Tracking movement, home range, and microhabitat use in a small terrestrial breeding frog using harmonic direction-finding technology
Jordy Groffen A * , Conrad J. Hoskin A , Matthew S. Siderhurst B and Myles H. M. Menz
A * , Conrad J. Hoskin A , Matthew S. Siderhurst B and Myles H. M. Menz  A C
A C
A
B
C
Abstract
Tracking the movements of an animal increases our understanding of its behaviour and ecological preferences.
This study aimed to assess the movements, home range, nesting sites, and microhabitat use of a very small, cryptic, terrestrial microhylid frog species (Austrochaperina robusta) in an upland rainforest, during the breeding season.
We used harmonic direction-finding (HDF) technology with ultra-light harnesses/tag combinations of two sizes (small 0.023 g and large 0.033 g) to track male A. robusta. These are substantially lighter than all tag/harness combinations previously used in amphibian tracking studies and represented a small proportion (1.8–2.58%) of the body mass of the very small study species, A. robusta (1.27 ± 0.20 g).
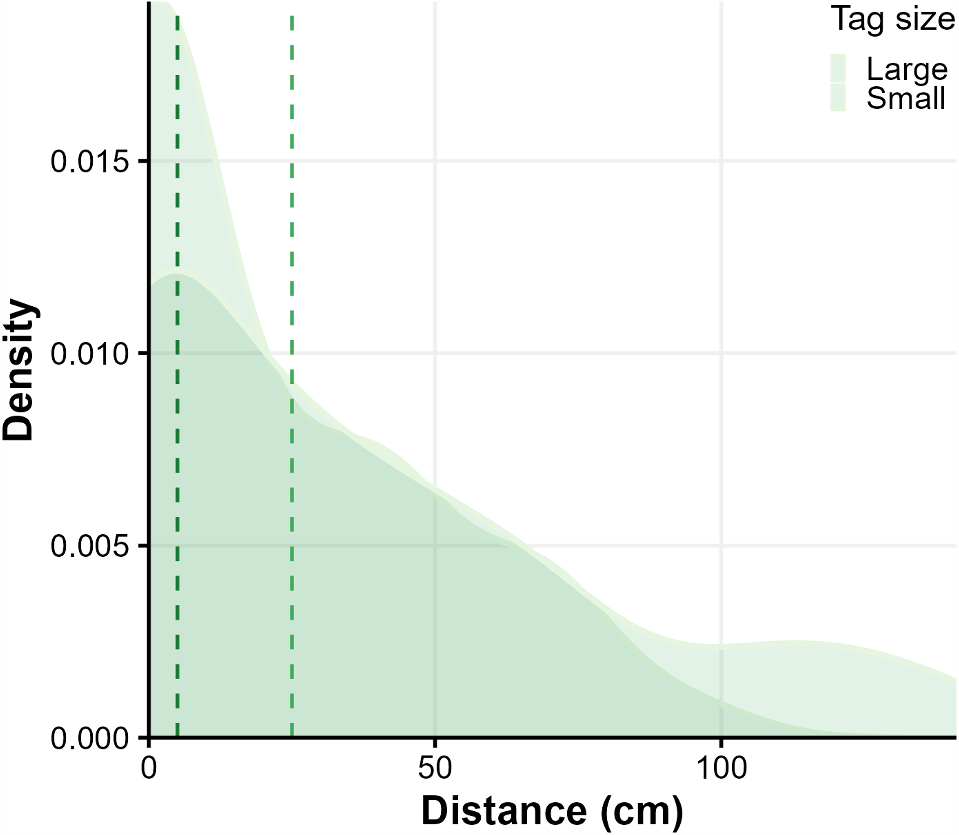
Both tag sizes were effective for tracking, and tag size did not change the distance moved or time until an individual was found. Males did not move far between surveys (average 58.2 ± 24.7 cm) and had small home ranges (0.46 ± 0.20 m2) over the 5-day tracking period.
Our study highlights that HDF can be used to track very small vertebrates in structurally complex environments. This method has the potential to fill important knowledge gaps regarding the ecology of small terrestrial breeding amphibians, providing insights that can inform conservation measures and population assessments for vulnerable species.
Keywords: amphibian, conservation, harmonic direction-finder, Microhylids, nursery frogs, oviposition site, site fidelity, telemetry, tracking.
Introduction
Understanding animal movement is key to better understanding a species’ behavioural and spatial ecology (Horne et al. 2020). The home range of individuals includes the space where daily activities are performed such as access to shelter, food resources, and breeding sites (Odum and Kuenzler 1955; Burt 1943; Gerking 1953). By tracking the movement of an animal, we can obtain information about their habitat preferences, use and requirements, as well as foraging strategies, behaviour, dispersal, homing abilities, and mating system (e.g. Koch and Hero 2007; Ludwig et al. 2013; Pašukonis et al. 2014; Kingsbury and Robinson 2016; Ayala et al. 2019; Garrido-Priego et al. 2024). This behavioural and spatial information provides basic ecological data for a species, and can be used to resolve microhabitat requirements (Rowley and Alford 2007), population sizes (Gupta et al. 2019), and develop effective conservation measures (Parsons 2016; Fraser et al. 2018; Garrido-Priego et al. 2024).
Amphibians are the most endangered group of vertebrates, with 40.7% of species under threat (Luedtke et al. 2023). Understanding their movement and microhabitat use is key to developing conservation measures for determining suitable areas for breeding and survival (Garrido-Priego et al. 2024). Biotelemetry is the most popular method for studying animal movement (McGowan et al. 2017). However, this is complicated in many amphibian species due to their small size. While the size and weight of radio transmitters has decreased considerably in the last decade (0.15 g smallest commercially available), they are still too heavy to attach to smaller species (Rowley and Alford 2007). Furthermore, the smaller transmitter constrains battery capacity and, therefore, the device’s operational lifespan (Rowley and Alford 2007; Altobelli et al. 2022).
Amphibian movement is studied using a variety of methods, often depending on species size and ecological context. The four methods predominantly used in amphibian movement studies are: radio telemetry (e.g. Pašukonis et al. 2019; Altobelli et al. 2022), harmonic direction finding (HDF, also referred to as harmonic radar, e.g. Moseley and Castleberry 2005; Rowley and Alford 2007; Pašukonis et al. 2014, 2019; Borzée et al. 2018;), thread-trailing or spooling (Lemckert and Brassil 2000; Schwenke 2016), and the least used method is fluorescent powder (e.g. Rittenhouse et al. 2006; Merino-Viteri 2018; reviewed by Altobelli et al. 2022). While radio telemetry tags use batteries and constantly transmit a signal, HDF tags modify and ‘reflect’ a signal emitted from a hand-held transceiver and require no batteries, which makes them light enough for tracking smaller species (Mascanzoni and Wallin 1986; Rowley and Alford 2007; Pašukonis et al. 2014; Miller et al. 2022; Siderhurst et al. 2024), and are much cheaper (US$3 in materials for HDF versus hundreds of dollars for radio telemetry). However, HDF has relatively lower detection distances (i.e. tens of metres versus hundreds of metres for radio telemetry; Langkilde and Alford 2002; decreasing to 1–2 m in densely vegetated or rocky environments; Sizun 2005; Rowley and Alford 2007) and does not allow for individual identification without extra alteration (Ramírez et al. 2017). While spooling can be effective for larger species in more open habitats, it only works for a relatively short amount of time (2–5 days depending on the length of the spool), and the pack or nylon can easily get tangled or caught (Lemckert and Brassil 2000). This technique involves attaching a lightweight spool of thread to the animal to track its movements by manually following the thread. Fluorescent powder can be effective for smaller species, but sufficient powder needs to stick to the animal, and powder dropped in the environment will only remain visible for 1–2 days, and less if it rains (Ramírez et al. 2017).
Based on the above information, the HDF method would be the best option for smaller species. Harmonic direction finding has been used in many studies of amphibian movement, but generally for species of moderate to larger size, typically in the range of 2–6 g (Pellet et al. 2006; Sapsford et al. 2014; Borzée et al. 2019), with tags being in the range of 0.07–0.27 g (Altobelli et al. 2022). The smallest amphibian tracked to date using HDF is Allobates femoralis (1.4–2.3 grams), with tags comprising 3–5% of their body mass (Pašukonis et al. 2014), compared to radio telemetry (Dendrobates tinctorius, body weight 3.5–4.2 g), with tags comprising 9.5–10% of their body mass (Pašukonis et al. 2019). Studies on insects have shown that lighter HDF tags (<1 mg) can be effective for studying movement in very small animals (e.g. Makinson et al. 2019; Miller et al. 2022; Moore and Siderhurst 2022; Siderhurst et al. 2024). Here, we use tags originally designed for insects to effectively track the movement of a very small frog species (<1.5 g weight) while remaining below the recommended threshold of 5–10% of the amphibian’s body weight (Richards et al. 1994; Altobelli et al. 2022).
Building on advancements in HDF technology for studying small animals, we applied this method to address knowledge gaps in the movement ecology and habitat use of Microhylidae in Australia. The family Microhylidae is represented by two genera: Cophixalus (18 species) and Austrochaperina (five species) (Zweifel 1985; Hoskin 2004, 2012). This family is increasingly of conservation concern, with five species listed as Critically Endangered and others listed as Endangered or Vulnerable (Gillespie et al. 2020; Geyle et al. 2021). However, despite their diversity and conservation concerns, there is very little known about their breeding biology and microhabitat use, primarily due to their typically restricted distributions, small size and cryptic nature. Using HDF, we aimed to determine the scale of movements, home range size, nesting sites, and microhabitat use of male robust whistling frogs (Austrochaperina robusta) during their breeding season.
Materials and methods
Study area and species
Cophixalus and Austrochaperina are terrestrial breeders with direct development (i.e. embryos develop to metamorphosis within the jelly capsules) and perform paternal care (Hoskin 2004). In this study, we use the common species Austrochaperina robusta (robust whistling frog) as a model for other microhylids of similar ecology. Austrochaperina robusta is small-sized (22–32 mm snout-vent length) and restricted to mid-elevation (~600–1000 m.a.s.l.) and upland rainforest of the Wet Tropics region of northeastern Queensland, Australia, where it has a cryptic life history among leaf litter and under logs and rocks (Hoskin and Hero 2008). Males call at night from the leaf litter, close to surface objects such as logs and roots and are mostly inactive during the day (Groffen et al. 2024). The clutch (9–15 eggs) is laid in leaf litter or under a log or rock and is attended by an adult male (Hoskin 2004; Anstis et al. 2011). Austrochaperina robusta can be found in high density, but because of their small size and cryptic life history, little is known of their home range, movement behaviour, and nesting sites. In this study, home range is defined as the spatial area used by the study species during a 5-day tracking period during the breeding season.
Tracking system and tag attachment
The harmonic direction-finding (HDF) system consists of a passive reflector/transponder diode ‘tag’ and an active directional transceiver, in this case, a RECCO hand-held transceiver (RECCO R9 Handheld Detector, www.recco.com) (Mascanzoni and Wallin 1986). The tags generally consist of one or, most commonly (this study), two wires attached to a Schottky diode. Harmonic tags do not use batteries. Instead, they modify (double) the signal emitted from the transceiver (~900 MHz in this case), which can then be used to triangulate the location of the animal (Leskovar and Sinsch 2005; Rowley and Alford 2007).
The most widely used method for tracking anurans via HDF involves fitting tags on top of a silicone tube, measuring 1.5 mm in diameter, to create a harness (Fig. 1a; reviewed by Altobelli et al. 2022). Due to the small size of our study species, we used 0.5 mm diameter medical-grade silicone tubing. We placed the harness in front of the frog’s hindlegs, at the narrowest part of the body, to prevent the harness from sliding forward up the body or backward off the hindlegs (Fig. 1a; Bartelt and Peterson 2000; Pašukonis et al. 2014; Fischer et al. 2020). The harness was fastened via a cotton thread fitted through the tube (Natural cotton C Ne #50 Gutermann), which was tied using a double reef knot. The cotton is expected to break and release the harness after approximately two to three weeks (Beck et al. 2017), in case an individual is unable to be recaptured. Each harness had a harmonic radar tag attached with super glue (Loctite Super Glue). Two sizes of tags were tested, both fabricated from a Schottky diode with two super elastic nitinol wire antennas attached to form a dipole (for more details see Miller et al. 2022; Siderhurst et al. 2024). The smaller tags were made with a small diode and two 4 cm lengths of 0.025 mm diameter nitinol wire (~1 mg total mass; Miller et al. 2022), while the larger tags had a larger diode and two 8 cm lengths of 0.076 mm diameter nitinol wire (~15 mg total mass; Siderhurst et al. 2024). The ‘hair like’ antennas were oriented transversely on the frog (protruding to the left and right) and could be bent in any direction so as not to obstruct the frog’s movement (Fig. 1a).
Locating frogs
Surveys for calling males were conducted in their breeding season between 8–15 January 2024, after sunset (19:00–23:30 h), along a 1.4 km existing trail in the rainforest uplands of the Paluma Range (‘H-Track’; 19.0107°S, 146.2055°E), north Queensland, Australia. Once a calling male was found, it was caught and placed in a Ziplock bag, with moist leaf litter added to minimise stress. Snout-vent length (SVL) was measured to the nearest 0.01 mm with callipers and the weight was taken to the nearest 0.01 g using a 10 g Pesola® spring scale. A small or large tag was fitted, as outlined above. Flagging tape with individual ID, date, and survey number was used to mark the capture location, and GPS coordinates were recorded. Handling time and tag size were recorded, and the captured individual was placed back at the exact capture location and covered with a leaf. Microhabitat notes were recorded for each capture location. The following environmental measurements were recorded: ambient temperature, humidity (%), precipitation (Yes/No), and number of other male A. robusta calling within 5 metres.
Each tagged individual was subsequently located a minimum of two times per 24-h period: once during daytime (10:30–19:00 h) and once during the night (20:00–00:00 h). Once we were in the vicinity (i.e. within 2 meters) of the last known location of a tagged frog, the RECCO hand-held transceiver was turned on and a timer was started as the start time for searching. The timer was stopped when we had a visual of the frog with harness or a detached harness. Once the frog was found, location, microhabitat notes (same as original capture, see paragraph above), search time and time of day were recorded. We stopped searching after 15 min (900 s) if the frog had not been located. If the tagged individual had moved from the previously recorded location, flagging tape with individual number, date, and survey number was placed at the new location. We recorded the distance and GPS bearing to previous capture location, and distance to closest object (log, rock, root or tree). If the transceiver suggested the same location as the previous survey (i.e. within 5 cm), we did not uncover the frog (to minimise disturbance), but if this repeated at the next survey, we searched for the frog to ensure it had not lost the harness. After 48 h, each tagged individual was captured and checked for potential injuries from the harness and tag. These were assessed by looking for visible surface wounds or abrasions around the harness area. The frog was then released at the exact point of capture. After 5 days, each tagged individual was recaptured and the harness removed. Following harness removal, each individual was checked again for potential injuries from the harness/tag, and handling time was once again recorded. Only individuals with three or more location records (regardless of whether each record involved actual movement) were used for calculations of movement distances and minimal home range size.
Home range estimation
At the end of the tracking period, photographs were taken from approximately 1.5 m looking down onto the forest floor, with all flagging tape markers of locations visible, to document the frog’s home range. A minimum convex polygon (MCP) method (Mohr 1947) was used to estimate home range by importing the images into ImageJ (Schindelin et al. 2012) and using the polygon measure function. Other studies also calculate kernel density estimates to complement the MCP method (e.g. Garrido-Priego et al. 2024). But for our home ranges, the precision of locations and location numbers were too small to use this or similar methods.
Statistical analyses
All analyses were conducted in R version 4.3.2. We used the lme4 package (Bates et al. 2015) to run all models and ggplot2 (Wickham 2016) to construct figures. To examine if tag size (fixed effect) influenced the search time until found (response variable), and distance travelled (response variable), we used a linear mixed model (gaussian distribution) and a generalised linear mixed model (gaussian distribution), respectively. When a tag was not found, a search time of 900 s was recorded in the dataset. A linear mixed model was used to examine if there was a difference between search time until located between daytime and nighttime surveys. The models included frog ID as a random effect to account for the repeated measures per individual.
Results
Twenty-two adult male A. robusta were tagged between 8–20 January 2024, of which 11 individuals (50%) lost their harnesses within the first 15 h. The average weight of the tagged frogs was 1.27 ± 0.20 g, and the average length was 22.45 ± 0.91 mm (SVL). One individual lost the harness after 3 days and another after 4 days, resulting in six and eight locations, respectively. The remaining nine frogs that were tracked for the full 5-day tracking period had locations recorded at least twice a day, resulting in a total of 10–13 locations per frog (Table 1). All frogs were hidden underneath the leaf litter (i.e. between the leaf litter and soil) or in small holes in the ground during daytime. A general pattern observed was that frogs often remained hidden under the leaf litter, in holes, or burrows during the day and moved to the upper layers of the leaf litter at night. On average, frogs were just as likely to be recorded at a different location between checks (54%, range 33–83%) as the same location. Average ambient temperature during the tracking period was 22.2 ± 4.8°C, humidity was always above 96%, and it rained to some degree every day (average daily precipitation was 15.3 ± 11.8 mm).
| ID | # check | Capture date | Size (mm) | Weight (g) | Tag size | % of body weight | Average search time (s) ± s.e. | Times moved | Average movement (cm) ± s.e. | Cumulative distance moved (cm) | Longest distance between surveys (cm) | Longest distance from capture (cm) | Home range (m2) | Net displacement (cm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 10 | 10/01/2024 | 21.2 | 1.15 | Large | 2.9 | 340 ± 35 | 7 | 38.6 ± 5.8 | 272 | 63 | 103 | 0.62 | 103 | |
| B | 13A | 10/01/2024 | 22.6 | 1.25 | Large | 2.6 | 287 ± 81 | 5 | 47.8 ± 15 | 191 | 70 | 90 | 0.32 | 69 | |
| C | 8A | 09/01/2024 | 21.6 | 1.5 | Small | 1.5 | 268 ± 158 | 3 | 110 ± 23 | 330 | 133 | 163 | 0.45 | 64 | |
| D | 12B | 12/01/2024 | 21.1 | 1.4 | Small | 1.6 | 477 ± 89 | 10 | 40 ± 6.6 | 400 | 75 | 92 | 0.54 | 45 | |
| E | 12 | 12/01/2024 | 21.5 | 0.84 | Small | 2.7 | 270 ± 66 | 8 | 51.4 ± 14.3 | 411 | 141 | 169 | 0.65 | 44 | |
| F | 12 | 12/01/2024 | 24.1 | 1.4 | Small | 1.6 | 184 ± 55 | 4 | 93.8 ± 13.7 | 375 | 123 | 173 | 0.66 | 173 | |
| G | 11 | 15/01/2024 | 23.1 | 1.4 | Small | 1.6 | 84 ± 15 | 6 | 48 ± 7.6 | 288 | 68 | 134 | 0.27 | 106 | |
| H | 11B | 15/01/2024 | 21.3 | 1.55 | Small | 1.5 | 210 ± 103 | 4 | 51.5 ± 20.9 | 206 | 114 | 114 | 0.19 | 98 | |
| I | 10 | 10/01/2024 | 22.9 | 1.6 | Large | 2.1 | 188 ± 54 | 6 | 49.5 ± 11.5 | 297 | 94 | 152 | 0.76 | 92 | |
| J | 6B | 12/01/2024 | 21.2 | 1.1 | Small | 2.1 | 316 ± 81 | 5 | 77.6 ± 15.8 | 388 | 107 | 29 | 0.28 | 47 | |
| K | 11 | 08/01/2024 | 22 | 1.25 | Large | 2.6 | 118 ± 33 | 4 | 32.4 ± 6.7 | 129.6 | 71 | 106 | 0.09 | 80 |
Tag size
Average frog weight (±s.d.) was 1.27 ± 0.20 g and harness/tag weights were 0.023 ± 0.005 g (n = 7) for the smaller tags (tag average 1.80% of body weight, range 1.5–2.1%) and 0.033 ± 0.004 g (n = 4) for the larger tag (tag average 2.58% of body weight, range 2.1–2.9%) (Table 1). In all cases, the combined weight of the harness and tag was under the standard threshold of 5–10% of the amphibian’s body weight recommended in the literature (Richards et al. 1994; Altobelli et al. 2022). Tagged individuals could not be located in seven of the 116 surveys (9.7% of location checks). Individuals with the smaller tags took on average 258 ± 106 s to be located and were unable to be located on five occasions (6.9%; all during daytime searches), while individuals with the larger tags took on average 233 ± 87 s to be located and were unable to be located on only two occasions (4.5% of location checks, once during daytime and once during nighttime). Although these numbers fit the expectation that larger tags are more readily found, there was no overall difference in search time between tag sizes (R2 = 0.21, β = 23.53, P = 0.76, Supplementary Fig. S1A). There was also no significant difference between the time it too to locate a frog and the time of day (R2 = 0.19, β = −17.88, P = 0.68, Fig. S1C).
Handling time for the original capture was an average (±s.d.) of 13.1 ± 5.6 min and 7.2 ± 4.0 min for removal of the harness at the end of the study. During the 48-h check, there were no signs of injuries, however, after the 5-day period one individual had two small lesions where the harness had been.
Home range and distance travelled
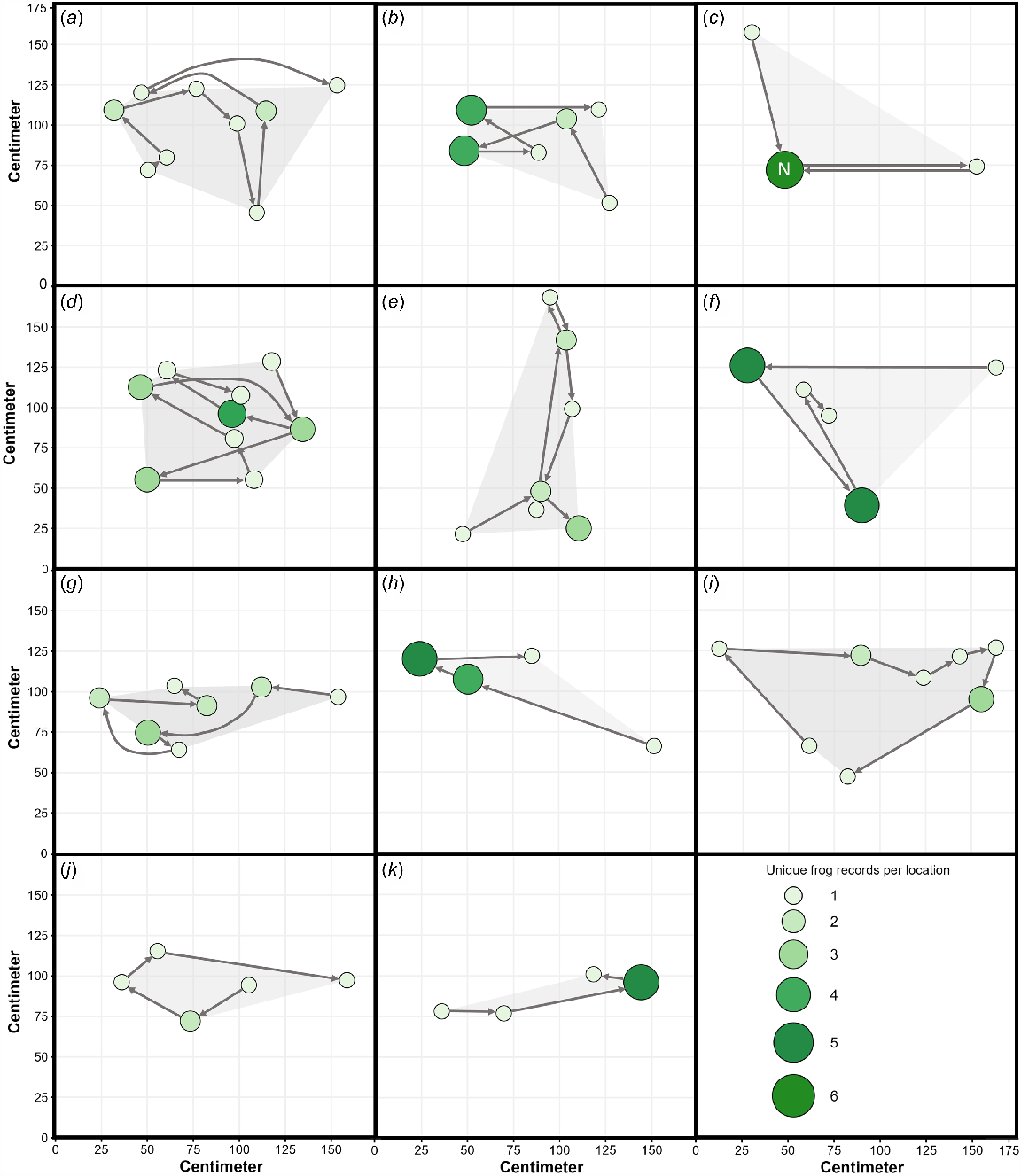
The average distance moved between two consecutive surveys was 58.2 ± 24.7 cm (mean ± s.d.; n = 62 movements, Fig. 2 and Fig. S1). The longest movement from the previous check was 141 cm, and the furthest distance moved from the original capture site was 173 cm (Table 1). The average home range across the 11 frogs was 0.46 ± 0.20 m2 (range 0.19–0.76 m2, Figs 2, 3). One frog had a nest with four eggs in terrestrial development stage 10–11 (Anstis 2013; Fig. 1b). During night surveys, tagged males had other A. robusta males (1–3 males) calling within 5 metres 57.4% of the time. On five occasions, a non-tagged individual was located within 50 cm of a tagged individual: three non-calling adults (sex unknown, 5 cm, 12.5 cm and 23 cm away), one calling male (46 cm), and one metamorph (9 cm). There was no significant difference between tag size and distance travelled (R2 = 0.02, β = 11, P = 0.17, Fig. S1). Out of 11 frogs, three individuals (27.3%) did not show an increase in their minimum convex polygon (MCP, minimal home range size) after the first three locations were recorded; others often moved away and then returned close to the original capture site but expanded their MCP (Fig. S2).
Density plot showing the distribution of movement distances between tracking locations, and the relationship between tag size (‘small’ tag = dark green; ‘large tag’ = light green) and movements for the eleven tracked males Austrochaperina robusta. Dashed lines are medians. Movement distances are for all frogs (n small tags = 7, n large tags = 4).
Movements and home range visualisation of 11 tagged A. robusta individuals in a 5-day period. The size and colour of the bubble indicates the number of unique frog records per location. The light grey polygons show the minimum estimated home ranges. The ‘N’ in panel (c) indicates the location of the nest. The letters of each panel relate to the Frog ID column in Table 1.
Discussion
In this study, we used an ultra-light tag and harness (0.023–0.033 g) to study the movements, home range, habitat use, and nesting sites of a small (22.45 ± 0.91 mm) and very light (1.27 ± 0.20 g) terrestrial breeding frog species, Austrochaperina robusta. Our primary ecological findings are (1) frogs moved remarkably small distances and had uniformly small home ranges, (2) frogs are on or near the surface at night and deeper in the leaf litter/soil during the day, and (3) the tracking can lead to the location of egg clutches. In terms of technique assessment, both the ‘small’ and ‘large’ harness/tag setups were highly effective for this species, and we saw no significant ethical issues for using the technique with frogs. Furthermore, to the best of our knowledge, these are the lightest harness/tag setups used in amphibian tracking studies to date, being over 50% lighter than previous systems (previous lightest harness/tag was 0.07 g; Altobelli et al. 2022).
Despite the minimal weight of our harness/tag setup, half of the tagged individuals lost their harness in the first 15 h. Like many other Anuran species, when A. robusta is under threat, they inflate themselves with air to look larger. When captured, A. robusta showed this defensive behaviour, resulting in some harnesses being attached too loosely. Additionally, we observed that they often pushed themselves through small spaces in the leaf litter and soil, potentially dislodging the harness. These are the suspected main causes of harness loss, especially at the start of the study (8 of the first 10 frogs lost their harness). Harnesses were subsequently tied tighter, which improved retention of the harnesses and caused little adverse effect to the frogs, aside from possibly two small lesions on one individual at the end of the 5-day tracking period.
There was no significant difference between tag size and search time, likely due to the small home range of the study species and the light weight of both the small and large tags. Furthermore, the small sample size might also influence this. However, the smaller tags were more often unlocated when the frogs were deeper in the leaf litter. Therefore, we recommend using the larger tag size for species with a larger home range or those that are more fossorial. The smaller tag size is suitable for smaller species to stay under the recommended 5% of their body weight, have a small home range, or are more epigeal.
Home range size and body size have been shown to be positively correlated in a number of frog species (Duellman and Trueb 1986). Other similarly small frogs tracked to date also have small home range sizes, but our home range size estimates for A. robusta (0.46 ± 0.20 m2, range 0.19–0.76 m2) are small even compared to these. For example, Rhinoderma darwinii (22–31 mm SVL) have an average home range of 1.82 ± 0.54 m2 (range 0.1–16 m2; Valenzuela-Sánchez et al. 2014), Hylodes dactylocinus (24–27 mm SVL) home ranges average 2.2 ± 2.1 m2 (0.12–13.12 m2; Narvaes and Rodrigues 2005), and Phyllobates vittatus (24 mm SVL) average 55.7 m2 (36.95–67.64 m2; Garrido-Priego et al. 2024). We tracked the individuals for a maximum of 5 days, whereas some of these studies mentioned above tracked frogs for longer, which may generate larger home range estimates. Additionally, we only tracked in the breeding season, and home ranges could be larger during the non-breeding season (Duellman and Trueb 1986; Wells 2007). While some individuals kept increasing their minimum convex polygon (MCP), the later direction was often towards the original capture site, showing high site fidelity. This might reflect only a snapshot of the individual’s spatial behaviour during this specific period and may not encompass their full annual or lifetime range. However, based on the strong site fidelity and extremely sedentary behaviour of the species in this study and others (Groffen and Hoskin 2024, unpubl. data), we do not expect that the home range will increase drastically.
The ability to follow individual frogs as they move in their environment allows observations of much more granular spatial/temporal observations of life history and behaviours. Individuals may defend their territory to secure limited resources such as food, calling sites, mates, eggs, and shelters (Duellman and Trueb 1986; Grant 1993; Wollenberg and Harvey 2010). Many terrestrial Anuran species with parental care show territorial behaviour to protect certain habitat requirements, resources, and eggs (Garrido-Priego et al. 2024). While locating tagged individuals, we sometimes found another adult A. robusta in very close proximity, and in 57.4% of the night checks a calling male was within 5 m of a tagged individual. These results suggest that A. robusta has very small territories and is tolerant of other individuals in close proximity. Adult male A. robusta were found deep under the leaf litter or in the soil during the daytime and in the top layer of the leaf litter after dark. This fits expectations, with the frogs sheltering in moist, cool, hidden conditions during the day and being on the surface during nocturnal activity (calling for females (Groffen et al. 2024), moving, and presumably foraging). Austrochaperina robusta does not have a clumped breeding habitat (moist leaf litter) and is a generalist feeder of small invertebrates (Williams et al. 2006), and these evenly distributed and abundant resources may limit territorial behaviour.
These behavioural insights raise further questions about the species’ reproductive strategies and nest-site fidelity. While A. robusta are abundant in their localised range, little is known of their ecology and breeding biology due to their small size, cryptic habits, and difficulty finding nests. In this study, one of the tagged individuals was found on a nest in a small depression (4 cm deep) in the soil under leaf litter and a small log. There were only four eggs (average clutch size is 12; Hoskin 2004; Anstis et al. 2011). This male was caught the night before calling 64 cm away from the nest. While the harness was lost after 3 days (133 cm away from the nest), the data showed that the male often spent time at the nest (Fig. 3c).
Using ultra-light harness and HDF – tag systems, we successfully tracked the fine-scale movements and habitat use of the small terrestrial frog Austrochaperina robusta. The technique proved effective and minimally invasive. Ecologically, we found that A. robusta has extremely small home ranges, strong site fidelity during the breeding season, and shows tolerance to conspecifics at close distances, likely due to abundant, evenly distributed resources. Tracking also enabled rare observations of nesting location.
Declaration of funding
This work was funded by the Holsworth Wildlife Research Endowment Grant (Ecological Society of Australia), the Skyrail Rainforest Research Fund (Skyrail Rainforest Foundation), and the Ric Nattrass Research Grant (Queensland Frog Society Inc.).
Author contributions
Conceptualization: JG, MHMM, CJH. Developing methods: JG, MSS, MHMM. Data analysis: JG. Preparation of Figures and tables: JG. Conducting the research, data interpretation, writing: JG, MSS, MHMM, CJH.
Acknowledgements
We would like to thank Scott Macor, Laure Senor, David Woods, Jacopo Bartholomew, and Jacqueline Richmond-Clay for their help in the field. This research was conducted under James Cook University animal ethics approval A2945 and Queensland Government scientific research permits WA0057056. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The USDA is an equal opportunity provider and employer.
References
Altobelli JT, Dickinson KJM, Godfrey SS, Bishop PJ (2022) Methods in amphibian biotelemetry: two decades in review. Austral Ecology 47, 1382-1395.
| Crossref | Google Scholar |
Anstis M, Parker F, Hawkes T, Morris I, Richards SJ (2011) Direct development in some Australopapuan microhylid frogs of the genera Austrochaperina, Cophixalus and Oreophryne (Anura: Microhylidae) from northern Australia and Papua New Guinea. Zootaxa 3052, 1-50.
| Crossref | Google Scholar |
Ayala C, Ramos AG, Merlo Á, Zambrano L (2019) Microhabitat selection of axolotls, Ambystoma mexicanum, in artificial and natural aquatic systems. Hydrobiologia 828, 11-20.
| Crossref | Google Scholar |
Bartelt PE, Peterson CR (2000) A description and evaluation of a plastic belt for attaching radio transmitters to western toads (Bufo boreas). Northwestern Naturalist 81, 122-128.
| Crossref | Google Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1-48.
| Crossref | Google Scholar |
Beck KB, Loretto MC, Ringler M, Hödl W, Pašukonis A (2017) Relying on known or exploring for new? movement patterns and reproductive resource use in a tadpole-transporting frog. PeerJ 2017, 1-24.
| Crossref | Google Scholar |
Borzée A, Kim YI, Kim K, Yikweon J (2018) Methodological development for harmonic direction finder tracking in salamanders. Herpetological Conservation and Biology 13, 473-478.
| Google Scholar |
Borzée A, Choi Y, Kim YE, Jablonski PG, Jang Y (2019) Interspecific variation in seasonal migration and brumation behaviour in two closely related species of treefrogs. Frontiers in Ecology and Evolution 7, 55.
| Crossref | Google Scholar |
Burt WH (1943) Territoriality and home range concepts as applied to mammals. Journal of Mammalogy 24, 346-352.
| Crossref | Google Scholar |
Fischer MT, Ringler M, Ringler E, Pašukonis A (2020) Reproductive behavior drives female space use in a sedentary Neotropical frog. PeerJ 8, e8920.
| Crossref | Google Scholar |
Fraser KC, Davies KTA, Davy CM, Ford AT, Flockhart DTT, Martins EG (2018) Tracking the conservation promise of movement ecology. Frontiers in Ecology and Evolution 6, 150.
| Crossref | Google Scholar |
Garrido-Priego M, Monge-Velázquez M, Whitworth A, Gomez-Mestre I (2024) Home range and notes about social interactions in the poison frog Phyllobates vittatus (Anura: Dendrobatidae). Evolutionary Ecology 38, 193-204.
| Crossref | Google Scholar |
Gerking SD (1953) Evidence for the concepts of home range and territory in stream fishes. Ecoloy 34, 347-365.
| Crossref | Google Scholar |
Geyle HM, Hoskin CJ, Bower DS, Catullo R, Clulow S, Driessen M, Daniels K, Garnett ST, Gilbert D, Heard GW, Hero JM, Hines HB, Hoffmann EP, Hollis G, Hunter DA, Lemckert F, Mahony M, Marantelli G, McDonald KR, Mitchell NJ, Newell D, Roberts JD, Scheele BC, Scroggie M, Vanderduys E, Wassens S, West M, Woinarski JCZ, Gillespie GR (2021) Red hot frogs: Identifying the Australian frogs most at risk of extinction. Pacific Conservation Biology 28(3), 211-223.
| Crossref | Google Scholar |
Gillespie GR, Roberts JD, Hunter D, Hoskin CJ, Alford RA, Heard GW, Hines H, Lemckert F, Newell D, Scheele BC (2020) Status and priority conservation actions for Australian frog species. Biological Conservation 247, 108543.
| Crossref | Google Scholar |
Grant JWA (1993) Whether or not to defend? The influence of resource distribution. Marine Behaviour and Physiology 23, 137-153.
| Crossref | Google Scholar |
Groffen J, Rush ER, Hoskin CJ (2024) Calling locations and courtship calls of the frogs Austrochaperina robusta Fry, 1912 and Pseudophryne covacevichae Ingram & Corben, 1994 in northern Australia. Herpetology Notes 17, 315-321.
| Google Scholar |
Gupta A, Dilkina B, Morin DJ, Fuller AK, Royle JA, Sutherland C, Gomes CP (2019) Reserve design to optimize functional connectivity and animal density. Conservation Biology 33, 1023-1034.
| Crossref | Google Scholar | PubMed |
Hoskin CJ (2004) Australian microhylid frogs (Cophixalus and Austrochaperina): phylogeny, taxonomy, calls, distributions and breeding biology. Australian Journal of Zoology 52, 237-269.
| Crossref | Google Scholar |
Hoskin CJ (2012) Two new frog species (Microhylidae: Cophixalus) from the Australian Wet Tropics region, and redescription of Cophixalus ornatus. Zootaxa 3271, 1-16.
| Crossref | Google Scholar |
Koch AJ, Hero JM (2007) The relationship between environmental conditions and activity of the giant barred frog (Mixophyes iteratus) on the Coomera River, south-east Queensland. Australian Journal of Zoology 55, 89-95.
| Crossref | Google Scholar |
Langkilde T, Alford RA (2002) The tail wags the frog: harmonic radar transponders affect movement behavior in Litoria lesueuri. Journal of Herpetology 36, 711-715.
| Crossref | Google Scholar |
Lemckert F, Brassil T (2000) Movements and habitat use of the endangered giant barred river frog (Mixophyes iteratus) and the implications for its conservation in timber production forests. Biological Conservation 96, 177-184.
| Crossref | Google Scholar |
Leskovar C, Sinsch U (2005) Harmonic direction finding: a novel tool to monitor the dispersal of small-sized anurans. Herpetological Journal 15, 173-180.
| Google Scholar |
Ludwig G, Sinsch U, Pelster B (2013) Migratory behaviour during autumn and hibernation site selection in common frogs (Rana temporaria) at high altitude. The Herpetological Journal 23, 121-124.
| Google Scholar |
Luedtke JA, Chanson J, Neam K, Hobin L, Maciel AO, Catenazzi A, Borzée A, Hamidy A, Aowphol A, Jean A, Sosa-Bartuano Á, Fong GA, de Silva A, Fouquet A, Angulo A, Kidov AA, Muñoz Saravia A, Diesmos AC, Tominaga A, Shrestha B, Gratwicke B, Tjaturadi B, Martínez Rivera CC, Vásquez Almazán CR, Señaris C, Chandramouli SR, Strüssmann C, Cortez Fernández CF, Azat C, Hoskin CJ, Hilton-Taylor C, Whyte DL, Gower DJ, Olson DH, Cisneros-Heredia DF, Santana DJ, Nagombi E, Najafi-Majd E, Quah ESH, Bolaños F, Xie F, Brusquetti F, Álvarez FS, Andreone F, Glaw F, Castañeda FE, Kraus F, Parra-Olea G, Chaves G, Medina-Rangel GF, González-Durán G, Ortega-Andrade HM, Machado IF, Das I, Dias IR, Urbina-Cardona JN, Crnobrnja-Isailović J, Yang JH, Jianping J, Wangyal JT, Rowley JJL, Measey J, Vasudevan K, Chan KO, Gururaja KV, Ovaska K, Warr LC, Canseco-Márquez L, Toledo LF, Díaz LM, Khan MMH, Meegaskumbura M, Acevedo ME, Napoli MF, Ponce MA, Vaira M, Lampo M, Yánez-Muñoz MH, Scherz MD, Rödel MO, Matsui M, Fildor M, Kusrini MD, Ahmed MF, Rais M, Kouamé NGG, García N, Gonwouo NL, Burrowes PA, Imbun PY, Wagner P, Kok PJR, Joglar RL, Auguste RJ, Brandão RA, Ibáñez R, von May R, Hedges SB, Biju SD, Ganesh SR, Wren S, Das S, Flechas SV, Ashpole SL, Robleto-Hernández SJ, Loader SP, Incháustegui SJ, Garg S, Phimmachak S, Richards SJ, Slimani T, Osborne-Naikatini T, Abreu-Jardim TPF, Condez TH, De Carvalho TR, Cutajar TP, Pierson TW, Nguyen TQ, Kaya U, Yuan Z, Long B, Langhammer P, Stuart SN (2023) Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 622, 308-314.
| Crossref | Google Scholar | PubMed |
Makinson JC, Woodgate JL, Reynolds A, Capaldi EA, Perry CJ, Chittka L (2019) Harmonic radar tracking reveals random dispersal pattern of bumblebee (Bombus terrestris) queens after hibernation. Scientific Reports 9, 1-11.
| Crossref | Google Scholar |
Mascanzoni D, Wallin H (1986) The harmonic radar: a new method of tracing insects in the field. Ecological Entomology 11, 387-390.
| Crossref | Google Scholar |
McGowan J, Beger M, Lewison RL, Harcourt R, Campbell H, Priest M, Dwyer RG, Lin HY, Lentini P, Dudgeon C, McMahon C, Watts M, Possingham HP (2017) Integrating research using animal-borne telemetry with the needs of conservation management. Journal of Applied Ecology 54, 423-429.
| Crossref | Google Scholar |
Merino-Viteri AR (2018) The vulnerability of microhylid frogs, Cophixalus spp., to climate change in the Australian Wet Tropics. PhD thesis. James Cook University. Available at http://doi.org/10.4225/28/5b0c8d84e69b2
Miller ND, Yoder TJ, Manoukis NC, Carvalho LAFN, Siderhurst MS (2022) Harmonic radar tracking of individual melon flies, Zeugodacus cucurbitae, in Hawaii: determining movement parameters in cage and field settings. PLoS ONE 17, e0276987.
| Crossref | Google Scholar |
Mohr CO (1947) Table of equivalent populations of North American small mammals. American Midland Naturalist 37, 223-249.
| Google Scholar |
Moore A, Siderhurst M (2022) Proposal for detecting coconut rhinoceros beetle breeding sites using harmonic radar. Research Ideas and Outcomes 8, e86422.
| Crossref | Google Scholar |
Moseley KR, Castleberry SB (2005) Assessment of subcutaneously implanted reflector tags for relocating mole salamanders (Ambystoma talpoideum). Georgia Journal of Science 63, 91-96.
| Google Scholar |
Narvaes P, Rodrigues MT (2005) Visual communication, reproductive behavior, and home range of Hylodes dactylocinus (Anura, Leptodactylidae). Phyllomedusa 4, 147-158.
| Crossref | Google Scholar |
Odum EP, Kuenzler EJ (1955) Measurement of territory and home range size in birds. Ornithology 72, 128-137.
| Crossref | Google Scholar |
Parsons ECM (2016) Why IUCN should replace “data deficient” conservation status with a precautionary “assume threatened” status—A cetacean case study. Frontiers in Marine Science 3, 193.
| Crossref | Google Scholar |
Pašukonis A, Loretto MC, Landler L, Ringler M, Hödl W (2014) Homing trajectories and initial orientation in a Neotropical territorial frog, Allobates femoralis (Dendrobatidae). Frontiers in Zoology 11, 29.
| Crossref | Google Scholar |
Pašukonis A, Loretto MC, Rojas B (2019) How far do tadpoles travel in the rainforest? Parent-assisted dispersal in poison frogs. Evolutionary Ecology 33, 613-623.
| Crossref | Google Scholar | PubMed |
Pellet J, Rechsteiner L, Skrivervik AK, Zürcher JF, Perrin N (2006) Use of the harmonic direction finder to study the terrestrial habitats of the European tree frog (Hyla arborea). Amphibia Reptilia 27, 138-142.
| Crossref | Google Scholar |
Ramírez PA, Bell BD, Germano JM, Bishop PJ, Nelson NJ (2017) Tracking a small cryptic amphibian with fluorescent powders. New Zealand Journal of Ecology 41, 134-138.
| Crossref | Google Scholar |
Rittenhouse TAG, Altnether TT, Semlitsch RD (2006) Fluorescent powder pigments as a harmless tracking method for ambystomatids and ranids. Herpetological Review 37, 188-191.
| Google Scholar |
Rowley JJL, Alford RA (2007) Techniques for tracking amphibians: the effects of tag attachment, and harmonic direction finding versus radio telemetry. Amphibia Reptilia 28, 367-376.
| Crossref | Google Scholar |
Sapsford SJ, Roznik EA, Alford RA, Schwarzkopf L (2014) Visible implant elastomer marking does not affect short-term movements or survival rates of the treefrog Litoria rheocola. Herpetologica 70, 23-33.
| Crossref | Google Scholar |
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676-682.
| Crossref | Google Scholar | PubMed |
Siderhurst MS, Murman KM, Kaye KT, Wallace MS, Cooperband MF (2024) Radio telemetry and harmonic radar tracking of the spotted lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae). Insects 15, 17.
| Crossref | Google Scholar |
Valenzuela-Sánchez A, Harding G, Cunningham AA, Chirgwin C, Soto-Azat C (2014) Home range and social analyses in a mouth brooding frog: testing the coexistence of paternal care and male territoriality. Journal of Zoology 294, 215-223.
| Crossref | Google Scholar |
Wickham H (2016) ‘ggplot2: Elegant Graphics for Data Analysis’. (Springer-Verlag: New York) Available at https://ggplot2.tidyverse.org
Williams YM, Williams SE, Alford RA, Waycott M, Johnson CN (2006) Niche breadth and geographical range: ecological compensation for geographical rarity in rainforest frogs. Biology Letters 2, 532-535.
| Crossref | Google Scholar | PubMed |
Wollenberg KC, Harvey J (2010) First assessment of the male territorial vocal behaviour of a Malagasy leaf litter frog (Gephyromantis thelenae). Herpetology Notes 3, 141-150.
| Google Scholar |
Zweifel RG (1985) Australian frogs of the family Microhylidae. Bulletin of the American Museum of Natural History 182, 265-388 Available at https://digitallibrary.amnh.org/items/e13048b0-5fc6-46bf-b5da-78d4a07bfd87.
| Google Scholar |



