Prevalence of pathogens important to human and companion animal health in an urban unowned cat population
Tamar Michaelian A , Lana Harriott B * , Matthew Gentle B , Tatiana Proboste A , Ian Kei Ho A and Rowland Cobbold A
B * , Matthew Gentle B , Tatiana Proboste A , Ian Kei Ho A and Rowland Cobbold A
A
B
Abstract
The deleterious impacts of cat predation on wildlife have been well documented. Additionally, unowned cats may act as reservoirs of disease important to public and companion animal health and their proclivity for roaming and fighting enables effective disease transmission. Urban environments support the highest human populations and companion animal densities, increasing the potential for disease transmission from unowned cats to people and pets. However, there is little data on the prevalence of pathogens in unowned cat populations.
This aim of this research was to establish baseline prevalence data for priority pathogens in an urban population of unowned cats.
One hundred unowned cat cadavers were collected from the Brisbane City Council region, Queensland, Australia. Blood and additional organ or tissue samples were collected post-mortem. Diagnostic methods for pathogen detection included use of real-time polymerase-chain reaction, commercially available rapid enzyme-linked-immunosorbent assay, lavage and faecal flotation.
Pathogen carriage was found in 79% (95% CI 71, 87%) of sampled cats. In total, 62% (95% CI 52, 72%) of cats showed evidence of co-carriage of two or more pathogenic organisms. The overall prevalence found for pathogens and parasites investigated were: Toxoplasma gondii, 7% (95% CI 2, 12%); Coxiella burnetii, 0.0% (95% CI 0, 0%); feline immunodeficiency virus, 12% (95% CI 6, 18%); feline leukaemia virus, 0.0% (95% CI 0, 0%); and gastrointestinal parasites, 76.8% (95% CI 68, 85%).
This study reports contemporary prevalence data for these pathogens that have not previously been available for unowned cats of south-east Queensland. High rates of gastrointestinal parasitism observed throughout the study population prompt concerns of a general increase in pathogenic prevalence, especially in comparison with that of owned domestic cats, as per previously published literature. The presence of signs of fighting is an important risk factor for increased likelihood of infection.
Data produced from this study contribute to informing cat management efforts throughout urban regions. Continued and expanded investigations, considering prevalence and risk factors of pathogens important to human and companion animal health, are recommended for the south-east Queensland area and beyond.
Keywords: disease, Feline Influenza Virus, feral cats, parasites, pathogens, Toxoplasma gondii, unowned cats, wildlife management, zoonoses.
Introduction
Urbanisation and human habitation have aided in supporting the dissemination of cats (Felis catus), leading to widespread owned, unowned and feral populations (Koch et al. 2015; Hanmer et al. 2017). Owned cats are domesticated animals that rely on, and belong to, a designated owner. Unowned cats are free ranging, without defined ownership but may have some or intermittent dependence on humans, and feral cats are free ranging with no dependence on humans, often in unmodified habitats (Australian Veterinary Association 2022). Today, feral cats are broadly distributed across Australia, with an estimated population between 2.1 and 6.3 million and occupation of more than 99.8% of Australia’s landmass (Legge et al. 2017). The highest densities of cats occur in urban environments, enabled by an abundance of resources and suitable living conditions (Gilot-Fromont et al. 2012). Likewise, urban environments support the highest densities of both human and companion animal populations, suggesting these regions hold the potential for considerable contact and subsequent spread of various diseases from unowned cats to people and pets.
The impacts of predation by cats on wildlife are well documented (Hardman et al. 2016; Doherty et al. 2017; Murphy et al. 2019; Woolley et al. 2019). Cats can also carry a variety of harmful infectious pathogens (Loss and Marra 2017; Legge et al. 2020a) and are known to harbour up to 50 zoonoses globally (Woinarski et al. 2019). Thirty-six pathogens have been identified in Australian feral cat populations (Doherty et al. 2017). Some notable pathogens include: (1) Toxoplasma gondii, a zoonotic protozoan parasite that causes toxoplasmosis. In humans, it can be asymptomatic or symptomatic, and is estimated to infect around one-third of the global population, with a remarkably diverse intermediate host range (Hill et al. 2005; Dickson 2018); (2) Coxiella burnetii, a highly infectious zoonotic bacterial pathogen, causing the notifiable disease Q fever (Kazar 2005; Malo et al. 2018; Ma et al. 2020); (3) Feline immunodeficiency virus (FIV), a lentivirus that induces a syndrome in cats similar to human acquired immunodeficiency syndrome (AIDS) (Norris et al. 2007; Elder et al. 2010); (4) Feline leukaemia virus (FeLV), a highly pathogenic γ-retrovirus, most frequently inducing anaemia or immunosuppression in infected cats (Hartmann 2012; Westman et al. 2019); and (5) a range of gastrointestinal parasites carried by cats, with the co-carriage of two or more species common, and of which several are zoonotic (Hill et al. 2000; Palmer et al. 2008; Paris et al. 2014; Yang and Liang 2015; Dybing et al. 2018).
Contemporary knowledge of disease prevalence throughout urban areas is essential to inform risk potential and the development of appropriate management strategies. There are limited data available regarding the prevalence and risks of pathogens carried by urban unowned cats. Efforts to educate community members on the seriousness of zoonotic pathogens can be hampered by lack of information, difficulty in disseminating relevant data to the public and how the public perceive the information provided (Nguyen et al. 2021; D. Franks, pers. comm.). Several factors also contribute to barriers for enacting efficient unowned cat management within urban regions. Resident contravention of animal management legislation, including interference with management actions and supplementary feeding of unowned cats, hinder management efforts (Deak et al. 2019; D. Franks, pers. comm.), leading to increased densities of unowned cats and, subsequently, the potential for heightened spread of disease. Therefore, the aim of this study was to determine the prevalence and risk factors of key pathogens that have importance to human or companion animal health in an urban unowned cat population in Brisbane, Queensland, Australia. The data reports contemporary baseline prevalence information for these pathogens in unowned cats. This knowledge is critical to inform future epidemiological studies, for public education campaigns, and most importantly to assist in building strategies for unowned and feral cat management.
Materials and methods
Study population
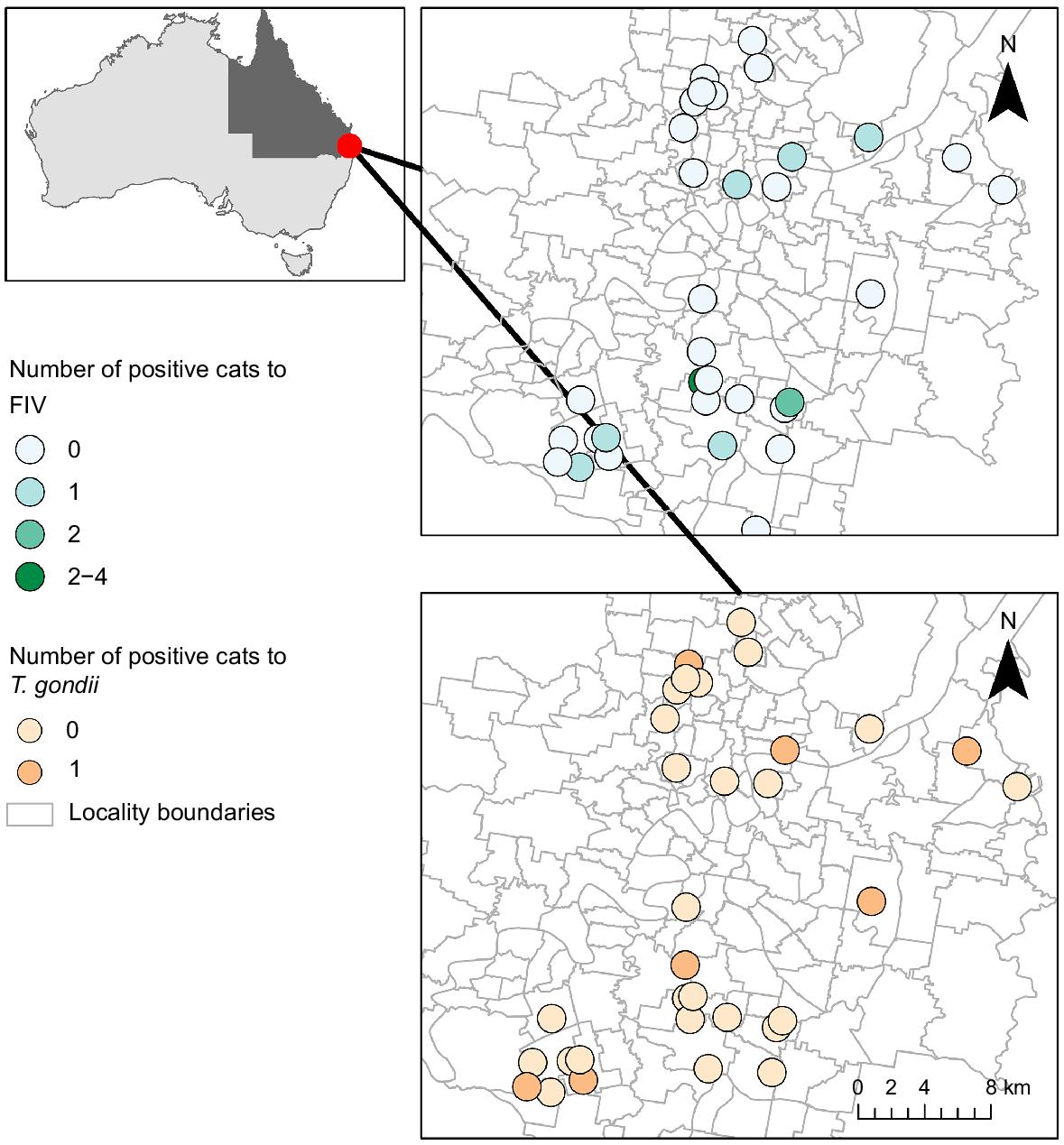
One hundred cat carcasses were contributed from an unowned cat management program conducted across the study area, situated in the Brisbane City Council local government area of south-east Queensland, Australia (Fig. 1). Cats were captured between November 2020 and February 2021 using cage traps baited with commercially available cat food. Trapping locations were chosen in response to public complaints, known resident feeder locations and recognised problem areas, and occurred widely across the local government area. No demographic selective criteria (i.e. preference for age, sex, body condition score or other) was stipulated in collection protocols. Cats categorised as unowned (no microchip, not desexed, no known owner or indication of ownership) and not suited to rehabilitation/rehoming were euthanised. All capture and euthanasia processes were performed by suitably qualified and experienced Brisbane City Council operators and conducted in compliance with University of Queensland animal ethics approval ANRFA/SVS/219/20.
Sample collection
Initial sampling was performed by Brisbane City Council operators. Whole blood and sera were collected via cardiac puncture and transferred to ethylenediaminetetraacetic acid (EDTA) tubes and serum separator tubes (SST). Samples and cadavers were then stored at −20°C until transport to the University of Queensland Anatomy Laboratory freezers. Cadavers were thawed at 4°C for 4–5 days prior to necropsy. Demographic data recorded included: cat type; sex; estimated age (juvenile, adult, elderly); signs of fighting (present, absent); body condition score (emaciated, underweight, ideal, overweight, obese); weight; and desexing or pregnancy status. Ectoparasites were collected and stored. The following samples were collected during necropsy: diaphragm; thigh (typically sartorius or gracilis); heart; tongue; kidney; spleen; liver; lung; brain; and mesenteric lymph nodes (where enlarged). A minimum of 2 cm × 2 cm of each sample was collected where possible. Brain (1–2 mL) was extracted from each carcass. Lymph nodes were stored refrigerated at 4°C, and all other remaining samples were stored frozen at −20°C. If present, faeces were removed from the colon and stored at 4°C. Stomach contents were assessed during necropsy and collected if present.
T. gondii
Frozen muscles were thawed at 4°C. Portions of contributing samples were pooled to a total of 6 g of tissue: 1 g each of diaphragm, thigh, heart and brain; and 2 g of tongue. Artificial tissue digestion and DNA extraction was performed as per Adriaanse et al. (2020), with minor modifications as follows. Tissues were cut into small pieces prior to addition of Tris-EDTA lysis buffer (TE) (molecular grade water, 40 mM Tris, 10 mM ETDA, The University of Queensland, Gatton, Qld, Australia). TE was added to pooled tissues to make up to a volume of 10 mL. Samples were then oven incubated at 90°C for 10 min. After being cooled to room temperature, 200 μL of Proteinase K (20 mg/mL) (Qiagen, Chadstone Centre, Vic., Australia; Bioline, Eveleigh, NSW, Australia) was added to each sample and oven incubated at 55°C overnight (~18 h). Following incubation, genomic DNA extraction was performed using Qiagen DNeasy Blood and Tissue kits (Qiagen, Chadstone Centre, Vic., Australia), following the manufacturer protocol. Total nucleic acid concentrations were assessed using a NanodropTM 2000 spectrophotometer (Thermo Fisher Scientific, Seventeen Mile Rocks, Qld, Australia).
DNA oligonucleotide primers and TaqMan probe (Adriaanse et al. 2020) were made and supplied by Sigma-Aldrich (Saint Louis, MO, United States). Amplification mixture consisted of 10 μL TaqMan™ Universal Master Mix II, no UNG (Thermo Fisher Scientific, Seventeen Mile Rocks, Qld, Australia), 0.3 μL of each T. gondii primer and probe (from 100 μM stock), 8.1 μL molecular grade water and 1 μL of DNA sample for a final reaction volume of 20 μL. Positive control was provided by Dr Steven Kopp (University of Queensland). A sample was considered positive when amplification occurred across all three wells with the Cq averaging ≤37. Due to time constraints and initial delay in product delivery, qPCR validation of tissue gDNA used feline glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers (Cattori et al. 2006) and PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Seventeen Mile Rocks, Qld, Australia). Amplification mixture consisted of 10 μL SYBR Green, 0.5 μL of each GAPDH primer, 7 μL molecular grade water and 2 μL of gDNA sample. All processing and analyses were performed in a PC2 Laboratory.
C. burnetii
Non-nucleated blood pre-treatment was undertaken as follows. EDTA tubes were vortexed to ensure homogenisation of whole blood contents; 200 μL blood was aliquoted with addition of 20 μL of Proteinase K (20 mg/mL) and 200 μL Buffer AL (Qiagen, Chadstone Centre, Victoria, Australia). Samples were incubated at 56°C for 10 min. DNA extraction was performed following manufacturer protocol (Qiagen DNeasy Blood and Tissue kit).
DNA oligonucleotide primer and probe sequences for targeted genes IS1111a and com1 were used as per Banazis et al. (2010) and Lockhart et al. (2011), respectively. qPCR cycling for target genes was run separately. Amplification mixtures consisted of 10 μL TaqMan™ Universal Master Mix II, no UNG (Thermo Fisher Scientific, Seventeen Mile Rocks, Qld, Australia), 0.3 μL of each primer and probe (from 200 μM stock), 8.1 μL molecular grade water (Thermo Fisher Scientific, Seventeen Mile Rocks, Qld, Australia) and 1 μL of DNA sample for a final reaction volume of 20 μL.
C. burnetii Phase I antigen (Nine Mile strain) (Institute Virion\Serion GmbH, Würzburg, Germany) was used to perform five ten-fold serial dilutions to mitigate the potential of inhibition due to high cell density and enable determination of a non-arbitrary Cq cut-off for qPCR testing. Duplicate antigen dilutions were subjected to qPCR using the same cycling protocol as described for T. gondii testing. Serial dilutions were run for both IS1111a and com1 target genes using TaqMan probe and primer sets as per Banazis et al. (2010) and Lockhart et al. (2011). C. burnetii DNA oligonucleotide primers and probes were made and supplied by GeneWorks (Thebarton, SA, Australia). Cq cut-off for C. burnetii testing was determined as 38: upper limit Cq value of dilutions (IS1111a) showed standard deviation of <0.3 between replicates, plus one standard deviation of Cq difference between dilution factors. The dilution factor selected for use as positive control was 1:10 000 C. burnetii Phase I antigen.
qPCR validation of whole blood gDNA used feline GAPDH primer and TaqMan probe sets as per Cattori et al. (2006). Validation amplification mixture consisted of 10 μL TaqMan™ Universal Master Mix II, no UNG, 0.5 μL of each GAPDH primer and probe (100 μM), 6.5 μL molecular grade water and 2 μL of gDNA sample for a total volume of 20 μL.
All qPCR pathogen detection reactions were carried out on gDNA extracts as per Adriaanse et al. (2020), with minor modifications as follows. qPCR run protocol was set to subject reaction mixture to an initial incubation of 95°C for 10 min followed by 45 cycles of denaturation: 95°C for 15 s; and annealing/extension: 60°C for 60 s. Genomic DNA and reaction inhibition status were validated using feline GAPDH as an internal control (Cattori et al. 2006). qPCR validation run protocol was set to subject reaction mixtures to an initial incubation of 95°C for 2 min followed by 45 cycles of denaturation: 95°C for 15 s; and annealing/extension: 60°C for 60 s. Cq values were considered up to 40 cycles, with a threshold set to 200. All reactions were performed separately, in triplicate. Positive (1 μL) and negative (1 μL of UltraPure™ Distilled Water) control wells were included in each run. Positive reactions were normalised using the Delta Delta Cq method (Livak and Schmittgen 2001). All processing and analyses were performed in a PC2 Laboratory. Bio-Rad CFX96 Real-time PCR system was used to complete qPCR reactions.
Feline immunodeficiency virus and feline leukaemia virus
SNAP Combo Plus FeLV Ag/FIV Ab enzyme-linked immunosorbent (ELISA) assays (IDEXX Laboratories, East Brisbane, Australia) utilising sera samples were used for detection of FIV and FeLV infection. Testing was performed as per manufacturer instructions.
Gastrointestinal parasites
The small intestine was removed at necropsy and lavage was performed by opening the tract and washing its contents. Any parasites found through visual observation were collected and stored in 70% ethanol for identification. Faecal flotations and egg counts were performed using the modified McMaster technique (Zajac and Conboy 2012). Identification of gastrointestinal parasites was based on Taylor et al. (2007). Identification of hookworm species from strongyle eggs and species-level identification of oocysts were not possible, due to lack of morphological distinction (Zajac and Conboy 2012).
Analyses
To explore the association between T. gondii and potential risk factors (sign of fighting, age, body condition, weight and presence of fleas), we conducted a General Additive Model (GAM) using mgcv package (Wood 2011). The same model was used to determine association between FIV and potential risk factors. For both models, the presence or absence of the pathogen was considered our response variable (binary). We excluded variables with insufficient data. For example, Body Condition Score did not have sufficient data for each level, so we used Weight (kg) instead. Because we had no female cats testing positive for T. gondii and only one pregnant cat tested positive for FIV, we excluded sex (from the T. gondii model only) and pregnancy variables from our analysis due to their lack of variability in the dataset. All models were optimised using Restricted Maximum Likelihood (REML) An effect was considered significant when P-value was <0.05. All statistical analysis were performed using R.
Prevalence and 95% confidence intervals (CI) were calculated for all pathogens. Prevalence was calculated by dividing the number of positive samples by the total number of samples tested, for each pathogen.
Results
The sample population consisted of 100 cats (52 males and 48 females), comprising one domestic medium-haired, 18 domestic long-haired and 81 domestic short haired cats. The group included 34 juveniles (≤12 weeks of age by visual assessment), 60 adults and six elderly cats. Signs of fighting were noted on 19 individuals. Body condition score was normally distributed across underweight, ideal and overweight categories, with six cats noted as emaciated and none as obese. Body weight ranged from 0.37 kg to 6.17 kg, with an average of 2.73 kg. All cats were entire (not neutered). 76 cats were found to have fleas and seven females (n = 48) were pregnant, at varying stages of gestation.
Although 100 unowned cats were contributed for examination, not all samples could be collected for the various tests. A total of 100 sets of muscle samples were tested for T. gondii. While 97 cat whole blood samples were available for processing, 81 samples were tested targeting the com1 gene and 85 samples were tested targeting the IS1111a gene for the detection of C. burnetii. SNAP Combo Plus FeLV Ag/FIV Ab ELISA testing was performed on 100 cat sera samples. Gastrointestinal parasite determination was performed via lavage on 92 cats, and faecal flotation was undertaken on 91 cats.
Pathogen carriage was found in 79% (95% CI 71, 87%) of unowned cats, and the presence of two or more pathogens was detected in 62% (95% CI 52, 72%) of the sampled population. Overall, males and females demonstrated similar carriage rates: 75% (95% CI 63, 87%) and 81.3% (95% CI 70, 93%), respectively. Occurrence of multiple pathogens was greater in females than in males: 72.9% (95% CI 60, 86%) and 51.9% (95% CI 38, 66%), respectively. Over two-thirds (70.6% (95% CI 54, 87%)) of the juvenile cats were positive for a pathogen, with 61.8% (95% CI 45, 79%) having more than one pathogen. Most adult cats (81.8% (95% CI 72, 91%)) were detected with pathogen carriage of some kind, and 62.1% (95% CI 50, 74%) were positive for more than one pathogen.
The total prevalence of T. gondii was 7% (95% CI 2, 12%), whereas C. burnetii was 0.0%. FIV had a prevalence of 12% (95% CI 6, 18%), whereas FeLV returned 0.0%. Only male cats were found to be positive for T. gondii, and more male than female cats were also FIV positive (Table 1). The prevalence of gastrointestinal parasites was 76.8% (95% CI 68, 85%), with the highest carriage found for strongyle eggs at 57.1% (95% CI 47, 68%) (Table 2).
| Cat risk factors | Sample size | Toxoplasma gondii | 95% CI | FIV | 95% CI | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 48 | 0 (0%) | – | 2 (4%) | (0.51, 14.25) | |
| Male | 52 | 7 (13%) | (5.59, 25.79) | 10 (19%) | (9.63, 32.53) | |
| Sign of fighting | ||||||
| Absence | 81 | 3 (4%) | (0.77, 10.44) | 6 (7%) | (2.77, 15.43) | |
| Presence | 19 | 4 (21%) | (6.05, 45.57) | 6 (32%) | (12.58, 56.55) | |
| Age | ||||||
| Juvenile | 34 | 1 (3%) | (0.07, 15.33) | 4 (12%) | (3.3, 27.45) | |
| Adult | 60 | 4 (7%) | (1.85, 16.2) | 7 (12%) | (1.85, 16.2) | |
| Elderly | 6 | 2 (33%) | (4.33, 77.72) | 1 (17%) | (0.42, 64.12) | |
| Body conditions score | ||||||
| Emaciated | 6 | 1 (17%) | (0.42, 64.12) | 0 (0%) | – | |
| Underweight | 19 | 1 (5%) | (0.13, 26.03) | 0 (0%) | – | |
| Ideal | 56 | 5 (9%) | (2.96, 19.62) | 10 (18%) | (8.91, 30.40) | |
| Overweight | 19 | 0 (0%) | – | 2 (11%) | (1.30, 33.14) | |
| Pregnancy | ||||||
| Pregnant | 7 | 0 (0%) | – | 1 (14%) | (0.36, 57.87) | |
| Non-pregnant | 93 | 7 (8%) | (3.08, 14.90) | 11 (12%) | (6.05, 20.18) | |
| Presence of fleas | ||||||
| Presence | 76 | 5 (7%) | (46.34, 67.47) | 9 (12%) | (5.56, 21.29) | |
| Absence | 24 | 2 (8%) | (1.03, 27.00) | 3 (13%) | (2.66, 32.36) | |
CI, confidence interval.
| Pathogen | Sample size (n) | Prevalence % (95% CI) | |
|---|---|---|---|
| Hookworm spp | 92 | 10.9 (4, 17) | |
| Cystoisospora rivolta | 92 | 37.4 (27, 47) | |
| Dipylidium caninum | 92 | 17.4 (9, 25) | |
| Spirometra erinacei | 92 | 19.6 (11, 28) | |
| Cystoisospora felis | 91 | 22.0 (13, 31) | |
| Toxocara cati | 91 | 19.8 (11, 28) | |
| Toxoplasma oocyst | 91 | 26.4 (17, 36) | |
| Strongyle egg | 91 | 57.1 (47, 68) |
CI, confidence interval.
The General Additive Model showed that no risk factors were statistically significant; however, signs of fighting and FIV showed a strong association, with animals that had signs of fighting being 1.8 times more likely to be FIV positive (P-value = 0.094) (Table 3). For T. gondii, elderly animals seems to have a weak association with the pathogen (P-value = 0.19) (Table 4).
| Variables | Estimate | s.e. | P-value | |
|---|---|---|---|---|
| Sex (ref female) | ||||
| Male | 1.214 | 0.890 | 0.173 | |
| Sign of fighting (ref absence) | ||||
| Presence of fight | 1.878 | 1.122 | 0.094 | |
| Age (ref juvenile) | ||||
| Adult | −0.861 | 1.512 | 0.569 | |
| Elderly | 0.269 | 0.776 | 0.728 | |
| Weight (kg) | −0.006 | 0.507 | 0.990 | |
| Fleas (ref presence) | ||||
| Absence of flea | −0.136 | 0.783 | 0.862 | |
| Variables | Estimate | s.e. | P-value | |
|---|---|---|---|---|
| Sign of fighting (ref absence) | ||||
| Presence of fight | 0.608 | 1.248 | 0.626 | |
| Age (ref juvenile) | ||||
| Adult | −0.331 | 1.679 | 0.844 | |
| Elderly | 1.168 | 0.906 | 0.197 | |
| Weight (kg) | 0.760 | 0.600 | 0.205 | |
| Fleas (ref presence) | ||||
| Absence of flea | 0.065 | 0.994 | 0.948 | |
Lymphadenitis was observed in 98.9% (95% CI 97, 100%) of cats for which mesenteric lymph nodes were examined. No indication of significant bacterial infection was found from preliminary bacteriological testing performed on a small subset of enlarged lymph nodes. Visual examination of dietary composition from stomachs throughout necropsy revealed that the broad majority of cats examined demonstrated recent consumption of commercial cat food, and/or human-sourced food scraps, (e.g. chicken, bread) or were empty, and were thus not examined further.
Discussion
This study established contemporary prevalence data for pathogens of importance to public and companion animal health in unowned cats of south-east Queensland (SEQ). Unowned cats were infected with T. gondii (7%), FIV (12%) and gastrointestinal parasites (77%). Over two-thirds of cats sampled were co-infected with two or more pathogens, but there was no detection of C. burnetii or FeLV. These data suggest that both feline-specific and zoonotic pathogens are present in the unowned cat population of SEQ, which could potentially compromise the health of community members and their pets.
The prevalence of the four main pathogens (T. gondii, C. burnetii, FIV and FeLV) examined in this study vary on a local and global scale. The presence of T. gondii infection in cats is highly variable across regions of Australia but, in general, it is relatively common. It has been estimated that an average of 41% of feral cats sampled nationwide have been infected with the pathogen (Dickson 2018). Island populations of feral cats have been demonstrated to have very high seroprevalence (Fancourt and Jackson 2014); for example, up to 96% on Christmas Island (Adams et al. 2008). Similarly high rates are reported globally, with an estimated average seroprevalence of 59% in wild felids (Montazeri et al. 2020). The lower prevalence of T. gondii reported in SEQ from this study (7%) is positive and could possibly reflect a high turnover of feral cats (short life expectancy) or the benefits of a long-term management program. Alternatively, the time of year samples were collected for this project (mostly summer) may have influenced detection – low mean annual temperatures are most favourable for T. gondii (Dickson 2018), and cats trapped in winter can have a higher probability of infection (Adriaanse et al. 2020). However, sampling and diagnostic inconsistencies among studies makes direct comparisons difficult. There are little data available on the presence of FIV in feral cats in Australia, but previous studies have reported a prevalence of 21% and 25% in two separate populations (Norris et al. 2007). Like other pathogens, prevalence fluctuates depending on region, with reports varying between 2 and 44% (Sprißler et al. 2022). Previously published research on urban owned cat populations reported the prevalence of FIV to be similar to that detected in this study (Malik et al. 1997; Chang-Fung-Martel et al. 2013). The absence of detecting FeLV in this study is reflective of the lower infection rates usually reported in comparison with FIV (Friend et al. 1990; Malik et al. 1997; Westman et al. 2019). A survey including a larger cross-section of the SEQ unowned cat population may improve the ability to detect or estimate prevalence for FeLV. Investigations into cats as a carrier of C. burnetii is relatively novel. International studies are yet to detect the presence of this pathogen in feral cat populations (Cyr et al. 2021; Neves 2021), but research conducted in Australia has detected domestic cats to be seropositive for C. burnetii (Ma et al. 2020), and approximately 39% of feral cats tested were also found to be seropositive (Cooper et al. 2012). In addition to this, outbreaks of Q-fever in humans have been linked to infected parturient cats (Kopecny et al. 2013; Malo et al. 2018). Although prevalence results from this current study may seem low, it is important to consider that estimates only provide a snapshot of the burden of a particular pathogen, within a population, at a specific point in time (Hanage 2016).
Gastrointestinal helminth infection is common in cats, and several are of zoonotic concern (Hill et al. 2000; Traversa 2012). The nematode Toxoxcara cati, for example, widely considered as important to cat and human health, has a variable global incidence, with studies finding prevalence rates ranging from 8.2% to 58.4% (Rostami et al. 2020). Compared with results of a comprehensive national survey on the prevalence of gastrointestinal parasitism in cats (Palmer et al. 2008), parasitism in unowned cats from this current study is greater than for refuge cats. This was the case for cestodes (Dipylidium caninum and Spirometra erinacei), hookworm (Ancylostoma spp.) and roundworm (T. cati) species. Overall, helminth carriage in unowned cats from this study was high (87.7%), but previously reported data on individual helminthic carriage in unowned cats of Brisbane were comparable (Wilson-Hanson and Prescott 1982). It is possible that Queensland’s climate contributes to heightened helminth prevalence. Hookworm, for example, prefers moistened, warmer soils within which to support survival of larvae (Gibbs 1982). A greater prevalence seen in unowned cats compared with refuge (owned domestic) cats may be due to the increased likelihood of exposure to infected faeces and environments resultant from overlapping home ranges and general aggregative activities of unowned cats (Gordon et al. 2016). In addition, the lack of anthelmintic treatment in unowned cats encourages increased prevalence of gastrointestinal parasites (Traversa 2012), including the absence of preventative treatment for ectoparasites, which heightens the risk of tapeworm infection (from fleas) and promotes continued parasite cycling (Beugnet et al. 2018). Unowned cats, exposed to an assortment of compromising factors are, without the measures afforded to typical domestic (owned) cats, at considerable risk of gastrointestinal parasitism.
The highest densities of unowned cats have been suggested as occurring within urban environments (Gilot-Fromont et al. 2012). The provision of supplementary food resources by residents encourages aggregation of unowned cats and supports increased population densities (McDonald and Skillings 2021). This, in turn, heightens potential burdens of environmental contamination, increases home-range sharing of unowned cats and can impact the overall risks of pathogen transmission to humans and animals (Hwang et al. 2018). Further, although the degree of direct risk posed to the health of resident feeders through handling and provision of raw meat to unowned cats has yet to be investigated, studies on owned domestic cats suggest this is a cause for concern (Brennan et al. 2020). Multiple individual level risk factors can be connected to the roaming of unowned cats, with risks of pathogen transmission exacerbated by various environmental factors. It has been predicted, for example, that the highest prevalence of T. gondii in unowned cats will be present across south-eastern coastal regions of Australia, where mean annual temperatures are low (Dickson 2018), coinciding with some of the most densely human-populated and highly urbanised cities. Widespread contamination of urban environments – through dissemination of oocysts/eggs shed by roaming unowned cats – compromises human, companion animal and wildlife health (Benenson et al. 1982; Eymann et al. 2006; Dubey et al. 2020).
The General Additive Model shows that the presence of signs of fighting was associated with carriage of FIV. The principal route of FIV transmission is conspecific biting, which generally accompanies territorial behaviours such as fighting (Courchamp et al. 2000; Norris et al. 2007). The ability to roam (and consequential likelihood of fighting) has been previously associated with increased risk of FIV infection (Malik et al. 1997; Tran et al. 2019). However, sex was not identified as risk factor for the carriage of T. gondii. This is likely due to the low sample size and the lack of female cats carrying T. gondii, which would also explain the high standard error. Reports of association between T. gondii infection and gender have been variable (Jakob-Hoff and Dunsmore 1983; Sumner and Ackland 1999; Brennan et al. 2020). General biological and behavioural characteristics of male cats may see them more prone to infection than females. It has been suggested that T. gondii transmission may be more frequent in male cats due to their larger bodyweight necessitating increased food requirements and subsequently heightened predative activities (Gilot-Fromont et al. 2012). The prevalence of T. gondii in intermediate hosts, such as rodents and lagomorphs, is positively correlated with increasing body mass (Afonso et al. 2007), and larger cats (males) tend to predate on larger prey (Kutt 2012). The lifestyle of unowned cats, especially their proclivity to roam and utilise shared habitats and resources (Kim et al. 2018), may provide reasoning for the significance of these risks, but further research will be needed to quantify the significance of these factors relative to unowned cats in general, rather than within this study population alone.
Zoonoses are of significant concern to humans, though the impacts of zoonotic pathogens are not limited to people alone (Loss and Marra 2017). Pathogen spillover from domestic to wild-type animal counterparts, wildlife and livestock is also not uncommon (Kellner et al. 2018; Chalkowski et al. 2019). Likewise, exposure risks to owned domestic cats should not be overlooked (Loss and Marra 2017; Chalkowski et al. 2019). Disease places considerable burden on the economy. The economic impact of only three zoonotic diseases found in Australian cats (cat scratch disease, sarcocystosis and toxoplasmosis) was estimated to be over AU$6.7 billion annually (Legge et al. 2020b). Disease transmission is facilitated via the roaming and fighting of cats, compromising both community and animal health (Malik et al. 1997; Gerhold and Jessup 2013). Our study provides important data that support the potential risks to human and companion animal health from unowned cats.
There were some limitations to the methods in this study. Notably, investigations of C. burnetii prevalence would benefit from a longer timeframe for analysis to optimise qPCR diagnostic protocols. Additionally, FeLV testing may benefit from a more diversified diagnostic strategy – for example, utilisation of a combination of diagnostic techniques. Both need to be considered when interpreting the results but were unlikely to significantly influence the prevalence recorded. The prevalence of pathogens may be influenced by a variety of factors associated with geographic location (e.g. climate, season, local prey densities, population density, demographics). Detailed analysis of location data, to evaluate spatial significance or association with pathogen carriage prevalence, is recommended for future studies. Longitudinal collection of data to examine the potential of temporal and seasonal trends would provide enhanced understanding of infection prevalence and risk factors. The ability to directly compare populations of cats across Australian urban environments, i.e. unowned versus domestically owned cats, remains as a gap in knowledge that should be addressed in future studies. The examination of risk factor and behavioural/quantitative research on human interactions with urban unowned cats, specifically resident ‘feeders’, would benefit investigations into public health risks and impacts. Finally, ongoing monitoring of pathogen prevalence within unowned cat populations is important to retain an overall understanding of potential risks to public health over time, and to monitor the impacts of any ongoing mitigation measures.
The results from this study provide contemporary prevalence data as a baseline for evaluating the effects of future interventions on pathogen prevalence. Information from this study can be used to support and evaluate cat management programs. Data providing insight into the prevalence of diseases carried by unowned cats should be strategically utilised to enhance regional control and compliance efforts. The ubiquitous nature of cats and disease throughout the world emphasises the aims and objectives of this current study as globally relevant. Understanding disease dynamics, including prevalence and transmission risks, is arguably one of the most important social health considerations of contemporary society. The knowledge of the prevalence of priority pathogens and parasites remains an important basis to improve epidemiological understanding and a key to continued improvement of risk mitigation strategies, both locally and on a global scale.
Data availability
All data generated and analysed throughout the duration of this study can be found within the published manuscript. The raw datasets used as the basis of this research are available from the corresponding author on reasonable request.
Declaration of funding
This research was conducted with financial support from the Queensland Department of Agriculture and Fisheries and the University of Queensland.
Author contributions
Conceptualisation, TM, LH, MG and RC; Methodology, TM, LH, TB, MG and RC; Software, TM and LH; Validation, TM; Formal Analysis, TM, LH, TB, MG and RC; Investigation, TM, LH, MG and RC; Data Curation, TM; Writing – Original Draft Preparation, TM; Writing – Review and Editing, TM, LH, TB, MG and RC; Visualisation, TM, LH, MG and RC; Supervision, LH, MG and RC; Project Administration, TM, LH, MG and RC; Funding Acquisition, MG and RC.
Acknowledgements
The authors acknowledge Dr. Lee McMichael for her extensive support in laboratory-based methodological input, Dr. Steven Kopp and Dr. Caitlin Wood for their product contributions, Ian Kei Ho for her assistance throughout the necropsy process and examination of gastrointestinal parasites, Dr Nicholas Clark for his statistical advice and Graham Panzram and Michael Cobbin for their kindness and support throughout our use of the anatomy laboratory facilities. Finally, the authors also acknowledge the contribution from the pest animal management staff at Brisbane City Council.
References
Adams PJ, Elliot AD, Algar D, Brazell RI (2008) Gastrointestinal parasites of feral cats from Christmas Island. Australian Veterinary Journal 86, 60-63.
| Crossref | Google Scholar | PubMed |
Adriaanse K, Firestone SM, Lynch M, Rendall AR, Sutherland DR, Hufschmid J, Traub R (2020) Comparison of the modified agglutination test and real-time PCR for detection of Toxoplasma gondii exposure in feral cats from Phillip Island, Australia, and risk factors associated with infection. International Journal for Parasitology: Parasites and Wildlife 12, 126-133.
| Crossref | Google Scholar | PubMed |
Afonso E, Thulliez P, Pontier D, Gilot-Fromont E (2007) Toxoplasmosis in prey species and consequences for prevalence in feral cats: not all prey species are equal. Parasitology 134, 1963-1971.
| Crossref | Google Scholar | PubMed |
Australian Veterinary Association (2022) Management of cats in Australia In ‘Companion animals – management and welfare’. Australian Veterinary Association Ltd (AVA). Available at https://www.ava.com.au/policy-advocacy/policies/companion-animals-management-and-welfare/management-of-cats-in-australia/
Banazis MJ, Bestall AS, Reid SA, Fenwick SG (2010) A survey of western Australian sheep, cattle and kangaroos to determine the prevalence of Coxiella burnetii. Veterinary Microbiology 143, 337-345.
| Crossref | Google Scholar | PubMed |
Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ (1982) Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. New England Journal of Medicine 307, 666-669.
| Crossref | Google Scholar | PubMed |
Beugnet F, Labuschagne M, Vos Cd, Crafford D, Fourie J (2018) Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas—Part 2. Distinct canine and feline host association with two different Dipylidum caninum genotypes. Parasite 25, 31.
| Crossref | Google Scholar | PubMed |
Brennan A, Hawley J, Dhand N, Boland L, Beatty JA, Lappin MR, Barrs VR (2020) Seroprevalence and risk factors for Toxoplasma gondii infection in owned domestic cats in Australia. Vector-Borne and Zoonotic Diseases 20, 275-280.
| Crossref | Google Scholar | PubMed |
Cattori V, Tandon R, Pepin A, Lutz H, Hofmann-Lehmann R (2006) Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Molecular and Cellular Probes 20, 172-181.
| Crossref | Google Scholar | PubMed |
Chalkowski K, Wilson AE, Lepczyk CA, Zohdy S (2019) Who let the cats out? A global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus). Biology Letters 15, 20180840.
| Crossref | Google Scholar | PubMed |
Chang-Fung-Martel J, Gummow B, Burgess G, Fenton E, Squires R (2013) A door-to-door prevalence study of feline immunodeficiency virus in an Australian suburb. Journal of Feline Medicine and Surgery 15, 1070-1078.
| Crossref | Google Scholar | PubMed |
Cooper A, Goullet M, Mitchell J, Ketheesan N, Govan B (2012) Serological evidence of Coxiella burnetii exposure in native marsupials and introduced animals in Queensland, Australia. Epidemiology and Infection 140, 1304-1308.
| Crossref | Google Scholar | PubMed |
Courchamp F, Say L, Pontier D (2000) Transmission of feline immunodeficiency virus in a population of cats (Felis catus). Wildlife Research 27, 603-611.
| Crossref | Google Scholar |
Cyr J, Turcotte M-È, Desrosiers A, Bélanger D, Harel J, Tremblay D, Leboeuf A, Gagnon CA, Côté J-C, Arsenault J (2021) Prevalence of Coxiella burnetii seropositivity and shedding in farm, pet and feral cats and associated risk factors in farm cats in Quebec, Canada. Epidemiology and Infection 149, e57.
| Crossref | Google Scholar | PubMed |
Deak BP, Ostendorf B, Taggart DA, Peacock DE, Bardsley DK (2019) The significance of social perceptions in implementing successful feral cat management strategies: a global review. Animals 9, 617.
| Crossref | Google Scholar | PubMed |
Doherty TS, Dickman CR, Johnson CN, Legge SM, Ritchie EG, Woinarski JCZ (2017) Impacts and management of feral cats Felis catus in Australia. Mammal Review 47, 83-97.
| Crossref | Google Scholar |
Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Yang Y, Su C (2020) Toxoplasma gondii infections in dogs: 2009–2020. Veterinary Parasitology 287, 109223.
| Crossref | Google Scholar | PubMed |
Dybing NA, Jacobson C, Irwin P, Algar D, Adams PJ (2018) Challenging the dogma of the ‘Island Syndrome’: a study of helminth parasites of feral cats and black rats on Christmas Island. Australasian Journal of Environmental Management 25, 99-118.
| Crossref | Google Scholar |
Elder JH, Lin Y-C, Fink E, Grant CK (2010) Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Current HIV Research 8, 73-80.
| Crossref | Google Scholar | PubMed |
Eymann J, Herbert CA, Cooper DW, Dubey JP (2006) Serologic survey for Toxoplasma gondii and Neospora caninum in the common brushtail possum (Trichosurus vulpecula) from urban Sydney, Australia. Journal of Parasitology 92, 267-272.
| Crossref | Google Scholar | PubMed |
Fancourt BA, Jackson RB (2014) Regional seroprevalence of Toxoplasma gondii antibodies in feral and stray cats (Felis catus) from Tasmania. Australian Journal of Zoology 62, 272-283.
| Crossref | Google Scholar |
Friend SCE, Birch CJ, Lording PM, Marshall JA, Studdert MJ (1990) Feline immunodeficiency virus: prevalence, disease associations and isolation. Australian Veterinary Journal 67, 237-243.
| Crossref | Google Scholar | PubMed |
Gerhold RW, Jessup DA (2013) Zoonotic diseases associated with free-roaming cats. Zoonoses and Public Health 60, 189-195.
| Crossref | Google Scholar | PubMed |
Gibbs HC (1982) Mechanisms of survival of nematode parasites with emphasis on hypobiosis. Veterinary Parasitology 11, 25-48.
| Crossref | Google Scholar | PubMed |
Hanage WP (2016) Pathogen epidemiology. In ‘Encyclopedia of evolutionary biology’. (Ed. RM Kliman) pp. 225–231. (Academic Press) doi:10.1016/b978-0-12-800049-6.00228-6
Hanmer HJ, Thomas RL, Fellowes MDE (2017) Urbanisation influences range size of the domestic cat (Felis catus): consequences for conservation. Journal of Urban Ecology 3, jux014.
| Crossref | Google Scholar |
Hardman B, Moro D, Calver M (2016) Direct evidence implicates feral cat predation as the primary cause of failure of a mammal reintroduction programme. Ecological Management & Restoration 17, 152-158.
| Crossref | Google Scholar |
Hartmann K (2012) Clinical aspects of feline retroviruses: a review. Viruses 4, 2684-2710.
| Crossref | Google Scholar | PubMed |
Hill SL, Cheney JM, Taton-Allen GF, Reif JS, Bruns C, Lappin MR (2000) Prevalence of enteric zoonotic organisms in cats. Journal of the American Veterinary Medical Association 216, 687-692.
| Crossref | Google Scholar | PubMed |
Hill DE, Chirukandoth S, Dubey JP (2005) Biology and epidemiology of Toxoplasma gondii in man and animals. Animal Health Research Reviews 6, 41-61.
| Crossref | Google Scholar | PubMed |
Hwang J, Gottdenker NL, Oh D-H, Nam H-W, Lee H, Chun M-S (2018) Disentangling the link between supplemental feeding, population density, and the prevalence of pathogens in urban stray cats. PeerJ 6, e4988.
| Crossref | Google Scholar | PubMed |
Jakob-Hoff RM, Dunsmore JD (1983) Epidemiological aspects of toxoplasmosis in southern Western Australia. Australian Veterinary Journal 60, 217-218.
| Crossref | Google Scholar | PubMed |
Kazar J (2005) Coxiella burnetii infection. Annals of the New York Academy of Sciences 1063, 105-114.
| Crossref | Google Scholar | PubMed |
Kellner A, Carver S, Scorza V, McKee CD, Lappin M, Crooks KR, VandeWoude S, Antolin MF (2018) Transmission pathways and spillover of an erythrocytic bacterial pathogen from domestic cats to wild felids. Ecology and Evolution 8, 9779-9792.
| Crossref | Google Scholar | PubMed |
Kim J-S, Kim J-U, Jeon J-H, Lee JK, Lee W-S (2018) Radio-tracking survey of stray cat home range in a suburban area: non-exclusive use of home ranges in stray cats (Felis catus). Mammal Study 44, 69-75.
| Crossref | Google Scholar |
Koch K, Algar D, Searle JB, Pfenninger M, Schwenk K (2015) A voyage to Terra Australis: human-mediated dispersal of cats. BMC Evolutionary Biology 15, 262.
| Crossref | Google Scholar | PubMed |
Kopecny L, Bosward KL, Shapiro A, Norris JM (2013) Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak. Journal of Feline Medicine and Surgery 15, 1037-1045.
| Crossref | Google Scholar | PubMed |
Kutt AS (2012) Feral cat (Felis catus) prey size and selectivity in North-Eastern Australia: implications for mammal conservation. Journal of Zoology 287, 292-300.
| Crossref | Google Scholar |
Legge S, Murphy BP, McGregor H, Woinarski JCZ, Augusteyn J, Ballard G, Baseler M, Buckmaster T, Dickman CR, Doherty T, Edwards G, Eyre T, Fancourt BA, Ferguson D, Forsyth DM, Geary WL, Gentle M, Gillespie G, Greenwood L, Hohnen R, Hume S, Johnson CN, Maxwell M, McDonald PJ, Morris K, Moseby K, Newsome T, Nimmo D, Paltridge R, Ramsey D, Read J, Rendall A, Rich M, Ritchie E, Rowland J, Short J, Stokeld D, Sutherland DR, Wayne AF, Woodford L, Zewe F (2017) Enumerating a continental-scale threat: how many feral cats are in Australia? Biological Conservation 206, 293-303.
| Crossref | Google Scholar |
Legge S, Woinarski JCZ, Dickman CR, Murphy BP, Woolley L-A, Calver MC (2020a) We need to worry about Bella and Charlie: the impacts of pet cats on Australian wildlife. Wildlife Research 47, 523-539.
| Crossref | Google Scholar |
Legge S, Taggart PL, Dickman CR, Read JL, Woinarski JCZ (2020b) Cat-dependent diseases cost Australia AU$6 billion per year through impacts on human health and livestock production. Wildlife Research 47, 731-746.
| Crossref | Google Scholar |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402-408.
| Crossref | Google Scholar | PubMed |
Lockhart MG, Graves SR, Banazis MJ, Fenwick SG, Stenos J (2011) A comparison of methods for extracting DNA from Coxiella burnetii as measured by a duplex qPCR assay. Letters in Applied Microbiology 52, 514-520.
| Crossref | Google Scholar | PubMed |
Loss SR, Marra PP (2017) Population impacts of free-ranging domestic cats on mainland vertebrates. Frontiers in Ecology and the Environment 15, 502-509.
| Crossref | Google Scholar |
Ma GC, Norris JM, Mathews KO, Chandra S, Šlapeta J, Bosward KL, Ward MP (2020) New insights on the epidemiology of Coxiella burnetii in pet dogs and cats from New South Wales, Australia. Acta Tropica 205, 105416.
| Crossref | Google Scholar | PubMed |
Malik R, Kendall K, Cridland J, Coulston S, Stuart AJ, Snow D, Love DN (1997) Prevalences of feline leukaemia virus and feline immunodeficiency virus infections in cats in Sydney. Australian Veterinary Journal 75, 323-327.
| Crossref | Google Scholar | PubMed |
Malo JA, Colbran C, Young M, Vasant B, Jarvinen K, Viney K, Lambert SB (2018) An outbreak of Q fever associated with parturient cat exposure at an animal refuge and veterinary clinic in southeast Queensland. Australian and New Zealand Journal of Public Health 42, 451-455.
| Crossref | Google Scholar | PubMed |
McDonald JL, Skillings E (2021) Human influences shape the first spatially explicit national estimate of urban unowned cat abundance. Scientific Reports 11, 20216.
| Crossref | Google Scholar | PubMed |
Montazeri M, Mikaeili Galeh T, Moosazadeh M, Sarvi S, Dodangeh S, Javidnia J, Sharif M, Daryani A (2020) The global serological prevalence of Toxoplasma gondii in felids during the last five decades (1967–2017): a systematic review and meta-analysis. Parasites & Vectors 13, 82.
| Crossref | Google Scholar | PubMed |
Murphy BP, Woolley L-A, Geyle HM, Legge SM, Palmer R, Dickman CR, Augusteyn J, Brown SC, Comer S, Doherty TS, Eager C, Edwards G, Fordham DA, Harley D, McDonald PJ, McGregor H, Moseby KE, Myers C, Read J, Riley J, Stokeld D, Trewella GJ, Turpin JM, Woinarski JCZ (2019) Introduced cats (Felis catus) eating a continental fauna: the number of mammals killed in Australia. Biological Conservation 237, 28-40.
| Crossref | Google Scholar |
Nguyen T, Clark N, Jones MK, Herndon A, Mallyon J, Soares Magalhaes RJ, Abdullah S (2021) Perceptions of dog owners towards canine gastrointestinal parasitism and associated human health risk in southeast Queensland. One Health 12, 100226.
| Crossref | Google Scholar | PubMed |
Norris JM, Bell ET, Hales L, Toribio J-ALML, White JD, Wigney DI, Baral RM, Malik R (2007) Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. Journal of Feline Medicine and Surgery 9, 300-308.
| Crossref | Google Scholar | PubMed |
Palmer CS, Thompson RCA, Traub RJ, Rees R, Robertson ID (2008) National study of the gastrointestinal parasites of dogs and cats in Australia. Veterinary Parasitology 151, 181-190.
| Crossref | Google Scholar | PubMed |
Paris JK, Wills S, Balzer H-J, Shaw DJ, Gunn-Moore DA (2014) Enteropathogen co-infection in UK cats with diarrhoea. BMC Veterinary Research 10, 13.
| Crossref | Google Scholar | PubMed |
Sprißler F, Jongwattanapisan P, Luengyosluechakul S, Pusoonthornthum R, Reese S, Bergmann M, Hartmann K (2022) Prevalence and risk factors of feline immunodeficiency virus and feline leukemia virus infection in healthy cats in Thailand. Frontiers in Veterinary Science 8, 764217.
| Crossref | Google Scholar |
Sumner B, Ackland ML (1999) Toxoplasma gondii antibody in domestic cats in Melbourne. Australian Veterinary Journal 77, 447-449.
| Crossref | Google Scholar | PubMed |
Tran V, Kelman M, Ward M, Westman M (2019) Risk of feline immunodeficiency virus (FIV) infection in pet cats in Australia is higher in areas of lower socioeconomic status. Animals 9, 592.
| Crossref | Google Scholar |
Traversa D (2012) Pet roundworms and hookworms: a continuing need for global worming. Parasites & Vectors 5, 91.
| Crossref | Google Scholar | PubMed |
Westman M, Norris J, Malik R, Hofmann-Lehmann R, Harvey A, McLuckie A, Perkins M, Schofield D, Marcus A, McDonald M, Ward M, Hall E, Sheehy P, Hosie M (2019) The diagnosis of feline leukaemia virus (FeLV) infection in owned and group-housed rescue cats in Australia. Viruses 11, 503.
| Crossref | Google Scholar | PubMed |
Wilson-Hanson SL, Prescott CW (1982) A survey for parasites in cats. Australian Veterinary Journal 59, 194.
| Crossref | Google Scholar |
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society Series B: Statistical Methodology 73, 3-36.
| Crossref | Google Scholar |
Woolley L-A, Geyle HM, Murphy BP, Legge SM, Palmer R, Dickman CR, Augusteyn J, Comer S, Doherty TS, Eager C, Edwards G, Harley DKP, Leiper I, McDonald PJ, McGregor HW, Moseby KE, Myers C, Read JL, Riley J, Stokeld D, Turpin JM, Woinarski JCZ (2019) Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed. Mammal Review 49, 354-368.
| Crossref | Google Scholar |
Yang Y, Liang H (2015) Prevalence and risk factors of intestinal parasites in cats from China. BioMed Research International 2015, 967238.
| Crossref | Google Scholar | PubMed |