Chum dine with me: assessing the effects of wildlife tourism on non-target fish assemblages
Sasha K. Whitmarsh A * , Thomas M. Clarke
A * , Thomas M. Clarke  B , Mollie Owens B , Jamie Hicks C , Danny Brock C , Caitlin J. Fox B , Lauren Meyer B and Charlie Huveneers B
B , Mollie Owens B , Jamie Hicks C , Danny Brock C , Caitlin J. Fox B , Lauren Meyer B and Charlie Huveneers B
A
B
C
Abstract
Wildlife tourism is becoming increasingly popular and often uses bait and berley to attract the target species and enhance customer experience. However, few studies have assessed the effects of food-based attractants on non-target species. Understanding the impacts of provisioning on the marine ecosystem, including non-target species, is required to assess the effects of wildlife tourism comprehensively.
We compared fish assemblages at a white shark cage-diving site to those at other offshore islands to assess whether any detectable differences could be observed and attributed to shark diving operations.
We used baited remote underwater video stations (BRUVS) to quantify and compare fish assemblages across a 6-year timespan at the Neptunes Islands Group (South Australia) and six reference locations. The Neptunes Islands Group consist of one high provisioning site, where most (~85%) of the bait and berley input takes place, and one lower provisioning site, where operators visit less frequently.
Fish assemblages at the Neptunes Islands Group and all other offshore islands had similar levels of variability. There was a higher total abundance of fish at both Neptune Islands sites than at reference sites, driven by higher abundances of horseshoe leatherjacket, Meuschenia hippocrepis, and barber perch, Caesioperca rasor. However, there were no detectable differences in species richness or evenness between the Neptunes Islands Group and other offshore islands.
Despite operators using food-based attractant on a near daily basis and some fishes feeding on bait and berley continuously, the cage-diving industry has minimal effects on demersal fish abundance and diversity.
This suggests that the current level of provisioning has limited ecological impacts on the reef fish community and highlights that the management regulations are currently suitable for non-target fish assemblages.
Keywords: anthropogenic stressor, baited cameras, BRUVS, cage-diving, fish assemblages, offshore island, shark tourism, temperate fish, white shark.
Introduction
Wildlife tourism is popular worldwide (Trave et al. 2017; Patroni et al. 2018), with the variety of opportunities to view and interact with wildlife growing rapidly, partly owing to the global growth of protected habitats and increased accessibility to remote areas where wildlife occurs (Orams 2002). When managed sustainably, it can provide substantial socio-economic benefits by offering a non-consumptive use of wildlife and contributes up to ~US$45 billion in annual global revenue (Rizzolo 2021). Wildlife tourism also provides employment opportunities for the local community, supports innovative research (Buckley 2009), and raises awareness for conservation and education (Apps et al. 2018).
Tourists’ expectations for memorable moments and unique experiences often relies on up-close encounters with wildlife (Hausmann et al. 2018), leading to increased pressure for operators to provide consistent and prolonged interactions with wild animals (Orams 2002). As a result, tourism operators target species that naturally aggregate and often use an attractant to provide up-close viewings (Knight 2009). Food-based attractants are the most common form of provisioning for wildlife tourism (Meyer et al. 2022) and can have various impacts on target species (Orams 2002; Patroni et al. 2018), but also on non-target species (Rizzari et al. 2017; Clarke et al. 2022, 2023; Dennis et al., in review). Food-based attractants can have various effects on fishes, including increased abundance of dominant species (Milazzo et al. 2005; Feitosa et al. 2012; Prinz et al. 2020); decreased diversity (Ilarri et al. 2008); changed distribution, daily movements, and residency, by enticing fish to occupy areas nearer the food source (Rizzari et al. 2017; Wen et al. 2019; Clarke et al. 2022; Pini-Fitzsimmons et al. 2023); habituation behaviour (Cole 1994; Hémery and McClanahan 2005; Milazzo et al. 2005; Albuquerque et al. 2014; Wen et al. 2019); aggression towards conspecifics and other species (Milazzo et al. 2005; Ilarri et al. 2008; Cruz de Paula et al. 2018; Wen et al. 2019) including humans (Hémery and McClanahan 2005; Ilarri et al. 2008; Cruz de Paula et al. 2018); anxiousness (Geffroy et al. 2018; Wen et al. 2019); or decreased foraging on natural preys (Prinz et al. 2020). For example, bread is regularly used to feed fish in the Cook Islands and has resulted in decreased richness during feeding times with an increase in abundance of certain trophic groups, leading to potential species dominance and reducing evenness (Prinz et al. 2020). Similarly, a study in the Abrolhos Bank also noted an increase in fish abundance at fish feeding sites, with some species dominating the assemblages (Feitosa et al. 2012). These studies highlight the changes fish feeding can have on community structures and by extension ecosystem function.
Delivering unnatural food source to fish through food-based provisioning can also affect fish health and condition through increased risk of crateriform lesions in the stomach wall, hepatic carcinomas, acarid parasites, and papillomas (Brookhouse et al. 2013) because of overhandling by tourists, increased exposure to bacteria, viruses, and fungi, and increased parasite loads causing lesions. Fish in feeding areas often have higher levels of corticosterone and cortisol, the two major stress hormones (Geffroy et al. 2018). Understanding the implications and health risks of food-based provisioning during wildlife tourism is vital for management strategies and tourism regulations, as well as being required for marine protected area and conservation strategies because many areas and species targeted are of conservation importance.
While provisioning aims to attract target (i.e. focal) species, non-target species can also be attracted and feed on bait. For example, non-target rodents, rabbits, and hares consumed up to 98% of the feed used to entice deer and quail into the viewing or hunting area (Donalty et al. 2003). Non-target fish and rays also consume food used to attract sharks (Meyer et al. 2020; Clarke et al. 2022, 2023; Dennis et al. 2024). Fish that inhabit the area but do not directly feed on the bait and berley can also be affected, owing to increased predation risk from more aggressive predators (Milazzo et al. 2006), which creates food competition (Prinz et al. 2020), and theft of local resources (Cruz de Paula et al. 2018). Despite the potential influence of provisioning on non-target species, most studies assessing the impacts of wildlife tourism have predominantly focussed on target species, with only 7% of wildlife tourism studies assessing the effects on non-target species or the broader ecosystem (Trave et al. 2017). Management frameworks have also identified the need to account for the impacts of feeding on non-target species to ensure a comprehensive assessment of the sustainability of wildlife tourism industries (Higginbottom et al. 2003; Meyer et al. 2021).
Shark-based tourism is conducted across the globe and often involves provisioning activities (Cisneros-Montemayor et al. 2013; Araujo et al. 2017). In Australia, the shark-diving industry generates at least AU$47 million per year (Huveneers et al. 2017), with white shark tourism in South Australia generating the second-largest revenue after the snorkelling with whale shark industry off Ningaloo Reef, Western Australia. White shark, Carcharodon carcharias, cage-diving is a sought-after tourism experience, because of their roles as top predators, threatened conservation status, and low probability of seeing them naturally in the wild (Huveneers et al. 2017). This form of tourism occurs in five countries, namely, Australia, New Zealand, South Africa, the United States of America (USA), and Canada, with all but the USA using food-based attractants (i.e. bait and berley) to bring white shark in proximity of the underwater cages. Mexico also previously offered white shark cage-diving experiences, but this industry closed in 2023 as a result of permitting restrictions on operators (Sosa-Nishizaki 2023). Similarly, the South African cage-diving industry has become unreliable as a result of orca predations displacing white sharks from the known aggregations (Towner et al. 2022). As a result, this has likely increased demand on the New Zealand and South Australian cage-diving locations for both tourists and documentary productions. The Neptune Islands Group Marine Park is currently the only location where white shark cage-diving is permitted in Australia. Two licensed operators are permitted to use food-based attractants. Both operators use tethered pieces of southern bluefin tuna, Thunnus maccoyii (referred to as bait), and berley (minced T. maccoyii) to attract white sharks to the cage-diving vessels. This bait and berley are sustainably sourced from the local tuna fisheries and is part of the bioregion’s natural fauna. Despite the industry targeting white sharks, smaller particles of bait and berley disperse into the water column and are being fed on by non-target species, with potential implications on the health and behaviour of these individuals (Meyer et al. 2020; Dennis 2024). Diet analysis indicated that several fish species feed on the bait and berley, including trevally, Pseudocaranx spp., horseshoe leatherjackets, Meuschenia hippocrepis, and yellowtail kingfish, Seriola lalandi, alongside visual observations of behavioural change, for example, demersal species coming from ~25 m deep to the surface to feed or species being attracted to the boat even before berley is released (Meyer et al. 2020). Other studies have found that operations lead to changes in the daily residency and movements of yellowtail kingfish at the Neptune Islands, in addition to raised activity during operating times (Clarke et al. 2022, 2023). However, body condition of yellowtail kingfish at the Neptune Islands was comparable to individuals from other control sites of similar oceanographic conditions. This suggests that behaviour changes did not lead to overall changes in body condition or raised energy expenditure was compensated by feeding on the southern bluefin tuna used as bait. Of the eight species assessed by Meyer et al. (2020), four showed different diets between the tourism and control sites. Whether this translates to effects on the abundance and composition of fish assemblages remains unknown, but has been identified as a research priority to assess the sustainability and acceptability of this industry (Meyer et al. 2021).
Our study serves as the first assessment of whether the food-based provisioning occurring at the Neptune Islands affects fish assemblages. Specifically, we tested whether food-based provisioning alters the overall fish assemblage, increases abundance of species that feed on the bait and berley, or decrease the number of species that either do not feed on the bait or could be prey of species attracted to the bait. We also assessed the evenness of the assemblage which, along with abundance and number of species, provides an indication of whether the bait and berley leads to some species dominating the assemblage. We assessed fish at two sites with different amount of food-based provisioning being used and compared those to six reference sites that have no berley or bait input.
Materials and methods
Study sites
The South Australian white shark cage-diving industry is restricted to the Neptune Islands Group (also known as the Ron and Valerie Taylor) Marine Park, which encompasses two island groups located ~70 km south of Port Lincoln, namely, North Neptune Islands Group (35.3375°S, E 136.1199°E; hereafter North Neptunes) and South Neptune Islands Group (35.3375°S, E 136.1199°E; hereafter South Neptunes) situated 11 km south of North Neptunes (Fig. 1). At these two island groups, two companies use a near-constant plume of food-based attractants, i.e. berley (minced southern bluefin tuna, Thunnus maccoyii, products, such as blood, offal, and oil) and tethered baits (gills and stomachs, or small ~3–5 kg body sections of T. maccoyii), to attract white sharks into the view of tourists (Huveneers et al. 2018). White shark cage-diving primarily (~85% of the time) occurs at North Neptunes, owing to it being closest to Port Lincoln, having reliable sightings historically, and to suitable anchoring locations in most weather conditions (Meyer et al. 2020). Because of the small size of the Neptune Islands, we could not consider any accessible areas to be unaffected by provisioning activities and, thus, chose reference locations at other island groups. This allowed us to develop a study with two levels of provisioning (high at North Neptunes, and low at South Neptunes), along with multiple reference locations that can be used to assess the natural variability in fish assemblages and how those assemblages compare to potentially affected assemblages at the Neptune Islands.
Map showing the study sites and their respective marine bioregions. Esri Service Layer Credits: Esri, TomTom, Garmin, The Food and Agriculture Organization of the United Nations, National Oceanic and Atmospheric Administration, The United States Geological Survey.
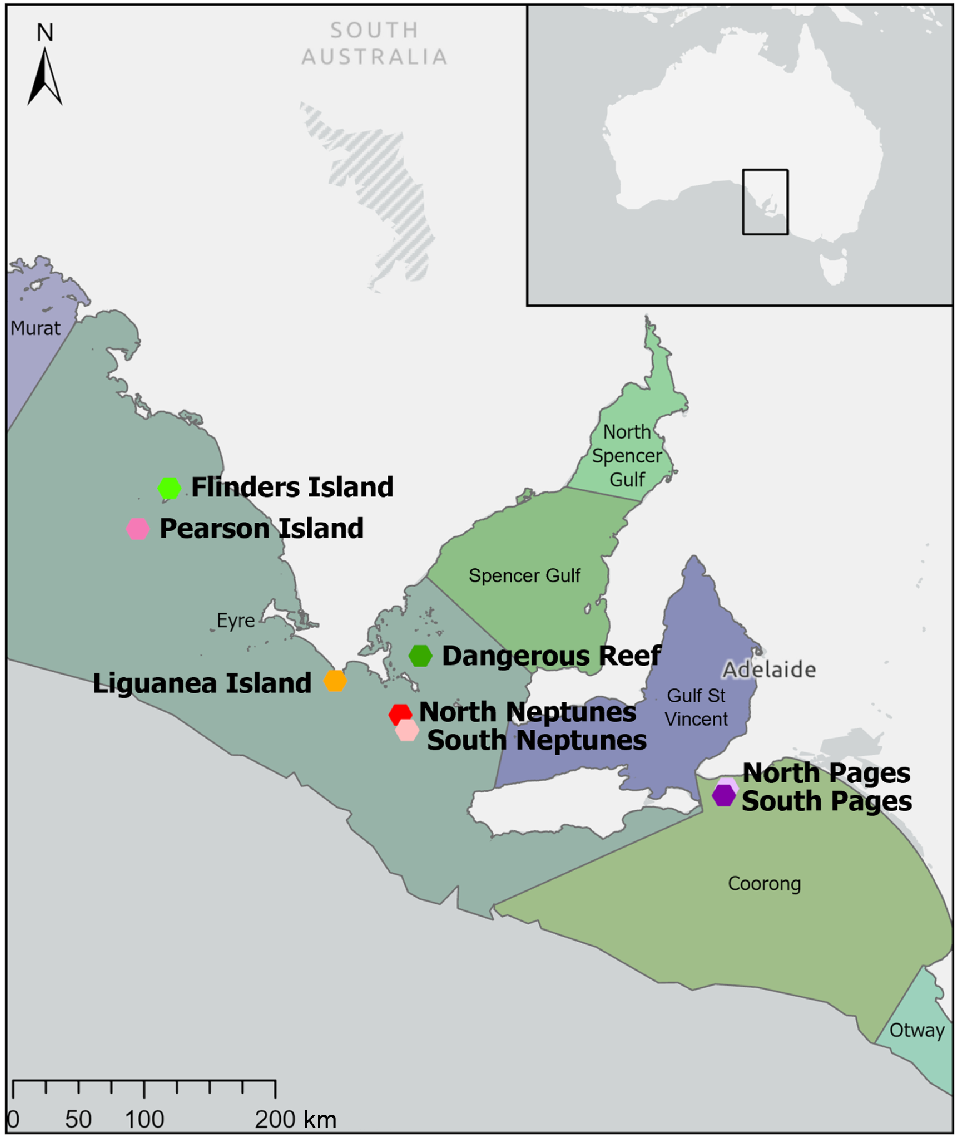
Fish assemblages were also recorded at the following six reference offshore islands with similar habitat and oceanographic conditions (Fig. 1), where commercial shark cage-diving is prohibited: Dangerous Reef (34.8156°S, 136.2125°E); Liguanea Island (34.9895°S, 135.6214°E); The Pages (North and South) (35.7771°S, 138.2919°E); Flinders Island (33.7166°S, 134.4842°E), and Pearson Island (33.9617°S, 134.2663°E). All locations, except for North and South Pages, are within the Eyre Bioregion of South Australia. The Pages (North and South) are within the Coorong Bioregion, but they are also temperate offshore islands with habitat structure similar to that in all other locations (i.e. macroalgae-dominated reefs and seagrass meadows) and have breeding populations of pinnipeds similar to those in the Neptune Islands (Goldsworthy and Page 2009). All locations should therefore contain similar assemblages of fishes and allow for comparisons of abundance and diversity to determine the influence of feeding from cage-diving operations.
Baited remote underwater video stations
We observed fish richness and abundance by using baited remote underwater video stations (BRUVS). BRUVS are a standard tool, used to quantify fish assemblages in a robust, non-invasive, and cost-effective manner (Whitmarsh et al. 2017; Langlois et al. 2020). BRUVS have previously been used for a broad range of environmental purposes, which includes reviewing the effectiveness of marine protected areas, understanding the spatial variation among fish assemblages, and determining human-induced anthropogenic influences (Whitmarsh et al. 2014, 2017; Clarke et al. 2019). Although BRUVS limitations have previously been documented (e.g. bias towards less cryptic species) (Langlois et al. 2010; Harvey et al. 2012; Whitmarsh et al. 2017), they are well suited to sample mobile species within reef and sand habitats (Langlois et al. 2020).
Our study used mono-BRUVS or stereo-BRUVS, which consisted of a large metal frame enclosing one or two cameras (usually GoPro 7+ Black edition) in waterproof housing, mounted horizontally to the ocean floor and set to record in 1080p at 60 or 30 frames per second with a wide field of view. A bait arm and a bait bag extended in front of the GoPro and was baited with 500 g of crushed sardines, Sardinops sagax. Units were deployed on the seafloor for a minimum of 1 h during daylight hours and set to continuous recording. The number of deployments varied among locations and habitat types as follows: North Neptunes (n = 70), South Neptunes (n = 55), Liguanea Island (n = 5), Dangerous Reef (n = 54), Pearson Island (n = 20), Flinders Island (n = 12), South Pages (n = 65), and North Pages (n = 65) (total = 347 BRUVS deployments). The low replication at Liguanea Island was due to logistical challenges of sampling at that site. BRUVS were usually deployed in depths ranging from 20 to 30 m. BRUVS were placed a minimum of 300 m away from each other to reduce the potential for overlapping bait plumes and for individual fish to move between replicates, and thus be recorded by different BRUVS (Langlois et al. 2020).
Video annotation
Videos were annotated using the specialised SeaGIS EventMeasure software (SeaGIS Pty Ltd, Bacchus Marsh, Vic, Australia; seagis.com.au/event.html). For each BRUVS deployment, taxa including fish, large mobile invertebrates (e.g. cephalopods and decapods), and marine mammals were identified to the lowest taxonomic level (Kuiter 1996; Gomon et al. 2008) and counted using the standard measure of abundance, MaxN. MaxN is the maximum number of individuals of a given species detected in a single frame throughout the duration of the deployment (Ellis and DeMartini 1995; Willis and Babcock 2000). This results in a conservative estimate of abundance, because it reduces the likelihood of counting the same individual twice (Langlois et al. 2020).
Habitat description
The habitat of each deployment was assessed using BenthoBox (https://benthobox.com/), which uses a 20-point 5 × 4 grid overlay set onto each still image. For each grid point, the broad habitat type (i.e. macroalgae, seagrass, invertebrate complex, consolidated [i.e. rocky bottom], or unconsolidated [i.e. sand/rubble]) was applied following the CATAMI classification scheme (Althaus et al. 2015). The dominant habitat (>50%) was recorded for grids that contained multiple habitat types.
Statistical analysis
Statistical analysis was performed using PRIMER (ver. 7, https://www.primer-e.com/software; Clarke and Gorley 2015) with the PERMANOVA+ add-on (Anderson et al. 2008). The data were first visualised via shade plots to assess the need for transformation (Clarke et al. 2014). Data were transformed using standard dispersion weighting by location, which accommodates for the schooling behaviour of fish species and down-weighs the influence of species that are highly abundant (Clarke et al. 2006) and, from this, a Bray–Curtis similarity matrix was constructed. To account for temporal variability, year was included as a covariate in PERMANOVA designs. The analysis design included a two-factor multivariate PERMANOVA, including impact (fixed factor, two levels – North and South Neptunes in one group vs all other Islands) and location (fixed factor, eight levels, nested within impact) and habitat (fixed factor, four levels). The discriminant function test of canonical analysis of principal coordinates (CAP) was first used on the full data set to visualise assemblages in a multivariate space and assess differences among groups for location and habitat. SIMilarity PERcentage (SIMPER) analyses were also used to assess the similarity between pairs of islands. Univariate total species abundance, richness, and evenness per replicate were analysed separately by using a PERMANOVA with a Euclidean resemblance matrix to test for differences using the above design.
To test whether bait and berley from cage-diving operations influenced key species, we conducted univariate analyses on the trevally, Pseudocaranx spp., horseshoe leatherjacket, Meuschenia hippocrepis, bluethroat wrasse, Notolabrus tetricus, and barber perch, Caesioperca rasor. These species were chosen owing to being common species across all study locations, in high abundances across the temperate southern coast of South Australia (Gomon et al. 2008), and also because of interactions with cage-diving vessels where they have been observed feeding on the bait and berley (Dennis 2024). Each species was analysed separately by using the same PERMANOVA design tested above, with a Euclidean resemblance matrix to test for differences among the island groups. We wanted to test whether the abundance of each species was significantly different between the Neptune Islands and the reference locations.
Results
Assemblage structure
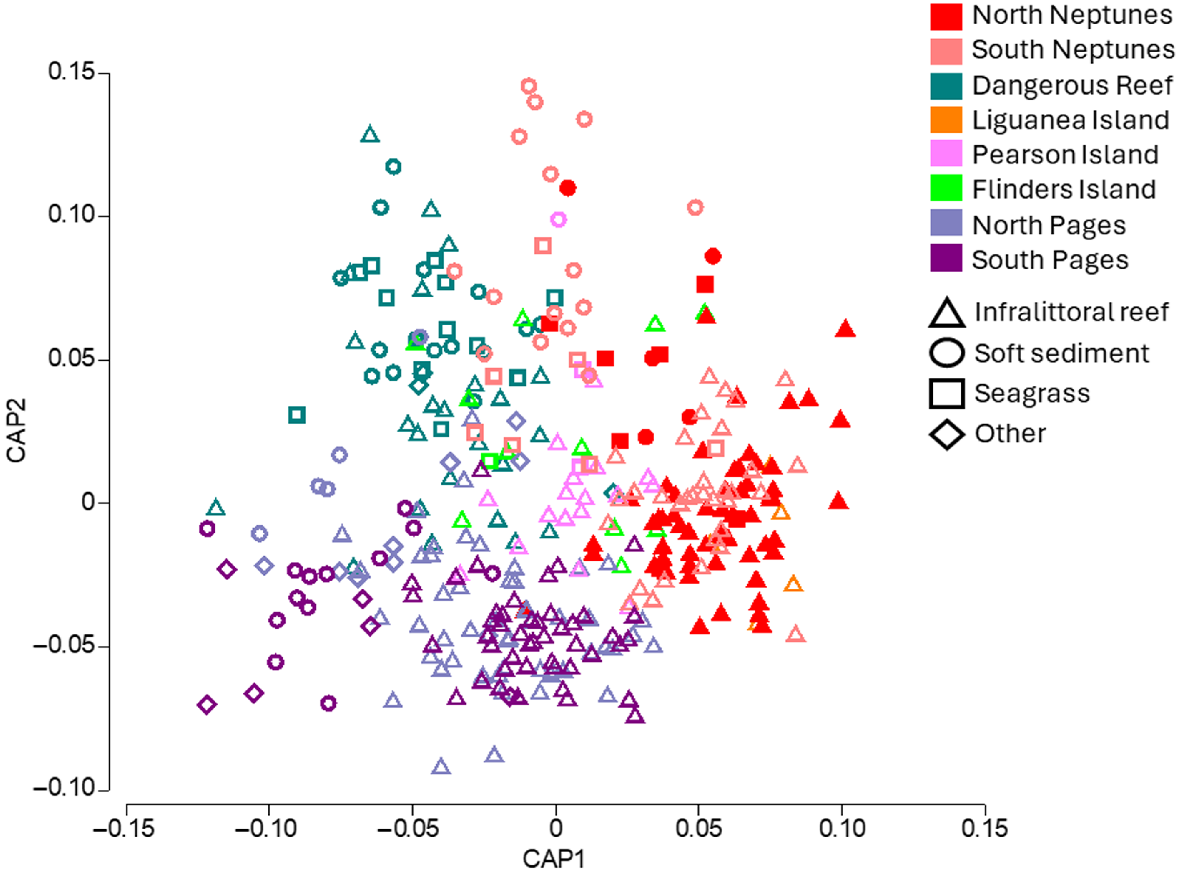
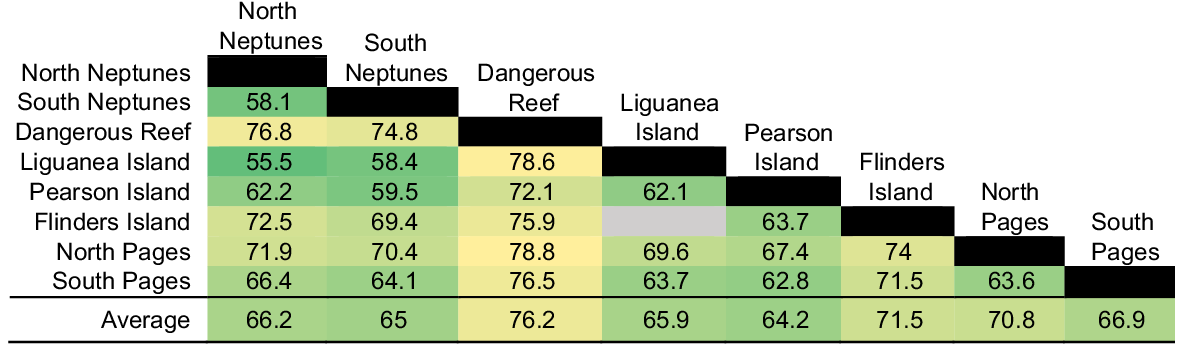
Each offshore island had different assemblage structures and were typically most similar to geographic neighbours (Fig. 2). We found that North and South Neptunes had significant overlap in assemblages, along with their closest neighbour Liguanea Island. Pearson Island and its closest neighbour Flinders Island also had a significant overlap. Dangerous Reef situated further north of the Neptunes and being more sheltered within the Spencer Gulf had a relatively distinct assemblages compared to other locations which had more overlap. Sites furthest to the east, i.e. North and South Pages, also had significant overlap and were the most distinct from the Neptunes. These were the only two islands sampled within in a separate bioregion from that of Neptunes. We observed significant differences between provisioning and reference areas for the fish assemblage across all habitat types (Table 1), but all island groups were equivalently distinct from each other for reef locations (Fig. 3). Because of lower numbers of deployments in other habitat types, e.g. sand, similarity values are not shown.
CAP ordination plot showing differences in fish assemblages among locations and habitat types. Choice of m = 30. Mis-classification error = 52%. Trace and delta P = 0.001.
| Factor | d.f. | MS | Pseudo-F | P | |
|---|---|---|---|---|---|
| Year (covariate) | 1 | 9752.2 | 4.36 | 0.001 | |
| Impact | 1 | 53,197 | 23.77 | 0.001 | |
| Habitat | 3 | 45,051 | 20.13 | 0.001 | |
| Location (impact) | 6 | 11,398 | 5.09 | 0.001 | |
| Impact × habitat A | 2 | 11,467 | 5.12 | 0.001 | |
| Location (impact) × habitat | 9 | 5576 | 2.49 | 0.001 | |
| Residual | 323 | 2238.2 |
Year was included as a covariate, unique permutations ranged from 996 to 999. Significant values are shown in bold. Pairwise tests for the interaction of impact and habitat were significant (P = 0.001) for all habitat types (except ‘other’ which did not run).
SIMPER dissimilarity values showing differences between location pairs for reef-only deployments (larger number represent more dissimilar pairs). Green indicates highly significant (P < 0.001), whereas yellow indicates significance of between P = 0 001–0.05. Grey indicates that no test was performed because of low sample size.
Abundance
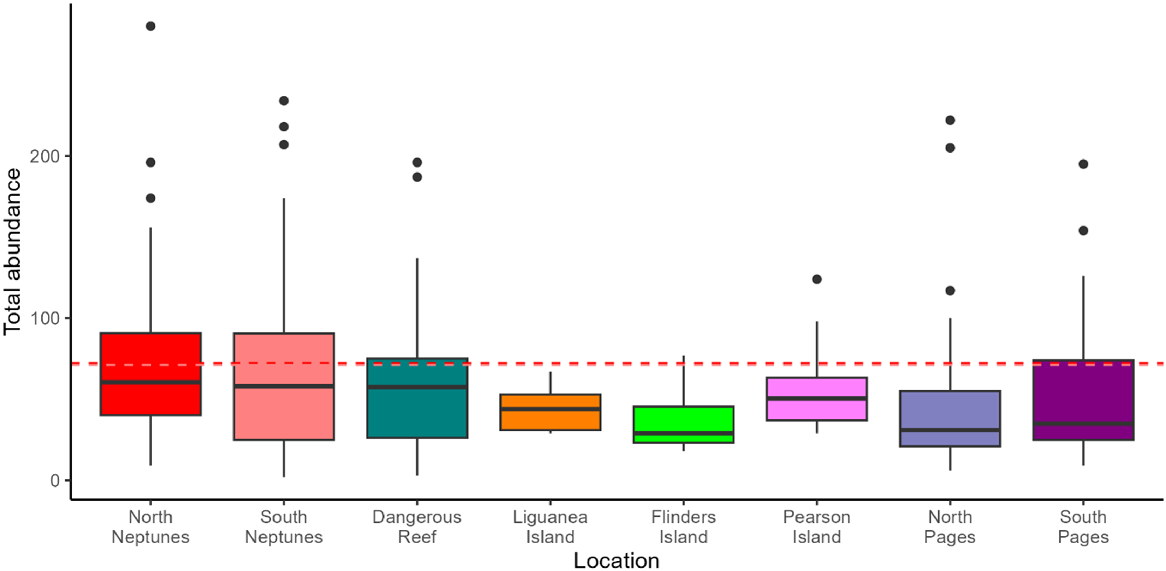
We found the average total abundance at North Neptunes (high provisioning; 72 ± 5.7 individuals per deployment) and South Neptunes (low provisioning; 71 ± 7.5) to be significantly higher than that of reference areas (no provisioning; 37–57 ± 4.6–7.1) (Table 2; Fig. 4).
| Factor | d.f. | Total abundance | Species richness | Evenness (J′) | ||||
|---|---|---|---|---|---|---|---|---|
| Pseudo-F | P | Pseudo-F | P | Pseudo-F | P | |||
| Year (covariate) | 1 | 43.1 | 0.001 | 0.1 | 0.815 | 25.2 | 0.001 | |
| Impact | 1 | 18.5 | 0.001 | 3.1 | 0.072 | 2.3 | 0.107 | |
| Habitat | 3 | 10.0 | 0.001 | 30.9 | 0.001 | 2.8 | 0.573 | |
| Location (impact) | 6 | 1.5 | 0.191 | 8.7 | 0.001 | 0.9 | 0.042 | |
| Impact × habitatA | 21 | 1.4 | 0.255 | 0.04 | 0.961 | 2.3 | 0.42 | |
| Location (impact) ×habitat | 9 | 0.4 | 0.888 | 3.2 | 0.001 | 1.1 | 0.011 | |
| Residual | 323 | |||||||
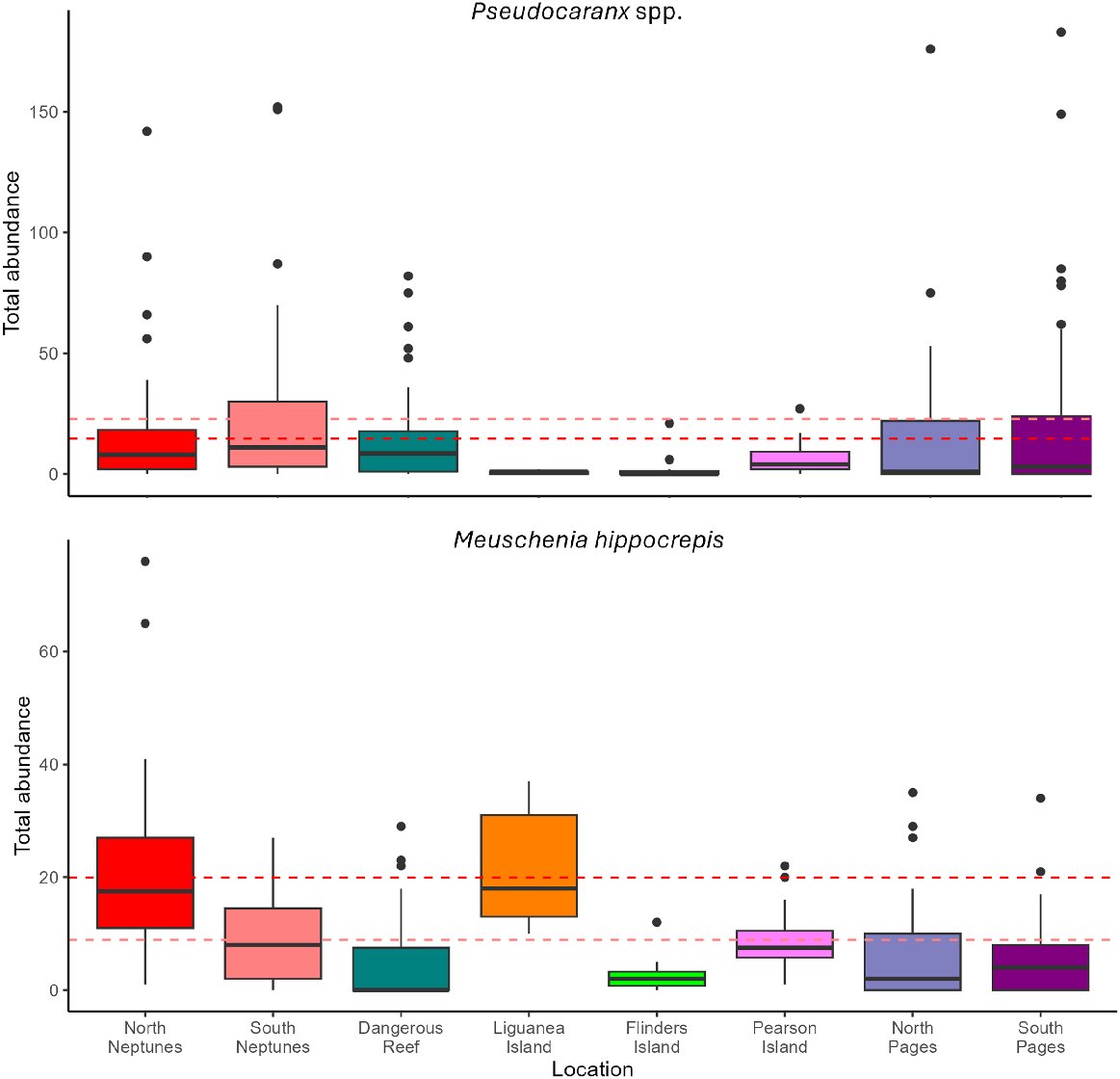
Large schools (~50–200 individuals) of trevally, Pseudocaranx spp., were observed sporadically at most sites, with smaller schools being more frequently observed at the Neptunes and The Pages (Fig. 5). Overall, trevally, Pseudocaranx spp., were observed on 73% of deployments, with a total of 5316 individuals being observed. We observed no significant differences in the abundances of trevally, Pseudocaranx spp., between provisioning and reference locations (Table 3).
Boxplots showing differences among locations for abundance for trevally, Pseudocaranx spp. (top), and horseshoe leatherjacket, Meuschenia hippocrepis (bottom), observed on BRUVS deployments from all habitat types. The dashed lines show the mean value for North Neptunes (red) and South Neptunes (light red).
| Factor | d.f. | Pseudocaranx spp. | Meuschenia hippocrepis | Notolabrus tetricus | Caesioperca rasor | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-F | P | Pseudo-F | P | Pseudo-F | P | Pseudo-F | P | |||
| Year (covariate) | 1 | 27.3 | 0.001 | 21.1 | 0.001 | 1.3 | 0.258 | 26.6 | 0.001 | |
| Impact | 1 | 0.8 | 0.38 | 109.8 | 0.001 | 0.1 | 0.786 | 41.6 | 0.001 | |
| Habitat | 3 | 0.9 | 0.438 | 33.4 | 0.001 | 21.2 | 0.001 | 6.3 | 0.005 | |
| Location (impact) | 6 | 1.5 | 0.182 | 13.1 | 0.001 | 0.5 | 0.762 | 4.0 | 0.005 | |
| Impact × habitatA | 2 | 1.7 | 0.183 | 1.5 | 0.214 | 3.4 | 0.036 | 6.5 | 0.004 | |
| Location (impact) × habitat | 9 | 0.8 | 0.493 | 0.4 | 0.886 | 0.3 | 0.962 | 0.3 | 0.949 | |
| Residual | 323 | |||||||||
| Provisioning vs reference | ||||||||||
| Infralittoral reef | – | – | – | – | 1.07 | 0.267 | 6.6 | 0.001 | ||
| Soft sediment | – | – | – | – | 3.4 | 0.001 | 0.3 | 0.806 | ||
| Seagrass | – | – | – | – | 0.9 | 0.336 | 0.3 | 0.783 | ||
Two sites had high abundances of horseshoe leatherjacket, Meuschenia hippocrepis (up to 76 individuals per BRUVS), namely, North Neptunes (high provisioning site) and Liguanea Island (reference site) (Fig. 5). The high abundance at North Neptunes contributed to the significant difference observed between provisioning and reference locations (Table 3). All other sites had comparable abundances of M. hippocrepis.
All sites had similar abundances of blue-throat wrasse, Notolabrus tetricus, with abundances ranging from 0 to 38 individuals per replicate. We observed no significant differences between reference and provisioning locations for reef and seagrass areas but did observe significantly higher numbers of N. tetricus in soft sediment provisioning sites than in reference locations (Fig. 6, Table 3).
Boxplots showing differences among locations for abundance of blue-throat wrasse, Notolabrus tetricus (left), and barber perch, Caesioperca rasor (right), observed on BRUVS deployments in reef and sand habitats (seagrass and other habitats are not shown because of low deployment numbers and non-significance). The dashed lines show the mean value for North Neptunes (red) and South Neptunes (light red).
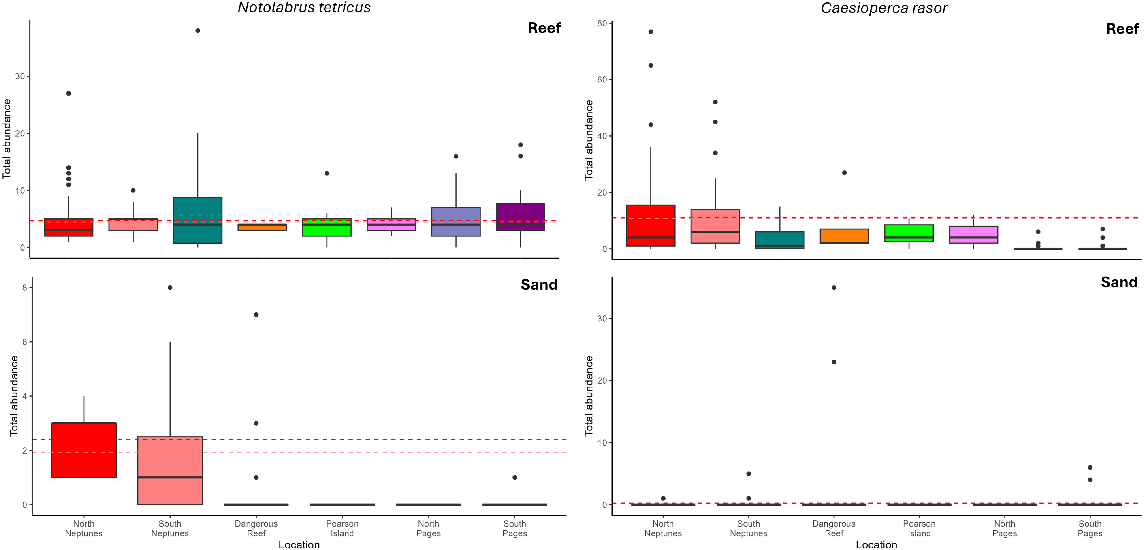
North and South Neptunes frequently had large schools of barber perch, Caesioperca rasor, whereas numbers of individuals were often lower in reference sites and very few individuals were observed at North and South Pages (Fig. 6). The range of individuals observed was from 0 to 77 across all sites. We found a significantly higher abundance of C. rasor in the provisioning sites than at reference sites for reef habitats only (Table 3), which was likely driven by the presence of sporadic large schools at the Neptune Islands. There was no significant difference in the abundance of C. rasor between North and South Neptunes (P = 0.22).
Diversity
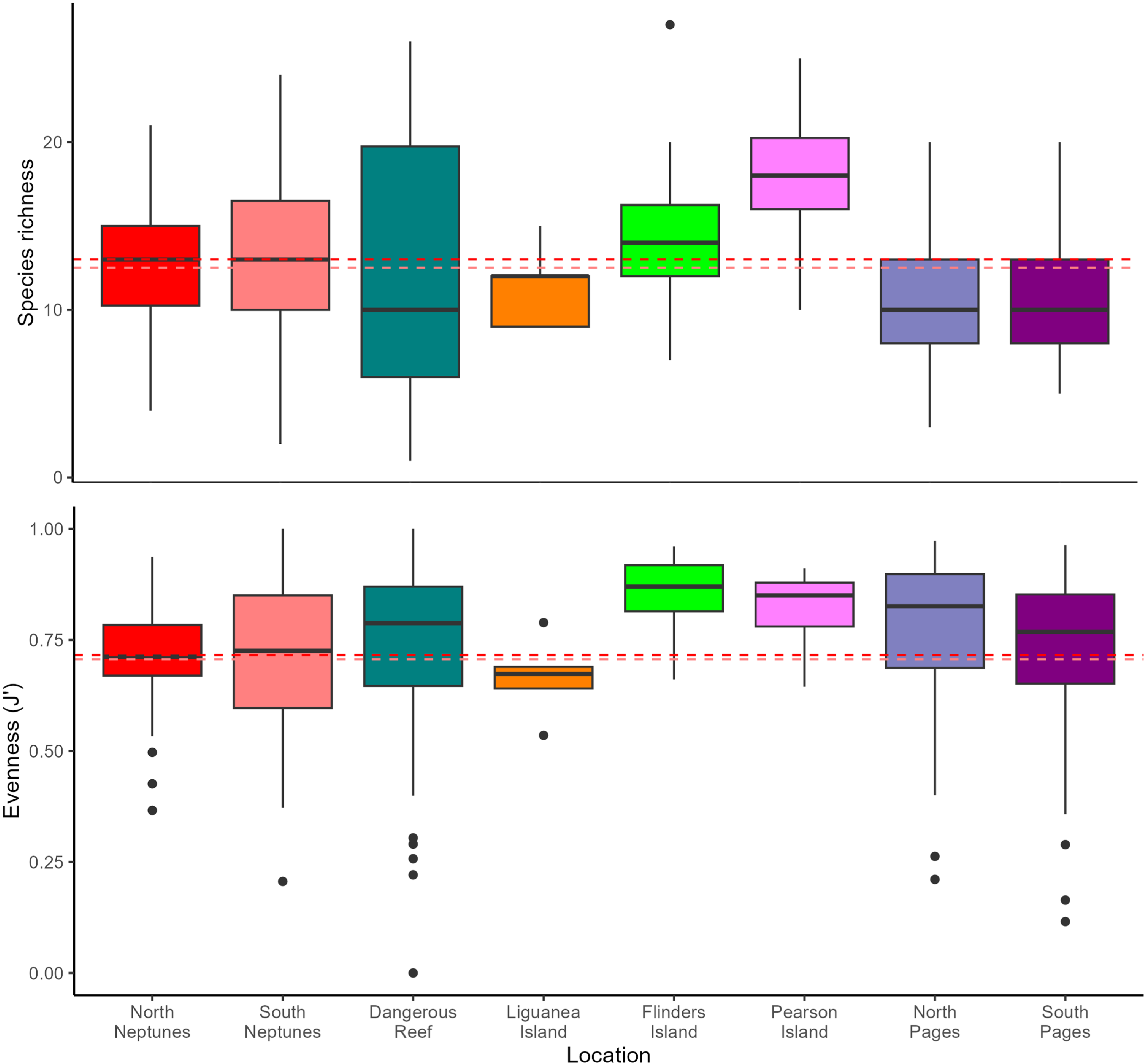
We found that species richness at North Neptunes (high bait and berley input) was similar to that at South Neptunes (low provisioning) and reference areas (no provisioning) with an average of 13 ± 0.4 taxa per deployment, compared with 12 ± 0.7 and 11–18 ± 0.5–1.5 respectively (Fig. 7). There was no significant difference between North and South Neptunes compared with the reference areas (Table 2).
Evenness was also comparable across sites, with North Neptunes (high provisioning) and South Neptunes (low provisioning) similar to reference areas (no provisioning), with an average J of 0.72 ± 0.01 and 0.71 ± 0.7, compared with 0.66–0.85 ± 0.02–0.04 respectively (Fig. 7). There was no significant difference between North and South Neptunes compared with the reference areas (Table 2).
Discussion
The use of food-based attractants during wildlife tourism can have a multitude of effects on target species (Trave et al. 2017; Patroni et al. 2018), yet, the impacts on non-target species are often unexplored and difficult to assess. At the Neptune Islands, non-target fish consume the bait and berley used by the South Australian white shark cage-diving industry (Meyer et al. 2020) and this affects fish growth (Dennis et al., in review), daily movements, and residency (Rizzari et al. 2017; Clarke et al. 2022, 2023), prompting an assessment of the effects of this industry on the fish assemblage, abundance, and diversity. Although our study was not specifically designed to test for the effects of provisioning, it serves as an initial assessment of fish communities at temperate offshore islands and can be used to monitor potential changes in the future. Overall, we did not find any evidence suggesting that bait and berley affected fish assemblages at North Neptune Islands. Each offshore island assessed had unique fish assemblages, with those at North Neptunes not being more distinct than other locations. Total fish abundance was slightly higher at North Neptunes but species richness and evenness were comparable to those at other islands. Slight differences in abundance were observed for the individual species analysed, with horseshoe leatherjacket, Meuschenia hippocrepis, having higher abundances at the provisioning locations and barber perch, Caesioperca rasor, and blue-throat wrasse, Notolabrus tetricus, having higher abundances in soft sediment at provisioning sites than at reference locations. Therefore, individual species might be affected by the cage-diving industry. However, the abundance of M. hippocrepis at North Neptunes was similar to that in one of the reference sites, Liguanea Island, indicating that the higher abundance of this species may not be linked to provisioning.
Variations in fish assemblages and species diversity among locations is more likely to be due to natural geographical differences among locations (Gaston et al. 2008). Separate offshore islands have their own environmental and oceanographic conditions, as well as different stressors and conservation measures (Wantiez et al. 1997; Velmurugan 2008), which makes it challenging to disentangle which factors are affecting fish assemblages. For example, the no-take Sanctuary Zone at North Neptunes may have affected the fish assemblage. However, recreational fishing pressure at this island prior to the Sanctuary Zone implementation in 2014 was very low, because this island is ~70 km from the closest boat ramp (Port Lincoln) and requires medium to large vessels that typically do not target reef fishes but focus on pelagic fishes such as southern bluefin tuna, Thunnus maccoyii. Before-after-control-impact (BACI) designs can be an ideal experimental design in ecology, especially those with multiple control locations (Underwood 1994), but as cage-diving started at the Neptune Islands in the early 1980s, information about fish assemblages prior to wildlife tourism is not available. The influence of geographic variation on fish assemblages is highlighted by the similar fish communities at the two geographically closest locations, namely, North and South Pages. Fish assemblages at North and South Pages are also distinct compared with other locations, which can be explained by this island group being in a different bioregion (Coorong bioregion) from the other locations (Eyre bioregion). Similarly, Dangerous Reef also had high dissimilarity values, potentially owing to geographic differences between this island and others (smaller, with less reef habitat). Comparing multiple reference locations helps account for these differing environmental, anthropogenic, and oceanographic conditions at each island group, and allow us to account for natural variation in fish assemblages when assessing whether fish communities at the Neptune Islands have been affected by the bait and berley used when cage-diving.
Large schools of trevally (up to ~3000 individuals), Pseudocaranx spp., can be observed actively feeding on the bait and berley next to the cage-diving boats (Dennis et al. 2024). It has been hypothesised that the large number of Pseudocaranx spp. may act as a buffer species by feeding on bait and berley at the surface, limiting access and consumption of the bait and berley by other species nearer the benthos (Meyer et al. 2020). This was suggested on the basis of a diet study, showing that only Pseudocaranx spp. and yellowtail kingfish, Seriola lalandi, had fatty acid signatures that reflected the consumption of the bait and berley (Meyer et al. 2020) at North Neptunes. In contrast, some benthic species also had fatty acid signatures indicating that the berley may reach to lower levels of the water column at South Neptunes where lower numbers of Pseudocaranx spp. are present at the cage-diving vessel. On the BRUVS, we observed similar abundances of Pseudocaranx spp. among all sites and were not able to detect the large schools dominating assemblages around the cage-diving vessels at North Neptunes. The lack of differences observed might be due to the conservative measure used to quantify abundance (MaxN), which is known to underestimate large abundances because of screen saturation (Stobart et al. 2015; Whitmarsh et al. 2018). As such, MaxN may not be sensitive enough in high-density environments to detect increases in species abundance beyond a maximum observable threshold (Whitmarsh et al. 2018). This would result in a hyper-stable relationship, whereby catchability decreases as true site abundance increases (Kilfoil et al. 2017; Whitmarsh et al. 2018). However, we did not observe large schools of Pseudocaranx spp. that would have saturated the field of view. Our benthic-BRUVS might, therefore, not adequately sample the large Pseudocaranx spp. schools typically seen around the surface at the cage-diving vessels. Although Pseudocaranx spp. are usually reef-associated or bentho-pelagic species, they have a tendency to school mid-water at North Neptunes and are more abundant on pelagic-BRUVS at North Neptunes than at South Neptunes and Dangerous Reef (S. K. Whitmarsh, unpubl. data). This highlights the need for more pelagic-based sampling to encompass pelagic or mid-water species (Rees et al. 2015, 2018; Clarke et al. 2019). However, presence of white sharks at the tourism site makes pelagic sampling challenging because of increased likelihood of interactions with sharks for pelagic-BRUVS than benthic-BRUVS. Further research on the Pseudocaranx spp. at Neptune Islands has been conducted recently, assessing their abundance by using mark–recapture methods, along with the fine-scale movement of individuals, and physiological aspects (Dennis 2024; Dennis et al. 2024). The swim pass method was assessed to be the best method to estimate Pseudocaranx spp. abundance (Dennis et al. 2024). This method lured Pseudocaranx spp. with bait past a camera. The fine-scale movement study also showed that the space use of Pseudocaranx spp. was much smaller in the presence of operators and that most Pseudocaranx spp. were occupying the upper 5 m of the water column (near the operator vessels). These results highlight the difficulty of assessing the Pseudocaranx spp. population by using traditional methods such as BRUVS or trawling, which, despite showing promise in other studies (e.g. Osgood et al. 2019; French et al. 2021), were not effective at capturing the Pseudocaranx spp. in the present study because of the modified behaviour of this species in the presence of operator vessels.
Meuschenia hippocrepis is also regularly observed feeding on bait and berley at the surface where cage-diving occurs (Meyer et al. 2020). Although the natural diet of M. hippocrepis is mostly based on algae and sponges (Jones 1992; Rodgers et al. 2013), it is known to be attracted to fish-based baits and is commonly caught as by-catch in the commercial rock-lobster fishery (Brock et al. 2007; Rodgers et al. 2013). It is, therefore, likely that this species is an opportunistic scavenger that will consume carnivorous baits when available. In addition to observations of M. hippocrepis feeding on bait and berley, biochemical analyses reflected the consumption of the bait and berley at South Neptunes (Meyer et al. 2020). Similar changes in the diet of a benthic feeder in relation to tourism provisioning has previously been shown in threadfin butterfly fish, Chaetodon auriga (Prinz et al. 2020). This species is normally corallivore, feeding on live coral (Cole and Pratchett 2014), but consumed bread during provisioning activities. The abundance of M. hippocrepis followed the expected trend for a species affected by wildlife tourism provisioning, with the highest abundance recorded at North Neptunes, followed by South Neptunes. However, high abundance of M. hippocrepis was also observed at Liguanea Island, and to some extent at Pearson Island, with large schools being sighted at several other locations. Therefore, the bait and berley use at North Neptunes might be affecting the abundance of this species, but not to the point of reaching unnatural levels.
Barber perch, Caesioperca rasor, also showed a response, with significantly higher abundance in reef habitats at the Neptune Islands Group than at other offshore islands. This species schools in large numbers over rocky outcrops and feeds on zooplankton (Gomon et al. 2008). This species has not been observed to feed directly on the bait and berley near the cage-diving vessels. Their increased abundance could be explained by either natural variation and habitat suitability at the Neptune Islands, because they are known to form large schools in other areas (e.g. Wilson’s Promontory in Victoria) (Turner and Norman 1998), or the uptake of bait and berley by other species could potentially increase food availability for C. rasor, therefore increasing their abundance. Trevally, Pseudocaranx spp., consume zooplankton as part of their natural diet (Meyer et al. 2020) and if these individuals are instead consuming bait and berley, there may be less competition for zooplankton, freeing up resources for other species such as C. rasor. Research into the diet of C. rasor at the Neptunes Islands may provide insight into whether they are directly feeding on bait and berley.
Provisioning at the Neptune Islands may also be affecting the behaviour of species, with higher abundances of blue-throat wrasse, Notolabrus tetricus, but only on soft sediment habitats. Provisioning activities may therefore be encouraging species to spend time in untypical areas, because N. tetricus was rarely sighted on soft sediment habitats at the reference sites. Provisioning can change space use and result in individuals inhabiting areas close to the food source that may be outside their usual habitat (Wen et al. 2019; Clarke et al. 2022; Pini-Fitzsimmons et al. 2023). Habitat heterogeneity at the Neptune Islands may have also influenced this result because reef was the primary target for most reference locations, leading to BRUVS deployments on soft sediment being mostly incidental and likely close to reef. Future research should generate detailed seafloor maps such as those derived from multibeam echo-sounders to determine habitat distributions and patch size. This information is critical to better understand the influence of habitat on demersal fish assemblages and how this may have affected our results.
Conclusions
Our study serves as a first look into whether food provisioning associated with the use of bait and berley by the white shark tour operators influences fish assemblages at the Neptune Islands by examining assemblage structure, species abundances, and diversity using demersal BRUVS. We found no evidence that the South Australian white shark cage-diving industry impacted demersal fish assemblages substantially, but it likely contributed to overall high fish abundance, driven by changes from a small number of species. Fish assemblages were distinct among island groups, but the variation between the Neptune Islands Group and the reference locations was similar to that among reference locations, supporting that food provisioning does not markedly alter the structure of the Neptune Islands fish community. Large schools of Pseudocaranx spp. that are commonly observed around the shark-cage diving vessels were not detected on our benthic BRUVS in high abundances, indicating that there may be other effects from food provisioning that we could not quantify. Overall, while some effects were observed, food provisioning at the Neptune Islands does not appear to alter demersal fish assemblages, nor increase the abundance of bait-feeding species beyond natural variability, or decrease species diversity at an ecosystem-wide level. This suggests that the current level of provisioning has limited observable ecological impacts on the demersal fish community and highlights that the management regulations may be suitable for non-target fish assemblages to ensure the sustainability of the white shark cage-diving industry.
Data availability
Data are available upon request of the authors. Metadata are available from https://globalarchive.org/.
Conflicts of interest
Lauren Meyer and Charlie Huveneers are Guest Editors of the ‘White Sharks Global proceedings and recent advances in white shark ecology and conservation’ collection of Wildlife Research but were not involved in the peer review or any decision-making process for this paper. The authors have no further conflicts of interest to declare.
Declaration of funding
Support for this project was provided by the Rodney Fox Shark Expeditions, Holsworth Wildlife Research Endowment, The Department of Environment and Water, and The Nature Foundation of South Australia.
Acknowledgements
We acknowledge the significant contributions and mentorship of the late Professor Peter Fairweather to the initial design and statistical analysis of this study and earlier versions of the paper. We thank the crew of the Princess II and MV Rodney Fox for their assistance with fieldwork, in particular A. Fox, R. John, and J. Parker. We also thank our fieldwork volunteers, especially C. Barry, J. Dennis, C. Roberts, as well as the crew of the Nature Films documentary company, Neiser Foundation, and Breakout Productions.
References
Albuquerque T, Loiola M, Nunes JdACC, Reis-Filho JA, Sampaio CLS, Leduc AOHC (2014) In situ effects of human disturbances on coral reef-fish assemblage structure: temporary and persisting changes are reflected as a result of intensive tourism. Marine and Freshwater Research 66(1), 23-32.
| Crossref | Google Scholar |
Althaus F, Hill N, Ferrari R, Edwards L, Przeslawski R, Schönberg CHL, Stuart-Smith R, Barrett N, Edgar G, Colquhoun J, Tran M, Jordan A, Rees T, Gowlett-Holmes K (2015) A standardised vocabulary for identifying benthic biota and substrata from underwater imagery: the CATAMI classification scheme. PLoS ONE 10(10), e0141039.
| Crossref | Google Scholar | PubMed |
Apps K, Dimmock K, Huveneers C (2018) Turning wildlife experiences into conservation action: can white shark cage-dive tourism influence conservation behaviour? Marine Policy 88, 108-115.
| Crossref | Google Scholar |
Araujo G, Vivier F, Labaja JJ, Hartley D, Ponzo A (2017) Assessing the impacts of tourism on the world’s largest fish Rhincodon typus at Panaon Island, Southern Leyte, Philippines. Aquatic Conservation: Marine and Freshwater Ecosystems 27(5), 986-994.
| Crossref | Google Scholar |
Brock DJ, Hawthorne PJ, Ward TM, Linnane AJ (2007) Two monitoring methods that assess species composition and spatio-temporal trends in bycatch from an important temperate rock lobster (Jasus edwardsii) fishery. Marine and Freshwater Research 58(3), 273-285.
| Crossref | Google Scholar |
Brookhouse N, Bucher DJ, Rose K, Kerr I, Gudge S (2013) Impacts, risks and management of fish feeding at Neds Beach, Lord Howe Island Marine Park, Australia: a case study of how a seemingly innocuous activity can become a serious problem. Journal of Ecotourism 12(3), 165-181.
| Crossref | Google Scholar |
Buckley R (2009) Evaluating the net effects of ecotourism on the environment: a framework, first assessment and future research. Journal of Sustainable Tourism 17(6), 643-672.
| Crossref | Google Scholar |
Cisneros-Montemayor AM, Barnes-Mauthe M, Al-Abdulrazzak D, Navarro-Holm E, Sumaila UR (2013) Global economic value of shark ecotourism: implications for conservation. Oryx 47(3), 381-388.
| Crossref | Google Scholar |
Clarke KR, Chapman MG, Somerfield PJ, Needham HR (2006) Dispersion-based weighting of species counts in assemblage analyses. Marine Ecology Progress Series 320, 11-27.
| Crossref | Google Scholar |
Clarke KR, Tweedley JR, Valesini FJ (2014) Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. Journal of the Marine Biological Association of the United Kingdom 94(1), 1-16.
| Crossref | Google Scholar |
Clarke TM, Whitmarsh SK, Fairweather PG, Huveneers C (2019) Overlap in fish assemblages observed using pelagic and benthic baited remote underwater video stations. Marine & Freshwater Research 70(6), 870-880.
| Crossref | Google Scholar |
Clarke TM, Whitmarsh SK, Dwyer RG, Udyawer V, Pederson H, Huveneers C (2022) Effects of shark tourism on the daily residency and movements of a non-focal pelagic teleost. Marine Ecology Progress Series 687, 133-146.
| Crossref | Google Scholar |
Clarke TM, Whitmarsh SK, Champion C, Pederson H, Meyer L, Dennis JD, Dwyer RG, Huveneers C (2023) Influence of shark tourism on the activity and physiological condition of a non-focal pelagic fish. ICES Journal of Marine Science 80(6), 1670-1682.
| Crossref | Google Scholar |
Cole RG (1994) Abundance, size structure, and diver-oriented behaviour of three large benthic carnivorous fishes in a marine reserve in northeastern New Zealand. Biological Conservation 70(2), 93-99.
| Crossref | Google Scholar |
Cruz de Paula Y, Schiavetti A, Sampaio CL, Calderon E (2018) The effects of fish feeding by visitors on reef fish in a Marine Protected Area open to tourism. Biota Neotropica 18(3), e20170339.
| Crossref | Google Scholar |
Dennis JD, Meyer L, Dudgeon CL, Huveneers C (2024) One fish, two fish, three fish, more: novel resighting method produces precise and cost-effective estimates of abundance. Journal of Fish Biology 105(6), 1603-1613.
| Crossref | Google Scholar | PubMed |
Dennis JD, Meyer L, Grammer G, Smart J, Clarke TM, Davey J, Huveneers C (in review) Impacts of supplemental feeding on the growth, body condition, and fatty acid profiles of a non-focal fish. 10.2139/ssrn.5023181
Donalty S, Henke SE, Kerr CL (2003) Use of winter food plots by nongame wildlife species. Wildlife Society Bulletin 31(3), 774-778.
| Google Scholar |
Ellis DM, DeMartini EE (1995) Evaluation of a video camera technique for indexing abundances of juvenile pink snapper, Pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Oceanographic Literature Review 93(42), 67-77.
| Google Scholar |
Feitosa CV, Chaves LdCT, Ferreira BP, de Araújo ME (2012) Recreational fish feeding inside Brazilian MPAs: impacts on reef fish community structure. Journal of the Marine Biological Association of the United Kingdom 92(7), 1525-1533.
| Crossref | Google Scholar |
French B, Wilson S, Holmes T, Kendrick A, Rule M, Ryan N (2021) Comparing five methods for quantifying abundance and diversity of fish assemblages in seagrass habitat. Ecological Indicators 124, 107415.
| Crossref | Google Scholar |
Gaston KJ, Chown SL, Evans KL (2008) Ecogeographical rules: elements of a synthesis. Journal of Biogeography 35(3), 483-500.
| Crossref | Google Scholar |
Geffroy B, Sadoul B, Bouchareb A, Prigent S, Bourdineaud J-P, Gonzalez-Rey M, Morais RN, Mela M, Nobre Carvalho L, Bessa E (2018) Nature-based tourism elicits a phenotypic shift in the coping abilities of fish. Frontiers in Physiology 9, 13.
| Crossref | Google Scholar |
Harvey ES, Newman SJ, McLean DL, Cappo M, Meeuwig JJ, Skepper CL (2012) Comparison of the relative efficiencies of stereo-BRUVs and traps for sampling tropical continental shelf demersal fishes. Fisheries Research 125–126, 108-120.
| Crossref | Google Scholar |
Hausmann A, Toivonen T, Slotow R, Tenkanen H, Moilanen A, Heikinheimo V, Di Minin E (2018) Social media data can be used to understand tourists’ preferences for nature-based experiences in protected areas. Conservation Letters 11(1), e12343.
| Crossref | Google Scholar |
Hémery G, McClanahan TR (2005) Effect of recreational fish feeding on reef fish community composition and behaviour. Western Indian Ocean Journal of Marine Science 4(2), 123-134.
| Google Scholar |
Higginbottom K, Green R, Northrope C (2003) A framework for managing the negative impacts of wildlife tourism on wildlife. Human Dimensions of Wildlife 8(1), 1-24.
| Crossref | Google Scholar |
Huveneers C, Meekan MG, Apps K, Ferreira LC, Pannell D, Vianna GMS (2017) The economic value of shark-diving tourism in Australia. Reviews in Fish Biology and Fisheries 27(3), 665-680.
| Crossref | Google Scholar |
Huveneers C, Watanabe YY, Payne NL, Semmens JM (2018) Interacting with wildlife tourism increases activity of white sharks. Conservation Physiology 6(1), coy019.
| Crossref | Google Scholar |
Ilarri MDI, de Souza AT, de Medeiros PR, Grempel RG, Rosa IML (2008) Effects of tourist visitation and supplementary feeding on fish assemblage composition on a tropical reef in the Southwestern Atlantic. Neotropical Ichthyology 6, 651-656.
| Crossref | Google Scholar |
Jones GP (1992) Interactions between herbivorous fishes and macro-algae on a temperate rocky reef. Journal of Experimental Marine Biology and Ecology 159(2), 217-235.
| Crossref | Google Scholar |
Kilfoil JP, Wirsing AJ, Campbell MD, Kiszka JJ, Gastrich KR, Heithaus MR, Zhang Y, Bond ME (2017) Baited remote underwater video surveys undercount sharks at high densities: insights from full-spherical camera technologies. Marine Ecology Progress Series 585, 113-121.
| Crossref | Google Scholar |
Knight J (2009) Making wildlife viewable: habituation and attraction. Society and Animals 17(2), 167-184.
| Crossref | Google Scholar |
Langlois TJ, Harvey ES, Fitzpatrick B, Meeuwig JJ, Shedrawi G, Watson DL (2010) Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquatic Biology 9(2), 155.
| Crossref | Google Scholar |
Langlois T, Goetze J, Bond T, Monk J, Abesamis RA, Asher J, Barrett N, Bernard ATF, Bouchet PJ, Birt MJ, Cappo M, Currey-Randall LM, Driessen D, Fairclough DV, Fullwood LAF, Gibbons BA, Harasti D, Heupel MR, Hicks J, Holmes TH, Huveneers C, Ierodiaconou D, Jordan A, Knott NA, Lindfield S, Malcolm HA, McLean D, Meekan M, Miller D, Mitchell PJ, Newman SJ, Radford B, Rolim FA, Saunders BJ, Stowar M, Smith ANH, Travers MJ, Wakefield CB, Whitmarsh SK, Williams J, Harvey ES (2020) A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods in Ecology and Evolution 11(11), 1401-1409.
| Crossref | Google Scholar |
Meyer L, Whitmarsh SK, Nichols PD, Revill AT, Huveneers C (2020) The effects of wildlife tourism provisioning on non-target species. Biological Conservation 241, 108317.
| Crossref | Google Scholar |
Meyer L, Apps K, Bryars S, Clarke T, Hayden B, Pelton G, Simes B, Vaughan LM, Whitmarsh SK, Huveneers C (2021) A multidisciplinary framework to assess the sustainability and acceptability of wildlife tourism operations. Conservation Letters 14(3), e12788.
| Crossref | Google Scholar |
Meyer L, Barry C, Araujo G, Barnett A, Brunnschweiler JM, Chin A, Gallagher A, Healy T, Kock A, Newsome D, Ponzo A, Huveneers C (2022) Redefining provisioning in marine wildlife tourism. Journal of Ecotourism 21(3), 210-229.
| Crossref | Google Scholar |
Milazzo M, Badalamenti F, Vega Fernández T, Chemello R (2005) Effects of fish feeding by snorkellers on the density and size distribution of fishes in a Mediterranean marine protected area. Marine Biology 146(6), 1213-1222.
| Crossref | Google Scholar |
Milazzo M, Anastasi I, Willis TJ (2006) Recreational fish feeding affects coastal fish behavior and increases frequency of predation on damselfish Chromis chromis nests. Marine Ecology – Progress Series 310, 165-172.
| Crossref | Google Scholar |
Orams MB (2002) Feeding wildlife as a tourism attraction: a review of issues and impacts. Tourism Management 23(3), 281-293.
| Crossref | Google Scholar |
Osgood GJ, McCord ME, Baum JK (2019) Using baited remote underwater videos (BRUVs) to characterize chondrichthyan communities in a global biodiversity hotspot. PLoS ONE 14(12), e0225859.
| Crossref | Google Scholar | PubMed |
Patroni J, Simpson G, Newsome D (2018) Feeding wild fish for tourism—a systematic quantitative literature review of impacts and management. International Journal of Tourism Research 20, 286-298.
| Crossref | Google Scholar |
Pini-Fitzsimmons J, Knott NA, Brown C (2023) Recreational fishery discard practices influence use of tidal estuary by a large marine mesopredator. Marine and Freshwater Research 74(4), 320-334.
| Crossref | Google Scholar |
Prinz N, Story R, Lyon S, Ferse SCA, Bejarano S (2020) To feed or not to feed? Coral Reef fish responses to artificial feeding and stakeholder perceptions in the Aitutaki Lagoon, Cook Islands. Frontiers in Marine Science 7, 145.
| Crossref | Google Scholar |
Rees M, Knott N, Fenech G, Davis A (2015) Rules of attraction: enticing pelagic fish to mid-water remote underwater video systems (RUVS). Marine Ecology Progress Series 529, 213-218.
| Crossref | Google Scholar |
Rees MJ, Knott NA, Davis AR (2018) Habitat and seascape patterns drive spatial variability in temperate fish assemblages: implications for marine protected areas. Marine Ecology Progress Series 607, 171-186.
| Crossref | Google Scholar |
Rizzari JR, Semmens JM, Fox A, Huveneers C (2017) Observations of marine wildlife tourism effects on a non-focal species. Journal of Fish Biology 91(3), 981-988.
| Crossref | Google Scholar | PubMed |
Rizzolo JB (2021) Wildlife tourism and consumption. Journal of Sustainable Tourism 31(5), 1181-1194.
| Crossref | Google Scholar |
Rodgers GG, Linnane AJ, Huveneers C (2013) Contrasting diet of two temperate reef fish species (Notolabrus tetricus and Meuschenia hippocrepis) as determined from commercial rock lobster bycatch samples. Transactions of the Royal Society of South Australia 137(1), 80-89.
| Crossref | Google Scholar |
Stobart B, Díaz D, Álvarez F, Alonso C, Mallol S, Goñi R (2015) Performance of baited underwater video: does it underestimate abundance at high population densities? PLoS ONE 10(5), e0127559.
| Crossref | Google Scholar |
Towner AV, Watson RGA, Kock AA, Papastamatiou Y, Sturup M, Gennari E, Baker K, Booth T, Dicken M, Chivell W, Elwen S, Kaschke T, Edwards D, Smale MJ (2022) Fear at the top: killer whale predation drives white shark absence at South Africa’s largest aggregation site. African Journal of Marine Science 44(2), 139-152.
| Crossref | Google Scholar |
Trave C, Brunnschweiler J, Sheaves M, Diedrich A, Barnett A (2017) Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism. Biological Conservation 209(5652), 211-222.
| Crossref | Google Scholar |
Turner ML, Norman MD (1998) Fishes of Wilsons Promontory and Corner Inlet, Victoria: composition and biogeographic affinities. Memoirs of the Museum of Victoria 57(1), 143-165.
| Crossref | Google Scholar |
Underwood AJ (1994) On beyond BACI: Sampling designs that might reliably detect environmental disturbances. Ecological Applications 4(1), 3-15.
| Crossref | Google Scholar |
Velmurugan A (2008) Chapter 1. The nature and characters of tropical islands. In ‘Biodiversity and climate change adaptation in tropical Islands’. (Eds C Sivaperuman, A Velmurugan, AK Singh, I Jaisankar) pp. 3–30. (Academic Press) 10.1016/B978-0-12-813064-3.00001-6
Wantiez L, Thollot P, Kulbicki M (1997) Effects of marine reserves on coral reef fish communities from five islands in New Caledonia. Coral Reefs 16(4), 215-224.
| Crossref | Google Scholar |
Wen CKC, Chen KS, Tung WC, Chao A, Wang CW, Liu SL, Ho MJ (2019) The influence of tourism-based provisioning on fish behavior and benthic composition. Ambio 48(7), 779-789.
| Crossref | Google Scholar | PubMed |
Whitmarsh SK, Fairweather PG, Brock DJ, Miller D (2014) Nektonic assemblages determined from baited underwater video in protected versus unprotected shallow seagrass meadows on Kangaroo Island, South Australia. Marine Ecology Progress Series 503, 205-218.
| Crossref | Google Scholar |
Whitmarsh SK, Fairweather PG, Huveneers C (2017) What is Big BRUVver up to? Methods and uses of baited underwater video. Reviews in Fish Biology and Fisheries 27, 53-73.
| Crossref | Google Scholar |
Whitmarsh SK, Huveneers C, Fairweather PG (2018) What are we missing? Advantages of more than one viewpoint to estimate fish assemblages using baited video. Royal Society Open Science 5(5), 171993.
| Crossref | Google Scholar | PubMed |
Willis TJ, Babcock RC (2000) A baited underwater video system for the determination of relative density of carnivorous reef fish. Marine and Freshwater Research 51(8), 755-763.
| Crossref | Google Scholar |