Localisation and function of glucose transporter GLUT1 in chicken (Gallus gallus domesticus) spermatozoa: relationship between ATP production pathways and flagellar motility
Rangga Setiawan A , Chathura Priyadarshana A , Atsushi Tajima B , Alexander J. Travis C and Atsushi Asano B D
B D
A Graduate School of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan.
B Faculty of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8572, Japan.
C Baker Institute for Animal Health, Cornell University, Hungerford Hill Road, Ithaca, NY 14853, USA.
D Corresponding author. Email: asano.atsushi.ft@u.tsukuba.ac.jp
Reproduction, Fertility and Development 32(7) 697-705 https://doi.org/10.1071/RD19240
Submitted: 4 July 2019 Accepted: 30 October 2019 Published: 28 January 2020
Abstract
Glucose plays an important role in sperm flagellar motility and fertility via glycolysis and oxidative phosphorylation, although the primary mechanisms for ATP generation vary between species. The glucose transporter 1 (GLUT1) is a high-affinity isoform and a major glucose transporter in mammalian spermatozoa. However, in avian spermatozoa, the glucose metabolic pathways are poorly characterised. This study demonstrates that GLUT1 plays a major role in glucose-mediated motility of chicken spermatozoa. Using specific antibodies and ligand, we found that GLUT1 was specifically localised to the midpiece. Sperm motility analysis showed that glucose supported sperm movement during incubation for 0–80 min. However, this was abolished by the addition of a GLUT1 inhibitor, concomitant with a substantial decrease in glucose uptake and ATP production, followed by elevated mitochondrial activity in response to glucose addition. More potent inhibition of ATP production and mitochondrial activity was observed in response to treatment with uncouplers of oxidative phosphorylation. Because mitochondrial inhibition only reduced a subset of sperm movements, we investigated the localisation of the glycolytic pathway and showed glyceraldehyde-3-phosphate dehydrogenase and hexokinase I at the midpiece and principal piece of the flagellum. The results of this study provide new insights into the mechanisms involved in ATP production pathways in avian spermatozoa.
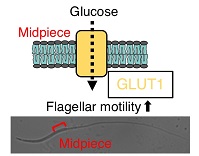
Additional keywords: sperm motility.
References
Angulo, C., Rauch, M. C., Droppelmann, A., Reyes, A. M., Slebe, J. C., Delgado-Lopez, F., Guaiquil, V. H., Vera, J. C., and Concha, I. I. (1998). Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J. Cell. Biochem. 71, 189–203.| Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C.Crossref | GoogleScholarGoogle Scholar | 9779818PubMed |
Asano, A., Selvaraj, V., Buttke, D. E., Nelson, J. L., Green, K. M., Evans, J. E., and Travis, A. J. (2009). Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts. J. Cell. Physiol. 218, 537–548.
| Biochemical characterization of membrane fractions in murine sperm: identification of three distinct sub-types of membrane rafts.Crossref | GoogleScholarGoogle Scholar | 19006178PubMed |
Bolton, A. E., and Linford, E. (1970). Presence of the dehydrogenase of the pentose phosphate pathway in boar spermatozoa. Reproduction 21, 353–354.
| Presence of the dehydrogenase of the pentose phosphate pathway in boar spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Bucci, D., Rodriguez-Gil, J. E., Vallorani, C., Spinaci, M., Galeati, G., and Tamanini, C. (2011). GLUTs and mammalian sperm metabolism. J. Androl. 32, 348–355.
| GLUTs and mammalian sperm metabolism.Crossref | GoogleScholarGoogle Scholar | 21088231PubMed |
Bunch, D. O., Welch, J. E., Magyar, P. L., Eddy, E. M., and O’Brien, D. A. (1998). Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis. Biol. Reprod. 58, 834–841.
| Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 9510974PubMed |
Burant, C. F., Takeda, J., Brot-Laroche, E., Bell, G. I., and Davidson, N. O. (1992). Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 267, 14523–14526.
| 1634504PubMed |
Burrows, W. H., and Quinn, J. P. (1937). The collection of spermatozoa from domestic fowl and turkey. Poult. Sci. 16, 19–24.
| The collection of spermatozoa from domestic fowl and turkey.Crossref | GoogleScholarGoogle Scholar |
Carlson, D., Black, D. L., and Howe, G. R. (1970). Oviduct secretion in the cow. J. Reprod. Fertil. 22, 549–552.
| Oviduct secretion in the cow.Crossref | GoogleScholarGoogle Scholar | 5465736PubMed |
Devaskar, S. U., and Mueckler, M. M. (1992). The mammalian glucose transporters. Pediatr. Res. 31, 1–13.
| The mammalian glucose transporters.Crossref | GoogleScholarGoogle Scholar | 1594323PubMed |
Dias, T. R., Alves, M. G., Silva, B. M., and Oliveira, P. F. (2014). Sperm glucose transport and metabolism in diabetic individuals. Mol. Cell. Endocrinol. 396, 37–45.
| Sperm glucose transport and metabolism in diabetic individuals.Crossref | GoogleScholarGoogle Scholar | 25128846PubMed |
Dupuy, V., and Blesbois, E. (1996). The effects of age on the composition of uterine fluid of broiler breeder hens and on maintenance of quality of fowl spermatozoa when stored in uterine fluid or in a synthetic medium. Theriogenology 45, 1221–1234.
| The effects of age on the composition of uterine fluid of broiler breeder hens and on maintenance of quality of fowl spermatozoa when stored in uterine fluid or in a synthetic medium.Crossref | GoogleScholarGoogle Scholar | 16727878PubMed |
Feiden, S., Wolfrum, U., Wegener, G., and Kamp, G. (2008). Expression and compartmentalisation of the glycolytic enzymes GAPDH and pyruvate kinase in boar spermatogenesis. Reprod. Fertil. Dev. 20, 713–723.
| Expression and compartmentalisation of the glycolytic enzymes GAPDH and pyruvate kinase in boar spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 18671919PubMed |
Ford, W. C. (2006). Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 12, 269–274.
| Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round?Crossref | GoogleScholarGoogle Scholar | 16407453PubMed |
Fraser, L. R., and Quinn, P. J. (1981). A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J. Reprod. Fertil. 61, 25–35.
| A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse.Crossref | GoogleScholarGoogle Scholar | 7452624PubMed |
Froman, D. P., Feltmann, A. J., Rhoads, M. L., and Kirby, J. D. (1999). Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus). Biol. Reprod. 61, 400–405.
| Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus).Crossref | GoogleScholarGoogle Scholar | 10411518PubMed |
Gardner, D. K., and Leese, H. J. (1990). Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J. Reprod. Fertil. 88, 361–368.
| Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro.Crossref | GoogleScholarGoogle Scholar | 2313649PubMed |
Goodson, S. G., Qiu, Y., Sutton, K. A., Xie, G., Jia, W., and O’Brien, D. A. (2012). Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol. Reprod. 87, 75.
| Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation.Crossref | GoogleScholarGoogle Scholar | 22837480PubMed |
Granchi, C., Fancelli, D., and Minutolo, F. (2014). An update on therapeutic opportunities offered by cancer glycolytic metabolism. Bioorg. Med. Chem. Lett. 24, 4915–4925.
| An update on therapeutic opportunities offered by cancer glycolytic metabolism.Crossref | GoogleScholarGoogle Scholar | 25288186PubMed |
Haber, R. S., Weinstein, S. P., O’Boyle, E., and Morgello, S. (1993). Tissue distribution of the human GLUT3 glucose transporter. Endocrinology 132, 2538–2543.
| Tissue distribution of the human GLUT3 glucose transporter.Crossref | GoogleScholarGoogle Scholar | 8504756PubMed |
Heilig, C. W., Saunders, T., Brosius, F. C., Moley, K., Heilig, K., Baggs, R., Guo, L., and Conner, D. (2003). Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc. Natl Acad. Sci. USA 100, 15613–15618.
| Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy.Crossref | GoogleScholarGoogle Scholar | 14673082PubMed |
Hyne, R. V., and Edwards, K. P. (1985). Influence of 2-deoxy-d-glucose and energy substrates on guinea-pig sperm capacitation and acrosome reaction. J. Reprod. Fertil. 73, 59–69.
| Influence of 2-deoxy-d-glucose and energy substrates on guinea-pig sperm capacitation and acrosome reaction.Crossref | GoogleScholarGoogle Scholar | 3968663PubMed |
Kim, S. T., and Moley, K. H. (2007). The expression of GLUT8, GLUT9a, and GLUT9b in the mouse testis and sperm. Reprod. Sci. 14, 445–455.
| The expression of GLUT8, GLUT9a, and GLUT9b in the mouse testis and sperm.Crossref | GoogleScholarGoogle Scholar | 17913964PubMed |
Kinet, S., Swainson, L., Lavanya, M., Mongellaz, C., Montel-Hagen, A., Craveiro, M., Manel, N., Battini, J. L., Sitbon, M., and Taylor, N. (2007). Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells. Retrovirology 4, 31.
| Isolated receptor binding domains of HTLV-1 and HTLV-2 envelopes bind Glut-1 on activated CD4+ and CD8+ T cells.Crossref | GoogleScholarGoogle Scholar | 17504522PubMed |
Manel, N., Kim, F. J., Kinet, S., Taylor, N., Sitbon, M., and Battini, J. L. (2003). The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115, 449–459.
| The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV.Crossref | GoogleScholarGoogle Scholar | 14622599PubMed |
McLean, D. J., Jones, L. G., and Froman, D. P. (1997). Reduced glucose transport in sperm from roosters (Gallus domesticus) with heritable subfertility. Biol. Reprod. 57, 791–795.
| Reduced glucose transport in sperm from roosters (Gallus domesticus) with heritable subfertility.Crossref | GoogleScholarGoogle Scholar | 9314582PubMed |
Miki, K., Qu, W., Goulding, E. H., Willis, W. D., Bunch, D. O., Strader, L. F., Perreault, S. D., Eddy, E. M., and O’Brien, D. A. (2004). Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl Acad. Sci. USA 101, 16501–16506.
| Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility.Crossref | GoogleScholarGoogle Scholar | 15546993PubMed |
Mújica, A., Moreno-Rodríguez, R., Naciff, J., Neri, L., and Tash, J. S. (1991). Glucose regulation of guinea-pig sperm motility. Reproduction 92, 75–87.
| Glucose regulation of guinea-pig sperm motility.Crossref | GoogleScholarGoogle Scholar |
Mukai, C., and Okuno, M. (2004). Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 71, 540–547.
| Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement.Crossref | GoogleScholarGoogle Scholar | 15084484PubMed |
Nakamura, N., Shibata, H., O’Brien, D. A., Mori, C., and Eddy, E. M. (2008). Spermatogenic cell-specific type 1 hexokinase is the predominant hexokinase in sperm. Mol. Reprod. Dev. 75, 632–640.
| Spermatogenic cell-specific type 1 hexokinase is the predominant hexokinase in sperm.Crossref | GoogleScholarGoogle Scholar | 17924400PubMed |
Nguyen, T. M., Alves, S., Grasseau, I., Metayer-Coustard, S., Praud, C., Froment, P., and Blesbois, E. (2014). Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 91, 121.
| Central role of 5′-AMP-activated protein kinase in chicken sperm functions.Crossref | GoogleScholarGoogle Scholar | 25297543PubMed |
Nichol, R., Hunter, R. H., Gardner, D. K., Leese, H. J., and Cooke, G. M. (1992). Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. J. Reprod. Fertil. 96, 699–707.
| Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period.Crossref | GoogleScholarGoogle Scholar | 1339849PubMed |
Parrish, J. J., Susko-Parrish, J. L., and First, N. L. (1989). Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH. Biol. Reprod. 41, 683–699.
| Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH.Crossref | GoogleScholarGoogle Scholar | 2620077PubMed |
Rogers, B. J., and Yanagimachi, R. (1975). Retardation of guinea pig sperm acrosome reaction by glucose: the possible importance of pyruvate and lactate metabolism in capacitation and the acrosome reaction. Biol. Reprod. 13, 568–575.
| Retardation of guinea pig sperm acrosome reaction by glucose: the possible importance of pyruvate and lactate metabolism in capacitation and the acrosome reaction.Crossref | GoogleScholarGoogle Scholar | 1203412PubMed |
Schurmann, A., Koling, S., Jacobs, S., Saftig, P., Krauss, S., Wennemuth, G., Kluge, R., and Joost, H. G. (2002). Reduced sperm count and normal fertility in male mice with targeted disruption of the ADP-ribosylation factor-like 4 (Arl4) gene. Mol. Cell. Biol. 22, 2761–2768.
| Reduced sperm count and normal fertility in male mice with targeted disruption of the ADP-ribosylation factor-like 4 (Arl4) gene.Crossref | GoogleScholarGoogle Scholar | 11909968PubMed |
Simpson, I. A., Dwyer, D., Malide, D., Moley, K. H., Travis, A., and Vannucci, S. J. (2008). The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 295, E242–E253.
| The facilitative glucose transporter GLUT3: 20 years of distinction.Crossref | GoogleScholarGoogle Scholar | 18577699PubMed |
Storey, B. T. (2008). Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int. J. Dev. Biol. 52, 427–437.
| Mammalian sperm metabolism: oxygen and sugar, friend and foe.Crossref | GoogleScholarGoogle Scholar | 18649255PubMed |
Travis, A. J., Foster, J. A., Rosenbaum, N. A., Visconti, P. E., Gerton, G. L., Kopf, G. S., and Moss, S. B. (1998). Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol. Biol. Cell 9, 263–276.
| Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 9450953PubMed |
Travis, A. J., Jorgez, C. J., Merdiushev, T., Jones, B. H., Dess, D. M., Diaz-Cueto, L., Storey, B. T., Kopf, G. S., and Moss, S. B. (2001). Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J. Biol. Chem. 276, 7630–7636.
| Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 11115497PubMed |
Urner, F., and Sakkas, D. (2005). Involvement of the pentose phosphate pathway and redox regulation in fertilization in the mouse. Mol. Reprod. Dev. 70, 494–503.
| Involvement of the pentose phosphate pathway and redox regulation in fertilization in the mouse.Crossref | GoogleScholarGoogle Scholar | 15685628PubMed |
Ushiyama, A., Priyadarshana, C., Setiawan, R., Miyazaki, H., Ishikawa, N., Tajima, A., and Asano, A. (2019). Membrane raft-mediated regulation of glucose signaling pathway leading to acrosome reaction in chicken sperm. Biol. Reprod. 100, 1482–1491.
| Membrane raft-mediated regulation of glucose signaling pathway leading to acrosome reaction in chicken sperm.Crossref | GoogleScholarGoogle Scholar | 30721935PubMed |
van Eck, J. H., and Vertommen, M. (1984). Biochemical changes in blood and uterine fluid of fowl following experimental EDS’76 virus infection. Vet. Q. 6, 127–134.
| Biochemical changes in blood and uterine fluid of fowl following experimental EDS’76 virus infection.Crossref | GoogleScholarGoogle Scholar | 6091320PubMed |
Welch, J. E., Schatte, E. C., O’Brien, D. A., and Eddy, E. M. (1992). Expression of a glyceraldehyde 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells. Biol. Reprod. 46, 869–878.
| Expression of a glyceraldehyde 3-phosphate dehydrogenase gene specific to mouse spermatogenic cells.Crossref | GoogleScholarGoogle Scholar | 1375514PubMed |
Westhoff, D., and Kamp, G. (1997). Glyceraldehyde 3-phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa. J. Cell Sci. 110, 1821–1829.
| 9264469PubMed |
Williams, A. C., and Ford, W. C. (2001). The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 22, 680–695.
| 11451366PubMed |
Wishart, G. J. (1982). Maintenance of ATP concentrations in and of fertilizing ability of fowl and turkey spermatozoa in vitro. J. Reprod. Fertil. 66, 457–462.
| Maintenance of ATP concentrations in and of fertilizing ability of fowl and turkey spermatozoa in vitro.Crossref | GoogleScholarGoogle Scholar | 7175802PubMed |
Wishart, G. J., and Palmer, F. H. (1986). Correlation of the fertilising ability of semen from individual male fowls with sperm motility and ATP content. Br. Poult. Sci. 27, 97–102.
| Correlation of the fertilising ability of semen from individual male fowls with sperm motility and ATP content.Crossref | GoogleScholarGoogle Scholar | 3708409PubMed |
Wood, T. E., Dalili, S., Simpson, C. D., Hurren, R., Mao, X., Saiz, F. S., Gronda, M., Eberhard, Y., Minden, M. D., Bilan, P. J., Klip, A., Batey, R. A., and Schimmer, A. D. (2008). A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol. Cancer Ther. 7, 3546–3555.
| A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death.Crossref | GoogleScholarGoogle Scholar | 19001437PubMed |


