The ‘canary of the estuary’, the contribution of Sydney rock oyster aquaculture to understanding and protecting Australian estuarine health
Michael C. Dove A , Laura M. Parker A B * , Anthony Zammit C , Hazel Farrell C , Penelope Ajani D , Shauna Murray D , Kirsten Benkendorff E , Geoff R. MacFarlane F and Wayne A. O’Connor
E , Geoff R. MacFarlane F and Wayne A. O’Connor  A F *
A F *
A
B
C
D
E
F
Handling Editor: Bridie Allan
Abstract
The Sydney rock oyster (Saccostrea glomerata) is an iconic Australian native species of great commercial and ecological significance, that has been farmed in New South Wales for over 150 years.
To highlight the role of S. glomerata industry in safeguarding Australia’s estuaries.
Literature review.
S. glomerata, more than any other species, has served to highlight emerging threats to estuaries, ranging from viral and bacterial contamination to chemical pollution, and climate change. Their use as biomonitors and in bioassays for pollutants (metals, PAHs, PFAS and pesticides) has been instrumental in identifying and quantifying potential threats. The oyster industry provides one of NSW’s largest and longest-running monitoring programs for estuarine environmental conditions. Currently, S. glomerata is at the forefront of remediation efforts, leading legislative change in environmental protection, and using ecoengineering, reef restoration and selective breeding programs to bolster oyster resilience.
Even though the community has long recognised the intrinsic link between oyster health and estuarine well-being and works with industry to advocate for estuarine ecosystem conservation and the species present, the contribution the industry makes is underestimated.
Amid debate over aquaculture expansion, greater consideration of the positives arising from culture activities is warranted.
Keywords: aquaculture, bioassay, climate change, estuarine health, monitoring, oyster, pollution, Saccostrea glomerata.
Introduction
The Sydney rock oyster (Saccostrea glomerata, formerly S. commercialis), holds a significant place as an iconic native oyster species along Australia’s southern coastline, stretching from southern Queensland to Albany in Western Australia (Snow et al. 2023). Historically, this once-abundant oyster served as a valuable food source for Aboriginal communities, before being used for sustenance and lime production during the colonial period in New South Wales (NSW). Its cultivation began in the 1880s, laying the foundation for one of Australia’s oldest aquaculture industries. Most S. glomerata culture occurs in NSW estuaries, although oyster farms are also established in Queensland, Victoria and Western Australia (O’Connor and Dove 2009). Unfortunately, although the industry grows, wild S. glomerata populations have declined by more than 90% (Gillies et al. 2018; McAfee et al. 2020), largely through overharvest, prompting efforts for reef-building interventions.
NSW is Australia’s most populous state, with over 85% of its population residing within 50 km of the coast, being primarily concentrated around estuarine areas (Planning NSW 2023). Consequently, the S. glomerata industry has evolved alongside significant anthropogenic impact, with sites such as Sydney Harbour once considered among the world’s most contaminated environments (Davis and Birch 2010, 2011). Given the economic and ecological significance of S. glomerata and its ability to accumulate pollutants, there has been extensive research into various anthropogenic threats (Avery et al. 1996: Ewere et al. 2019a, 2019b: Islam et al. 2024). Oysters are often called the ‘lungs of the estuaries’ or ‘canaries of the estuary’, with their health serving as an indicator of river health issues (Healthy Rivers Commission 2003). They are well suited as biomonitors, because most oysters (including S. glomerata) are broadcast spawners and their embryonic and larval stages are exposed to general environmental conditions. Both the larvae and adult stages are exposed to high volumes of seawater in proportion to their size, with adult S. glomerata capable of filtering over 100 L day−1 (7.2 L day−1 g−1 of oyster; Bayne 2002).
Initially, concerns over pollutant threats to S. glomerata focused on bacterial contamination and metals, including tributyltin (Batley et al. 1989). However, studies have since expanded to encompass the impacts of polycyclic aromatic hydrocarbons (PAHs), endocrine-disrupting chemicals (EDCs), and emerging threats such as pesticides, perfluoroalkyl substances (PFAS) and microplastics. S. glomerata has also played a crucial role in assessing key threatening processes such as climate-change-induced ocean warming and acidification, with this research contributing to the resilience of industry production and supporting reef restoration initiatives (Pereira et al. 2020).
Beyond its role as a beloved seafood staple contributing to NSW’s most valuable fishery (valued at A$77 million in 2022–23, NSW Department of Primary Industries and Regional Development 2024), S. glomerata has been instrumental in driving environmental protection efforts for NSW’s estuarine environments. In the Sydney Morning Herald, Lockwood (2014) reported that
Oysters are the canaries in the mine or marine world, and less oysters mean less fish. But they are just the tip of the iceberg when it comes to pollution woes and researchers fear other marine critters are under great stress.
Concerns for Sydney rock oyster health have spurred legislative changes since the 1870s, and, thanks to a proactive industry, continue today. Larval S. glomerata have been used widely in bioassays to assess the impacts of pollutants, and adults have been used as biomonitors for diffuse and point-source pollution events (Avery et al. 1996; Scanes 1996; van Dam et al. 2008). Monitoring S. glomerata in estuarine environments has uncovered emerging pollution problems (Jamal et al. 2024), and when new problems are discovered, they were among the first species tested to assess biological impacts. While other estuarine species such as mangroves and seagrasses have been focal points of protection efforts, few have been as influential in advocating for NSW estuary preservation as S. glomerata. This review is not an attempt to chronicle the myriad studies of anthropogenic impacts on S. glomerata, rather it seeks to highlight the sometimes-undervalued role that cultured species can have in protecting the environments they rely on.
History
For at least 10,000 years, oysters and other foods harvested from Australian estuaries have served as vital sources of sustenance and nutrition for Aboriginal communities (Gibbs et al. 2024). From the earliest European observations, the richness of NSW estuarine resources was recognised and prized. Early settlers relied on these resources for food, and in the case of oysters, as a source of lime for construction. Consequently, there exists a longstanding tradition of valuing our estuarine ecosystems and making efforts to safeguard them.
During colonial Australia, Royal Commissions emerged as powerful instruments to investigate public issues, often preceding legislative reform (Gilligan 2002). One of the earliest Royal Commissions convened in NSW (1865–1866) focused on an ‘Inquiry into the Condition of the Harbour of Port Jackson’ (Sydney, NSW), which included an examination of the impacts of city sewage on the harbour environment. This concern for the estuarine environment, particularly for S. glomerata, resurfaced little more than a decade later in 1876 when the Royal Commission on Oyster Culture was established to explore methods for maintaining the colony’s natural oyster beds. Subsequently, in 1884, an Act of Parliament was made for the preservation and cultivation of oysters, laying the groundwork to develop the oyster industry as we know it today. Areas within estuaries were leased to farmers for the cultivation of oysters, which were to become the sole source of oysters for commercial exploitation, preventing large-scale natural oyster harvest. Since then, oysters have played a pivotal role in identifying and raising awareness of various threats to the health of our estuaries, serving as indicators of the efficacy of conservation efforts (Fig. 1).
Celebrating the return of oysters to Sydney Harbour, front page, Sydney Morning Herald on 25 January 2002 (Morris 2002), at the time, one of the highest-circulation papers in Australia’s largest city.

The threat posed to S. glomerata, particularly its safety as a food product, was a regular source of concern to Sydney’s residents and was frequently discussed in newspapers. Following the 1856 Royal Commission, sewage pollution of Sydney Harbour, and later Botany Bay, remained topical for well over a century. The closure of Middle Harbour (in Sydney Harbour) to oyster harvest in the early 1900s led to headlines such as ‘Poison in the local oysters. A remarkable revelation’ (Anon. 1903) and charges being laid for illegal oyster collection (Anon. 1906).
As Sydney developed, new sources of pollution were recognised. Proposals to build an oil refinery in Botany Bay in the late 1930s raised new fears. Mr T. C. Roughley, Special Research Officer of the Fisheries Department, warned ‘that if effluent from such works reached Botany Bay it would be of great detriment to oyster farms’, and went on to say, ‘We have been too complacent in the past about pollution of our bays and rivers’ (Anon. 1940).
In 1995, the NSW Government established the Healthy Rivers Commission to conduct independent public inquiries into selected NSW rivers and propose long-term strategies to achieve environmental, social and economic goals. These inquiries included major oyster-producing estuaries, such as Georges River, Hawkesbury River and Shoalhaven River. Oysters were so topical, that the NSW Government subsequently requested that the Healthy Rivers Commission specifically examine the relationship between river health and the cultivation of oysters safe for human consumption. The widespread recognition of the correlation between healthy estuaries and safe oysters has led to continued legislative support for the NSW oyster industry. In 2006, the Oyster Industry Sustainable Aquaculture Strategy (OISAS) was publicly announced, along with amendments to NSW State Environmental Planning Policy 62 – Sustainable Aquaculture (State Environmental Planning Policy, SEPP 62). OISAS identified Priority Oyster Aquaculture Areas (POAAs) in each estuary, established water quality objectives for these areas based on Healthy Rivers Commission recommendations (Healthy Rivers Commission 2003), and placed obligations on oyster farmers for improved farming practices. Subsequent legislation, including SEPP Primary Production and Rural Development 2019 and the current SEPP Primary Production 2021, require consent authorities considering development applications affecting POAA or other oyster aquaculture areas to follow specific procedures, including notifying the Secretary of the Department of Industry, considering OISAS provisions, and evaluating any proposed measures to address compatibility issues with oyster aquaculture.
Whether addressing the historical threat of sewage pollution in Port Jackson nearly 170 years ago or confronting the myriad pollutants of the Anthropocene era, S. glomerata has consistently played a pivotal role in detecting, highlighting and addressing environmental threats.
Environmental change
Physical environmental variables
Marine environments are highly susceptible to fluctuations in salinity and temperature, and these are among the first variables considered in attempting to understand the biology and ecology of estuarine species. The NSW oyster industry has been a source of these data since the mid-1960s (Wolf and Collins 1979) and continues to support investigative programs. As part of the comprehensive commercial shellfish program monitoring in NSW, salinity and temperature measurements are systematically collected alongside microbiological, phytoplankton and biotoxin samples. This results in the acquisition of up to 20,000 environmental data points annually. With the increasing availability of real-time monitoring sensors, recent initiatives, such as the projects spanning from 2017 to 2021 and from 2021 to 2024 (Murray et al. 2024), have enhanced baseline monitoring in major oyster-producing estuaries (Ajani et al. 2024). These projects have intensified sampling intervals to 15–30 min for temperature and salinity, with all data being publicly accessible and utilised across a diverse array of estuarine investigations (see https://salinity.research.uts.edu.au). In some estuaries, extensive sensor arrays have been installed and have been coupled to terrestrial weather stations to enhance the data provided and the range of applications (Bates et al. 2021; Hornsby Shire Council, see https://mhl.nsw.gov.au/users/HornsbyShireCouncil/). Without the oyster industry, the quantity, duration and availability of foundational environmental data to support estuarine research would be significantly reduced. Particularly, in the face of climate change, where 50 years of data will likely be of great value.
Algal toxins
Phytoplankton play a crucial role at the foundation of most marine food chains and serve as a primary food source for oysters; however, some naturally occurring phytoplankton produce toxins with potentially severe impacts on the wider marine ecosystem and seafood consumers. Because these toxins can accumulate to levels toxic for humans relatively quickly (days to weeks, Farrell et al. 2015), there is a regulatory requirement for monitoring in shellfish safety guidelines (i.e. Australian Shellfish Quality Assurance Advisory Committee 2024). Because of its size, the majority of marine phytoplankton and algal toxin monitoring on Australia’s eastern coast is undertaken by the S. glomerata aquaculture industry. This comprehensive effort involves up to 3000 tests year−1 that are co-funded and collected by the S. glomerata industry. Although the primary objective of this monitoring is to safeguard consumers and ensure human health from algal biotoxins, it also provides valuable insights into estuary health and the impacts of changing climate conditions.
The data gathered from monitoring S. glomerata have led to the detection of new potentially harmful algal species and highlighted recent changes in their frequency of occurrence and the intensity of blooms. The first observations of toxic Pseudo-nitzschia delicatissima blooms in the southern hemisphere were from oyster-growing areas in the Hawkesbury River (Ajani et al. 2020), whereas data from the same area were used to provide the first insights into the dynamics of toxic Dinophysis acuminata blooms in Australia and elucidate the potential mechanism(s) for bloom development (Ajani et al. 2016).
Comprehensive datasets of harmful algal blooms in Australian waters are rare (Ajani et al. 2017), with the largest and longest-running dataset arising from testing in S. glomerata. Here alone, a total of 54 amnesic shellfish toxin (AST) detections (max. 20 in 2013), 27 diarrhetic shellfish toxin detections (max. 11 in 2013) and 102 paralytic shellfish toxin detections (max. 30 in 2010) were recorded from 2004 to 2015 (Ajani et al. 2017). At that time, all but one of these events, an AST detection (20 samples) in Wagonga Inlet in 2010, remained below the regulatory limit and posed little threat. Since 2016, more frequent and larger-scale blooms of Alexandrium pacificum causing toxins above the safe limit for human consumption appear to be occurring (Barua et al. 2020) with the highest recorded levels of paralytic shellfish toxins in S. glomerata observed during late 2022. This event also affected other seafood species and prompted the government to advise all recreational seafood consumers of the potential threat (NSW Department of Primary Industries and Regional Development 2022).
The importance of rapid and sensitive detection of toxins in S. glomerata has been the impetus for the development of new detection methods. For example, the detection of a bloom of mixed Pseudo-nitzschia species in oyster leases at Wagonga Inlet, NSW, led to the development of a quantitative real-time polymerase chain-reaction (qPCR) assay to detect only species belonging to the toxin-producing P. pseudodelicatissima complex. The data from this assay were then used alongside high-resolution water temperature and salinity sensor data from oyster leases to develop predictive models of the abundance of P. delicatissima within the estuary to warn of future blooms (Ajani et al. 2021).
Ocean acidification and warming
Climate change poses a significant threat, with the potential to severely disrupt and alter marine and estuarine environments worldwide. Human activities have led to an increase in the concentration of carbon dioxide (CO2) in the atmosphere, causing estuaries and oceans to undergo acidification and warming at an unprecedented rate on geological timescales. This trend is projected to persist throughout this century (Collins et al. 2013; Lee et al. 2021). In Australia, these changes are already observable in estuarine environments. Data collected from 166 estuaries along the Australian coastline showed significant warming and acidification, in particular, with the eastern Australian estuaries, which are a home to the largest S. glomerata populations, warming, acidifying and freshening more quickly than predicted by global models (Scanes et al. 2020).
For nearly two decades, the concern has centred on the potential impacts of these changes on marine and estuarine organisms and the ecosystem services they provide. S. glomerata was among the first species globally to be assessed for the impacts of ocean acidification and warming (Parker et al. 2009, 2010; Watson et al. 2009) and has been at the forefront of marine climate-change research since then. Early studies focussed predominantly on early-life history stages and showed detrimental impacts on larval growth and development, the percentage of abnormal larvae, and larval survival. Further studies found that like many other calcifying marine and estuarine species, S. glomerata is vulnerable across each stage in its life cycle, with reductions in juvenile and adult shell growth (Parker et al. 2010; Scanes et al. 2017; Fitzer et al. 2018, 2019) and reproductive capacity (Parker et al. 2018), changes in physiology (e.g. acid–base balance, clearance rate and metabolism; Parker et al. 2012, 2024; Stapp et al. 2018) and alterations in their microbiome (Scanes et al. 2021, 2023). This research has highlighted how pervasive the impacts can be, how varied the mechanisms are, and provided an impetus for similar research in other local molluscan species (e.g. Scanes et al. 2014; Cole et al. 2016; Pereira et al. 2020).
Owing to the severity of climate-change impacts, research on S. glomerata quickly shifted to estimating the capacity for acclimation or adaptation (Parker et al. 2011, 2012, 2015). Parker et al. (2011) demonstrated that genetically distinct pair-mated families of S. glomerata differed in their growth response to ocean acidification, with growth being unaffected in some families. This provided the first evidence that tolerant genotypes may exist within populations of S. glomerata, potentially facilitating genetic adaptation. Following this, it was found that wild populations of S. glomerata can rapidly acclimate to ocean acidification, by a process known as transgenerational plasticity (TGP), which has since been demonstrated in oysters for other stressors such as injections of the immunostimulant poly(I:C) and low salinity (Green et al. 2016; Griffiths et al. 2021). Exposure of adult S. glomerata to ocean acidification during reproductive conditioning in a hatchery setting resulted in positive TGP effects being passed to their offspring. These effects primed the offspring for acidified conditions, enabling them to grow and develop faster with fewer abnormalities than do the offspring from non-exposed parents (Parker et al. 2012). More recently, it was found that adult S. glomerata exhibited considerable resilience to simulated moderate marine heat waves and elevated temperatures (Ewere et al. 2021a). Although some minor changes were observed in the micronutrient and elemental composition of the flesh, there were no significant changes in parameters such as haemocyte numbers, condition index, gene expression, flesh protein, or fatty acid profiles relative to ambient controls. Collectively, these studies have played an important role in shifting the focus of global marine and estuarine climate-change research from solely assessing impacts to considering the capacity for resilience, acclimation and adaptation.
In 2020, Oysters Australia, the national body for oyster grower advocacy, research and development, identified investigating the impacts of climate change on the oyster industry and implementing adaptation measures as a priority in their 2020–25 Strategic Plan (Oysters Australia Strategy Plan 2020–2025). Reflective of this, S. glomerata has become one of the few estuarine aquaculture species undergoing selective breeding for resilience to climate change (Parker et al. 2024). Concurrently, genetic, epigenetic and physiological tools are being employed to identify the underlying mechanisms conferring resilience, leading the way in understanding how climate-resilient organisms cope (Goncalves et al. 2016; Scanes et al. 2017; Stapp et al. 2018; Parker et al. 2024). Climate change-resilient S. glomerata individuals produced through selective breeding are also being trialled for their potential in oyster reef restoration. Current reef restoration efforts in Australia primarily focus on replacing lost substrate to facilitate increased oyster settlement and recruitment. Despite initial success, future oyster reef restoration efforts may face challenges because of interactions among ocean acidification, warming and cumulative pressures (e.g. overharvesting, sedimentation, pollution, introduced pests and disease) that historically led to declines in oyster reefs (Ogburn et al. 2007; O’Connor and Dove 2009). Seeding oyster reefs with climate-resilient oysters could enhance oyster survival and reduce the risk of future restoration failures.
Environmental contamination
Biomonitoring and ecotoxicology
Oysters are widely used as a sentinel species to monitor estuarine pollution because both the larvae and adult stages are exposed to high volumes of seawater in proportion to their size. Studies using oyster embryos as test species have been conducted for over 50 years, with the US EPA documenting its first larval development test almost 30 years ago in 1995. Simultaneously, the first molluscan larval bioassays were developed in Australia on the basis of S. glomerata and Chlamys asperrima (Krassoi 1995). These assays have been commercialised and extensively utilised to assess the impacts of various pollutants, including chemicals, effluents, leachates, groundwater and sediments (Ecotox Services Australasia 2024). S. glomerata larvae have shown sensitivity to metals (Krassoi 1995; Wilson and Hyne 1997), ammonia, petroleum hydrocarbons and dispersants (Smith et al. 2004). Additionally, they have been instrumental in investigating and improving hatchery production for this species (Dove and O’Connor 2007). Indeed, S. glomerata has been the primary species used in most molluscan toxicity assessments in Australia, and despite being a temperate species, it has also been regularly used in tropical toxicity assessments (van Dam et al. 2008).
The filtration capacity of adult oysters offers the potential to strategically deploy adult oysters as ‘passive samplers’ to detect and monitor pollution hotspots in estuaries receiving run-off from intensive agriculture, industrial and urban development, and further test the impacts of flood and fire. There is also potential to expand field translocation/relocation experiments within estuaries to test depuration rates for key contaminants and maximise the use of grow-out and harvest areas within affected estuaries.
Viral and bacterial contamination
While the quantity, treatment and discharge locations of sewage in NSW have changed over the past two centuries, sewage continues to affect NSW coastal waters. Sewage comprises a complex mixture of various pollutants, but it is often the viral and bacterial load that raises the most concern among the public. Oysters, as filter feeders, can accumulate bacteria such as faecal coliforms and Vibrio species, as well as viruses such as norovirus and hepatitis, all of which can pose health risks to humans. This risk has been recognised for many years, with historical references noting that ‘Oysters have been shown to be a very certain source of typhoid’ (Anon. 1902). Oysters have thus long been regarded as indicators of seafood safety and estuarine health, akin to canaries in the mine.
The consequences of viral or bacterial food poisoning can be severe. In 1978, over 2000 cases of norovirus illness were recorded after the consumption of oysters harvested from the Georges River, NSW (Kraa 1990), with a further 1750 cases being recorded in 1990 (Bird and Kraa 1992). Tragically, in 1996–97, Australia experienced its largest recorded outbreak of hepatitis A following the consumption of oysters from Wallis Lake, NSW (Anon. 1997), affecting almost 500 individuals and resulting in one fatality. These events significantly affected the oyster industry, leading to immediate declines in oyster (Fig. 2) and general seafood sales (Handmer and Hillman 2004). Although these incidents were not the fault of the oyster industry, they received extensive media coverage and greatly affected public perceptions of waterway health. However, they also galvanised efforts among oyster farmers and the public to drive change.
Annual production of Sydney rock oysters (1930–2023) and food-borne illness incidents influencing oyster sales: A, 1978 Georges River norovirus outbreak; B, 1990 Georges River norovirus outbreak; C, 1996 Wallis Lake hepatitis outbreak.
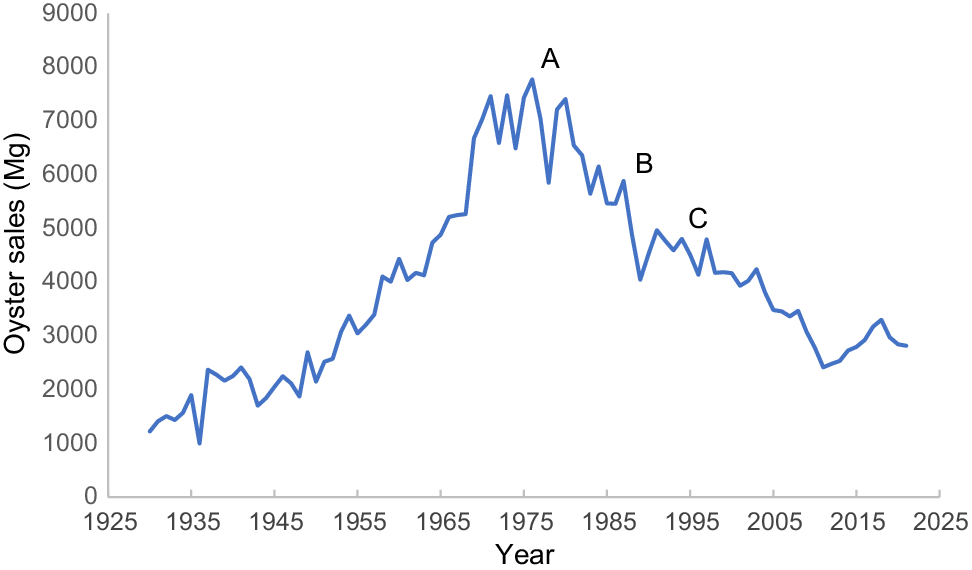
A decade after the Wallis Lake outbreak, the response was lauded as ‘a model for how to handle an environmental public health crisis’ (Grennan 2008). Through collaborative efforts involving the oyster industry, fishing industry, local council, businesses and the public, the Wallis Lakes Catchment Management Plan was implemented. This initiative led to significant improvements, including the construction of wetlands to control urban water quality, shoreline fencing for riparian protection, and rehabilitation of acid sulfate soils in wetlands. Furthermore, it paved the way for NSW’s first coastal catchment initiative to enhance water quality (Grennan 2008), demonstrating a successful public health response to an urgent environmental issue. Not only did this protect public health and enhance the environment, but it also facilitated the recovery and prosperity of the local oyster industry (Kardamanidis et al. 2009).
Commercial oyster production and the preservation of estuarine and marine environments are inherently intertwined. Today, the classification of NSW oyster-producing estuaries is regulated under the NSW Food Act 2003, Food Regulation 2015 (see https://legislation.nsw.gov.au/view/html/inforce/current/sl-2015-0622) and the Food Standards Australia New Zealand (2025), with assessments based on potential contamination risks (Australian Shellfish Quality Assurance Advisory Committee 2024). Since the establishment of the NSW Shellfish Program in 2003, up to 30 estuaries have undergone continuous and routine monitoring, co-funded by the NSW government and the industry. This program constitutes one of the most extensive environmental contamination monitoring efforts in NSW. Trained shellfish industry samplers, supported by local industry committees, conduct dedicated sampling regimes to ensure the safety and quality of shellfish products while also contributing positively to environmental conservation. On average, ~7000 microbiological environmental samples are collected and analysed annually, including tests for E. coli in shellfish meat and faecal coliforms in water, serving as primary indicators of potential microbial contamination, including sewage impacts.
In addition to general E. coli monitoring, microbial source tracking of bacterial estuarine water contamination (human, avian, bovine; Geary et al. 2015; Ajani et al. 2024) in key S. glomerata-producing estuaries is underway, with over 11,000 samples having been collected in the past 5 years alone (Ajani et al. 2024). This enhanced testing has provided greater insights into food safety risks and helped identify at-risk areas. Where E. coli from human sources are apparent, targeted efforts are directed towards addressing failing septic or sewerage systems and supporting network upgrades. Similarly, high levels of bovine faecal coliforms have highlighted regions at risk of riparian damage from cattle and promoted remediation through improved stock management practices and fencing upgrades.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) constitute a diverse group of organic compounds formed through the incomplete combustion of organic materials and various natural processes, such as carbonisation (World Health Organization 1998). Concerns regarding the impact of PAHs on aquatic ecosystems have long been recognised (Anon. 1940). Many PAHs exhibit high lipophilicity, leading to bioconcentration factors exceeding 930 in fish and 130,240 in Daphnia (Canadian Council of Resource and Environment Ministers 1987). Given their threat to a wide array of aquatic organisms (Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand 2000) and human health, the occurrence of PAHs in oysters is routinely monitored.
Aquatic organisms face exposure to PAHs from numerous sources. Although oil refineries have long been feared as significant contributors (Anon. 1940), they are not the sole concern. Recent bushfires in New South Wales (NSW) have introduced substantial ash into coastal waterways, and activities such as farming and recreational boating with two-stroke motors pose additional risks. Amidst this, the historical use of coal tar and creosote to safeguard wooden maritime structures against marine borers has drawn attention to the oyster industry. Despite the industry’s voluntary shift away from tar-based products for well over a decade, PAHs persist in the environment, with a considerable legacy of tar-treated infrastructure on land and in certain NSW estuaries. Consequently, PAH accumulation and its effects on S. glomerata have undergone assessment (Ertl et al. 2016a; Melwani et al. 2016). Unlike many other species, the spectrum of PAHs detected in S. glomerata, the correlation between sediment and oyster tissue levels, and their capacity to depurate PAHs have also been scrutinised (Idowu et al. 2020a, 2020b). Furthermore, investigations have expanded to include Australia’s first evaluation of the concentration and distribution of parent and polar PAHs within an aquatic species, encompassing oxygenated PAHs, nitrated PAHs and heterocyclic PAHs (Idowu et al. 2020a). Overall, the threat to S. glomerata and the environment from PAHs remains, but the contribution of the oyster industry to the threat has increasingly reduced.
Metals
The capacity of bivalves to accumulate metals and the potential repercussions for both consumers and the organisms themselves have been well documented (Roesijadi 1996). Consequently, monitoring for metal contamination in oysters has been established in the USA for over 50 years (Lauenstein et al. 1990) and for over three decades in S. glomerata populations in Australia (Scanes 1996). Large-scale routine monitoring of metals, alongside PAHs and pesticides, in S. glomerata has been conducted triennially since the 1990s across all major oyster-producing areas in NSW. While general monitoring is increasing, this program stands as one of the most extensive of its type for aquatic life within NSW estuaries.
Numerous studies have examined the impacts of a broad range of metals on various life stages of S. glomerata (Table 1), collectively informing and advocating for estuarine protection. A notable instance is tributyltin (TBT), an antifoulant used on boats. As early as the mid-1980s, international concerns regarding the effects of TBT on oysters were raised (Alzieu et al. 1986), and subsequent research confirmed its threat to S. glomerata. Correlations between abnormal shell growth and recreational boating were observed in 1987 (Scammell 1987), followed by evidence demonstrating the adverse effects of TBT on shell growth, deformity rates and survival (Scammell 1992). In 1998, the Australian federal government banned the use of TBT on vessels repainted in Australia, specifically acknowledging the detrimental effects on marine organisms such as oysters (House of Representatives Committees 2003).
| Metal | Authors | Examples of impacts detected | |
|---|---|---|---|
| Iron (Fe) | Dove and Sammut (2007a, 2007b) (A) | Together with reduced pH and elevated Al, Fe was associated with reduced growth and survival, and thinner shells in adults (Dove and Sammut 2007a). | |
| Copper (Cu) | Wisely and Blick (1967)(L), Nell and Holliday (1986)(L), Butterfield (1987)(S), Muralidharan et al. (2012), Taylor et al. (2013) (A), Yingprasertchai et al. (2017) (L), Ertl et al. (2016b) (A), Yingprasertchai et al. (2019) (A) and Scanes et al. (2018) (A) | Elevated larval mortality, (Wisely and Blick 1967), and reduced settlement and survival. Tissue-specific changes in adult transcriptomes increased metallothionein expression (Ertl et al. 2016b) and a reduction in defensin (Taylor et al. 2013). Metal dependent changes in the proteome of adult oysters, specifically including histones (Muralidharan et al. 2012). | |
| Lead (Pb) | Butterfield (1987) (S), Avery et al. (1996) (A), Muralidharan et al. (2012) (A), Taylor et al. (2013) (A) and Jamal et al. (2022) (A) | Metal dependent changes in the proteome of adult oysters, including NADH dehydrogenase (Muralidharan et al. 2012). | |
| Zinc (Zn) | Wisely and Blick (1967) (L), Butterfield (1987) (S), Muralidharan et al. (2012) (A), Taylor et al. (2013) (A), Lee et al. (2015) (A), Yingprasertchai et al. (2017) (L), Yingprasertchai et al. (2019) (A) and Jamal et al. (2022) (A) | Elevated larval mortality (Wisely and Blick 1967). Increased adult metallothionein expression (Yingprasertchai et al. 2019). Metal dependent changes in the proteome of adult oysters, specifically including tubulins and histones (Muralidharan et al. 2012). | |
| Tin (Sn) | Batley et al. (1989) (A) | Reduced growth and shell deformity in adult oysters. | |
| Aluminium (Al) | Wilson and Hyne (1997) (L), Dove and Sammut (2007b) (A) and Jamal et al. (2022) (A) | Disruption in embryonic development (Wilson and Hyne 1997) with higher mortality, reduced condition index and reduced growth in adults (Dove and Sammut 2007b; Jamal et al. 2022). | |
| Cadmium (Cd) | Ward (1982) (A), Taylor et al. (2013) (A), Schmitz et al. (2015) (A) and Yingprasertchai et al. (2019) (A) | Increased expression of metallothionein (Yingprasertchai et al. 2019), and HSP90, and a reduction in defensin (Taylor et al. 2013) in adults. Reduced total antioxidant capacity, increased lipid peroxidation and lysosome destabilisation (Schmitz et al. 2015). | |
| Mercury (Hg) | Wisely and Blick (1967) (L) | Elevated larval mortality (Wisely and Blick 1967). | |
| Nickel (Ni) | Jamal et al. (2022) (A) | Increased flesh Ni concentrations correlated with reduced body weight (Jamal et al. 2022). | |
| Magnesium (Mg) | Jamal et al. (2022) (A) | Increased flesh Mg concentrations correlated with reduced condition index (Jamal et al. 2022). | |
| Silver (Ag) | Carrazco-Quevedo et al. (2019) (A) | Increased DNA damage, and elevated lipid peroxidation and glutathione reductase in adults. |
The success of TBT control was evident in the recovery of S. glomerata populations and the return of the ecosystem services they provide (Fig. 1). Two years after the partial ban of TBT-based antifouling paints for vessels in NSW, assessments conducted in Sydney Harbour showed the absence of reduced growth and shell deformities seen before the ban. Healthy oysters exhibited significantly lower TBT concentrations, nearing the detection limit (0.2 μg Sn kg−1), with previously high tissue concentrations of copper and zinc also substantially reduced (Batley et al. 1992). Surveys conducted between 1995 and 2005 indicated a notable increase in oyster abundance and density, being particularly evident in areas with high shipping activity, suggesting that the TBT ban may have significantly contributed to the resurgence of S. glomerata in the estuary (Birch et al. 2013).
Estuarine acidification caused by outflows from drained landscapes containing acid sulfate soils (ASS) is a recurrent problem in many NSW estuaries, including those used for S. glomerata cultivation. ASS are characterised by soils and sediments containing oxidisable and already oxidised sulfides, predominantly iron pyrite (FeS2) (White and Melville 1996). Elements commonly associated with ASS-affected waters include SO4, Ca, Si, Mg, Na, Al, K, Fe, Zn, Cu, B, Mn and Cl (Sammut et al. 1996). The concentration of these elements in areas of S. glomerata production depends on the dilution of outflows by upland flows, mixing with estuary waters, and the pH-dependent solubility of the element, particularly Zn, Al and Fe (Sammut et al. 1996). In the 1990s, the S. glomerata industry first linked acidic, floodplain outflows with poor oyster production, prompting action from government authorities and scientists. Studies demonstrated that ASS-affected waters detrimentally affected the survival, growth and reproduction of S. glomerata (Dove and Sammut 2007a, 2013). Exposure to ASS-affected waters resulted in changes in the gills and mantle of S. glomerata, reduced filtration rates because of acid and Al gill damage, and Fe accumulation on gill surfaces (Dove and Sammut 2007b). Iron precipitates could also coat the substrate of the intertidal zone, affecting S. glomerata natural recruitment patterns (Bishop 2000), reducing wild oyster populations and the ecosystem services they provide.
Further examples of S. glomerata driving impactful research followed the recent catastrophic bushfires in NSW, including the ‘black summer’ fires, which raised concerns over the impact of ash on estuarine environments. These concerns were exacerbated by the potential for increased incidence and severity of fires with climate change. Bushfires can volatilise nutrients and metals, including Fe, Mn, As, Cu, Al, Pb, Cr Cd and Ni (Ito et al. 2020; Barros et al. 2022), leading to significant influxes into estuaries. Long-term, real-time environmental monitoring established for major S. glomerata production estuaries in 2018 (Ajani et al. 2024), coupled with regular oyster tissue and phytoplankton sampling, is now providing unique and valuable insights into the impacts of bushfires on estuarine biota. Specifically, the accumulation of Fe in oysters and its impacts on algal blooms, including potentially harmful Dinophysis spp. (Penny Ajani, pers. comm., 2024). These data and preserved biological samples now serve as a valuable archive for investigating and understanding new and emerging threats to NSW estuaries.
Typically, studies delving into the impacts of metals and metalloids on aquatic organisms focus on individual elements in isolation, or at most, consider simultaneous exposure to two or three related metals (Pan et al. 2015). However, organisms in the environment face mixtures of metals, which may be elevated in contaminated areas or following events such as floods and fires. Chronic exposure to certain metals has been demonstrated to enhance the bioaccumulation of other metals or metalloids in oysters (Liu and Wang 2014). A recent investigation in the Richmond River showed that combinations of multiple elements were robust predictors of S. glomerata health (Jamal et al. 2022). Specifically, the amalgamated concentrations of trace elements such as Hg, Ni, Cr, Cu, Cd, or Pb in the flesh exhibited strong correlations with oyster condition index and mortality rates across various sites, whereas individually, these elements did not display significant relationships. This study underscores the importance of the consideration of the synergistic impacts of all stressors, and that heightened mortality and diminished physical condition within natural S. glomerata populations can serve as valuable indicators of potential chronic pollution, aiding in prioritising areas for further investigation.
Overall, the study of metal impacts is one of the clearer examples of S. glomerata’s role in protecting our estuarine ecosystems. The comparative number of studies (Table 1) and their continuation for over 50 years is testament to their importance. The understanding developed has led to the use of oysters as bioindicators and in ecotoxicology assays to monitor pollutant inputs to protect estuaries. Critically this work has underpinned change, as was the case for TBT, and that has to led wider ecosystem benefits.
Pesticides
Efforts to increase food security, along with rapid growth in the human population, have led to increased production and use of a diversity of synthetic pesticides (Popp et al. 2013), including herbicides, insecticides and fungicides. Notably, in Australia, pesticide use is estimated to have increased 255% from 17,866 Mg in 1990 to 63,416 Mg in 2020 (Ritchie et al. 2022). These chemicals, when applied on land, can seep into water bodies and accrue in estuarine environments through surface run-off, spray drift and groundwater infiltration (Shishaye et al. 2021; Laicher et al. 2022; Warne et al. 2023). S. glomerata has been pivotal in our endeavours to monitor pesticide pollution and has been the subject of numerous studies scrutinising pesticide impacts on aquatic organisms.
The ability of bivalves to accumulate some chemical contaminants in their flesh makes them useful for environmental monitoring of pesticides. S. glomerata have been used to detect pesticide residues since 1970, when chlorinated hydrocarbon pesticide residue concentrations were monitored over 2 years in Morton Bay (Clegg 1974). Subsequently, Scanes (1996) developed the NSW Oyster Watch program where S. glomerata was deployed in Sydney ocean effluent outfalls to detect contaminants, whereas others, such as Hunter Water, have undertaken similar exercises (Andrew-Priestly et al. 2012). Scanes and Gibson (1996) also sampled wild S. glomerata to investigate organochlorine residues more broadly in estuaries on the NSW coast. An experimental field study using S. glomerata demonstrated the uptake of several organochlorine compounds from a contaminated location in Sydney Harbour, followed by depuration in a clean location (Scanes 1997). The biological half-lives of these pesticides were modelled and employed to inform the Mussel Watch and NSW EPA Oyster Watch programs for biomonitoring. Pesticide run-off into water bodies also presents risks to human consumer health. Consequently, under the NSW Shellfish Program, ongoing triennial surveys for persistent organic pollutants and pesticides have been conducted since 1999. This monitoring has indicated that oysters supplied for consumption have not been significantly affected by pesticide run-off. Nonetheless, this monitoring program persists while the oyster industry continues harvesting from estuarine environments, offering ongoing surveillance to safeguard both the industry and the estuarine ecosystem.
In the 1990s, a new class of neuroactive insecticides called neonicotinoids was registered for agricultural use and these rapidly became the most widely used insecticides worldwide (Ewere et al. 2021b). Extremely high environmental concentrations of the neonicotinoid imidacloprid (up to 294 μg L−1) were detected in non-oyster-producing intensive agricultural catchment draining into the Solitary Islands Marine Park in northern NSW (Laicher et al. 2022). Even so, S. glomerata uptake and depuration of imidacloprid was demonstrated and its sublethal effects elucidated through transcriptomics (Ewere et al. 2019a). At environmentally relevant concentrations, imidacloprid was found to affect haemocyte aggregation, haemolymph protein expression, acetylcholine esterase and oxidative stress enzyme activity of S. glomerata (Ewere et al. 2019b, 2020). Furthermore, exposure to both imidacloprid and a commercially available formulated product, caused alterations in fatty acid biosynthesis, leading to a reduction in healthy polyunsaturated fatty acids and thus lowering the nutritional quality of the flesh (Ewere et al. 2019b, 2020). The co-ingredients in commercial pesticide products, such as surfactants, dyes and antifoaming agents, are rarely considered in ecotoxicology testing, but can modify the bioavailability and toxicity of pesticides (Mesnage and Antoniou 2018).
Within complex agricultural and urban catchments, surface run-off is likely to transport numerous pesticides and associated contaminants simultaneously into estuarine environments. A recent study in the Richmond River on the North Coast of NSW found wild S. glomerata to be effective passive samplers, accumulating 13 different pesticides, compared with just five in water samples collected concurrently from the same location (Jamal et al. 2024). Benomyl, a pesticide banned in Australia, was detected, triggering further investigation by the NSW EPA. Chlorpyrifos, an insecticide currently subject to regulatory action restricting its use in Australia (Australian Pesticide and Veterinary Medicine Authority 2023), was also detected in the oysters, but not in the water samples (Jamal et al. 2024). Furthermore, pesticides, including the herbicides atrazine, diuron and hexazinone, that have been banned, severely restricted or not approved for use in the European Union because of ubiquitous water contamination concerns, were detected. These pesticides have also been found in surface run-off and groundwater in intensive agricultural catchments of the Great Barrier Reef (Lewis et al. 2009; Shishaye et al. 2021; Warne et al. 2023), thus highlighting the need for regulatory review of their use in Australia. None of the pesticides detected in S. glomerata by Jamal et al. (2024) have maximum residue limits (MRLs) for seafood and five do not have MRLs for meat products. Although they may not have been expected to contaminate seafood or meat, oysters have led the way and chlorpyrifos, atrazine, diuron and hexazinone have now been added to routine monitoring programs in NSW. Collectively, the pesticides found in S. glomerata represent 10 different chemical classes with seven distinct modes of action (Jamal et al. 2024), underscoring the potential for cumulative stress from synergistic or accumulative effects.
Endocrine disrupting chemicals (EDCs)
EDCs encompass a diverse range of environmentally persistent synthetic chemicals that mimic the structure of endocrine hormones and induce similar effects on accumulation by organisms. Among these compounds are oestrogenic contaminants, structurally resembling the female sex hormone oestrogen and capable of mimicking its effects. Examples include ethinyloestradiol (EE2), a component of contraceptive pills, bisphenol A (BPA) and breakdown products of alkylphenol ethoxylates such as nonylphenol (NP) and octophenol (OP). Oestrogenic contaminants enter water bodies from various sources, including industrial discharge and effluents from wastewater treatment plants, and are frequently detected in Australian wastewater and receiving waters (Leusch et al. 2005; Tan et al. 2007; Scott et al. 2017; Islam et al. 2021). Although oestrogenic EDC concentrations in Australian waterways are lower than those internationally reported, they still exceed predicted no-effect concentrations for various aquatic biota (e.g. 0.1 L−1 for EE2, Tran et al. 2019). With total effluent estimates from individual wastewater treatment plants in NSW reported at a staggering rate of 10,585 ML year−1, and most of these effluents directly being discharged into marine or estuarine environments, potential effects raise significant concern (Islam et al. 2021).
Several studies employing vertebrate models have been made, predominantly examining the role of EDCs in feminisation and vitellogenin induction in fish (e.g. Batty and Lim 1999; Codi King et al. 2008). Vitellogenin, a gonadal egg-yolk protein precursor, is an appropriate oestrogenic biomarker, because its production increases on exposure to oestrogens (Andrew et al. 2008). Vitellogenin offers a measure of the presence of complex oestrogenic mixtures where individual contaminants may be unknown and can demonstrate biologically significant impacts on the organism of interest. Remarkably, vitellogenin expression in males has been observed, serving as direct evidence of endocrine disruption because males typically do not produce vitellogenin under normal conditions (Tran et al. 2019). Exposure to EE2 and NP increases gonadal vitellogenin production up to three- and two-fold respectively, in female S. glomerata, and significant increases in vitellogenin were also observed in males exposed to 50 ng L−1 EE2 (Andrew et al. 2008). Further experiments showed that S. glomerata adults exposed to environmentally relevant concentrations of EE2 (0, 6.25, 12.5, 25 or 50 ng L−1) for 49 days exhibited dose-dependent increases in vitellogenin concentrations for both females and males (Andrew et al. 2010). Histological examination of gonads indicated a significant shift towards females over time, with the presence of intersex (ovotestis) individuals suggesting that oestrogenic exposure can facilitate the transition of protandric males towards female gametal status. These laboratory studies demonstrated the potential of S. glomerata as a biomonitor for detecting exposure and impacts of oestrogenic EDCs. In field studies, oysters deployed adjacent to an ocean outfall with effluent containing oestrogenic compounds exhibited elevated vitellogenin gene and protein expression compared with numerous reference deployments in the region (Andrew-Priestly et al. 2012). Additionally, females at the ocean outfall site showed more advanced stages of oocyte development than those at reference locations.
Perfluoroalkyl substances
Among the emerging threats to NSW estuaries and oyster cultivation in NSW are the increasing number of sites contaminated with perfluoroalkyl substances (PFAS). PFAS are persistent environmental pollutants used in a broad range of industrial applications and consumer goods that are now of worldwide concern for their potential adverse effects on human health, such as cardiovascular disease and cancer (Lindstrom et al. 2011). PFAS were initially detected in S. glomerata in Sydney Harbour (Thompson et al. 2011), but oyster farming did not occur in the area at the time, and commercial and recreational fishing had previously been banned (Roach et al. 2009). The gravity of the situation became more apparent when the first impacts on farmed oysters emerged in Port Stephens, one of the largest oyster-producing estuaries in NSW. The Tilligerry Creek farming area was affected by PFAS-contaminated runoff from a nearby airfield. Prompt testing commenced, with S. glomerata among the initial species monitored. Perfluorooctane sulfonate (PFOS) was detected in both S. glomerata and, the Pacific oyster, Crassostrea gigas. Fortunately, direct harvesting of oysters from the area was prohibited without depuration or relocation to an approved open harvest area for a period of 14 days. Both methods were found to result in significant reductions in oyster tissue PFOS concentrations (O’Connor et al. 2018). The level of concern for the environment and the industry was so high that this research, including depuration monitoring, was completed within 3 weeks of notification of the PFAS threat. Preliminary results were promptly disseminated to industry stakeholders and the public, and despite allaying some concerns, they added impetus and urgency for investigation in other estuarine species.
Microplastics
Microplastics are recognised globally as a threat to the marine environment, and discussions on their impacts on marine organisms, and ultimately on their consumers, are widespread (Carbery et al. 2018). Once again, research has utilised S. glomerata as a model organism to assess the risk to estuarine organisms in NSW and, in this case, to explore the response of the oyster industry to this threat.
Like many marine organisms, S. glomerata has been found to ingest and absorb microplastics, both in laboratory settings (Scanes et al. 2019) and when sampled from Australian estuaries (Wootton et al. 2022; Boyd 2023). On ingestion, microplastics may cause physical harm and serve as vectors for a variety of chemicals leaching from the plastic particles or adsorbed onto their surfaces, subsequently being released (Wright et al. 2013; Carbery et al. 2020; Bhagwat et al. 2021a, 2021b). Furthermore, the microbiome that swiftly develops on the surface of microplastic particles varies with plastic type, and can pose additional risks to oysters (Bhagwat et al. 2021a, 2021c) and other estuarine species.
As NSW oyster farmers transitioned from tar-treated timber sticks and trays to recyclable plastics in response to the threats posed by PAHs, the potential for oyster exposure to microplastics increased. Despite the minimal plastic usage by the industry compared with other sources, it has actively supported microplastic research. Efforts have commenced to monitor the degradation of plastics commonly employed in the oyster industry, to better comprehend the specific threats posed and aid in the selection of the most suitable plastic types for use in cultivation equipment (Bhagwat et al. 2021a). In a research first, the impact of long-term plastic aging through weathering and biofouling on plastic behaviour and fate in the marine environment utilised S. glomerata cultivation equipment to characterise contaminant profiles and bacterial communities. Using 10-year-old plastic infrastructure from intertidal, subtidal and sediment-buried segments of an oyster lease, assessments evaluated the risk to oysters, their consumers and the broader environment from microplastics of all origins finding high-density polyethylene plastics harboured high concentrations of metal(loid)s, PAHs and PFAS. Metagenomic analysis showed that the bacterial composition at the plastic surface differed by habitat type, and included potentially pathogenic bacterial taxa including Vibrio, Shewanella and Psychrobacter (Bhagwat et al. 2021a). This research helped broaden the understanding of the wider threat posed by microplastics beyond their accumulation in the food chain, to include their role as a vector for contaminants through the ecosystem.
Oyster industry
The success of efforts to restore and maintain estuarine health hinges on the values held by the community and stakeholders, their collective vision, and their enduring dedication to sustained action and investment (Thom et al. 2023). In this context, the NSW oyster industry emerges as a pivotal player in shaping this shared community vision. With its longstanding presence, the oyster industry has become deeply ingrained in the identity of many coastal communities in NSW, and the unwavering passion of farmers for both the industry and the waterways it relies on is widely recognised (Barclay et al. 2016; Fig. 3).
When surveying public perceptions of aquaculture in NSW, where oyster production dominates, Barclay et al. (2016) uncovered a high level of community trust in the value of aquaculture, owing to its social, economic and environmental contributions. Specifically for the oyster industry, the public expressed concerns about threats posed by residential development, river pollution, flooding, acid sulfate soils and toxic algal blooms. Reflecting the community belief in the interconnectedness of healthy oysters and healthy rivers, Barclay et al. (2016) found that the industry played a significant role in promoting environmental awareness, particularly in areas such as managing estuarine health, improving water quality and understanding estuarine ecology.
The ongoing efforts of oyster farmers to safeguard the resources they depend on are evident through their proactive identification of threats, advocacy efforts and adherence to self-imposed environmental protection measures outlined in their Environmental Management Systems (EMS), which are all publicly available (see https://www.nswoysters.com.au/environmental-management-systems.html). These EMS are estuary-specific and encourage farmers to work with other stakeholders to actively contribute to marine environmental protection and conservation through pollution reduction, habitat protection and sustainable resource management. Moreover, their commitment to adopting best practice guidelines underscores their dedication to environmental stewardship.
The future and natural recovery
The outlook for the S. glomerata industry and research remains promising, with a continued focus on enhancing estuarine environmental health. Economic incentives for research are growing, paralleling the increasing value of the industry (now exceeding A$80 million, NSW DPI, unpubl. data), which rivals the combined value of all commercial fisheries in NSW (BDO EconSearch 2023). Research priorities are evolving to address emerging threats in estuaries, including the complex interplay of stressors such as climate change, and flood and fire-related contaminants.
Whereas the NSW government remains committed to selective breeding for S. glomerata, the focus has broadened beyond commercial priorities to encompass traits relevant to broader environmental recovery and longevity, such as ocean acidification and warming resistance. Research efforts increasingly target the restoration of once-extensive oyster reefs (Cole et al. 2022). Projects aim to understand factors facilitating reef establishment and expansion, improve interventions in aquatic environments to support ecosystem maintenance and develop direct reef restoration techniques (Hart et al. 2024; Leong et al. 2024). Moreover, research continues to quantify the ecosystem benefits derived from reefs (Cole et al. 2022; Filippini et al. 2023), whether for community or commercial purposes (Bishop et al. 2023). Research on the ecosystem benefits of commercial oyster leases is also warranted; although natural oyster reef ecosystems have been largely absent from NSW estuaries for over a century (McAfee et al. 2022), active oyster farming has ensured that substantial populations of oysters have been maintained in most large waterways. It is important to understand better the role oyster farming can play in delivering ecosystem services such as improved water quality, and habitat provision to preserve the diversity and abundance of native estuarine species.
Innovative projects utilising eco-engineering techniques are underway to enrich native species biodiversity through habitat enhancement (Strain et al. 2018; Bishop et al. 2022). For instance, the ‘Living Seawalls: Bring Back the Sydney Rock Oyster’ project has demonstrated the benefits of seeding oysters onto engineered surfaces, resulting in increased abundance, richness and diversity of other taxa, as well as enhanced suspended particle removal rates from the water (Vozzo et al. 2021).
Although awareness of the scale of loss of S. glomerata reefs is by no means new, dedicated restoration efforts for Australian shellfish reefs began only in 2015 (McAfee et al. 2022). Importantly, the sustained research and environmental monitoring of the aquaculture industry have influenced government management of coastal water quality (Schrobback et al. 2014) and generated the knowledge to underpin restoration efforts (McAfee et al. 2022). Currently, 14 reef restoration projects are underway across southern Australia, with plans to expand to 60 projects by 2030 (The Nature Conservancy 2024).
In addition to reef restoration, ‘oyster gardening’ has been a popular initiative elsewhere in the world (Brumbaugh and Coen 2009; Oesterling and Petrone 2012), allowing citizen scientists to broaden the scale and scope of restoration activities. It can be achieved cheaply and allows participants to contribute to community needs (Toomey et al. 2020; Agnello et al. 2022). This cost-effective approach not only provides adult oysters for restoration programs, but also supports other ecosystem services such as habitat provision for fishes and invertebrates, while fostering community involvement (Boström-Einarsson et al. 2022). Australia’s first oyster gardening program began in a canal estate on Bribie Island in Moreton Bay in 2016–17, and was undertaken using S. glomerata that was later used in nearby reef restoration initiatives (Boström-Einarsson et al. 2022).
Aligned with the oyster industry commitment to sustainability and maintaining social license, efforts are underway to map its impacts and quantify the ecosystem benefits of farming S. glomerata. These assessments draw on decades-old physiological studies of S. glomerata, such as those of Bayne and Svensson (2006), but are expanding on this work and considering them in a broader ecosystem services framework. For example, the role of the NSW oyster industry in nutrient removal from estuaries has recently been assessed, with an estimated A$5.2 million worth of nitrogen and phosphorus being assimilated (BDO EconSearch 2023). The value of S. glomerata farm infrastructure as habitat for other marine organisms has been confirmed, with rack and basket culture both found to support at least as many species of fish as adjacent biogenic habitats such as oyster reefs, seagrass and mangroves (Martínez-Baena et al. 2022). These authors noted that the ecosystem services S. glomerata should be considered in estuarine habitat enhancement, conservation and restoration, and the oyster industry is engaging further with the concept of regenerative aquaculture to promote net positive environmental outcomes, as explored by Alleway et al. (2019) and Gentry et al. (2020).
Conclusions
S. glomerata is unique among the many animals that inhabit NSW estuaries. It is a keystone species in the ecology of our estuaries, it forms the basis of NSW’s most valuable fishery, is NSW’s oldest aquaculture industry, and is considered one of our greatest gastronomic delicacies. In the minds of the NSW public, it has been strongly linked with the health of our estuaries for 150 years. Whether S. glomerata is ‘the canary in the mine’, the ‘doormat of the estuary’ (Ogburn 2011) or the ‘lungs of healthy estuaries’ (Diggles 2015), they have been, and most likely will continue to be, a focus for research and a rallying point for the protection of one of NSW’s most valuable assets, namely, its estuarine ecosystems and inhabitants.
Globally, S. glomerata farming is not unique among mollusc aquaculture industries for its wider social, economic and ecological contribution. The body of research around other commonly cultivated species such as Crassostrea gigas and C. virginica, is substantially larger. These species have similarly been used for pollutant impact evaluations (Alzieu et al. 1986), biomonitoring (Lauenstein et al. 1990) and climate-change impact assessment (Gazeau et al. 2013). C. virginica has been the subject of reef restoration efforts for decades (Howie and Bishop 2021), which are supported by an increasing public awareness of the ecosystem services provided by oysters. However, aquaculture still faces issues with social license and as we develop concepts such as regenerative aquaculture (Alleway et al. 2019), the full extent of the benefits arising from commercial interest in a species need to be elaborated. Here, we have tried to describe the range of benefits that have arisen from the S. glomerata industry, to assist industry in furthering social license and in the hope that it may encourage other industries to chronicle their contributions.
Data availability
All data used in the preparation of this review was obtained from published literature listed in the references.
References
Agnello G, Vercammen A, Knight AT (2022) Understanding citizen scientists’ willingness to invest in, and advocate for, conservation. Biological Conservation 265, 109422.
| Crossref | Google Scholar |
Ajani P, Larsson ME, Rubio A, Bush S, Brett S, Farrell H (2016) Modelling bloom formation of the toxic dinoflagellates Dinophysis acuminata and Dinophysis caudata in a highly modified estuary, south eastern Australia. Estuarine, Coastal and Shelf Science 183, 95-106.
| Crossref | Google Scholar |
Ajani P, Harwood DT, Murray S (2017) Recent trends in marine phycotoxins from Australian coastal waters. Marine Drugs 15(2), 33.
| Crossref | Google Scholar |
Ajani PA, Larsson ME, Woodcock S, Rubio A, Farrell H, Brett S, Murray SA (2020) Fifteen years of Pseudo-nitzschia in an Australian estuary, including the first potentially toxic P. delicatissima bloom in the southern hemisphere. Estuarine Coastal and Shelf Science 236, 106651.
| Crossref | Google Scholar |
Ajani PA, Verma A, Kim JH, Woodcock S, Nishimura T, Farrell H, Zammit A, Brett S, Murray SA (2021) Using qPCR and high-resolution sensor data to model a multi-species Pseudo-nitzschia (Bacillariophyceae) bloom in southeastern Australia. Harmful Algae 108, 102095.
| Crossref | Google Scholar |
Ajani P, Dove M, Farrell H, O’Connor W, Tesoreiro M, Verma A, Zammit A, Hughes B, Murray SA (2024) High-resolution temperature, salinity and depth data from southeastern Australian estuaries, 2018-2021. Scientific Data 11, 968.
| Crossref | Google Scholar |
Alleway HK, Gillies CL, Bishop MJ, Gentry RR, Theuerkauf SJ, Jones R (2019) The ecosystem services of marine aquaculture: valuing benefits to people and nature. BioScience 69, 59-68.
| Crossref | Google Scholar |
Alzieu CL, Sanjuan J, Deltreil JP, Borel M (1986) Tin contamination in Arcachon Bay: effects on oyster shell anomalies. Marine Pollution Bulletin 17(11), 494-498.
| Crossref | Google Scholar |
Andrew-Priestly MN, O’Connor WA, Dunstan RH, Van Zwieten L, Tyler T, Kumar A, Macfarlane GR (2012) Estrogen mediated effects in the Sydney rock oyster, Saccostrea glomerata, following field exposures to sewage effluent containing estrogenic compounds and activity. Aquatic Toxicology 120-121, 99-108.
| Crossref | Google Scholar |
Andrew MN, Dunstan RH, O’Connor WA, Van Zwieten L, Nixon B, MacFarlane GR (2008) Effects of 4-nonylphenol and 17α-ethynylestradiol exposure in the Sydney rock oyster, Saccostrea glomerata: vitellogenin induction and gonadal development. Aquatic Toxicology 88(1), 39-47.
| Crossref | Google Scholar | PubMed |
Andrew MN, O’Connor WA, Dunstan RH, MacFarlane GR (2010) Exposure to 17α-ethynylestradiol causes dose and temporally dependent changes in intersex, females and vitellogenin production in the Sydney rock oyster. Ecotoxicology 19, 1440-1451.
| Crossref | Google Scholar | PubMed |
Anon. (1902) Contaminated oysters. Poisoning and typhoid fever. In Sydney Morning Herald, 19 December 1902, p. 5. Available at https://trove.nla.gov.au/newspaper/article/14493749
Anon. (1903) Middle Harbour sewage. Poison in the local oysters. In Sydney Morning Herald, 15 October 1903, p. 3. Available at https://trove.nla.gov.au/newspaper/article/14566114
Anon. (1906) Oysters from Crown Lands. In Sydney Morning Herald, 6 July 1906, p. 3. Available at http://nla.gov.au/nla.news-article14784017
Anon. (1940) Proposed oil refinery. Effects on Oyster beds. Farmers fear pollution. In Sydney Morning Herald, 27 March 1940, p. 14. Available at http://nla.gov.au/nla.news-article17658135
Anon. (1997) Hepatitis A outbreak in New South Wales. Communicable Disease Intelligence 21(4), 46.
| Google Scholar |
Australian Pesticide and Veterinary Medicine Authority (2023) Chlorpyrifos. (APVMA) Available at https://www.apvma.gov.au/resources/chemicals-news/chlorpyrifos [Verified May 2024]
Australian Shellfish Quality Assurance Advisory Committee (2024) Australian Shellfish Quality Assurance Program – Operations Manual (Version 8). (Safefish) Available at https://safefish.com.au/report/https-safefish-com-au-wp-content-uploads-2024-12-asqaac-manual-v8-2024-pdf/
Avery EL, Dunstan RH, Nell JA (1996) The detection of pollutant impact in marine environments: condition index, oxidative DNA damage, and their associations with metal bioaccumulation in the Sydney rock oyster Saccostrea commercialis. Archives of Environmental Contamination and Toxicology 31, 192-198.
| Crossref | Google Scholar | PubMed |
Barclay K, McIlgorm A, Mazur N, Voyer M, Schnierer S, Payne AM (2016) Social and economic evaluation of NSW coastal aquaculture. FRDC 2015/302. (Fisheries Research and Development Corporation) Available at https://www.frdc.com.au/project/2015-302
Barros TL, Bracewell SA, Mayer-Pinto M, Dafforn KA, Simpson SL, Farrell M, Johnston EL (2022) Wildfires cause rapid changes to estuarine benthic habitat. Environmental Pollution 308, 119571.
| Crossref | Google Scholar |
Barua A, Ajani PA, Ruvindy R, Farrell H, Zammit A, Brett S, Hill D, Sarowar C, Hoppenrath M, Murray SA (2020) First detection of paralytic shellfish toxins from Alexandrium pacificum above the regulatory limit in blue mussels (Mytilus galloprovincialis) in New South Wales, Australia. Microorganisms 8, 905.
| Crossref | Google Scholar |
Bates H, Pierce M, Benter A (2021) Real-time environmental monitoring for aquaculture using a LoRaWAN-based IoT sensor network. Sensors 21(23), 7963.
| Crossref | Google Scholar |
Batley GE, Fuhua C, Brockbank CI, Flegg KJ (1989) Accumulation of tributyltin by the sydney rock oyster, Saccostrea commercialis. Australian Journal of Marine and Freshwater Research 40, 49-54.
| Crossref | Google Scholar |
Batley GE, Scammell MS, Brockbank CI (1992) The impact of the banning of tributyltin-based antifouling paints on the Sydney rock oyster, Saccostrea commercialis. Science of The Total Environment 122(3), 301-314.
| Crossref | Google Scholar |
Batty J, Lim R (1999) Morphological and reproductive characteristics of male mosquitofish (Gambusia affinis holbrooki) inhabiting sewage-contaminated waters in New South Wales, Australia. Archives of Environmental Contamination and Toxicology 36, 301-307.
| Crossref | Google Scholar | PubMed |
Bayne BL (2002) A physiological comparison between Pacific oysters Crassostrea gigas and Sydney rock oysters Saccostrea glomerata: food, feeding and growth in a shared estuarine habitat. Marine Ecology Progress Series 232, 163-178.
| Crossref | Google Scholar |
Bayne BL, Svensson S (2006) Seasonal variability in feeding behaviour, metabolic rates and carbon and nitrogen balances in the Sydney oyster, Saccostrea glomerata (Gould). Journal of Experimental Marine Biology and Ecology 332(1), 12-26.
| Crossref | Google Scholar |
BDO EconSearch (2023) Economic contribution of aquaculture to New South Wales. A report for the NSW Department of Primary Industries. (BDO: Adelaide, SA, Australia) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0008/1499606/NSW-Aquaculture-Economic-Contribution_Final-2023.pdf
Bhagwat G, Carbery M, Anh Tran TK, Grainge I, O’Connor W, Palanisami T (2021a) Fingerprinting plastic-associated inorganic and organic matter on plastic aged in the marine environment for a decade. Environmental Science & Technology 55, 7407-7417.
| Crossref | Google Scholar |
Bhagwat G, Tran TKA, Lamb D, Senathirajah K, Grainge I, O’Connor W, Juhasz A, Palanisami T (2021b) Biofilms enhance the adsorption of toxic contaminants on plastic microfibres under environmentally relevant conditions. Environmental Science & Technology 55, 8877-8887.
| Crossref | Google Scholar |
Bhagwat G, Zhu Q, O’Connor W, Subashchandrabose S, Grainge I, Knight R, Palanisami T (2021c) Exploring the composition and functions of plastic microbiome using whole-genome sequencing. Environmental Science & Technology 55, 4899-4913.
| Crossref | Google Scholar |
Birch GF, Apostolatos C, Taylor SE (2013) A remarkable recovery in the Sydney rock oyster (Saccostrea glomerata) population in a highly urbanised estuary (Sydney Estuary, Australia). Journal of Coastal Research 29(5), 1009-1015.
| Crossref | Google Scholar |
Bishop MJ, Vozzo ML, Mayer-Pinto M, Dafforn KA (2022) Complexity–biodiversity relationships on marine urban structures: reintroducing habitat heterogeneity through eco-engineering. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 377, 20210393.
| Crossref | Google Scholar |
Bishop MJ, Lanham BS, Esquivel-Muelbert JR, Cole VJ, Faelnar KM, Jenkins C, Keating J, Martínez-Baena F, O’Connor WA (2023) Oyster reef restoration – aquaculture interactions: maximizing positive synergies. Frontiers in Marine Science 10, 1162487.
| Crossref | Google Scholar |
Boström-Einarsson L, Martínez-Baena F, Diggles B, Firby L, McLeod IM (2022) An ecological assessment of Australia’s first community oyster gardens. Ecological Management & Restoration 23, 244-251.
| Crossref | Google Scholar |
Brumbaugh RD, Coen LD (2009) Contemporary approaches for small-scale oyster reef restoration to address substrate versus recruitment limitation: a review and comments relevant for the Olympia oyster, Ostrea lurida Carpenter 1864. Journal of Shellfish Research 28, 147-161.
| Crossref | Google Scholar |
Canadian Council of Resource and Environment Ministers (1987) Canadian water quality guidelines. (CCREM: Ottawa, ON, Canada) Available at chrome-https://alarmmyanmar.org/pdf/2.CanadianWater.pdf
Carbery M, O’Connor W, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environment International 115, 400-409.
| Crossref | Google Scholar | PubMed |
Carbery M, Macfarlane GR, O’Connor W, Afrose S, Taylor H, Palanisami T (2020) Baseline analysis of metal(loid)s on microplastics collected from the Australian shoreline using citizen science. Marine Pollution Bulletin 152, 110914.
| Crossref | Google Scholar |
Carrazco-Quevedo A, Römer I, Salamanca MJ, Poynter A, Lynch I, Valsami-Jones E (2019) Bioaccumulation and toxic effects of nanoparticulate and ionic silver in Saccostrea glomerata (rock oyster). Ecotoxicology and Environmental Safety 179, 127-134.
| Crossref | Google Scholar | PubMed |
Clegg DE (1974) Chlorinated hydrocarbon pesticide residues in oysters (Crassostrea commercialis) in Moreton Bay, Queensland, Australia. Pesticides Monitoring Journal 8(3), 162-166.
| Google Scholar | PubMed |
Codi King S, Hassell K, Nugegoda D, Kristiansen SI (2008) The assessment of vitellogenin as a biomarker of exposure to estrogenic compounds in two Australian perciformes. Marine Environmental Research 66, 116-118.
| Crossref | Google Scholar | PubMed |
Cole VJ, Parker LM, O’Connor SJ, O’Connor WA, Scanes E, Byrne M, Ross PM (2016) Effects of multiple climate change stressors: ocean acidification interacts with warming, hyposalinity, and low food supply on the larvae of the brooding flat oyster Ostrea angasi. Marine Biology 163(5), 125.
| Crossref | Google Scholar |
Cole VJ, Harasti D, Lines R, Stat M (2022) Estuarine fishes associated with intertidal oyster reefs characterized using environmental DNA and baited remote underwater video. Environmental DNA 4(1), 50-62.
| Crossref | Google Scholar |
Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski W, Johns T, Krinner G (2013) Long-term climate change: projections, commitments and irreversibility. In ‘Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley) pp. 1029–1136. (Cambridge University Press: Cambridge, UK, and New York, NY, USA)
Davis B, Birch G (2010) Comparison of heavy metal loads in stormwater runoff from major and minor urban roads using pollutant yield rating curves. Environmental Pollution 158(8), 2541-2545.
| Crossref | Google Scholar | PubMed |
Davis BS, Birch GF (2011) Spatial distribution of bulk atmospheric deposition of heavy metals in metropolitan Sydney, Australia. Water, Air, & Soil Pollution 214(1–4), 147-162.
| Crossref | Google Scholar |
Diggles B (2015) Protection and repair of Australia’s shellfish reefs, Southern Queensland Report. Client Report DF15-01, prepared for National Environmental Science Program. (DigsFish Services: Bribie Island, Qld, Australia) Available at http://restorepumicestonepassage.org/wp-content/uploads/2014/04/NESP-Project-Southern-Queensland-Report-DigsFish.pdf
Dove MC, O’Connor WA (2007) Ecotoxicological evaluations of common hatchery substances and procedures used in the production of Sydney rock oysters Saccostrea glomerata (Gould 1850). Journal of Shellfish Research 26, 501-508.
| Crossref | Google Scholar |
Dove MC, Sammut J (2007a) Impacts of estuarine acidification on survival and growth of sydney rock oysters Saccostrea glomerata (Gould 1850). Journal of Shellfish Research 26(2), 519-527.
| Crossref | Google Scholar |
Dove MC, Sammut J (2007b) Histological and feeding response of Sydney rock oysters, Saccostrea glomerata, to acid sulfate soil outflows. Journal of Shellfish Research 26(2), 509-518.
| Crossref | Google Scholar |
Dove MC, Sammut J (2013) Acid sulfate soil induced acidification of estuarine areas used for the production of Sydney rock oysters, Saccostrea glomerata. Journal of Water Resource and Protection 5, 320-335.
| Crossref | Google Scholar |
Ecotox Services Australasia (2024) Toxicity Test Fact Sheet #7. (ESA: Sydney, NSW, Australia) Available at https://www.ecotox.com.au/wp-content/uploads/2018/11/Testfactsheet7.pdf
Ertl NG, O’Connor WA, Brooks P, Keats M, Elizur A (2016a) Combined exposure to pyrene and fluoranthene and their molecular effects on the Sydney rock oyster, Saccostrea glomerata. Aquatic Toxicology 177, 136-145.
| Crossref | Google Scholar |
Ertl NG, O’Connor WA, Papanicolaou A, Wiegand AN, Elizur A (2016b) Transcriptome analysis of the Sydney rock oyster, Saccostrea glomerata: insights into molluscan immunity. PLoS ONE 11, e0156649.
| Crossref | Google Scholar |
Ewere EE, Powell D, Rudd D, Reichelt-Brushett A, Mouatt P, Voelcker NH, Benkendorff K (2019a) Uptake, depuration and sublethal effects of the neonicotinoid, imidacloprid, exposure in Sydney rock oysters. Chemosphere 230, 1-13.
| Crossref | Google Scholar |
Ewere EE, Reichelt-Brushett A, Benkendorff K (2019b) Imidacloprid and formulated product impacts the fatty acids and enzymatic activities in tissues of Sydney rock oysters, Saccostrea glomerata. Marine Environmental Research 151, 104765.
| Crossref | Google Scholar |
Ewere EE, Reichelt-Brushett A, Benkendorff K (2020) The neonicotinoid insecticide imidacloprid, but not salinity, impacts the immune system of Sydney rock oyster, Saccostrea glomerata. Science of The Total Environment 742, 140538.
| Crossref | Google Scholar |
Ewere EE, Rosic N, Bayer PE, Ngangbam A, Edwards D, Kelaher BP, Mamo LT, Benkendorff K (2021a) Marine heatwaves have minimal influence on the quality of adult Sydney rock oyster flesh. Science of The Total Environment 795, 148846.
| Crossref | Google Scholar |
Ewere EE, Reichelt-Brushett A, Benkendorff K (2021b) Impacts of neonicotinoids on molluscs: what we know and what we need to know. Toxics 9, 21.
| Crossref | Google Scholar |
Farrell H, Seebacher F, O’Connor W, Zammit A, Harwood DT, Murray S (2015) Warm temperature acclimation impacts metabolism of paralytic shellfish toxins from Alexandrium minutum in commercial oysters. Global Change Biology 21(9), 3402-3413.
| Crossref | Google Scholar | PubMed |
Filippini G, Bugnot AB, Ferguson A, Gribben PE, Palmer J, Erickson K, Dafforn KA (2023) The influence of oyster reefs and surrounding sediments on nitrogen removal – an in situ study along the east coast of Australia. Environmental Research 237(5), 116947.
| Crossref | Google Scholar |
Fitzer SC, Torres Gabarda S, Daly L, Hughes B, Dove M, O’Connor W, Potts J, Scanes P, Byrne M (2018) Coastal acidification impacts on shell mineral structure of bivalve mollusks. Ecology and Evolution 8(17), 8973-8984.
| Crossref | Google Scholar | PubMed |
Fitzer SC, McGill RAR, Torres Gabarda S, Hughes B, Dove M, O’Connor W, Byrne M (2019) Selectively bred oysters can alter their biomineralization pathways, promoting resilience to environmental acidification. Global Change Biology 25, 4105-4115.
| Crossref | Google Scholar | PubMed |
Food Standards Australia New Zealand (2025) Food Standards Code: guidance and resources. (FSANZ) Available at https://www.foodstandards.gov.au/food-standards-code
Gazeau F, Parker LM, Comeau S, Gattuso J-P, O’Connor WA, Martin S, Pörtner H-O, Ross PM (2013) Impacts of ocean acidification on marine shelled molluscs. Marine Biology 160, 2207-2245.
| Crossref | Google Scholar |
Geary PM, Evans CA, Maswabi TM, Lee C CC, Zammit A, Webster G, Hunter M (2015) Monitoring and tracking contaminant sources in estuaries. Water Practice and Technology 10(3), 601-608.
| Crossref | Google Scholar |
Gentry RR, Alleway HK, Bishop MJ, Gillies CL, Waters T, Jones R (2020) Exploring the potential for marine aquaculture to contribute to ecosystem services. Reviews in Aquaculture 12, 499-512.
| Crossref | Google Scholar |
Gibbs MC, Parker LM, Scanes E, Ross PM (2024) Recognising the importance of shellfish to First Nations peoples, Indigenous and Traditional Ecological Knowledge in aquaculture and coastal management in Australia. Marine and Freshwater Research 75, MF23193.
| Crossref | Google Scholar |
Gillies CL, McLeod IM, Alleway HK, Cook P, Crawford C, Creighton C, et al. (2018) Australian shellfish ecosystems: past distribution, current status and future direction. PLoS ONE 13(2), e0190914.
| Crossref | Google Scholar |
Gilligan G (2002) Royal commissions of inquiry. Australia & New Zealand Journal of Criminology 35(3), 289-307.
| Crossref | Google Scholar |
Goncalves P, Anderson K, Thompson EL, Melwani A, Parker LM, Ross PM, Raftos DA (2016) Rapid transcriptional acclimation following transgenerational exposure of oysters to ocean acidification. Molecular Ecology 25(19), 4836-4849.
| Crossref | Google Scholar | PubMed |
Green TJ, Helbig K, Speck P, Raftos DA (2016) Primed for success: oyster parents treated with poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Molecular Immunology 78, 113-120.
| Crossref | Google Scholar | PubMed |
Grennan H (2008) The world is now their oyster. In Sydney Morning Herald, 22 July 2008. Available at https://www.smh.com.au/national/the-world-is-now-their-oyster-20080722-gdsn5l.html
Griffiths JS, Johnson KM, Sirovy KA, Yeats MS, Pan FTC, La Peyre JF, Kelly MW (2021) Transgenerational plasticity and the capacity to adapt to low salinity in the eastern oyster, Crassostrea virginica. Proceedings of the Royal Society of London – B. Biological Sciences 288, 20203118.
| Crossref | Google Scholar |
Handmer J, Hillman M (2004) Economic and financial recovery from disaster. The Australian Journal of Emergency Management 19(4), 44-50.
| Google Scholar |
Hart JL, Smith RL, Poore AGB, Erickson KR, Figueira WF, Bishop MJ, O’Connor WA, Gribben PE (2024) Habitat-setting affects biodiversity while predation determines oyster survival on experimental oyster reefs. Restoration Ecology 32, e14133.
| Crossref | Google Scholar |
Healthy Rivers Commission (2003) Healthy Rivers for Tomorrow. Final report, November 2003. (HRC: Sydney, NSW, Australia) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0006/634254/HRC_Healthy-Rivers-for-Tomorrow_2003.pdf
House of Representatives Committee (2003) International convention on the control of harmful anti-fouling systems on ships (the AFS convention). In ‘Report 52: treaties tabled in March 2003’. pp. 73–81. (Commonwealth of Australia) Available at https://www.aph.gov.au/~/media/wopapub/house/committee/jsct/march2003/report/chap5_pdf.ashx
Howie AH, Bishop MJ (2021) Contemporary oyster reef restoration: responding to a changing world. Frontiers in Ecology and Evolution 9, 689915.
| Crossref | Google Scholar |
Idowu O, Tran TKA, Baker P, Farrel H, Zammit A, Semple KT, O’Connor W, Thavamani P (2020a) Bioavailability of polycyclic aromatic compounds (PACs) to the Sydney rock oyster (Saccostrea glomerata) from sediment matrices of an economically important Australian estuary. Science of The Total Environment 736, 139574.
| Crossref | Google Scholar |
Idowu O, Tran TKA, Webster G, Chapman I, Baker P, Farrel H, Zammit A, Semple KT, Hansbro PM, O’Connor W, Thavamani P (2020b) Quantitative biomonitoring of polycyclic aromatic compounds (PACs) using the Sydney rock oyster (Saccostrea glomerata). Science of The Total Environment 742, 140497.
| Crossref | Google Scholar |
Islam R, Yu RMK, Andrew-Priestley M, Smith N, Rahman MM, Tran TKA, O’Connor WA, MacFarlane GR (2021) Secondary treatment phase of tertiary wastewater treatment works significantly reduces estrogenic load. Water Research 200, 117257.
| Crossref | Google Scholar |
Islam R, Yu RMK, O’Connor WA, Lin X, Lai KP, Leusch FDL, Macfarlane GR (2024) Intergenerational toxicity of 17α-ethinylestradiol (EE2): effects of parental exposure on early larval development and transcriptomic profiles in the Sydney rock oyster, Saccostrea glomerata. Journal of Hazardous Materials 475, 134876.
| Crossref | Google Scholar |
Ito A, Perron MMG, Proemse BC, Strzelec M, Gault-Ringold M, Boyd PW, Bowie AR (2020) Evaluation of aerosol iron solubility over Australian coastal regions based on inverse modeling: implications of bushfires on bioaccessible iron concentrations in the Southern Hemisphere. Progress in Earth and Planetary Science 7, 42.
| Crossref | Google Scholar |
Jamal E, Reichelt-Brushett A, Benkendorff K (2022) Exposure to multiple elements reduces the health of Saccostrea glomerata: an assessment of the Richmond River Estuary, NSW Australia. Marine Pollution Bulletin 184, 114177.
| Crossref | Google Scholar |
Jamal E, Reichelt-Brushett A, Gillmore M, Pearson B, Benkendorff K (2024) Pesticide occurrence in a subtropical estuary, Australia: complementary sampling methods. Environmental Pollution 342, 123084.
| Crossref | Google Scholar |
Kardamanidis K, Corbett SJ, Zammitt AP (2009) Bug breakfast in the bulletin: hepatitis a: Wallis Lake revisited. NSW Public Health Bulletin 20(2), 29-30.
| Crossref | Google Scholar |
Kraa E (1990) Oyster related food poisoning. New South Wales Public Health Bulletin 1(6), 11.
| Crossref | Google Scholar |
Krassoi R (1995) Salinity adjustment of effluents for use with marine bioassays: effects on the larvae of the doughboy scallop Chlamys asperrimus and the Sydney rock oyster Saccostrea commercialis. Australasian Journal of Ecotoxicology 1, 143-148.
| Google Scholar |
Laicher D, Benkendorff K, White S, Conrad S, Woodrow RL, Butcherine P, Sanders CJ (2022) Pesticide occurrence in an agriculturally intensive and ecologically important coastal aquatic system in Australia. Marine Pollution Bulletin 180, 113675.
| Crossref | Google Scholar |
Lauenstein GG, Robertson A, O’Connor TP (1990) Comparison of trace metal data in mussels and oysters from a mussel watch programme of the 1970s with those from a 1980s programme. Marine Pollution Bulletin 21, 440-447.
| Crossref | Google Scholar |
Lee J-H, Birch GF, Cresswell T, Johansen MP, Adams MS, Simpson SL (2015) Dietary ingestion of fine sediments and microalgae represent the dominant route of exposure and metal accumulation for Sydney rock oyster (Saccostrea glomerata): a biokinetic model for zinc. Aquatic Toxicology 167, 46-54.
| Crossref | Google Scholar | PubMed |
Lee JY, Marotzke J, Bala G, Cao L, Corti AA, Dunne J, Engelbrecht F, Fischer E, Fyfe J, Jones C, Maycock A, Mutemi J, Ndiaye O, Panickal S, Zhou T (2021) Future global climate: scenario-based projections and near-term information. In ‘Climate Change 2021: the Physical Science Basis. Working Group I Contribution to the Intergovernmental Panel on Climate Change (IPCC) Sixth Assessment Report (ARC-WG1)’. (Eds V Masson-Delmotte, P Zhai, A Pirani, CL Connors, C Pean, Y Chen, L Goldfarb, MI Gomis, JBR Matthews, S Berger, M Hurang, O Yelekci, R Yu, B Zhou, L Lonnoy, TK Maycock, T Waterfield, K Leitzell, N Caud) pp. 553–672. (Cambridge University Press: Cambridge, UK, and New York, NY, USA) 10.1017/9781009157896.006
Leong RC, Bugnot AB, Ross PM, Erickson KR, Gibbs MC, Marzinelli EM, O’Connor WA, Parker LM, Poore AGB, Scanes E, Gribben PE (2024) Recruitment of a threatened foundation oyster species varies with large and small spatial scales. Ecological Applications 34, e2968.
| Crossref | Google Scholar |
Leusch FDL, Chapman HF, Körner W, Gooneratne SR, Tremblay LA (2005) Efficacy of an advanced sewage treatment plant in southeast Queensland, Australia, to remove estrogenic chemicals. Environmental Science & Technology 39(15), 5781-5786.
| Crossref | Google Scholar | PubMed |
Lewis SE, Brodie JE, Bainbridge ZT, Rohde KW, Davis AM, Masters BL, Maughan M, Devlin MJ, Mueller JF, Schaffelke B (2009) Herbicides: a new threat to the Great Barrier Reef. Environmental Pollution 157(8-9), 2470-2484.
| Crossref | Google Scholar | PubMed |
Lindstrom AB, Strynar MJ, Libelo EL (2011) Polyfluorinated compounds: past, present, and future. Environmental Science & Technology 45, 7954-7961.
| Crossref | Google Scholar | PubMed |
Liu F, Wang WX (2014) Differential influences of Cu and Zn chronic exposure on Cd and Hg bioaccumulation in an estuarine oyster. Aquatic Toxicology 148, 204-210.
| Crossref | Google Scholar |
Lockwood D (2014) Waterways pollution remains a problem in Sydney. In Sydney Morning Herald, 17 April 2014. Available at https://www.smh.com.au/sport/waterways-pollution-remains-a-problem-in-sydney-20140417-zqvxs.html
Martínez-Baena F, Lanham BS, McLeod I, Taylor MD, McOrrie S, Bishop MJ (2022) De novo reefs: fish habitat provision by oyster aquaculture varies with farming method. Aquaculture Environment Interactions 14, 71-84.
| Crossref | Google Scholar |
McAfee D, McLeod IM, Boström-Einarsson L, Gillies CL (2020) The value and opportunity of restoring Australia’s lost rock oyster reefs. Restoration Ecology 28, 304-314.
| Crossref | Google Scholar |
McAfee D, McLeod IM, Alleway HK, Bishop MJ, Branigan S, Connell SD, Copeland C, Crawford CM, Diggles BK, Fitzsimons JA, Gilby BL, Hamer P, Hancock B, Pearce R, Russell K, Gillies CL (2022) Turning a lost reef ecosystem into a national restoration program. Conservation Biology 36, e13958.
| Crossref | Google Scholar |
Melwani AR, Thompson EL, Raftos DA (2016) Differential proteomic response of Sydney rock oysters (Saccostrea glomerata) to prolonged environmental stress. Aquatic Toxicology 173, 53-62.
| Crossref | Google Scholar | PubMed |
Mesnage R, Antoniou MN (2018) Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Frontiers in Public Health 5(5), 361.
| Crossref | Google Scholar |
Muralidharan S, Thompson E, Raftos D, Birch G, Haynes PA (2012) Quantitative proteomics of heavy metal stress responses in Sydney rock oysters. Proteomics 12, 906-921.
| Crossref | Google Scholar | PubMed |
Murray S, Ajani P, Zammit A, Farrell H, O’Connor W (2024) Transforming Australian Shellfish Production. Project Number FA 076. (Food Agility CRC) Available at https://www.foodagility.com/research/transforming-australian-shellfish-production
Nell JA, Holliday JE (1986) Effects of potassium and copper on the settling rate of Sydney rock oyster (Saccostrea commercialis) larvae. Aquaculture 58, 263-267.
| Crossref | Google Scholar |
NSW Department of Primary Industries and Regional Development (2022) Toxic algae blooms detected on NSW coastline. 21 Oct 2022. (NSW Government) Available at https://www.dpi.nsw.gov.au/about-us/media-centre/releases/2022/general/toxic-algae-blooms-detected-on-nsw-coastline
NSW Department of Primary Industries and Regional Development (2024) Aquaculture in New South Wales: Facts and Figures 2024. (NSW Government) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0020/1432208/Aquaculture-facts-figures-2024.pdf
Ogburn DM (2011) The NSW oyster industry: a risk indicator of sustainable coastal policy and practice. PhD dissertation, The Australian National University, Canberra, ACT, Australia. 10.25911/5d7a266d782dc
Ogburn DM, White I, McPhee DP (2007) The disappearance of oyster reefs from eastern Australian estuaries – impact of colonial settlement or mudworm invasion? Coastal Management 35(2–3), 271-287.
| Crossref | Google Scholar |
O’Connor WA, Dove MC (2009) The changing face of oyster culture in New South Wales, Australia. Journal of Shellfish Research 28, 803-811.
| Crossref | Google Scholar |
O’Connor WA, Zammit A, Dove MC, Stevenson G, Taylor MD (2018) First observations of perfluorooctane sulfonate occurrence and depuration from Sydney rock oysters, Saccostrea glomerata, in Port Stephens NSW Australia. Marine Pollution Bulletin 127, 207-210.
| Crossref | Google Scholar |
Pan J, Pan J-F, Diao M (2015) Trace metal mixture toxicity in aquatic organism reviewed from a biotoxicity perspective. Human and Ecological Risk Assessment: An International Journal 21, 2155-2169.
| Crossref | Google Scholar |
Parker LM, Ross PM, O’Connor WA (2009) The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould, 1850). Global Change Biology 15(9), 2123-2136.
| Crossref | Google Scholar |
Parker LM, Ross PM, O’Connor WA (2010) Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Marine Biology 157, 2435-2452.
| Crossref | Google Scholar |
Parker LM, Ross PM, O’Connor WA (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Marine Biology 158, 689-697.
| Crossref | Google Scholar |
Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner H-O (2012) Adult exposure influences offspring response to ocean acidification in oysters. Global Change Biology 18(1), 82-92.
| Crossref | Google Scholar |
Parker LM, O’Connor WA, Raftos DA, Pörtner H-O, Ross PM (2015) Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE 10(7), e0132276.
| Crossref | Google Scholar |
Parker LM, O’Connor WA, Byrne M, Dove M, Coleman RA, Pörtner HO, Scanes E, Virtue P, Gibbs M, Ross PM (2018) Ocean acidification but not warming alters sex determination in the Sydney rock oyster, Saccostrea glomerata. Proceedings of the Royal Society of London – B. Biological Sciences 285(1872), 2017286914.
| Crossref | Google Scholar |
Parker LM, Scanes E, O’Connor WA, Dove M, Elizur A, Pörtner H-O, Ross PM (2024) Resilience against the impacts of climate change in an ecologically and economically significant native oyster. Marine Pollution Bulletin 198, 115788.
| Crossref | Google Scholar |
Pereira RRC, Scanes E, Gibbs M, Byrne M, Ross PM (2020) Can prior exposure to stress enhance resilience to ocean warming in two oyster species? PLoS ONE 15(4), e0228527.
| Crossref | Google Scholar |
Planning NSW (2023) Coastal management. Coastal and marine management. (NSW Government) Available at https://www.planning.nsw.gov.au/policy-and-legislation/coastal-and-marine-management/coastal-management
Popp J, Pető K, Nagy J (2013) Pesticide productivity and food security. A review. Agronomy for Sustainable Development 33, 243-255.
| Crossref | Google Scholar |
Ritchie H, Roser M, Rosado P (2022) Pesticides: pesticides are used to protect plants from weeds, fungi, or insects. Where are they used? What is their impact? In OurWorldInData.org, 2022. Available at https://ourworldindata.org/pesticides
Roach AC, Muller R, Komarova T, Symons R, Stevenson GJ, Mueller JF (2009) Using SPMDS to monitor water column concentrations of PCDDS, PCDFS and dioxin-like PCBS in Port Jackson (Sydney Harbour), Australia. Chemosphere 75(9), 1243-1251.
| Crossref | Google Scholar | PubMed |
Sammut J, White I, Melville MD (1996) Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils. Marine and Freshwater Research 47, 669-684.
| Crossref | Google Scholar |
Scanes P (1996) ‘Oyster Watch’: monitoring trace metal and organochlorine concentrations in Sydney’s coastal waters. Marine Pollution Bulletin 33, 226-238.
| Crossref | Google Scholar |
Scanes PR (1997) Uptake and depuration of organochlorine compounds in Sydney rock oysters (Saccostrea commercialis). Marine and Freshwater Research 48, 1-6.
| Crossref | Google Scholar |
Scanes E, Parker LM, O’Connor WA, Ross PM (2014) Mixed effects of elevated pCO2 on fertilisation, larval and juvenile development and adult responses in the mobile subtidal scallop Mimachlamys asperrima (Lamarck, 1819). PLoS ONE 9(4), e93649.
| Crossref | Google Scholar |
Scanes E, Parker LM, O’Connor WA, Stapp LS, Ross PM (2017) Intertidal oysters reach their physiological limit in a future high-CO2 world. Journal of Experimental Biology 220, 765-774.
| Crossref | Google Scholar | PubMed |
Scanes E, Parker LM, O’Connor WA, Gibbs MC, Ross PM (2018) Copper and ocean acidification interact to lower maternal investment, but have little effect on adult physiology of the Sydney rock oyster Saccostrea glomerata. Aquatic Toxicology 203, 51-60.
| Crossref | Google Scholar | PubMed |
Scanes E, Wood H, Ross P (2019) Microplastics detected in haemolymph of the Sydney rock oyster Saccostrea glomerata. Marine Pollution Bulletin 149, 110537.
| Crossref | Google Scholar |
Scanes E, Scanes PR, Ross PM (2020) Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 11, 1803.
| Crossref | Google Scholar |
Scanes E, Parker LM, Seymour JR, Siboni N, Dove MC, O’Connor WA, Ross PM (2021) Microbiomes of an oyster are shaped by metabolism and environment. Scientific Reports 11, 21112.
| Crossref | Google Scholar |
Scanes E, Ross PM, Seymour JR, Siboni N, Dove MC, O’Connor WA, Dittes C, Parker LM (2023) Transgenerational transfer of the microbiome is altered by ocean acidification in oyster larvae. Aquaculture 565, 739153.
| Crossref | Google Scholar |
Schmitz HA, Maher WA, Taylor AM, Krikowa F (2015) Effects of cadmium accumulation from suspended sediments and phytoplankton on the oyster Saccostrea glomerata. Aquatic Toxicology 160, 22-30.
| Crossref | Google Scholar | PubMed |
Schrobback P, Pascoe S, Coglan L (2014) History, status and future of Australia’s native Sydney rock oyster industry. Aquatic Living Resources 27(3-4), 153-165.
| Crossref | Google Scholar |
Scott PD, Coleman HM, Colville A, Lim R, Matthews B, McDonald JA, Miranda A, Neale PA, Nugegoda D, Tremblay LA, Leusch FDL (2017) Assessing the potential for trace organic contaminants commonly found in Australian rivers to induce vitellogenin in the native rainbowfish (Melanotaenia fluviatilis) and the introduced mosquitofish (Gambusia holbrooki). Aquatic Toxicology 185(2017), 105-120.
| Crossref | Google Scholar |
Shishaye HA, Tait DR, Maher DT, Befus KM, Erler D, Jeffrey L, Reading MJ, Morgenstern U, Kaserzon S, Mueller J, De Verelle-Hill W (2021) The legacy and drivers of groundwater nutrients and pesticides in an agriculturally impacted Quaternary aquifer system. Science of The Total Environment 753, 142010.
| Crossref | Google Scholar |
Snow M, Fotedar S, Wilson NG, Kirkendale LA (2023) Clarifying the natural distribution of Saccostrea Dollfus & Dautzenberg, 1920 (edible rock oyster) species in Western Australia to guide development of a fledgling aquaculture industry. Aquaculture 566, 739202.
| Crossref | Google Scholar |
Stapp LS, Parker LM, O’Connor WA, Bock C, Ross PM, Pörtner HO, Lannig G (2018) Sensitivity to ocean acidification differs between populations of the Sydney rock oyster: role of filtration and ion-regulatory capacities. Marine Environmental Research 135, 103-113.
| Crossref | Google Scholar | PubMed |
Strain EMA, Olabarria C, Mayer-Pinto M, Cumbo V, Morris RL, Bugnot AB, Dafforn KA, Heery E, Firth LB, Brooks PR, Bishop MJ (2018) Eco-engineering urban infrastructure for marine and coastal biodiversity: which interventions have the greatest ecological benefit? Journal of Applied Ecology 55, 426-441.
| Crossref | Google Scholar |
Tan BLL, Hawker DW, Müller JF, Leusch FD, Tremblay LA, Chapman HF (2007) Modelling of the fate of selected endocrine disruptors in a municipal wastewater treatment plant in South East Queensland, Australia. Chemosphere 69(4), 644-654.
| Crossref | Google Scholar | PubMed |
Taylor DA, Thompson EL, Nair SV, Raftos DA (2013) Differential effects of metal contamination on the transcript expression of immune- and stress-response genes in the Sydney rock oyster, Saccostrea glomerata. Environmental Pollution 178, 65-71.
| Crossref | Google Scholar | PubMed |
The Nature Conservancy (2024) Reef builder: rebuilding Australia’s lost shellfish reefs. (TNC) Available at https://www.natureaustralia.org.au/what-we-do/our-priorities/oceans/ocean-stories/restoring-shellfish-reefs/
Thom B, Hudson J, Dean-Jones P (2023) Estuary contexts and governance models in the new climate era, New South Wales, Australia. Frontiers in Environmental Science 11, 1127839.
| Crossref | Google Scholar |
Thompson J, Roach A, Eaglesham G, Bartkow ME, Edge K, Mueller JF (2011) Perfluorinated alkyl acids in water, sediment and wildlife from Sydney Harbour and surroundings. Marine Pollution Bulletin 62, 2869-2875.
| Crossref | Google Scholar | PubMed |
Toomey AH, Strehlau-Howay L, Manzolillo B, Thomas C (2020) The place-making potential of citizen science: creating social-ecological connections in an urbanized world. Landscape and Urban Planning 200, 103824.
| Crossref | Google Scholar |
Tran TKA, Yu RMK, Islam R, Nguyen THT, Bui TLH, Kong RYC, O’Connor WA, Leusch FDL, Andrew-Priestley M, MacFarlane GR (2019) The utility of vitellogenin as a biomarker of estrogenic endocrine disrupting chemicals in molluscs. Environmental Pollution 248, 1067-1078.
| Crossref | Google Scholar | PubMed |
van Dam RA, Harford AJ, Houston MA, Hogan AC, Negri AP (2008) Tropical marine toxicity testing in Australia: a review and recommendations. Australasian Journal of Ecotoxicology 14, 55-88.
| Google Scholar |
Vozzo ML, Mayer-Pinto M, Bishop MJ, Cumbo VR, Bugnot AB, Dafforn KA, Johnston EL, Steinberg PD, Strain EMA (2021) Making seawalls multifunctional: the positive effects of seeded bivalves and habitat structure on species diversity and filtration rates. Marine Environmental Research 165, 105243.
| Crossref | Google Scholar |
Ward TJ (1982) Effect of cadmium on particle clearence by the Sydney rock oyster, Saccostrea commercialis (I.&R.). Australian Journal of Marine and Freshwater Research 33, 711-715.
| Crossref | Google Scholar |
Warne MSJ, Neelamraju C, Strauss J, Turner RDR, Smith RA, Mann RM (2023) Estimating the aquatic risk from exposure to up to twenty-two pesticide active ingredients in waterways discharging to the Great Barrier Reef. Science of The Total Environment 892, 164632.
| Crossref | Google Scholar |
Watson SA, Southgate PC, Tyler PA, Peck LS (2009) Early larval development of the Sydney rock oyster Saccostrea glomerata under near-future predictions of CO2-driven ocean acidification. Journal of Shellfish Research 28(3), 431-437.
| Crossref | Google Scholar |
Wilson SP, Hyne RV (1997) Toxicity of acid-sulfate soil leachate and aluminum to embryos of the sydney rock oyster. Ecotoxicology and Environmental Safety 37(1), 30-36.
| Crossref | Google Scholar | PubMed |
Wisely B, Blick RAP (1967) Mortality of marine invertebrate larvae in mercury, copper, and zinc solutions. Australian Journal of Marine and Freshwater Research 18, 63-72.
| Crossref | Google Scholar |
Wootton N, Sarakinis K, Varea R, Reis-Santos P, Gillanders BM (2022) Microplastic in oysters: a review of global trends and comparison to southern Australia. Chemosphere 307(4), 136065.
| Crossref | Google Scholar |
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environmental Pollution 178, 483-492.
| Crossref | Google Scholar | PubMed |
Yingprasertchai T, Yu RMK, O’Connor WA, Hopwood T, MacFarlane GR (2017) Acclimatory processes are likely responsible for metal tolerance in oyster embryos. Marine Environmental Research 127, 49-61.
| Crossref | Google Scholar | PubMed |
Yingprasertchai T, Yu RMK, Tran TKA, Chong Kong RY, O’Connor WA, MacFarlane GR (2019) Characterisation of the metallothionein gene in the Sydney rock oyster and its expression upon metal exposure in oysters with different prior metal exposure histories. Marine Environmental Research 151, 104775.
| Crossref | Google Scholar |



