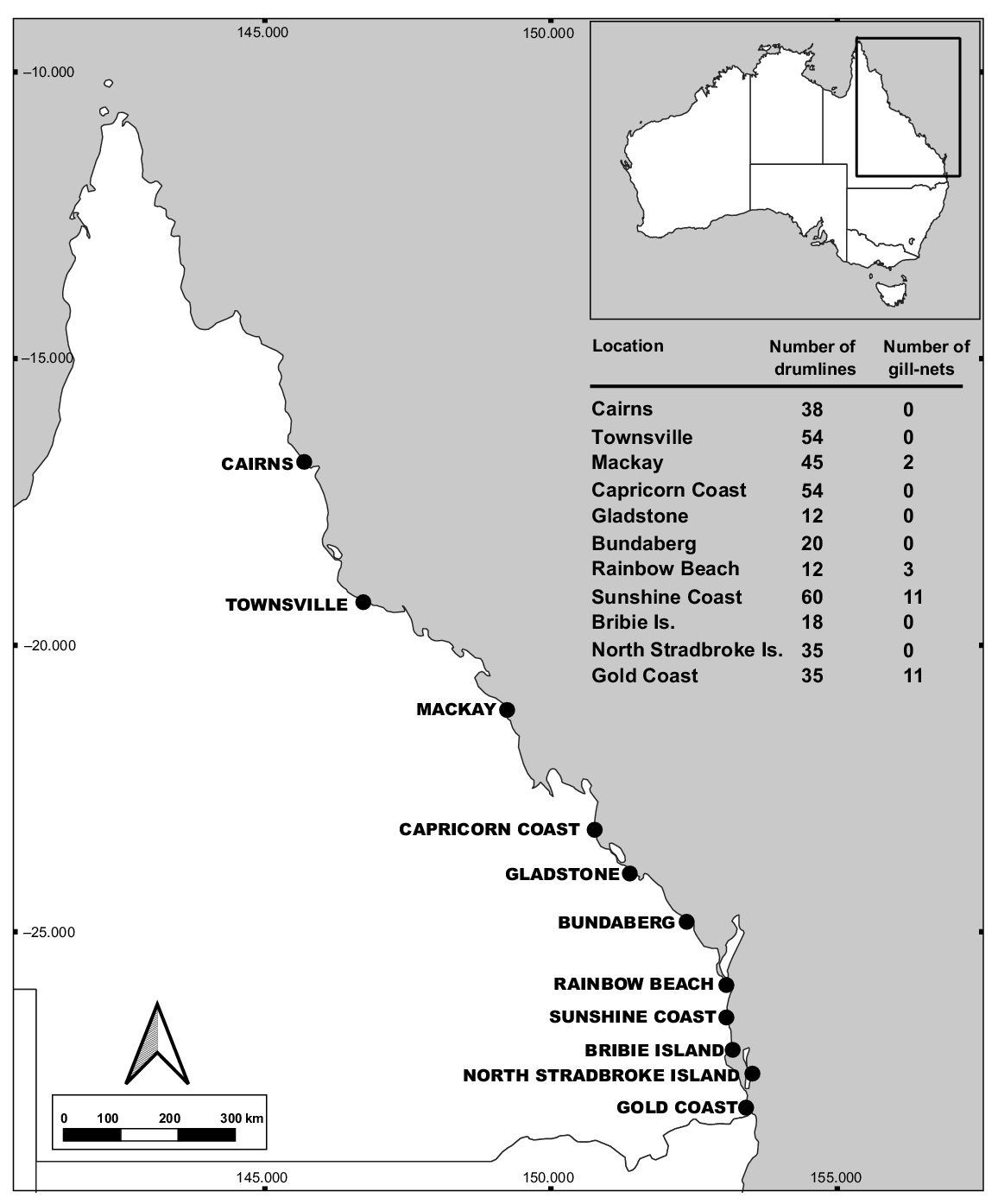
The QSCP extends along the eastern seaboard of Qld, from the Gold Coast to Cairns, using year-round deployments of a combination of gill-nets and drumlines along 85 beaches (Fig. 1). The drumlines are positioned ~500–1000 m from the shoreline and equipped with a single 14/0 J stainless steel hook (Sumpton et al. 2011), baited with ~2 kg of sea mullet (Mugil cephalus) or shark flesh. Gill-nets are deployed at the same distances from shore, running 186 m parallel to the shoreline, with a gauge of 50 cm and a 6-m drop (Holmes et al. 2012; Haig et al. 2018; Werry et al. 2018). All gill-nets used within this program are surface set to minimise entanglements with non-target benthic species (e.g. stingrays) (Sumpton et al. 2011).
The program comprises 10 contract areas, with Bribie Island being placed under the Sunshine Coast contract, with each region consisting of several beaches (Holmes et al. 2012). Independent shark contractors must service the gear every 1–3 days, with all gear being checked 15–20 times monthly (Holmes et al. 2012; Haig et al. 2018; Werry et al. 2018). Each time the gear is serviced, the contractor completes an activity report (Department of Agriculture and Fisheries 2024). When a shark is caught, contractors record key information, including species, sex (presence of claspers in males), total length (m TL), fate, location and gear type (Department of Agriculture and Fisheries 2024). The total length is measured from the tip of the nose to the end of the tail’s upper lobe in a natural position, measured on the dorsal side of the shark. During routine checks, any damaged gear and missing bait are replaced (Haig et al. 2018).
Size classes were identified and split into three life-stage categories. Adults were identified as larger than or equal to 2.2 m TL; this was chosen because the size at maturity for bull sharks was estimated between 2.04 and 2.25 m TL for females and between 1.90 and 2.20 m TL for males (Branstetter and Stiles 1987; Cruz-Martínez et al. 2005). Subadults were classified as those ≥1.75 m TL (but <2.2 m), because this is when bull sharks on the eastern coast of Australia are shown to make greater use of coastal habitats (Niella et al. 2022; Smoothey et al. 2023); juveniles subsequently were categorised as sharks <1.75 m TL, because they are often still site attached to riverine ecosystems (Branstetter and Stiles 1987; Werry et al. 2011).
Throughout a significant portion of its operating history, the species identification process within the QSCP has been considered unreliable, primarily owing to confusion among morphologically similar species commonly caught (Macbeth et al. 2018). For instance, the pig-eye shark (Carcharhinus amboinensis), which is also present in Queensland waters, closely resembles the bull shark and shares extensive habitat overlaps (Last 2009). Consequently, after a systematic review, the QSCP underwent significant changes in species identification protocols in 1996 (34 years after its establishment). Evaluations conducted in 1992 and 1996 by two Ministerial Committees highlighted substantial misidentifications, leading to recommendations for improved training in species-level identification (Gribble et al. 1998). Following these reviews, several specialised courses were introduced to enhance the accuracy of species identification within the program, particularly for whaler sharks (Family Carcharhinidae), to address long-standing classification challenges (Werry et al. 2018).
Given these issues, the data analysed herein were collected post-1995, when identification protocols were improved. However, this presents a significant limitation in reconstructing historical baselines, as the initial population structure and catch composition before these improvements remain uncertain. The lack of reliable species-level data from the program’s first three decades complicates efforts to assess long-term population trends and the full impact of the QSCP. If bull sharks were misidentified at a substantial rate during the early years of the program, the historical catch records may not accurately represent the species’ true abundance and size structure at the time. This uncertainty underscores the need for caution when interpreting long-term declines and highlights the importance of considering potential biases in historical data when assessing population trends.
Since its establishment, the QSCP has undergone significant variations in fishing configurations and effort. Initially, the program employed a large proportion of gill-nets throughout its operating beaches. However, because of unacceptable bycatch rates of non-target species, primarily turtles and dugongs (Sumpton et al. 2011), and a decrease in shark catch (Paterson 1990), they were changed to single-hook drumlines in many regions. To appropriately model CPUE metrics for each gear type, it was essential to adjust for these changes in effort (Fig. S1 of the Supplementary material). Effort data were acquired from the Queensland Department of Agriculture and Fisheries (QDAF), taking into consideration seasonal gear lifting and reconfigurations. To improve the accuracy of the analysis, data for several regions were also filtered and omitted. Specifically, data from the Capricorn Coast region were excluded post-2021 owing to the initiation of the Catch Alert Drumline (CAD) trial, which uses gear configurations different from those of the historical setups (Campbell and Scott-Holland 2023). Additionally, the period immediately preceding this trial (2019–2021) was omitted because of inconsistencies in gear configuration, deployment and servicing, as documented in the CAD trial report (Campbell and Scott-Holland 2023). Rainbow Beach was also excluded from the catch analyses owing to data quality concerns (Holmes et al. 2012), and therefore, the catch rate results here were not considered further. The gear type was recorded into the following three categories: ‘drumline’, ‘gill-net’ and ‘other’. However, the ‘other’ category was omitted because of uncertainty regarding the specific gear used when a shark catch was not properly assigned a gear type. Additionally, effort was not standardised across gear types, meaning that differences in the number of deployed drumlines and gill-nets (Fig. 1) were not accounted for in the analysis. This variation in effort should be considered when interpreting differences in catch rates among gear types.
Statistical analyses
To identify trends within the annual CPUE of bull sharks within the QSCP, the catch information (total sharks caught per year for each region) was fit into generalised linear models (GLMs) within R (ver. 4.3.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/). The significance level of all statistical tests was set at alpha = 0.05. Individual beaches were aggregated by region, allowing for the following nine study regions: Cairns, Townsville, Mackay, Capricorn Coast, Gladstone, Bundaberg, Sunshine Coast, North Stradbroke Island and Gold Coast. The QSCP regions of Bribie Island, Sunshine Coast North and Sunshine Coast South were aggregated to form the Sunshine Coast region. Each gear type (gill-net or drumline) and location were modelled independently. The dependent variable, the number of sharks caught per year, was assumed to follow a negative binomial distribution (Yates et al. 2015). This dependent variable was modelled against year for each location and gear type by using additive models to identify inter-annual trends and patterns within the catch, resulting in nine models assessed. Models included an offset term employed to represent the logarithm effort, quantified as sharks per gill-net per day or sharks per drumline per day. This offset enabled the response variable to be transformed into an adjusted CPUE pooled by year level. The annual adjusted CPUE for tropical and subtropical regions was investigated utilising the same methodology, although beaches were aggregated by latitude. The tropics were considered any location from Cairns to Bundaberg, whereas subtropics were classified as any area from Sunshine Coast to Gold Coast. Bundaberg was included within the tropical region because of the land bridge to the continental shelf, separating it from other subtropical regions; this is also consistent with Werry (2010) to enable direct comparisons.
Linear regression models were also utilised to analyse average length shifts for bull sharks within the QSCP. Sharks were pooled by region, with each gear type being modelled separately. Time-series means were employed to investigate shifts in the annual average length of sharks caught for each gear type for each location. To analyse relationships among gear type, sex, region and size, average lengths were compared across gear, sex and region by using independent-sample Student’s t-tests. Size distributions were grouped by gear type and were compared using a non-parametric Kolmogorov–Smirnov test (Chakravarti et al. 1967). The sex ratios of state-wide aggregated shark catches were tested using a Chi-Square test (χ2).
Results
Over the 27-year (1996–2022) study period, 2352 Carcharhinus leucas individuals were captured within the QSCP. Of these, 55.5% were female and 44.5% were male, with sex ratios significantly deviating from unity for both drumline (χ2 = 13.7, d.f. = 1, P < 0.001) and net catches (χ2 = 20.8, d.f. = 1, P < 0.001), displaying female to male ratios of 1.85:1 and 1.53:1 respectively (Table 1). The size of captured sharks across all gear types and locations ranged from 0.6 to 4.0 m TL, with a mean total length (TL) of 1.8 ± 0.5 m. The largest individual recorded was a 4.0 m TL female, caught on a drumline at the Sunshine Coast. Across age classes, the sex ratio varied, with 0.94:1 for juveniles (χ2 = 0.99, d.f. = 1, P = 0.32), 1.43:1 for subadults (χ2 = 21.37, d.f. = 1, P < 0.001) and 1.87:1 for adults (χ2 = 51.75, d.f. = 1, P < 0.001). Most drumline regions, except for the Capricorn Coast, Sunshine Coast and Townsville, captured significantly more females (Table 1). The capture of sharks on drumlines at North Stradbroke Island was predominantly female (85.1%), and similar trends were observed in the Gold Coast and Bundaberg regions. QSCP nets also showed a higher proportion of females across all regions, although no major skewing towards either sex was observed (i.e. <70% skew) (Table 1). In Townsville, where nets were removed in 2005, both sharks caught were female.
| Location | Drumline catch composition | Gill-net catch composition | ||||||
|---|---|---|---|---|---|---|---|---|
| ♂ (%) | ♀ (%) | Total (n) | ♂ (%) | ♀ (%) | Total (n) | |||
| Tropics | Cairns | 36.9 | 63.1 | 198 | 38.9 | 61.1 | 18 | |
| Townsville | 44.2 | 55.8 | 294 | 0 | 100 | 2 | ||
| Mackay | 43.3 | 56.7 | 261 | 42.2 | 57.8 | 204 | ||
| Capricorn Coast | 55.4 | 44.6 | 697 | |||||
| Gladstone | 44.4 | 55.6 | 196 | |||||
| Bundaberg | 27.1 | 72.9 | 85 | |||||
| Subtropical | Sunshine Coast | 47.4 | 52.6 | 79 | 40.9 | 59.1 | 149 | |
| Point Lookout | 14.9 | 85.1 | 47 | |||||
| Gold Coast | 24.2 | 75.8 | 33 | 32.3 | 67.7 | 96 | ||
| Queensland | 45.8 | 54.2 | 1883 | 39.45 | 60.55 | 469 | ||
Empty rows indicate regions with no gear deployed.
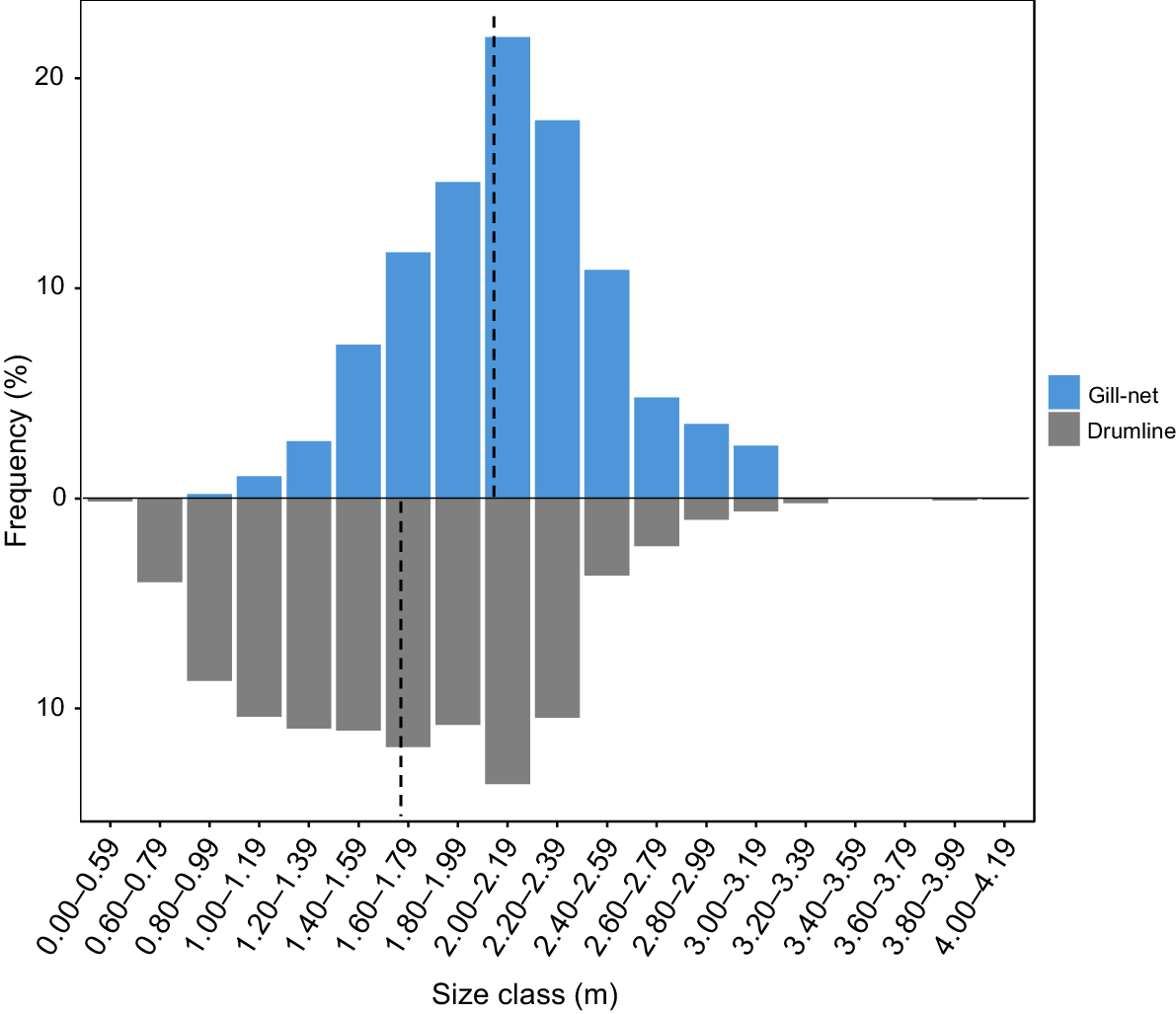
Gill-nets and drumlines exhibited significant differences in their catch composition. Approximately 19.9% (n = 469) of all sharks were caught within gill-nets, and 80.1% (n = 1883) were caught on drumlines (not correcting for effort among gear types). The total length distributions between the two gear types differed significantly (Z, P < 0.001). Mean TL was significantly larger for gill-net-captured sharks (mean TL = 2.04 ± 0.02 m) than for drumline-captured sharks (mean TL = 1.68 ± 0.01 m) (Fig. 2). Gill-nets primarily selected for adults (40.3%) and subadults (38%), whereas drumlines predominantly captured juveniles (53.5%) (Fig. 3). Regionally, the Capricorn Coast accounted for 49.28% (n = 515) of all juveniles caught during the study period (Fig. 3).
Size frequency distribution of C. leucas catch within drumline and gill-nets within the Queensland Sharks Control Program (QSCP), between 1996 and 2022. Mean length for each gear type is denoted by the dashed vertical line.
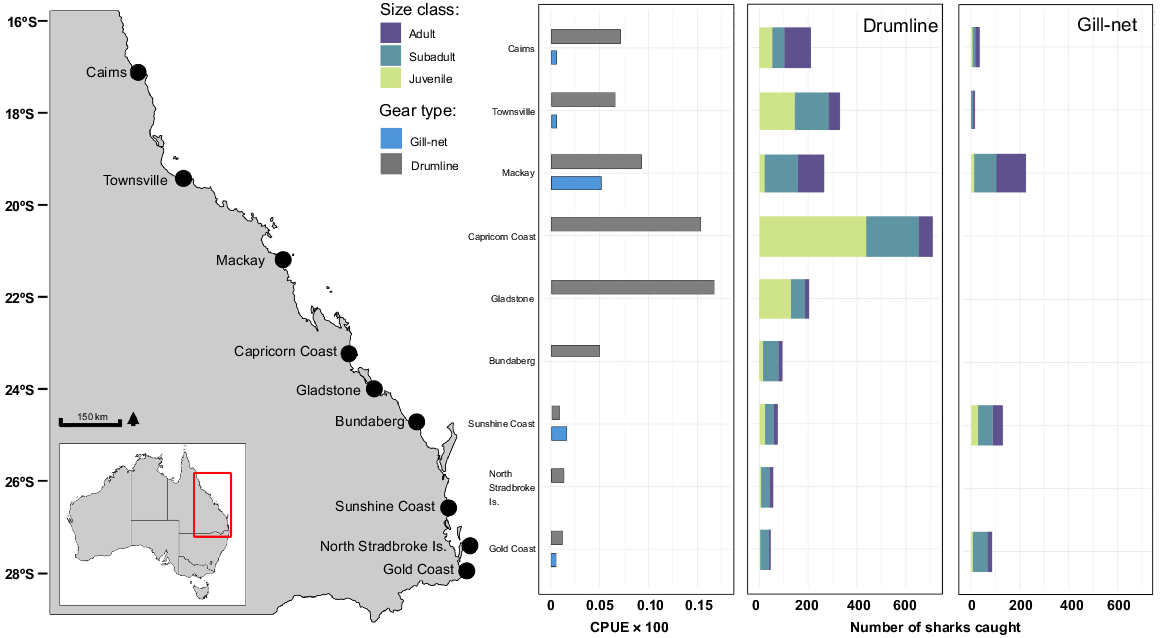
Catch per unit effort of C. leucas (CPUE; number of sharks caught per gear per day × 100) for each deployment area within the Queensland Shark Control Program (QSCP) and total number of sharks caught per each region between 1996 and 2022.
There was a significant interaction between total length and year for drumline and net catches, with notable differences among locations. Declines in annual mean length were observed at Capricorn Coast, Gladstone and Sunshine Coast (Table 2). Further investigation of gill-net catches at specific locations showed significant declines at Cairns, Sunshine Coast and Gold Coast, and a small but significant increase in mean length was detected in the gill-net catch from Mackay (Table 2).
| Area | Location | Gill-net | Drumline | Mean annual length | Mean annual length | Mean annual catch | Gear selectivity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r CPUE | r CPUE | r (net) | r (drumline) | ♂ n | ♀ n | Gill-net | Drumline | |||||
| Min. size (m TL) | Max. size (m TL) | Min. size (m TL) | Max. size (m TL) | |||||||||
| Tropics | Cairns | −0.089 | −0.065 | −0.049 | −0.003 | 3.14 | 5.59 | 0.90 | 2.90 | 0.70 | 3.20 | |
| Townsville | −0.270 | +0.024 | −0.050 | −0.002 | 4.58 | 5.72 | 2.15 | 2.20 | 0.70 | 3.15 | ||
| Mackay | −0.066 | −0.051 | +0.013 | +0.004 | 7.00 | 8.81 | 1.00 | 3.00 | 0.80 | 3.10 | ||
| Capricorn Coast | −0.001 | −0.027 | 14.96 | 12.52 | 0.50 | 3.20 | ||||||
| Gladstone | +0.033 | −0.021 | 3.50 | 3.72 | 0.60 | 2.70 | ||||||
| Bundaberg | +0.019 | −0.007 | 1.40 | 2.50 | 0.96 | 3.00 | ||||||
| Subtropics | Sunshine Coast | +0.032 | −0.001 | −0.026 | −0.047 | 3.52 | 4.00 | 1.00 | 3.10 | 1.00 | 4.00 | |
| Point Lookout | −0.062 | −0.002 | 1.17 | 1.85 | 1.30 | 3.30 | ||||||
| Gold Coast | −0.006 | −0.009 | −0.005 | −0.003 | 1.67 | 3.43 | 1.20 | 2.80 | 1.00 | 2.74 | ||
| Tropics | −0.029 | −0.013 | +0.010 | −0.015 | 27.24 | 29.15 | 0.90 | 3.00 | 0.50 | 3.20 | ||
| Subtropics | +0.017 | −0.019 | −0.015 | −0.021 | 14.96 | 8.84 | 1.00 | 2.80 | 1.00 | 4.00 | ||
| Queensland | −0.009 | −0.016 | −0.008 | −0.015 | 34.69 | 43.54 | 0.90 | 3.10 | 0.50 | 4.00 | ||
Min., mimimum; Max., maximum; TL, total length measured in metres; r, value of regression coefficient. Bold indicates significance (P < 0.05), + or − highlighting the direction of slope.
Analysis of annual trends in drumline CPUE indicated significant differences among areas (P < 0.001). When aggregated into subtropical and tropical zones, CPUE differed significantly between zones (P < 0.001) and years (P < 0.05). Significant declines in drumline CPUE were detected in Cairns, Mackay, North Stradbroke Island and the aggregated tropical areas, whereas increases were observed in Townsville and Gladstone (Fig. 4).
Trends in annual drumline CPUE for C. leucas (as the number of sharks caught per gear per day, with shaded area showing ±s.e.) within the Queensland Shark Control Program (QSCP), displaying regions that showed significant declines or increases in annual CPUE between 1996 and 2022.

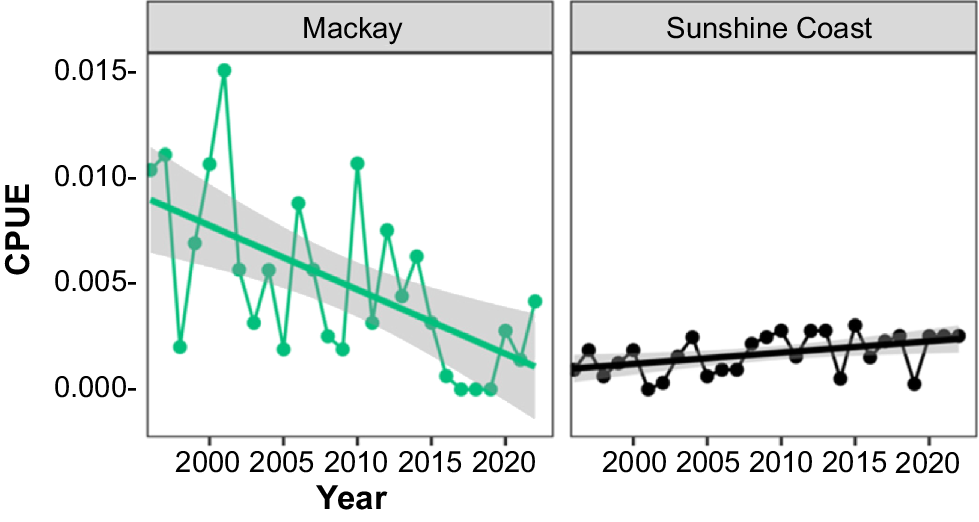
Gill-net CPUE varied significantly among areas (P < 0.001). When aggregated into subtropical and tropical zones, there were significant regional differences (P < 0.001), but no significant trend was detected in state-wide gill-net catches (P = 0.307). However, Mackay experienced significant declines in CPUE over the study period (P < 0.001), whereas the Sunshine Coast showed a significant increase (P < 0.05) (Fig. 5).
Discussion
Shark control programs aim to reduce the risk of shark encounters in nearshore areas by culling or capturing potentially dangerous sharks by using methods such as drumlines and gill-nets. By lowering shark numbers near popular beaches, these programs seek to minimise the chance of negative interactions with humans. However, removing large apex predators can significantly affect ecosystem functioning and ecological balance (Estes et al. 2011; Ripple et al. 2014; Henderson et al. 2024; Hammerschlag et al. 2025). Despite the widespread distribution of sharks globally, comprehensive data on their long-term population trends and the extent of their declines remain limited (Clarke et al. 2006; Ferretti et al. 2010; Roff et al. 2016; Dulvy et al. 2024). This lack of knowledge makes it challenging to effectively monitor and understand the broader ecological consequences of their removal.
Analysing catch size and composition changes over time can provide insights into the exploitation or recovery of fish stocks (Jennings and Kaiser 1998; Guillemin et al. 2025). Additionally, assessing catch rates alongside biological characteristics offers a deeper understanding of a population trends (Maunder et al. 2006; Pacoureau et al. 2021), whereas variations in catch composition can indicate ecosystem status and community change (Greenstreet and Rogers 2006; Hammerschlag et al. 2019). Understanding the stock composition for important coastal species is critical given the burgeoning global shift in climate baselines (McHenry et al. 2019; Niella et al. 2022). By analysing 27 years of catch data from a state-wide shark control program, significant long-term trends in catch rates of bull sharks have been elucidated herein.
Gear-specific trends and catchability of bull shark catch
Gear selectivity significantly influences shark catch composition in fisheries and shark control programs (Dudley and Simpfendorfer 2006; Sumpton et al. 2011). A critical finding of this study is that the QSCP, despite targeting large, potentially dangerous sharks, predominantly captures juveniles and subadult bull sharks. This discrepancy between target (large adult sharks) and actual catch (primarily juveniles) raises important questions about the program’s effectiveness and ecological impact. In the QSCP, drumlines captured a higher proportion of juveniles (53.7%) and accounted for 80.1% of bull shark catches; however, effort was not standardised across gear types. Similar patterns were observed by Haig et al. (2018), where drumlines caught more bull sharks but selected for smaller individuals. By contrast, KwaZulu–Natal (KZN) South African’s shark control program found that gill-nets were far more effective for bull sharks than drumlines (Cliff and Dudley 2011), aligning with Sumpton et al. (2011), who identified bull sharks as the most susceptible target species in Queensland gill-nets. Standardised catch rates in south-eastern Queensland also support this trend (Lopes et al. 2024). Additionally, in the KZN shark control program, drumlines tended to catch smaller bull sharks than did gill-nets (Cliff and Dudley 2011), reinforcing global trends in gear efficiency and selectivity.
The higher number of smaller bull sharks caught on drumlines is likely to reflect a combination of species-specific behavioural traits, environmental factors and gear configurations. Bull sharks are highly opportunistic predators with strong olfactory sensitivity (Tricas and Sisneros 2004; Heupel and Simpfendorfer 2008), potentially making them susceptible to baited gear. Additionally, drumlines are often positioned near estuarine-influenced areas where juveniles are more common (Heupel and Simpfendorfer 2011). Seasonal movements, competition and environmental factors such as turbidity and water temperature may also drive catch selectivity (Lopes et al. 2024). Moreover, the larger number of beaches with drumlines than those with gill-nets may also help explain the higher catch rates.
This juvenile-biased selectivity has ecological implications, as sustained removals could contribute to recruitment overfishing (Diekert 2012) and alter population structures over time (Shin et al. 2005; Greenstreet and Rogers 2006; Svedäng and Hornborg 2017). The gear disparity in catch composition has key management implications. Drumlines appear more effective at targeting bull sharks, but if the goal is to remove larger, potentially more dangerous individuals, modifications such as adjusting hook size, bait type, or deployment depth may be necessary. Similar adjustments have been made in South Africa to gear deployments to help further refine selectivity (Cliff and Dudley 2011). Recent work by Lopes et al. (2024) on bull shark catch rates along eastern Australia further supports the need for strategic gear deployment based on seasonal habitat use patterns.
Length trends and composition across sites
Size trends in shark populations provide key insights into population dynamics and potential shifts in species composition over time (Field et al. 2012). In the QSCP, the predominance of juvenile bull sharks suggests that (1) gear type influences selectivity (e.g. hook size on drumlines), (2) estuarine habitats used by juveniles are near QSCP deployment sites, or (3) the recruiting stock is dominated by younger individuals. Similar declines in the mean total length (TL) of sharks have been documented in multiple regions, including in KZN program (Cliff and Dudley 1991), the QSCP up to 2012 (Haig et al. 2018), and the Gulf of Mexico, where bull shark length decreased in fishing tournaments over an 80-year period (Powers et al. 2013). These findings suggest a widespread trend of a declining shark size in response to fishing pressure and habitat changes.
Our study observed significant declines in the mean total length (TL) of bull sharks in Cairns, Sunshine Coast, Gladstone and Capricorn Coast, suggesting shifts in community composition towards smaller individuals and providing insights into the contemporary dynamics of these coastal shark populations. Annual length declines of 1.5 cm (cm) per year have been identified from statewide QSCP catch, with consistent patterns of decline shown throughout subtropical and tropical regions, as identified herein. This declining average TL aligns with global trends across several shark species in both commercial fisheries and shark control programs (blacknose shark, Carcharhinus acronotus; dusky shark, Carcharhinus obscurus; silky shark, Carcharhinus falciformis; sandbar shark, Carcharhinus plumbeus, Benavides et al. 2021; tiger sharks, Galeocerdo cuvier; whaler sharks, Roff et al. 2018). Similar reductions in the annual average length of bull sharks have been reported by Cliff and Dudley (1991) in the KZN program and by Haig et al. (2018) in the QSCP (up to 2012). Additionally, research in the Gulf of Mexico has indicated that bull shark length decreased during fishing tournaments over an 80-year period (Powers et al. 2013). Our findings indicated that the length trends Haig et al. (2018) observed have continued to decline over the past 10 years, indicating that there are ongoing shifts in the eastern Australian bull shark population that interact with the QSCP. Although the QSCP has been operational since 1962, these continued trends may reflect delayed or compounding effects of gear interaction on population structure. The consistent removal of larger and older individuals can truncate a stock’s structure, leading to fluctuations in abundances and making populations more volatile and less resilient (Anderson et al. 2008; Botsford et al. 2014; Secor et al. 2015). Interestingly, we identified significant increases in the average length of bull sharks in Mackay, a central Qld location known for high prey productivity and large number of estuaries (Haig et al. 2018). It may be that habitats such as these have been integral to maintaining the viability of the broader population, facilitating recovery of the stock from the individuals removed.
The observed declines in average length across the QSCP have significant ecological implications. Smaller average lengths indicate younger sharks, reducing the population’s overall reproductive output and resilience. This trend can lead to trophic downgrading, where the loss of large apex predators disrupts the balance of marine ecosystems, potentially causing an increase in the mesopredator population and subsequent declines in biodiversity (Hammerschlag et al. 2025). Shifts towards smaller sizes in shark populations have been linked to anthropogenic exploitation (Rago et al. 1998; Bradshaw et al. 2008; Roff et al. 2018), possibly influenced by the size-selectivity of fishing gear and improved technologies (e.g. side sonar, electric reels) (McLoughlin and Stevens 1994; Stevens et al. 2000). Our results are consistent with long-term declines in abundance and average length of coastal shark species in QSCP (Roff et al. 2018; Henderson et al. 2024) and highlight the need for adaptive management strategies. The observed changes within nearshore communities are likely to indicate anthropogenically induced trophic cascades (Henderson et al. 2024). Specifically, the shifts composition of juveniles may signal changes in population structure or habitat use, suggesting that these habitats serve as critical nursery regions. Localised shifts in catch composition necessitate targeted coastal management actions, such as refining spatial management zones, adjusting gear types or implementing additional protections in these important shark habitats.
Previous studies have established linkages between nearshore fisheries production and estuarine habitat structures (Lee 2004; Manson et al. 2005; Lefcheck et al. 2019), including relationships with the extent of intertidal habitat and mangrove cover (Lee 2004; Heithaus et al. 2009). Juvenile bull sharks rely on nearshore coastal environments as nursery habitats, providing protection and foraging opportunities (Simpfendorfer et al. 2005; Heupel and Simpfendorfer 2008; Heupel et al. 2010; Curtis et al. 2011; Bangley et al. 2018; Niella et al. 2022). Here, we found that catch of juvenile bull sharks on drumlines in waters off the Capricorn Coast waters was significantly higher than at all other QSCP locations. The Capricorn Coast region supports the greatest wetland area of all tropical sites and the second-highest area within all QSCP sites (Haig et al. 2018). The positive influence of wetlands on bull shark CPUE, as documented by Haig et al. (2018), indicates that the proximity of the QSCP gear to this area is likely to be driving the high juvenile catch rates.
Trends in CPUE
Bull sharks were caught in all QSCP locations, indicating a widespread distribution along the coastline. Regions within the tropics (north of Bundaberg <24.8°S) exhibited consistently greater CPUE of bull sharks than regions south of this point. A similar relationship can be seen in the northern hemisphere, where higher abundances were observed within the lower latitudinal waters off the Gulf of Mexico (~25°N) than in the higher latitudinal regions off Florida (~28°N) (Curtis et al. 2011). The nearshore tropical regions of eastern Australia are characterised by consistently warm temperatures, shallow waters (<50 m deep), and a distinct monsoon period. Various factors such as prey availability (Hammerschlag et al. 2012; Lubitz et al. 2023), water temperature (Smoothey et al. 2016; Niella et al. 2020; Lopes et al. 2024), rainfall (Werry et al. 2018) and habitat features (Haig et al. 2018) have been shown to influence the distribution of bull sharks. These sharks prefer warm waters at all life stages, with juveniles being observed to shift nursery areas in response to global climate change (Bangley et al. 2018; Mullins et al. 2024). Water temperature influences both the fine-scale habitat selections and large-scale migrations of bull sharks (Werry et al. 2018; Espinoza et al. 2021; Smoothey et al. 2023). Notwithstanding, changes in ocean conditions owing to climate-induced warming waters may affect these patterns. A study by Niella et al. (2020) off eastern Australia indicated a 3-month increase in the availability of favourable water temperatures for bull sharks at higher latitudes by 2030 under modelled future climate scenarios. These shifts in habitat use may lead to an increase within CPUE for higher latitudes within the QSCP and, consequently, possible increases in human–shark interactions within south-eastern Qld.
Local population fluctuations with significant inter-annual CPUE variations were observed across regions and gear types. Notably, over the study period, CPUE declines were seen in Cairns, Mackay and North Stradbroke Island, whereas Gladstone, Sunshine Coast and Townsville displayed significant CPUE increases. As noted in other studies, inter-annual bull shark catch rates are highly variable, influenced by environmental changes, prey availability and female philopatry (Dudley and Simpfendorfer 2006; Hammerschlag et al. 2012; Haig et al. 2018). In an unpublished study by Werry (2010), the reported QSCP bull shark catch rates from 1996 to 2006 had several key similarities to our findings herein. First, we identified CPUE increases within Townsville, as well as increases in catch at several tropical locations, which were attributed to several key environmental factors (Werry 2010). Werry (2010) suggested that unusually low rainfall between 1991 and 1995 (annual average 368 mm for the Ross River, Townsville) followed by substantial rainfall from 1996 to 2006 (annual average 995.4 mm for the Ross River) could account for the significant increases in juvenile catch. Werry (2010) also showed that peak catches for Cairns, Townsville, Mackay and Gladstone were attributed to peak rainfall years. Environmental drivers, such as rainfall, significantly influence bull shark occurrences, with salinity a known driver of fine-scale habitat use within juveniles (Schlaff et al. 2014). Both adult and juvenile bull sharks may utilise these lower salinity habitats to a higher extent after significant rain events to reduce inter-species competition, because they can tolerate low salinity for prolonged periods (Pillans et al. 2008). These relationships may contribute to our observed widespread inter-annual variations in catch rates, but our understanding of these drivers remains limited to very few empirical studies.
The observed fluctuations in localised CPUE are likely to be influenced, at least partly, by the highly complex and variable occurrence patterns of bull sharks, driven by several intrinsic and extrinsic factors (Werry et al. 2011; Heupel et al. 2015; Espinoza et al. 2016). These occurrence patterns involve large-scale migration (Heupel et al. 2015), residency (Heupel and Simpfendorfer 2008; Brunnschweiler and Barnett 2013), site fidelity (Brunnschweiler and Baensch 2011), regional philopatry (Tillett et al. 2012; Sandoval Laurrabaquio-A et al. 2019) and partial migrations (Espinoza et al. 2016). Intra-specific variations and the complex behaviours of bull sharks are likely to influence trends in CPUE within a fixed-effort fishery. Acoustic tracking studies have shown that bull sharks undergo partial migrations, meaning that some individuals will display residency whereas others undertake large migrations (Espinoza et al. 2016, 2021). This partial migration phenomenon is also seen in other large shark species along the eastern coast of Australia (e.g. tiger sharks, Galeocerdo cuvier; Holmes et al. 2014; Werry et al. 2014) and could explain some of the inter-annual variations displayed within nearshore catch rates of bull sharks. Additional data are needed to accurately elucidate the drivers behind these significant inter-annual trends within CPUE. Correlating tracking data with environmental information (e.g. rainfall, temperature) and fishing mortality is key to identifying potential drivers causing these trends. Although observed reductions in catch rates are consistent with localised stock depletions, cautious interpretations are needed, given the significant inter-annual variations in CPUE in the shark control data. Owing to the unknown levels of connectivity between coastal shark populations in Qld and accurate descriptions of the key drivers, interpretation of local catch data in isolation could result in very different conclusions than those made from data across wider regions.
Sex ratios
Sexual and size segregation have been identified in numerous oceanic and coastal shark species, such as bull, tigers, scalloped hammerheads (Sphyrna lewini), shortfin mako (Isurus oxyrinchus), blue shark (Prionace glauca) and blacktip reef sharks (Carcharhinus melanopterus) (Mucientes et al. 2009; Noriega et al. 2011; Werry et al. 2011; Werry and Clua 2013). Such intra-specific variability in behaviour and movement occurs for various reasons, including predator avoidance, reduced competition, differing nutritional requirements, or reproduction; however, these are highly context dependent (Lubitz et al. 2022). This study observed a significant sex bias in the state-wide program, with the catch of bull sharks predominately consisting of females across all life stages except juvenile. Female bias has been observed within whaler shark populations throughout the NSW Shark Meshing Program (Reid et al. 2011) and the KZN program (Cliff and Dudley 1991). One possible explanation for the observed sex bias is the unique reproductive strategy of bull sharks. Previous studies have shown that females return to rivers to pup bi-annually (Werry et al. 2011, 2012; Tillett et al. 2012), with gravid adult females using shallow waters (<5 m) for parturition (Lea et al. 2015). Espinoza et al. (2016) highlighted intra-specific differences, reporting that mature female bull sharks in the central Great Barrier Reef undertake wider migratory movements than do males. Our data indicated that sexual segregation occurs at the point nearing reproductive maturity, suggesting reproduction could be driving intra-specific variation in habitat use on the Australian eastern coast. Mating and courtship in sharks are often aggressive, with males inflicting significant bite wounds on females (Klimley 1987). This aggression may lead to sexual segregation in some species, because it can increase female fitness by reducing mating frequency (Mucientes et al. 2009). It is possible that bull shark harassment by males influences female sharks to display avoidance behaviours, which Mucientes et al. (2009) proposed to cause geographic-scale segregation within shortfin makos. Significant sex-based spatial segregation has also been observed in other shark species in eastern Australia (e.g. scalloped hammerheads, Noriega et al. 2011; tiger sharks, Holmes et al. 2012), and understanding the fine-scale dynamics of this phenomenon highlights the need for further research. Migrations and habitat use are complex and context-dependent, influenced by environmental variability and prey dynamics (Lubitz et al. 2023). Long-term tracking can help elucidate how factors such as size, sex and environmental context drive habitat choice and spatial segregation in sharks.
Management implications and future research
The observed declines in CPUE, average length and the significant sex bias in catch, highlight the need for further research to assess the complexities of assessing stock status of bull shark populations off the Australian eastern coast. Understanding gear selectivity and CPUE trends is essential for effective fisheries management (McClanahan and Mangi 2004; Sampson 2014; Maunder et al. 2020). Despite shark control programs being designed to catch and remove large, potentially dangerous, sharks from the population, the majority of bull sharks caught and killed in the QSCP during the study period were juveniles or immature subadults. This discrepency between target (large adults) and actual (primarily juveniles) catch suggests that current methods are not optimally addressing the program’s primary objective of reducing human–shark conflict.
On the basis of our findings, we recommend several management actions to improve the effectiveness of the program and reduce ecological impacts, including the following:
Modify gear specifications. Increasing hook sizes on drumlines could reduce juvenile captures while maintaining effectiveness for larger target sharks.
Alternative technologies. Integrate non-lethal alternatives such as expanding catch alert drumline (CAD) trial (which allow live release), electromagnetic deterrents and drone surveillance in high shark occurrence areas identified in this study.
Targeted research program. Implement a comprehensive tagging and tracking program for released individuals to better understand movement patterns and habitat use, which could inform more precise gear placement.
Regular review of effectiveness. Establish ongoing assessment of whether catch composition aligns with program objectives, with metrics to evaluate both safety outcomes and ecological impacts.
Local eastern coast commercial shark fishers also predominantly target juvenile bull sharks for flake and are fished for and landed within estuaries (Niella et al. 2020). Queensland’s shark fisheries operate under a gauntlet-style approach, in which only a subset of the population (small sharks) are subject to fisheries harvest (Kinney and Simpfendorfer 2009). This mangement style is strategised to conserve populations of long-lived, slow-to-reproduce, shark species (Simpfendorfer 1999). However, regional depletion in catch and long-term shifts in shark length indicate that the extraction of mature bull sharks is still frequent enough that these declines are being observed.
In the Gulf of Mexico, juvenile bull sharks have shown significant population increases in recent years, thriving in response to the region’s warming climate (Bangley et al. 2018; Mullins et al. 2024). Given that both QSCP and shark fisheries in the region are reporting large proportions of juveniles within catches, we must consider that these shifts may be driven by several successful pupping and recruitment pulses to the fishery over the past few years. Additionally, with south-eastern Queensland having been identified as a global climate change hotspot (Hughes and Steffen 2017), population shifts similar to those observed in the USA may also be occurring here. Anecdotally, local fishers are also reporting increases in shark abundance and depredation events in both recreational and commercial fishing pursuits. Future research should focus on identifying the species responsible for depredating catches, along with improved local population estimates and relatedness to gain insights into how bull sharks use nearshore regions of Qld. This information will be critical in determining whether the observed fluctuations within regional CPUE indicate population-level changes in abundance.
It is important to acknowledge that the reliance on catch data from control programs may introduce bias because of non-random sampling methods. Additionally, the analysis did not account for environmental variables, which influence shark distributions (Smoothey et al. 2023). The limitation of data starting from 1996, rather than the program’s inception in 1962, means we may be observing only part of the population response to long-term fishing pressure. Comparisons with the KZN shark control program, which documented significant catch declines in the first 5 years of implementation (Cliff and Dudley 1991), suggest that our analysis may underestimate the true extent of population impacts. Contemporary research efforts should incorporate these external factors to assess the drivers of bull shark CPUE shifts more accurately. Given the projected range shifts driven by water temperature for marine species, investigations into environmental drivers and ecological baselines for the species in Qld waters are advised, particularly considering the displayed variations in CPUE of the species temporally and spatially.
Species identification
A notable limitation of this study is the inherent difficulty in distiniguishing between bull sharks (C. leucas) and pig-eye sharks (C. amboinensis) at sea. Despite training provided to contractors, morphological similarities between these species, particularly in juvenile stages, create potential for misidentification. This challenge has been documented in shark control programs globally (Cliff and Dudley 2011; Reid et al. 2011) and may influence the accuracy of our species-specific catch data and subsequent trends.
To address this limitation in future research and strengthen confidence in species-specific trends, we recommend implementing validation protocols, including the following:
Genetic verification through tissue sampling of a subset of captured individuals, particularly in regions where both species co-occur.
Standardised photographic documentation of key diagnostic features to allow for expert verification.
Regular assessment of identification accuracy through blind tests with contractors to quantify and potentially correct for misidentification rates.
These validation measures would strengthen confidence in species-specific catch data and trends, providing more robust information for management decisions. The implementation of these methods would be particularly valuable given the different ecological roles and conservation statuses of these two species and the need for species-specific management approaches.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This work was funded under the Sunshine Coast Bull Shark Program, led by the University of the Sunshine Coast, with partners the Queensland Department of Agriculture and Fisheries, Sunshine Coast Council, Noosa Biosphere Reserve Foundation and Sea Life Mooloolaba.
Acknowledgements
We acknowledge Elders past and present, who are the traditional custodians of the lands and waters, on which this work was conducted. We thank the QSCP contractors throughout Queensland for their efforts in collecting the data utilised within this study. We also thank Dr Tracey Scott-Holland and Dr Matthew Campbell from the Queensland Government: Department of Agriculture and Fisheries, for access to the historical QSCP data, historical effort information, and insights into the program’s operational history.
References
Anderson CNK, Hsieh C-H, Sandin SA, Hewitt R, Hollowed A, Beddington J, May RM, Sugihara G (2008) Why fishing magnifies fluctuations in fish abundance. Nature 452(7189), 835-839.
| Crossref | Google Scholar | PubMed |
Bangley CW, Paramore L, Shiffman DS, Rulifson RA (2018) Increased abundance and nursery habitat use of the bull shark (Carcharhinus leucas) in response to a changing environment in a warm-temperate estuary. Scientific Reports 8(1), 6018.
| Crossref | Google Scholar |
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299(5605), 389-392.
| Crossref | Google Scholar | PubMed |
Benavides MT, Fodrie FJ, Fegley SR, Bargione G (2021) Size changes within a southeastern United States coastal shark assemblage: 1975–2018. Marine and Coastal Fisheries 13(3), 228-239.
| Crossref | Google Scholar |
Botsford LW, Holland MD, Field JC, Hastings A (2014) Cohort resonance: a significant component of fluctuations in recruitment, egg production, and catch of fished populations. ICES Journal of Marine Science 71(8), 2158-2170.
| Crossref | Google Scholar |
Bradshaw CJA, Fitzpatrick BM, Steinberg CC, Brook BW, Meekan MG (2008) Decline in whale shark size and abundance at Ningaloo reef over the past decade: the world’s largest fish is getting smaller. Biological Conservation 141(7), 1894-1905.
| Crossref | Google Scholar |
Branstetter S, Stiles R (1987) Age and growth estimates of the bull shark, Carcharhinus leucas, from the northern Gulf of Mexico. Environmental Biology of Fishes 20(3), 169-181.
| Crossref | Google Scholar |
Brunnschweiler JM, Baensch H (2011) Seasonal and long-term changes in relative abundance of bull sharks from a tourist shark feeding site in Fiji. PLoS ONE 6(1), e16597.
| Crossref | Google Scholar |
Brunnschweiler JM, Barnett A (2013) Opportunistic visitors: long-term behavioural response of bull sharks to food provisioning in Fiji. PLoS ONE 8(3), e58522.
| Crossref | Google Scholar |
Campbell MJ, Scott-Holland TB (2023) Queensland shark control program: catch alert drumline trial 2022–2023. Technical Report. (State of Queensland: Brisbane, Qld, Australia) Avaliable at https://www.publications.qld.gov.au/dataset/shark-control-research/resource/1b3116fd-f8f5-45a3-8a14-fe4f09b9c53a
Clarke SC, McAllister MK, Milner-Gulland EJ, Kirkwood GP, Michielsens CGJ, Agnew DJ, Pikitch EK, Nakano H, Shivji MS (2006) Global estimates of shark catches using trade records from commercial markets. Ecology Letters 9(10), 1115-1126.
| Crossref | Google Scholar | PubMed |
Cliff G, Dudley SFJ (1991) Sharks caught in the protective gill nets off Natal, South Africa. 4. The bull shark Carcharhinus leucas Valenciennes. South African Journal of Marine Science 10(1), 253-270.
| Crossref | Google Scholar |
Cliff G, Dudley SFJ (2011) Reducing the environmental impact of shark-control programs: a case study from KwaZulu–Natal, South Africa. Marine and Freshwater Research 62(6), 700-709.
| Crossref | Google Scholar |
Collareta A, Kindlimann R, Baglioni A, Landini W, Sarti G, Altamirano A, Urbina M, Bianucci G (2022) Dental morphology, palaeoecology and palaeobiogeographic significance of a new species of requiem shark (genus Carcharhinus) from the lower Miocene of Peru (east Pisco Basin, chilcatay formation). Journal of Marine Science and Engineering 10(10), 1466.
| Crossref | Google Scholar |
Cruz-Martínez A, Chiappa-Carrara X, Arenas-Fuentes V (2005) Age and growth of the bull shark, Carcharhinus leucas, from southern gulf of Mexico. Journal of Northwest Atlantic Fishery Science 35(13), 367-374.
| Crossref | Google Scholar |
Curtis TH, Adams DH, Burgess GH (2011) Seasonal distribution and habitat associations of bull sharks in the Indian river lagoon, Florida: a 30-year synthesis. Transactions of the American Fisheries Society 140(5), 1213-1226.
| Crossref | Google Scholar |
Department of Agriculture and Fisheries (2024) QFish. (Department of Agriculture and Fisheries, State of Queensland: Brisbane, Qld, Australia) Available at https://qfish.fisheries.qld.gov.au/Help [Verified 10 September 2024]
Devloo-Delva F, Burridge CP, Kyne PM, Brunnschweiler JM, Chapman DD, Charvet P, Chen X, Cliff G, Daly R, Drymon JM, Espinoza M, Fernando D, Barcia LG, Glaus K, González-Garza BI, Grant MI, Gunasekera RM, Hernandez S, Hyodo S, Jabado RW, Jaquemet S, Johnson G, Ketchum JT, Magalon H, Marthick JR, Mollen FH, Mona S, Naylor GJP, Nevill JEG, Phillips NM, Pillans RD, Postaire BD, Smoothey AF, Tachihara K, Tillet BJ, Valerio-Vargas JA, Feutry P (2023) From rivers to ocean basins: the role of ocean barriers and philopatry in the genetic structuring of a cosmopolitan coastal predator. Ecology and Evolution 13(2), 1-22.
| Crossref | Google Scholar |
Diekert FK (2012) Growth overfishing: the race to fish extends to the dimension of size. Environmental and Resource Economics 52, 549-572.
| Crossref | Google Scholar |
Dudley SFJ (1997) A comparison of the shark control programs of New South Wales and Queensland (Australia) and Kwazulu–Natal (South Africa). Ocean & Coastal Management 34(1), 1-27.
| Crossref | Google Scholar |
Dudley SFJ, Simpfendorfer CA (2006) Population status of 14 shark species caught in the protective gillnets off KwaZulu–Natal beaches, South Africa, 1978–2003. Marine and Freshwater Research 57(2), 225-240.
| Crossref | Google Scholar |
Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C, Martinez J, Musick JA, Soldo A, Stevens JD, Valenti S (2008) You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems 18(5), 459-482.
| Crossref | Google Scholar |
Dulvy NK, Simpfendorfer CA, Davidson LNK, Fordham SV, Bräutigam A, Sant G, Welch DJ (2017) Challenges and priorities in shark and ray conservation. Current Biology 27(11), R565-R572.
| Crossref | Google Scholar | PubMed |
Dulvy NK, Pacoureau N, Rigby CL, Pollom RA, Jabado RW, Ebert DA, Finucci B, Pollock CM, Cheok J, Derrick DH, Herman KB, Sherman CS, VanderWright WJ, Lawson JM, Walls RHL, Carlson JK, Charvet P, Bineesh KK, Fernando D, Ralph GM, Matsushiba JH, Hilton-Taylor C, Fordham SV, Simpfendorfer CA (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Current Biology 31(21), 4773-4787.e8.
| Crossref | Google Scholar | PubMed |
Dulvy NK, Pacoureau N, Matsushiba JH, Yan HF, VanderWright WJ, Rigby CL, Finucci B, Sherman CS, Jabado RW, Carlson JK, Pollom RA, Charvet P, Pollock CM, Hilton-Taylor C, Simpfendorfer CA (2024) Ecological erosion and expanding extinction risk of sharks and rays. Science 386(6726), eadn1477.
| Crossref | Google Scholar |
Espinoza M, Heupel MR, Tobin AJ, Simpfendorfer CA (2016) Evidence of partial migration in a large coastal predator: opportunistic foraging and reproduction as key drivers? PLoS ONE 11(2), e0147608.
| Crossref | Google Scholar |
Espinoza M, Lédée EJI, Smoothey AF, Heupel MR, Peddemors VM, Tobin AJ, Simpfendorfer CA (2021) Intra-specific variation in movement and habitat connectivity of a mobile predator revealed by acoustic telemetry and network analyses. Marine Biology 168(6), 80.
| Crossref | Google Scholar |
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333(6040), 301-306.
| Crossref | Google Scholar | PubMed |
Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK (2010) Patterns and ecosystem consequences of shark declines in the ocean. Ecology Letters 13(8), 1055-1071.
| Crossref | Google Scholar | PubMed |
Field IC, Meekan MG, Buckworth RC, Bradshaw CJA (2009) Protein mining the world’s oceans: Australasia as an example of illegal expansion-and-displacement fishing. Fish and Fisheries 10(3), 323-328.
| Crossref | Google Scholar |
Field IC, Buckworth RC, Yang G-J, Meekan MG, Johnson G, Stevens JD, Pillans RD, McMahon CR, Bradshaw CJA (2012) Changes in size distributions of commercially exploited sharks over 25 years in northern Australia using a bayesian approach. Fisheries Research 125-126, 262-271.
| Crossref | Google Scholar |
Greenstreet SPR, Rogers SI (2006) Indicators of the health of the North Sea fish community: identifying reference levels for an ecosystem approach to management. ICES Journal of Marine Science 63(4), 573-593.
| Crossref | Google Scholar |
Gribble NA, McPherson G, Lane B (1998) Effect of the Queensland shark control program on non-target species: whale, dugong, turtle and dolphin: a review. Marine and Freshwater Research 49, 645-651.
| Crossref | Google Scholar |
Guillemin TA, Pepperell JG, Schilling HT, Williamson JE (2025) 90 years of catch data reveal changes in catch composition in the Australian east coast recreational marlin fishery. Reviews in Fish Biology and Fisheries 35, 371-389.
| Crossref | Google Scholar |
Haig JA, Lambert GI, Sumpton WD, Mayer DG, Werry JM (2018) Habitat features influence catch rates of near-shore bull shark (Carcharhinus leucas) in the Queensland shark control program, Australia 1996–2012. Estuarine, Coastal and Shelf Science 200, 289-300.
| Crossref | Google Scholar |
Hammerschlag N, Luo J, Irschick DJ, Ault JS (2012) A comparison of spatial and movement patterns between sympatric predators: bull sharks (Carcharhinus leucas) and Atlantic tarpon (Megalops atlanticus). PLoS ONE 7(9), e45958.
| Crossref | Google Scholar |
Hammerschlag N, Schmitz OJ, Flecker AS, Lafferty KD, Sih A, Atwood TB, Gallagher AJ, Irschick DJ, Skubel R, Cooke SJ (2019) Ecosystem function and services of aquatic predators in the Anthropocene. Trends in Ecology & Evolution 34(4), 369-383.
| Crossref | Google Scholar | PubMed |
Hammerschlag N, Herskowitz Y, Fallows C, Couto TBA (2025) Evidence of cascading ecosystem effects following the loss of white sharks from False Bay, South Africa. Frontiers in Marine Science 12, 1530362.
| Crossref | Google Scholar |
Harry AV, Tobin AJ, Simpfendorfer CA, Welch DJ, Mapleston A, White J, Williams AJ, Stapley J (2011) Evaluating catch and mitigating risk in a multispecies, tropical, inshore shark fishery within the Great Barrier Reef World Heritage Area. Marine and Freshwater Research 62(6), 710-721.
| Crossref | Google Scholar |
Heithaus M, Dill L, Marshall G, Buhleier B (2002) Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Marine Biology 140, 237-248.
| Crossref | Google Scholar |
Heithaus MR, Delius BK, Wirsing AJ, Dunphy-Daly MM (2009) Physical factors influencing the distribution of a top predator in a subtropical oligotrophic estuary. Limnology and Oceanography 54(2), 472-482.
| Crossref | Google Scholar |
Henderson CJ, Gilby BL, Turschwell MP, Goodridge Gaines LA, Mosman JD, Schlacher TA, Borland HP, Olds AD (2024) Long term declines in the functional diversity of sharks in the coastal oceans of eastern Australia. Communications Biology 7(1), 611.
| Crossref | Google Scholar |
Heupel MR, Simpfendorfer CA (2008) Movement and distribution of young bull sharks Carcharhinus leucas in a variable estuarine environment. Aquatic Biology 1, 277-289.
| Crossref | Google Scholar |
Heupel MR, Simpfendorfer CA (2011) Estuarine nursery areas provide a low-mortality environment for young bull sharks Carcharhinus leucas. Marine Ecology Progress Series 433, 237-244.
| Crossref | Google Scholar |
Heupel MR, Yeiser BG, Collins AB, Ortega L, Simpfendorfer CA (2010) Long-term presence and movement patterns of juvenile bull sharks, Carcharhinus leucas, in an estuarine river system. Marine and Freshwater Research 61(1), 1-10.
| Crossref | Google Scholar |
Heupel MR, Knip DM, Simpfendorfer CA, Dulvy NK (2014) Sizing up the ecological role of sharks as predators. Marine Ecology Progress Series 495, 291-298.
| Crossref | Google Scholar |
Heupel MR, Simpfendorfer CA, Espinoza M, Smoothey AF, Tobin A, Peddemors V (2015) Conservation challenges of sharks with continental scale migrations. Frontiers in Marine Science 2, 12.
| Crossref | Google Scholar |
Holmes BJ, Sumpton WD, Mayer DG, Tibbetts IR, Neil DT, Bennett MB (2012) Declining trends in annual catch rates of the tiger shark (Galeocerdo cuvier) in Queensland, Australia. Fisheries Research 129-130, 38-45.
| Crossref | Google Scholar |
Holmes BJ, Pepperell JG, Griffiths SP, Jaine FRA, Tibbetts IR, Bennett MB (2014) Tiger shark (Galeocerdo cuvier) movement patterns and habitat use determined by satellite tagging in eastern Australian waters. Marine Biology 161, 2645-2658.
| Crossref | Google Scholar |
Hughes L, Steffen W (2017) Climate change in Australia: trends, projections and impacts. In ‘The future of the Great Barrier Reef: the need for a new social contract’. (Eds G Lawrence, S Baum, D Chase) pp. 437–461. (Springer) doi:10.1007/978-3-319-50094-2_25
Jennings S, Kaiser MJ (1998) The effects of fishing on marine ecosystems. Advances in Marine Biology 34, 201-352.
| Crossref | Google Scholar |
Kinney MJ, Simpfendorfer CA (2009) Reassessing the value of nursery areas to shark conservation and management. Conservation Letters 2(2), 53-60.
| Crossref | Google Scholar |
Klimley AP (1987) The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini. Environmental Biology of Fishes 18(1), 27-40.
| Crossref | Google Scholar |
Lea JSE, Humphries NE, Clarke CR, Sims DW (2015) To Madagascar and back: long-distance, return migration across open ocean by a pregnant female bull shark Carcharhinus leucas. Journal of Fish Biology 87(6), 1313-1321.
| Crossref | Google Scholar | PubMed |
Lee SY (2004) Relationship between mangrove abundance and tropical prawn production: a re-evaluation. Marine Biology 145, 943-949.
| Crossref | Google Scholar |
Lee KA, Roughan M, Harcourt RG, Peddemors VM (2018) Environmental correlates of relative abundance of potentially dangerous sharks in nearshore areas, southeastern Australia. Marine Ecology Progress Series 599, 157-179.
| Crossref | Google Scholar |
Lee KA, Smoothey AF, Harcourt RG, Roughan M, Butcher PA, Peddemors VM (2019) Environmental drivers of abundance and residency of a large migratory shark, Carcharhinus leucas, inshore of a dynamic western boundary current. Marine Ecology Progress Series 622, 121-137.
| Crossref | Google Scholar |
Lefcheck JS, Hughes BB, Johnson AJ, Pfirrmann BW, Rasher DB, Smyth AR, Williams BL, Beck MW, Orth RJ (2019) Are coastal habitats important nurseries? A meta-analysis. Conservation Letters 12, e12645.
| Crossref | Google Scholar |
Lopes SM, Williamson JE, Lambreghts Y, Allen AP, Brown C (2024) Predicting whaler shark presence and interactions with humans in southern Queensland, Australia. Science of The Total Environment 934, 172957.
| Crossref | Google Scholar |
Lubitz N, Bradley M, Sheaves M, Hammerschlag N, Daly R, Barnett A (2022) The role of context in elucidating drivers of animal movement. Ecology and Evolution 12, e9128.
| Crossref | Google Scholar |
Lubitz N, Daly R, Filmalter JD, Sheaves M, Cowley PD, Næsje TF, Barnett A (2023) Context drives movement patterns in a mobile marine predator. Movement Ecology 11, 28.
| Crossref | Google Scholar |
Macbeth WG, Butcher PA, Collins D, McGrath SP, Provost SC, Bowling AC, Geraghty PT, Peddemors VM (2018) Improving reliability of species identification and logbook catch reporting by commercial fishers in an Australian demersal shark longline fishery. Fisheries Management and Ecology 25, 186-202.
| Crossref | Google Scholar |
Manson FJ, Loneragan NR, Harch BD, Skilleter GA, Williams L (2005) A broad-scale analysis of links between coastal fisheries production and mangrove extent: a case-study for northeastern Australia. Fisheries Research 74(1-3), 69-85.
| Crossref | Google Scholar |
Marinac AS (2022) Jailing Indonesians for shark finning in Australian waters doesn’t solve the real driver – poverty. In The Conversation, 7 December 2022. Available at https://theconversation.com/jailing-indonesians-for-shark-finning-in-australian-waters-doesnt-solve-the-real-driver-poverty-195909 [Verified 28 May 2024]
Maunder MN, Sibert JR, Fonteneau A, Hampton J, Kleiber P, Harley SJ (2006) Interpreting catch per unit effort data to assess the status of individual stocks and communities. ICES Journal of Marine Science 63(8), 1373-1385.
| Crossref | Google Scholar |
Maunder MN, Thorson JT, Xu H, Oliveros-Ramos R, Hoyle SD, Tremblay-Boyer L, Lee HH, Kai M, Chang S-K, Kitakado T, Albertsen CM, Minte-Vera CV, Lennert-Cody CE, Aires-da-Silva AM, Piner KR (2020) The need for spatio-temporal modeling to determine catch-per-unit effort based indices of abundance and associated composition data for inclusion in stock assessment models. Fisheries Research 229, 105594.
| Crossref | Google Scholar |
McClanahan TR, Mangi SC (2004) Gear-based management of a tropical artisanal fishery based on species selectivity and capture size. Fisheries Management and Ecology 11(1), 51-60.
| Crossref | Google Scholar |
McHenry J, Welch H, Lester SE, Saba V (2019) Projecting marine species range shifts from only temperature can mask climate vulnerability. Global Change Biology 25, 4208-4221.
| Crossref | Google Scholar | PubMed |
McLoughlin KJ, Stevens JD (1994) Gill-net mesh selectivities for two species of commercial carcharhinid shark taken in northern Australia. Marine and Freshwater Research 45(4), 521-534.
| Crossref | Google Scholar |
Mitchell JD, McLean DL, Collin SP, Langlois TJ (2018) Shark depredation in commercial and recreational fisheries. Reviews in Fish Biology and Fisheries 28(4), 715-748.
| Crossref | Google Scholar |
Mitchell JD, Drymon JM, Vardon J, Coulson PG, Simpfendorfer CA, Scyphers SB, Kajiura SM, Hoel K, Williams S, Ryan KL, Barnett A, Heupel MR, Chin A, Navarro M, Langlois T, Ajemian MJ, Gilman E, Prasky E, Jackson G (2023) Shark depredation: future directions in research and management. Reviews in Fish Biology and Fisheries 33(2), 475-499.
| Crossref | Google Scholar | PubMed |
Mucientes GR, Queiroz N, Sousa LL, Tarroso P, Sims DW (2009) Sexual segregation of pelagic sharks and the potential threat from fisheries. Biology Letters 5(2), 156-159.
| Crossref | Google Scholar | PubMed |
Mullins L, Cartwright J, Dykstra SL, Evans K, Mareska J, Matich P, Plumlee JD, Sparks E, Drymon JM (2024) Warming waters lead to increased habitat suitability for juvenile bull sharks (Carcharhinus leucas). Scientific Reports 14(1), 4100.
| Crossref | Google Scholar |
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315(5820), 1846-1850.
| Crossref | Google Scholar | PubMed |
Niella Y, Smoothey AF, Peddemors V, Harcourt R (2020) Predicting changes in distribution of a large coastal shark in the face of the strengthening East Australian Current. Marine Ecology Progress Series 642, 163-177.
| Crossref | Google Scholar |
Niella Y, Peddemors VM, Green M, Smoothey AF, Harcourt R (2021) A ‘wicked problem’ reconciling human–shark conflict, shark bite mitigation, and threatened species. Frontiers in Conservation Science 2, 720741.
| Crossref | Google Scholar |
Niella Y, Raoult V, Gaston T, Goodman K, Harcourt R, Peddemors V, Smoothey AF (2022) Reliance of young sharks on threatened estuarine habitats for nutrition implies susceptibility to climate change. Estuarine, Coastal and Shelf Science 268, 107790.
| Crossref | Google Scholar |
Noriega R, Werry JM, Sumpton W, Mayer D, Lee SY (2011) Trends in annual CPUE and evidence of sex and size segregation of Sphyrna lewini: management implications in coastal waters of northeastern Australia. Fisheries Research 110(3), 472-477.
| Crossref | Google Scholar |
O’Connell MT, Shepherd TD, O’Connell AMU, Myers RA (2007) Long-term declines in two apex predators, bull sharks (Carcharhinus leucas) and alligator gar (Atractosteus spatula), in Lake Pontchartrain, an oligohaline estuary in southeastern Louisiana. Estuaries and Coasts 30(4), 567-574.
| Crossref | Google Scholar |
Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H, Carlson JK, Fordham SV, Barreto R, Fernando D, Francis MP, Jabado RW, Herman KB, Liu K-M, Marshall AD, Pollom RA, Romanov EV, Simpfendorfer CA, Yin JS, Kindsvater HK, Dulvy NK (2021) Half a century of global decline in oceanic sharks and rays. Nature 589, 567-571.
| Crossref | Google Scholar | PubMed |
Paterson RA (1990) Effects of long-term anti-shark measures on target and non-target species in Queensland, Australia. Biological Conservation 52(2), 147-159.
| Crossref | Google Scholar |
Pillans RD, Good JP, Anderson WG, Hazon N, Franklin CE (2005) Freshwater to seawater acclimation of juvenile bull sharks (Carcharhinus leucas): plasma osmolytes and Na+/K+-ATPase activity in gill, rectal gland, kidney and intestine. Journal of Comparative Physiology Biology 175, 37-44.
| Crossref | Google Scholar |
Pillans RD, Good JP, Anderson WG, Hazon N, Franklin CE (2008) Rectal gland morphology of freshwater and seawater acclimated bull sharks Carcharhinus leucas. Journal of Fish Biology 72, 1559-1571.
| Crossref | Google Scholar |
Pimiento C, Albouy C, Silvestro D, Mouton TL, Velez L, Mouillot D, Judah AB, Griffin JN, Leprieur F (2023) Functional diversity of sharks and rays is highly vulnerable and supported by unique species and locations worldwide. Nature Communications 14, 7691.
| Crossref | Google Scholar |
Powers SP, Fodrie FJ, Scyphers SB, Drymon JM, Shipp RL, Stunz GW (2013) Gulf-wide decreases in the size of large coastal sharks documented by generations of fishermen. Marine and Coastal Fisheries 5(1), 93-102.
| Crossref | Google Scholar |
Rago PJ, Sosebee KA, Brodziak JKT, Murawski SA, Anderson ED (1998) Implications of recent increases in catches on the dynamics of northwest Atlantic spiny dogfish (Squalus acanthias). Fisheries Research 39(2), 165-181.
| Crossref | Google Scholar |
Reid DD, Robbins WD, Peddemors VM (2011) Decadal trends in shark catches and effort from the New South Wales, Australia, Shark Meshing Program 1950–2010. Marine and Freshwater Research 62, 676-693.
| Crossref | Google Scholar |
Rigby C, Espinoza M, Derrick D, Pacoureau N, Matt D (2021) Bull shark Carcharhinus leucas. In ‘The IUCN Red List of Threatened Species 2021’. e.T39372A2910670. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/39372/2910670
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ (2014) Status and ecological effects of the world’s largest carnivores. Science 343(6167), 1241484.
| Crossref | Google Scholar |
Roff G, Doropoulos C, Rogers A, Bozec Y-M, Krueck NC, Aurellado E, Priest M, Birrell C, Mumby PJ (2016) The ecological role of sharks on coral reefs. Trends in Ecology & Evolution 31(5), 395-407.
| Crossref | Google Scholar | PubMed |
Roff G, Brown CJ, Priest MA, Mumby PJ (2018) Decline of coastal apex shark populations over the past half century. Communications Biology 1(1), 223.
| Crossref | Google Scholar |
Sampson DB (2014) Fishery selection and its relevance to stock assessment and fishery management. Fisheries Research 158, 5-14.
| Crossref | Google Scholar |
Sandoval Laurrabaquio-A N, Islas-Villanueva V, Adams DH, Uribe-Alcocer M, Alvarado-Bremer JR, Díaz-Jaimes P (2019) Genetic evidence for regional philopatry of the bull shark (Carcharhinus leucas), to nursery areas in estuaries of the Gulf of Mexico and western north Atlantic ocean. Fisheries Research 209, 67-74.
| Crossref | Google Scholar |
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Reviews in Fish Biology and Fisheries 24(4), 1089-1103.
| Crossref | Google Scholar |
Secor DH, Rooker JR, Gahagan BI, Siskey MR, Wingate RW (2015) Depressed resilience of bluefin tuna in the western atlantic and age truncation. Conservation Biology 29, 400-408.
| Crossref | Google Scholar | PubMed |
Shin Y-J, Rochet M-J, Jennings S, Field JG, Gislason H (2005) Using size-based indicators to evaluate the ecosystem effects of fishing. ICES Journal of Marine Science 62(3), 384-396.
| Crossref | Google Scholar |
Simpfendorfer CA (1999) Demographic analysis of the dusky shark fishery in southwestern Australia. In ‘Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals. American Fisheries Society Symposium 23’, 26–30 September 2023, Évian, France. (Ed. JA Muscik) pp. 149–160. (American Fisheries Society) 10.47886/9781888569155.ch11
Simpfendorfer CA, Freitas GG, Wiley TR, Heupel MR (2005) Distribution and habitat partitioning of immature bull sharks (Carcharhinus leucas) in a southwest Florida estuary. Estuaries 28(1), 78-85.
| Crossref | Google Scholar |
Smoothey AF, Gray CA, Kennelly SJ, Masens OJ, Peddemors VM, Robinson WA (2016) Patterns of occurrence of sharks in Sydney Harbour, a large urbanised estuary. PLoS ONE 11(1), e0146911.
| Crossref | Google Scholar |
Smoothey AF, Lee KA, Peddemors VM (2019) Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia. Scientific Reports 9(1), 18864.
| Crossref | Google Scholar |
Smoothey AF, Niella Y, Brand C, Peddemors VM, Butcher PA (2023) Bull shark (Carcharhinus leucas) occurrence along beaches of south-eastern Australia: understanding where, when and why. Biology 12(9), 1189.
| Crossref | Google Scholar |
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES Journal of Marine Science 57(3), 476-494.
| Crossref | Google Scholar |
Sumaila UR, Alder J, Keith H (2006) Global scope and economics of illegal fishing. Marine Policy 30(6), 696-703.
| Crossref | Google Scholar |
Sumpton WD, Taylor SM, Gribble NA, McPherson G, Ham T (2011) Gear selectivity of large-mesh nets and drumlines used to catch sharks in the Queensland shark control program. African Journal of Marine Science 33(1), 37-43.
| Crossref | Google Scholar |
Svedäng H, Hornborg S (2017) Historic changes in length distributions of three Baltic cod (Gadus morhua) stocks: evidence of growth retardation. Ecology and Evolution 7, 6089-6102.
| Crossref | Google Scholar | PubMed |
Thomerson JE, Thorson TB, Hempel RL (1977) The bull shark, Carcharhinus leucas, from the upper Mississippi River near Alton, Illinois. Copeia 1977, 166-168.
| Crossref | Google Scholar |
Thorson TB (1972) The status of the bull shark, Carcharhinus leucas, in the Amazon River. Copeia 1972(3), 601-605.
| Crossref | Google Scholar |
Tillett BJ, Meekan MG, Field IC, Thorburn DC, Ovenden JR (2012) Evidence for reproductive philopatry in the bull shark Carcharhinus leucas. Journal of Fish Biology 80(6), 2140-2158.
| Crossref | Google Scholar | PubMed |
Tricas TC, Sisneros JA (2004) Ecological functions and adaptations of the elasmobranch electrosense. In ‘The senses of fish’. (Eds G von der Emde, J Mogdans, BG Kapoor) pp. 308–329. (Springer: Dordrecht, Netherlands) doi:10.1007/978-94-007-1060-3_14
Vardon JL, Williams SM, Bucher DJ, Morgan JAT (2021) Identifying shark species responsible for fisheries depredation off southeast Queensland, Australia. Molecular Biology Reports 48(5), 4961-4965.
| Crossref | Google Scholar | PubMed |
Ward-Paige CA, Keith DM, Worm B, Lotze HK (2012) Recovery potential and conservation options for elasmobranchs. Journal of Fish Biology 80(5), 1844-1869.
| Crossref | Google Scholar | PubMed |
Werry JM (2010) Habitat ecology of the bull shark, Carcharhinus leucas, on urban coasts in eastern Queensland, Australia. PhD thesis, Griffith University, Qld, Australia. 10.25904/1912/2913
Werry JM, Clua E (2013) Sex-based spatial segregation of adult bull sharks, Carcharhinus leucas, in the New Caledonian great lagoon. Aquatic Living Resources 26, 281-288.
| Crossref | Google Scholar |
Werry JM, Lee SY, Otway NM, Hu Y, Sumpton W (2011) A multi-faceted approach for quantifying the estuarine–nearshore transition in the life cycle of the bull shark, Carcharhinus leucas. Marine and Freshwater Research 62(12), 1421-1431.
| Crossref | Google Scholar |
Werry JM, Lee SY, Lemckert CJ, Otway NM (2012) Natural or artificial? Habitat use by the bull shark, Carcharhinus leucas. PLoS ONE 7(11), e49796.
| Crossref | Google Scholar |
Werry JM, Planes S, Berumen ML, Lee KA, Braun CD, Clua E (2014) Reef fidelity and migration of tiger sharks, Galeocerdo cuvier, across the Coral Sea. PLoS ONE 9(1), e83249.
| Crossref | Google Scholar |
Werry JM, Sumpton W, Otway NM, Lee SY, Haig JA, Mayer DG (2018) Rainfall and sea surface temperature: key drivers for occurrence of bull shark, Carcharhinus leucas, in beach areas. Global Ecology and Conservation 15, e00430.
| Crossref | Google Scholar |
Yates PM, Heupel MR, Tobin AJ, Simpfendorfer CA (2015) Ecological drivers of shark distributions along a tropical coastline. PLoS ONE 10(4), e0121346.
| Crossref | Google Scholar |