A mouse that rocks: camera trapping shows that mound assessment criteria do not reliably predict pebble mouse (Pseudomys chapmani) activity
Renée C. Firman A * , Rachelle E. Buckley A , William De Angelis A and Dustin R. Rubenstein B
A * , Rachelle E. Buckley A , William De Angelis A and Dustin R. Rubenstein B
A
B
Abstract
The western pebble mound mouse (Pseudomys chapmani) is an understudied Australian rodent that tunnels in rocky substrate to excavate a complex subterranean burrow system that is topped with a ‘fortress style’ pebble mound. Most of the species’ current distribution occurs in the Pilbara region of Western Australia, which is a hub of anthropogenic disturbance, primarily because of iron-ore mining. In 1996, a scoring system based on external mound features was developed to allow for quick assessment of whether a mound was ‘active’ or not. However, we identified that the investigation that developed the scoring system had several shortcomings, including a lack of a test of repeatability across investigators and minimal trapping effort to validate the criteria. Because this scoring system has been applied to ‘determine’ presence or absence of this species in environmental surveys, there are concerns about the accuracy of population data that have informed the management of this species.
The aim of our investigation was to provide a rigorous assessment of the established western pebble mouse mound scoring system.
We obtained independent mound activity scores from three investigators and intensively camera trapped western pebble mouse mounds during their most active period of the year.
Our analyses produced four key results: (1) western pebble mouse activity is highly variable across mounds; (2) activity scores derived from using the mound assessment criteria were not reproducible among investigators; (3) there was a lack of congruency in mound status (active or not active) as determined by the mound activity scores (predicted activity) and camera detections (actual activity); and (4) mounds that had higher predicted activity scores tended to have a greater number of camera detections.
Our investigation demonstrates that the use of external mound structures to determine western pebble mouse activity is not reliable. We show that high variation in activity across mounds is likely to be a population trait and suggest that mounds with low or no activity are critical resources, either to be occupied by locally dispersing females or as temporary respites for males that move transiently across the landscape.
We provide recommendations on how to adequately assess western pebble mouse activity, the application of which will be important in defining the current and future conservation status of this important Pilbara ecoengineer.
Keywords: Australian rodent, ecological index, Karijini National Park, mound assessment, pebble mound, Pilbara region, Pseudomys chapmani, species presence, western pebble mouse.
Introduction
Arid regions, which are characterised by hot days, cold nights and sporadic rainfall and support substantial biodiversity, occupy over one-third of the earth’s land surface (Mirzabaev et al. 2019). Arid-dwelling animals have evolved physiological, morphological and behavioural adaptations to cope with these environments where evapotranspiration greatly exceeds precipitation during most of the year (Walsberg 2000; Fuller et al. 2014). As a behavioural adaptation, burrowing results in engineered shelters that provide critical refuge from temperature extremes, as well as protection from fire and predators (Kinlaw 1999). Burrows created by arid-dwelling mammals have been shown to have positive effects on geomorphology, hydrology, soil dynamics and biodiversity at scales ranging from microsites to landscapes (Walsberg 2000; Davidson and Lightfoot 2008). Burrowing mammals are therefore recognised to be important ecosystem engineers in their ability to modify habitats and, either directly or indirectly, regulate resource availability for other species (Davidson and Lightfoot 2008; Fleming et al. 2014; Lacher et al. 2019).
As the driest inhabited continent in the world, Australia has a diverse assemblage of arid-dwelling mammals. Equally diverse, in terms of depth, diameter and complexity, are the burrows or burrow systems that these species create. For example, the greater bilby (Macrotis lagotis) constructs a spiral-shaped burrow of up to 3 m long and 2 m deep (Southgate and Johnson 2023), whereas the common wombat (Vombatus ursinus) excavates a long, multichambered burrow (>15 m) (Browne et al. 2021). On a global scale, rodents form the major group of burrowing mammals in semiarid and arid regions. Despite this, Australia is limited to only a few rodent species. Of the 17 Australian burrowing rodent species that occur in the semiarid and arid regions, only seven create burrows considered to be ‘complex’ (all others create simple burrows of <30 cm deep; Baker and Gynther 2023). One of these complex burrowing rodent species is the western pebble mouse (Pseudomys chapmani), which excavates a complex subterranean burrow system in rocky substrate that, at ground level, is topped with a ‘fortress style’ pebble mound (Fig. 1a). Although the adaptive significance of the mound-burrow system has not been adequately investigated, researchers have hypothesised that it functions in providing protection from the extreme environment, including cooling during high temperatures, warming during cold temperatures, refuge from wildfires or flooding (e.g. diversion of heavy rainfall around burrow entrances), and potentially predators (e.g. closing burrow entrances with pebbles to inhibit the passage of snakes) (e.g. see Firman and Rubenstein 2025).
(a) An image of a pebble mound displaying prominent mound structures and the post-camera design (photograph credit: R. Firman); (b) a mouse holds a pebble in its mouth as it cleared a burrow entrance after being released from a trap (photograph credit: A. Gibson Vega); the western pebble mouse distribution that mostly falls within the Pilbara region of Western Australia (image adapted from Start 2023 with permission). The dark shading represents the current distribution, and the light shading represents the historic distribution (pre-European colonisation of Australia). The Pilbara region is approximately represented by the hashed lines and the study location is indicated by the dark dot.
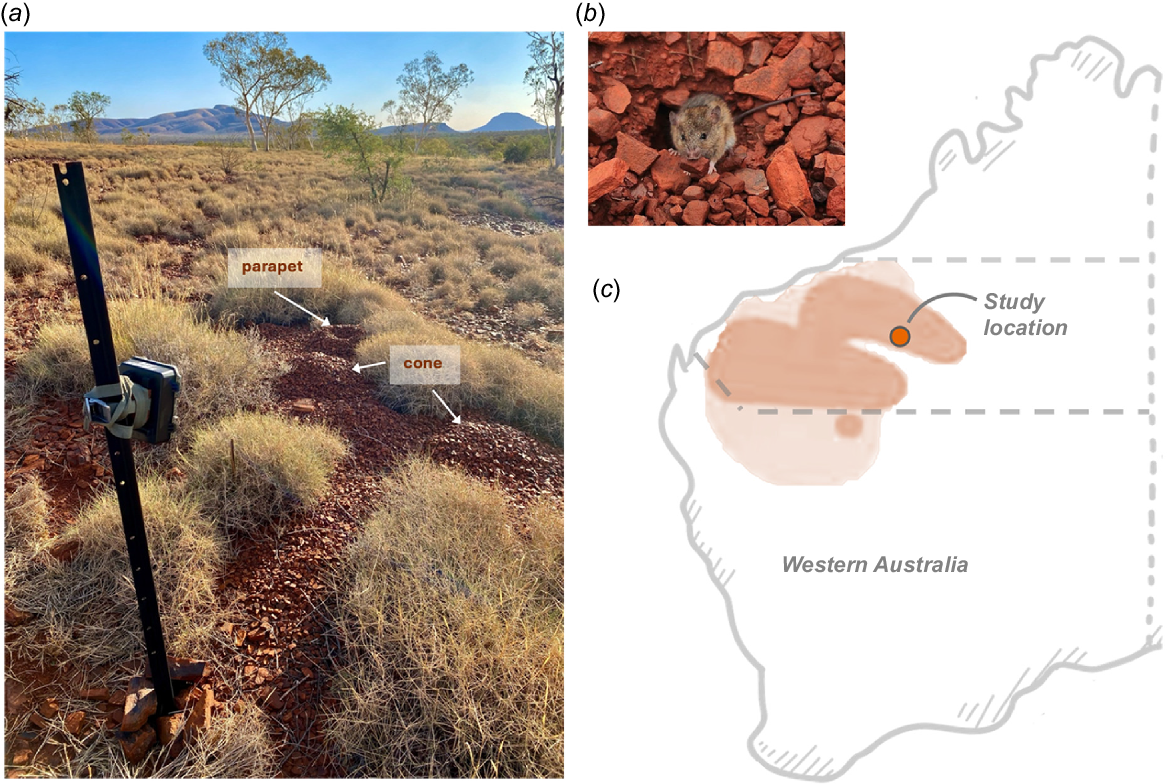
The western pebble mouse is reliant on the mound-burrow system for survival and is consequently restricted to rocky substrates mostly within the Pilbara Bioregion of Western Australia (Fig. 1c). Within the Hamersley Range, a mountainous area of the Pilbara, pebble mounds are common on gentle colluvial slopes that occur between steep-sided ridges (Start et al. 2000). Mounds are also found at the tops of hills and, in some instances, on the steep sides of hills (R. Firman, pers. obs.). Western Australia’s Pilbara region is also a hub of anthropogenic disturbance, primarily because of iron-ore mining, which depletes and degrades the natural landscape (Majer 2014). The western pebble mouse was only first described in 1980, some 8–16 years after the first iron-ore mines were established in the Pilbara (Start et al. 2000). At this time, the species was declared to be ‘fauna that is likely to become extinct or is rare’ (Start et al. 2000, p. 125). Research funded by mining companies resulted in a series of papers being published in the 1990s and early 2000s. The outcome of this research culminated in a review that ultimately led to the western pebble mouse being delisted as a conservation significant species (Start et al. 2000). The Western Australian Department of Biodiversity, Conservation and Attractions currently classifies the western pebble mouse as ‘Rare, Near Threatened and other species in need of monitoring’. Mining activity in the Pilbara has intensified over the past three decades (Sinclair and Coe 2024). Although this has likely negatively affected the western pebble mouse, there has been no targeted research on this species in recent times.
One of the outcomes of the research focus on the western pebble mouse in the 1990s was the development of ‘a system of scoring pebble mounds on their external features to determine whether the mound is occupied’ (Anstee 1996, p. 429). This study concluded that the scoring system was ‘an effective predictor’ of western pebble mouse mound activity and use (Anstee 1996, p. 429). However, we propose that Anstee (1996) had several significant shortcomings in terms of how the activity criteria were assessed and how activity status (active or not active) was verified. Specifically, we identified that there was no assessment of score reproducibility across investigators, which is critical in defining criteria ‘objectiveness’ and the suitability of the application of the criteria by different investigators with varying experience and across different contexts. Further, in the Anstee (1996) investigation, insufficient trapping effort for the species was applied to validate the criteria. This scoring system has been applied as a reliable method for ‘determining’ presence or absence of this species in environmental surveys (e.g. Knuckey and Heidrich 2014), which raises concerns about the accuracy of population data that have informed preservation management and conservation classification. Here, by obtaining mound activity scores from three investigators and intensively camera trapping western pebble mouse mounds for ~3 months over the southern hemisphere winter when the mice are most active, we provide a rigorous assessment of the established mound scoring system.
Materials and methods
Study area and data collection
The study was conducted in Karijini National Park (KNP), located in the Pilbara Bioregion of Western Australia. KNP is 6274 km2 in area and is characterised by a rugged terrain of hills (700–800 m above sea level) that form part of the Hamersley Range. KNP has deep gorges that permanently hold water. The climate is semiarid, with summer temperatures ranging from 18 to 48°C and winter from 4 to 37°C (Bureau of Meterology, Climate Data Online, see http://www.bom.gov.au/climate/data/index.shtml). Characteristic of the Pilbara region is highly variable annual rainfall that mainly falls in the summer months of December to March (past 5 years = 180–450 mm year−1 see http://www.bom.gov.au/climate/data/index.shtml). Summer is characterised by heavy rainfall driven by cyclonic activity, and temperatures that reach >40°C. KNP covers areas of the traditional homeland of the Banjima, Kurrama and Innawonga Aboriginal Peoples; this study was conducted in the south-western area of KNP on Banjima Country. The vegetation cover is mainly composed of spinifex (Triodia spp.), as well as having a sparse overstorey of eucalypts and shrubs, typically Acacia, Senna and Ptilotus. The individual mounds (n = 30) that were studied in this investigation were far enough apart to allow for spatial independence according to reported home-range sizes of ~300 m for males and ~150 m for females (Anstee et al. 1997) (Supplementary Fig. S1).
Mound assessments were performed on 10 and 11 July 2024. Two investigators (A, B) had never encountered a western pebble mouse mound prior to this investigation; the third investigator (C) had been studying pebble mice for ~12 months but had no knowledge of the activity status of the mounds when they were assessed. Each investigator used the mound activity criteria established in Anstee (1996) to provide an independent activity score for each of the 30 mounds; that is, each investigator had their own copy of the three mound activity criteria as laminated cards. Although the three investigators made their assessments at the same time, there was no communication about the mound activity criteria or the appearance of the mound at this time. Discussions occurred only after all 30 mounds had been assessed.
In Anstee (1996), the pebble mounds (n = 26) that were studied for the development of the activity criteria were also located within KNP, but in a different area from the mounds studied here (up to 30 km apart). The mound activity criteria are provided in Anstee (1996) and presented in Fig. S2. Briefly, an activity index for each mound was calculated by scoring the following three features of mound structure: (1) general structure in relation to the number and condition of prominent pebble cones and degree that surface features are cemented together; (2) condition of the access holes with respect to whether or not burrow entrances are visible, whether they are evident by a shallow depression or are deeper, and whether they are blocked (by pebbles, cobwebs or plant material) or open; and (3) condition of the access parapet in terms of how prominent a feature the parapets are, degree of weathering and the steepness of the sides (Anstee 1996; Fig. 1a, S2). In Anstee (1996), the presence or absence of mice was determined by trapping on the mounds for 5 consecutive nights:
Mounds were encircled by a 30 cm high drift fence of aluminium fly-wire and 8–10 medium Elliott traps were set on the mound (within the circle of fly-wire) [Anstee 1996, p. 432].
It was said that ‘a mound with a score of 6 or higher can be regarded as presently or recently active’ (Anstee 1996, p. 434). We adopted the same criteria; mounds with an activity score of ≥6 were defined as ‘active’ and those with ≤5 as ‘inactive’.
We utilised two methods of camera trapping to assess mound activity, which included (1) post cameras (n = 30) that remained in a fixed position for the duration of the season and (2) ground cameras (n = 6) that were positioned on the mound surface for 7 nights. The same camera model was used for each camera-trapping method (Enduro Swift Regular Wide-angle; Outdoor Cameras, Toowoomba, Qld, Australia). Because pebble mice are a strictly nocturnal species (R. Firman, unpubl. data), all cameras were set to capture activity between 17:00 and 07:00 hours. Each post camera was fixed to a 130-cm steel stake at a height of ~70–90 cm from ground, which was positioned at the edge of the mound on a tilted angle (Fig. 1a). Dummy triggers were conducted to ensure that the camera was positioned appropriately with the entire mound surface being displayed in the images. The post cameras were deployed following the mound assessment on either 10 or 11 July 2024 and retrieved on 3 or 4 October 2024, providing 84–86 nights of trapping data. Post cameras were set to capture a burst of five images per trigger. These images were loaded into Colorado Parks and Wildlife (CPW) Photo Warehouse for western pebble mouse identification (Ivan and Newkirk 2016; Newkirk 2016). The CPW Photo Warehouse built-in activity analysis was then applied to extract activity data, which included ‘location’ (mound), ‘image date’ and ‘time’ (decimal time) of each image that contained a western pebble mouse (Ivan and Newkirk 2016; Newkirk 2016). Because we were interested in total mound activity (i.e. irrespective of the activity of discreet individuals), we did not apply a ‘quiet period’ (Newkirk 2016). We were unable to distinguish whether the mouse that had been captured in the image was a western pebble mouse or a different Pseudomys mouse species of a similar size. However, our extensive trapping effort during 2023–24 showed that other Pseudomys mouse species occur on the mounds at an extremely low rate (i.e. only a single sandy inland mouse, Pseudomys hermannsburgensis, captured in ~4000 trap nights; R. Firman, unpubl. data). Therefore, although possible, it is extremely unlikely that other Pseudomys species triggered the cameras in this investigation.
The ground cameras were placed on the mound surface in the position deemed best to detect activity (e.g. in front of an open burrow entrance; between spinifex bushes if no open burrows) and left for 7 nights. Six ground cameras were rotated among the 30 mounds between the period of 7 August and 30 September 2024. Ground cameras were set to capture 10-s videos. The videos were analysed, and the time of western pebble mouse activity was recorded. To avoid data replication, only ground-camera detections that had not been detected by the post camera were added to the data set. This was achieved by cross-referencing date and time data from the ground-camera detections with date and time data from the post-camera detections.
Ethics
This research followed the guidelines for the Australian Code for the Care and Use of Animals for Scientific Purposes under UWA Animal Ethics Committee approval (2022/ET000045) and permitted under a DBCA ‘Fauna taking (scientific or other purposes) licence’ (F025000600).
Statistical analyses
We tested whether the mound assessment scores were statistically repeatable across the three investigators by using the library(rptR) in the ‘devtools’ package (ver. 2.4.5, see https://cran.r-project.org/package=devtools) with the argument datatype function as ‘binary’. We also performed generalised linear models (GLMs) fitted with ‘binomial’ distributions as a test of pairwise mound status (active or not active) congruency between investigators. A GLM fitted with a ‘binomial’ distribution was applied to test whether the score assessments (predictor; active or not active) could statistically predict the actual activity status of mounds (response; active or not active). A GLM fitted with a ‘Poisson’ distribution was applied to assess the relationship between mound activity score (predictor) and camera activity level (response; count of western pebble mouse camera detections). These GLMs were performed as tests on the median activity score of all three investigators, as well as individual tests for each investigator. All plots were constructed using ggplot (ver. 3.5.2, see https://CRAN.R-project.org/package=ggplot2; Wickham 2016). When appropriate, points were jittered to account for overlapping data. Analyses were performed in R (ver. 4.2.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/).
Results
Mound scores
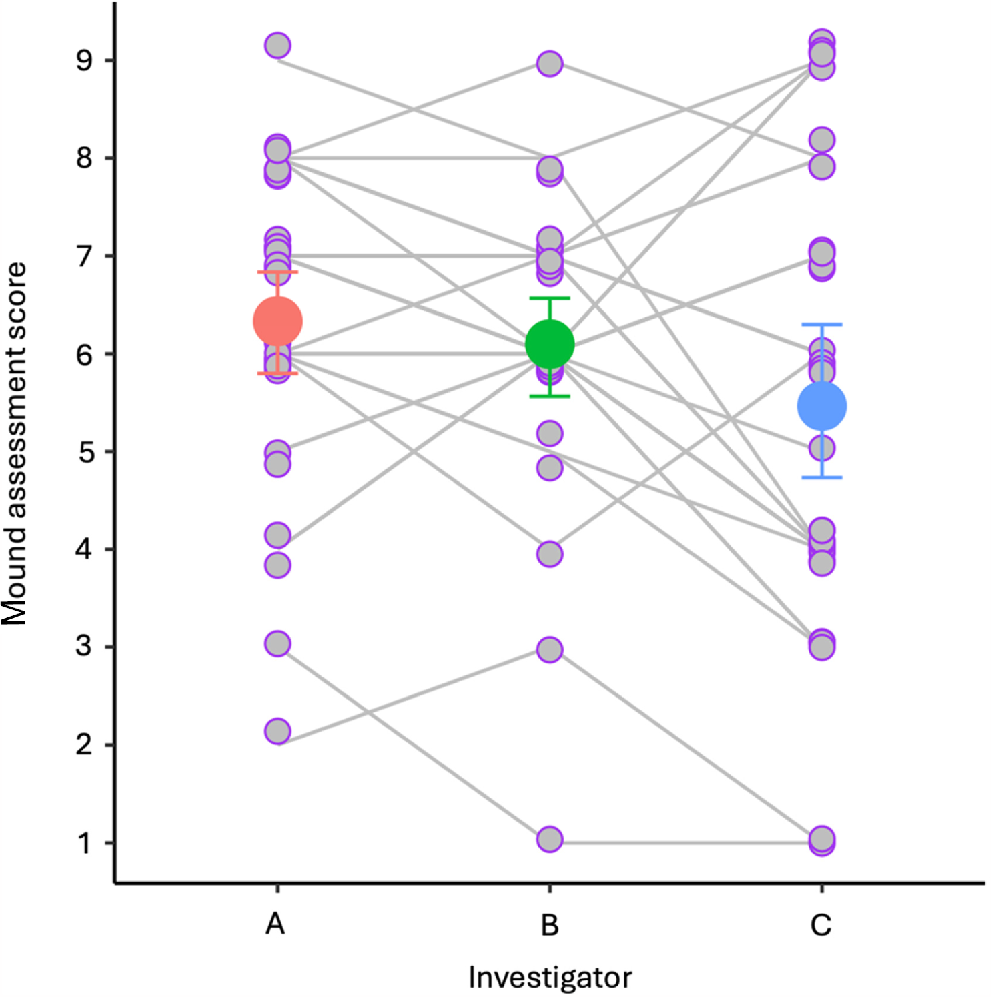
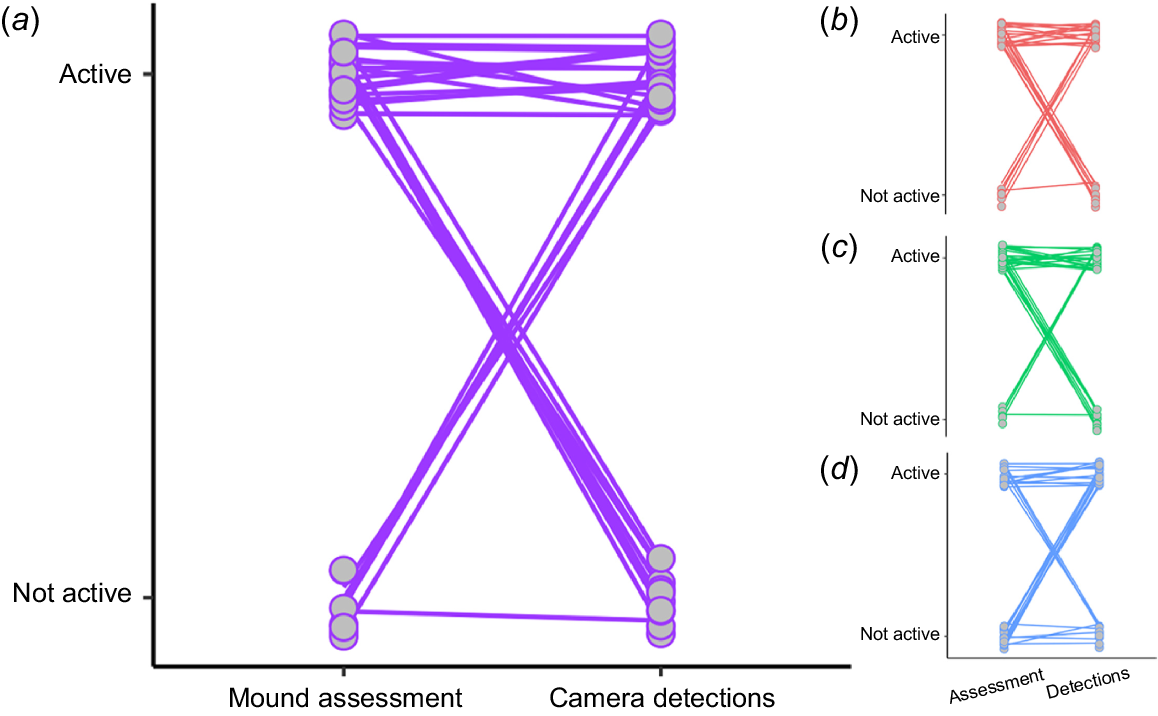
The range of the median scores fell between 1 and 8, with the modal score being 7. As defined by the mound scoring system, 23 mounds were ‘active’ and 7 mounds were ‘not active’ (median score). Although the three investigators had similar levels of variation in their score set (min., max., mode: A = 2, 9, 6; B = 1, 9, 6; C = 1, 9, 4), the repeatability analysis showed that mound assessment scores were not statistically repeatable across the three investigators (D = 0.285, d.f. = 1, P = 0.297; Fig. 2). This was supported by the lack of congruence observed in the plotted score pathways between investigators A–B and B–C (Fig. 2). In addition, the pairwise GLM comparisons showed that the activity status of one investigator did not reliably predict the activity status of another investigator (all combinations; Table 1).
Camera detections
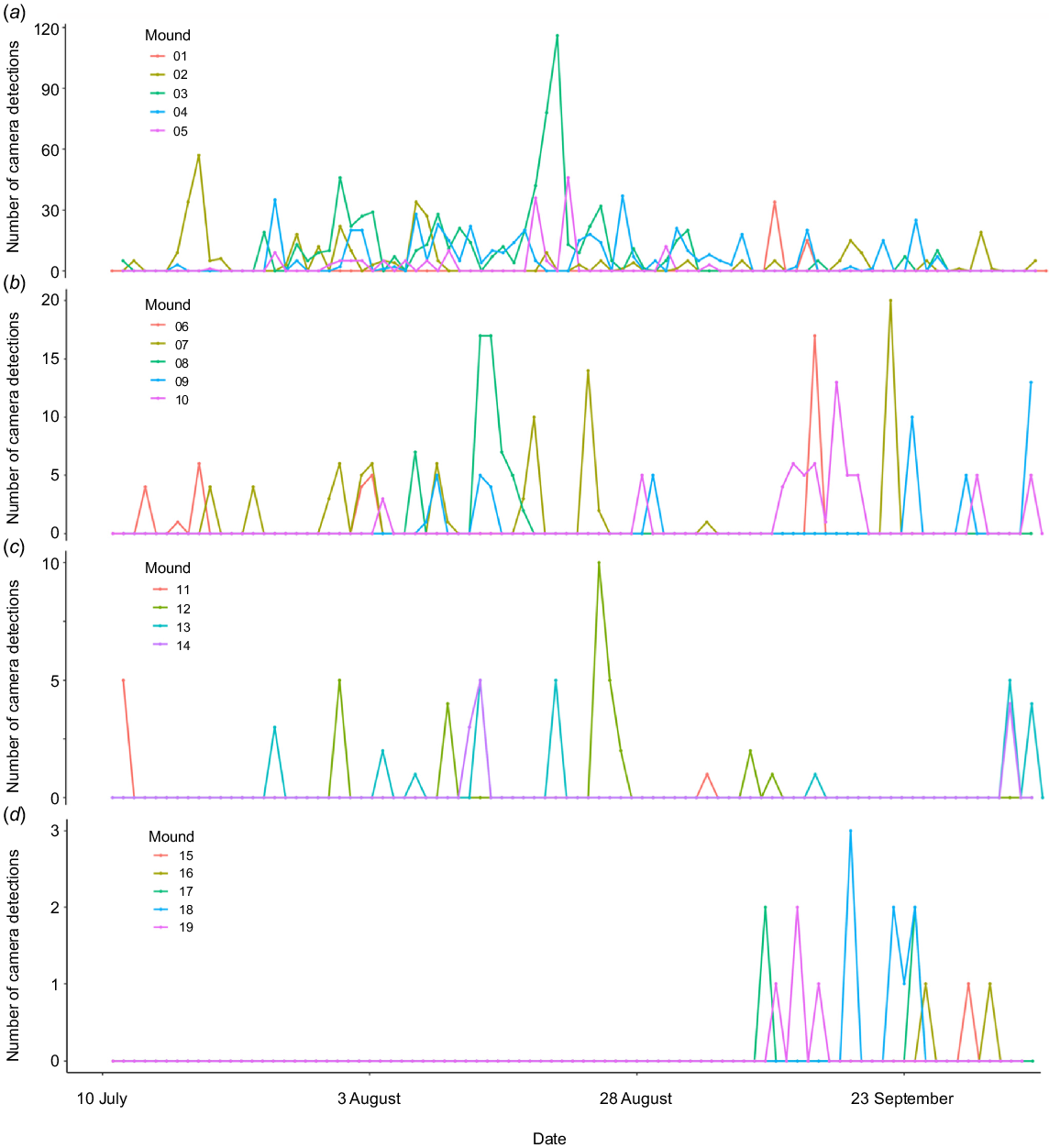
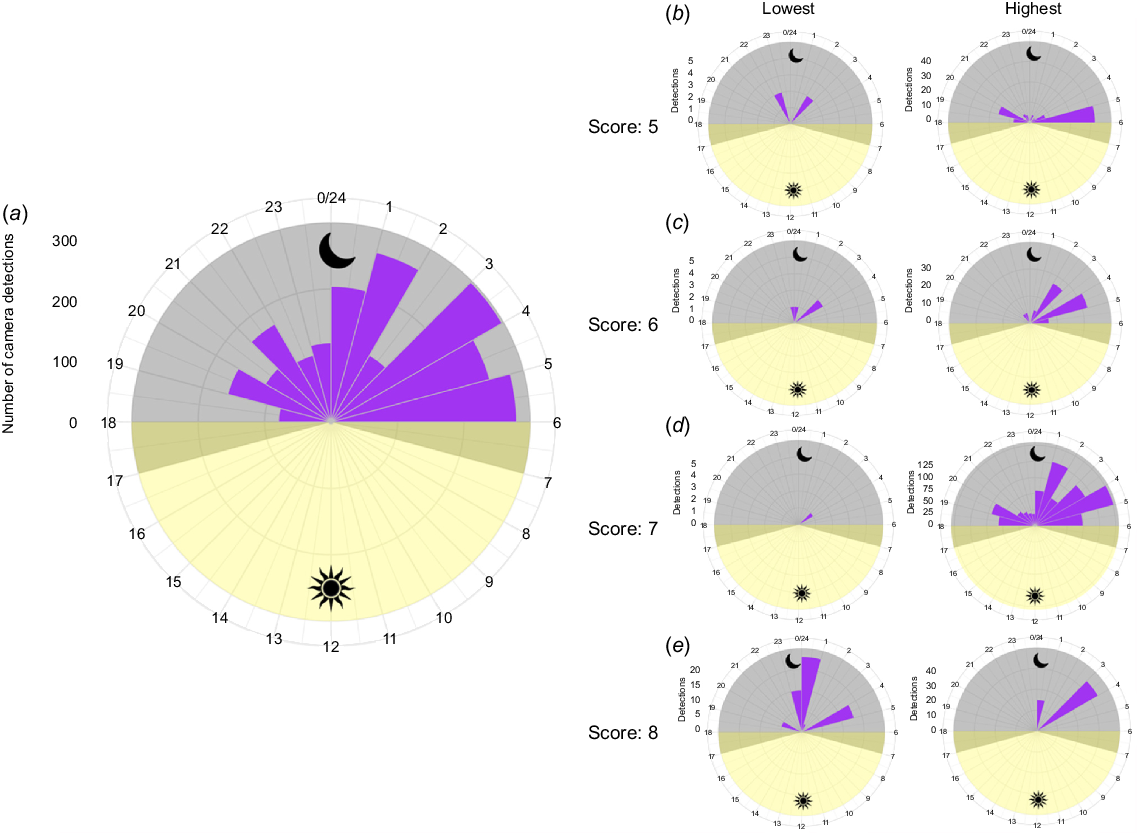
The post-camera images (2019 total detections) provided evidence of western pebble mouse activity on 15 of the 30 mounds. The ground cameras detected activity on four additional mounds at low frequency (detections on these mounds are 1, 2, 4 and 8). Thus, camera trapping showed that 19 mounds were ‘active’ and 11 were ‘not active’. There was large variation among mounds in the total number of detections (min. = 1, max. = 723, mean ± s.e. = 113.42 ± 44.87) (Fig. 3, 4b–e, S3). Most activity was detected between 01:00 and 06:00 hours, with the highest concentration of activity occurring between 03:00 and 04:00 hours (Fig. 4a). Patterns of activity did vary among mounds; however, those showing the highest levels of activity displayed the same general pattern, with most activity occurring between 01:00 and 06:00 hours (Fig. 4b–e, S3).
Assessment of mound activity score reliability
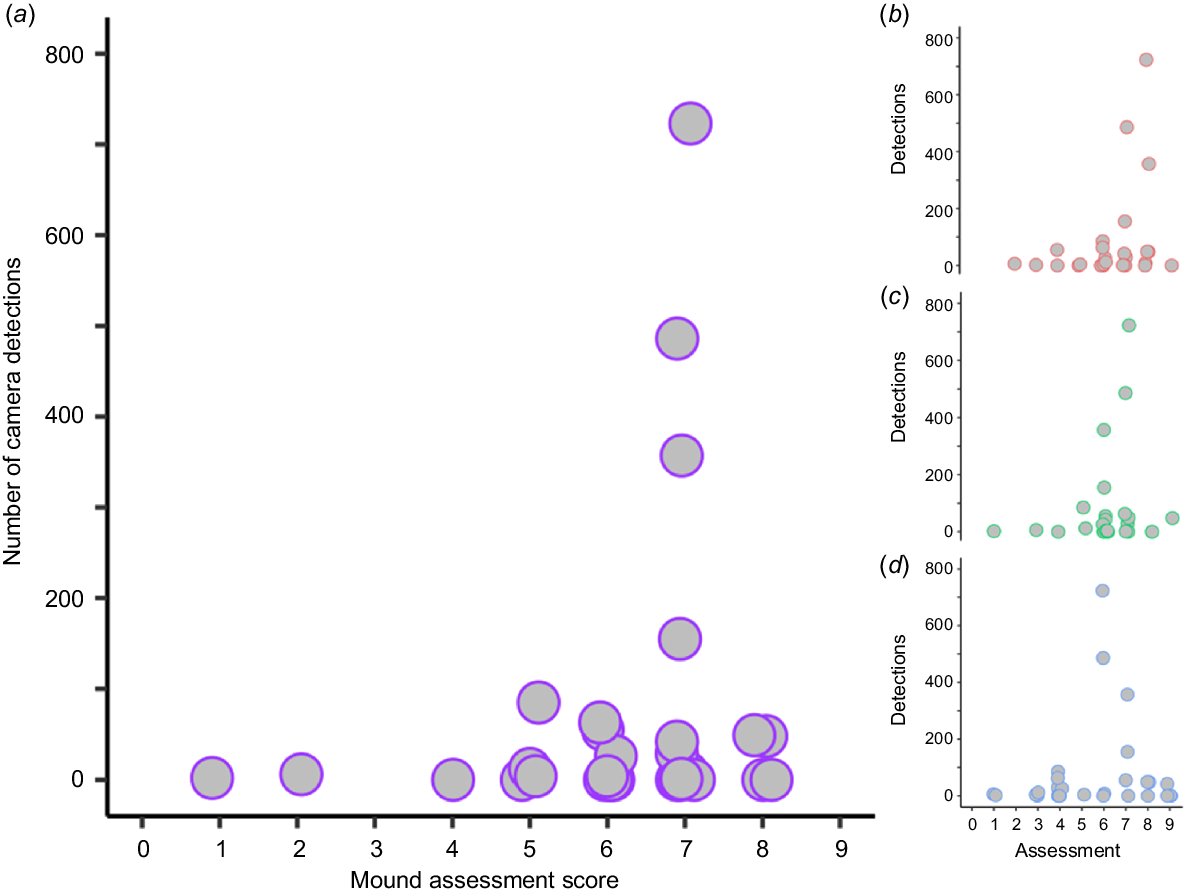
The binomial GLM showed that the activity scores did not reliably predict whether a mound was active (Table 2, Fig. 5). This was true for both the GLM performed on the median activity score, as well as for the GLMs applied individually to each investigator (Table 2, Fig. 5). The Poisson GLMs revealed that there was a significant association between the mound activity score and the number of camera detections (Table 2); those with higher activity scores typically had higher numbers of detections (Fig. 6). This significant relationship persisted when the dataset was restricted to (i) only those mounds that had detections (n = 19) and (ii) mounds with a total number of detections of <100 (n = 26) (both median score and individual investigator scores tested; analyses not shown).
| Item | Estimate | s.e. | Z-value | P-value | |
|---|---|---|---|---|---|
| (a) GLM (‘binomial’) | |||||
| Median score | −1.163 | 1.165 | −0.998 | 0.318 | |
| A’s score | −0.916 | 1.178 | −0.778 | 0.437 | |
| B’s score | −0.633 | 1.197 | −0.528 | 0.597 | |
| C’s score | 0.319 | 0.801 | 0.398 | 0.691 | |
| (b) GLM (‘Poisson’) | |||||
| Median score | 0.468 | 0.022 | 21.570 | <0.001 | |
| A’s score | 0.614 | 0.020 | 30.974 | <0.001 | |
| B’s score | 0.319 | 0.018 | 17.570 | <0.001 | |
| C’s score | 0.139 | 0.010 | 14.37 | <0.001 |
Letters A–C refer to the three investigators. Probabilities in bold are significant at P < 0.05.
Paired plots of the status of mounds (active or not active) according to mound activity scores and camera detections (n = 30). (a) Mound status based on the median mound activity score from three independent investigators (purple). Mound status based on the mound activity scores of (b) Investigator A (pink), (c) Investigator B (green), and (d) Investigator C (blue). Data points have been jittered.
Plot of the mound activity scores against the count of camera detections for pebble mouse mounds (n = 30). (a) The median mound assessment score from three independent investigators (purple). Mound assessment scores of (b) Investigator A (pink), (c) Investigator B (green), and (d) Investigator C (blue). Data points have been jittered.
Discussion
Burrows are critical resources, especially in semiarid and arid regions where they provide protection from environmental extremes, fire and predation (Kinlaw 1999). Burrowers can be classified into the following three categories: primary excavators (‘tunnelers’) are dependent on digging burrows for their survival; secondary modifiers further transform the burrows of primary excavators; and dwellers occupy the burrows of other species but do not contribute to their construction (Kinlaw 1999). As a primary excavator, the western pebble mouse is an important ecoengineer that creates subterranean habitat used by other species, including insects (e.g. crickets, ants), spiders, reptiles (e.g. Stimson’s python, Antaresia stimsoni; ring-tailed dragon, Ctenophorus caudicinctus) and other mammals (e.g. sandy inland mice, Pilbara ningaui, Ningaui timealeyi) (R. Firman, R. Buckley, W. De Angelis, pers. obs.). At least in terms of providing protection from temperature extremes, the burrow-mound system is recognised to be integral to the survival and reproduction of western pebble mice (Firman and Rubenstein 2025). One common misconception is that western pebble mice ‘decorate’ or ‘scatter’ the burrow entrances with pebbles by collecting them from the surrounding area (Dickman 1999; Breed and Ford 2007); in fact, mounds appear to be formed only by the extraction of pebbles during borrow construction (R. Firman, pers. obs.). The expansive size of the mounds (on average 3 by 2.5 m) is likely to be due to the continual excavation by successive generations of individuals inhabiting the mounds (Start et al. 2000; Firman and Rubenstein 2025). Although we cannot age the mounds, it is quite possible that many are tens or hundreds of years old.
Pebble extraction and subsequent movement result in the formation of prominent mound structures (Fig. 1a, S2). Here, we have provided a rigorous test of established mound criteria that score (0–3) these, and other structures, which are summed to produce an overall ‘activity’ index (Anstee 1996). Scoring systems need to engender confidence as reliable decision-making indicators. A shortcoming in Anstee (1996) was the lack of appropriate score validity; the extent to which scores rendered consensual agreement and were based on appropriate criteria was not tested. This is of critical importance for ensuring objective, reproducible outcomes and conclusions. Our repeatability analysis showed that activity scores derived from using published mound assessment criteria (Anstee 1996) were not reproducible among investigators. Although all investigators utilised the full range of scores, consistency was rare (Fig. 2), which is suggestive of subjectivity in the assessment criteria. The investigators identified that the criteria were highly subjective in the following three main ways: (i) ‘mound complexity’ was not specifically defined and therefore required interpretation; (ii) there was no consideration of how to average mounds that had structures that were both in ‘disrepair’ and that appeared well-maintained; and (iii) there was a lack of clarity on the definition of some characteristics (e.g. ‘cones fused together’). Critically, whether a mound was classified as active (≥6) or not (≤5) varied among investigators. This result is indicative of a lack of reproducibility, which raises concerns on the outcomes and conclusions that have been derived from mound assessments performed.
Our statistical analyses showed that there was also a lack of congruency in mound status (active or not active) as determined by the mound activity scores (estimation) and camera detections (actuality). The paired plots indicated that there were cases where the mound assessment produced the wrong outcome (in both directions; Fig. 5). When there was congruency, this was most often when mounds were active, which is also reflected in an association between activity scores and number of camera detections (Fig. 6). This result suggests that a high activity score may be useful for estimating heavily visited or used mounds. When considering how variable the activity on high-scoring mounds was detected to be (e.g. scores 5 to 8 in Fig. 4b–e), it is apparent that this prediction is not infallible. Indeed, variation in detected activity was highly variable among mounds of all activity scores (Fig. 4b–e, S3).
Although high camera detection rates may be indicative of mound ‘occupancy’, here we can only speak of ‘activity’ or ‘presence’. In Anstee (1996), ‘activity’, ‘presence’ and ‘occupancy’ were used interchangeably (Anstee 1996), but in fact these terms mean different things. Occupancy implies that an individual has fidelity to a specific mound and ‘occupies’ it on a regular basis (Anstee et al. 1997). Additionally, there was discussion of the scoring system defining ‘mound occupancy’, but it was stated that the ‘presence or absence’ of western pebble mice ‘was verified by intensive trapping and radio-tracking [details not provided] of mice on the mound’ (Anstee 1996, p. 432). It was later explained that ‘Trapping was conducted for 5 consecutive nights at each mound’ (Anstee 1996, p. 432). Our investigation indicated that this is insufficient trapping effort to effectively determine whether a burrow-mound system is active owing to the presence of (or being occupied by) pebble mice (Fig. 3). We found that mound activity typically occurs over a burst of a few nights following a lull period of no activity over several days to weeks (Fig. 3). The significance of low levels of activity on mounds should not be underestimated. Female western pebble mice are reported to routinely visit and maintain neighbouring mounds that are in close proximity to their home mound (Anstee et al. 1997; Start et al. 2000) in preparation for use by locally dispersing offspring (Firman et al. 2019). Our observations have indicated that males do not show fidelity to a specific mound (R. Firman, unpubl. data), which supports evidence that male offspring disperse further from the natal mound than do female offspring (Firman et al. 2019). Thus, ‘empty’ mounds are likely to play a critical role in western pebble mouse population viability and persistence by providing essential refuge for breeding females and males as they move across the landscape.
Overall, our investigation demonstrated that the use of external mound structures cannot reliably predict whether or not a western pebble mouse mound is active. For future monitoring of this species, we recommend that a form of trapping, either live capture or camera trapping, is performed to establish presence or absence of western pebble mouse. Further, our research here has shown that a single camera in a fixed position may not always be sufficient for detecting activity, but multiple cameras, ideally placed at different heights, are necessary to confidently determine the activity status of a mound. On the basis of what we now know about western pebble mouse activity across days and weeks, it will also be important to ensure that trapping is performed over a sufficient timeline. Our data showed that activity occurs in lulls and peaks, and that trapping should be conducted over several weeks to accurately assess the activity status of a mound. It is important to note that our investigation was conducted from June to September when the highest activity levels are expected because of breeding activity (R. Firman, unpubl. data). Over these months, camera trapping for 2–3 weeks seems appropriate. Camera trapping at different times of the year has shown that activity can be extremely low, especially during the summer months (R. Firman, unpubl. data). Therefore, extended monitoring periods of 4–6 weeks will likely be necessary to accurately assess mound activity status outside of this peak period of activity (i.e. between October and May).
Conclusions
The western pebble mouse is an understudied, elusive species that resides in a hotspot of mining activity. The species has been monitored through environmental surveys associated with mining activity; however, for the most part this information is unavailable. In these surveys, the external appearance of the western pebble mouse mounds is often applied as a proxy for identifying whether the mound is ‘occupied’, or pebble mice are present within the area. Here, we provided a rigorous test of a mound activity scoring system that was designed to act as a substitute for trapping on the basis that trapping is a ‘time-consuming process requiring several nights of effort’ (Anstee 1996, p. 429). Despite a lack of objective assessment, it was concluded that the scoring system was an ‘effective predictor of the presence of P. chapmani in pebble mounds’ (Anstee 1996, p. 429). Our investigation suggests that this is not the case. We found that (i) mound scores were not reproducible among investigators and (ii) mound scores did not consistently predict whether mounds were active or not. We did find that high levels of activity tended to be associated with higher numbers of camera detections. However, we also found that there is a large degree of variation among mounds that achieve the same activity scores. We suggest that mounds not heavily used presently may still represent critical resources in the future. We provide guidelines for the purpose that mound activity is adequately assessed in the future. Our hope is that these guidelines will provide quality data that will be used to inform conservation managers when it comes to defining the status of this important Pilbara ecoengineer.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This investigation falls under the Pilbara Pebble Mouse Project, which is funded by an Australian Research Council Discovery Project (DP220103484) awarded to R. C. Firman and D. R. Rubenstein.
Acknowledgements
The authors acknowledge that the Banjima People are the Traditional Owners of the land on which this research was conducted; we thank them for supporting our work and allowing this research to be conducted on Banjima Country. We are extremely thankful to the Department of Biodiversity, Conservation and Attractions (DBCA) Parks and Wildlife Karijini Rangers, especially Jay Crossman and Steph Whitehouse, for their unwavering support and assistance with our pebble mouse investigations.
References
Anstee SD (1996) Use of external mound structures as indicators of the presence of the pebble-mound mouse, Pseudomys chapmani, in mound systems. Wildlife Research 23, 429-434.
| Crossref | Google Scholar |
Anstee SD, Roberts JD, O’Shea JE (1997) Social structure and patterns of movement of the western pebble-mound mouse, Pseudomys chapmani, at Marandoo, Western Australia. Wildlife Research 24, 295-305.
| Crossref | Google Scholar |
Browne E, Driessen MM, Ross R, Roach M, Carver S (2021) Environmental suitability of bare-nosed wombat burrows for Sarcoptes scabiei. International Journal for Parasitology: Parasites and Wildlife 16, 37-47.
| Crossref | Google Scholar | PubMed |
Davidson AD, Lightfoot DC (2008) Burrowing rodents increase landscape heterogeneity in a desert grassland. Journal of Arid Environments 72(7), 1133-1145.
| Crossref | Google Scholar |
Firman RC, Rubenstein DR (2025) The thermal benefits of a mound-burrow system in a semi-desert Australian landscape: will this pebble fortress provide refuge from climate change? bioRxiv 2025, 2025.05.14.654155 [Preprint, published 18 May 2025].
| Crossref | Google Scholar |
Firman RC, Ottewell KM, Fisher DO, Tedeschi JN (2019) Range-wide genetic structure of a cooperative mouse in a semi-arid zone: evidence for panmixia. Journal of Evolutionary Biology 32, 1014-1026.
| Crossref | Google Scholar | PubMed |
Fleming PA, Anderson H, Prendergast AS, Bretz MR, Valentine LE, Hardy GES (2014) Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function? Mammal Review 44, 94-108.
| Crossref | Google Scholar |
Fuller A, Hetem RS, Maloney SK, Mitchell D (2014) Adaptation to heat and water shortage in large, arid-zone mammals. Physiology 29(3), 159-167.
| Crossref | Google Scholar | PubMed |
Ivan JS, Newkirk ES (2016) CPW Photo Warehouse: a custom database to facilitate archiving, identifying, summarizing and managing photo data collected from camera traps. Methods in Ecology and Evolution 7, 499-504.
| Crossref | Google Scholar |
Kinlaw A (1999) A review of burrowing by semi-fossorial vertebrates in arid environments. Journal of Arid Environments 41(2), 127-145.
| Crossref | Google Scholar |
Knuckey C, Heidrich A (2014) Fortescue Metals Group Ltd, Investigator Project. Terrestrial Vertebrate Fauna Assessment. (Fortescue Metals Group: Perth, WA, Australia) Available at https://library.dbca.wa.gov.au/FullTextFiles/925442.pdf
Lacher TE, Jr, Davidson AD, Fleming TH, Gómez-Ruiz EP, McCracken GF, Owen-Smith N, Peres CA, Vander Wall SB (2019) The functional roles of mammals in ecosystems. Journal of Mammalogy 100(3), 942-964.
| Crossref | Google Scholar |
Mirzabaev A, Wu J, Evans J, García-Oliva F, Hussein IAG, Iqbal MH, Kimutai J, Knowles T, Meza F, Nedjraoui D, Tena F, Türkeş M, Vázquez RJ, Weltz M (2019) Desertification. In ‘Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems’. (Eds PR Shukla, J Skea, E Calvo Buendia, V Masson-Delmotte, H-O Pörtner, DC Roberts, P Zhai, R Slade, S Connors, R van Diemen, M Ferrat, E Haughey, S Luz, S Neogi, M Pathak, J Petzold, J Portugal Pereira, P Vyas, E Huntley, K Kissick, M Belkacemi, J Malley) pp. 249–343. (Intergovernmental Panel on Climate Change) doi:10.1017/9781009157988.005
Newkirk ES (2016) CPW Photo Warehouse. (Colorado Parks and Wildlife: Fort Collins, CO, USA) Available at http://cpw.state.co.us/learn/Pages/ResearchMammalSofware.aspx
Sinclair L, Coe NM (2024) Critical mineral strategies in Australia: Industrial upgrading without environmental or social upgrading. Resources Policy 91, 104860.
| Crossref | Google Scholar |
Start AN, Anstee SD, Endersby M (2000) A review of the biology and conservation status of the Ngadji, Pseudomys chapmani Kitchener, 1980 (Rodentia: Muridae). CALMScience 3, 125-147.
| Google Scholar |
Walsberg GE (2000) Small mammals in hot deserts: some generalizations revisited. BioScience 50(2), 109-120.
| Crossref | Google Scholar |