How does secondary hypogonadism affect the spermatozoa proteome? Lessons from a porcine animal model
Ana P. B. Souza A , Tayná N. Lopes A , Anna F. T. Silva A , Lucélia Santi B , Walter O. Beys-da-Silva B , John R. Yates A D
A D
A Laboratório de Biotecnologia, Universidade do Vale do Taquari, Rua Avelino Tallini, 171, Lajeado, RS 9514-014, Brazil.
B Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, Avenida Ipiranga, 2752, Porto Alegre, RS 90610-000, Brazil.
C Scripps Research Institute, SR11, Department of Molecular Medicine, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
D Corresponding author. Email: ivan.bustamante@pq.cnpq.br
Reproduction, Fertility and Development 32(13) 1125-1144 https://doi.org/10.1071/RD20017
Submitted: 15 January 2020 Accepted: 30 June 2020 Published: 4 August 2020
Abstract
Secondary hypogonadism is a consequence of congenital or acquired diseases that affect the hypothalamus and/or pituitary gland, impairing secretion of gonadotrophin-releasing hormone (GnRH). Androgen deficiency resulting from reduced GnRH secretion is likely to have disrupting effects in epididymal epithelial cells, impairing the sperm maturation process. The aim of this study was to describe changes in the proteome of epididymal spermatozoa in a porcine model of secondary hypogonadism. Cauda epididymal spermatozoa were obtained from 10 boars previously immunised against GnRH (Vivax; Pfizer) and from 10 healthy boars. Protein extracts were analysed by multidimensional protein identification technology. In all, 1322 unique proteins were identified in the protein extracts of cauda epididymal spermatozoa, with significant changes in the abundance of key proteins involved in sperm metabolism (enolase, pyruvate dehydrogenase), acrosome reaction and capacitation (oxoprolinase, acrosomal protein SP-10, dihydrolipoyl dehydrogenase) and sperm–oocyte interactions (zona pellucida-binding protein, zonadhesin, sperm adhesion molecule 1). In addition, the abundance of mitochondrial proteins was severely affected, with significant changes in proteins of Complex I and II, as well as ATPase of the oxidative phosphorylation chain. The proteins identified in this study are potential sperm biomarkers of testicular and epididymal dysfunction related to disruption of the hypothalamus–pituitary–testis axis.
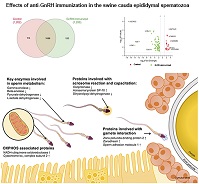
Additional keywords: epididymis, fertility, spermatozoa.
References
Aguiar, G. B., Caldas-Bussiere, M. C., Maciel, V. L., Carvaho, C. S. P., and Souza, C. L. M. (2019). Association of L-arginine with heparin on the sperm capacitation improves in vitro embryo production in bovine. Anim. Reprod. 16, 938–944.| Association of L-arginine with heparin on the sperm capacitation improves in vitro embryo production in bovine.Crossref | GoogleScholarGoogle Scholar | 32368274PubMed |
Aguilera-Aguirre, L., Bacsi, A., Saavedra-Molina, A., Kurosky, A., Sur, S., and Boldogh, I. (2009). Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 183, 5379–5387.
| Mitochondrial dysfunction increases allergic airway inflammation.Crossref | GoogleScholarGoogle Scholar | 19786549PubMed |
Akintayo, A., Legare, C., and Sullivan, R. (2015). Dicarbonyl l-xylulose reductase (DCXR), a ‘moonlighting protein’ in the bovine epididymis. PLoS One 10, e0120869.
| Dicarbonyl l-xylulose reductase (DCXR), a ‘moonlighting protein’ in the bovine epididymis.Crossref | GoogleScholarGoogle Scholar | 25815750PubMed |
Almagro Armenteros, J. J., Tsirigos, K. D., Sonderby, C. K., Petersen, T. N., Winther, O., Brunak, S., von Heijne, G., and Nielsen, H. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423.
| SignalP 5.0 improves signal peptide predictions using deep neural networks.Crossref | GoogleScholarGoogle Scholar | 30778233PubMed |
Amaral, A., Lourenco, B., Marques, M., and Ramalho-Santos, J. (2013). Mitochondria functionality and sperm quality. Reproduction 146, R163–R174.
| Mitochondria functionality and sperm quality.Crossref | GoogleScholarGoogle Scholar | 23901129PubMed |
Araujo, A. B., Esche, G. R., Kupelian, V., O’Donnell, A. B., Travison, T. G., Williams, R. E., Clark, R. V., and McKinlay, J. B. (2007). Prevalence of symptomatic androgen deficiency in men. J. Clin. Endocrinol. Metab. 92, 4241–4247.
| Prevalence of symptomatic androgen deficiency in men.Crossref | GoogleScholarGoogle Scholar | 17698901PubMed |
Arce, J. C., De Souza, M. J., Pescatello, L. S., and Luciano, A. A. (1993). Subclinical alterations in hormone and semen profile in athletes. Fertil. Steril. 59, 398–404.
| Subclinical alterations in hormone and semen profile in athletes.Crossref | GoogleScholarGoogle Scholar | 8425638PubMed |
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., Davis, A. P., Dolinski, K., Dwight, S. S., Eppig, J. T., Harris, M. A., Hill, D. P., Issel-Tarver, L., Kasarskis, A., Lewis, S., Matese, J. C., Richardson, J. E., Ringwald, M., Rubin, G. M., and Sherlock, G. (2000). Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29.
| Gene ontology: tool for the unification of biology. The Gene Ontology Consortium.Crossref | GoogleScholarGoogle Scholar | 10802651PubMed |
Baker, M. A., Reeves, G., Hetherington, L., Muller, J., Baur, I., and Aitken, R. J. (2007). Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin. Appl. 1, 524–532.
| Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis.Crossref | GoogleScholarGoogle Scholar | 21136703PubMed |
Basaria, S. (2014). Male hypogonadism. Lancet 383, 1250–1263.
| Male hypogonadism.Crossref | GoogleScholarGoogle Scholar | 24119423PubMed |
Bedford, J. M. (1983). Significance of the need for sperm capacitation before fertilization in eutherian mammals. Biol. Reprod. 28, 108–120.
| Significance of the need for sperm capacitation before fertilization in eutherian mammals.Crossref | GoogleScholarGoogle Scholar | 6338941PubMed |
Belleannee, C., Belghazi, M., Labas, V., Teixeira-Gomes, A. P., Gatti, J. L., Dacheux, J. L., and Dacheux, F. (2011). Purification and identification of sperm surface proteins and changes during epididymal maturation. Proteomics 11, 1952–1964.
| Purification and identification of sperm surface proteins and changes during epididymal maturation.Crossref | GoogleScholarGoogle Scholar | 21472858PubMed |
Belleannée, C., Thimon, V., and Sullivan, R. (2012). Region-specific gene expression in the epididymis. Cell Tissue Res. 349, 717–731.
| Region-specific gene expression in the epididymis.Crossref | GoogleScholarGoogle Scholar | 22427067PubMed |
Bi, M., Hickox, J. R., Winfrey, V. P., Olson, G. E., and Hardy, D. M. (2003). Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem. J. 375, 477–488.
| Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome.Crossref | GoogleScholarGoogle Scholar | 12882646PubMed |
Bilskis, R., Sutkeviciene, N., Riskeviciene, V., Januskauskas, A., and Zilinskas, H. (2012). Effect of active immunization against GnRH on testosterone concentration, libido and sperm quality in mature AI boars. Acta Vet. Scand. 54, 33.
| Effect of active immunization against GnRH on testosterone concentration, libido and sperm quality in mature AI boars.Crossref | GoogleScholarGoogle Scholar | 22640725PubMed |
Bollwein, H., and Bittner, L. (2018). Impacts of oxidative stress on bovine sperm function and subsequent in vitro embryo development. Anim. Reprod. 15, 703–710.
| Impacts of oxidative stress on bovine sperm function and subsequent in vitro embryo development.Crossref | GoogleScholarGoogle Scholar |
Brunius, C., Zamaratskaia, G., Andersson, K., Chen, G., Norrby, M., Madej, A., and Lundstrom, K. (2011). Early immunocastration of male pigs with Improvac® – effect on boar taint, hormones and reproductive organs. Vaccine 29, 9514–9520.
| Early immunocastration of male pigs with Improvac® – effect on boar taint, hormones and reproductive organs.Crossref | GoogleScholarGoogle Scholar | 22008824PubMed |
Carvalho, P. C., Yates III, J. R., and Barbosa, V. C. (2010). Analyzing shotgun proteomic data with PatternLab for proteomics. Curr. Protoc. Bioinform. 30, 13.13.1–13.13.15.
| Analyzing shotgun proteomic data with PatternLab for proteomics.Crossref | GoogleScholarGoogle Scholar |
Carvalho, P. C., Yates, J. R., and Barbosa, V. C. (2012). Improving the TFold test for differential shotgun proteomics. Bioinformatics 28, 1652–1654.
| Improving the TFold test for differential shotgun proteomics.Crossref | GoogleScholarGoogle Scholar | 22539673PubMed |
Carvalho, P. C., Lima, D. B., Leprevost, F. V., Santos, M. D. M., Fischer, J. S. G., Aquino, P. F., Moresco, J. J., Yates, J. R., and Barbosa, V. C. (2016). Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat. Protoc. 11, 102–117.
| Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0.Crossref | GoogleScholarGoogle Scholar | 26658470PubMed |
Choi, Y. J., Uhm, S. J., Song, S. J., Song, H., Park, J. K., Kim, T., Park, C., and Kim, J. H. (2008). Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: linking proteome analysis. J. Reprod. Dev. 54, 68–83.
| Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: linking proteome analysis.Crossref | GoogleScholarGoogle Scholar | 18094529PubMed |
Conesa, A., Gotz, S., Garcia-Gomez, J. M., Terol, J., Talon, M., and Robles, M. (2005). Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676.
| Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research.Crossref | GoogleScholarGoogle Scholar | 16081474PubMed |
Crofts, A. R. (2004). The cytochrome bc1 complex: function in the context of structure. Annu. Rev. Physiol. 66, 689–733.
| The cytochrome bc1 complex: function in the context of structure.Crossref | GoogleScholarGoogle Scholar | 14977419PubMed |
Dacheux, J. L., Castella, S., Gatti, J. L., and Dacheux, F. (2005). Epididymal cell secretory activities and the role of proteins in boar sperm maturation. Theriogenology 63, 319–341.
| Epididymal cell secretory activities and the role of proteins in boar sperm maturation.Crossref | GoogleScholarGoogle Scholar | 15626402PubMed |
Darby, E., and Anawalt, B. D. (2005). Male hypogonadism: an update on diagnosis and treatment. Treat. Endocrinol. 4, 293–309.
| Male hypogonadism: an update on diagnosis and treatment.Crossref | GoogleScholarGoogle Scholar | 16185098PubMed |
Einarsson, S., Brunius, C., Wallgren, M., Lundstrom, K., Andersson, K., Zamaratskaia, G., and Rodriguez-Martinez, H. (2011). Effects of early vaccination with Improvac® on the development and function of reproductive organs of male pigs. Anim. Reprod. Sci. 127, 50–55.
| Effects of early vaccination with Improvac® on the development and function of reproductive organs of male pigs.Crossref | GoogleScholarGoogle Scholar | 21802872PubMed |
Erickson, D. W., Way, A. L., Bertolla, R. P., Chapman, D. A., and Killian, G. J. (2007). Influence of osteopontin, casein and oviductal fluid on bovine sperm capacitation. Anim. Reprod. 4, 103–112.
Foster, J. A., Klotz, K. L., Flickinger, C. J., Thomas, T. S., Wright, R. M., Castillo, J. R., and Herr, J. C. (1994). Human SP-10: acrosomal distribution, processing, and fate after the acrosome reaction. Biol. Reprod. 51, 1222–1231.
| Human SP-10: acrosomal distribution, processing, and fate after the acrosome reaction.Crossref | GoogleScholarGoogle Scholar | 7888499PubMed |
Fraietta, R., Zylberstejn, D. S., and Esteves, S. C. (2013). Hypogonadotropic hypogonadism revisited. Clinics (São Paulo) 68, 81–88.
| Hypogonadotropic hypogonadism revisited.Crossref | GoogleScholarGoogle Scholar |
França, L. R., Avelar, G. F., and Almeida, F. F. (2005). Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63, 300–318.
| Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs.Crossref | GoogleScholarGoogle Scholar | 15626401PubMed |
Frapsauce, C., Pionneau, C., Bouley, J., Delarouziere, V., Berthaut, I., Ravel, C., Antoine, J. M., Soubrier, F., and Mandelbaum, J. (2014). Proteomic identification of target proteins in normal but nonfertilizing sperm. Fertil. Steril. 102, 372–380.
| Proteomic identification of target proteins in normal but nonfertilizing sperm.Crossref | GoogleScholarGoogle Scholar | 24882558PubMed |
Gao, Z., and Garbers, D. L. (1998). Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J. Biol. Chem. 273, 3415–3421.
| Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains.Crossref | GoogleScholarGoogle Scholar | 9452463PubMed |
Garrido, N., Meseguer, M., Alvarez, J., Simon, C., Pellicer, A., and Remohi, J. (2004). Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil. Steril. 82, 1059–1066.
| Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men.Crossref | GoogleScholarGoogle Scholar | 15474074PubMed | 15474074PubMed |
Gibb, Z., and Aitken, R. J. (2016). The impact of sperm metabolism during in vitro storage: the stallion as a model. BioMed Res. Int. 2016, 9380609.
| The impact of sperm metabolism during in vitro storage: the stallion as a model.Crossref | GoogleScholarGoogle Scholar | 26881234PubMed | 26881234PubMed |
Griffith, R. O., Dressendorfer, R. H., Fullbright, C. D., and Wade, C. E. (1990). Testicular function during exhaustive endurance training. Phys. Sportsmed. 18, 54–64.
| Testicular function during exhaustive endurance training.Crossref | GoogleScholarGoogle Scholar | 27424583PubMed | 27424583PubMed |
Gu, N. H., Zhao, W. L., Wang, G. S., and Sun, F. (2019). Comparative analysis of mammalian sperm ultrastructure reveals relationships between sperm morphology, mitochondrial functions and motility. Reprod. Biol. Endocrinol. 17, 66.
| Comparative analysis of mammalian sperm ultrastructure reveals relationships between sperm morphology, mitochondrial functions and motility.Crossref | GoogleScholarGoogle Scholar | 31416446PubMed | 31416446PubMed |
Guan, S. S., Sheu, M. L., Wu, C. T., Chiang, C. K., and Liu, S. H. (2015). ATP synthase subunit-beta down-regulation aggravates diabetic nephropathy. Sci. Rep. 5, 14561.
| ATP synthase subunit-beta down-regulation aggravates diabetic nephropathy.Crossref | GoogleScholarGoogle Scholar | 26449648PubMed | 26449648PubMed |
Guo, Y., Wang, A., Liu, X., and Li, E. (2019). Effects of resveratrol on reducing spermatogenic dysfunction caused by high-intensity exercise. Reprod. Biol. Endocrinol. 17, 42.
| Effects of resveratrol on reducing spermatogenic dysfunction caused by high-intensity exercise.Crossref | GoogleScholarGoogle Scholar | 31060552PubMed | 31060552PubMed |
Hamada, A., Esteves, S. C., Nizza, M., and Agarwal, A. (2012). Unexplained male infertility: diagnosis and management. Int. Braz J Urol 38, 576–594.
| Unexplained male infertility: diagnosis and management.Crossref | GoogleScholarGoogle Scholar | 23131516PubMed | 23131516PubMed |
Hamzeh, M., and Robaire, B. (2011). Androgens activate mitogen-activated protein kinase via epidermal growth factor receptor/insulin-like growth factor 1 receptor in the mouse PC-1 cell line. J. Endocrinol. 209, 55–64.
| Androgens activate mitogen-activated protein kinase via epidermal growth factor receptor/insulin-like growth factor 1 receptor in the mouse PC-1 cell line.Crossref | GoogleScholarGoogle Scholar | 21220406PubMed | 21220406PubMed |
Han, X., Zhou, Y., Zeng, Y., Sui, F., Liu, Y., Tan, Y., Cao, X., Du, X., Meng, F., and Zeng, X. (2017). Effects of active immunization against GnRH versus surgical castration on hypothalamic-pituitary function in boars. Theriogenology 97, 89–97.
| Effects of active immunization against GnRH versus surgical castration on hypothalamic-pituitary function in boars.Crossref | GoogleScholarGoogle Scholar | 28583614PubMed | 28583614PubMed |
Hardy, D. M., and Garbers, D. L. (1994). Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J. Biol. Chem. 269, 19000–19004.
| 8034657PubMed |
| 8034657PubMed |
Hardy, D. M., and Garbers, D. L. (1995). A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J. Biol. Chem. 270, 26025–26028.
| A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor.Crossref | GoogleScholarGoogle Scholar | 7592795PubMed | 7592795PubMed |
Heberle, H., Meirelles, G. V., da Silva, F. R., Telles, G. P., and Minghim, R. (2015). InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16, 169.
| InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams.Crossref | GoogleScholarGoogle Scholar | 25994840PubMed | 25994840PubMed |
Herr, J. C., Wright, R. M., John, E., Foster, J., Kays, T., and Flickinger, C. J. (1990). Identification of human acrosomal antigen SP-10 in primates and pigs. Biol. Reprod. 42, 377–382.
| Identification of human acrosomal antigen SP-10 in primates and pigs.Crossref | GoogleScholarGoogle Scholar | 2337631PubMed | 2337631PubMed |
Ho, C. C., and Tan, H. M. (2013). Treatment of the hypogonadal infertile male – a review. Sex. Med. Rev. 1, 42–49.
| Treatment of the hypogonadal infertile male – a review.Crossref | GoogleScholarGoogle Scholar | 27784559PubMed | 27784559PubMed |
Holland, A., and Ohlendieck, K. (2015). Comparative profiling of the sperm proteome. Proteomics 15, 632–648.
| Comparative profiling of the sperm proteome.Crossref | GoogleScholarGoogle Scholar | 24909132PubMed | 24909132PubMed |
Intasqui, P., Camargo, M., Del Giudice, P. T., Spaine, D. M., Carvalho, V. M., Cardozo, K. H., Cedenho, A. P., and Bertolla, R. P. (2013). Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation. J. Assist. Reprod. Genet. 30, 1187–1202.
| Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation.Crossref | GoogleScholarGoogle Scholar | 23893156PubMed | 23893156PubMed |
Kaplan, M., Russell, L. D., Peterson, R. N., and Martan, J. (1984). Boar sperm cytoplasmic droplets: their ultrastructure, their numbers in the epididymis and at ejaculation and their removal during isolation of sperm plasma membranes. Tissue Cell 16, 455–468.
| Boar sperm cytoplasmic droplets: their ultrastructure, their numbers in the epididymis and at ejaculation and their removal during isolation of sperm plasma membranes.Crossref | GoogleScholarGoogle Scholar | 6464007PubMed | 6464007PubMed |
Kato, S., Shibukawa, T., Harayama, H., and Kannan, Y. (1996). The timing of shedding and disintegration of cytoplasmic droplets from boar and goat. J. Reprod. Dev. 42, 237–241.
| The timing of shedding and disintegration of cytoplasmic droplets from boar and goat.Crossref | GoogleScholarGoogle Scholar |
Katz, D. J., Nabulsi, O., Tal, R., and Mulhall, J. P. (2012). Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 110, 573–578.
| Outcomes of clomiphene citrate treatment in young hypogonadal men.Crossref | GoogleScholarGoogle Scholar | 22044663PubMed | 22044663PubMed |
Kruljac, M., Finnbogadóttir, H., Bobjer, J., Giraldi, A., Fugl-Meyer, K., and Giwercman, A. (2020). Symptoms of sexual dysfunction among men from infertile couples: prevalence and association with testosterone deficiency. Andrology 8, 160–165.
| Symptoms of sexual dysfunction among men from infertile couples: prevalence and association with testosterone deficiency.Crossref | GoogleScholarGoogle Scholar | 31325248PubMed | 31325248PubMed |
Kuster, C. E., and Althouse, G. C. (2003). A technique for preserving retained distal cytoplasmic droplets in situ for immunofluorescence evaluation of ejaculated porcine spermatozoa. Prep. Biochem. Biotechnol. 33, 301–310.
| A technique for preserving retained distal cytoplasmic droplets in situ for immunofluorescence evaluation of ejaculated porcine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 14606687PubMed | 14606687PubMed |
Kwon, W. S., Rahman, M. S., Ryu, D. Y., Park, Y. J., and Pang, M. G. (2015). Increased male fertility using fertility-related biomarkers. Sci. Rep. 5, 15654.
| Increased male fertility using fertility-related biomarkers.Crossref | GoogleScholarGoogle Scholar | 26489431PubMed | 26489431PubMed |
Landim-Alvarenga, F. C., Graham, J. K., Alvarenga, M. A., and Squires, E. L. (2004). Calcium influx into equine and bovine spermatozoa during in vitro capacitation. Anim. Reprod. 1, 96–105.
Lea, I. A., Sivashanmugam, P., and O’Rand, M. G. (2001). Zonadhesin: characterization, localization, and zona pellucida binding. Biol. Reprod. 65, 1691–1700.
| Zonadhesin: characterization, localization, and zona pellucida binding.Crossref | GoogleScholarGoogle Scholar | 11717130PubMed | 11717130PubMed |
Leemans, B., Stout, T. A. E., De Schauwer, C., Heras, S., Nelis, H., Hoogewijs, M., Van Soon, A., and Gadella, B. M. (2019). Update on mammalian sperm capacitation: how much does the horse differ from other species? Reproduction 157, R181–R197.
| Update on mammalian sperm capacitation: how much does the horse differ from other species?Crossref | GoogleScholarGoogle Scholar | 30721132PubMed | 30721132PubMed |
Li, L. Y., Seddon, A. P., Meister, A., and Risley, M. S. (1989). Spermatogenic cell–somatic cell interactions are required for maintenance of spermatogenic cell glutathione. Biol. Reprod. 40, 317–331.
| Spermatogenic cell–somatic cell interactions are required for maintenance of spermatogenic cell glutathione.Crossref | GoogleScholarGoogle Scholar | 2720029PubMed | 2720029PubMed |
Liberzon, A., Birger, C., Thorvaldsdottir, H., Ghandi, M., Mesirov, J. P., and Tamayo, P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425.
| The Molecular Signatures Database (MSigDB) hallmark gene set collection.Crossref | GoogleScholarGoogle Scholar | 26771021PubMed | 26771021PubMed |
Liu, H., Sadygov, R. G., and Yates, J. R. (2004). A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201.
| A model for random sampling and estimation of relative protein abundance in shotgun proteomics.Crossref | GoogleScholarGoogle Scholar | 15253663PubMed | 15253663PubMed |
Lundgren, D. H., Hwang, S. I., Wu, L., and Han, D. K. (2010). Role of spectral counting in quantitative proteomics. Expert Rev. Proteomics 7, 39–53.
| Role of spectral counting in quantitative proteomics.Crossref | GoogleScholarGoogle Scholar | 20121475PubMed | 20121475PubMed |
McDonald, W. H., Tabb, D. L., Sadygov, R. G., MacCoss, M. J., Venable, J., Graumann, J., Johnson, J. R., Cociorva, D., and Yates, J. R. (2004). MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 18, 2162–2168.
| MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications.Crossref | GoogleScholarGoogle Scholar | 15317041PubMed | 15317041PubMed |
Meybodi, A. M., Mozdarani, H., Moradi, Sh. Z., and Akhoond, M. R. (2012). Importance of sperm gluthatione treatment in ART. J. Assist. Reprod. Genet. 29, 625–630.
| Importance of sperm gluthatione treatment in ART.Crossref | GoogleScholarGoogle Scholar | 22492222PubMed | 22492222PubMed |
Mitra, K., and Shivaji, S. (2004). Novel tyrosine-phosphorylated post-pyruvate metabolic enzyme, dihydrolipoamide dehydrogenase, involved in capacitation of hamster spermatozoa. Biol. Reprod. 70, 887–899.
| Novel tyrosine-phosphorylated post-pyruvate metabolic enzyme, dihydrolipoamide dehydrogenase, involved in capacitation of hamster spermatozoa.Crossref | GoogleScholarGoogle Scholar | 14645106PubMed | 14645106PubMed |
Mitra, K., Rangaraj, N., and Shivaji, S. (2005). Novelty of the pyruvate metabolic enzyme dihydrolipoamide dehydrogenase in spermatozoa: correlation of its localization, tyrosine phosphorylation, and activity during sperm capacitation. J. Biol. Chem. 280, 25743–25753.
| Novelty of the pyruvate metabolic enzyme dihydrolipoamide dehydrogenase in spermatozoa: correlation of its localization, tyrosine phosphorylation, and activity during sperm capacitation.Crossref | GoogleScholarGoogle Scholar | 15888450PubMed | 15888450PubMed |
Montiel, E. E., Huidobro, C. C., and Castellon, E. A. (2003). Glutathione-related enzymes in cell cultures from different regions of human epididymis. Arch. Androl. 49, 95–105.
| Glutathione-related enzymes in cell cultures from different regions of human epididymis.Crossref | GoogleScholarGoogle Scholar | 12623745PubMed | 12623745PubMed |
Nakamura, N., Dai, Q., Williams, J., Goulding, E. H., Willis, W. D., Brown, P. R., and Eddy, E. M. (2013). Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility. Biol. Reprod. 88, 90.
| Disruption of a spermatogenic cell-specific mouse enolase 4 (eno4) gene causes sperm structural defects and male infertility.Crossref | GoogleScholarGoogle Scholar | 23446454PubMed | 23446454PubMed |
National Research Council (2011). ‘Guide for the Care and Use of laboratory Animals.’ 8th edn. (The National Academies Press: Washington, DC.)
National Research Council (2012). ‘Nutrient Requirements of Swine.’ 11th edn. (The National Academies Press: Washington, DC.)
Olson, G. E., Winfrey, V. P., Bi, M., Hardy, D. M., and NagDas, S. K. (2004). Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis. Biol. Reprod. 71, 1128–1134.
| Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis.Crossref | GoogleScholarGoogle Scholar | 15175237PubMed | 15175237PubMed |
Park, Y. J., Kwon, W. S., Oh, S. A., and Pang, M. G. (2012). Fertility-related proteomic profiling bull spermatozoa separated by percoll. J. Proteome Res. 11, 4162–4168.
| Fertility-related proteomic profiling bull spermatozoa separated by percoll.Crossref | GoogleScholarGoogle Scholar | 22794312PubMed | 22794312PubMed |
Patrão, M. T., Silva, E. J., and Avellar, M. C. (2009). Androgens and the male reproductive tract: an overview of classical roles and current perspectives. Arq. Bras. Endocrinol. Metabol 53, 934–945.
| Androgens and the male reproductive tract: an overview of classical roles and current perspectives.Crossref | GoogleScholarGoogle Scholar | 20126845PubMed | 20126845PubMed |
Perez-Patiño, C., Barranco, I., Li, J., Padilla, L., Martinez, E. A., Rodriguez-Martinez, H., Roca, J., and Parrilla, I. (2019a). Cryopreservation differentially alters the proteome of epididymal and ejaculated pig spermatozoa. Int. J. Mol. Sci. 20, 1791.
| Cryopreservation differentially alters the proteome of epididymal and ejaculated pig spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Perez-Patiño, C., Li, J., Barranco, I., Martinez, E. A., Rodriguez-Martinez, H., Roca, J., and Parrilla, I. (2019b). The proteome of frozen–thawed pig spermatozoa is dependent on the ejaculate fraction source. Sci. Rep. 9, 705.
| The proteome of frozen–thawed pig spermatozoa is dependent on the ejaculate fraction source.Crossref | GoogleScholarGoogle Scholar | 30679492PubMed | 30679492PubMed |
Perez-Patiño, C., Parrilla, I., Li, J., Barranco, I., Martinez, E. A., Rodriguez-Martinez, H., and Roca, J. (2019c). The proteome of pig spermatozoa is remodeled during ejaculation. Mol. Cell. Proteomics 18, 41–50.
| The proteome of pig spermatozoa is remodeled during ejaculation.Crossref | GoogleScholarGoogle Scholar | 30257877PubMed | 30257877PubMed |
Perobelli, J. E., Patrao, M. T., Fernandez, C. D., Sanabria, M., Klinefelter, G. R., Avellar, M. C., and Kempinas, W. D. (2013). Androgen deprivation from pre-puberty to peripuberty interferes in proteins expression in pubertal and adult rat epididymis. Reprod. Toxicol. 38, 65–71.
| Androgen deprivation from pre-puberty to peripuberty interferes in proteins expression in pubertal and adult rat epididymis.Crossref | GoogleScholarGoogle Scholar | 23541399PubMed | 23541399PubMed |
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45.
| A new mathematical model for relative quantification in real-time RT-PCR.Crossref | GoogleScholarGoogle Scholar | 11328886PubMed | 11328886PubMed |
Ramakers, C., Ruijter, J. M., Deprez, R. H., and Moorman, A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66.
| Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data.Crossref | GoogleScholarGoogle Scholar | 12618301PubMed | 12618301PubMed |
Reddi, P. P., Shore, A. N., Acharya, K. K., and Herr, J. C. (2002). Transcriptional regulation of spermiogenesis: insights from the study of the gene encoding the acrosomal protein SP-10. J. Reprod. Immunol. 53, 25–36.
| Transcriptional regulation of spermiogenesis: insights from the study of the gene encoding the acrosomal protein SP-10.Crossref | GoogleScholarGoogle Scholar | 11730901PubMed | 11730901PubMed |
Robaire, B., and Hamzeh, M. (2011). Androgen action in the epididymis. J. Androl. 32, 592–599.
| Androgen action in the epididymis.Crossref | GoogleScholarGoogle Scholar | 21764895PubMed | 21764895PubMed |
Ruijter, J. M., Ramakers, C., Hoogaars, W. M., Karlen, Y., Bakker, O., van den Hoff, M. J., and Moorman, A. F. (2009). Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45.
| Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data.Crossref | GoogleScholarGoogle Scholar | 19822576PubMed | 19822576PubMed |
Santi, L., Beys-da-Silva, W. O., Berger, M., Calzolari, D., Guimaraes, J. A., Moresco, J. J., and Yates, J. R. (2014). Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. J. Proteome Res. 13, 1545–1559.
| Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes.Crossref | GoogleScholarGoogle Scholar | 24467693PubMed | 24467693PubMed |
Scheer, H., and Robaire, B. (1980). Steroid delta 4–5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase in the rat epididymis during development. Endocrinology 107, 948–953.
| Steroid delta 4–5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase in the rat epididymis during development.Crossref | GoogleScholarGoogle Scholar | 6931815PubMed | 6931815PubMed |
Schorr-Lenz, A. M., Alves, J., Henckes, N. A., Seibel, P. M., Benham, A. M., and Bustamante-Filho, I. C. (2016). GnRH immunization alters the expression and distribution of protein disulfide isomerases in the epididymis. Andrology 4, 957–963.
| GnRH immunization alters the expression and distribution of protein disulfide isomerases in the epididymis.Crossref | GoogleScholarGoogle Scholar | 27323298PubMed | 27323298PubMed |
Sharma, R., Agarwal, A., Mohanty, G., Hamada, A. J., Gopalan, B., Willard, B., Yadav, S., and du Plessis, S. (2013). Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod. Biol. Endocrinol. 11, 48.
| Proteomic analysis of human spermatozoa proteins with oxidative stress.Crossref | GoogleScholarGoogle Scholar | 23688036PubMed | 23688036PubMed |
Shukla, K. K., Kwon, W. S., Rahman, M. S., Park, Y. J., You, Y. A., and Pang, M. G. (2013). Nutlin-3a decreases male fertility via UQCRC2. PLoS One 8, e76959.
| Nutlin-3a decreases male fertility via UQCRC2.Crossref | GoogleScholarGoogle Scholar | 24130818PubMed | 24130818PubMed |
Sipilä, P., Krutskikh, A., Pujianto, D. A., Poutanen, M., and Huhtaniemi, I. (2011). Regional expression of androgen receptor coregulators and androgen action in the mouse epididymis. J. Androl. 32, 711–717.
| Regional expression of androgen receptor coregulators and androgen action in the mouse epididymis.Crossref | GoogleScholarGoogle Scholar | 21764902PubMed | 21764902PubMed |
Siva, A. B., Panneerdoss, S., Sailasree, P., Singh, D. K., Kameshwari, D. B., and Shivaji, S. (2014). Inhibiting sperm pyruvate dehydrogenase complex and its E3 subunit, dihydrolipoamide dehydrogenase affects fertilization in Syrian hamsters. PLoS One 9, e97916.
| Inhibiting sperm pyruvate dehydrogenase complex and its E3 subunit, dihydrolipoamide dehydrogenase affects fertilization in Syrian hamsters.Crossref | GoogleScholarGoogle Scholar | 24852961PubMed | 24852961PubMed |
Sutovsky, P. (2018). Review: sperm–oocyte interactions and their implications for bull fertility, with emphasis on the ubiquitin–proteasome system. Animal 12, s121–s132.
| Review: sperm–oocyte interactions and their implications for bull fertility, with emphasis on the ubiquitin–proteasome system.Crossref | GoogleScholarGoogle Scholar | 29477154PubMed | 29477154PubMed |
Szklarczyk, D., Franceschini, A., Wyder, S., Forslund, K., Heller, D., Huerta-Cepas, J., Simonovic, M., Roth, A., Santos, A., Tsafou, K. P., Kuhn, M., Bork, P., Jensen, L. J., and von Mering, C. (2015). STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452.
| STRING v10: protein–protein interaction networks, integrated over the tree of life.Crossref | GoogleScholarGoogle Scholar | 25352553PubMed | 25352553PubMed |
Szklarczyk, D., Santos, A., von Mering, C., Jensen, L. J., Bork, P., and Kuhn, M. (2016). STITCH 5: augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380–D384.
| STITCH 5: augmenting protein–chemical interaction networks with tissue and affinity data.Crossref | GoogleScholarGoogle Scholar | 26590256PubMed | 26590256PubMed |
Tabb, D. L., McDonald, W. H., and Yates, J. R. (2002). DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome Res. 1, 21–26.
| DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics.Crossref | GoogleScholarGoogle Scholar | 12643522PubMed | 12643522PubMed |
Tardif, S., Wilson, M. D., Wagner, R., Hunt, P., Gertsenstein, M., Nagy, A., Lobe, C., Koop, B. F., and Hardy, D. M. (2010). Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 285, 24863–24870.
| Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida.Crossref | GoogleScholarGoogle Scholar | 20529856PubMed | 20529856PubMed |
Thimon, V., Belghazi, M., Dacheux, J. L., and Gatti, J. L. (2006). Analysis of furin ectodomain shedding in epididymal fluid of mammals: demonstration that shedding of furin occurs in vivo. Reproduction 132, 899–908.
| Analysis of furin ectodomain shedding in epididymal fluid of mammals: demonstration that shedding of furin occurs in vivo.Crossref | GoogleScholarGoogle Scholar | 17127750PubMed | 17127750PubMed |
Travis, A. J., Jorgez, C. J., Merdiushev, T., Jones, B. H., Dess, D. M., Diaz-Cueto, L., Storey, B. T., Kopf, G. S., and Moss, S. B. (2001). Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J. Biol. Chem. 276, 7630–7636.
| Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 11115497PubMed | 11115497PubMed |
Tulsiani, D. R. (2003). Glycan modifying enzymes in luminal fluid of rat epididymis: are they involved in altering sperm surface glycoproteins during maturation? Microsc. Res. Tech. 61, 18–27.
| Glycan modifying enzymes in luminal fluid of rat epididymis: are they involved in altering sperm surface glycoproteins during maturation?Crossref | GoogleScholarGoogle Scholar | 12672119PubMed | 12672119PubMed |
Vaamonde, D., Algar-Santacruz, C., Abbasi, A., and Garcia-Manso, J. M. (2018). Sperm DNA fragmentation as a result of ultra-endurance exercise training in male athletes. Andrologia 50, e12793.
| Sperm DNA fragmentation as a result of ultra-endurance exercise training in male athletes.Crossref | GoogleScholarGoogle Scholar | 28295487PubMed | 28295487PubMed |
Verhoeven, G., Denolet, E., Swinnen, J. V., Willems, A., Saunders, P. T. K., Sharpe, R. M., and Gendt, K. D. (2007). The role of androgens in the control of spermatogenesis: lessons from transgenic models involving a Sertoli cell-selective knockout of the androgen receptor. Anim. Reprod. 4, 3–14.
Wang, G., Guo, Y., Zhou, T., Shi, X., Yu, J., Yang, Y., Wu, Y., Wang, J., Liu, M., Chen, X., Tu, W., Zeng, Y., Jiang, M., Li, S., Zhang, P., Zhou, Q., Zheng, B., Yu, C., Zhou, Z., Guo, X., and Sha, J. (2013). In-depth proteomic analysis of the human sperm reveals complex protein compositions. J. Proteomics 79, 114–122.
| In-depth proteomic analysis of the human sperm reveals complex protein compositions.Crossref | GoogleScholarGoogle Scholar | 23268119PubMed | 23268119PubMed |
Watanabe, K., Taskesen, E., van Bochoven, A., and Posthuma, D. (2017). Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826.
| Functional mapping and annotation of genetic associations with FUMA.Crossref | GoogleScholarGoogle Scholar | 29184056PubMed | 29184056PubMed |
Weber, A., Alves, J., Abujamra, A. L., and Bustamante-Filho, I. C. (2018). Structural modeling and mRNA expression of epididymal beta-defensins in GnRH immunized boars: a model for secondary hypogonadism in man. Mol. Reprod. Dev. 85, 921–933.
| Structural modeling and mRNA expression of epididymal beta-defensins in GnRH immunized boars: a model for secondary hypogonadism in man.Crossref | GoogleScholarGoogle Scholar | 30307666PubMed | 30307666PubMed |
Weber, A., Argenti, L. E., de Souza, A. P. B., Santi, L., Beys-da-Silva, W. O., Yates, J. R., and Bustamante-Filho, I. C. (2020). Ready for the journey: a comparative proteome profiling of porcine cauda epididymal fluid and spermatozoa. Cell Tissue Res. 379, 389–405.
| Ready for the journey: a comparative proteome profiling of porcine cauda epididymal fluid and spermatozoa.Crossref | GoogleScholarGoogle Scholar | 31444576PubMed | 31444576PubMed |
Wolters, D. A., Washburn, M. P., and Yates, J. R. (2001). An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 73, 5683–5690.
| An automated multidimensional protein identification technology for shotgun proteomics.Crossref | GoogleScholarGoogle Scholar | 11774908PubMed | 11774908PubMed |
Xu, T., Venable, J. D., Park, S. K., Cociorva, D., Lu, B., Liao, L., Wohlschlegel, J., Hewel, J., and Yates, J. R. (2006). ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Mol. Cell. Proteomics 5, S174.
Yeung, C. H., and Cooper, T. G. (2002) Acquisition and development of sperm motility upon maturation in the epididymis. In ‘The Epididymis – From Molecules To Clinical Practice’. Vol. 1. (Eds B. Robaire and B. T. Hinton.) pp. 417–434. (Kluwer Academic/Plenum Press: New York.)
Zamaratskaia, G., Andersson, H. K., Chen, G., Andersson, K., Madej, A., and Lundstrom, K. (2008). Effect of a gonadotropin-releasing hormone vaccine (Improvac) on steroid hormones, boar taint compounds and performance in entire male pigs. Reprod. Domest. Anim. 43, 351–359.
| Effect of a gonadotropin-releasing hormone vaccine (Improvac) on steroid hormones, boar taint compounds and performance in entire male pigs.Crossref | GoogleScholarGoogle Scholar | 18086253PubMed | 18086253PubMed |
Zigo, M., Jonakova, V., Manaskova-Postlerova, P., Kerns, K., and Sutovsky, P. (2019). Ubiquitin–proteasome system participates in the de-aggregation of spermadhesins and DQH protein during boar sperm capacitation. Reproduction 157, 283.
| Ubiquitin–proteasome system participates in the de-aggregation of spermadhesins and DQH protein during boar sperm capacitation.Crossref | GoogleScholarGoogle Scholar | 30620719PubMed | 30620719PubMed |
Zirkin, B. R., Santulli, R., Awoniyi, C. A., and Ewing, L. L. (1989). Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043–3049.
| Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis.Crossref | GoogleScholarGoogle Scholar | 2498065PubMed | 2498065PubMed |


