Evidence of fish community fragmentation in a tropical river upstream and downstream of a dam, despite the presence of a fishway
Dwi Atminarso A B C * , Lee J. Baumgartner A , Robyn J. Watts A C , Meaghan L. Rourke A D , Jennifer Bond A C and Arif Wibowo
A B C * , Lee J. Baumgartner A , Robyn J. Watts A C , Meaghan L. Rourke A D , Jennifer Bond A C and Arif Wibowo  B
B
A Gulbali Institute For Agriculture, Water and Environment, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
B Research Center for Conservation of Marine and Inland Water Resources, National Research and Innovation Agency, Jalan Raya Jakarta-Bogor Km 48, Cibinong, West Java 16911, Indonesia.
C School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
D Department of Primary Industries, Narrandera Fisheries Centre, PO Box 182, Narrandera, NSW 2700, Australia.
Abstract
Rapid human population growth has increased demand for water supply, food security, electricity, and flood mitigation worldwide. To address these challenges, governments have invested heavily in the expansion of water infrastructure. However, there is substantial evidence that globally, this infrastructure impacts aquatic ecosystems and can have a significant impact on the persistence of fish species. Despite being well understood globally, the impacts of dams on fish have been given scant attention in Indonesia. Thus, considerations for fish are rarely included in river development planning frameworks.
To document the impact of riverine barriers on Indonesian freshwater fish, we surveyed multiple sites, using three different kinds of gear (gillnets, castnets, and bait traps), upstream and downstream of Perjaya Dam in the Komering River.
The study revealed 13 of 36 species were found only downstream of the dam and five of 36 species were found only above the dam. There were significant differences in fish community composition between upstream and downstream regions using either fish abundance (Pseudo-F = 4.495, d.f. = 1, P < 0.05), species richness (Pseudo-F = 15.837, d.f. = 1, P < 0.05) or species diversity as the response metrics (Pseudo-F = 8.3256, d.f. = 1, P < 0.05).
The local extirpation of many species from upstream areas suggests that the Perjaya Dam hinders fish migration.
Despite containing a fishway, the results indicate that fish are not successfully recolonising upstream reaches.
Keywords: fish community, fish movement, fishway, fragmentation, Indonesia, Komering River, Perjaya Dam, tropical river.
Introduction
River regulation has negatively impacted fish populations globally (Dynesius and Nilsson 1994; Gehrke et al. 2002). Both large dams and small instream barriers have contributed to a reduction in population size and species diversity (Poff and Hart 2002; Alexandre and Almeida 2010). Barriers to migration can physically prevent fishes from completing their life cycle and can also have negative genetic consequences for fish populations. For example, population fragmentation can lead to a reduction in gene flow, genetic diversity and adaptive potential and ultimately, population viability (Gehrke et al. 2002; Argentina et al. 2018). Migratory freshwater fishes are particularly susceptible to population fragmentation where artificial barriers such as dams restrict mixing (Rourke et al. 2019; Vu et al. 2020; Stoffels et al. 2022). River regulation has also significantly changed free flowing rivers into still water habitats in the upstream of impoundments that can benefit introduced species. The subsequent increase in populations of introduced species in reservoirs can provide competition for local native species (Mercado-Silva et al. 2009). Identifying populations at risk of negative impacts of barriers can allow action to be taken to reduce the risk of local extinction (Rourke et al. 2019; Ovidio et al. 2020).
The development of dams is necessary to meet human needs. However, there needs to be strict criteria governing the construction of new dams to minimise negative impacts on the aquatic fauna including fish. One of the solutions to minimise negative consequences of dam construction is through the provision of fishways (Oldani and Baigún 2002; Baumgartner et al. 2012; Baumgartner et al. 2013; Rourke et al. 2019). Fishways are structures designed to slow the passage of water over the barrier to the extent that migrating fish can pass upstream (or downstream). There are several types of fishway designs that are used including Denil fishways, Vertical slot, and Pool and weir (Stuart and Berghuis 2002; Baumgartner et al. 2012). But simply installing a fishway does not ensure the recovery of migratory species. Fishway design must consider the swimming ability of target fish species, in the context of local ecology and hydrology, to maximise success and to ensure those dependent upon fish derive benefits (Kowarsky and Ross 1981; Harris et al. 2017).
Thousands of fish migration barriers have been installed in Indonesian rivers for many purposes including hydropower, irrigation infrastructure, water storage, and flood mitigation but only four fishways have been constructed in the entire country (Baumgartner and Wibowo 2018); largely due to a paucity of information on how these can be effectively constructed. Consequently, these technologies are still considered relatively new in Indonesia. The construction of these fishways were not tailored to the local fish community (Nizar 2014) and effectiveness is either unknown or they have been suggested to be ineffective. But considering that Indonesia is embarking on a refurbishment program of past barriers, there is an opportunity to ensure that new structures contain appropriate mitigation strategies to protect migratory fish. In order to facilitate such an outcome, there is a need to ensure that the impacts of dams on fisheries resources is documented and acknowledged.
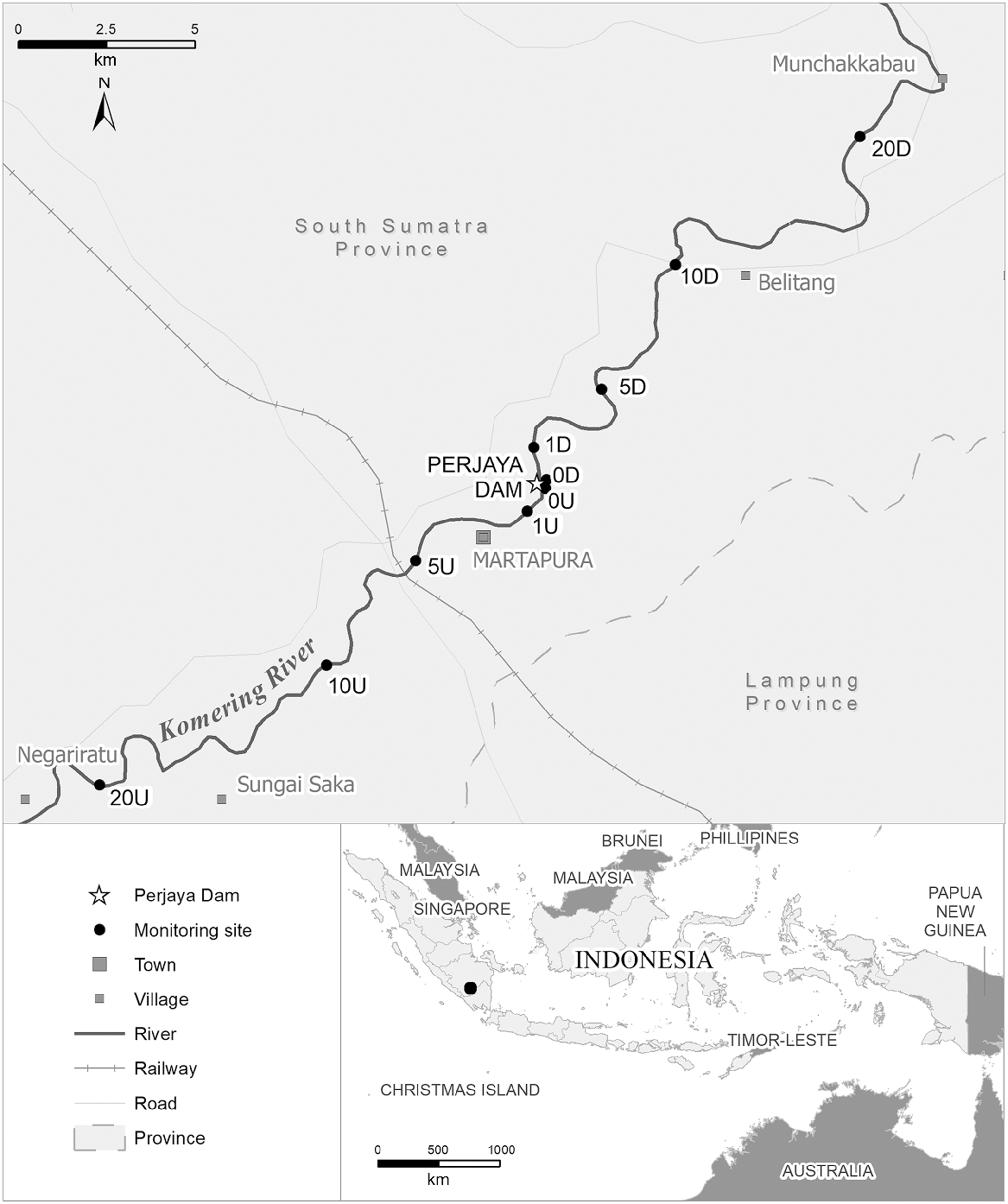
The Komering River is one of nine large tributaries of the Musi River in South Sumatra. It is approximately 145 km long (Aida et al. 2010) and it is under four different local government administrations (South Ogan Komering Ulu, East Ogan Komering Ulu, Ogan Komering Ilir, and Banyuasin) (Nizar 2014). At least two diadromous species, freshwater sole (Achiroides leucorhynchos) and giant freshwater prawn (Macrobrachium sp.) have been documented in this river. Masheer (Tor sp.), which migrate long distances in freshwater, have also been caught in this river. Previously, masheer were not collected, while the other two diadromous species existed at very low numbers and with smaller total length (Husnah et al. 2007; Nizar 2014). In 1991, the Perjaya Dam was constructed to provide water for irrigation; to mitigate impact on fish, it was equipped with a 75 m long slot and submerged orifice fishway consisting of 18 baffles. Perjaya Dam is 6.45 m high. Prior to the construction of the dam, 55 fish species were recorded in the river both upstream and downstream of the dam (Gaffar and Utomo 1991), but 16 years later, local fishers were finding it difficult to catch some species (Husnah et al. 2007). By 2006, the number of species had declined to 48 (Husnah et al. 2007), and then further declined to 40 species in 2014 (Husnah et al. 2007; Nizar 2014). These results suggested that the dam altered the hydrological regime in the Komering River, that the local ecology is impacted and suggests that the fishway was not completely effective in allowing upstream and downstream movement. It also suggests that, with time, the fish community has entered a state of continual decline.
Although previous studies have revealed the fish species were in decline in the Komering River, these studies also indicated that the fish community continued to decline 30 years following construction of the dam despite the presence of a fishway. There have been no surveys conducted since, and the broad aim of this study was to investigate if the impacts of the dam still persist 30 years after construction; and several years since the last survey. We used standardised experimental fishing methods to examine the fish community differences upstream and downstream of Perjaya Dam to determine if there were significant differences in fish community structure that may be attributed to the presence of the dam. In addition, we also predict that if the Perjaya Dam has hindered fish spawning movement routes, there will be differences in size classes between upstream and downstream sites.
Materials and methods
Study area and sample collection
The Perjaya Dam is located in the upper Komering River. The upriver section is about 65 km long, while downriver is around 80 km long. Two barriers have been constructed at about 80 km and 85 km upstream of the Perjaya Dam in Selabung River, which is one of the Komering tributaries. The Musi River, which the Komering flows into, is unregulated. Ten sampling sites were chosen to collect fish upstream (n = 5 sites) and downstream (n = 5 sites) of the Perjaya Dam in the upper Komering River, Sumatra, Indonesia (Fig. 1). Sampling sites were at 0 km, 1 km, 5 km, 10 km, and 20 km from the dam, both upstream and downstream. Spacing the sites at this distance sought to ensure the reservoir effect did not bias data collection. Experimental fishing included three types of fishing gear (two sets of multi-panel gillnets, 10 collapsible bait traps and 20 castnets). Each gillnet comprised six different mesh sizes (19.05, 25.4, 38.1, 50.8, 76.2, and 101.6 mm). The dimension of the bait trap was 400 × 220 × 220 mm (length × width × height) with 60 mm entry diameter. In addition, 2 m diameter castnets with 19.05 mm mesh size were used. We chose these three types of fishing gear because of their selectivity and because they were a mix of active (castnets) and passive methods (gillnets and bait traps). These kinds of gear are used by the locals and were known to be reliable. We implemented a standardised procedure to ensure the results were comparable. Previous studies did not implement a standardised monitoring regime. In addition, we aimed to catch both pelagic fish (gillnet) and bottom fish (bait trap) to capture a wide range of fish species. Gillnets and bait traps were set in the river for 2 h, while castnets were thrown 20 times with 1 min between casts. We used 100 g chicken intestine sourced from a local traditional market for bait. All experimental fishing was conducted between 8:00 am and 2:00 pm. All sites were sampled twice during the rainy season (1–18 February 2020 and 25 April – 7 May 2021) and twice during the dry season (10–23 November 2020 and 18–29 November 2021) to capture any potential seasonal variations in fish numbers. All fish collected were photographed, measured (total length, to 1 mm) and weighed (to 0.1 g). Individual fish were identified to species where possible using an identification book (Kottelat et al. 1993), and the fishbase website (www.fishbase.org).
Data analysis
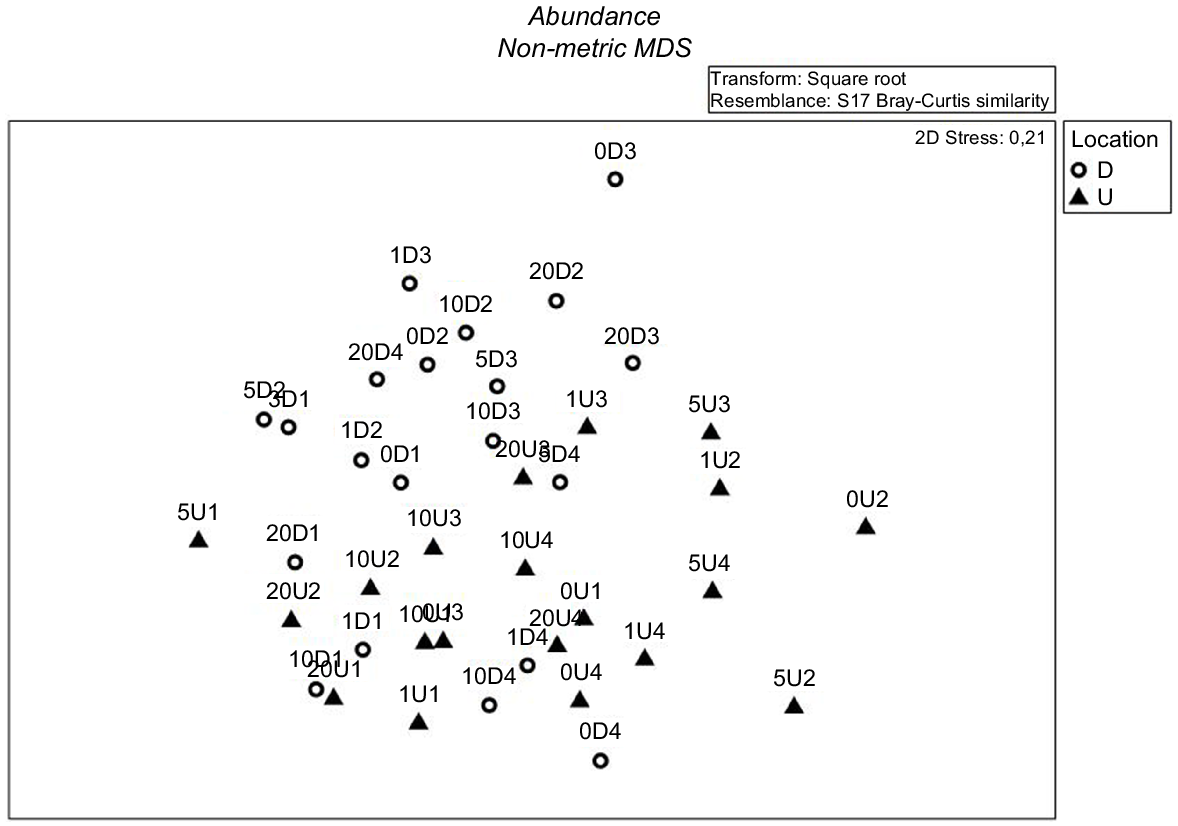
Because we employed a more comprehensive fish collection protocol than previous studies, abundance, species richness, and species diversity data approach to fish species were used to analyse fish community differences temporally (rainy and dry seasons) and spatially (upstream and downstream). All analyses were conducted using Primer v7 (Clarke and Gorley 2015). Permutational analysis of variance (PERMANOVA) was used to examine if there was a significant difference in the fish community samples between different seasons (dry and rainy) and locations (upstream and downstream) among the 10 sampling sites. The number of fish caught was loge (X + 1) transformed and Bray–Curtis similarities were calculated. Two factors (location and season) were included in the model and the significance values calculated based on 9999 unrestricted permutations of the raw data. Multi-dimensional scaling (MDS) was utilised to visualise the differences in the fish community structure between locations and seasons.
Length frequency distribution analyses were conducted in excel to identify and visualise any differences in total length between fish communities upstream and downstream. The Kolmogorov–Smirnov (S-M) test was used to test for any significant difference in length distribution between upstream and downstream reaches. If the dam has blocked fish spawning migration routes, we predict there will be differences in size classes between upstream and downstream sites. Only species that had more than 25 individuals in both upstream and downstream reaches were used in this analysis.
Comparison of presence and absence of species between a previous study in 2014 (Nizar 2014) and the current study was undertaken by comparing presence/absence of species at a site that both studies had surveyed. Two locations downstream (0 km and 5 km) in the current study were able to be compared with two of the sites (0 km and 5 km) in the study by Nizar (2014). This enables a comparison of the changes in the presence of fish species between surveys conducted 22 years and 30 years after the construction of the dam.
Results
The experimental fishing resulted in the capture of 882 individual fish representing 36 species (Table 1). Three dominant species made up 54.05% of the catch: Mystaceloucus marginatus – common barb (31.58 % of total catch), Rasbora argyrotaenia – silver rasbora (11.74 %), and Macrobrachium sp. – giant freshwater prawn (10.73%). The Cyprinidae family dominated the total catch with 20 species caught (Table 1). There were three non-native species (Erimyzon sucetta, Hypostomus sp., and Tetraodon sp.) captured, representing 0.34% of the catch. Hypostomus sp. was the most abundant non-native species but it was only found downstream of the Perjaya Dam. The sampling site that contributed most to the overall species collection was 5 km downstream (20 species).
| No. | Family | Species name | Local name | Total caught upstream | Total caught downstream | Type of migration | |
|---|---|---|---|---|---|---|---|
| 1. | Bagridae | Hemibagrus nemurus | Baung | 1 | 3 | Potamodromous | |
| 2. | Catostomidae | Erimyzon sucetta* | 2 | 0 | Unknown | ||
| 3. | Cobitidae | Acantopsis dialuzona | Julung-julung | 2 | 0 | Unknown | |
| 4. | Syncrossus hymenophysa | Langli | 5 | 7 | Unknown | ||
| 5. | Cyprinidae | Anematichthys repasson | Kepras | 11 | 6 | Potamodromous | |
| 6. | Barbichthys laevis | Nilem | 0 | 3 | Unknown | ||
| 7. | Barbichthys sp. | Timah | 6 | 6 | Potamodromous | ||
| 8. | Barbonymus gonionotus | Batu ulu | 0 | 6 | Unknown | ||
| 9. | Barbonymus schwanenfeldii | Kepiat | 25 | 15 | Potamodromous | ||
| 10. | Barbonymus sp. | Tawes | 1 | 13 | Unknown | ||
| 11. | Crossocheilus nigriloba | Nilom batu | 0 | 12 | Unknown | ||
| 12. | Cyclocheilichthys sp. | 2 | 3 | Unknown | |||
| 13. | Hampala macrolepidota | Sebarau | 8 | 5 | Potamodromous | ||
| 14. | Labiobarbus leptocheilus | Umbut | 0 | 33 | Potamodromous | ||
| 15. | Labiobarbus ocellatus | Lambak | 0 | 1 | Potamodromous | ||
| 16. | Luciosoma setigerum | Sejuar | 0 | 4 | Unknown | ||
| 17. | Luciosoma trinema | Seluang batang | 0 | 26 | Unknown | ||
| 18. | Mystacoleucus marginatus | Baru | 207 | 87 | Unknown | ||
| 19. | Osteochillus microcephalus | Nilom kayu | 0 | 1 | Unknown | ||
| 20. | Osteochillus vittatus | Nilom | 8 | 12 | Potamodromous | ||
| 21. | Puntigrus tetrazona | Sumatera | 25 | 16 | Unknown | ||
| 22. | Puntius waandersi | Mata balak | 3 | 16 | Unknown | ||
| 23. | Rasbora argyrotaenia | Seluang | 27 | 53 | Unknown | ||
| 24. | Thynnichthys thynnoides | Luma | 0 | 4 | Potamodromous | ||
| 25. | Eleotrididae | Oxyeleotris marmorata | Betutu | 3 | 0 | Potamodromous | |
| 26. | Loricariidae | Hypostomus sp.* | Sapu-sapu | 0 | 9 | Unknown | |
| 27. | Mastacembelidae | Macrognathus aculeatus | Piluk | 1 | 1 | Potamodromous | |
| 28. | Osphronemidae | Betta sp. | Cupang | 1 | 0 | Unknown | |
| 29. | Palaeomonidae | Palaemon sp. | Udang beras | 36 | 38 | Unknown | |
| 30. | Macrobrachium sp. | Udang satang | 52 | 51 | Diadromous | ||
| 31. | Pristolepididae | Pristolepis fasciata | Kepor | 3 | 2 | Potamodromous | |
| 32. | Sisoridae | Bagarius lica | Dalum | 0 | 1 | Potamodromous | |
| 33. | Soleidae | Achiroides leucorhynchos | Lidah | 0 | 5 | Diadromous | |
| 34. | Unionidae | Pilsbryoconcha exilis | 3 | 0 | Unknown | ||
| 35. | Tetraodontidae | Tetraodon sp.* | Buntal | 4 | 5 | Unknown | |
| 36. | Zenarchopteridae | Dermogenys sp. | 1 | 0 | Unknown |
An asterisk indicates introduced species.
Thirteen of the 36 fish species collected were found only in the downstream sites, five species were caught only in the upstream sites, and 18 species were collected from both downstream and upstream sites (Table 1). The Cyprinidae family dominated the species that were caught downstream and upstream. In contrast, the five species that were found only upstream represented five families. Two of three non-native fish species (E. sucetta and H. sp.) were found only in the downstream site and another non-native species (T. sp.) was found in both sites. The species caught only downstream of the dam were A. leucorhynchos, Barbichthys sp., B. laevis, Crossocheilus nigriloba, E. sucetta, Bagarius lica, Hypostomus sp, Labiobarbus leptocheilus, L. ocellatus, Luciosoma setigerum, L. trinema, Osteochillus microcephalus, Thynnichthys thynnoides.
There were significant differences in fish community composition between upstream and downstream locations using either fish abundance (Pseudo-F = 4.495, d.f. = 1, P < 0.05; Table 2, Fig. 2), species richness (Pseudo-F = 15.837, d.f. = 1, P < 0.05; Table 3, Fig. 2) or species diversity as the response metrics (Pseudo-F = 8.3256, d.f. = 1, P < 0.05; Table 4, Fig. 2). Moreover, there were significant differences in fish community between seasons (dry and rainy) using species richness (Pseudo-F = 10.292, d.f. = 1, P < 0.05; Table 3, Fig. 2) and species diversity (Pseudo-F = 3.6764, d.f. = 1, P < 0.05; Table 4, Fig. 2) but not using fish abundance (Pseudo-F = 1.933, d.f. = 1, P > 0.05; Table 2, Fig. 2).
| Source | d.f. | SS | MS | Pseudo-F | P(perm) | Perms | |
|---|---|---|---|---|---|---|---|
| Location | 1 | 11 183 | 11 183 | 4.495 | 0.0002* | 9944 | |
| Season | 1 | 4809.7 | 4809.7 | 1.9332 | 0.061 | 9956 | |
| Location × season | 1 | 2819 | 2819 | 1.1334 | 0.3543 | 9936 | |
| Residual | 36 | 89 567 | 2488 | ||||
| Total | 39 | 1.0838E + 05 |
The comparison between upstream and downstream (location) of the Perjaya Dam are shown by a star (*) and significant value (α = 0.05) P values indicated in bold.
d.f., degree of freedom; SS, sum of squares; MS, mean squares, Perms, number of permutations.
Multi-dimensional scaling (MDS) ordination of differences in fish communities from sites upstream (triangle) and downstream (circle) of Perjaya Dam, Indonesia. Labels indicate the sampling sites (number depicting kilometres and U or D referring to upstream or downstream).
| Source | d.f. | SS | MS | Pseudo-F | P(perm) | Perms | |
|---|---|---|---|---|---|---|---|
| Location | 1 | 1384.8 | 1384.8 | 15.837 | 0.0004* | 9937 | |
| Season | 1 | 899.86 | 899.86 | 10.292 | 0.0016 | 9953 | |
| Location × season | 1 | 544.14 | 544.14 | 6.2233 | 0.0128 | 9927 | |
| Residual | 36 | 3147.7 | 87.436 | ||||
| Total | 39 | 5506.7 |
The comparison between upstream and downstream (location) of the Perjaya Dam are shown by a star (*) and significant value (α = 0.05) P values indicated in bold.
d.f., degree of freedom; SS, sum of squares; MS, mean squares; Perms, number of permutations.
| Source | d.f. | SS | MS | Pseudo-F | P(perm) | Perms | |
|---|---|---|---|---|---|---|---|
| Location | 1 | 3170.7 | 3170.7 | 8.3256 | 0.0001* | 9936 | |
| Season | 1 | 1400.1 | 1400.1 | 3.6764 | 0.0188 | 9926 | |
| Location × season | 1 | 1660.5 | 1660.5 | 4.3602 | 0.0066 | 9935 | |
| Residual | 36 | 13 710 | 380.84 | ||||
| Total | 39 | 18 678 |
The comparison between upstream and downstream (location) of the Perjaya Dam are shown by a star (*) and significant value (α = 0.05) P values indicated in bold.
d.f., degree of freedom; SS, sum of squares; MS, mean squares, Perms, number of permutations.
Species that contributed to the overall dissimilarity in the upstream and downstream group were M. marginatus, R. argyrotaenia, P. sp., M. sp., L. leptocheilus, Puntigrus tetrazona, Barbonymus schwanenfeldii. Those seven species were responsible for 53.32 % dissimilarity, while M. marginatus itself was the highest contributor with 12.89% (SIMPER [similarity percentage analysis]; Table 5).
| Species | Downstream vs upstream. Average dissimilarity = 79.60 | ||||||
|---|---|---|---|---|---|---|---|
| Av. abund group downstream | Av. abund group upstream | Av.Diss | Diss/s.d. | Contrib% | Cum.% | ||
| Mystacoleucus marginatus | 1.13 | 1.51 | 10.26 | 1.24 | 12.89 | 12.89 | |
| Rasbora argyrotaenia | 0.79 | 0.37 | 6.64 | 0.96 | 8.34 | 21.23 | |
| Palaemon sp. | 0.43 | 0.67 | 5.54 | 0.92 | 6.96 | 28.19 | |
| Macrobrachium sp. | 0.57 | 0.46 | 5.53 | 0.79 | 6.94 | 35.13 | |
| Labiobarbus leptocheilus | 0.70 | 0.00 | 5.23 | 0.94 | 6.57 | 41.70 | |
| Puntigrus tetrazona | 0.33 | 0.61 | 5.01 | 1.02 | 6.30 | 48.00 | |
| Barbonymus schwanenfeldii | 0.32 | 0.42 | 4.24 | 0.77 | 5.32 | 53.32 | |
| Crossocheilus nigriloba | 0.35 | 0.00 | 3.08 | 0.68 | 3.87 | 57.20 | |
| Puntius waandersi | 0.39 | 0.09 | 2.99 | 0.72 | 3.75 | 60.95 | |
| Osteochillus vittatus | 0.28 | 0.26 | 2.76 | 0.84 | 3.47 | 64.42 | |
Av. Abund, Average abundance; Av. Diss, Average dissimilarity upstream and downstream; Contrib%, indicates the percentage of dissimilarity that a species contributes to the total dissimilarity between upstream and downstream group; Cum.%, cumulative percentage.
Two of 36 species collected were considered diadromous and 13 species were considered potamodromous, while the remaining 21 species were unknown (Table 1). One of two diadromous species (M. sp.) was collected in both upstream and downstream sites, while one species (A. leucorhynchos) was only found downstream. In addition, eight of 13 potamodromous species collected were found both upstream and downstream, while five potamodomous species were found either upstream or downstream, but not in both habitats.
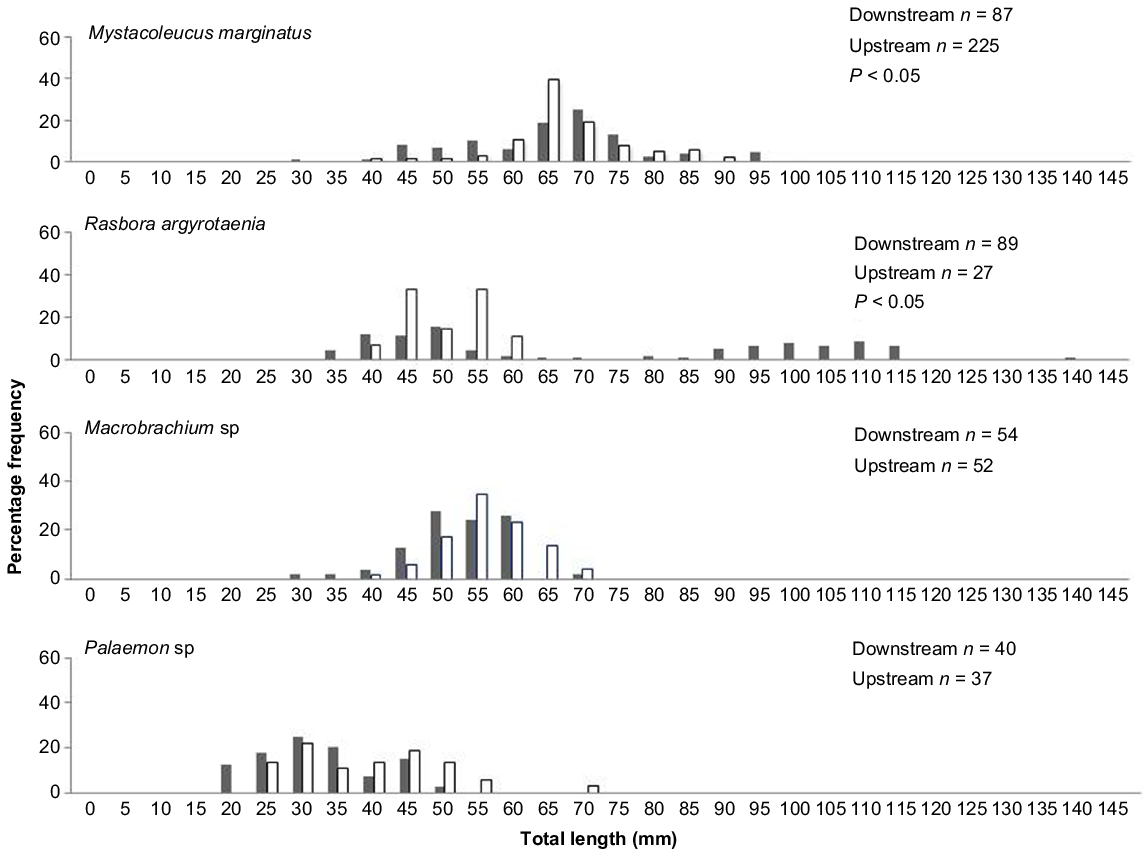
Four species, M. marginatus, R. argyrotaenia, Macrobrachium sp., and Palaemon sp., were caught in adequate numbers to allow length frequency distribution analysis. Significant differences (KS (Kolmogorov–Smirnov): P < 0.05) in length frequency distributions between upstream and downstream locations were found in M. marginatus (P = 0.0091) and R. argyrotaenia (P = 0.001) (Fig. 3). There were more individuals of larger size classes of M. marginatus at upstream sites than downstream sites, while larger size classes of R. argyrotaenia were found downstream than upstream. In addition, there were no significant differences in length frequency distribution between upstream and downstream of Palaemon sp. (P = 0.07) and Macrobrachium sp. (P = 0.117).
Length frequency distribution of four species between downstream and upstream fish community. These four species have adequate numbers of individuals (25 or more individuals) to perform the analysis. Black indicates downstream sample and white is upstream sample.
Comparison of fish species between the 2014 study and the current study showed that since 2014 there has been a decline in the total number of species in both locations (0 km and 5 km downstream). Total number of species documented at 0 km downstream in 2014 was 27 species, whereas the 2022 study found 15 species in the same location. In addition, the location at 5 km downstream in 2014 revealed 35 species while the current research documented 20 species (Table 6).
| No | Species name | 0 km downstream | 5 km downstream | |||
|---|---|---|---|---|---|---|
| 2014 | 2022 | 2014 | 2022 | |||
| 1. | Acanthopsis dialuzoma | ✓ | – | ✓ | – | |
| 2. | Achiroides leucorhynchus | – | ✓ | ✓ | – | |
| 3. | Anematichthys repasson | – | – | – | ✓ | |
| 4. | Bagarius lica | ✓ | – | – | – | |
| 5. | Bagroides melapterus | – | – | ✓ | ||
| 6. | Barbichthys laevis | ✓ | – | ✓ | ✓ | |
| 7. | Barbichthys sp. | – | – | – | ✓ | |
| 8. | Barbonymus gonionotus | ✓ | – | – | ✓ | |
| 9. | Barbonymus schwanenfeldii | ✓ | – | ✓ | ✓ | |
| 10. | Barbonymus sp. | – | – | – | ✓ | |
| 11. | Channa striata | – | – | ✓ | – | |
| 12. | Parachela oxygastroides | ✓ | – | – | – | |
| 13. | Chitala chitala | – | – | ✓ | – | |
| 14. | Crossocheilus nigriloba | ✓ | ✓ | ✓ | ✓ | |
| 15. | Crossocheilus oblongus | ✓ | – | ✓ | – | |
| 16. | Crossocheilus sp. | ✓ | ✓ | ✓ | – | |
| 17. | Cyclocheilichthys repasson | ✓ | – | ✓ | – | |
| 18. | Epalzheorhynchus kallopterus | – | – | ✓ | – | |
| 19. | Fluta alba | ✓ | – | – | – | |
| 20. | Glyptothorax platypogonides | ✓ | – | ✓ | – | |
| 21. | Hampala macrolepidota | – | – | ✓ | ✓ | |
| 22. | Hemibagrus nemurus | ✓ | ✓ | ✓ | ✓ | |
| 23. | Hemibagrus nigriceps | ✓ | – | ✓ | – | |
| 24. | Homaloptera ocellata | – | – | ✓ | – | |
| 25. | Hypostomus sp. | – | ✓ | – | ✓ | |
| 26. | Kryptopterus sp. | ✓ | – | ✓ | – | |
| 27. | Labeo chrysopekadion | ✓ | – | ✓ | – | |
| 28. | Labeobarbus leptocheilus | ✓ | ✓ | ✓ | ✓ | |
| 29. | Laides hexanema | ✓ | ✓ | – | ||
| 30. | Luciosoma setigerum | – | – | – | ✓ | |
| 31. | Luciosoma trinema | – | – | ✓ | – | |
| 32. | Macrobrachium sp. | ✓ | ✓ | ✓ | – | |
| 33. | Mystacoleucus marginatus | ✓ | ✓ | ✓ | ✓ | |
| 34. | Osteochillus microcephalus | – | – | ✓ | – | |
| 35. | Osteochillus sp. | – | – | ✓ | – | |
| 36. | Osteochillus vittatus | ✓ | ✓ | ✓ | ✓ | |
| 37. | Oxyeleotris marmorata | – | – | ✓ | – | |
| 38. | Palaemon sp. | ✓ | ✓ | ✓ | ✓ | |
| 39. | Pristolepis fasciatus | ✓ | – | – | – | |
| 40. | Puntius schwanenfeldii | ✓ | – | ✓ | – | |
| 41. | Puntius tetrazona | – | ✓ | – | ✓ | |
| 42. | Puntius waandersi | ✓ | ✓ | ✓ | ✓ | |
| 43. | Rasbora argyrotaenia | ✓ | ✓ | ✓ | ✓ | |
| 44. | Syncrossus hymenophysa | – | ✓ | ✓ | ✓ | |
| 45. | Tetraodon sp. | – | ✓ | – | ✓ | |
| 46. | Thynnichtys thynnoides | ✓ | – | ✓ | – | |
| 47. | Trichogaster trichopterus | – | – | ✓ | – | |
| Total number of species | 27 | 15 | 35 | 20 | ||
Symbol thick (✓), present; symbol dash (–), absent.
Discussion
The study provides the first evidence of the long-term sustained impact of a migration barrier to fish communities in Indonesia. The results confirm that Perjaya Dam has created a significant barrier to fish migration and has affected the fish community structure in the Komering River between sites upstream and downstream of the dam. This supports previous studies that have also shown a decline in the number of fish species in the Komering River since dam construction (Husnah et al. 2007; Nizar 2014). In addition, the specific comparison of the 2014 study and this recent study of two downstream sampling sites at 0 km and 5 km showed species diversity has declined further. Our study is consistent with previous research that has implicated the development of water infrastructure in the loss of fish species (Townsend 1975; Fjellheim and Raddum 1996; Holmquist et al. 1998; Rivinoja et al. 2001). Barriers restrict fish migration (Buisson et al. 2008; De Leeuw and Winter 2008; Taylor et al. 2008) and convert lotic water bodies to lentic water bodies thus altering critical feeding and spawning habitat (Cadwallader 1978; McKay et al. 2017). In Australia, a significant decline in the abundance and distribution of macquarie perch (Macquaria australasica), bony herring (Nematalosa erebi), silver perch (Bidyanus bidyanus), golden perch (Macquaria ambigua), trout cod (Maccullochella macquariensis) and murray cod (Maccullochella peelii) were believed to be associated with dam construction (Cadwallader 1978; Allan and Flecker 1993; Mallen-Cooper 1996; McDowall 1996; Harris and Gehrke 1997; Allen et al. 2002).
Although the drawbacks of water infrastructure development have been well acknowledged globally, other human activities may add more pressures to fish communities such as overfishing, water pollution, habitat degradation, introduction of non-native species, and damaging interactions between wild and hatchery fish (Allan and Flecker 1993). It is estimated that migratory freshwater fish experienced dramatic declines of about 76% between 1970 and 2016 (Deinet et al. 2020). About one half of the pressures come from river changes, habitat degradation, and loss, while around 33% was contributed by overexploitation (Deinet et al. 2020). Dams and weirs have been implicated in changes in fish assemblage composition, particularly in rivers where diadromous species are present (Baumgartner 2005). Here we found the greatest species diversity was immediately downstream from the dam. This suggests an accumulation of migratory fish species waiting to move upstream but being unable to use the fishway. Small catadromous fish commonly accumulate downstream of weirs or dams in Australian coastal rivers. High relative abundances of striped gudgeon (Gobiomorphus australis) and freshwater herring (Potamalosa richmondia) have been documented downstream of Tallowa Dam (Bishop and Bell 1978; Gehrke et al. 2002). Similarly, the accumulation of western carp gudgeon (Hypseleotris klunzingeri) and empire gudgeon (H. compressa) species downstream of a tidal barrage on the sub-tropical Fitzroy River has been recorded (Stuart and Mallen-Cooper 1999).
The ongoing decline in migratory species from upstream reaches suggests that the Perjaya Dam fishway is not effectively facilitating fish movement. Baumgartner and Wibowo (2018), have identified some failures in the design of the fishway in the Perjaya Dam. First, the entrance is placed a significant distance from the dam and fish are unlikely to find it. Second, the internal baffles were designed for strong fish swimmers and create high velocity and turbulence that prevent fish from ascending. Third, the exit of the fishway is located near the irrigation offtake, which increases the chance of fish that have ascended being diverted into the channel system. The impact of the Perjaya Dam is likely to be greater for potamodromous species than diadromous species. A study from Australia revealed the impact of barriers to diadromous species was greater than for potamodromous taxa, as 10 diadromous species disappeared from the upstream reaches of the Shoalhaven River in Australia due to the construction of the Tallowa dam (Gehrke et al. 2002).
Results from our study show that of the species collected both upstream and downstream of the dam, the majority were considered potamodromous. The disappearance of potamodromous species after the construction of weirs and dams is less frequent than the loss of diadromous species, because of the potential ability of the former to develop self-sustaining populations both upstream and downstream, if circumstances support recruitment and spawning (Baumgartner 2005). Nevertheless, some potamodromous species undertake large migrations wholly within freshwater environments to spawn (Bhatt and Pandit 2016) and their offspring may rely on the rich food sources of floodplain habitat to survive (Fernandes 1997). For example, the endangered Colorado squawfish (Ptychoceilus lucius) has disappeared from the White River, Colorado because the construction of the Taylor Draw Dam prevented their upstream migration (Martinez et al. 1994). The Komering River contains a population of recently recognised B. lica, (Ng and Kottelat 2021), which are poorly understood but are potentially potamodromous and likely to undertake spawning migrations, similar to their congener (B. yarelli) (Ng and Kottelat 2021). Only a single B. lica was collected during this study, and eight individuals in the previous study, and thus it is possible that the Perjaya Dam has interfered with the reproductive needs of this species, potentially resulting in its decline in numbers. It is not known if larvae of this species, or indeed many of the other species in the Komering River, migrate downstream to estuaries to develop. Future, otolith microchemistry studies using strontium isotope ratios could be very important to aid our understanding of the migratory requirements of the Komering River fish community (Vu et al. 2022). This will inform future management by determining if improved fish passage facilities are needed to promote upstream and downstream migration.
The difference in size classes upstream and downstream suggests that the barrier may have modified fish habitats upstream and downstream and affected fish spawning and recruitment success. This is particularly true for Macrobrachium species that are known to be diadromous. Observing smaller Macrobrachium from downstream sites is consistent with the ecology of this species as they are likely juvenile individuals seeking to recolonise upstream reaches. The construction of dams and weirs can also affect habitat availability especially through the reduction of river flows. For example, the availability of appropriate spawning habitats for many freshwater species is known to be impacted by the intentional creation of a lentic system upstream of dams/weirs (Sullivan et al. 2020). In addition, sand mining activities in the upstream area of the dam (1–5 km upstream) may also impact fish reproduction. The surface and groundwater quality of rivers can be impacted by sand and gravel mining activities through the alteration of the standard levels of physicochemical parameters like acidity and dissolved oxygen (Bayram and Önsoy 2015; Mercado-Garcia et al. 2018). Sand mining was undertaken prior to the Perjaya Dam construction and has very likely increased pressure on the health of fish environment including macroinvertebrate drift, community structure, food web dynamics and fish movement (Koehnken et al. 2020). So, in addition to the barrier effect, it is important to note that dams and weirs can also alter habitat and this can reduce fish populations.
Conclusion
Our results show that the Perjaya Dam has negatively impacted the fish community in the Komering River, with differences in the fish community upstream and downstream of the dam. Although the dam has been equipped with a fishway, the results suggest that the operation of the fishway is not appropriate for many species of local fish; or is operating at an insufficient level to recover upstream populations. Multidisciplinary studies including the socio-economic impact of the dams, genetic fragmentation, and studies of the effectiveness of the engineering operation of the fishway are urgently needed to provide comprehensive evidence of the dam’s impact in Indonesia and propose solutions to minimise the impact. Our follow-on study of fishway effectiveness will evaluate upstream fish movement and will help to determine the operational adjustments needed to improve its efficiency.
Data availability
The data used in this manuscript were arranged by the first author (Dwi Atminarso). Readers can consult with him for access to the data.
Acknowledgements
This study was funded by The Australian Centre for International Agricultural Research (ACIAR-FIS-153) – Translating fish passage research outcome into policy and legislation across South East Asia and also Australia Awards – Department Foreign Affairs and Trade Australia. Supports were also made from institution partners including Research Institute for Inland Fisheries and Extension (RIIFE), River Basin Institute of Sumatera VIII, Department of Fisheries and Livestock of Ogan Komering Ulu Timur, and Bogor Research Institute for Freshwater Aquaculture and Fisheries Extension (RIFAFE). This research also forms part of a doctoral thesis at Charles Sturt University, Australia. Assistance in the field was also made by some individuals (Mr Nizar, Prof. Husnah, Dian, Rezki, Sigit, Mersi, Armun, Mr Udin, Fadli, Kuniawan, Deni, Hendra, Mrs Ira, Mrs Desuneti). The research was conducted under the Charles Sturt University Animal Care and Ethics approval (A19357).
References
Alexandre CM, Almeida PR (2010) The impact of small physical obstacles on the structure of freshwater fish assemblages. River Research and Applications 26, 977-994.
| Google Scholar |
Allan JD, Flecker AS (1993) Biodiversity conservation in running waters. BioScience 43, 32-43.
| Crossref | Google Scholar |
Argentina JE, Angermeier PL, Hallerman EM, Welsh SA (2018) Spatial extent of analysis influences observed patterns of population genetic structure in a widespread darter species (Percidae). Freshwater Biology 63, 1185-1198.
| Crossref | Google Scholar |
Baumgartner LJ, Wibowo A (2018) Addressing fish-passage issues at hydropower and irrigation infrastructure projects in Indonesia. Marine and Freshwater Research 69, 1805-1813.
| Crossref | Google Scholar |
Baumgartner LJ, Marsden T, Singhanouvong D, Phonekhampheng O, Stuart IG, Thorncraft G (2012) Using an experimental in situ fishway to provide key design criteria for lateral fish passage in tropical rivers: a case study from the Mekong River, central Lao PDR. River Research and Applications 28, 1217-1229.
| Crossref | Google Scholar |
Bayram A, Önsoy H (2015) Sand and gravel mining impact on the surface water quality: a case study from the city of Tirebolu (Giresun Province, NE Turkey). Environmental Earth Science 73, 1997-2011.
| Crossref | Google Scholar |
Bhatt JP, Pandit MK (2016) Endangered Golden mahseer Tor putitora Hamilton: a review of natural history. Reviews in Fish Biology and Fisheries 26, 25-38.
| Crossref | Google Scholar |
Bishop KA, Bell JD (1978) Observations on the fish fauna below Tallowa Dam (Shoalhaven River, New South Wales) during river flow stoppages. Marine and Freshwater Research 29, 543-549.
| Crossref | Google Scholar |
Buisson L, Thuiller W, Lek S, Lim P, Grenouillet G (2008) Climate change hastens the turnover of stream fish assemblages. Global Change Biology 14, 2232-2248.
| Crossref | Google Scholar |
Cadwallader PL (1978) Some causes in the decline in range and abundance of native fish in the Murray-Darling River system. Proceedings of the Royal Society of Victoria 90, 211-224.
| Google Scholar |
Deinet S, Scott-Gatty K, Rotton H, Twardek WM, Marconi V, Mcrae L, Baumgartner LJ, Brink K, Claussen JE, Cooke SJ, Darwall W, Eriksson BK, Garcia de Leaniz C, Hogan Z, Royte J, Silva LGM, Thieme ML, Tickner D, Waldman J, Wanningen H, Weyl OLF, Berkhuysen A (2020) The living planet index (LPI) for migratory freshwater fish: technical report. World Fish Migration Foundation, The Netherlands.
De Leeuw JJ, Winter HV (2008) Migration of rheophilic fish in the large lowland rivers Meuse and Rhine, the Netherlands. Fisheries Management and Ecology 15, 409-415.
| Crossref | Google Scholar |
Dynesius M, Nilsson C (1994) Fragmentation and flow regulation of river systems in the northern third of the world. Science 266, 753-762.
| Crossref | Google Scholar |
Fernandes CC (1997) Lateral migration of fishes in Amazon floodplains. Ecology of Freshwater Fish 6, 36-44.
| Crossref | Google Scholar |
Fjellheim A, Raddum GG (1996) Weir building in a regulated west Norwegian river: long-term dynamics of invertebrates and fish. Regulated Rivers: Research & Management 12, 501-508.
| Crossref | Google Scholar |
Gaffar A, Utomo A (1991) Fisheries resources in Komering River. Bulletin Penelitian Perikanan Darat 10, 1-6.
| Google Scholar |
Gehrke PC, Gilligan DM, Barwick M (2002) Changes in fish communities of the Shoalhaven River 20 years after construction of Tallowa Dam, Australia. River Research and Applications 18, 265-286.
| Crossref | Google Scholar |
Harris JH, Kingsford RT, Peirson W, Baumgartner LJ (2017) Mitigating the effects of barriers to freshwater fish migrations: the Australian experience. Marine and Freshwater Research 68, 614-628.
| Crossref | Google Scholar |
Holmquist JG, Schmidt-Gengenbach JM, Yoshioka BB (1998) High dams and marine-freshwater linkages: effects on native and introduced fauna in the Caribbean. Conservation Biology 12, 621-630.
| Crossref | Google Scholar |
Husnah, Wijaya D, Arsyad MN (2007) The Perjaya dam (upper Komering): roles and problems for fisheries resources in Komering River. In ‘Proceeding of science congress of Western of Indonesia 2007, Palembang, 3–5 June 2007’. pp. 10–20. (Sriwijaya University and Indonesian Institute of Sciences: Palembang)
Koehnken L, Rintoul MS, Goichot M, Tickner D, Loftus A-C, Acreman MC (2020) Impacts of riverine sand mining on freshwater ecosystems: a review of the scientific evidence and guidance for future research. River Research and Applications 36, 362-370.
| Crossref | Google Scholar |
Kowarsky J, Ross AH (1981) Fish movement upstream through a central Queensland (Fitzroy River) coastal fishway. Marine and Freshwater Research 32, 93-109.
| Crossref | Google Scholar |
Martinez PJ, Chart TE, Trammell MA, Wullschleger JG, Bergersen EP (1994) Fish species composition before and after construction of a main stem reservoir on the White River, Colorado. Environmental Biology of Fishes 40, 227-239.
| Crossref | Google Scholar |
McKay SK, Cooper AR, Diebel MW, Elkins D, Oldford G, Roghair C, Wieferich D (2017) Informing watershed connectivity barrier prioritization decisions: a synthesis. River Research and Applications 33, 847-862.
| Crossref | Google Scholar |
Mercado-Garcia D, Wyseure G, Goethals P (2018) Freshwater ecosystem services in mining regions: modelling options for policy development support. Water 10, 531.
| Crossref | Google Scholar |
Mercado-Silva N, Helmus MR, Zanden MJV (2009) The effects of impoundment and non-native species on a river food web in Mexico’s central plateau. River Research and Applications 25, 1090-1108.
| Crossref | Google Scholar |
Ng HH, Kottelat M (2021) Description of Bagarius vegrandis, a new species of sisorid catfish from Indochina (Actinopterygii: Siluriformes), with notes on the identity of Bagarius bagarius. Zootaxa 4926, 134-146.
| Crossref | Google Scholar |
Oldani NO, Baigún CRM (2002) Performance of a fishway system in a major South American dam on the Parana River (Argentina–Paraguay). River Research and Applications 18, 171-183.
| Crossref | Google Scholar |
Ovidio M, Sonny D, Watthez Q, Goffaux D, Detrait O, Orban P, Nzau Matondo B, Renardy S, Dierckx A, Benitez J-P (2020) Evaluation of the performance of successive multispecies improved fishways to reconnect a rehabilitated river. Wetlands Ecology and Management 28, 641-654.
| Crossref | Google Scholar |
Poff NL, Hart DD (2002) How dams vary and why it matters for the emerging science of dam removal: an ecological classification of dams is needed to characterize how the tremendous variation in the size, operational mode, age, and number of dams in a river basin influences the potential for restoring regulated rivers via dam removal. BioScience 52, 659-668.
| Crossref | Google Scholar |
Rivinoja P, McKinnell S, Lundqvist H (2001) Hindrances to upstream migration of Atlantic salmon (Salmo salar) in a northern Swedish river caused by a hydroelectric power-station. Regulated Rivers: Research & Management: An International Journal Devoted to River Research and Management 17, 101-115.
| Google Scholar |
Rourke ML, Robinson W, Baumgartner LJ, Doyle J, Growns I, Thiem JD (2019) Sequential fishways reconnect a coastal river reflecting restored migratory pathways for an entire fish community. Restoration Ecology 27, 399-407.
| Crossref | Google Scholar |
Stoffels RJ, Humphries P, Bond NR, Price AE (2022) Fragmentation of lateral connectivity and fish population dynamics in large rivers. Fish and Fisheries 23, 680-696.
| Crossref | Google Scholar |
Stuart IG, Berghuis AP (2002) Upstream passage of fish through a vertical-slot fishway in an Australian subtropical river. Fisheries Management and Ecology 9, 111-122.
| Crossref | Google Scholar |
Stuart IG, Mallen-Cooper M (1999) An assessment of the effectiveness of a vertical-slot fishway for non-salmonid fish at a tidal barrier on a large tropical/subtropical river. Regulated Rivers: Research & Management 15, 575-590.
| Crossref | Google Scholar |
Sullivan CJ, Weber MJ, Pierce CL, Camacho CA (2020) A comparison of Grass Carp population characteristics upstream and downstream of Lock and Dam 19 of the Upper Mississippi River. Journal of Fish and Wildlife Management 11, 99-111.
| Crossref | Google Scholar |
Taylor CM, Millican DS, Roberts ME, Slack WT (2008) Long-term change to fish assemblages and the flow regime in a southeastern U.S. river system after extensive aquatic ecosystem fragmentation. Ecography 31, 787-797.
| Crossref | Google Scholar |
Townsend GH (1975) Impact of the Bennett Dam on the Peace-Athabasca Delta. Journal Fisheries Research Board of Canada 32, 171-176.
| Google Scholar |
Vu AV, Baumgartner LJ, Mallen-Cooper M, Howitt JA, Robinson WA, So N, Cowx IG (2020) Diadromy in a large tropical river, the Mekong: more common than assumed, with greater implications for management. Journal of Ecohydraulics 1-13.
| Google Scholar |
Vu AV, Baumgartner LJ, Limburg KE, Doran GS, Mallen-Cooper M, Gillanders BM, Thiem JD, Howitt JA, Kewish CM, Reinhardt J, Cowx IG (2022) Life history strategies of Mekong pangasiid catfishes revealed by otolith microchemistry. Fisheries Research 249, 106239.
| Crossref | Google Scholar |